Abstract
Medication-related osteonecrosis of the jaw (MRONJ) is one of the most interesting diseases in the field of maxillofacial surgery. In addition to bisphosphonates, the use of antiresorptive and antiangiogenic agents is known to be the leading cause. However, the exact pathogenesis of MRONJ has not been established, and various hypotheses have been proposed, such as oxidative stress-related theory. As a result, a definitive treatment protocol for MRONJ has not been identified, while various therapeutic approaches are applied to manage patients with MRONJ. Although the surgical approach to treat osteomyelitis of the jaw has been proven to be most effective, there are limitations, such as recurrence and delayed healing. Many studies and clinical trials are being conducted to develop another effective therapeutic modality. The use of some materials, including platelet concentrates and bone morphogenetic proteins, showed a positive effect on MRONJ. Among them, teriparatide is currently the most promising material, and it has shown encouraging results when applied to patients with MRONJ. Furthermore, cell therapy using mesenchymal stem cells showed promising results, and it can be the new therapeutic approach for the treatment of MRONJ. This review presents various treatment methods for MRONJ and their limitations while investigating newly developed and researched molecular and cellular therapeutic approaches along with a literature review.
1. Introduction
Medication-related osteonecrosis of the jaw (MRONJ) is one of the refractory diseases in the maxillofacial area. Since it was first reported by Marx in 2003 [1], there has been no gold standard treatment for MRONJ. At first, it was noticed under the name of bisphosphonate-related osteonecrosis of the jaw (BRONJ). However, as more drugs were reported to induce osteonecrosis of the jaw, it has been renamed MRONJ in the position paper of the American Association of Oral and Maxillofacial Surgeons (AAOMS) [2]. Afterward, the definition of the disease and the classification of the stages were further refined. With the increase in the elderly population, the use of drugs that may cause MRONJ continues to increase. However, the development of treatment for MRONJ remains stagnant despite this trend. This may be attributed to the fact that neither the exact pathogenesis of MRONJ nor the definitive pathognomonic biomarkers have been identified. As a result, various studies and research for a precise diagnosis and treatment of MRONJ are continuously in progress.
Surgical treatment is considered to be the most standard treatment of MRONJ, although some differences exist in the treatment approach depending on the stage. Since the possibility of recurrence due to bone remodeling changes caused by the remaining MRONJ-inducing drugs in the body cannot be entirely excluded, therapies focusing on this point are being studied. Various treatment modalities such as hyperbaric oxygen (HBO) therapy, ozone therapy (OT), and low-level laser therapy (LLLT) have been suggested. However, they are not considered the primary therapeutic modality but are rather considered an adjunctive treatment. At present, studies on molecular and cellular approaches targeted to hypotheses that are presumed to be the mechanism of MRONJ are being conducted. Among them, teriparatide has recently been proven effective through a randomized controlled trial [3], showing the possibility as a promising therapeutic agent in the future. Furthermore, bone morphogenetic protein (BMP) and autologous platelet concentrates (APC) are applied in clinical and research environments as adjunctive agents. Mesenchymal stem cell (MSC) therapy is also drawing attention as another therapeutic approach.
This review aims to present various therapies for MRONJ and its limitations. In addition, it examines newly developed and researched molecular and cellular therapeutic approaches along with a literature review.
2. Staging and Possible Hypothesis of the Pathogenesis of MRONJ
2.1. Staging of MRONJ and Treatment Strategies
The AAOMS first presented the staging system for BRONJ to establish a reasonable treatment guideline and collect data to evaluate patients’ prognosis with the history of bisphosphonate usage in 2007 [4]. At this point, BRONJ was classified into at risk, Stage 1, 2, and 3, with several clinical traits presented for each stage. However, as the demand for further classification had been raised, the first staging system had to be changed. As a result, Stage 0 was added to the staging system in 2009 [5]. Although the risk of progressing from Stage 0 to a higher stage was still unknown at that time, it was necessary to include patients presenting with nonspecific symptoms or clinical findings that may be due to bisphosphate exposure. Besides, the definition of Stage 3 was revised in this updated position paper to include advanced maxillary diseases such as oroantral/oronasal communication, exposed and necrotic bone extending to the maxillary sinus, and osteolysis extending to the sinus floor [5]. As knowledge and experience of BRONJ accumulated, and as other drugs thought to induce osteonecrosis were added, a new position paper by AAOMS was proposed in 2014 [2]. From this time on, the name MRONJ began to be commonly used since other antiresorptive and antiangiogenic agents besides bisphosphonate were included as drugs that could induce osteonecrosis. The drugs known to be associated with MRONJ are shown in Table 1. Although there was no remarkable change in staging, cutaneous or mucosal fistula, which is probed to the bone, was included in the definition of the exposed bone and was added as a clinical manifestation in Stages 1, 2, and 3. Additionally, the revised position paper stated that Stage 0 in the previous position paper was appropriate in that it was possible to detect patients with the prodromal disease. Therefore, Stage 0 was maintained in the staging system. The latest staging system suggested by AAOMS is presented in Table 2, and the clinical cases for Stages 0, 1, 2, and 3 are shown in Figure 1, Figure 2, Figure 3 and Figure 4, respectively.

Table 1.
Drugs are known to be associated with MRONJ.

Table 2.
Staging of MRONJ [2].
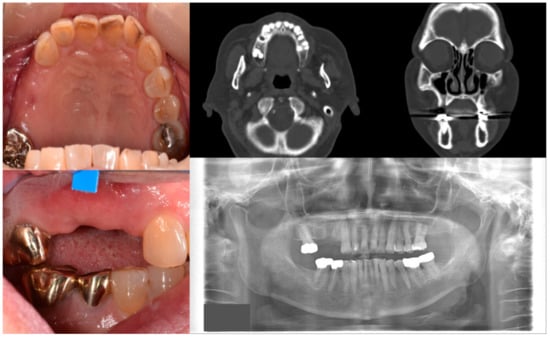
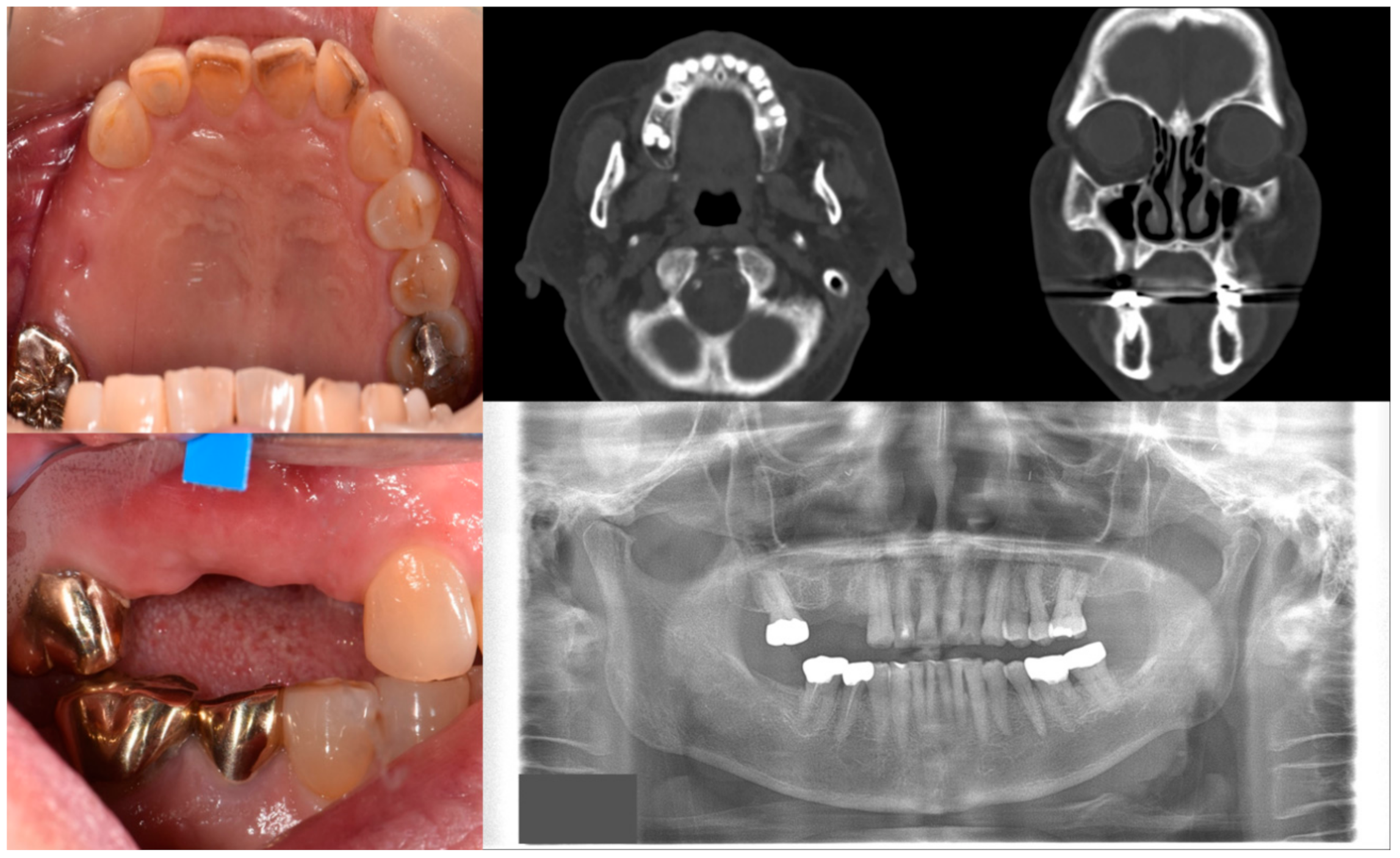
Figure 1.
Case 1 with MRONJ Stage 0: Clinical photos, panoramic view, and computed tomography imaging showing non-significant findings on the maxillary right posterior area. An 81-year-old female patient with a history of taking oral alendronate for five years complained of pain after removing the right upper second premolar five months before. There were no specific findings other than slight soft tissue depression in the area and hard tissue defect presumed to be an extraction socket.
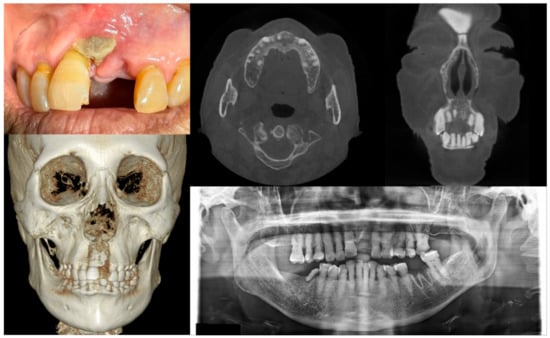
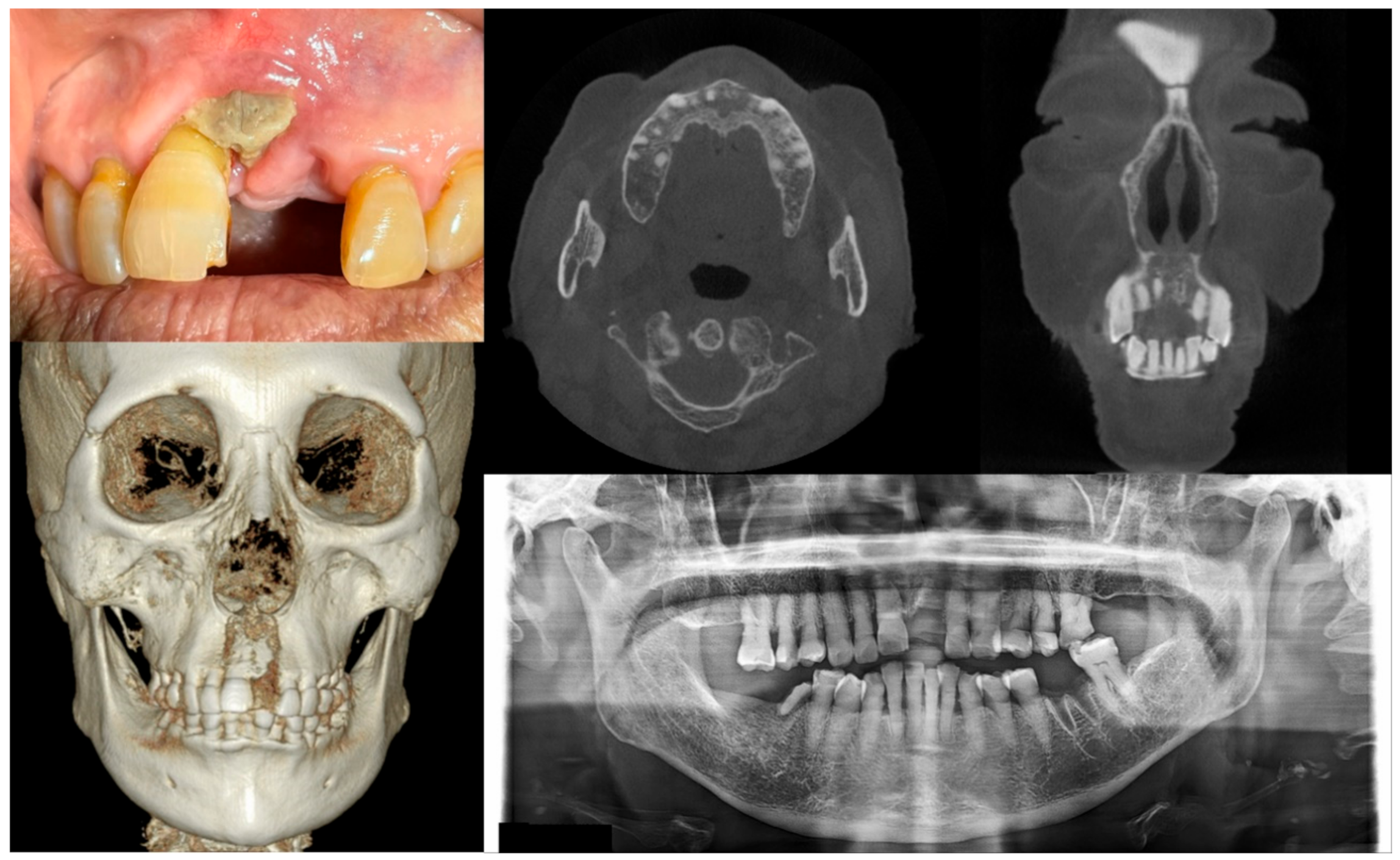
Figure 2.
