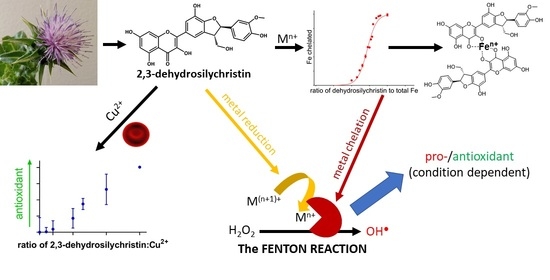
Dehydroflavonolignans from Silymarin Potentiate Transition Metal Toxicity In Vitro but Are Protective for Isolated Erythrocytes Ex Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. pH Conditions
2.3. Iron and Copper Chelation Assessment
2.3.1. Ferrozine Method
2.3.2. Hematoxylin Method
2.3.3. BCS Method
2.4. Effect on the Fenton Reaction Generated Hydroxyl Radicals (HPLC Method)
2.5. Erythrocyte Lysis Assay
2.6. Mathematical and Statistical Analysis
3. Results
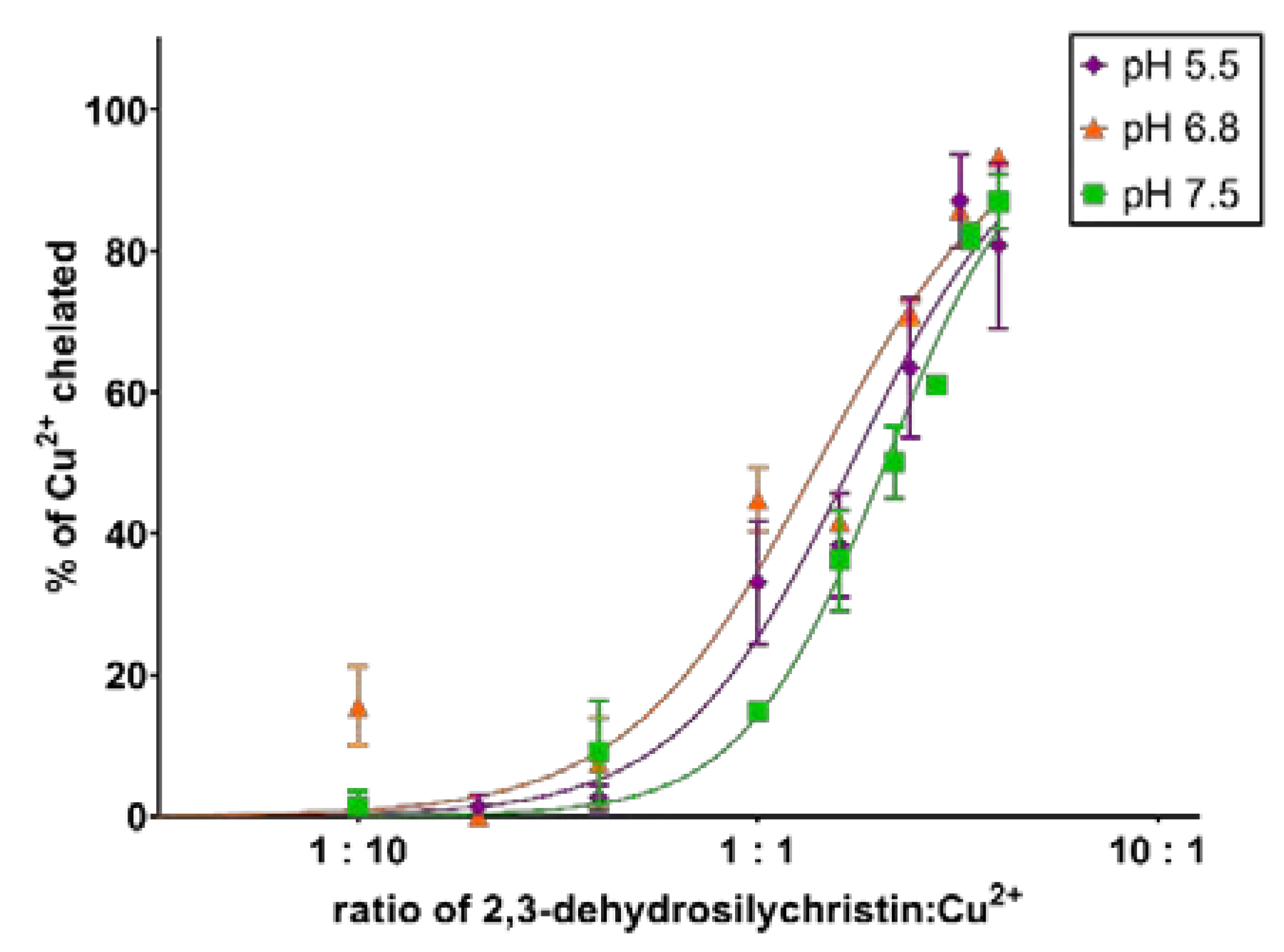
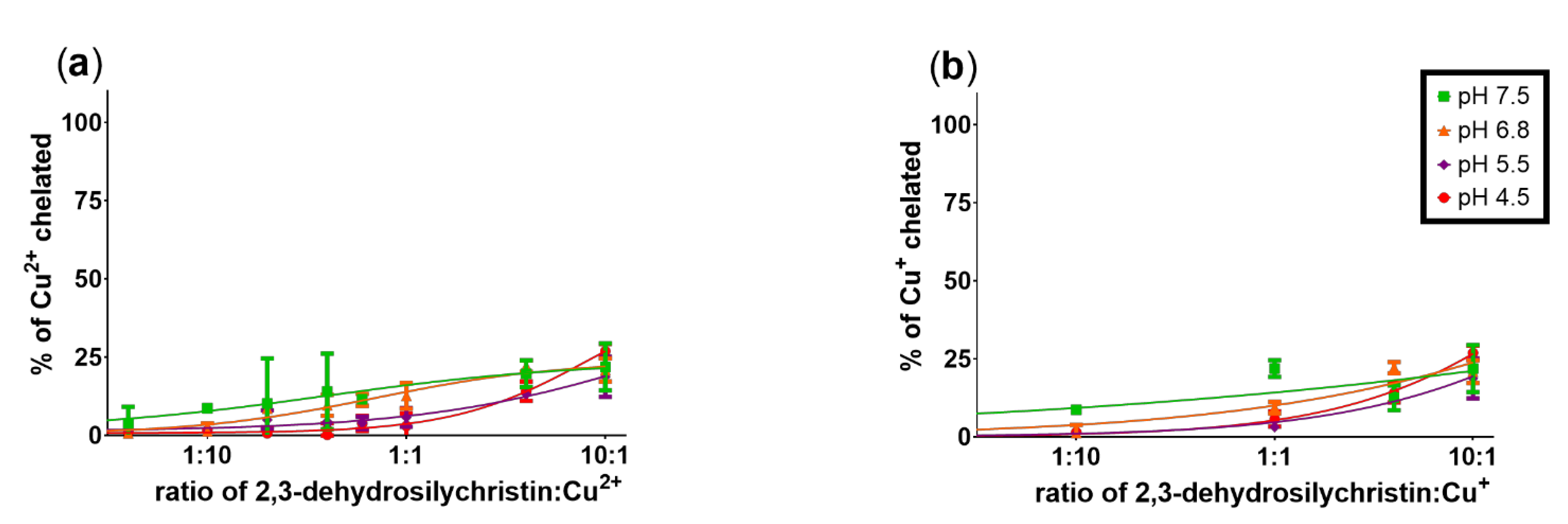
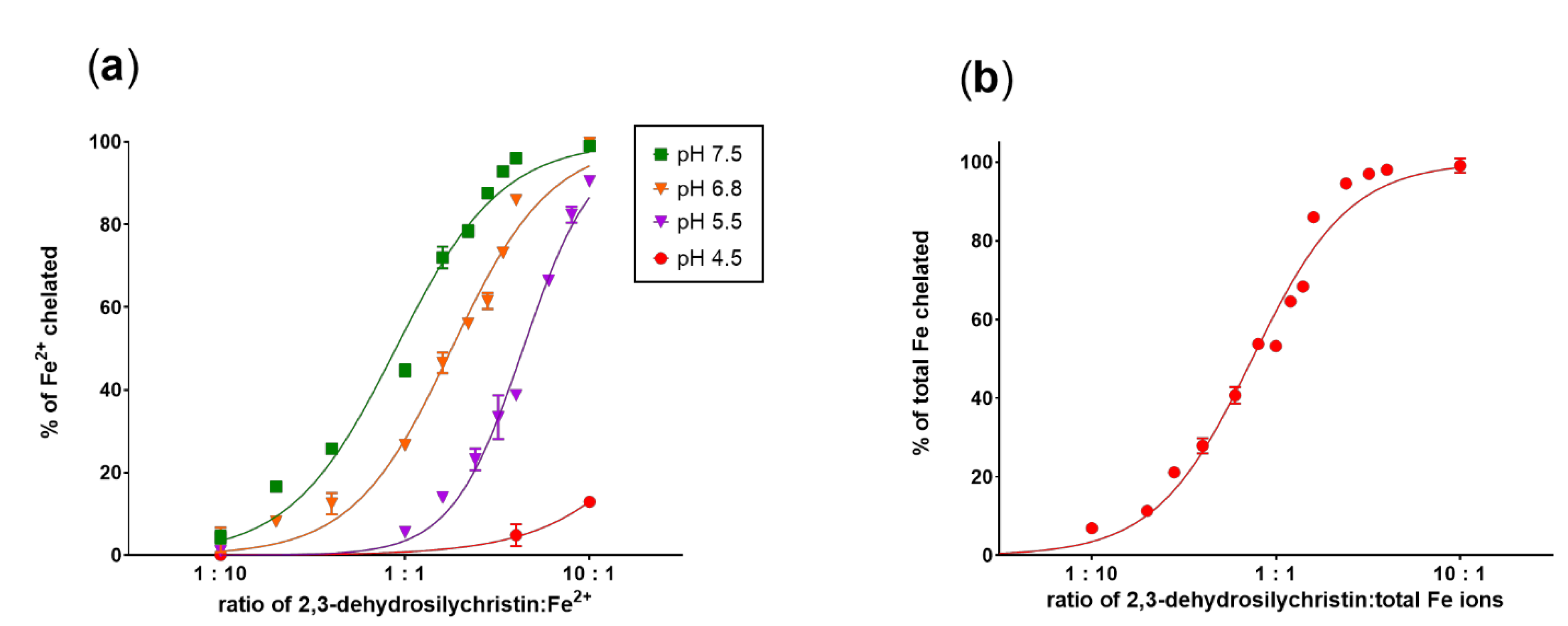
3.1. Copper and Iron Chelation
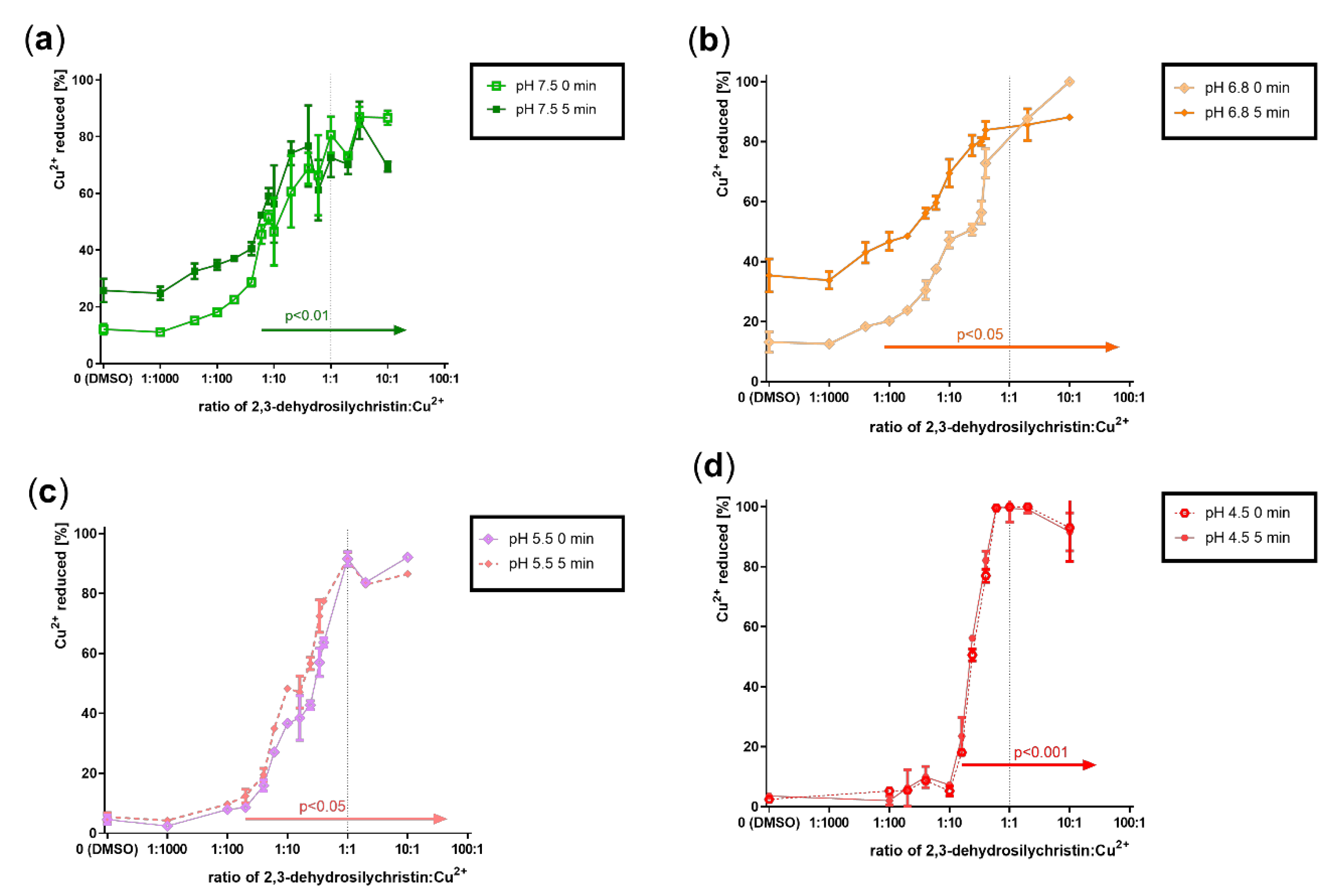
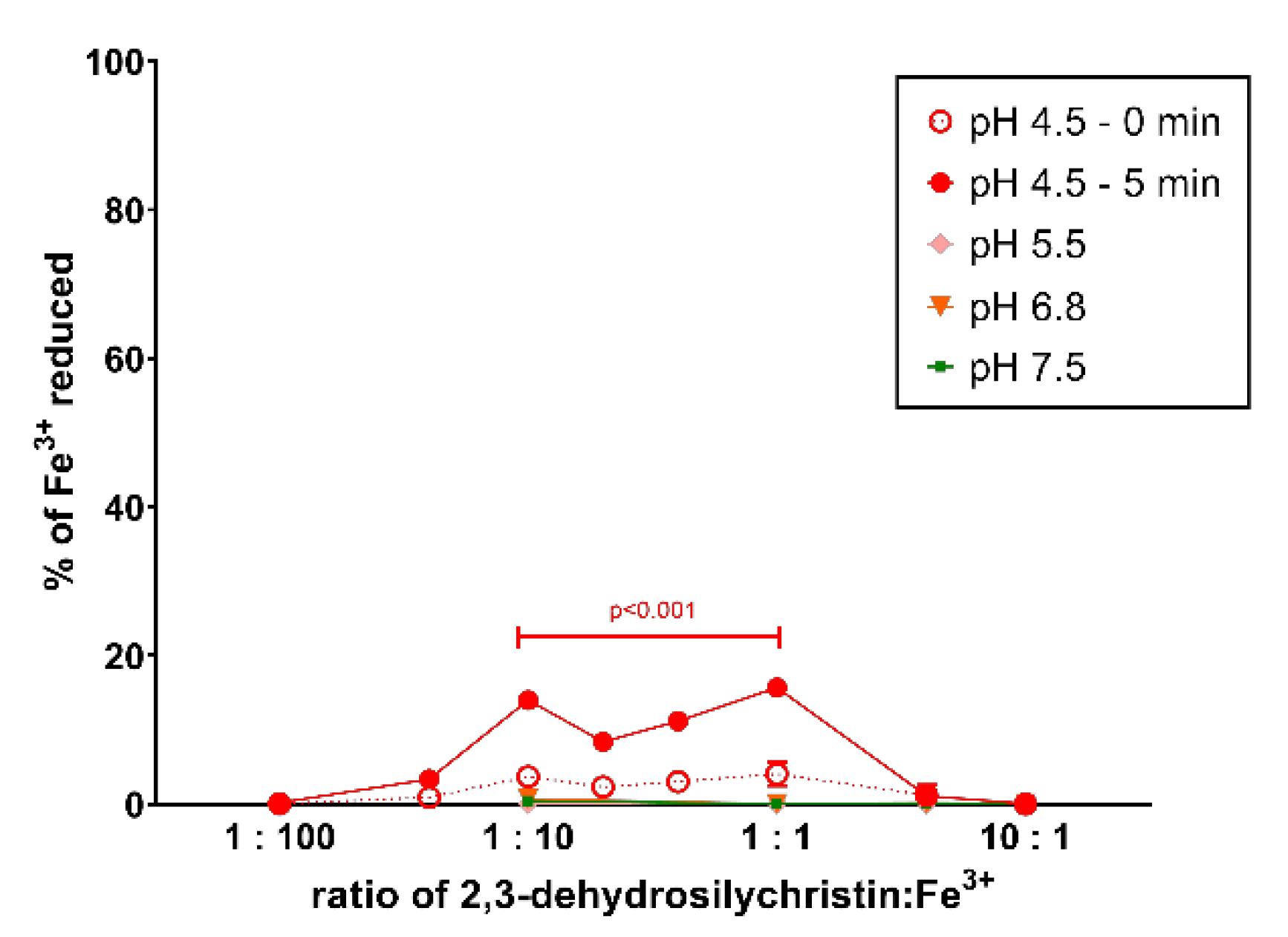
3.2. Cupric and Ferric Ion Reduction
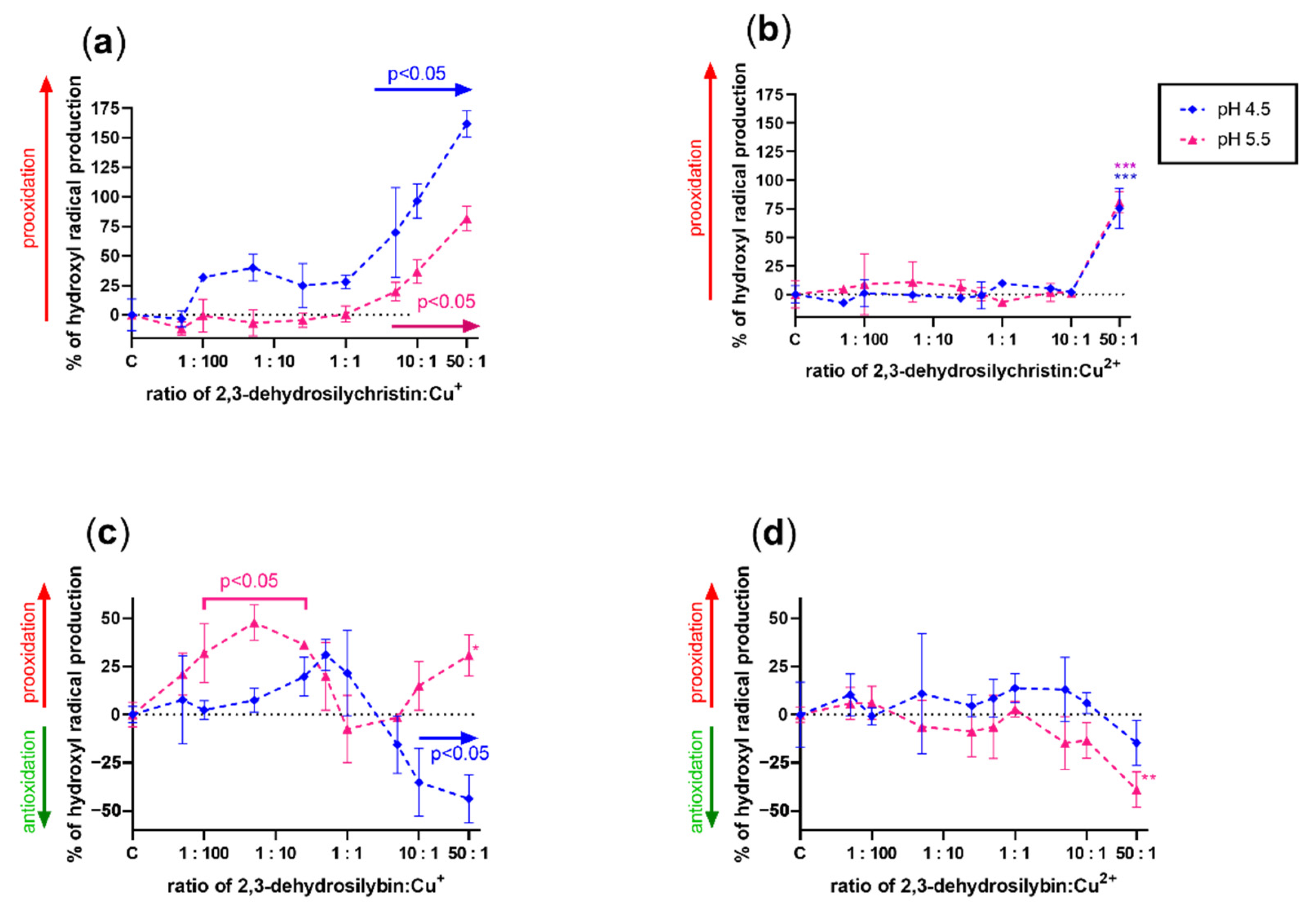
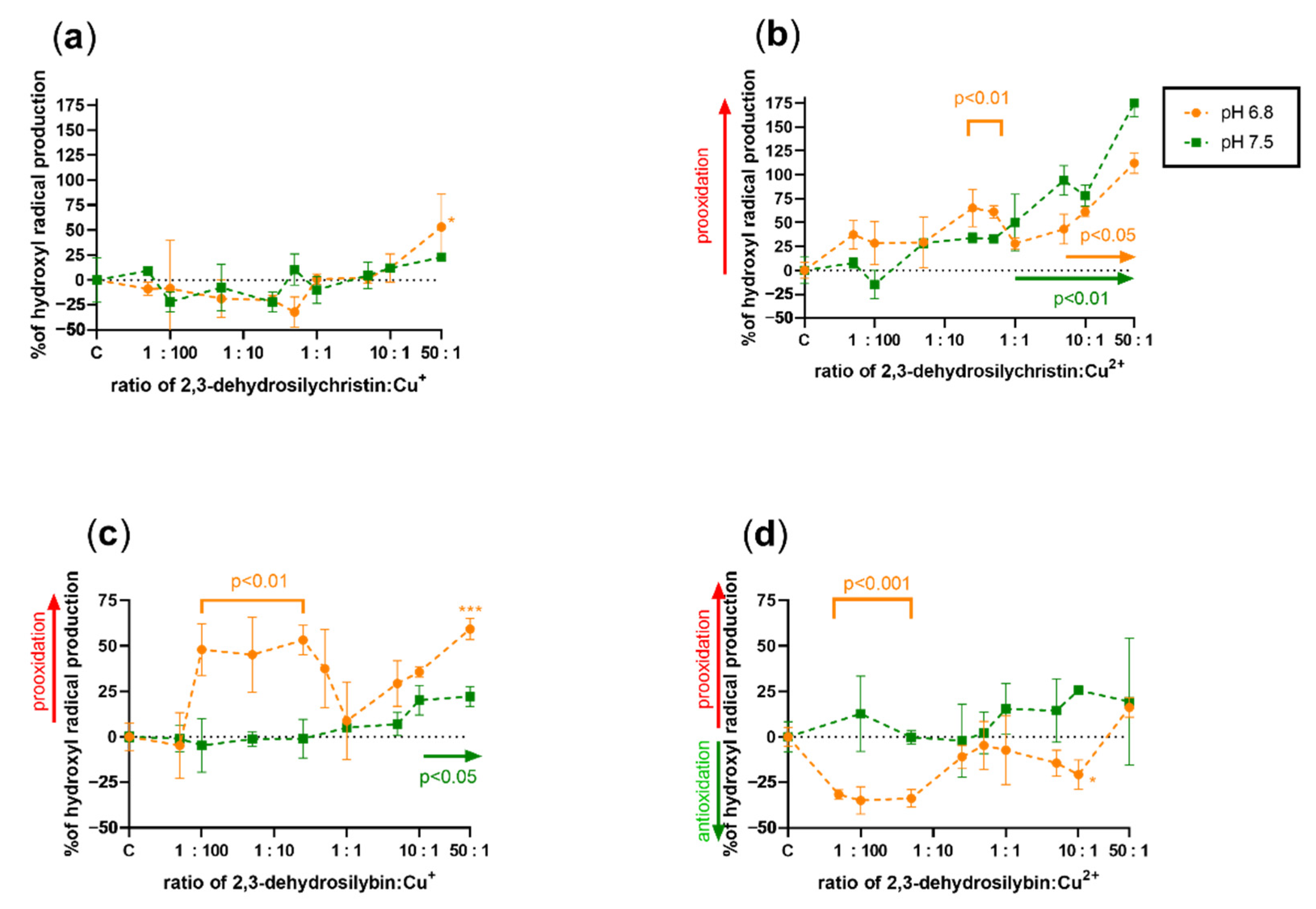
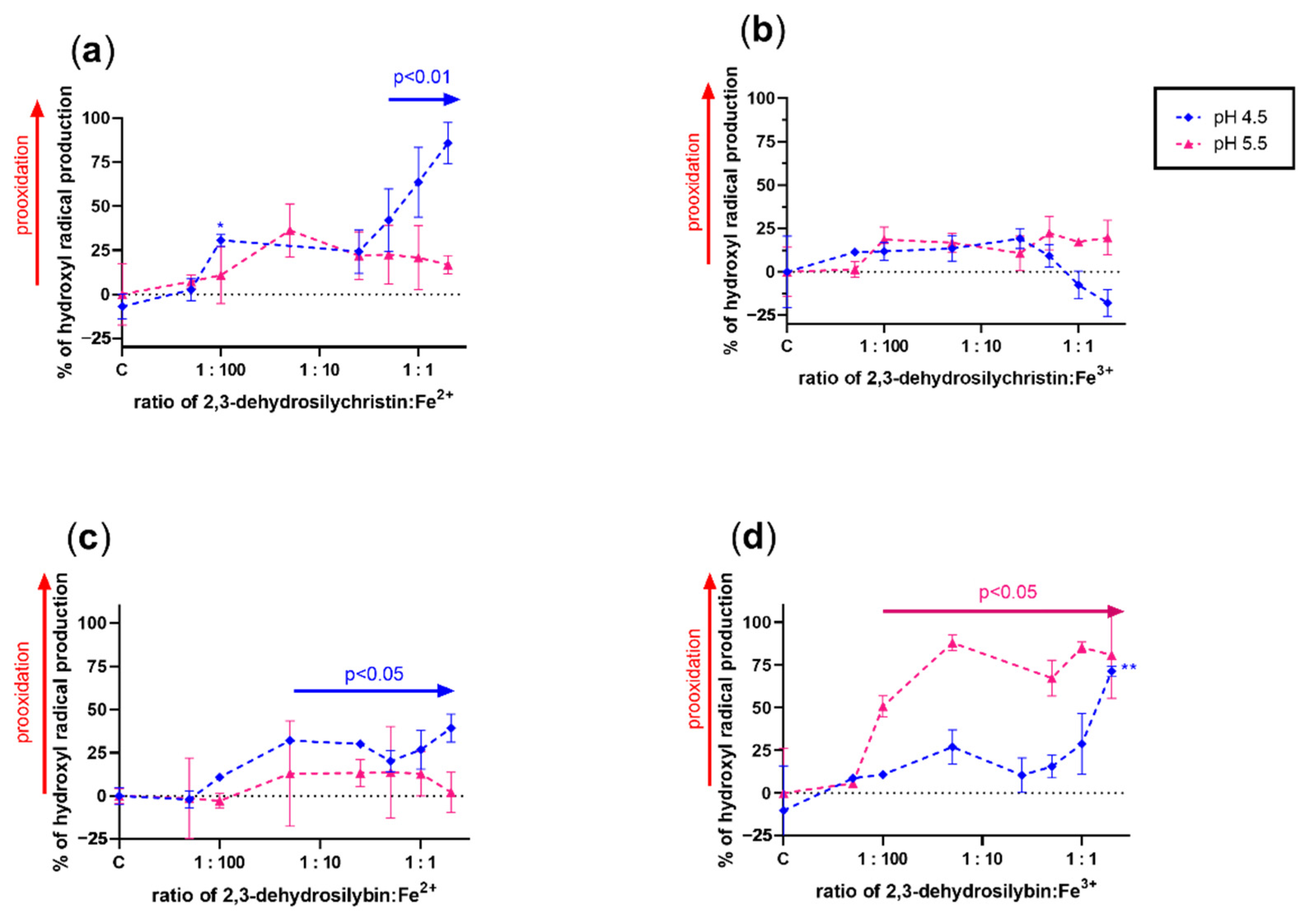
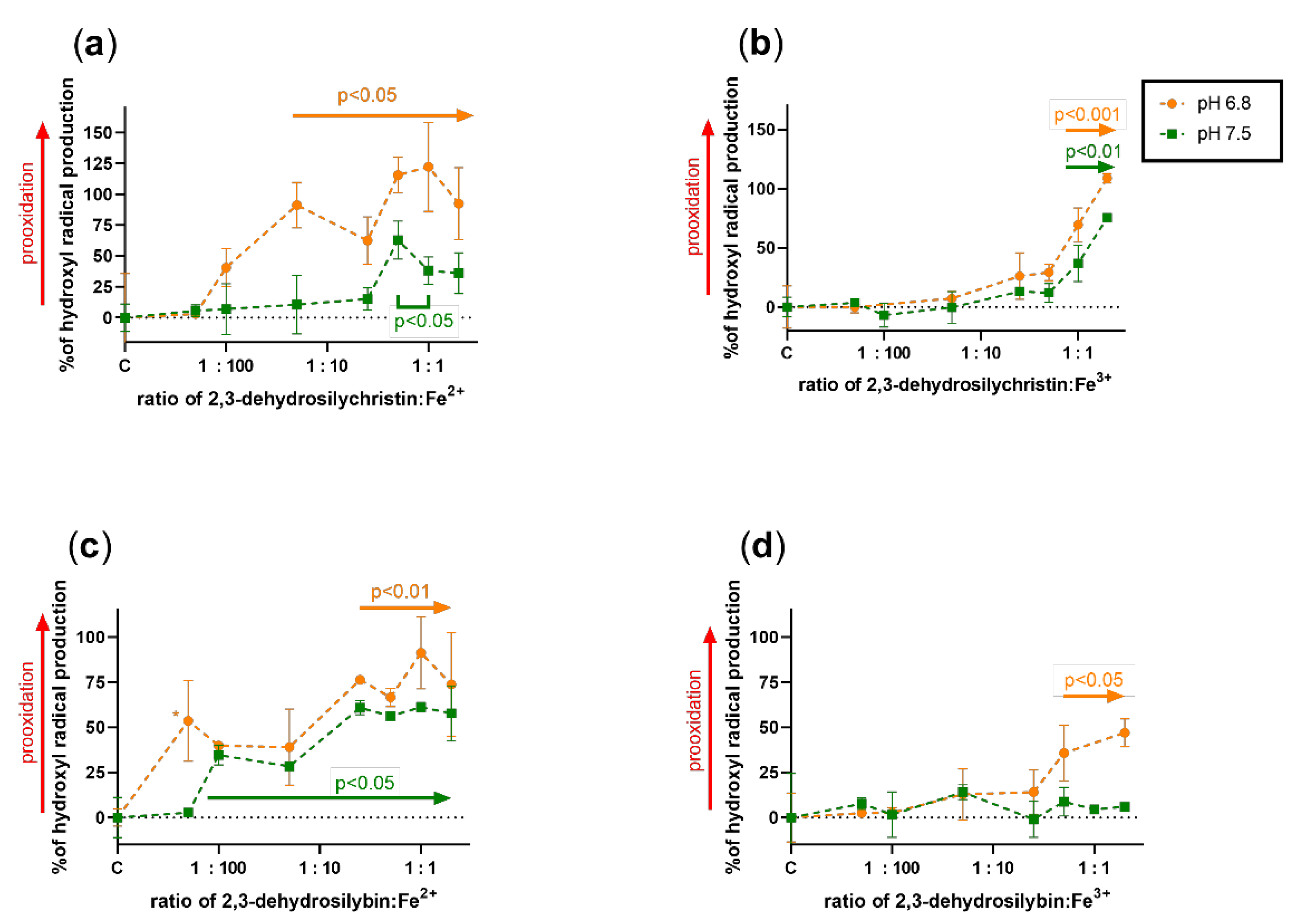
3.3. Effect on Copper and Iron-Catalyzed Hydroxyl Radical Generation
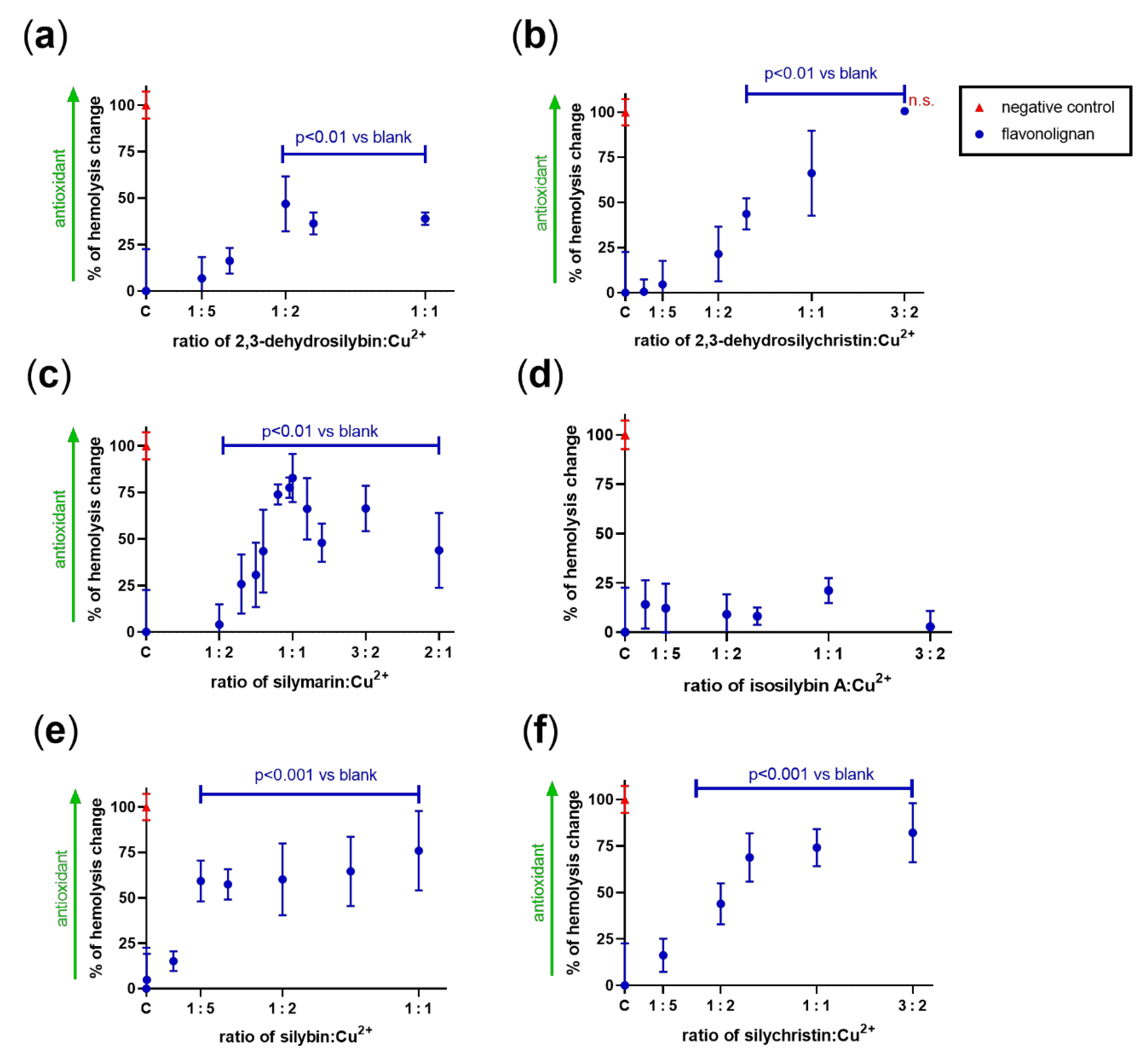
3.4. Effect on Copper-Based Oxidation of Isolated Rat Red Blood Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| •OH | hydroxyl radicals |
| 2,3-DHBA | 2,3-dihydroxybenzoic acid |
| 2,5-DHBA | 2,5-dihydroxybenzoic acid |
| β-NAD | β-nicotinamide adenine dinucleotide |
| BCS | disodium bathocuproine disulfonate |
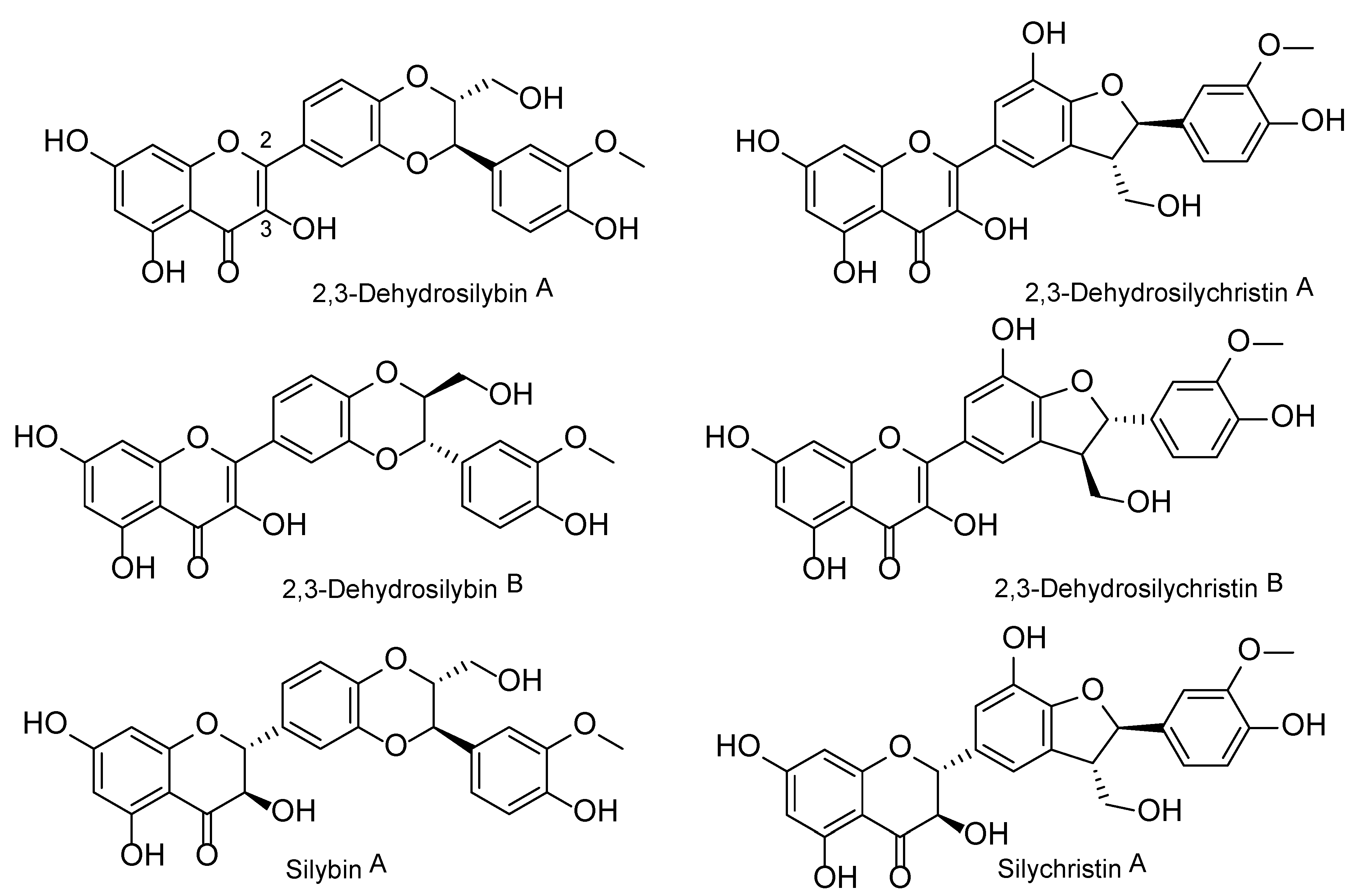
| DHS | 2,3-dehydrosilybin |
| DHSCH | 2,3-dehydrosilychristin |
| DMSO | dimethylsulfoxide |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| DTT | dithiothreitol |
| EDTA | ethylenediaminetetraacetic acid |
| FRAP | ferric reducing antioxidant power |
| HA | hydroxylamine hydrochloride |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| HPLC | high-performance liquid chromatography |
| ROS | reactive oxygen species |
| TRIS | tris(hydroxymethyl)aminomethane |
