Amino Acid Signature of Oxidative Stress in Patients with Type 2 Diabetes: Targeted Exploratory Metabolomic Research
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Study Assessments and Data Collection
2.3. Serum AA Levels Measurement
2.4. Statistical Analysis
3. Results
3.1. Participants’ Characteristics
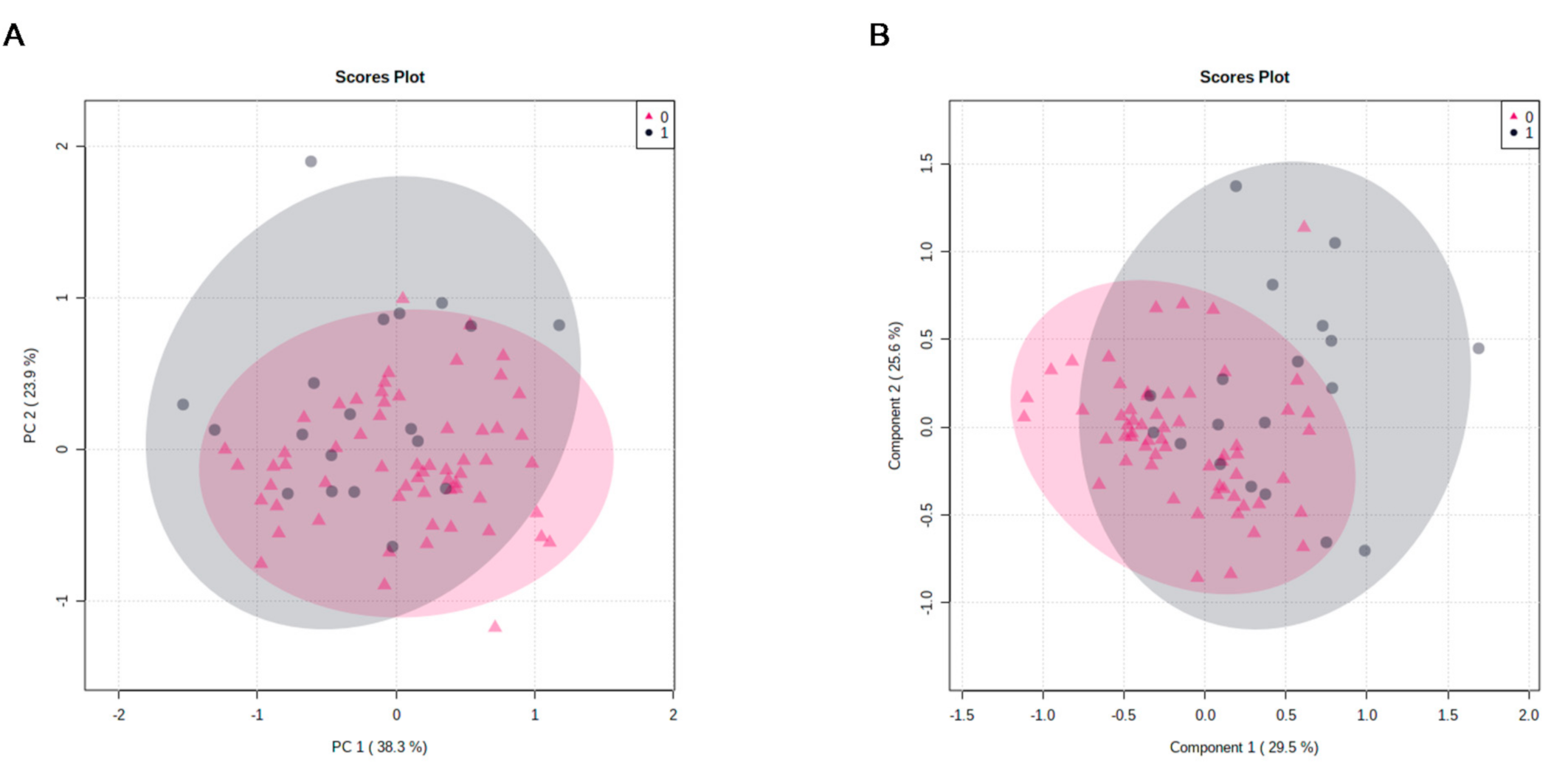
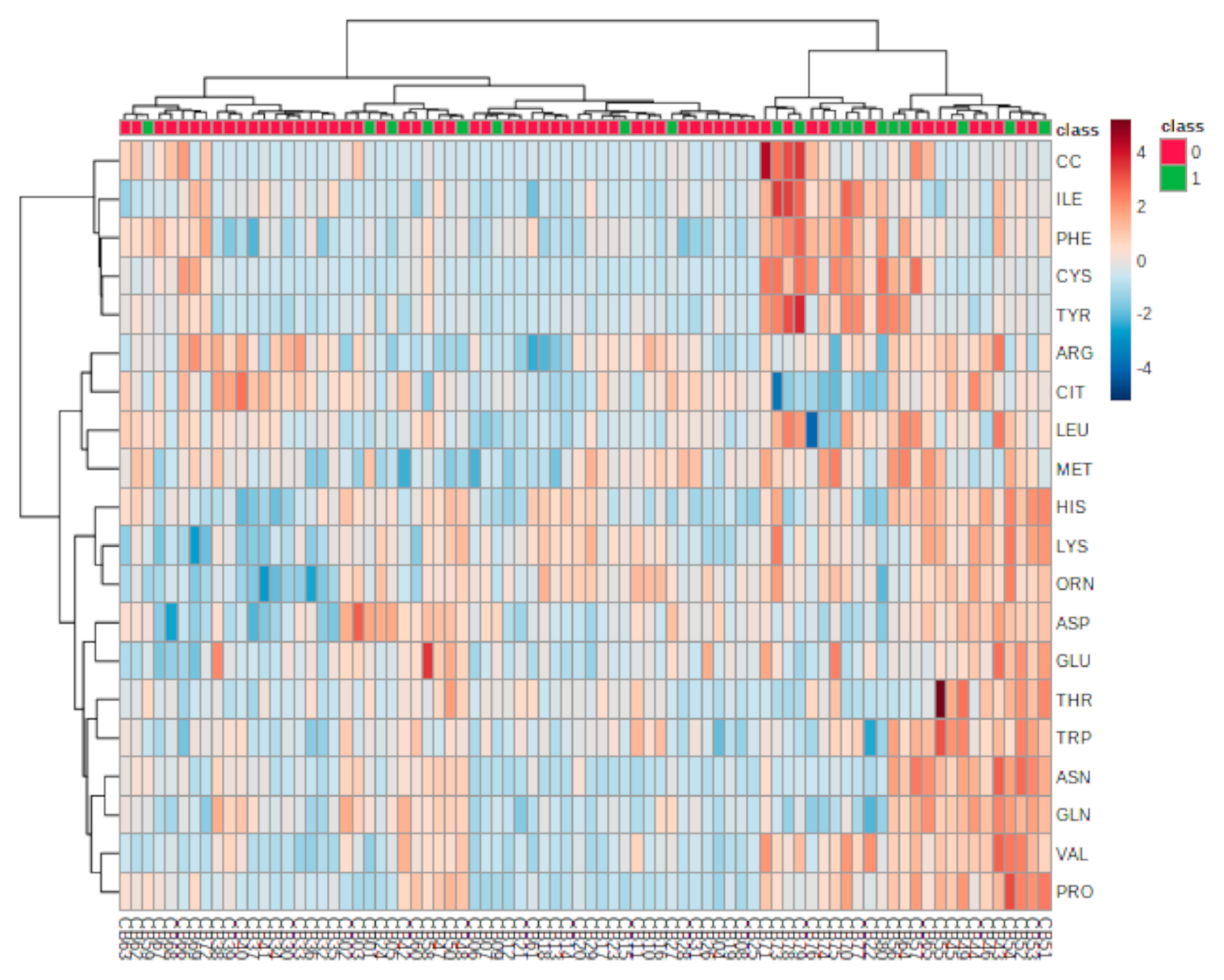
3.2. Metabolites’ Identification
3.3. Metabolic Analysis Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas. Ninth Edition. 2019. Available online: https://www.diabetesatlas.org/en/resources/ (accessed on 1 March 2021).