Case 2 with MRONJ Stage 1: Clinical photo, panoramic view, and computed tomography imaging including three-dimensional reconstruction showing exposed alveolar bone on the anterior maxillary area. An 89-year-old female patient with a history of oral alendronate for more than four years and intravenous administration of denosumab for one year showed the exposed bone after extracting the left upper central incisor three months ago. She reported no symptoms such as pain other than exposed bones. There was no sign of infection, including pus discharge on the exposed bone.
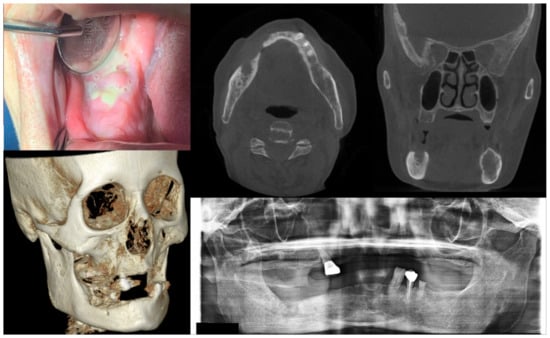
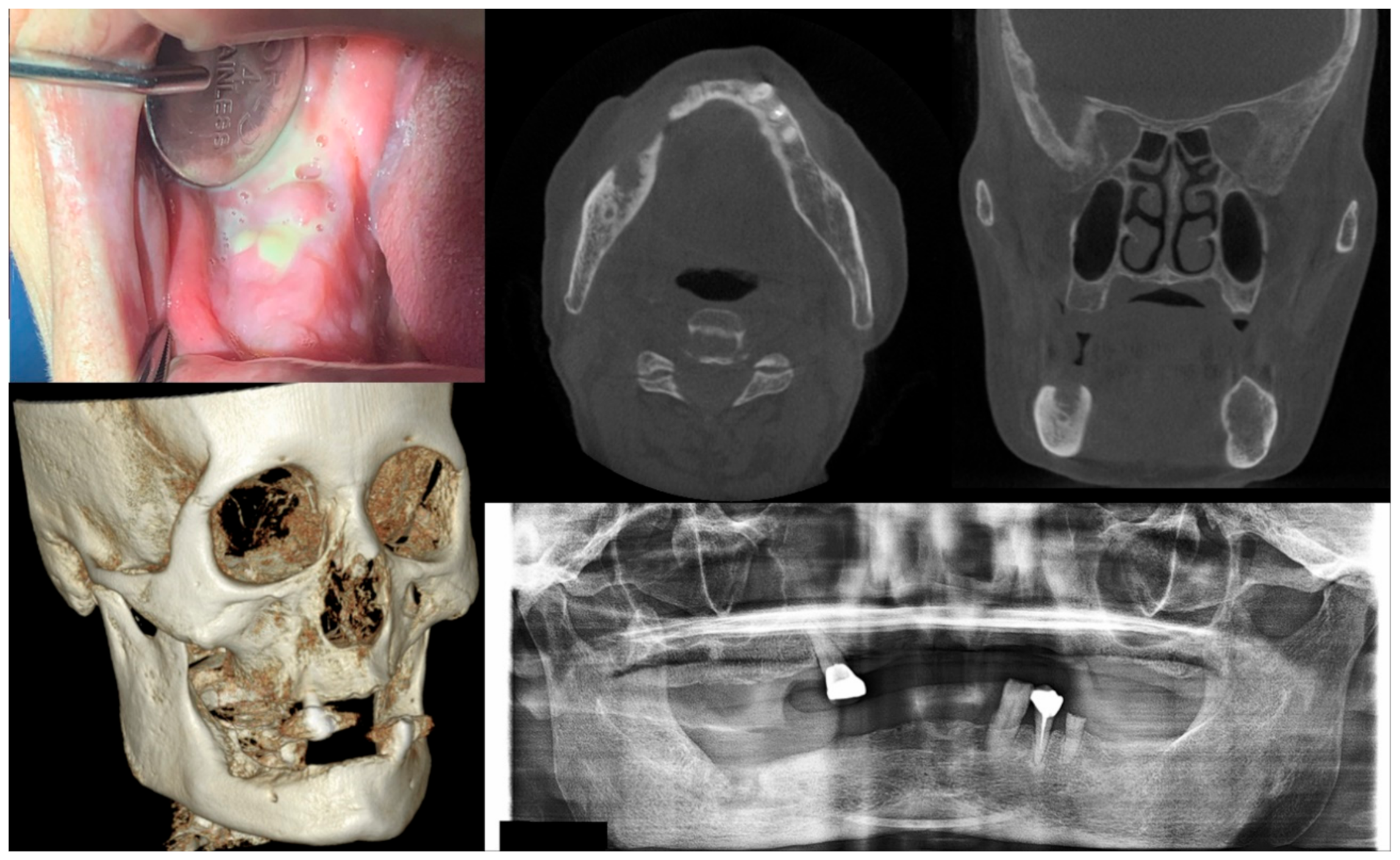
Figure 3.
Case 3 with MRONJ Stage 2: Clinical photo, panoramic view, and computed tomography imaging including three-dimensional reconstruction showing exposed alveolar bone with pus discharge on the right mandibular posterior area. A 68-year-old female patient with a history of intravenous administration of ibandronate for eight years and intravenous administration of denosumab for the last year complained of facial swelling, severe pain, and pus discharge. She had undergone extraction of the right lower second molar at the private dental clinic to relieve the facial swelling and pain. Symptoms were relieved slightly after extraction but worsened again after two weeks. As a result of the examination, exposed bone and infection signs were observed.
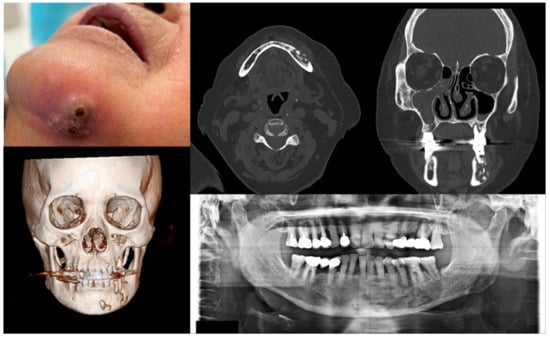
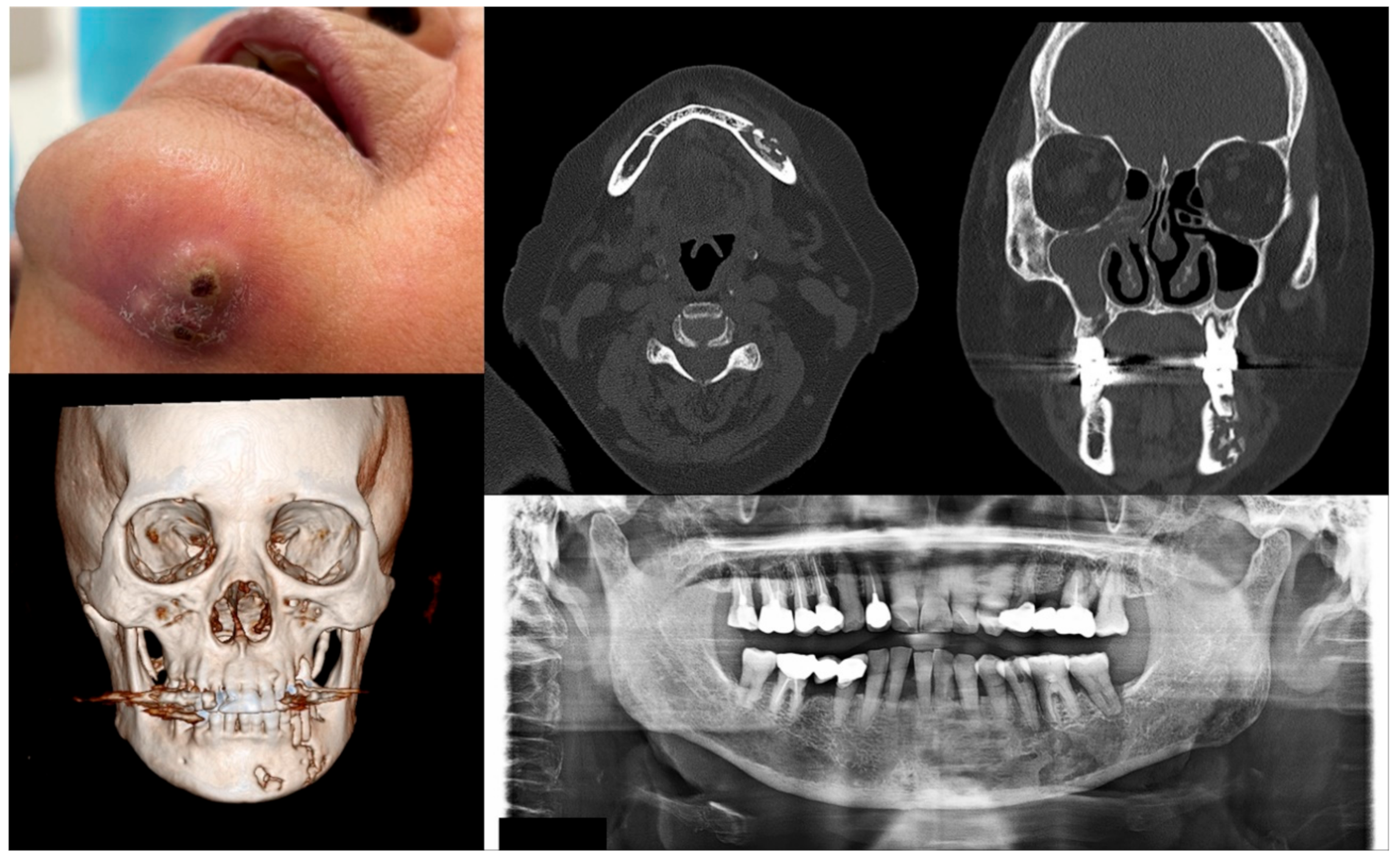
Figure 4.
Case 4 with MRONJ Stage 3: Clinical photo, panoramic view, and computed tomography imaging including three-dimensional reconstruction showing extraoral fistula and necrotic bone on the mandibular left anteroposterior area. An 80-year-old female patient with a history of intravenous administration of ibandronate and denosumab for one year was presented with inflammation and skin fistula on the chin area. She had undergone periodontal treatment on the left lower posterior teeth three months ago due to a periodontal abscess, but she was referred for recurrence with facial swelling from a private dental clinic. Bony lesions involving the inferior border of the mandible and the presence of extraoral fistula were observed.
2.2. Possible Hypothesis of Pathogenesis
The pathophysiological mechanisms of MRONJ have not been fully elucidated, although the first MRONJ cases were reported nearly 20 years ago. It is estimated that inhibition of osteoclastic activity in bone resorption and remodeling and/or inhibition of angiogenesis, which are related to the function of the osteonecrosis-induced drugs, plays a significant role in the occurrence of MRONJ. However, there are various hypotheses concerning the pathogenic mechanism of MRONJ.
2.2.1. Suppression of Bone Remodeling
Antiresorptive agents, including bisphosphonate and denosumab, inhibit osteoclast differentiation and function, increase apoptosis of osteoclast, and finally lead to decreased bone remodeling and resorption [6,7,8,9,10]. These conditions may be affected by triggering factors such as dental surgery, including tooth extraction, microtrauma to the jaw, and local inflammation, resulting in necrosis and jaw bone exposure [11]. It is not clear why MRONJ frequently occurs in the maxilla and mandible. The high rate of bone remodeling in the jaw may predispose to osteonecrosis compared to other bones. Since the bone turnover rate of the alveolar bone is more than ten times faster than that of the long bone, it seems to be related to the ability to contain much more bisphosphonates in the alveolar bone compared to the bones in other sites [12]. Although there are objections that bone turnover is not reduced in osteonecrosis lesions [13], and active bone resorption occurs due to the presence of osteoclasts in the osteonecrotic areas [14,15], this hypothesis is further strengthened by the favorable results of teriparatide, which stimulates the osteoclast activity [3,16].