References
- Fenclová, M.; Stránská-Zachariášová, M.; Beneš, F.; Novaková, A.; Jonatová, P.; Křen, V.; Vítek, L.; Hajslová, J. Liquid chromatography–drift tube ion mobility–mass spectrometry as a new challenging tool for the separation and characterization of silymarin flavonolignans. Anal. Bioanal. Chem. 2020, 412, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Petrásková, L.; Káňová, K.; Biedermann, D.; Křen, V.; Valentová, K. Simple and rapid HPLC separation and quantification of flavonoid, flavonolignans, and 2,3-dehydroflavonolignans in silymarin. Foods 2020, 9, 116. [Google Scholar] [CrossRef]
- Qin, N.; Sasaki, T.; Li, W.; Wang, J.; Zhang, X.; Li, D.; Li, Z.; Cheng, M.; Hua, H.; Koike, K. Identification of flavonolignans from Silybum marianum seeds as allosteric protein tyrosine phosphatase 1B inhibitors. J. Enzym. Inhib. Med. Chem. 2018, 33, 1283–1291. [Google Scholar] [CrossRef]
- Biedermann, D.; Buchta, M.; Holečková, V.; Sedlák, D.; Valentová, K.; Cvačka, J.; Bednárová, L.; Křenková, A.; Kuzma, M.; Škuta, C.; et al. Silychristin: Skeletal alterations and biological activities. J. Nat. Prod. 2016, 79, 3086–3092. [Google Scholar] [CrossRef] [PubMed]
- Pyszková, M.; Biler, M.; Biedermann, D.; Valentová, K.; Kuzma, M.; Vrba, J.; Ulrichová, J.; Sokolová, R.; Mojovic, M.; Popovic-Bijelic, A.; et al. Flavonolignan 2,3-dehydroderivatives: Preparation, antiradical and cytoprotective activity. Free Radic. Biol. Med. 2016, 90, 114–125. [Google Scholar] [CrossRef]
- Biedermann, D.; Moravcová, V.; Valentová, K.; Kuzma, M.; Petrásková, L.; Císařová, I.; Křen, V. Oxidation of flavonolignan silydianin to unexpected lactone-acid derivative. Phytochem. Lett. 2019, 30, 14–20. [Google Scholar] [CrossRef]
- Valentová, K.; Purchartová, K.; Rydlová, L.; Roubalová, L.; Biedermann, D.; Petrásková, L.; Křenková, A.; Pelantová, H.; Holečková-Moravcová, V.; Tesařová, E.; et al. Sulfated metabolites of flavonolignans and 2,3-dehydroflavonolignans: Preparation and properties. Int. J. Mol. Sci. 2018, 19, 2349. [Google Scholar] [CrossRef]