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44 (Suppl. 1), S15–S33. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Gao, H.Y.; Fan, Z.Y.; He, Y.; Yan, Y.X. Metabolomics Signatures in Type 2 Diabetes: A Systematic Review and Integrative Analysis. J. Clin. Endocrinol. Metab. 2020, 105, dgz240. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxid. Med. Cell. Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ojeda, F.; Olza, J.; Gil, A.; Aguilera, C. Oxidative Stress and Inflammation in Obesity and Metabolic Syndrome. In Obesity: Oxidative Stress and Dietary Antioxidants; Del Moral, A., Aguilera García, C., Eds.; Academic Press: London, UK, 2018; pp. 1–15. [Google Scholar]
- Ahsan, H. 3-Nitrotyrosine: A biomarker of nitrogen free radical species modified proteins in systemic autoimmunogenic conditions. Hum. Immunol. 2013, 74, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Mercuri, F.; Quagliaro, L.; Assaloni, R.; Motz, E.; Tonutti, L.; Taboga, C. Detection of nitrotyrosine in the diabetic plasma: Evidence of oxidative stress. Diabetologia 2001, 44, 834–838. [Google Scholar] [PubMed]
- Bartesaghi, S.; Radi, R. Fundamentals on the biochemistry of peroxynitrite and protein tyrosine nitration. Redox Biol. 2018, 14, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Franquesa, A.; Burkart, A.M.; Isganaitis, E.; Patti, M.E. What Have Metabolomics Approaches Taught Us About Type 2 Diabetes? Curr. Diab. Rep. 2016, 16, 74. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Song, Z.; Hong, Y.; Yang, Z.; Song, Y.; Chen, Z.; Chen, Z.; Cai, Z. Large-scale targeted metabolomics method for metabolite profiling of human samples. Anal. Chim. Acta 2020, 1125, 144–151. [Google Scholar] [CrossRef]
- Yun, J.H.; Lee, H.S.; Yu, H.Y.; Kim, Y.J.; Jeon, H.J.; Oh, T.; Kim, B.J.; Choi, H.J.; Kim, J.M. Metabolomics profiles associated with HbA1c levels in patients with type 2 diabetes. PLoS ONE 2019, 14, e0224274. [Google Scholar] [CrossRef]
- Arneth, B.; Arneth, R.; Shams, M. Metabolomics of Type 1 and Type 2 Diabetes. Int. J. Mol. Sci. 2019, 20, 2467. [Google Scholar] [CrossRef]
- Bala, C.; Rusu, A.; Ciobanu, D.M.; Craciun, A.E.; Roman, G. The association study of high-sensitivity C-reactive protein, pentraxin 3, nitrotyrosine, and insulin dose in patients with insulin-treated type 2 diabetes mellitus. Clin. Risk Manag. 2018, 14, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Rehman, T.; Shabbir, M.A.; Inam-Ur-Raheem, M.; Manzoor, M.F.; Ahmad, N.; Liu, Z.W.; Ahmad, M.H.; Siddeeg, A.; Abid, M.; Aadil, R.M. Cysteine and homocysteine as biomarker of various diseases. Food Sci. Nutr. 2020, 8, 4696–4707. [Google Scholar] [CrossRef] [PubMed]
- Mohorko, N.; Petelin, A.; Jurdana, M.; Biolo, G.; Jenko-Pražnikar, Z. Elevated serum levels of cysteine and tyrosine: Early biomarkers in asymptomatic adults at increased risk of developing metabolic syndrome. Biomed. Res. Int. 2015, 2015, 418681. [Google Scholar] [CrossRef] [PubMed]