2.2.2. Inhibition of Angiogenesis
Inhibition of angiogenesis is another central hypothesis that is thought to be a possible cause in the pathogenesis of MRONJ [17,18,19,20]. Because the interruption of the blood supply has classically been thought to cause osteonecrosis, it is natural for this hypothesis to draw attention as the leading cause of MRONJ. However, the exact etiopathogenesis of MRONJ associated with antiangiogenic agents is not well known. It is presumed that angiogenesis inhibition adversely affects the bone regeneration capacity after bone damage, delays bone remodeling or healing, and may increase susceptibility to superinfection [21,22]. Besides, antiangiogenic agents have other effects that may be presumed to be the cause of MRONJ. The anti-vascular endothelial growth factor (VEGF) agents such as bevacizumab and rituximab can inhibit osteoblast differentiation and macrophage chemotaxis [23], and the tyrosine kinase inhibitors (TKI) such as sunitinib and sorafenib act on macrophages and interferes with the osteoclast differentiation [24]. Interestingly, zoledronate can also cause a decrease in the circulating VEGF levels, leading to a reduction in angiogenesis [25]. However, it is thought that denosumab, another antiresorptive agent, does not cause inhibition of angiogenesis.
2.2.3. Infection and Inflammation
There is an opinion that the jaw is relatively vulnerable to infection because it is separated from oral pathogens with the help of only a thin mucoperiosteum, unlike other bones protected by thick soft tissues and skin [26]. Indeed, several animal studies have shown that the combination of inflammation or bacterial infections with systemic antiresorptive agents causes osteonecrosis [27,28,29,30,31,32]. However, in the progression of MRONJ, it is unclear whether osteonecrosis occurs first or whether infection and the resulting inflammation first occur. Although bisphosphonates have been shown to have bacterial biofilm-forming effects in osteonecrosis lesions [13,33], no specific infection focus was observed in most patients with MRONJ. Therefore, it is difficult to clearly state whether infection and inflammation are essential factors for osteonecrosis occurrence and progression.
2.2.4. Toxicity to Soft Tissues
Bisphosphonates, in addition to their effects on the osteoclast, have direct toxicity to soft tissues. Proliferation and transportation of oral keratinocytes can be inhibited by bisphosphonates [19,20,34], resulting in persistent bone exposure and infection. Therefore, trauma or procedures that cause intraoral wounds may be associated with this toxicity and induce osteonecrosis. However, since most bisphosphonates are excreted through the kidneys several hours after reaching the bloodstream, the concentration of bisphosphonates in soft tissues has been reported to be lower than that in bone [35]. Therefore, this hypothesis needs to be further elucidated.
2.2.5. Oxidative Stress
Another hypothesis for the pathophysiological mechanism of MRONJ is an oxidative stress-related theory. Oxidative stress refers to tissue damage caused by the loss of balance between reactive oxygen species (ROS) within tissue/cell and antioxidant mechanisms, resulting in a relatively excessive production of ROS. ROS target proteins, unsaturated phospholipids, and nucleic acids of DNA or RNA can weaken a cell’s structure and viability [36]. Therefore, it is not surprising that oxidative stress has been reported in cancer patients with advanced metastasis [37]. Moreover, it is well known that bisphosphonate treatment can cause oxidative stress [38] and that persistent local inflammation and associated infection processes can produce ROS [39,40,41].
Oxidative stress is related to osteonecrosis, mainly the one induced by steroids [42,43,44]. Since steroids in isolation were not regarded as a factor inducing MRONJ, oxidative stress was not initially presumed to be the inducing mechanism of MRONJ. However, the possibility of an association between oxidative stress and MRONJ has been suggested due to the relationship between bisphosphonates and oxidative stress. Starting with a study by Bagan et al. [45] in 2014, which reported that oxidative stress was detected in the MRONJ patients, animal experiments and clinical studies focusing on oxidative stress as a pathophysiological mechanism of MRONJ have been actively conducted in recent years. It is noteworthy that oxidative stress and bisphosphonate may induce osteonecrosis of the jaw after an invasive dentoalveolar surgery in mice [46] and that bisphosphonate-induced ROS could delay wound healing by preventing the growth and migration of oral fibroblasts [47]. Besides, it is interesting that hypotaurine, which acts as an antioxidant associated with cellular defense against oxidative stress, was significantly elevated in patients’ saliva with MRONJ and thus showed potential as a new biomarker for the early detection of MRONJ [48]. It is also important to note that [4-(methylthio) phenylthio] methan bisphosphonate, the most potent antioxidant among bisphosphonates, exhibited fewer adverse effects in patients with inflammatory bone disease, including rheumatoid arthritis and periodontitis, showing the potential as a “rescue” bisphosphonate [49]. At present, it is the beginning step of finding out that oxidative stress may cause MRONJ. However, the accurate identification of the relationship would be worthwhile because the antioxidants that can reverse oxidative stress can be applied to treat the MRONJ.
3. Various Therapeutic Methods for MRONJ
3.1. Medication
Medication, which may be referred to as conservative medical treatment, is the primary method currently available in the early stages of MRONJ. According to the position paper of AAOMS in 2014 [2], patients with MRONJ Stage 0 and 1 can benefit from medical treatments such as systematic antibiotics and/or antimicrobial rinse. It can also be applied as adjuvant therapy in Stages 2 and 3 when evidence of infection begins to appear. However, the conservative medical treatment may take the lead even in later stages that require surgical treatment despite these recommendations. For example, if the patient is reluctant to undergo surgery or the patient’s general condition may not allow surgery, conservative medical treatment might be a good alternative [50].
The most widely used antibiotics for the systematic medication in patients with MRONJ appear to be penicillin, amoxicillin, amoxicillin/clavulanate, metronidazole, or a combination thereof [51]. These drugs are also used as medications for common oral infections. Although the association between oral microflora and MRONJ has not been established, it is thought that these drugs are being applied because bacterial migration in the progression of MRONJ can cause inflammation or infection [52]. When it comes to local antimicrobial use for the management of MRONJ, there seems to be no disagreement that chlorhexidine is the first choice.
Stage-specific therapy has been a consensus among clinicians, and surgical treatment is considered effective treatment at the later stages of MRONJ. However, there is an opinion that conservative medical treatment can be applied at all stages. Ramaglia et al. [53] recommended starting with two weeks of conservative medical treatment for the management of MRONJ in their systematic review and meta-analysis because it showed effectiveness at all stages. Although the medical treatment had a lower treatment success rate in the advanced stages, 13 selected studies in Ragmalia et al.’s review [53] showed success rates of 63.6–100% in Stage 2 and 73% in Stage 3. Another advantage of conservative medical therapy is that it may also reduce the possibility of unnecessary surgical treatment by preventing progression to the higher stage of the disease. Besides, it may relieve the wound, even if it cannot achieve a complete cure of MRONJ, making it possible to perform less invasive surgery when performing a surgical treatment.
Medication therapy still has some limitations. First, there is no consensus on which antibiotic is the most effective and the total duration of application. Besides, since most of the papers did not specifically indicate the dosage of antibiotics, the dosage for each antibiotic has not been established. Second, although conservative medical treatment often works in the advanced stages, most clinicians agree that it only serves an auxiliary role to control infection in the later stages. They claim that, eventually, surgical treatment is necessary for the complete healing of the MRONJ wound. Lastly, there is a possibility of adverse events that may arise from the long-term use of antibiotics. Possible adverse events from long-term use of antibiotics consist of gastrointestinal events, end-organ toxic effects, multidrug-resistant organism colonization, and Clostridium difficile infections [54,55]. Although the probability of these adverse drug events is low, clinicians should consider this risk and apply long-term use of antibiotics with care.
Although the efficacy and scope of application have not been fully established, conservative medical treatment is a safe method that can be tried at first and remains the most commonly applied method in the treatment of MRONJ.
3.2. Surgical Treatment
Surgical treatment is known to be an essential method for the management of the later stages of MRONJ. According to AAOMS in 2014 [2], surgical treatment such as debridement was suggested from Stage 2, and its necessity was emphasized. The surgical treatment for MRONJ consists of debridement, curettage, sequestrectomy, and resection with or without microvascular reconstruction [2,56]. Although there are some differences according to the literature, debridement of the soft tissue, curettage of the bone with no extension and sequestrectomy belong to conservative surgical treatment, and major or complete resection of the necrotic lesion with or without microvascular reconstruction belongs to aggressive surgical treatment [57,58].
As mentioned above, surgical treatment is close to the most standard treatment in the management of MRONJ. Its effectiveness has already been widely proven in Stages 2 and 3 [59,60,61,62,63]. Recently, a prospective cohort study has reported that it is also useful in early stages such as Stage 1. Giudice et al. [64] evaluated the time to restore mucosal integrity and downstage lesions after surgical treatment in 57 patients with Stage 1 and 72 patients with Stage 2. Compared to patients with Stage 2, the time taken for mucosal coverage in patients with Stage 1 was significantly shorter (56.4 ± 54.5 days for MRONJ Stage 1 vs. 83.1 ± 74.9 days for MRONJ Stage 2) [64]. However, the time taken to achieve downstaging was shorter in Stage 2 than in Stage 1 patients, and this is presumed to be because Stage 2 patients can achieve downstaging even if the exposed bone is not completely removed and only the infection disappears, but Stage 1 patients must show complete wound healing for the downstaging.
In summary, Giudice et al. [64] suggested that surgical therapy has a high success rate and a short treatment period in the early stages of MRONJ. Surgical treatment provides pain and infection control, relief of soft tissue irritation, and reduced osteolysis [56,65]. In addition to the therapeutic effect of surgical treatment, surgical treatment has an additional advantage that enables histopathological examination of the removed tissues to exclude metastasis in oncologic patients in whom primary neoplasm is possible [66].
In the treatment of MRONJ, the extent of surgical treatment and which surgical method is most useful have not yet been established. Recently, a systematic review was published comparing conservative and aggressive surgery [58]. As a result of reviewing 13 studies, extensive resection to have a viable bleeding margin showed the best results in patients in Stage 3 [58]. When comparing the aggressive surgery and simultaneous microvascular flap reconstruction with the aggressive surgery only, the former showed complete mucosal healing (97% vs. 85%) [58]. However, since such surgery may cause more severe morbidity in patients, a judgment considering risk/benefit is required. There is no study comparing the effectiveness of conservative surgery and aggressive surgery in Stage 2.
Unique techniques worth mentioning in surgical treatment are the buccal fat pad flap in the maxilla and the mylohyoid flap in the mandible. In the surgical treatment for MRONJ, removal of the necrotic bone and soft tissue covering using flaps are required. In that case, if the closure is performed using only local mucosal flaps, a high failure rate is shown, which is due to poor vascularity of the MRONJ lesion [67]. Therefore, microvascular flaps can be used when a large soft-tissue defect is expected, but it cannot always be the first option due to donor site morbidity and the prolonged operation time. As a suboptimal solution, buccal fat pat flap or mylohyoid flap could be used, and when an appropriate case selection was accompanied, favorable results with minimal complications and no recurrence were shown [68,69].
Surgical treatment with additional surgical tools has been reported. Ultrasonic bone surgery, also known as piezoelectric surgery, has been actively performed in the field of maxillofacial surgery, and it has the advantage of enabling less invasive bone cutting without damaging soft tissues. Blus et al. [70] reported a complete soft tissue closure within postoperative one month and no recurrence during a 10–54 month follow-up period after performing surgical removal of necrotic bone and debridement using a piezoelectric device in 18 patients with MRONJ. In addition to piezoelectric surgery, there is also autofluorescence-guide bone surgery in which surgery is performed using fluorescence detection devices to determine the resection margin of necrotic bone during surgery. Since the incomplete sequestrectomy can cause recurrence, complete removal of the necrotic bone is essential during surgical treatment for MRONJ, and it is not easy to determine the margin of osteonecrosis. To overcome these difficulties, Giudice et al. [71] published a prospective study comparing the efficacy of surgery using the VELscope (visually enhanced lesion scope) fluorescence lamp and conventional surgery. However, autofluorescence-guided surgery did not show superior results to conventional surgery in mucosal healing and the quality of life [71]. As another method, a case report was recently published that applied dynamic navigation for the surgical treatment of MRONJ [72]. Dynamic navigation is a technology that enables the tracking of the position of surgical instruments while viewing the cone-beam computed tomography images in real-time and has also recently been applied in the field of dental implants. Pellegrino et al. [72] reported a case of successfully treating a patient with MRONJ Stage 2 involving inferior alveolar nerves by combining dynamic navigation with ultrasonic surgery and suggested that more clinical trials are needed to evaluate the effectiveness and safety of this technology in the management of MRONJ. Although surgical treatment using these additional surgical tools mentioned above requires more studies and evidence, it is meaningful that the surgeon can perform a more straightforward and comfortable operation in the surgical treatment for MRONJ by using these tools.
There are some disadvantages to the surgical treatment, although it has been proven to be effective. Most of those are related to the operation itself and are associated with complications after surgery. Some authors reported aggravation of the symptoms, pathological fractures, and loss of parts of the jaw after the surgical treatment [73,74]. However, these complications are preventable, and there is no need to fear or hesitate to perform surgical treatment when the surgery is indicated.
Conclusively, surgical treatment has not yet been proven to treat patients in the early stages of MRONJ, but it has been established as a necessary treatment for later stages. Attempts on surgical equipment and surgical methods for accurate and safe surgery are in progress, and attention is paid to continuing follow-up studies.
3.3. Application of Regenerative Materials
In general, the application of specific materials is additionally performed during the surgical treatment of MRONJ. However, it is necessary to discuss the types, mechanisms, and effects of these materials separately. Currently, regenerative materials widely used for the treatment of MRONJ include BMP and APC.