- Viktorová, J.; Dobiasová, S.; Řehořová, K.; Biedermann, D.; Káňová, K.; Šeborová, K.; Václavíková, R.; Valentová, K.; Ruml, T.; Křen, V.; et al. Antioxidant, anti-inflammatory, and multidrug resistance modulation activity of silychristin derivatives. Antioxidants 2019, 8, 303. [Google Scholar] [CrossRef] [PubMed]
- Viktorová, J.; Stránská-Zachariášová, M.; Fenclová, M.; Vítek, L.; Hajšlová, J.; Křen, V.; Ruml, T. Complex evaluation of antioxidant capacity of Milk thistle dietary supplements. Antioxidants 2019, 8, 317. [Google Scholar] [CrossRef]
- Huber, A.; Thongphasuk, P.; Erben, G.; Lehmann, W.-D.; Tuma, S.; Stremmel, W.; Chamulitrat, W. Significantly greater antioxidant anticancer activities of 2,3-dehydrosilybin than silybin. BBA Gen. Subj. 2008, 1780, 837–847. [Google Scholar] [CrossRef]
- Gabrielová, E.; Křen, V.; Jabůrek, M.; Modrianský, M. Silymarin component 2,3-dehydrosilybin attenuates cardiomyocyte damage following hypoxia/reoxygenation by limiting oxidative stress. Physiol. Res. 2015, 64, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Thongphasuk, P.; Stremmel, W.; Chamulitrat, W. Potent direct or TNF-α-promoted anticancer effects of 2,3-dehydrosilybin: Comparison study with silybin. Chemotherapy 2008, 54, 23–30. [Google Scholar] [CrossRef]
- Thongphasuk, P.; Stremmel, W.; Chamulitrat, W. 2,3-Dehydrosilybin is a better DNA topoisomerase I inhibitor than its parental silybin. Chemotherapy 2009, 55, 42–48. [Google Scholar] [CrossRef]
- Juráňová, J.; Aury-Landas, J.; Boumediene, K.; Baugé, C.; Biedermann, D.; Ulrichová, J.; Franková, J. Modulation of skin inflammatory response by active components of silymarin. Molecules 2019, 24, 123. [Google Scholar] [CrossRef]
