Seed Germination Behavior, Growth, Physiology and Antioxidant Metabolism of Four Contrasting Cultivars under Combined Drought and Salinity in Soybean
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Plant Materials
2.2. Seeds’ Germination Treatment
2.3. Seedlings’ Treatments
2.4. Seed Germination Calculation
2.5. Fresh and Dry Biomass, Plant Height, and Relative Water Content (RWC) Measurements
2.6. Chlorophyll Fluorescence, Photosynthetic Pigments, and Anthocyanin Content
2.7. Determination of Proline, Sugars, and Free Amino Acid and Soluble Protein
2.8. Lipid Peroxidation and Electrolyte Leakage (EL)
2.9. Determination of Phenol, Flavonoids
2.10. Determination of the Activity Levels of Antioxidant Enzymes
2.11. Statistical Analysis
3. Results
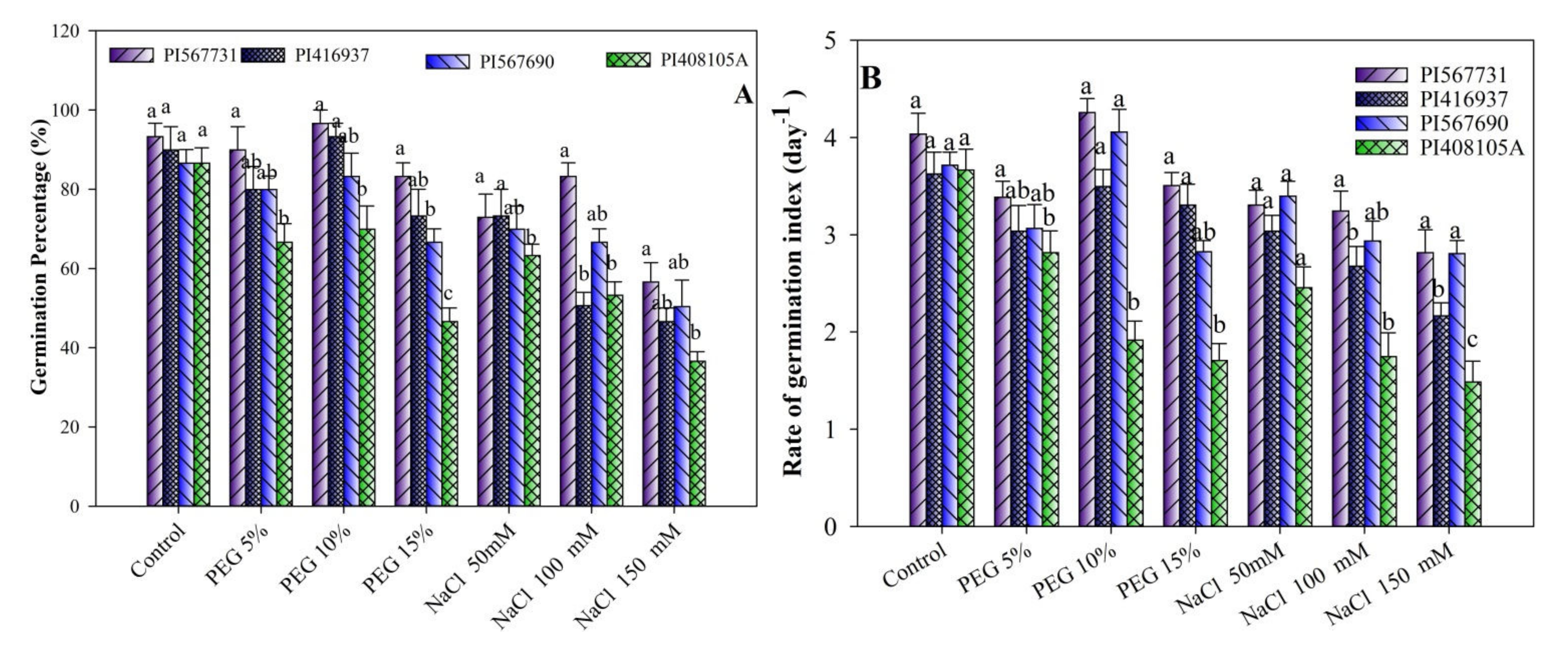
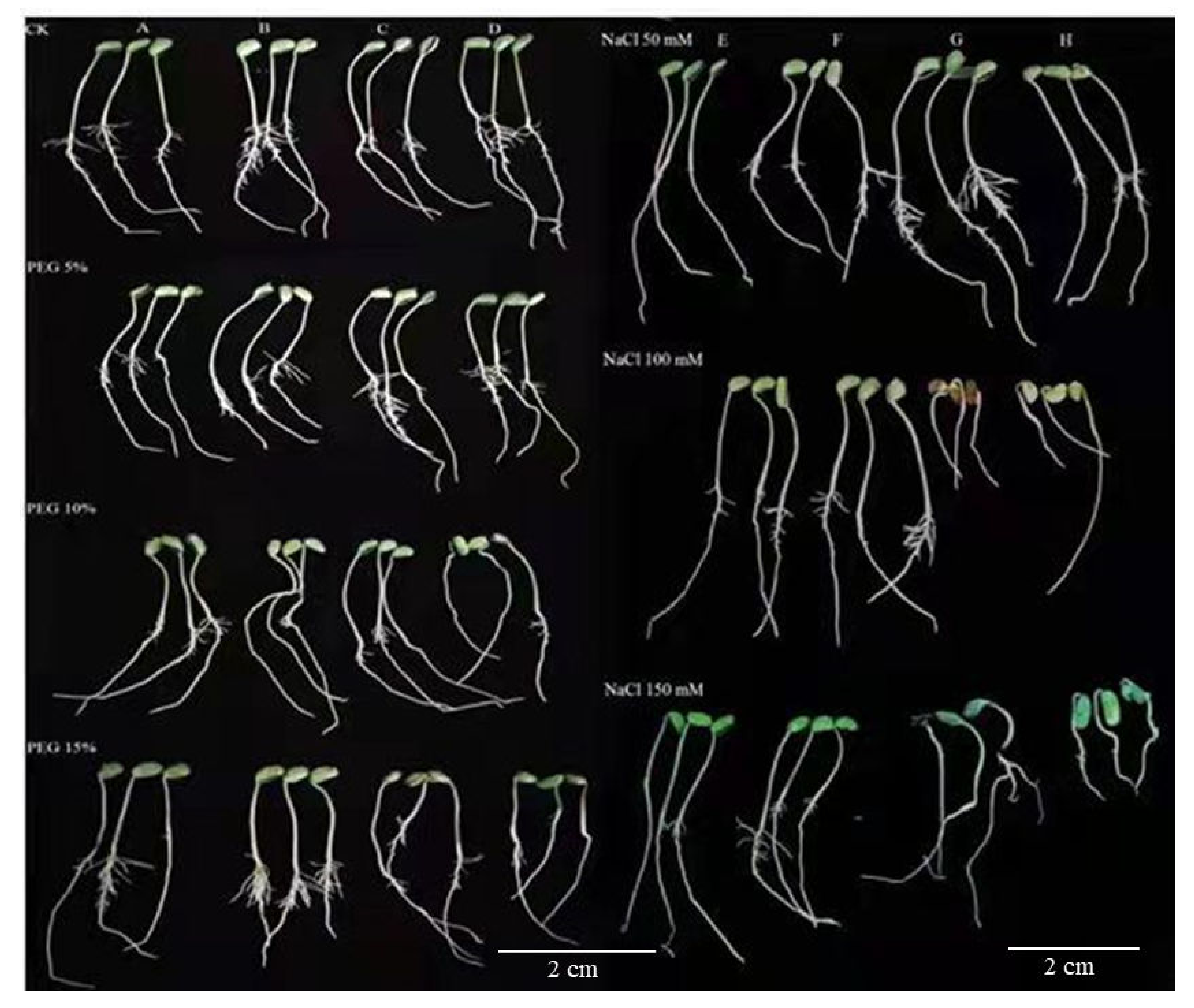
3.1. Seed Germination Behavior under Different Drought and Salinity Conditions
3.2. Germination Energy
3.3. Plant Height and Biomass and RWC under Drought and Salinity Conditions
3.4. Chlorophyll, Carotenoid, and Anthocyanin Content, and Chlorophyll Fluorescence
3.5. Sugars, Proline, Free Amino Acids, and Protein
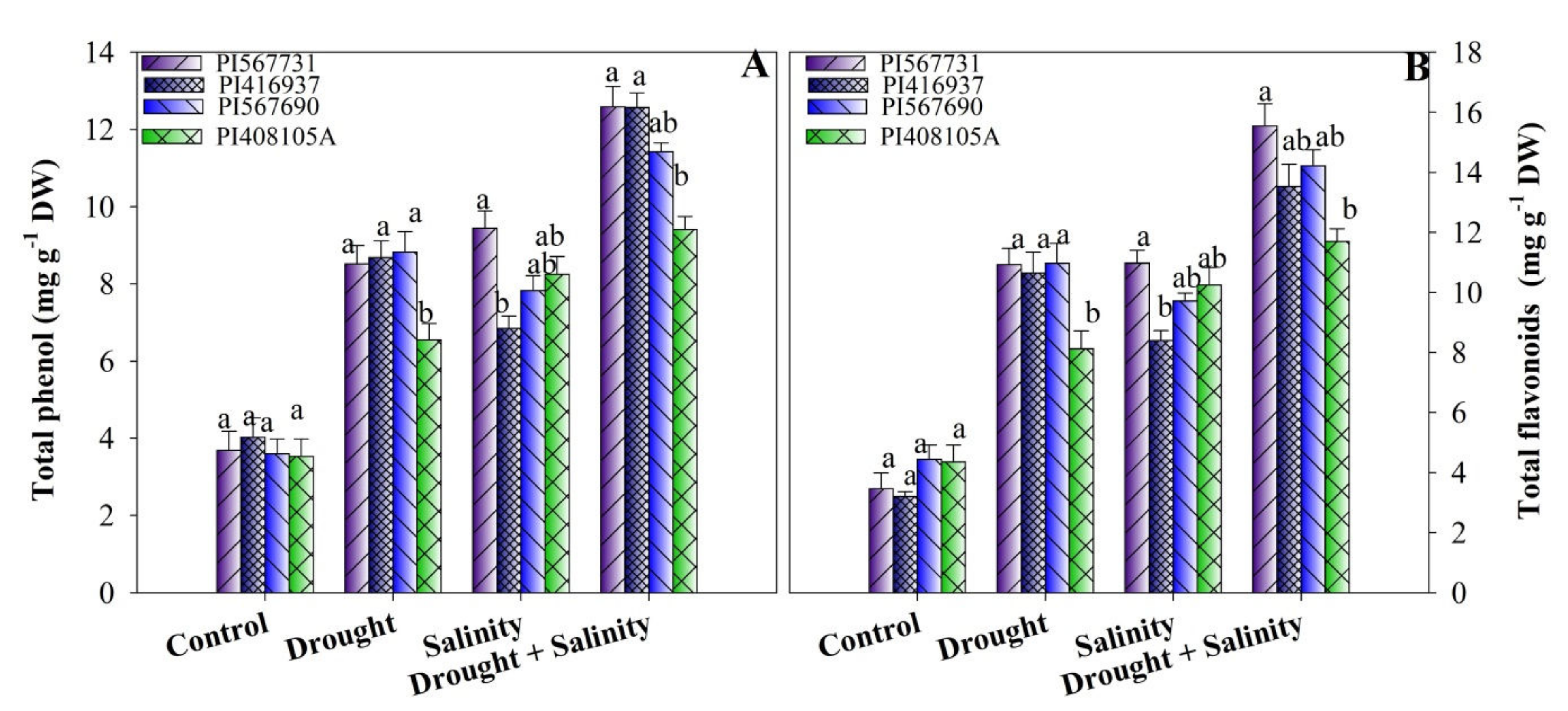
3.6. Total Phenolic and Flavonoid Contents
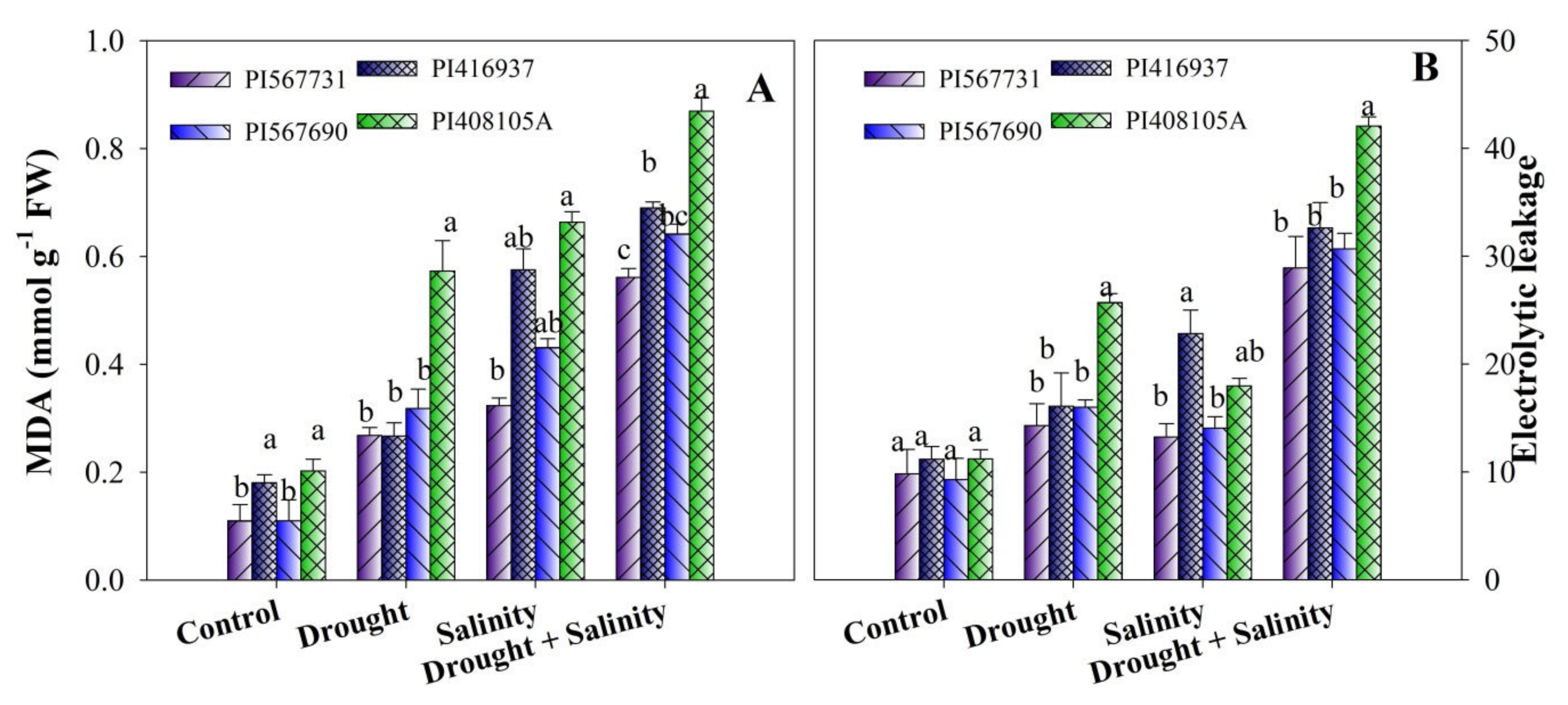
3.7. Oxidative Stress Indicators
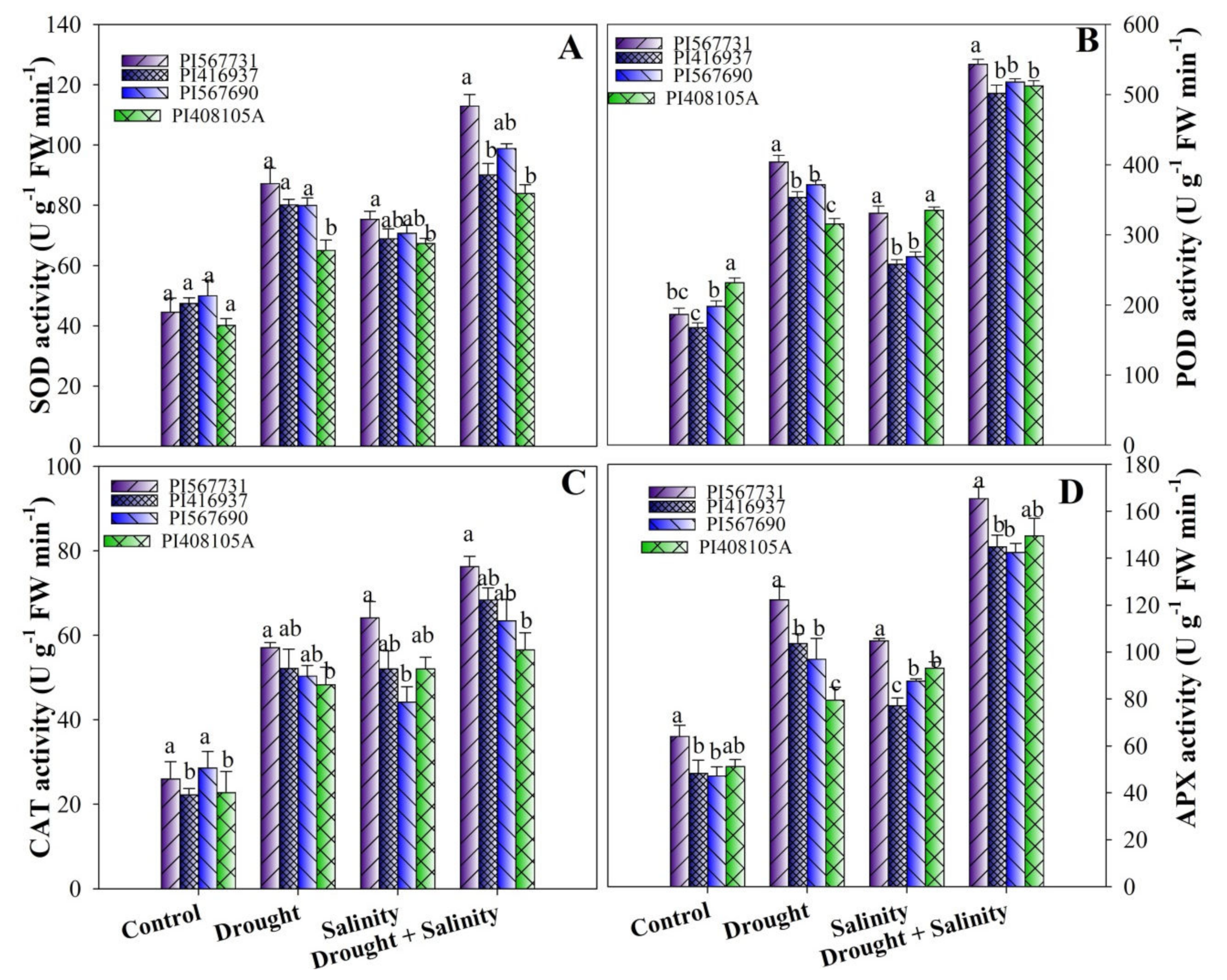
3.8. Activity Levels of Antioxidant Enzymes
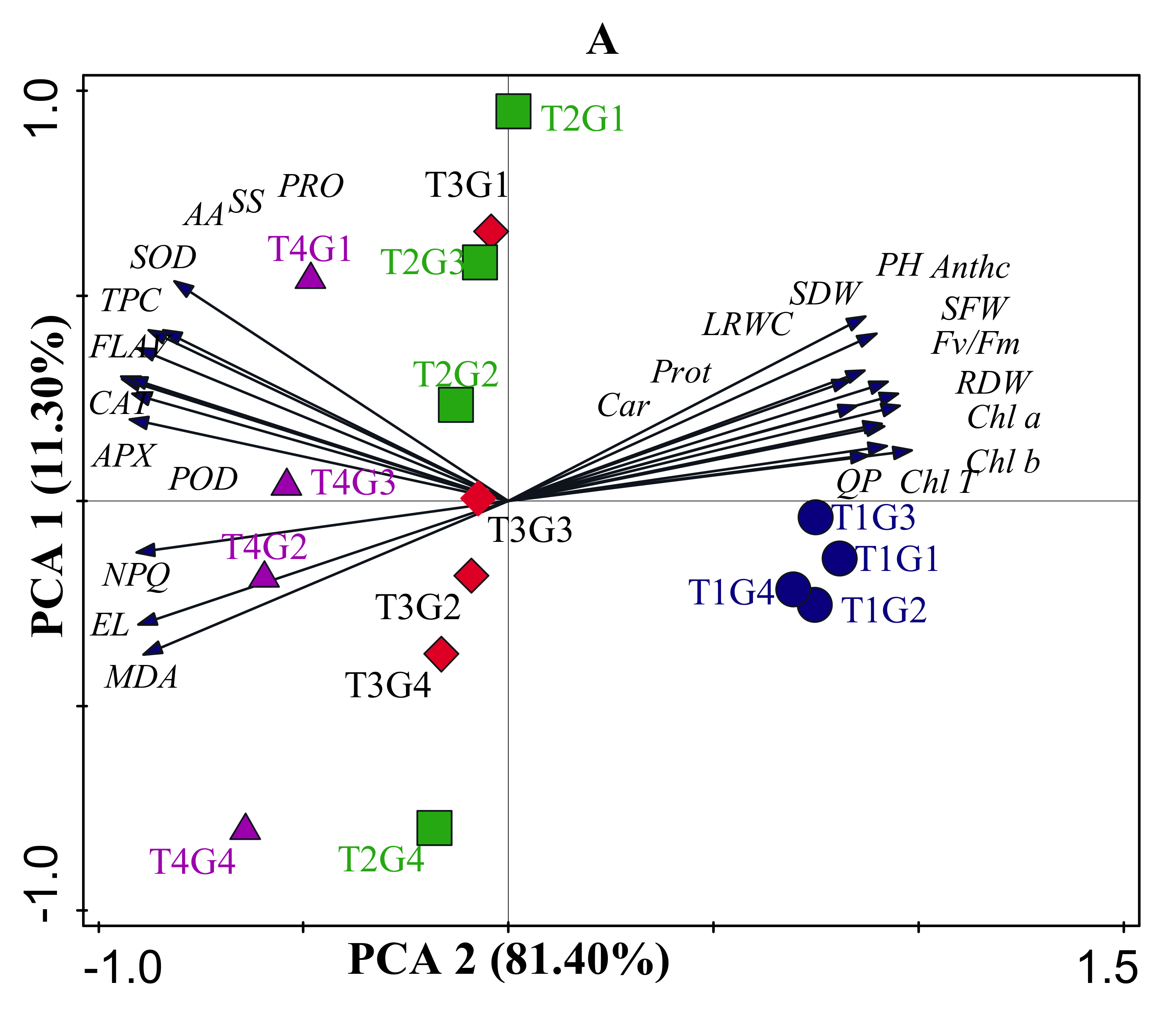
3.9. Relationships among Physiological Traits under Drought Stress, Salinity, and Combined Stress Conditions
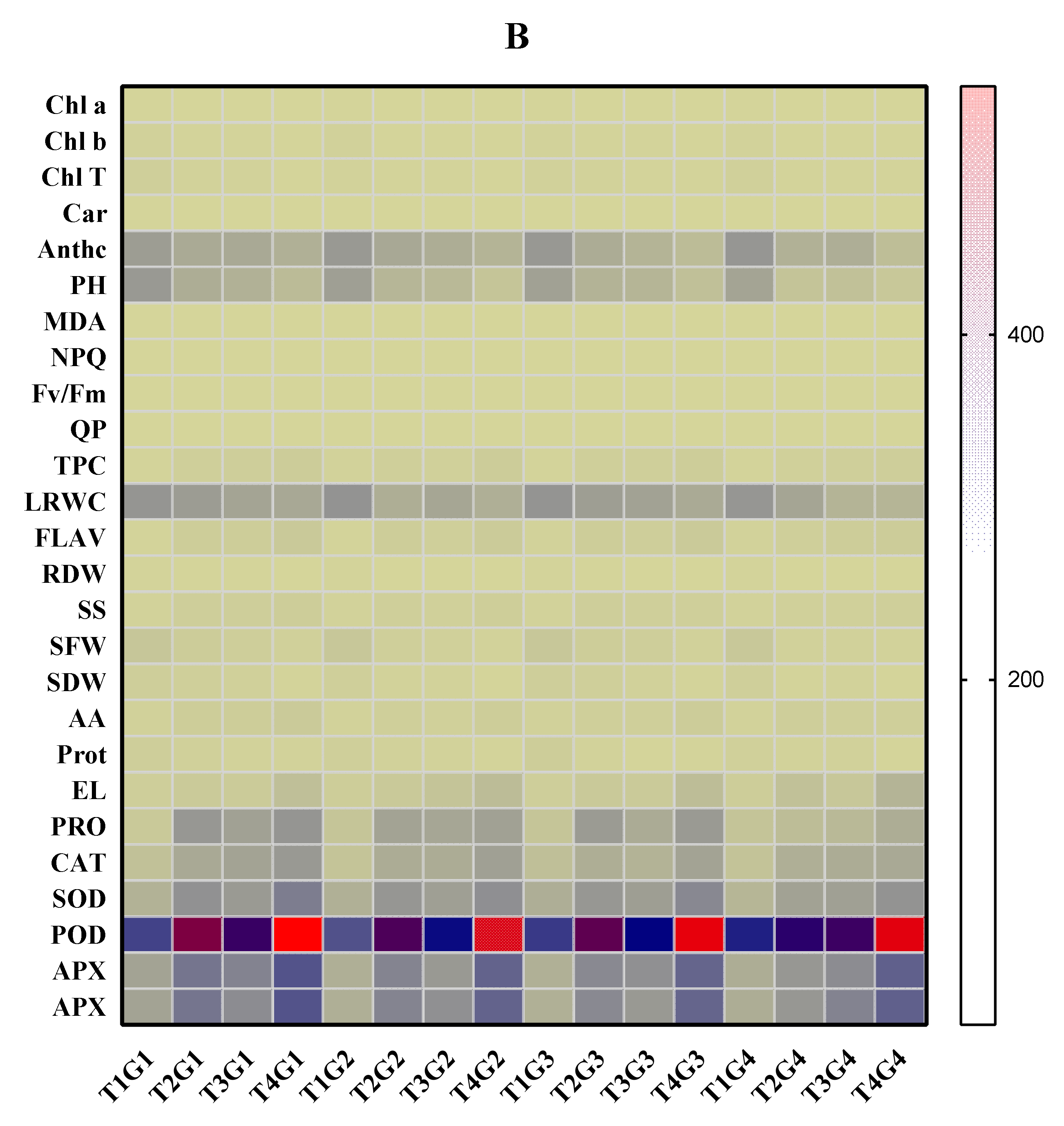
3.10. Correlation Analysis in Soybean Cultivars under Drought Stress, Salinity, and Combined Stress Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Begum, N.; Ahanger, M.A.; Zhang, L. AMF inoculation and phosphorus supplementation alleviates drought induced growth and photosynthetic decline in Nicotiana tabacum by up-regulating antioxidant metabolism and osmolyte accumulation. Environ. Exp. Bot. 2020, 176, 104088. [Google Scholar] [CrossRef]
- Sahin, U.; Ekinci, M.; Ors, S.; Turan, M.; Yildiz, S.; Yildirim, E. Effects of individual and combined effects of salinity and drought on physiological, nutritional and biochemical properties of cabbage (Brassica oleracea var. capitata). Sci. Hortic. 2018, 240, 196–204. [Google Scholar] [CrossRef]
- Chastain, D.R.; Snider, J.L.; Collins, G.D.; Perry, C.D.; Whitaker, J.; Byrd, S.A. Water deficit in field-grown Gossypium hirsutum primarily limits net photosynthesis by decreasing stomatal conductance, increasing photorespiration, and increasing the ratio of dark respiration to gross photosynthesis. J. Plant Physiol. 2014, 171, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alamri, S.A.; Alam, P.; Ashraf, M.; Ahmad, P. Potential of exogenously sourced kinetin in protecting Solanum lycopersicum from NaCl-induced oxidative stress through up-regulation of the antioxidant system, ascorbate-glutathione cycle and glyoxalase system. PLoS ONE 2018, 13, e0202175. [Google Scholar] [CrossRef]
