Melatonin for the Management of Preeclampsia: A Review
Abstract
1. Introduction
2. The Search for Novel Therapies
3. Melatonin in Normal Pregnancy and Preeclampsia
4. Melatonin as an Antihypertensive
5. Beyond Melatonin: Other Antioxidants for Preeclampsia
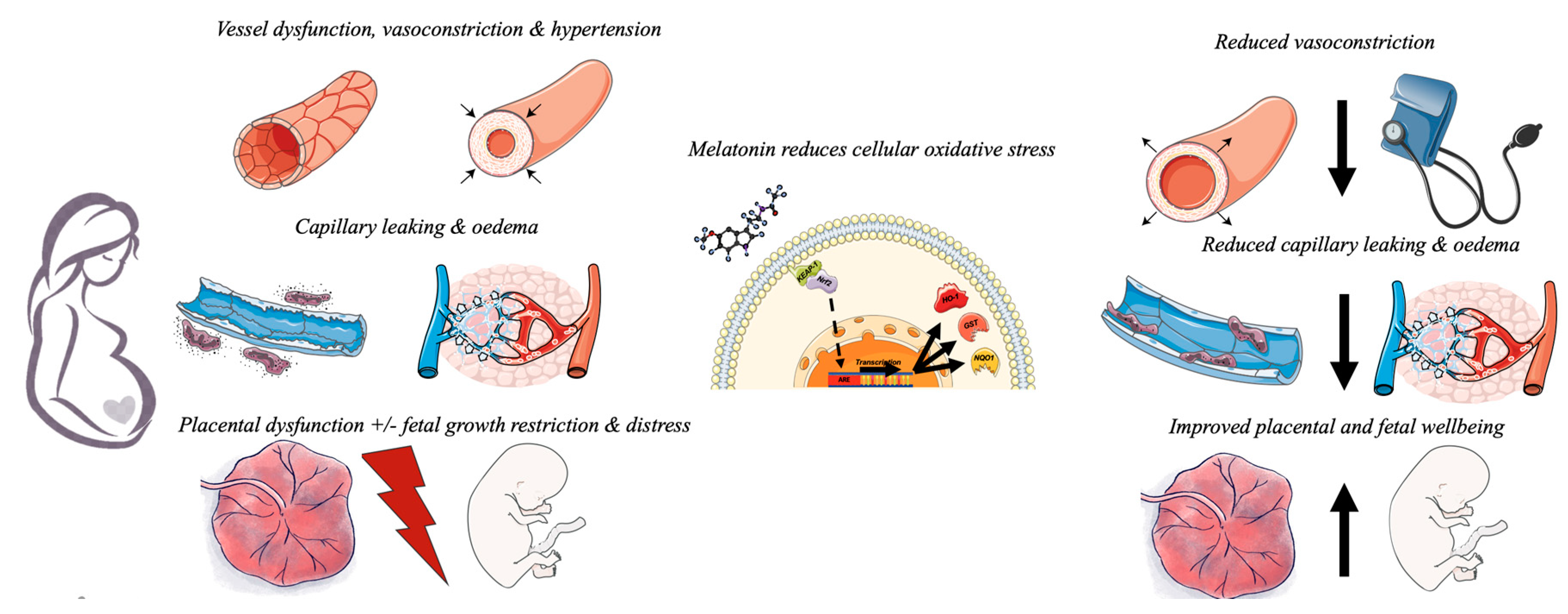
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lowe, S.A.; Bowyer, L.; Lust, K.; McMahon, L.P.; Morton, M.; North, R.A.; Paech, M.; Said, J.M. SOMANZ guidelines for the management of hypertensive disorders of pregnancy 2014. Aust. N. Z. J. Obstet. Gynaecol. 2015, 55, e1–e29. [Google Scholar] [CrossRef]
- Maynard, S.E.; Karumanchi, S.A. Angiogenic Factors and Preeclampsia. Semin Nephrol. 2011, 31, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Goulopoulou, S.; Davidge, S.T. Molecular mechanisms of maternal vascular dysfunction in preeclampsia. Trends Mol. Med. 2015, 21, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.M.; Hubel, C.A. Is oxidative stress the link in the two-stage model of pre-eclampsia? Lancet 1999, 354, 788–789. [Google Scholar] [CrossRef]
- Sibai, B.; Dekker, G.; Kupferminc, M. Pre-eclampsia. Lancet 2005, 365, 785–799. [Google Scholar] [CrossRef]
- National Collaborating Centre for Women’s and Children’s Health. Hypertension in Pregnancy: The Management of Hypertensive Disorders during Pregnancy; RCOG Press: London, UK, 2011. [Google Scholar]
- Ghulmiyyah, L.; Sibai, B. Maternal mortality from preeclampsia/eclampsia. Semin. Perinatol. 2012, 36, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Australian Institute of Health and Welfare. Maternal Deaths in Australia 2008–2012; AIHW: Canberra, Australia, 2015.