- Vostálová, J.; Tinková, E.; Biedermann, D.; Kosina, P.; Ulrichová, J.; Rajnochová Svobodová, A. Skin protective activity of silymarin and its flavonolignans. Molecules 2019, 24, 1022. [Google Scholar] [CrossRef]
- Dvořák, Z.; Vrzal, R.; Ulrichová, J. Silybin and dehydrosilybin inhibit cytochrome P450 1A1 catalytic activity: A study in human keratinocytes and human hepatoma cells. Cell Biol. Toxicol. 2006, 22, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Filippopoulou, K.; Papaevgeniou, N.; Lefaki, M.; Paraskevopoulou, A.; Biedermann, D.; Křen, V.; Chondrogianni, N. 2,3-Dehydrosilybin A/B as a pro-longevity and anti-aggregation compound. Free Radic. Biol. Med. 2017, 103, 256–267. [Google Scholar] [CrossRef]
- Tilley, C.; Deep, G.; Agarwal, C.; Wempe, M.F.; Biedermann, D.; Valentová, K.; Křen, V.; Agarwal, R. Silibinin and its 2,3-dehydro-derivative inhibit basal cell carcinoma growth via suppression of mitogenic signaling and transcription factors activation. Mol. Carcinog. 2016, 55, 3–14. [Google Scholar] [CrossRef]
- Zarrelli, A.; Sgambato, A.; Petito, V.; De Napoli, L.; Previtera, L.; Di Fabio, G. New C-23 modified of silybin and 2,3-dehydrosilybin: Synthesis and preliminary evaluation of antioxidant properties. Bioorg. Med. Chem. Lett. 2011, 21, 4389–4392. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Sekhon, A.; Oka, Y.; Chen, G.; Alrubati, N.; Kaur, J.; Orozco, A.; Zhang, Q.; Wang, G.; Chen, Q.-H. 23-O-Substituted-2,3-dehydrosilybins selectively suppress androgen receptor-positive LNCaP prostate cancer cell proliferation. Nat. Prod. Commun. 2020, 15, 1934578X20922326. [Google Scholar] [CrossRef]
- Wen, Y.-J.; Zhou, Z.-Y.; Zhang, G.-L.; Lu, X.-X. Metal coordination protocol for the synthesis of-2,3-dehydrosilybin and 19-O-demethyl-2,3-dehydrosilybin from silybin and their antitumor activities. Tetrahedron Lett. 2018, 59, 1666–1669. [Google Scholar] [CrossRef]