- Khan, A.; Anwar, Y.; Hasan, M.; Iqbal, A.; Ali, M.; Alharby, H.; Hakeem, K.; Hasanuzzaman, M. Attenuation of drought stress in Brassica seedlings with exogenous application of Ca2+ and H2O2. Plants 2017, 6, 20. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.J.; Souissi, A.; Souissi, S.; Hwang, J.S. Effects of salinity on the reproductive performance of Apocyclops royi (Copepoda, Cyclopoida). J. Exp. Mar. Bio. Ecol. 2016, 475, 108–113. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [Green Version]
- Oukarroum, A.; Bussotti, F.; Goltsev, V.; Kalaji, H.M. Correlation between reactive oxygen species production and photochemistry of photosystems I and II in Lemna gibba L. plants under salt stress. Environ. Exp. Bot. 2015, 109, 80–88. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Asgher, M.; Khan, N.A. Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycinebetaine and ethylene in mungbean (Vigna radiata L.). Plant Physiol. Biochem. 2014, 80, 67–74. [Google Scholar] [CrossRef]
- Ahmad, P.; Latef, A.A.A.; Hashem, A.; Abd Allah, E.F.; Gucel, S.; Tran, L.S.P. Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front. Plant Sci. 2016, 7, 11. [Google Scholar] [CrossRef] [Green Version]
- Hanin, M.; Ebel, C.; Ngom, M.; Laplaze, L.; Masmoudi, K. New insights on plant salt tolerance mechanisms and their potential use for breeding. Front. Plant Sci. 2016, 7, 1787. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Zhang, J.; Pei, D.; Yu, L. Combined effects of water stress and salinity on growth, physiological, and biochemical traits in two walnut genotypes. Physiol. Plant. 2021, 172, 176–187. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Yang, S.L.; Dao, J.M.; Deng, J.; Shahzad, A.N.; Fan, X.; Li, R.D.; Quan, Y.J.; Bukhari, S.A.H.; Zeng, Z.H. Drought-induced alterations in photosynthetic, ultrastructural and biochemical traits of contrasting sugarcane genotypes. PLoS ONE 2020, 15, e0235845. [Google Scholar] [CrossRef]
- Le Gall, H.; Philippe, F.; Domon, J.-M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell wall metabolism in response to abiotic stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Zhang, W.; Zhang, B.; Xie, F. Effect of drought stress on sugar metabolism in leaves and roots of soybean seedlings. Plant Physiol. Biochem. 2020, 146, 1–12. [Google Scholar] [CrossRef]
- Turner, N.C.; Saxena, N.P.; Johansen, C.; Chauhan, Y.S.; Rao, R.C. Drought resistance: A comparison of two frameworks. In Management of Agriculture and Drought: Agronomic and Genetic Options; Saxena, N.P., Johansen, C., Chauhan, Y.S., Rao, R.C.N., Eds.; Science Publishers: Enfield, CT, USA, 2000; pp. 89–102. [Google Scholar]
- Kebede, H.; Fisher, D.K.; Sui, R.; Reddy, K.N. Irrigation methods and scheduling in the Delta region of Mississippi: Current status and strategies to improve irrigation efficiency. Am. J. Plant Sci. 2014, 5, 2917–2928. [Google Scholar] [CrossRef] [Green Version]
- Margenot, A. The State of Soybean in Africa: Soybean Diseases. Farmdoc Dly. 2019, 9, 1–4. [Google Scholar]