BMP-2, a group of signaling molecules that belongs to the superfamily of transforming growth factor-β (TGF-β), has been used in orthopedic and maxillofacial surgery to increase bone remodeling. Due to its osteoinductive property [75], it is thought to have a potential effect of reversing the remodeling suppression in MRONJ and is currently being used to treat MRONJ [76,77,78]. BMP-2 is not generally applied as a single treatment modality but is applied in conjunction with surgical treatment, and it is delivered with a carrier to the defect from which the necrotic bone has been removed. Absorbable collagen sponges are frequently used as carriers for BMP-2, and some studies have shown that a rapid osteoinductive process is derived when these are applied to the jaw bone [79,80]. However, BMP-2 could result in some significant side effects such as swelling, inflammation, seroma, and carcinogenicity, although these could be dose dependent [81]. Therefore, it is considered that additional studies are needed to prove the efficacy and safety of BMP-2 further.
APC is also applied along with the surgical treatment of MRONJ, and its effect is achieved through the local application. APC is rich in several growth factors secreted from platelets, such as platelet-derived growth factor, TGF-β, endothelial growth factor, and vascular endothelial growth factors [82]. Therefore, since they can stimulate and accelerate tissue healing, the application of APC has been reported in the treatment of MRONJ. According to the definition of Dohan Ehrenfest et al. [83], APC is composed of 4 groups depending on the fibrin and leukocyte content. Among them, platelet-rich plasma (PRP) is the most frequently applied for treatment for MRONJ [84], and the application of leukocyte-rich and platelet-rich fibrin (L-PRF) has also been reported [85]. In particular, L-PRF has been in the spotlight as an adjunctive agent in recent years because it releases growth factors for a longer time at the application site compared to PRP. PRP is known to release 95% or more of the presynthesized growth factors within 1 h [86], while PRF is known to sustain the release of these growth factors for at least one week [87]. Besides, L-PRF has the effect of preventing infection by leukocytes and has the function of regulating the immune system [83]. According to a systematic review published in 2016 by Lopez-Jornet et al. [84], 85.98% of the complete resolution was reported when APC was applied with a combination of surgery to treat MRONJ. However, according to another systematic review by Fortunato et al. [88], the application of APC combined with surgery showed a better healing rate than the surgery alone (87.8% vs. 63.8%), but it was not statistically significant. Although sufficient scientific evidence to prove the effectiveness of APC in the treatment of MRONJ is lacking, it is considered promising as an adjunctive agent considering their local immunomodulatory properties and potential of accelerated tissue healing. The main limitation of APC is that there are no specific guidelines or protocols in the application. Besides, in the treatment of MRONJ, which is thought to be a bone disease, the question remains about the direct therapeutic effect of APC. These points will have to be solved in order for APC to be widely used for MRONJ.
3.4. HBO and OT
HBO and OT have been considered effective adjunctive treatment modalities in situations where normal bone healing is impaired, such as osteoradionecrosis and chronic osteomyelitis of the jaw [89,90,91]. Since MRONJ also exhibits the characteristics of reduced bone remodeling and impaired wound healing, HBO and OT are being applied with an anticipation that they might be helpful in the treatment of MRONJ.
HBO was traditionally thought to produce beneficial oxygen gradients [90,92] or correct pathogen killing of hypoxic-impaired leukocytes [89,93]. However, it was later discovered that HBO increases ROS and reactive nitrogen species (RNS), affecting the signaling processes important in wound healing [94,95,96,97,98,99,100,101]. ROS and RNS are known to influence osteoclast differentiation, activity and participate in regulating bone metabolism [102,103,104]. Along with this expectation, Freiberger et al. [105] published a case series in which HBO was applied to MRONJ patients, and remission or improvement was found in 62.5% of patients. Later, Freiberger et al. [106] performed a randomized controlled trial to evaluate HBO’s effectiveness based on their previous experience, but the HBO group did not show any significant difference from the control group in terms of complete gingival coverage. According to a recent systematic review [107], HBO mainly was applied as a neoadjuvant and/or adjuvant therapy in addition to the surgery rather than being used alone. Moreover, 5.17% of cases showed complete remission, while in the majority (48.27%) of the cases, slight benefits such as improvement or stability of symptoms were reported [107]. Therefore, it can be seen that HBO remains in its position as an adjunctive treatment method in the treatment of MRONJ at present.
Ozone generally exists in the form of gas composed of three oxygen atoms and positively affects hard and soft tissues by stimulating endogenous antioxidants [108,109,110]. Therefore, OT has been applied to various indications, including infectious disease, immune dysfunctions, ischemic pathologies, and neurodegenerative pathologies [108,109,110]. When ozone was applied intraorally, infection and inflammation were reduced in animal studies [108,111]. Agrillo et al. [112] first introduced OT as a new therapeutic protocol for patients with MRONJ by combining local minor curettage and antibiotic use. They noted that ozone exerts a positive effect on bone defects through oxidative preconditioning, which stimulates endogenous antioxidant systems and blocks the xanthine/xanthine oxidase pathway for ROS generation [112]. They also stated that ozone has beneficial effects on blood circulation by increasing the concentration of red blood cells and the rate of hemoglobin, which has a biological effect on oxygen, calcium, phosphorus, and iron metabolism germicidal properties [112]. After that, Agrillo et al. [113] reported that 131 patients with MRONJ had a 90% success rate after applying OT with minimally invasive surgery and antibiotic use. In a recent systematic review by Sacco et al. [107], OT was also most commonly used as neoadjuvant and/or adjuvant therapy related to surgery, as was HBO, and a complete resolution was reported in 44.58% of cases, and some symptom improvement was reported in 22.94%. Therefore, OT is also considered an adjunctive therapy for MRONJ treatment that requires more evidence at present.
The main limitation of HBO and OT is that there is no precise protocol besides the relatively low complete resolution rate. For HBO, 2.0–2.5 atm and 90–120 min have been reported for application pressure and application time [106,114,115], but it is currently unclear whether these values are appropriate and optimal. Moreover, guidelines for preoperative and postoperative application and the appropriate number of sessions have not been established. Likewise, in the case of the OT, the delivery method, dose, and frequency of ozone application were not established, and in many studies used, it was often unclearly mentioned. Agrillo et al. [112] initially applied ozone for 20 days at a frequency of application of twice a week for 5 min. In their subsequent study [116], one cycle of OT was performed each before and after surgery, and one cycle consisted of 8 sessions applied for 3 min. Ripamonti et al. [117] applied ozone oil to the lesion once every three days for 10 min and performed a maximum of 10 applications. The inconsistent results due to different application methods for each study can be a disadvantage of OT. Furthermore, in the case of HBO, special facilities such as a pressure chamber are required, and in the case of the OT, special equipment that delivers ozone gas is required. These are considered substantial obstacles to the wide use of HBO and OT for the management of MRONJ.3.5. Laser Therapy
Laser therapy has recently been used in the treatment of MRONJ in isolation or in combination with other treatment modalities, and its beneficial effects on tissue healing are the main reasons for its use [118,119]. The most widely used type of laser is LLLT, which is thought to be a promising adjuvant therapy in the treatment of MRONJ. It is known to regulate cellular metabolism, relieve pain, and improve wound healing. In addition, laser irradiation induces biostimulant effects that increase the number of differentiated osteoblasts and their activity, and the effect of LLLT on bone regeneration has also been reported in the field of dentistry [120]. Laser’s unique non-invasive properties and antibacterial effects on soft and hard tissues are also considered advantages of laser therapy.
As another kind of laser, the Er:YAG laser, which belongs to the family of high-power lasers, is thought to be a promising treatment modality for MRONJ. Because it has an affinity for water and hydroxyapatite and is well absorbed by them, it may be helpful in the management of bone tissues. More conservative surgery is possible because it produces an ablative surface conducive to cell attachment while less damaging the surrounding tissues [121]. When Er:Yag laser surgery was applied to the MRONJ lesion combined with LLLT, excellent mucosal healing was shown, and in the long-term follow-up, most cases showed complete healing [121,122]. Vescovi et al. [122] reported that the early laser-assisted conservative surgical approach with LLLT is a more reliable treatment than conservative medical therapy. However, since there were very few patients in Stage 3 treated with an Er:YAG laser in Vescovi et al.’s study [122], the application in the later stage of MRONJ seems to be lacking evidence.
Regarding the effectiveness of laser therapy, Weber et al. [123] published a systematic review in 2016 to investigate the positive effects of laser therapy in the management of MRONJ, and treatment modalities including laser therapy showed superior results compared to the conventional surgical therapy and/or the conservative medical therapy in terms of cure and improvement of lesions in the early stages of MRONJ. However, since they could not find a randomized controlled trial related to laser therapy, careful interpretation is considered necessary.
Among the limitations of laser therapy, the most critical is the lack of consistent reports on laser parameters. Due to the diversity of variables such as laser type, output power, output frequency, application time, and distance between the laser light source and the applied tissue, it is not easy to establish a standard protocol and collect results from every study. This may cause clinicians to hesitate to apply laser therapy to MRONJ treatment. Moreover, the need for a separate laser device and the need to wear safety glasses to protect the eyes from lasers are annoying disadvantages of laser treatment. If these shortcomings are addressed and additional results of higher-level research accumulate, laser therapy may play a more significant role in the treatment of MRONJ.3.6. Drug Holiday
As much as the debate over effective MRONJ treatment modality, the drug holiday is provoking a fierce debate among clinicians. Strictly speaking, drug holidays cannot be a treatment modality, but it is worth mentioning in this paper because it is an essential consideration in the prevention or treatment of MRONJ. A position paper of AAOMS in 2009 recommended discontinuation of oral bisphosphonates for three months before and three months after invasive dental surgery [5]. However, in a later position paper in 2014 [2], the modified drug holiday strategy was suggested by Damm and Jones [124]. That is, for patients who have been administered bisphosphonates for an extended period of more than four years, it is suggested that having a drug holiday for two months before invasive dental procedures is theoretically advantageous. Besides, drug holidays of the same period were recommended for patients who were administered bisphosphonate for less than four years and were also administered corticosteroids or antiangiogenic agents. Since it has been reported that a high total accumulation dose of bisphosphonate is associated with a significantly poor prognosis in the treatment of MRONJ [125], a drug holiday is considered to be necessary as the duration of bisphosphonate administration increases. However, there is no data on a drug holiday for denosumab and antiangiogenic agents, so, in this case, the clinician faces a difficult situation. Moreover, there is no explicit mention of the postoperative drug holiday in the 2014 position paper [2]. It only states that antiresorptive therapy should not be resumed until osseous healing has occurred. In the surgical treatment of MRONJ, the postoperative drug-free period is also crucial in that postoperative antiresorptive therapy or antiangiogenic therapy must be temporarily stopped not only because the bone exposure is accompanied during surgery but also because the bone healing must be expected after surgery.
There are two main views on the drug holiday for MRONJ: effective [126,127,128] or ineffective [129,130,131]. A recent systematic review [132], including three prospective studies and 11 retrospective studies, concluded that a drug holiday discontinuing high-dose antiresorptive therapy was unnecessary. However, since the studies included in the previous systematic review were heterogeneous, the meta-analysis could not be performed, and there is a limitation that the conclusion of only one controlled prospective study was asserted as it is. Therefore, at present, it is not possible to conclude whether or not a drug holiday is necessary for the management of MRONJ and the exact duration of suspension for each drug. Additional position papers based on data accumulation from more studies are expected in the future.
4. Promising Molecular and Cellular Therapeutic Methods for MRONJ
4.1. Teriparatide
Teriparatide is an analog of the human parathyroid hormone (PTH), consisting of the first 34 amino acids produced by genetic recombination. It was first approved for use in the treatment of osteoporosis by the United States Food and Drug Administration in 2002. When administered intermittently at low doses, PTH has an osteoanabolic effect, but when applied continuously, it exhibits an osteocatabolic effect, as seen in hyperparathyroidism [133]. In addition to its basic anti-fracture effect, it is widely used because its efficacy has been proven in low bone turnover conditions seen in patients with diabetes or patients undergoing long-term use of bisphosphonates [134,135,136].
In MRONJ, the expected effects of teriparatide might be to activate bone remodeling and increase bone formation, thereby inducing bone healing and necrotic bone removal. There is also an opinion that teriparatide shows its efficacy by activating WNT signaling and suppressing sclerostin production [133]. The osteoanabolic effect of teriparatide was confirmed in the craniofacial area [3,16], and in the field of dentistry, it was also found to promote bone growth and healing in patients with chronic periodontitis [137]. Due to this tangible osteoanabolic effect, expectations for the application of teriparatide for the management of MRONJ are increasing.
Among the studies that applied teriparatide for the treatment of MRONJ, the most interesting study is the randomized controlled study conducted by Sim et al. [3] in 2020. In their study, 34 patients with a total of 47 MRONJ lesions randomly received teriparatide (20μg/day) injection or placebo injection for eight weeks and were observed for 12 months. Teriparatide healed 45.4% of lesions at 52 weeks, showing a higher resolution rate than the placebo group in which 33.3% of lesions were healed. Teriparatide also increased bone volume, reducing bone defect size in a larger proportion of patients at 52 weeks (80.0% teriparatide versus 31.3% placebo). Besides, teriparatide increased the levels of P1NP and CTX, which are bone turnover markers. P1NP increased by three times compared to placebo at 4 and 8 weeks after receiving teriparatide, and CTX increased by 30% at eight weeks after. Sim et al.’s study [3] is significant because it is the first study to apply teriparatide in the treatment of MRONJ with a double-blind, randomized design. It also presents a new perspective and instills hope in the treatment of MRONJ.