- Gažák, R.; Valentová, K.; Fuksová, K.; Marhol, P.; Kuzma, M.; Medina, M.Á.; Oborná, I.; Ulrichová, J.; Křen, V. Synthesis and antiangiogenic activity of new silybin galloyl esters. J. Med. Chem. 2011, 54, 7397–7407. [Google Scholar] [CrossRef]
- Karas, D.; Gažák, R.; Valentová, K.; Chambers, C.S.; Pivodová, V.; Biedermann, D.; Křenková, A.; Oborná, I.; Kuzma, M.; Cvačka, J.; et al. Effects of 2,3-dehydrosilybin and its galloyl ester and methyl ether derivatives on human umbilical vein endothelial cells. J. Nat. Prod. 2016, 79, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Tvrdý, V.; Catapano, M.C.; Rawlik, T.; Karlíčková, J.; Biedermann, D.; Křen, V.; Mladěnka, P.; Valentová, K. Interaction of isolated silymarin flavonolignans with iron and copper. J. Inorg. Biochem. 2018, 189, 115–123. [Google Scholar] [CrossRef]
- Křenek, K.; Marhol, P.; Peikerová, Ž.; Křen, V.; Biedermann, D. Preparatory separation of the silymarin flavonolignans by Sephadex LH-20 gel. Food Res. Int. 2014, 65, 115–120. [Google Scholar] [CrossRef]
- Gažák, R.; Fuksová, K.; Marhol, P.; Kuzma, M.; Agarwal, R.; Křen, V. Preparative method for isosilybin isolation based on enzymatic kinetic resolution of silymarin mixture. Process Biochem. 2013, 48, 184–189. [Google Scholar] [CrossRef]
- Gažák, R.; Trouillas, P.; Biedermann, D.; Fuksová, K.; Marhol, P.; Kuzma, M.; Křen, V. Base-catalyzed oxidation of silybin and isosilybin into 2,3-dehydro derivatives. Tetrahedron Lett. 2013, 54, 315–317. [Google Scholar] [CrossRef]
- Mladěnka, P.; Macáková, K.; Zatloukalová, L.; Řeháková, Z.; Singh, B.K.; Prasad, A.K.; Parmar, V.S.; Jahodář, L.; Hrdina, R.; Saso, L. In vitro interactions of coumarins with iron. Biochimie 2010, 92, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Říha, M.; Karlíčková, J.; Filipský, T.; Macáková, K.; Hrdina, R.; Mladěnka, P. Novel method for rapid copper chelation assessment confirmed low affinity of D-penicillamine for copper in comparison with trientine and 8-hydroxyquinolines. J. Inorg. Biochem 2013, 123, 80–87. [Google Scholar] [CrossRef]