- Elshorbagy, A.K.; Smith, A.D.; Kozich, V.; Refsum, H. Cysteine and obesity. Obesity 2012, 20, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Elshorbagy, A.K.; Nurk, E.; Gjesdal, C.G.; Tell, G.S.; Ueland, P.M.; Nygård, O.; Tverdal, A.; Vollset, S.E.; Refsum, H. Homocysteine, cysteine, and body composition in the Hordaland Homocysteine Study: Does cysteine link amino acid and lipid metabolism? Am. J. Clin. Nutr. 2008, 88, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Parodi, O.; De Chiara, B.; Baldassarre, D.; Parolini, M.; Caruso, R.; Pustina, L.; Parodi, G.; Campolo, J.; Sedda, V.; Baudo, F.; et al. Plasma cysteine and glutathione are independent markers of postmethionine load endothelial dysfunction. Clin. Biochem. 2007, 40, 188–193. [Google Scholar] [CrossRef]
- Saez, G.; Thornalley, P.J.; Hill, H.A.; Hems, R.; Bannister, J.V. The production of free radicals during the autoxidation of cysteine and their effect on isolated rat hepatocytes. Biochim. Biophys. Acta 1982, 719, 24–31. [Google Scholar] [CrossRef]
- Jia, L.; Furchgott, R.F. Inhibition by sulfhydryl compounds of vascular relaxation induced by nitric oxide and endothelium-derived relaxing factor. J. Pharm. Exp. 1993, 267, 371–378. [Google Scholar]
- De Chiara, B.; Sedda, V.; Parolini, M.; Campolo, J.; De Maria, R.; Caruso, R.; Pizzi, G.; Disoteo, O.; Dellanoce, C.; Corno, A.R.; et al. Plasma total cysteine and cardiovascular risk burden: Action and interaction. Sci. World J. 2012, 2012, 303654. [Google Scholar] [CrossRef]
- Würtz, P.; Soininen, P.; Kangas, A.J.; Rönnemaa, T.; Lehtimäki, T.; Kähönen, M.; Viikari, J.S.; Raitakari, O.T.; Ala-Korpela, M. Branched-chain and aromatic amino acids are predictors of insulin resistance in young adults. Diabetes Care 2013, 36, 648–655. [Google Scholar] [CrossRef]
- Vangipurapu, J.; Stancáková, A.; Smith, U.; Kuusisto, J.; Laakso, M. Nine Amino Acids Are Associated with Decreased Insulin Secretion and Elevated Glucose Levels in a 7.4-Year Follow-up Study of 5181 Finnish Men. Diabetes 2019, 68, 1353–1358. [Google Scholar] [CrossRef]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef]
- Welsh, P.; Rankin, N.; Li, Q.; Mark, P.B.; Würtz, P.; Ala-Korpela, M.; Marre, M.; Poulter, N.; Hamet, P.; Chalmers, J.; et al. Circulating amino acids and the risk of macrovascular, microvascular and mortality outcomes in individuals with type 2 diabetes: Results from the ADVANCE trial. Diabetologia 2018, 61, 1581–1591. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.; Luo, H.; Fang, Z.Z.; Ai, H. Role of aromatic amino acids in pathogeneses of diabetic nephropathy in Chinese patients with type 2 diabetes. J. Diabetes Complicat. 2020, 34, 107667. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Chen, T.; Zhang, Q.; Zhang, H.; Zhu, Z.; Chai, Y.; Zhang, J. Metabolomic study of the protective effect of Gandi capsule for diabetic nephropathy. Chem. Biol. Interact. 2019, 314, 108815. [Google Scholar] [CrossRef]
- Werner, E.R.; Blau, N.; Thöny, B. Tetrahydrobiopterin: Biochemistry and pathophysiology. Biochem. J. 2011, 438, 397–414. [Google Scholar] [CrossRef]
- Kopple, J.D. Phenylalanine and tyrosine metabolism in chronic kidney failure. J. Nutr. 2007, 137 (Suppl. 1), 1586S–1590S; discussion, 97S–98S. [Google Scholar] [CrossRef]
- Syslová, K.; Böhmová, A.; Mikoška, M.; Kuzma, M.; Pelclová, D.; Kačer, P. Multimarker screening of oxidative stress in aging. Oxid. Med. Cell. Longev. 2014, 2014, 562860. [Google Scholar] [CrossRef]
- Citrulline. Compound Summary. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Citrulline (accessed on 5 March 2021).