- Lopez, M.A.; Xavier, A.; Rainey, K.M. Phenotypic variation and genetic architecture for photosynthesis and water use efficiency in soybean (Glycine max l. Merr). Front. Plant Sci. 2019, 10, 680. [Google Scholar] [CrossRef] [Green Version]
- Hudak, C.M.; Patterson, R.P. Vegetative growth analysis of a drought-resistant soybean plant introduction. Crop Sci. 1995, 35, 464–471. [Google Scholar] [CrossRef]
- Li, W.; Sun, Y.; Wang, B.; Xie, H.; Wang, J.; Nan, Z. Transcriptome analysis of two soybean cultivars identifies an aluminum respon-sive antioxidant enzyme GmCAT1. Biosci. Biotechnol. Biochem. 2020, 84, 1394–1400. [Google Scholar] [CrossRef]
- Pathan, S.M.; Lee, J.D.; Sleper, D.A.; Fritschi, F.B.; Sharp, R.E.; Carter, T.E., Jr.; Nelson, R.L.; King, C.A.; Schapaugh, W.T.; Ellersieck, M.R.; et al. Two soybean plant introductions display slow leaf wilting and reduced yield loss under drought. J. Agron. Crop Sci. 2014, 200, 231–236. [Google Scholar] [CrossRef] [Green Version]
- Mutava, R.N.; Prince, S.J.K.; Syed, N.H.; Song, L.; Valliyodan, B.; Chen, W.; Nguyen, H.T. Understanding abiotic stress tolerance mechanisms in soybean: A comparative evaluation of soybean response to drought and flooding stress. Plant Physiol. Biochem. 2015, 86, 109–120. [Google Scholar] [CrossRef]
- Ye, H.; Song, L.; Schapaugh, W.T.; Ali, M.L.; Sinclair, T.R.; Riar, M.K.; Mutava, R.N.; Li, Y.; Vuong, T.; Valliyodan, B.; et al. The importance of slow canopy wilting in drought tolerance in soybean. J. Exp. Bot. 2020, 71, 642–652. [Google Scholar] [CrossRef]
- Wu, C.; Chen, P.; Hummer, W.; Zeng, A.; Klepadlo, M. Effect of flood stress on soybean seed germination in the field. Am. J. Plant Sci. 2017, 8, 53–68. [Google Scholar] [CrossRef] [Green Version]
- Saleem, M.H.; Ali, S.; Seleiman, M.F.; Rizwan, M.; Rehman, M.; Akram, N.A.; Liu, L.; Alotaibi, M.; Al-Ashkar, I.; Mubushar, M. Assessing the correlations between different traits in copper-sensitive and copper-resistant varieties of jute (Corchorus capsularis L.). Plants 2019, 8, 545. [Google Scholar] [CrossRef] [Green Version]
- International Seed Testing Association. The Germination Test in: International Rules for Seed Testing; International Seed Testing Association: Bassersdorf, Switzland, 2015; pp. 5-1–5-6. [Google Scholar]
- Weatherley, P.E. Studies in the water relations of the cotton plant: I. the field measurement of water deficits in leaves. New Phytol. 1950, 49, 81–97. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Hughes, N.M.; Smith, W.K. Attenuation of incident light in Galax urceolata (Diapensiaceae): Concerted influence of adaxial and abaxial anthocyanic layers on photoprotection. Am. J. Bot. 2007, 94, 784–790. [Google Scholar] [CrossRef] [Green Version]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Fong, J.; Schaffer, F.L.; Kirk, P.L. The ultramicrodetermination of glycogen in liver. A comparison of the anthrone and reducing-sugar methods. Arch. Biochem. Biophys. 1953, 45, 319–326. [Google Scholar] [CrossRef]