- Catapano, M.C.; Protti, M.; Fontana, T.; Mandrioli, R.; Mladěnka, P.; Mercolini, L. An original HPLC method with coulometric detection to monitor hydroxyl radical generation via Fenton chemistry. Molecules 2019, 24, 3066. [Google Scholar] [CrossRef] [PubMed]
- Mladěnka, P.; Karlíčková, J.; Hrubša, M.; Veljović, E.; Muratović, S.; Carazo, A.; Shivling Mali, A.; Špirtović-Halilović, S.; Saso, L.; Pour, M.; et al. Interaction of 2,6,7-trihydroxy-xanthene-3-ones with iron and copper, and biological effect of the most active derivative on breast cancer cells and erythrocytes. Appl. Sci. 2020, 10, 4846. [Google Scholar] [CrossRef]
- Chan, F.K.; Moriwaki, K.; De Rosa, M.J. Detection of necrosis by release of lactate dehydrogenase activity. Methods Mol. Biol. 2013, 979, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Macáková, K.; Catapano, M.C.; Tvrdý, V.; Klimková, K.; Karlíčková, J.; Mladěnka, P. Hematoxylin assay of cupric chelation can give false positive results. J. Trace Elem. Med. Biol. 2019, 52, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Svobodová, A.; Zdařilová, A.; Walterová, D.; Vostálová, J. Flavonolignans from Silybum marianum moderate UVA-induced oxidative damage to HaCaT keratinocytes. J. Dermatol. Sci. 2007, 48, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, C.; Wadhwa, R.; Deep, G.; Biedermann, D.; Gažák, R.; Křen, V.; Agarwal, R. Anti-cancer efficacy of silybin derivatives—A structure-activity relationship. PLoS ONE 2013, 8, e60074. [Google Scholar] [CrossRef]
- Gažák, R.; Svobodová, A.; Psotová, J.; Sedmera, P.; Přikrylová, V.; Walterová, D.; Křen, V. Oxidised derivatives of silybin and their antiradical and antioxidant activity. Bioorg. Med. Chem. 2004, 12, 5677–5687. [Google Scholar] [CrossRef] [PubMed]