Even with this promising teriparatide, there are still several aspects to overcome. In a toxicity study using rats [138], teriparatide increases osteosarcoma incidence, and there is a concern about the potential risk for osteosarcoma. However, it is difficult for this result to be applied equally to humans since much larger doses have been administered throughout their entire life (about 24 months) than those applied to humans. Besides, these findings were not confirmed in the subsequent studies of rats and monkeys with long-term treatment [139,140]. Moreover, during the period of use after being marketed for nearly 18 years, according to data from clinical studies or post-marketing surveillance, the association between teriparatide treatment and osteosarcoma has not been observed in humans [133,141].
Nevertheless, since the osteoanabolic effect of teriparatide can theoretically cause the proliferation of dormant malignant cells in bone, it is considered that the safety of its use in oncologic patients should continue to be verified. Second, there are no results for long-term treatment. Therefore, large-scale and high-quality clinical studies about the long-term effect of teriparatide are continuously needed. Furthermore, the relatively high price of teriparatide and the need for good patient cooperation due to subcutaneous injection may be a limitation in the widespread use of teriparatide. Therefore, the use of teriparatide for the treatment of MRONJ should be determined based on an individual patient risk assessment by clinicians and should be applied in consideration of proven beneficial effects and potential safety issues.
4.2. MSC Therapy
MSCs are multipotent cells that have been widely applied in regenerative medicine in recent decades. MSCs are involved in organogenesis during embryogenesis, and after that, in the maintenance of adult tissues.
It is well known that MSCs can differentiate into tissue-forming cells, such as osteoblasts, chondrocytes, and adipocytes. Based on their capacity to differentiate into osteoblasts and their immunomodulatory properties, MSCs might be used as a graft material for osteonecrosis areas [142]. MSCs are generally obtained from bone marrow or adipose tissue in adults, but they also exist in various other tissues [143].
MSCs have been mainly applied in the form of grafts to verify the therapeutic potential of MRONJ, and MSC grafts have obtained good results in mice, pigs, and humans [144,145,146]. However, these results seem to be due to their immunomodulatory properties rather than their ability to differentiate into osteoblasts. The efficacy of an MSC graft was associated with its ability to increase IL-10, TGF-β1, and regulatory T cells and decrease IL-6, IL-17, and c-reactive proteins [145,146]. There is no evidence that MSC grafts are directly related to bone regeneration by their osteoblast differentiation. Since the average survival time of the grafted MSCs is from 1 to 2 weeks, this period is insufficient for direct bone regeneration, but rather it is estimated to be indirectly related to bone regeneration [147]. The indirect effect of MSC graft is presumed to stimulate regional endogenous cells to differentiate into osteoblasts, thereby affecting bone regeneration [2]. It was found that the jaw bone cells arise from cranial neural crest stem cells (NCSCs), and the jaw defects are recovered by neural crest cell recruitment [148]. Therefore, it is thought that graft-containing NCSC is beneficial for the healing of jaw lesions, and it was recently shown that adult bone marrow MSC and adipose-derived MSC are populations containing NSCS. The effectiveness of MSCs related to NSCS is a part that requires further research, but it is worth continuing studies.
Although there are many animal studies to investigate the effect of MSC on MRONJ treatment, few studies have applied MSC to humans to treat MRONJ, and only studies at the level of the case report or pilot study have been reported. Cella et al. [144] first published a case report in 2011 that achieved complete healing of the lesion after applying autologous bone marrow stem cells to a patient with MRONJ Stage 3 who did not respond to the conventional treatment. They injected bone marrow stem cells harvested from the posterior superior iliac crest in a fibrin sponge carrier, along with PRP, and applied it to the bone lesion. The resolution of symptoms and improvement of the lesion were obtained after two weeks, while bone healing and concentric ossification were observed through computed tomography at postoperative 15 months. Complete healing was confirmed 30 months later. The case report published by Gonzalvez-Garcia et al. [149] in 2013 did not differ significantly from that of Cella et al. [144], but the application of autologous MSC together with β-tricalcium phosphate, demineralized bone matrix, and PRP yielded good results of cure after six months. A pilot study conducted by Voss et al. [150] in 2017 reported satisfactory healing after applying autologous MSC to the site where the necrotic bone was removed, and autologous thrombin was applied in 6 patients with MRONJ Stage 2, covering it with a collagen membrane. They mentioned that MSC transplantation with surgical treatment is a promising treatment method for the treatment of MRONJ. The most recent report by Santis et al. [151] showed a successful treatment result in two MRONJ patients by applying autologous MSC expanded ex vivo for 3–4 weeks together with a bone substitute. In contrast to the autologous MSC, studies in which adipose-derived MSC or allogenic bone marrow MSC is applied to the treatment of MRONJ are limited to animal studies.
In the treatment of MRONJ, the limitation of MSC therapy is that it is not known precisely whether the primary mechanism of MSC is due to bone regeneration associated with osteoblast differentiation (direct or indirect) or immunomodulatory properties, or both. Moreover, there are some drawbacks to MSC therapy, including a bothersome procedure of collecting MSC from other parts of the body and the need for additional equipment such as a centrifuge. However, this shortcoming already exists in the application of PRP or PRF, and it is expected to be sufficiently overcome. Lastly, similar to other treatment methods using graft, it is challenging to apply MSC as a sole treatment modality in the treatment of MRONJ. Instead, it seems to be an adjunctive treatment method that requires surgical treatment. However, despite these limitations, treatment using MSC is considered to be one of the most promising treatment methods along with teriparatide due to the regenerative potential of MSC itself.
5. Conclusions
MRONJ is currently a refractory disease that must be overcome in the maxillofacial area, and the gold standard treatment has still not been identified. Various treatment modalities have been developed, but they are insufficient to heal MRONJ lesions as a single treatment method. Teriparatide is in the spotlight as a new therapeutic agent and has shown potential as a single therapeutic solution for the MRONJ, but it needs to be verified through more clinical studies to establish itself as a standard treatment. Besides, many studies developing new cellular therapeutic approaches such as MSC therapy are ongoing. A new path of MRONJ treatment may be built through various combinations of existing treatment methods or those of the existing and the new treatment methods. In the management of the MRONJ, prevention is also crucial along with the treatment. For the prevention of MRONJ, patients scheduled to be administered antiresorptive or antiangiogenic agents should undergo thorough intraoral screening and appropriate dental care such as patient motivation and education to maintain oral hygiene, fluoride application, and chlorhexidine rinses. Moreover, multidisciplinary discussions with doctors in other medical fields and biological scientists are needed on topics such as the appropriate drug-free period of each triggered drug and the risk evaluation regarding the accumulation period. It is also believed that further research and studies for the development of effective treatment modalities for MRONJ are required, along with the cooperation between researchers and clinicians.
Author Contributions
Conceptualization, S.-W.O. and B.-E.Y.; methodology, S.-W.O. and B.-E.Y.; Investigation, S.-W.O.; writing—original draft preparation, S.-W.O.; writing—review and editing, S.-H.B., S.-W.C., and B.-E.Y.; visualization, S.-W.O.; supervision, S.-H.B. and B.-E.Y.; project administration, B.-E.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This review received no external funding.
Acknowledgments
This work was supported by the Medical Device Technology Development Program (20006006, development of artificial intelligence-based augmented reality surgery system for oral and maxillofacial surgery) funded by the Ministry of Trade, Industry, and Energy, Republic of Korea.
Conflicts of Interest
The authors declare no competing interests.
References
- Marx, R.E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic. J. Oral Maxillofac. Surg. 2003, 61, 1115–1117. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 Update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef] [PubMed]
- Sim, I.W.; Borromeo, G.L.; Tsao, C.; Hardiman, R.; Hofman, M.S.; Papatziamos Hjelle, C.; Siddique, M.; Cook, G.J.R.; Seymour, J.F.; Ebeling, P.R. Teriparatide Promotes Bone Healing in Medication-Related Osteonecrosis of the Jaw: A Placebo-Controlled, Randomized Trial. J. Clin. Oncol. 2020, 38, 2971–2980. [Google Scholar] [CrossRef] [PubMed]
- Advisory Task Force on Bisphosphonate-Related Ostenonecrosis of the Jaws, American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J. Oral Maxillofac. Surg. 2007, 65, 369–376. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Assael, L.A.; Landesberg, R.; Marx, R.E.; Mehrotra, B. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws—2009 Update. J. Oral Maxillofac. Surg. 2009, 67, 2–12. [Google Scholar] [CrossRef]
- Baron, R.; Ferrari, S.; Russell, R.G. Denosumab and bisphosphonates: Different mechanisms of action and effects. Bone 2011, 48, 677–692. [Google Scholar] [CrossRef]
- Lacey, D.L.; Boyle, W.J.; Simonet, W.S.; Kostenuik, P.J.; Dougall, W.C.; Sullivan, J.K.; San Martin, J.; Dansey, R. Bench to bedside: Elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat. Rev. Drug Discov. 2012, 11, 401–419. [Google Scholar] [CrossRef]
- Roelofs, A.J.; Thompson, K.; Gordon, S.; Rogers, M.J. Molecular mechanisms of action of bisphosphonates: Current status. Clin. Cancer Res. 2006, 12, 6222s–6230s. [Google Scholar] [CrossRef]
- Russell, R.G.; Rogers, M.J. Bisphosphonates: From the laboratory to the clinic and back again. Bone 1999, 25, 97–106. [Google Scholar] [CrossRef]
- Russell, R.G.; Watts, N.B.; Ebetino, F.H.; Rogers, M.J. Mechanisms of action of bisphosphonates: Similarities and differences and their potential influence on clinical efficacy. Osteoporos. Int. 2008, 19, 733–759. [Google Scholar] [CrossRef]
- Hoefert, S.; Schmitz, I.; Tannapfel, A.; Eufinger, H. Importance of microcracks in etiology of bisphosphonate-related osteonecrosis of the jaw: A possible pathogenetic model of symptomatic and non-symptomatic osteonecrosis of the jaw based on scanning electron microscopy findings. Clin. Oral Investig. 2010, 14, 271–284. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Carlson, E.R.; Assael, L.A. Comprehensive review of bisphosphonate therapy: Implications for the oral and maxillofacial surgery patient. J. Oral Maxillofac. Surg. 2009, 67, 1. [Google Scholar] [CrossRef]