- Esper, R.J.; Nordaby, R.A.; Vilariño, J.O.; Paragano, A.; Cacharrón, J.L.; Machado, R.A. Endothelial dysfunction: A comprehensive appraisal. Cardiovasc. Diabetol. 2006, 5, 4. [Google Scholar] [CrossRef]
- Mannarino, E.; Pirro, M. Endothelial injury and repair: A novel theory for atherosclerosis. Angiology 2008, 59 (Suppl. 2), 69S–72S. [Google Scholar] [CrossRef]
- Mallika, V.; Goswami, B.; Rajappa, M. Atherosclerosis pathophysiology and the role of novel risk factors: A clinicobiochemical perspective. Angiology 2007, 58, 513–522. [Google Scholar] [CrossRef]
- Förstermann, U.; Münzel, T. Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation 2006, 113, 1708–1714. [Google Scholar] [CrossRef]
- Ceriello, A. Oxidative stress and diabetes-associated complications. Endocr. Pract. 2006, 12 (Suppl. 1), 60–62. [Google Scholar] [CrossRef]
- Marfella, R.; Quagliaro, L.; Nappo, F.; Ceriello, A.; Giugliano, D. Acute hyperglycemia induces an oxidative stress in healthy subjects (Letter). J. Clin. Investig. 2001, 108, 635–636. [Google Scholar] [CrossRef]
- Ceriello, A.; Esposito, K.; Piconi, L.; Ihnat, M.A.; Thorpe, J.E.; Testa, R.; Boemi, M.; Giugliano, D. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 2008, 57, 1349–1354. [Google Scholar] [CrossRef]
- Monnier, L.; Mas, E.; Ginet, C.; Michel, F.; Villon, L.; Cristol, J.P.; Colette, C. Activation of oxidative stress by acute glu-cose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006, 295, 1681–1687. [Google Scholar] [CrossRef]
- Oh, Y.S.; Jun, H.S. Effects of Glucagon-Like Peptide-1 on Oxidative Stress and Nrf2 Signaling. Int. J. Mol. Sci. 2017, 19, 26. [Google Scholar] [CrossRef]
- McGavin, J.K.; Perry, C.M.; Goa, K.L. Gliclazide modified release. Drugs 2002, 62, 1357–1364. [Google Scholar] [CrossRef]
- O’Brien, R.C.; Luo, M.; Balazs, N.; Mercuri, J. In vitro and in vivo antioxidant properties of gliclazide. J. Diab. Compl. 2000, 14, 201–206. [Google Scholar] [CrossRef]
- Prato, S.D.; Pulizzi, N. The place of sulfonylureas in the therapy for type 2 diabetes mellitus. Metab. Clin. Exp. 2006, 55 (Suppl. 1), S20–S27. [Google Scholar] [CrossRef]


| Q4 Nitrotyrosine N = 19 | Q1–Q3 Nitrotyrosine N = 61 | p-Value | |
|---|---|---|---|
| Women, n (%) | 13 (68.4%) | 34 (55.7%) | 0.427 |
| Smoking, n (%) | 1 (5.3%) | 8 (13.1%) | 0.678 |
| BMI, kg/m2 | 32.0 ± 4.6 | 32.7 ± 6.0 | 0.668 |
| Diabetes duration, years | 14.5 ± 7.7 | 12.3 ± 6.8 | 0.251 |
| FPG, mg/dL | 187.9 ± 62.1 | 177.5 ± 71.2 | 0.567 |
| HbA1c, % | 8.1 ± 1.2 | 8.5 ± 1.7 | 0.301 |
| LDL cholesterol, mg/dL | 86.6 ± 39.7 | 95.7 ± 34.9 | 0.340 |
| HDL cholesterol, mg/dL | 45.1 ± 11.6 | 46.7 ± 11.0 | 0.580 |