- Abourashed, E.A.; Mikell, J.R.; Khan, I.A. Bioconversion of silybin to phase I and II microbial metabolites with retained antioxidant activity. Bioorg. Med. Chem. 2012, 20, 2784–2788. [Google Scholar] [CrossRef] [PubMed]
- Theodosiou, E.; Purchartová, K.; Stamatis, H.; Kolisis, F.; Křen, V. Bioavailability of silymarin flavonolignans: Drug formulations and biotransformation. Phytochem. Rev. 2014, 13, 1–18. [Google Scholar] [CrossRef]
- Harper, E.; Seifter, S. Studies on the mechanism of action of collagenase: Inhibition by cysteine and other chelating agents. Isr. J. Chem. 1974, 12, 515–528. [Google Scholar] [CrossRef]
- Zatloukalová, M.; Vavříková, E.; Pontinha, A.D.R.; Coufal, J.; Křen, V.; Fojta, M.; Ulrichová, J.; Oliveira-Brett, A.M.; Vacek, J. Flavonolignan conjugates as DNA-binding ligands and topoisomerase i inhibitors: Electrochemical and electrophoretic approaches. Electroanalysis 2016, 28, 2866–2874. [Google Scholar] [CrossRef]
- Laughton, M.J.; Halliwell, B.; Evans, P.J.; Hoult, J.R. Antioxidant and pro-oxidant actions of the plant phenolics quercetin, gossypol and myricetin. Effects on lipid peroxidation, hydroxyl radical generation and bleomycin-dependent damage to DNA. Biochem. Pharmacol. 1989, 38, 2859–2865. [Google Scholar] [CrossRef]
- Goldman, M.; Ali, M. Wilson’s disease presenting as Heinz-body hemolytic anemia. Can. Med. Assoc. J. 1991, 145, 971–972. [Google Scholar]
- Iser, J.H.; Stevens, B.J.; Stening, G.F.; Hurley, T.H.; Smallwood, R.A. Hemolytic anemia of Wilson’s disease. Gastroenterology 1974, 67, 290–293. [Google Scholar] [CrossRef]
- Saran, M.; Michel, C.; Stettmaier, K.; Bors, W. Arguments against the significance of the Fenton reaction contributing to signal pathways under in vivo conditions. Free Radic. Res. 2000, 33, 567–579. [Google Scholar] [CrossRef]