- Lesclous, P.; Abi Najm, S.; Carrel, J.P.; Baroukh, B.; Lombardi, T.; Willi, J.P.; Rizzoli, R.; Saffar, J.L.; Samson, J. Bisphosphonate-associated osteonecrosis of the jaw: A key role of inflammation? Bone 2009, 45, 843–852. [Google Scholar] [CrossRef]
- Hansen, T.; Kunkel, M.; Weber, A.; James Kirkpatrick, C. Osteonecrosis of the jaws in patients treated with bisphosphonates—Histomorphologic analysis in comparison with infected osteoradionecrosis. J. Oral Pathol. Med. 2006, 35, 155–160. [Google Scholar] [CrossRef]
- O’Ryan, F.S.; Khoury, S.; Liao, W.; Han, M.M.; Hui, R.L.; Baer, D.; Martin, D.; Liberty, D.; Lo, J.C. Intravenous bisphosphonate-related osteonecrosis of the jaw: Bone scintigraphy as an early indicator. J. Oral Maxillofac. Surg. 2009, 67, 1363–1372. [Google Scholar] [CrossRef]
- Cheung, A.; Seeman, E. Teriparatide therapy for alendronate-associated osteonecrosis of the jaw. N. Engl. J. Med. 2010, 363, 2473–2474. [Google Scholar] [CrossRef]
- Allen, M.R.; Burr, D.B. The pathogenesis of bisphosphonate-related osteonecrosis of the jaw: So many hypotheses, so few data. J. Oral Maxillofac. Surg. 2009, 67, 61–70. [Google Scholar] [CrossRef]
- Kim, H.K. Introduction to osteonecrosis of the femoral head (OFH) and osteonecrosis of the jaw (ONJ). J. Musculoskelet. Neuronal Interact. 2007, 7, 350–353. [Google Scholar]
- Landesberg, R.; Woo, V.; Cremers, S.; Cozin, M.; Marolt, D.; Vunjak-Novakovic, G.; Kousteni, S.; Raghavan, S. Potential pathophysiological mechanisms in osteonecrosis of the jaw. Ann. N. Y. Acad. Sci. 2011, 1218, 62–79. [Google Scholar] [CrossRef]
- Yamashita, J.; McCauley, L.K. Antiresorptives and osteonecrosis of the jaw. J. Evid.-Based Dent. Pract. 2012, 12, 233–247. [Google Scholar] [CrossRef]
- Chang, J.; Hakam, A.E.; McCauley, L.K. Current Understanding of the Pathophysiology of Osteonecrosis of the Jaw. Curr. Osteoporos. Rep. 2018, 16, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Pimolbutr, K.; Porter, S.; Fedele, S. Osteonecrosis of the Jaw Associated with Antiangiogenics in Antiresorptive-Naive Patient: A Comprehensive Review of the Literature. BioMed Res. Int. 2018, 2018, 8071579. [Google Scholar] [CrossRef]
- Aldridge, S.E.; Lennard, T.W.; Williams, J.R.; Birch, M.A. Vascular endothelial growth factor receptors in osteoclast differentiation and function. Biochem. Biophys. Res. Commun. 2005, 335, 793–798. [Google Scholar] [CrossRef]
- Pazianas, M. Osteonecrosis of the jaw and the role of macrophages. J. Natl. Cancer Inst. 2011, 103, 232–240. [Google Scholar] [CrossRef]
- Santini, D.; Vincenzi, B.; Dicuonzo, G.; Avvisati, G.; Massacesi, C.; Battistoni, F.; Gavasci, M.; Rocci, L.; Tirindelli, M.C.; Altomare, V.; et al. Zoledronic acid induces significant and long-lasting modifications of circulating angiogenic factors in cancer patients. Clin. Cancer Res. 2003, 9, 2893–2897. [Google Scholar]
- Lombard, T.; Neirinckx, V.; Rogister, B.; Gilon, Y.; Wislet, S. Medication-Related Osteonecrosis of the Jaw: New Insights into Molecular Mechanisms and Cellular Therapeutic Approaches. Stem Cells Int. 2016, 2016, 8768162. [Google Scholar] [CrossRef]
- Aghaloo, T.L.; Kang, B.; Sung, E.C.; Shoff, M.; Ronconi, M.; Gotcher, J.E.; Bezouglaia, O.; Dry, S.M.; Tetradis, S. Periodontal disease and bisphosphonates induce osteonecrosis of the jaws in the rat. J. Bone Miner. Res. 2011, 26, 1871–1882. [Google Scholar] [CrossRef]
- Aguirre, J.I.; Akhter, M.P.; Kimmel, D.B.; Pingel, J.E.; Williams, A.; Jorgensen, M.; Kesavalu, L.; Wronski, T.J. Oncologic doses of zoledronic acid induce osteonecrosis of the jaw-like lesions in rice rats (Oryzomys palustris) with periodontitis. J. Bone Miner. Res. 2012, 27, 2130–2143. [Google Scholar] [CrossRef]
- Gotcher, J.E.; Jee, W.S. The progress of the periodontal syndrome in the rice rat. I. Morphometric and autoradiographic studies. J. Periodontal Res. 1981, 16, 275–291. [Google Scholar] [CrossRef]
- Kang, B.; Cheong, S.; Chaichanasakul, T.; Bezouglaia, O.; Atti, E.; Dry, S.M.; Pirih, F.Q.; Aghaloo, T.L.; Tetradis, S. Periapical disease and bisphosphonates induce osteonecrosis of the jaws in mice. J. Bone Miner. Res. 2013, 28, 1631–1640. [Google Scholar] [CrossRef]
- Lopez-Jornet, P.; Camacho-Alonso, F.; Martinez-Canovas, A.; Molina-Minano, F.; Gomez-Garcia, F.; Vicente-Ortega, V. Perioperative antibiotic regimen in rats treated with pamidronate plus dexamethasone and subjected to dental extraction: A study of the changes in the jaws. J. Oral Maxillofac. Surg. 2011, 69, 2488–2493. [Google Scholar] [CrossRef] [PubMed]
- Mawardi, H.; Treister, N.; Richardson, P.; Anderson, K.; Munshi, N.; Faiella, R.A.; Woo, S.B. Sinus tracts—An early sign of bisphosphonate-associated osteonecrosis of the jaws? J. Oral Maxillofac. Surg. 2009, 67, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Sedghizadeh, P.P.; Kumar, S.K.; Gorur, A.; Schaudinn, C.; Shuler, C.F.; Costerton, J.W. Microbial biofilms in osteomyelitis of the jaw and osteonecrosis of the jaw secondary to bisphosphonate therapy. J. Am. Dent. Assoc. 2009, 140, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Allam, E.; Allen, M.; Chu, T.M.; Ghoneima, A.; Jack Windsor, L. In vivo effects of zoledronic acid on oral mucosal epithelial cells. Oral Dis. 2011, 17, 291–297. [Google Scholar] [CrossRef]
- Reid, I.R.; Cornish, J. Epidemiology and pathogenesis of osteonecrosis of the jaw. Nat. Rev. Rheumatol. 2011, 8, 90–96. [Google Scholar] [CrossRef]
- Garcia-Heredia, A.; Kensicki, E.; Mohney, R.P.; Rull, A.; Triguero, I.; Marsillach, J.; Tormos, C.; Mackness, B.; Mackness, M.; Shih, D.M.; et al. Paraoxonase-1 deficiency is associated with severe liver steatosis in mice fed a high-fat high-cholesterol diet: A metabolomic approach. J. Proteome Res. 2013, 12, 1946–1955. [Google Scholar] [CrossRef]
- Battisti, V.; Maders, L.D.; Bagatini, M.D.; Reetz, L.G.; Chiesa, J.; Battisti, I.E.; Goncalves, J.F.; Duarte, M.M.; Schetinger, M.R.; Morsch, V.M. Oxidative stress and antioxidant status in prostate cancer patients: Relation to Gleason score, treatment and bone metastasis. Biomed. Pharmacother. 2011, 65, 516–524. [Google Scholar] [CrossRef]
- Kocer, G.; Naziroglu, M.; Celik, O.; Onal, L.; Ozcelik, D.; Kocer, M.; Sonmez, T.T. Basic fibroblast growth factor attenuates bisphosphonate-induced oxidative injury but decreases zinc and copper levels in oral epithelium of rat. Biol. Trace Elem. Res. 2013, 153, 251–256. [Google Scholar] [CrossRef]
- Awodele, O.; Olayemi, S.O.; Nwite, J.A.; Adeyemo, T.A. Investigation of the levels of oxidative stress parameters in HIV and HIV-TB co-infected patients. J. Infect. Dev. Ctries. 2012, 6, 79–85. [Google Scholar] [CrossRef]
- Lebreton, F.; van Schaik, W.; Sanguinetti, M.; Posteraro, B.; Torelli, R.; Le Bras, F.; Verneuil, N.; Zhang, X.; Giard, J.C.; Dhalluin, A.; et al. AsrR is an oxidative stress sensing regulator modulating Enterococcus faecium opportunistic traits, antimicrobial resistance, and pathogenicity. PLoS Pathog. 2012, 8, e1002834. [Google Scholar] [CrossRef]
- Moye-Rowley, W.S. Transcription factors regulating the response to oxidative stress in yeast. Antioxid. Redox Signal. 2002, 4, 123–140. [Google Scholar] [CrossRef]
- Ichiseki, T.; Kaneuji, A.; Katsuda, S.; Ueda, Y.; Sugimori, T.; Matsumoto, T. DNA oxidation injury in bone early after steroid administration is involved in the pathogenesis of steroid-induced osteonecrosis. Rheumatology 2005, 44, 456–460. [Google Scholar] [CrossRef]
- Ichiseki, T.; Matsumoto, T.; Nishino, M.; Kaneuji, A.; Katsuda, S. Oxidative stress and vascular permeability in steroid-induced osteonecrosis model. J. Orthop. Sci. 2004, 9, 509–515. [Google Scholar] [CrossRef]
- Kuribayashi, M.; Fujioka, M.; Takahashi, K.A.; Arai, Y.; Ishida, M.; Goto, T.; Kubo, T. Vitamin E prevents steroid-induced osteonecrosis in rabbits. Acta Orthop. 2010, 81, 154–160. [Google Scholar] [CrossRef]
- Bagan, J.; Saez, G.T.; Tormos, M.C.; Gavalda-Esteve, C.; Bagan, L.; Leopoldo-Rodado, M.; Calvo, J.; Camps, C. Oxidative stress in bisphosphonate-related osteonecrosis of the jaws. J. Oral Pathol. Med. 2014, 43, 371–377. [Google Scholar] [CrossRef]
- Tamaoka, J.; Takaoka, K.; Hattori, H.; Ueta, M.; Maeda, H.; Yamamura, M.; Yamanegi, K.; Noguchi, K.; Kishimoto, H. Osteonecrosis of the jaws caused by bisphosphonate treatment and oxidative stress in mice. Exp. Ther. Med. 2019, 17, 1440–1448. [Google Scholar] [CrossRef]
- Taniguchi, N.; Osaki, M.; Onuma, K.; Ishikawa, M.; Ryoke, K.; Kodani, I.; Okada, F. Bisphosphonate-induced reactive oxygen species inhibit proliferation and migration of oral fibroblasts: A pathogenesis of bisphosphonate-related osteonecrosis of the jaw. J. Periodontol. 2020, 91, 947–955. [Google Scholar] [CrossRef]
- Yatsuoka, W.; Ueno, T.; Miyano, K.; Uezono, Y.; Enomoto, A.; Kaneko, M.; Ota, S.; Soga, T.; Sugimoto, M.; Ushijima, T. Metabolomic profiling reveals salivary hypotaurine as a potential early detection marker for medication-related osteonecrosis of the jaw. PLoS ONE 2019, 14, e0220712. [Google Scholar] [CrossRef]
- Suzuki, K.; Takeyama, S.; Murakami, S.; Nagaoka, M.; Chiba, M.; Igarashi, K.; Shinoda, H. Structure-Dependent Effects of Bisphosphonates on Inflammatory Responses in Cultured Neonatal Mouse Calvaria. Antioxidants 2020, 9, 503. [Google Scholar] [CrossRef]
- Ristow, O.; Ruckschloss, T.; Muller, M.; Berger, M.; Kargus, S.; Pautke, C.; Engel, M.; Hoffmann, J.; Freudlsperger, C. Is the conservative non-surgical management of medication-related osteonecrosis of the jaw an appropriate treatment option for early stages? A long-term single-center cohort study. J. Cranio-Maxillofac. Surg. 2019, 47, 491–499. [Google Scholar] [CrossRef]
- Bermudez-Bejarano, E.B.; Serrera-Figallo, M.A.; Gutierrez-Corrales, A.; Romero-Ruiz, M.M.; Castillo-de-Oyague, R.; Gutierrez-Perez, J.L.; Torres-Lagares, D. Prophylaxis and antibiotic therapy in management protocols of patients treated with oral and intravenous bisphosphonates. J. Clin. Exp. Dent. 2017, 9, e141–e149. [Google Scholar] [CrossRef] [PubMed]
- Hallmer, F.; Bjornland, T.; Andersson, G.; Becktor, J.P.; Kristoffersen, A.K.; Enersen, M. Bacterial diversity in medication-related osteonecrosis of the jaw. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 123, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Ramaglia, L.; Guida, A.; Iorio-Siciliano, V.; Cuozzo, A.; Blasi, A.; Sculean, A. Stage-specific therapeutic strategies of medication-related osteonecrosis of the jaws: A systematic review and meta-analysis of the drug suspension protocol. Clin. Oral Investig. 2018, 22, 597–615. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Avdic, E.; Li, D.X.; Dzintars, K.; Cosgrove, S.E. Association of Adverse Events With Antibiotic Use in Hospitalized Patients. JAMA Intern. Med. 2017, 177, 1308–1315. [Google Scholar] [CrossRef]
- Werner, N.L.; Hecker, M.T.; Sethi, A.K.; Donskey, C.J. Unnecessary use of fluoroquinolone antibiotics in hospitalized patients. BMC Infect. Dis. 2011, 11, 187. [Google Scholar] [CrossRef]
- Khan, A.A.; Morrison, A.; Hanley, D.A.; Felsenberg, D.; McCauley, L.K.; O’Ryan, F.; Reid, I.R.; Ruggiero, S.L.; Taguchi, A.; Tetradis, S.; et al. Diagnosis and management of osteonecrosis of the jaw: A systematic review and international consensus. J. Bone Miner. Res. 2015, 30, 3–23. [Google Scholar] [CrossRef]
- Nisi, M.; Karapetsa, D.; Gennai, S.; Ramaglia, L.; Graziani, F.; Gabriele, M. Conservative surgical treatment of medication related osteonecrosis of the jaw (MRONJ) lesions in patients affected by osteoporosis exposed to oral bisphosphonates: 24 months follow-up. J. Cranio-Maxillofac. Surg. 2018, 46, 1153–1158. [Google Scholar] [CrossRef]
- Vanpoecke, J.; Verstraete, L.; Smeets, M.; Ferri, J.; Nicot, R.; Politis, C. Medication-related osteonecrosis of the jaw (MRONJ) stage III: Conservative and conservative surgical approaches versus an aggressive surgical intervention: A systematic review. J. Cranio-Maxillofac. Surg. 2020, 48, 435–443. [Google Scholar] [CrossRef]