- Sadasivam, S.; Manickam, A. Biochemical Methods; New Age International (P) Limited Publishers: New Delhi, India, 2004. [Google Scholar]
- Bradford, K.J. A water relations analysis of seed germination rates. Plant Physiol. 1990, 94, 840–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Sullivan, C.Y.; Ross, W.M. Selecting for drought and heat resistance in grain sorghum. In Stress Physiology in Crop Plants; Mussell, H., Staples., R.C., Eds.; John Wiley and Sons: New York, NY, USA, 1979; pp. 263–281. [Google Scholar]
- Singleton, V.L.; Rossi, J.A., Jr. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-dhindsa, P.; Thorpe, T.A. Leaf Senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Hori, M.; Kondo, H.; Ariyoshi, N.; Yamada, H.; Hiratsuka, A.; Watabe, T.; Oguri, K. Changes in the hepatic glutathione peroxidase redox system produced by coplanar polychlorinated biphenyls in Ah-responsive and -less-responsive strains of mice: Mechanism and implications for toxicity. Environ. Toxicol. Pharmacol. 1997, 3, 267–275. [Google Scholar] [CrossRef]
- Ilyas, M.; Nisar, M.; Khan, N.; Hazrat, A.; Khan, A.H.; Hayat, K.; Fahad, S.; Khan, A.; Ullah, A. Drought tolerance strategies in plants: A mechanistic approach. J. Plant Growth Regul. 2021, 40, 926–944. [Google Scholar] [CrossRef]
- Kakar, N.; Jumaa, S.H.; Redoña, E.D.; Warburton, M.L.; Reddy, K.R. Evaluating rice for salinity using pot-culture provides a systematic tolerance assessment at the seedling stage. Rice 2019, 12, 57. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Yuan, L.I.; Xin-Ming, W.U.; Gao, H.W.; Sun, G.Z. Study on germination characteristics and drought-resistance evaluation of Dactylis glomerata L. under osmotic stress. Chin. J. Grassl. 2008, 30, 50–54. [Google Scholar]
- ZhenPeng, X.U.; Wan, T.; Cai, P.; Zhang, Y.R.; Jing, Y.U.; Meng, C. Effects of PEG simulated drought stress on germination and physiological properties of Apocynum venetum seeds. Chin. J. Grassl. 2015, 37, 75–80. [Google Scholar]
- Yang, Z.Y.; Zhou, B.Z.; Zhou, Y.; Ge, X.G.; Wang, X.M.; Cao, Y.H. Effects of drought stress simulated by PEG on seed germination and growth physiological characteristics of Phyllostachys edulis. For. Res. 2018, 31, 47–54. [Google Scholar]
- Guo, J.M.; Liu, J.; Dong, K.H. Effect of PEG-6000 stress on seed germination of Bothriochica ischaemum. Chin. J. Grassl. 2015, 37, 58–62. [Google Scholar]
- Kumar, A.; Rodrigues, V.; Verma, S.; Singh, M.; Hiremath, C.; Shanker, K.; Shukla, A.K.; Sundaresan, V. Effect of salt stress on seed germination, morphology, biochemical parameters, genomic template stability, and bioactive constituents of Andrographis paniculata Nees. Acta Physiol. Plant. 2021, 43, 1–14. [Google Scholar] [CrossRef]
- Bajji, M.; Kinet, J.M.; Lutts, S. Osmotic and ionic effects of NaCl on germination, early seedling growth, and ion content of Atriplex halimus (Chenopodiaceae). Can. J. Bot. 2002, 80, 297–304. [Google Scholar] [CrossRef]