- Aaseth, J.; Skaug, V.; Alexander, J. Haemolytic activity of copper as influenced by chelating agents, albumine and chromium. Acta Pharmacol. Toxicol. 1984, 54, 304–310. [Google Scholar] [CrossRef]
- Khan, H.Y.; Zubair, H.; Ullah, M.F.; Ahmad, A.; Hadi, S. Oral administration of copper to rats leads to increased lymphocyte cellular DNA degradation by dietary polyphenols: Implications for a cancer preventive mechanism. Biometals 2011, 24, 1169–1178. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lomozová, Z.; Tvrdý, V.; Hrubša, M.; Catapano, M.C.; Macáková, K.; Biedermann, D.; Kučera, R.; Křen, V.; Mladěnka, P.; Valentová, K. Dehydroflavonolignans from Silymarin Potentiate Transition Metal Toxicity In Vitro but Are Protective for Isolated Erythrocytes Ex Vivo. Antioxidants 2021, 10, 679. https://doi.org/10.3390/antiox10050679
Lomozová Z, Tvrdý V, Hrubša M, Catapano MC, Macáková K, Biedermann D, Kučera R, Křen V, Mladěnka P, Valentová K. Dehydroflavonolignans from Silymarin Potentiate Transition Metal Toxicity In Vitro but Are Protective for Isolated Erythrocytes Ex Vivo. Antioxidants. 2021; 10(5):679. https://doi.org/10.3390/antiox10050679
Chicago/Turabian StyleLomozová, Zuzana, Václav Tvrdý, Marcel Hrubša, Maria Carmen Catapano, Kateřina Macáková, David Biedermann, Radim Kučera, Vladimír Křen, Přemysl Mladěnka, and Kateřina Valentová. 2021. "Dehydroflavonolignans from Silymarin Potentiate Transition Metal Toxicity In Vitro but Are Protective for Isolated Erythrocytes Ex Vivo" Antioxidants 10, no. 5: 679. https://doi.org/10.3390/antiox10050679
APA StyleLomozová, Z., Tvrdý, V., Hrubša, M., Catapano, M. C., Macáková, K., Biedermann, D., Kučera, R., Křen, V., Mladěnka, P., & Valentová, K. (2021). Dehydroflavonolignans from Silymarin Potentiate Transition Metal Toxicity In Vitro but Are Protective for Isolated Erythrocytes Ex Vivo. Antioxidants, 10(5), 679. https://doi.org/10.3390/antiox10050679