- Eguchi, T.; Kanai, I.; Basugi, A.; Miyata, Y.; Inoue, M.; Hamada, Y. The assessment of surgical and non-surgical treatment of stage II medication-related osteonecrosis of the jaw. Med. Oral Patol. Oral Cir. Bucal 2017, 22, e788–e795. [Google Scholar] [CrossRef]
- El-Rabbany, M.; Sgro, A.; Lam, D.K.; Shah, P.S.; Azarpazhooh, A. Effectiveness of treatments for medication-related osteonecrosis of the jaw: A systematic review and meta-analysis. J. Am. Dent. Assoc. 2017, 148, 584–594. [Google Scholar] [CrossRef]
- Hayashida, S.; Soutome, S.; Yanamoto, S.; Fujita, S.; Hasegawa, T.; Komori, T.; Kojima, Y.; Miyamoto, H.; Shibuya, Y.; Ueda, N.; et al. Evaluation of the Treatment Strategies for Medication-Related Osteonecrosis of the Jaws (MRONJ) and the Factors Affecting Treatment Outcome: A Multicenter Retrospective Study with Propensity Score Matching Analysis. J. Bone Miner. Res. 2017, 32, 2022–2029. [Google Scholar] [CrossRef]
- Lopes, R.N.; Rabelo, G.D.; Rocha, A.C.; Carvalho, P.A.; Alves, F.A. Surgical Therapy for Bisphosphonate-Related Osteonecrosis of the Jaw: Six-Year Experience of a Single Institution. J. Oral Maxillofac. Surg. 2015, 73, 1288–1295. [Google Scholar] [CrossRef]
- Marciano, A.; Rubino, E.; Peditto, M.; Mauceri, R.; Oteri, G. Oral Surgical Management of Bone and Soft Tissues in MRONJ Treatment: A Decisional Tree. Life 2020, 10, 99. [Google Scholar] [CrossRef]
- Giudice, A.; Barone, S.; Diodati, F.; Antonelli, A.; Nocini, R.; Cristofaro, M.G. Can Surgical Management Improve Resolution of Medication-Related Osteonecrosis of the Jaw at Early Stages? A Prospective Cohort Study. J. Oral Maxillofac. Surg. 2020, 78, 1986–1999. [Google Scholar] [CrossRef]
- Lesclous, P.; Grabar, S.; Abi Najm, S.; Carrel, J.P.; Lombardi, T.; Saffar, J.L.; Samson, J. Relevance of surgical management of patients affected by bisphosphonate-associated osteonecrosis of the jaws. A prospective clinical and radiological study. Clin. Oral Investig. 2014, 18, 391–399. [Google Scholar] [CrossRef]
- Favia, G.; Tempesta, A.; Limongelli, L.; Crincoli, V.; Maiorano, E. Medication-related osteonecrosis of the jaw: Surgical or non-surgical treatment? Oral Dis. 2018, 24, 238–242. [Google Scholar] [CrossRef]
- Lorenzo, S.D.; Trapassi, A.; Corradino, B.; Cordova, A. Histology of the Oral Mucosa in Patients With BRONJ at III Stage: A Microscopic Study Proves the Unsuitability of Local Mucosal Flaps. J. Clin. Med. Res. 2013, 5, 22–25. [Google Scholar] [CrossRef][Green Version]
- Mucke, T.; Koerdt, S.; Jung, M.; Mitchell, D.A.; Wolff, K.D.; Kesting, M.R.; Loeffelbein, D.J. The role of mylohyoid flap in the treatment of bisphosphonate-related osteonecrosis of the jaws. J. Cranio-Maxillofac. Surg. 2016, 44, 369–373. [Google Scholar] [CrossRef]
- Rotaru, H.; Kim, M.K.; Kim, S.G.; Park, Y.W. Pedicled buccal fat pad flap as a reliable surgical strategy for the treatment of medication-related osteonecrosis of the jaw. J. Oral Maxillofac. Surg. 2015, 73, 437–442. [Google Scholar] [CrossRef]
- Blus, C.; Giannelli, G.; Szmukler-Moncler, S.; Orru, G. Treatment of medication-related osteonecrosis of the jaws (MRONJ) with ultrasonic piezoelectric bone surgery. A case series of 20 treated sites. Oral Maxillofac. Surg. 2017, 21, 41–48. [Google Scholar] [CrossRef]
- Giudice, A.; Bennardo, F.; Barone, S.; Antonelli, A.; Figliuzzi, M.M.; Fortunato, L. Can Autofluorescence Guide Surgeons in the Treatment of Medication-Related Osteonecrosis of the Jaw? A Prospective Feasibility Study. J. Oral Maxillofac. Surg. 2018, 76, 982–995. [Google Scholar] [CrossRef]
- Pellegrino, G.; Pavanelli, F.; Ferri, A.; Lizio, G.; Parrulli, R.; Marchetti, C. Ultrasonic Navigation for the Treatment of Medication-Related Jaw Osteonecrosis Involving the Inferior Alveolar Nerve: A Case Report and Protocol Review. Methods Protoc. 2020, 3, 70. [Google Scholar] [CrossRef]
- Marx, R.E.; Sawatari, Y.; Fortin, M.; Broumand, V. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: Risk factors, recognition, prevention, and treatment. J. Oral Maxillofac. Surg. 2005, 63, 1567–1575. [Google Scholar] [CrossRef]
- Pichardo, S.E.; Kuijpers, S.C.; van Merkesteyn, J.P. Bisphosphonate-related osteonecrosis of the jaws: Cohort study of surgical treatment results in seventy-four stage II/III patients. J. Cranio-Maxillofac. Surg. 2016, 44, 1216–1220. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, M.; Mundy, G.R. Bone morphogenetic proteins. Growth Factors 2004, 22, 233–241. [Google Scholar] [CrossRef]
- Cicciu, M.; Herford, A.S.; Juodzbalys, G.; Stoffella, E. Recombinant human bone morphogenetic protein type 2 application for a possible treatment of bisphosphonates-related osteonecrosis of the jaw. J. Craniofacial Surg. 2012, 23, 784–788. [Google Scholar] [CrossRef]
- Gerard, D.A.; Carlson, E.R.; Gotcher, J.E.; Pickett, D.O. Early inhibitory effects of zoledronic acid in tooth extraction sockets in dogs are negated by recombinant human bone morphogenetic protein. J. Oral Maxillofac. Surg. 2014, 72, 61–66. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, J.W.; Kim, S.J. Does the Addition of Bone Morphogenetic Protein 2 to Platelet-Rich Fibrin Improve Healing After Treatment for Medication-Related Osteonecrosis of the Jaw? J. Oral Maxillofac. Surg. 2017, 75, 1176–1184. [Google Scholar] [CrossRef]
- Pelaez, M.; Susin, C.; Lee, J.; Fiorini, T.; Bisch, F.C.; Dixon, D.R.; McPherson, J.C., 3rd; Buxton, A.N.; Wikesjo, U.M. Effect of rhBMP-2 dose on bone formation/maturation in a rat critical-size calvarial defect model. J. Clin. Periodontol. 2014, 41, 827–836. [Google Scholar] [CrossRef]
- Selvig, K.A.; Sorensen, R.G.; Wozney, J.M.; Wikesjo, U.M. Bone repair following recombinant human bone morphogenetic protein-2 stimulated periodontal regeneration. J. Periodontol. 2002, 73, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Carreira, A.C.; Lojudice, F.H.; Halcsik, E.; Navarro, R.D.; Sogayar, M.C.; Granjeiro, J.M. Bone morphogenetic proteins: Facts, challenges, and future perspectives. J. Dent. Res. 2014, 93, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E. Platelet-rich plasma: Evidence to support its use. J. Oral Maxillofac. Surg. 2004, 62, 489–496. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Rasmusson, L.; Albrektsson, T. Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Jornet, P.; Sanchez Perez, A.; Amaral Mendes, R.; Tobias, A. Medication-related osteonecrosis of the jaw: Is autologous platelet concentrate application effective for prevention and treatment? A systematic review. J. Cranio-Maxillofac. Surg. 2016, 44, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Kim, S.J.; Kim, M.R. Leucocyte-rich and platelet-rich fibrin for the treatment of bisphosphonate-related osteonecrosis of the jaw: A prospective feasibility study. Br. J. Oral Maxillofac. Surg. 2014, 52, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kwon, O.H.; Kim, T.K.; Cho, Y.K.; Choi, K.Y.; Chung, H.Y.; Cho, B.C.; Yang, J.D.; Shin, J.H. Platelet-rich plasma: Quantitative assessment of growth factor levels and comparative analysis of activated and inactivated groups. Arch. Plast. Surg. 2013, 40, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part II: Platelet-related biologic features. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e45–e50. [Google Scholar] [CrossRef]
- Fortunato, L.; Bennardo, F.; Buffone, C.; Giudice, A. Is the application of platelet concentrates effective in the prevention and treatment of medication-related osteonecrosis of the jaw? A systematic review. J. Cranio-Maxillofac. Surg. 2020, 48, 268–285. [Google Scholar] [CrossRef]
- Knighton, D.R.; Fiegel, V.D.; Halverson, T.; Schneider, S.; Brown, T.; Wells, C.L. Oxygen as an antibiotic. The effect of inspired oxygen on bacterial clearance. Arch. Surg. 1990, 125, 97–100. [Google Scholar] [CrossRef]
- Marx, R.E. A new concept in the treatment of osteoradionecrosis. J. Oral Maxillofac. Surg. 1983, 41, 351–357. [Google Scholar] [CrossRef]
- Seidler, V.; Linetskiy, I.; Hubalkova, H.; Stankova, H.; Smucler, R.; Mazanek, J. Ozone and its usage in general medicine and dentistry. A review article. Prague Med. Rep. 2008, 109, 5–13. [Google Scholar]
- Marx, R.E. Osteoradionecrosis: A new concept of its pathophysiology. J. Oral Maxillofac. Surg. 1983, 41, 283–288. [Google Scholar] [CrossRef]
- Knighton, D.R.; Halliday, B.; Hunt, T.K. Oxygen as an antibiotic. The effect of inspired oxygen on infection. Arch. Surg. 1984, 119, 199–204. [Google Scholar] [CrossRef]
- Asano, T.; Kaneko, E.; Shinozaki, S.; Imai, Y.; Shibayama, M.; Chiba, T.; Ai, M.; Kawakami, A.; Asaoka, H.; Nakayama, T.; et al. Hyperbaric oxygen induces basic fibroblast growth factor and hepatocyte growth factor expression, and enhances blood perfusion and muscle regeneration in mouse ischemic hind limbs. Circ. J. 2007, 71, 405–411. [Google Scholar] [CrossRef]
- Boykin, J.V., Jr.; Baylis, C. Hyperbaric oxygen therapy mediates increased nitric oxide production associated with wound healing: A preliminary study. Adv. Skin Wound Care 2007, 20, 382–388. [Google Scholar] [CrossRef]
- Cabigas, B.P.; Su, J.; Hutchins, W.; Shi, Y.; Schaefer, R.B.; Recinos, R.F.; Nilakantan, V.; Kindwall, E.; Niezgoda, J.A.; Baker, J.E. Hyperoxic and hyperbaric-induced cardioprotection: Role of nitric oxide synthase 3. Cardiovasc. Res. 2006, 72, 143–151. [Google Scholar] [CrossRef]
- Elayan, I.M.; Axley, M.J.; Prasad, P.V.; Ahlers, S.T.; Auker, C.R. Effect of hyperbaric oxygen treatment on nitric oxide and oxygen free radicals in rat brain. J. Neurophysiol. 2000, 83, 2022–2029. [Google Scholar] [CrossRef]
- Gallagher, K.A.; Goldstein, L.J.; Thom, S.R.; Velazquez, O.C. Hyperbaric oxygen and bone marrow-derived endothelial progenitor cells in diabetic wound healing. Vascular 2006, 14, 328–337. [Google Scholar] [CrossRef]
- Gutsaeva, D.R.; Suliman, H.B.; Carraway, M.S.; Demchenko, I.T.; Piantadosi, C.A. Oxygen-induced mitochondrial biogenesis in the rat hippocampus. Neuroscience 2006, 137, 493–504. [Google Scholar] [CrossRef]
- Tandara, A.A.; Mustoe, T.A. Oxygen in wound healing--more than a nutrient. World J. Surg. 2004, 28, 294–300. [Google Scholar] [CrossRef]
- Thom, S.R.; Bhopale, V.M.; Velazquez, O.C.; Goldstein, L.J.; Thom, L.H.; Buerk, D.G. Stem cell mobilization by hyperbaric oxygen. American journal of physiology. Heart Circ. Physiol. 2006, 290, H1378–H1386. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.C.; Lu, D.; Liu, A.L.; Zhang, Z.M.; Li, X.M.; Zou, Z.P.; Zeng, W.S.; Cheng, B.L.; Luo, S.Q. Reactive oxygen species stimulates receptor activator of NF-kappaB ligand expression in osteoblast. J. Biol. Chem. 2005, 280, 17497–17506. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Choi, Y.G.; Baik, J.Y.; Han, S.Y.; Jeong, D.W.; Bae, Y.S.; Kim, N.; Lee, S.Y. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood 2005, 106, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.V. Regulatory mechanisms operative in osteoclasts. Crit. Rev. Eukaryot. Gene Expr. 2004, 14, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Freiberger, J.J.; Padilla-Burgos, R.; Chhoeu, A.H.; Kraft, K.H.; Boneta, O.; Moon, R.E.; Piantadosi, C.A. Hyperbaric oxygen treatment and bisphosphonate-induced osteonecrosis of the jaw: A case series. J. Oral Maxillofac. Surg. 2007, 65, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Freiberger, J.J.; Padilla-Burgos, R.; McGraw, T.; Suliman, H.B.; Kraft, K.H.; Stolp, B.W.; Moon, R.E.; Piantadosi, C.A. What is the role of hyperbaric oxygen in the management of bisphosphonate-related osteonecrosis of the jaw: A randomized controlled trial of hyperbaric oxygen as an adjunct to surgery and antibiotics. J. Oral Maxillofac. Surg. 2012, 70, 1573–1583. [Google Scholar] [CrossRef]