- Talei, D.; Yusop, M.K.; Kadir, M.A.; Valdiani, A.; Abdullah, M.P. Response of king of bitters (Andrographis paniculata Nees.) seedlings to salinity stress beyond the salt tolerance threshold. Aust. J. Crop Sci. 2012, 6, 1059–1067. [Google Scholar]
- Grieco, M.; Roustan, V.; Dermendjiev, G.; Rantala, S.; Jain, A.; Leonardelli, M.; Neumann, K.; Berger, V.; Engelmeier, D.; Bachmann, G. Adjustment of photosynthetic activity to drought and fluctuating light in wheat. Plant. Cell Environ. 2020, 43, 1484–1500. [Google Scholar] [CrossRef] [Green Version]
- Shawon, R.A.; Kang, B.S.; Sang, G.L.; Kim, S.K.; Yang, G.K. Influence of drought stress on bioactive compounds, antioxidant enzymes and glucosinolate contents of Chinese cabbage (Brassica rapa). Food Chem. 2019, 308, 125657. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Duan, B.; Zhang, S.; Korpelainen, H.; Niinemets, U.; Li, C. Growth, biomass allocation and photosynthetic responses are related to intensity of root severance and soil moisture conditions in the plantation tree Cunninghamia lanceolata. Tree Physiol. 2016, 36, 807–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.; Dong, H.; Li, Z.; Han, Z.; Korpelainen, H.; Li, C. Species-specific responses to drought, salinity and their interactions in Populus euphratica and P. pruinosa seedlings. J. Plant Ecol. 2020, 13, 563–573. [Google Scholar] [CrossRef]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Miao, Y.; Bi, Q.; Qin, H.; Zhang, X.; Tan, N. Moderate drought followed by re-watering initiates beneficial changes in the photosynthesis, biomass production and Rubiaceae-type cyclopeptides (RAs) accumulation of Rubia yunnanensis. Ind. Crops Prod. 2020, 148, 112284. [Google Scholar] [CrossRef]
- Hussain, T.; Koyro, H.W.; Zhang, W.; Liu, X.; Gul, B.; Liu, X. Low salinity improves photosynthetic performance in Panicum antidotale under drought stress. Front. Plant Sci. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef]
- Nezhadahmadi, A.; Prodhan, Z.H.; Faruq, G. Drought tolerance in wheat. Sci. World J. 2013, 2013, 610721. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Zhu, Y.; Song, X.; Song, F. Negative effects of long-term moderate salinity and short-term drought stress on the photosynthetic performance of Hybrid Pennisetum. Plant Physiol. Biochem. 2020, 155, 93–104. [Google Scholar] [CrossRef]
- Suresh, K.; Beena, A.S.; Monika, A.; Archana, S. Physiological, biochemical, epigenetic and molecular analyses of wheat (Triticum aestivum) genotypes with contrasting salt tolerance. Front. Plant Sci. 2017, 8, 1151. [Google Scholar]
- Martínez-Vilalta, J.; Sala, A.; Asensio, D.; Galiano, L.; Hoch, G.; Palacio, S.; Piper, F.I.; Lloret, F. Dynamics of non-structural carbohydrates in terrestrial plants: A global synthesis. Ecol. Monogr. 2016, 86, 495–516. [Google Scholar] [CrossRef]
- Tomasella, M.; Nardini, A.; Hesse, B.D.; MacHlet, A.; Matyssek, R.; Häberle, K.H. Close to the edge: Effects of repeated severe drought on stem hydraulics and non-structural carbohydrates in European beech saplings. Tree Physiol. 2019, 39, 717–728. [Google Scholar] [CrossRef]