| Triglycerides, mg/dL | 196.6 ± 98.9 | 174.5 ± 78.5 | 0.319 |
| Diabetic neuropathy, n (%) | 15 (78.9%) | 36 (59.0%) | 0.172 |
| Diabetic retinopathy, n (%) | 6 (31.6%) | 16 (26.2%) | 0.651 |
| Diabetic kidney disease, n (%) | 7 (36.8%) | 30 (49.2%) | 0.148 |
| HBP, n (%) | 15 (78.9%) | 54 (88.5%) | 0.281 |
| DLP, n (%) | 15 (78.9%) | 48 (78.7%) | 1.00 |
| CVD, n (%) | 8 (42.1%) | 25 (41.0%) | 1.00 |
| Diabetes therapy, n (%) | |||
| Insulin | 19 (100.0%) | 61 (100.0%) | - |
| Metformin | 14 (73.7%) | 39 (63.9%) | 0.581 |
| Sulfonylurea | 3 (15.8%) | 1 (1.6%) | 0.040 |
| GLP1 RA | 4 (21.1%) | 2 (3.3%) | 0.026 |
| DPP-4i | 0 (0.0%) | 1 (1.6%) | 1.00 |
| α-glucosidase inhibitor | 0 (0.0%) | 2 (3.3%) | 1.00 |
| Thiazolidinediones | 1 (5.3%) | 0 (0.0%) | 0.237 |
| Number of hypoglycemia in the previous 30 days | 0 (0; 1) | 0 (0; 1) | 0.971 |
| Nitrotyrosine, nmol/ml | 66.5 (48.5; 96.0) | 22.9 (19.1; 26.7) | <0.001 |
| Q4 Nitrotyrosine N = 19 | Q1–Q3 Nitrotyrosine N = 61 | p-Value | FDR Corrected p-Value | |
|---|---|---|---|---|
| Tyrosine | 45.1 (39.4; 116.7) | 36.0 (33.2; 41.6) | 1.7693e-05 | 0.00035385 |
| Phenylalanine | 89.2 (64.3; 150.5) | 59.3 (44.7; 77.9) | 0.00025327 | 0.0025327 |
| Cysteine | 84.7 (69.7; 213.0) | 67.7 (65.3; 78.7) | 0.0013475 | 0.0089834 |
| Citrulline | 11.5 (4.9; 14.8) | 17.0 (9.7; 25.5) | 0.0040269 | 0.020134 |
| Isoleucine | 46.3 (40.5; 69.4) | 40.8 (37.7; 47.0) | 0.0068384 | 0.027354 |
| Proline | 163.3 (140.7; 179.4) | 139.0 (130.8; 157.4) | 0.0083898 | 0.027966 |
| Methionine | 13.1 (11.7; 15.4) | 11.9 (10.2; 13.6) | 0.012433 | 0.035522 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bala, C.G.; Rusu, A.; Ciobanu, D.; Bucsa, C.; Roman, G. Amino Acid Signature of Oxidative Stress in Patients with Type 2 Diabetes: Targeted Exploratory Metabolomic Research. Antioxidants 2021, 10, 610. https://doi.org/10.3390/antiox10040610
Bala CG, Rusu A, Ciobanu D, Bucsa C, Roman G. Amino Acid Signature of Oxidative Stress in Patients with Type 2 Diabetes: Targeted Exploratory Metabolomic Research. Antioxidants. 2021; 10(4):610. https://doi.org/10.3390/antiox10040610
Chicago/Turabian StyleBala, Cornelia G., Adriana Rusu, Dana Ciobanu, Camelia Bucsa, and Gabriela Roman. 2021. "Amino Acid Signature of Oxidative Stress in Patients with Type 2 Diabetes: Targeted Exploratory Metabolomic Research" Antioxidants 10, no. 4: 610. https://doi.org/10.3390/antiox10040610
APA StyleBala, C. G., Rusu, A., Ciobanu, D., Bucsa, C., & Roman, G. (2021). Amino Acid Signature of Oxidative Stress in Patients with Type 2 Diabetes: Targeted Exploratory Metabolomic Research. Antioxidants, 10(4), 610. https://doi.org/10.3390/antiox10040610