- Sacco, R.; Leeson, R.; Nissan, J.; Olate, S.; Bettoni Cruz de Castro, C.H.; Acocella, A.; Lalli, A. A systematic review of oxygen therapy for the management of medication-related osteonecrosis of the jaw (MRONJ). Appl. Sci. 2019, 9, 1026. [Google Scholar] [CrossRef]
- Bilge, A.; Ozturk, O.; Adali, Y.; Ustebay, S. Could Ozone Treatment Be a Promising Alternative for Osteomyelitis? An Experimental Study. Acta Ortop. Bras. 2018, 26, 67–71. [Google Scholar] [CrossRef]
- Bocci, V.A. Scientific and medical aspects of ozone therapy. State of the art. Arch. Med. Res. 2006, 37, 425–435. [Google Scholar] [CrossRef]
- Stubinger, S.; Sader, R.; Filippi, A. The use of ozone in dentistry and maxillofacial surgery: A review. Quintessence Int. 2006, 37, 353–359. [Google Scholar]
- Grigor’ian, A.S.; Grigor’iants, L.A.; Guchetl, M.N. Experimental-morphological study of the anti-inflammatory action of ozone-perfluorane complex application. Stomatologiia 2008, 87, 4–9. [Google Scholar]
- Agrillo, A.; Petrucci, M.T.; Tedaldi, M.; Mustazza, M.C.; Marino, S.M.; Gallucci, C.; Iannetti, G. New therapeutic protocol in the treatment of avascular necrosis of the jaws. J. Craniofacial Surg. 2006, 17, 1080–1083. [Google Scholar] [CrossRef]
- Agrillo, A.; Filiaci, F.; Ramieri, V.; Riccardi, E.; Quarato, D.; Rinna, C.; Gennaro, P.; Cascino, F.; Mitro, V.; Ungari, C. Bisphosphonate-related osteonecrosis of the jaw (BRONJ): 5 year experience in the treatment of 131 cases with ozone therapy. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 1741–1747. [Google Scholar]
- Bedogni, A.; Blandamura, S.; Lokmic, Z.; Palumbo, C.; Ragazzo, M.; Ferrari, F.; Tregnaghi, A.; Pietrogrande, F.; Procopio, O.; Saia, G.; et al. Bisphosphonate-associated jawbone osteonecrosis: A correlation between imaging techniques and histopathology. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 105, 358–364. [Google Scholar] [CrossRef]
- Lee, C.Y.; David, T.; Nishime, M. Use of platelet-rich plasma in the management of oral biphosphonate-associated osteonecrosis of the jaw: A report of 2 cases. J. Oral Implantol. 2007, 33, 371–382. [Google Scholar] [CrossRef]
- Agrillo, A.; Ungari, C.; Filiaci, F.; Priore, P.; Iannetti, G. Ozone therapy in the treatment of avascular bisphosphonate-related jaw osteonecrosis. J. Craniofacial Surg. 2007, 18, 1071–1075. [Google Scholar] [CrossRef]
- Ripamonti, C.I.; Cislaghi, E.; Mariani, L.; Maniezzo, M. Efficacy and safety of medical ozone (O(3)) delivered in oil suspension applications for the treatment of osteonecrosis of the jaw in patients with bone metastases treated with bisphosphonates: Preliminary results of a phase I-II study. Oral Oncol. 2011, 47, 185–190. [Google Scholar] [CrossRef]
- da Guarda, M.G.; Paraguassu, G.M.; Cerqueira, N.S.; Cury, P.R.; Farias, J.G.; Ramalho, L.M. Laser GaAlAs (lambda860 nm) photobiomodulation for the treatment of bisphosphonate-induced osteonecrosis of the jaw. Photomed. Laser Surg. 2012, 30, 293–297. [Google Scholar] [CrossRef]
- Kan, B.; Altay, M.A.; Tasar, F.; Akova, M. Low-level laser therapy supported teeth extractions of two patients receiving IV zolendronate. Lasers Med. Sci. 2011, 26, 569–575. [Google Scholar] [CrossRef]
- Scoletta, M.; Arduino, P.G.; Reggio, L.; Dalmasso, P.; Mozzati, M. Effect of low-level laser irradiation on bisphosphonate-induced osteonecrosis of the jaws: Preliminary results of a prospective study. Photomed. Laser Surg. 2010, 28, 179–184. [Google Scholar] [CrossRef]
- Vescovi, P.; Manfredi, M.; Merigo, E.; Meleti, M.; Fornaini, C.; Rocca, J.P.; Nammour, S. Surgical approach with Er:YAG laser on osteonecrosis of the jaws (ONJ) in patients under bisphosphonate therapy (BPT). Lasers Med. Sci. 2010, 25, 101–113. [Google Scholar] [CrossRef]
- Vescovi, P.; Manfredi, M.; Merigo, E.; Guidotti, R.; Meleti, M.; Pedrazzi, G.; Fornaini, C.; Bonanini, M.; Ferri, T.; Nammour, S. Early surgical laser-assisted management of bisphosphonate-related osteonecrosis of the jaws (BRONJ): A retrospective analysis of 101 treated sites with long-term follow-up. Photomed. Laser Surg. 2012, 30, 5–13. [Google Scholar] [CrossRef]
- Weber, J.B.; Camilotti, R.S.; Ponte, M.E. Efficacy of laser therapy in the management of bisphosphonate-related osteonecrosis of the jaw (BRONJ): A systematic review. Lasers Med. Sci. 2016, 31, 1261–1272. [Google Scholar] [CrossRef]
- Damm, D.D.; Jones, D.M. Bisphosphonate-related osteonecrosis of the jaws: A potential alternative to drug holidays. Gen. Dent. 2013, 61, 33–38. [Google Scholar]
- Son, H.J.; Kim, J.W.; Kim, S.J. Pharmacoepidemiology and clinical characteristics of medication-related osteonecrosis of the jaw. Maxillofac. Plast. Reconstr. Surg. 2019, 41, 26. [Google Scholar] [CrossRef]
- Hinson, A.M.; Siegel, E.R.; Stack, B.C., Jr. Temporal correlation between bisphosphonate termination and symptom resolution in osteonecrosis of the jaw: A pooled case report analysis. J. Oral Maxillofac. Surg. 2015, 73, 53–62. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, H.K.; Song, S.I.; Lee, J.K. Drug holiday as a prognostic factor of medication-related osteonecrosis of the jaw. J. Korean Assoc. Oral Maxillofac. Surg. 2014, 40, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.S.; Correia, J.A.; Salvado, F.; Caldas, C.; Santos, N.; Capelo, A.; Palmela, P. Relevant factors for treatment outcome and time to healing in medication-related osteonecrosis of the jaws—A retrospective cohort study. J. Cranio-Maxillofac. Surg. 2017, 45, 1736–1742. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, S.; Yanamoto, S.; Fujita, S.; Hasegawa, T.; Komori, T.; Kojima, Y.; Miyamoto, H.; Shibuya, Y.; Ueda, N.; Kirita, T.; et al. Drug holiday clinical relevance verification for antiresorptive agents in medication-related osteonecrosis cases of the jaw. J. Bone Miner. Metab. 2020, 38, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.Y.; Suh, H.S.; Park, J.W.; Kwon, J.W. Drug holiday patterns and bisphosphonate-related osteonecrosis of the jaw. Oral Dis. 2019, 25, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Park, S.J.; Kim, M.K. The effect of bisphosphonate discontinuation on the incidence of postoperative medication-related osteonecrosis of the jaw after tooth extraction. J. Korean Assoc. Oral Maxillofac. Surg. 2020, 46, 78–83. [Google Scholar] [CrossRef]
- Ottesen, C.; Schiodt, M.; Gotfredsen, K. Efficacy of a high-dose antiresorptive drug holiday to reduce the risk of medication-related osteonecrosis of the jaw (MRONJ): A systematic review. Heliyon 2020, 6, e03795. [Google Scholar] [CrossRef]
- Cipriani, C.; Irani, D.; Bilezikian, J.P. Safety of osteoanabolic therapy: A decade of experience. J. Bone Miner. Res. 2012, 27, 2419–2428. [Google Scholar] [CrossRef]
- Langdahl, B.L.; Silverman, S.; Fujiwara, S.; Saag, K.; Napoli, N.; Soen, S.; Enomoto, H.; Melby, T.E.; Disch, D.P.; Marin, F.; et al. Real-world effectiveness of teriparatide on fracture reduction in patients with osteoporosis and comorbidities or risk factors for fractures: Integrated analysis of 4 prospective observational studies. Bone 2018, 116, 58–66. [Google Scholar] [CrossRef]
- Napoli, N.; Strollo, R.; Paladini, A.; Briganti, S.I.; Pozzilli, P.; Epstein, S. The alliance of mesenchymal stem cells, bone, and diabetes. Int. J. Endocrinol. 2014, 2014, 690783. [Google Scholar] [CrossRef]
- Silverman, S.; Langdahl, B.L.; Fujiwara, S.; Saag, K.; Napoli, N.; Soen, S.; Enomoto, H.; Melby, T.E.; Disch, D.P.; Marin, F.; et al. Reduction of Hip and Other Fractures in Patients Receiving Teriparatide in Real-World Clinical Practice: Integrated Analysis of Four Prospective Observational Studies. Calcif. Tissue Int. 2019, 104, 193–200. [Google Scholar] [CrossRef]
- Bashutski, J.D.; Eber, R.M.; Kinney, J.S.; Benavides, E.; Maitra, S.; Braun, T.M.; Giannobile, W.V.; McCauley, L.K. Teriparatide and osseous regeneration in the oral cavity. N. Engl. J. Med. 2010, 363, 2396–2405. [Google Scholar] [CrossRef]
- Vahle, J.L.; Sato, M.; Long, G.G.; Young, J.K.; Francis, P.C.; Engelhardt, J.A.; Westmore, M.S.; Linda, Y.; Nold, J.B. Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1-34) for 2 years and relevance to human safety. Toxicol. Pathol. 2002, 30, 312–321. [Google Scholar] [CrossRef]
- Vahle, J.L.; Long, G.G.; Sandusky, G.; Westmore, M.; Ma, Y.L.; Sato, M. Bone neoplasms in F344 rats given teriparatide [rhPTH(1-34)] are dependent on duration of treatment and dose. Toxicol. Pathol. 2004, 32, 426–438. [Google Scholar] [CrossRef]
- Vahle, J.L.; Zuehlke, U.; Schmidt, A.; Westmore, M.; Chen, P.; Sato, M. Lack of bone neoplasms and persistence of bone efficacy in cynomolgus macaques after long-term treatment with teriparatide [rhPTH(1-34)]. J. Bone Miner. Res. 2008, 23, 2033–2039. [Google Scholar] [CrossRef]
- Andrews, E.B.; Gilsenan, A.W.; Midkiff, K.; Sherrill, B.; Wu, Y.; Mann, B.H.; Masica, D. The US postmarketing surveillance study of adult osteosarcoma and teriparatide: Study design and findings from the first 7 years. J. Bone Miner. Res. 2012, 27, 2429–2437. [Google Scholar] [CrossRef]
- Di Ianni, M.; Del Papa, B.; De Ioanni, M.; Moretti, L.; Bonifacio, E.; Cecchini, D.; Sportoletti, P.; Falzetti, F.; Tabilio, A. Mesenchymal cells recruit and regulate T regulatory cells. Exp. Hematol. 2008, 36, 309–318. [Google Scholar] [CrossRef]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef]
- Cella, L.; Oppici, A.; Arbasi, M.; Moretto, M.; Piepoli, M.; Vallisa, D.; Zangrandi, A.; Di Nunzio, C.; Cavanna, L. Autologous bone marrow stem cell intralesional transplantation repairing bisphosphonate related osteonecrosis of the jaw. Head Face Med. 2011, 7, 16. [Google Scholar] [CrossRef]
- Kikuiri, T.; Kim, I.; Yamaza, T.; Akiyama, K.; Zhang, Q.; Li, Y.; Chen, C.; Chen, W.; Wang, S.; Le, A.D.; et al. Cell-based immunotherapy with mesenchymal stem cells cures bisphosphonate-related osteonecrosis of the jaw-like disease in mice. J. Bone Miner. Res. 2010, 25, 1668–1679. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.; Mao, L.; Liu, Y.; Gao, R.; Zheng, Z.; Chen, W.; Le, A.; Shi, S.; Wang, S. Allogeneic mesenchymal stem cell therapy for bisphosphonate-related jaw osteonecrosis in swine. Stem Cells Dev. 2013, 22, 2047–2056. [Google Scholar] [CrossRef]
- Zimmermann, C.E.; Gierloff, M.; Hedderich, J.; Acil, Y.; Wiltfang, J.; Terheyden, H. Survival of transplanted rat bone marrow-derived osteogenic stem cells in vivo. Tissue Eng. Part A 2011, 17, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Leucht, P.; Kim, J.B.; Amasha, R.; James, A.W.; Girod, S.; Helms, J.A. Embryonic origin and Hox status determine progenitor cell fate during adult bone regeneration. Development 2008, 135, 2845–2854. [Google Scholar] [CrossRef]
- Gonzalvez-Garcia, M.; Rodriguez-Lozano, F.J.; Villanueva, V.; Segarra-Fenoll, D.; Rodriguez-Gonzalez, M.A.; Onate-Sanchez, R.; Blanquer, M.; Moraleda, J.M. Cell therapy in bisphosphonate-related osteonecrosis of the jaw. J. Craniofacial Surg. 2013, 24, e226–e228. [Google Scholar] [CrossRef] [PubMed]
- Voss, P.J.; Matsumoto, A.; Alvarado, E.; Schmelzeisen, R.; Duttenhofer, F.; Poxleitner, P. Treatment of stage II medication-related osteonecrosis of the jaw with necrosectomy and autologous bone marrow mesenchymal stem cells. Odontology 2017, 105, 484–493. [Google Scholar] [CrossRef] [PubMed]
- De Santis, G.C.; de Macedo, L.D.; Orellana, M.D.; Innocentini, L.; Ferrari, T.C.; Ricz, H.M.A.; Caruso, S.R.; Fernandes, T.R.; Covas, D.T. Mesenchymal stromal cells administration for osteonecrosis of the jaw caused by bisphosphonate: Report of two cases. Acta Oncol. 2020, 59, 789–792. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).