- De Baerdemaeker, N.J.F.; Salomón, R.L.; De Roo, L.; Steppe, K. Sugars from woody tissue photosynthesis reduce xylem vulnerability to cavitation. New Phytol. 2017, 216, 720–727. [Google Scholar] [CrossRef] [Green Version]
- Boldizsár, Á.; Simon-Sarkadi, L.; Szirtes, K.; Soltész, A.; Szalai, G.; Keyster, M.; Ludidi, N.; Galiba, G.; Kocsy, G. Nitric oxide affects salt-induced changes in free amino acid levels in maize. J. Plant Physiol. 2013, 170, 1020–1027. [Google Scholar] [CrossRef] [Green Version]
- Waskiewicz, A.; Muzolf-Panek, M.; Goliński, P. Phenolic Content Changes in Plants under Salt Stress; Ahmad, P., Azooz, M., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2013; pp. 283–314. [Google Scholar]
- Bourgou, S.; Bettaieb, I.; Tounsi, M.S.; Marzouk, B. Fatty acids, essential oil, and phenolics modifications of black cumin fruit under NaCl stress conditions. J. Agric. Food Chem. 2010, 58, 12399–12406. [Google Scholar] [CrossRef]
- Choudhury, S.; Panda, P.; Sahoo, L.; Panda, S.K. Reactive oxygen species signaling in plants under abiotic stress. Plant Signal. Behav. 2013, 8, e23681. [Google Scholar] [CrossRef] [Green Version]
- Ray, R.L.; Ampim, P.A.Y.; Gao, M. Crop protection under drought stress. In Crop Protection under Changing Climate; Jabran, K., Florentine, S., Chauhan, B., Eds.; Springer: Cham, Switzerland, 2020; pp. 145–170. [Google Scholar]
- Farooq, M.A.; Ali, S.; Hameed, A.; Ishaque, W.; Mahmood, K.; Iqbal, Z. Alleviation of cadmium toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes; suppressed cadmium uptake and oxidative stress in cotton. Ecotoxicol. Environ. Saf. 2013, 96, 242–249. [Google Scholar] [CrossRef]
- Song, J.; Wang, Y.; Pan, Y.; Pang, J.; Zhang, X.; Fan, J.; Zhang, Y. The influence of nitrogen availability on anatomical and physiological responses of Populus alba × P. glandulosa to drought stress. BMC Plant Biol. 2019, 19, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Jia, J.; Zhang, C.; Li, H.; Liu, T.; Jiang, X.; Polle, A.; Peng, C.; Luo, Z. Bin Anatomical, physiological and transcriptional responses of two contrasting poplar genotypes to drought and re-watering. Physiol. Plant. 2014, 151, 480. [Google Scholar] [CrossRef]
- Petrov, V.; Hille, J.; Mueller-Roeber, B.; Gechev, T.S. ROS-mediated abiotic stress-induced programmed cell death in plants. Front. Plant Sci. 2015, 6, 69. [Google Scholar] [CrossRef] [Green Version]
- Goswami, B.; Rankawat, R.; Gadi, B.R. Physiological and antioxidative responses associated with drought tolerance of Lasiurus sindicus Henr. endemic to Thar desert, India. Rev. Bras. Bot. 2020, 43, 761–773. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, S.; Wang, B.; Zhao, J. Physiological and biochemical changes in different drought-tolerant alfalfa (Medicago sativa L.) varieties under PEG-induced drought stress. Acta Physiol. Plant. 2018, 40, 25. [Google Scholar] [CrossRef]
- Anjum, S.A.; Ashraf, U.; Tanveer, M.; Khan, I.; Hussain, S.; Shahzad, B.; Zohaib, A.; Abbas, F.; Saleem, M.F.; Ali, I.; et al. Drought induced changes in growth, osmolyte accumulation and antioxidant metabolism of three maize hybrids. Front. Plant Sci. 2017, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, S.; Zhao, H.; Korpelainen, H.; Li, C. Sex-related adaptive responses to interaction of drought and salinity in Populus yunnanensis. Plant Cell Environ. 2010, 33, 1767–1778. [Google Scholar] [CrossRef] [PubMed]
- Kirova, E.; Pecheva, D.; Simova-Stoilova, L. Drought response in winter wheat: Protection from oxidative stress and mutagenesis effect. Acta Physiol. Plant. 2021, 43, 1–11. [Google Scholar] [CrossRef]



| Sl. No. | Accession Name of the Cultivar | Cultivar | Origin/Country | Stress Tolerance | References |
|---|---|---|---|---|---|
| 1 | PI5A | KAS 633-19 | Korea, South | Flooding tolerance | [27] |
| 2 | PI31 | Fu yang (56) | China | Drought tolerance | [26] |
| 3 | PI90 | Fu yang (7) | China | Drought tolerance | [25] |
| 4 | PI37 | Houjaku Kuwazu | Japan | Aluminum-resistant | [23] |
| Cultivars | Treatments | Plant Height (cm) | Shoot Fresh Weight (mg g−1 FW) | Shoot Dry Weight (mg g−1 FW) | Root Dry Weight (mg g−1 FW) | LRWC (%) |
|---|---|---|---|---|---|---|
| PI31 | Control | 76.663 ± 0.95 a | 19.603 ± 0.65 a | 8.937 ± 0.30 a | 3.297 ± 0.40 a | 81.376 ± 2.37 a |
| Drought | 51.410 ± 3.20 a | 12.173 ± 0.37 a | 7.840 ± 0.32 a | 2.217 ± 0.06 a | 73.443 ± 2.55 a | |
| Salinity | 45.840 ± 0.76 a | 9.743 ± 0.36 a | 7.037 ± 0.76 a | 1.55 ± 0.28 a | 63.09 ± 2.62 a | |
| drought + Salinity | 33.93 ± 1.06 a | 6.800 ± 0.46 a | 3.270 ± 0.03 a | 1.043 ± 0.07 a | 58.45 ± 1.56 a | |
| PI90 | Control | 66.567 ± 2.27 b | 18.233 ± 0.32 a | 9.433 ± 0.65 a | 3.20 ± 0.07 a | 83.119 ± 2.57 a |
| Drought | 43.91± 1.02 b | 10.300 ± 0.34 a | 7.633 ± 0.55 ab | 1.280 ± 0.55 b | 70.96 ± 1.89 a | |
| Salinity | 40.85 ± 2.40 ab | 8.933 ± 0.40 a | 5.27 ± 0.17 b | 0.920 ± 0.18 b | 65.831 ± 1.57 a | |
| Drought + Salinity | 27.68 ± 0.76 b | 5.657 ± 0.18 ab | 2.867 ± 0.18 ab | 0.727 ± 0.18 ab | 55.514 ± 2.81 ab | |
| PI37 | Control | 69.233 ± 1.12 ab | 18.100 ± 0.22 a | 8.833 ± 0.22 a | 3.100 ± 0.11 a | 83.92 ± 3.21 a |
| Drought | 39.43 ± 1.06 b | 7.927 ± 0.30 b | 4.143 ± 0.64 b | 1.190 ± 0.14 bc | 50.5 1± 1.27 b | |
| Salinity | 36.267 ± 1.20 b | 6.7733 ± 0.38 b | 4.410 ± 0.46 b | 1.243 ± 0.12 ab | 60.79 ± 1.35 a | |
| Drought + Salinity | 20.367 ± 0.03 c | 4.680 ± 0.20 b | 2.263 ± 0.26 b | 0.497 ± 0.26 c | 48.47 ± 2.09 bc | |
| PI5A | Control | 62.93 ± 2.90 b | 16.74 ± 0.90 a | 8.267 ± 0.40 a | 2.897 ± 0.08 a | 80.39 ± 4.44 a |
| Drought | 22.03 ± 1.11 c | 5.480 ± 0.30 c | 2.573 ± 0.33 c | 0.887 ± 0.03 c | 61.78 ± 3.52 ab | |
| Salinity | 25.43 ± 1.93 c | 6.28 ± 0.23 b | 4.023 ± 0.86 c | 1.030 ± 0.08 ab | 42.48 ± 2.45 b | |
| Drought + Salinity | 17.33 ± 1.40 c | 4.657 ± 0.23 b | 1.633 ± 0.22 c | 0.347 ± 0.22 c | 41.39 ± 1.53 c |
| Cultivars | Treatments | Chl a (mg g−1 FW) | Chl b(mg g−1 FW) | Total Chl (mg g−1 FW) | Carotenoid (mg g−1 FW) | Anthocyanin (μg g−1 FW) |
|---|---|---|---|---|---|---|
| PI31 | Control | 1.414 ± 0.67 a | 5.278 ± 0.16 a | 6.692 ± 1.09 a | 1.283 ± 0.16 a | 71.490 ± 1.9 a |
| Drought | 0.816 ± 0.015 a | 3.202 ± a 0.18 a | 4.018 ± 1.21 a | 0.476 ± 0.05 a | 55.385 ± 4.46 a | |
| Salinity | 0.774 ± 0.02 a | 2.964 ± 0.2 a | 3.738 ± 0.72 a | 0.475 ± 0.22 a | 56.956 ± 1.87 a | |
| Drought + Salinity | 0.449 ± 0.04 a | 1.982 ± 0.19 a | 2.431 ± 0.12 a | 0.451 ± 0.34 a | 48.267 ± 3.24 a | |
| PI37 | Control | 1.388 ± 0.88 a | 5.149 ± 0. 10 a | 6.537 ± 0.43 a | 0.944 ± 0.27a | 76.596 ± 1.12 a |
| Drought | 0.625± 0.03 a | 2.380 ± 0.09 bc | 3.005 ± 0.64 b | 0.2887 ± 0.18 ab | 57.676 ± 1.33 a | |
| Salinity | 0.475 ± 0.01 b | 2.231± 0.36 b | 2.706± 0.57c | 0.256 ± 0.45 b | 42.815 ± 4.48 b | |
| Drought + Salinity | 0.231± 0.07 b | 1.728 ± 0.17 ab | 1.959 ± 0.54c | 0.174 ± 0.33 bc | 32.144 ± 4.67 b | |
| PI90 | Control | 1.437 ± 0.44 a | 4.994 ± 0.14 a | 6.431 ± 1.13 a | 1.0172 ± 1.11a | 78.691 ± 2.85 a |
| Drought | 0.316 ± 0.06 b | 2.771 ± 0.28 ab | 3.087 ± 0.74 a | 0.337 ± 0.55 ab | 52.544 ± 0.79 ab | |
| Salinity | 0.251 ± 0.08 c | 2.883 ± 0.19 a | 3.134 ± 0.22 b | 0.423 ± 0.13 ab | 51.784 ± 2.74 ab | |
| Drought + Salinity | 0.114 ± 0.03 b | 1.922 ± 0.11 a | 2.063 ± 0.29 b | 0.272 ± 0.65 b | 42.750 ± 3.53 ab | |
| PI5A | Control | 1.388 ± 0.055a | 4.902 ± 0.29 a | 6.29 ± 1.08 b | 0.967 ± 0.88 a | 80.262 ± 6.17 a |
| Drought | 0.215± 0.03b | 2.225 ± 0.15 c | 2.44 ± 0.98b | 0.181 ± 0.57 b | 46.94 ± 3.88 b | |
| Salinity | 0.233 ± 0.21c | 2.356 ± 0.17 b | 2.589 ± 0.65c | 0.254 ± 0.14 b | 50.082 ± 5.57 ab | |
| Drought + Salinity | 0.158 ±0.14 b | 1.421 ± 0.12 b | 1.579 ± 0.54ab | 0.1284 ± 0.44 c | 30.311 ± 0.74 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Begum, N.; Hasanuzzaman, M.; Li, Y.; Akhtar, K.; Zhang, C.; Zhao, T. Seed Germination Behavior, Growth, Physiology and Antioxidant Metabolism of Four Contrasting Cultivars under Combined Drought and Salinity in Soybean. Antioxidants 2022, 11, 498. https://doi.org/10.3390/antiox11030498
Begum N, Hasanuzzaman M, Li Y, Akhtar K, Zhang C, Zhao T. Seed Germination Behavior, Growth, Physiology and Antioxidant Metabolism of Four Contrasting Cultivars under Combined Drought and Salinity in Soybean. Antioxidants. 2022; 11(3):498. https://doi.org/10.3390/antiox11030498
Chicago/Turabian StyleBegum, Naheeda, Mirza Hasanuzzaman, Yawei Li, Kashif Akhtar, Chunting Zhang, and Tuanjie Zhao. 2022. "Seed Germination Behavior, Growth, Physiology and Antioxidant Metabolism of Four Contrasting Cultivars under Combined Drought and Salinity in Soybean" Antioxidants 11, no. 3: 498. https://doi.org/10.3390/antiox11030498
APA StyleBegum, N., Hasanuzzaman, M., Li, Y., Akhtar, K., Zhang, C., & Zhao, T. (2022). Seed Germination Behavior, Growth, Physiology and Antioxidant Metabolism of Four Contrasting Cultivars under Combined Drought and Salinity in Soybean. Antioxidants, 11(3), 498. https://doi.org/10.3390/antiox11030498