- Craici, I.; Wagner, S.; Garovic, V.D. Preeclampsia and future cardiovascular risk: Formal risk factor or failed stress test? Ther. Adv. Cardiovasc. Dis. 2008, 2, 249–259. [Google Scholar] [CrossRef]
- Bhorat, I.; Naidoo, D.P.; Moodley, J. Maternal cardiac haemodynamics in severe pre-eclampsia complicated by acute pulmonary oedema: A review. J. Matern. Fetal Neonatal Med. 2017, 30, 2769–2777. [Google Scholar] [CrossRef]
- Hakim, J.; Senterman, M.K.; Hakim, A.M. Preeclampsia is a biomarker for vascular disease in both mother and child: The need for a medical alert system. Int. J. Pediatr. 2013, 2013, 953150. [Google Scholar] [CrossRef]
- Amaral, L.M.; Cunningham, M.W.; Cornelius, D.C.; LaMarca, B. Preeclampsia: Long-term consequences for vascular health. Vasc. Health Risk Manag. 2015, 11, 403–415. [Google Scholar] [CrossRef] [PubMed]
- McCormick, M.C.; Litt, J.S.; Smith, V.C.; Zupancic, J.A. Prematurity: An overview and public health implications. Annu. Rev. Public Health 2011, 32, 367–379. [Google Scholar] [CrossRef]
- Chehade, H.; Simeoni, U.; Guignard, J.P.; Boubred, F. Preterm birth: Long term cardiovascular and renal consequences. Curr. Pediatr. Rev. 2018, 14, 219–226. [Google Scholar] [CrossRef]
- Abitbol, C.L.; Rodriguez, M.M. The long-term renal and cardiovascular consequences of prematurity. Nat. Rev. Nephrol. 2012, 8, 265–274. [Google Scholar] [CrossRef]
- Cox, A.G.; Marshall, S.A.; Palmer, K.R.; Wallace, E.M. Current and emerging pharmacotherapy for emergency management of preeclampsia. Expert Opin. Pharmacother. 2019, 20, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Fenton, C.; Hobson, S.R.; Wallace, E.M.; Lim, R. Future therapies for pre-eclampsia: Beyond treading water. Aust. N. Z. J. Obstet. Gynaecol. 2014, 54, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Karumanchi, S.A. Angiogenic factors in preeclampsia: From diagnosis to therapy. Hypertension 2016, 67, 1072–1079. [Google Scholar] [CrossRef]
- Roberts, J.M.; Taylor, R.N.; Musci, T.J.; Rodgers, G.M.; Hubel, C.A.; McLaughlin, M.K. Preeclampsia: An endothelial cell disorder. Am. J. Obstet. Gynecol. 1989, 161, 1200–1204. [Google Scholar] [CrossRef]
- Roberts, J.M.; Redman, C.W. Pre-eclampsia: More than pregnancy-induced hypertension. Lancet 1993, 341, 1447–1451. [Google Scholar] [CrossRef]
- Levine, R.J.; Maynard, S.E.; Qian, C.; Lim, K.H.; England, L.J.; Yu, K.F.; Schisterman, E.F.; Thadhani, R.; Sachs, B.P.; Epstein, F.H.; et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 2004, 350, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Venkatesha, S.; Toporsian, M.; Lam, C.; Hanai, J.-I.; Mammoto, T.; Kim, Y.M.; Bdolah, Y.; Lim, K.-H.; Yuan, H.-T.; Libermann, T.A.; et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat. Med. 2006, 12, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.; Acharya, R.; Delpachitra, P.; Hobson, S.; Sobey, C.G.; Drummond, G.R.; Wallace, E.M. Activin and NADPH-oxidase in preeclampsia: Insights from in vitro and murine studies. Am. J. Obstet. Gynecol. 2015, 212, 86.e1–86.e12. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Karumanchi, S.A.; Levine, R.J.; Venkatesha, S.; Rauh-Hain, J.A.; Tamez, H.; Thadhani, R. Sequential changes in antiangiogenic factors in early pregnancy and risk of developing preeclampsia. Hypertension 2007, 50, 137–142. [Google Scholar] [CrossRef]
- Anderson, C.M.; Lopez, F.; Zhang, H.-Y.; Shirasawa, Y.; Pavlish, K.; Benoit, J.N. Mesenteric vascular responsiveness in a rat model of pregnancy-induced hypertension. Exp. Biol. Med. 2006, 231, 1398–1402. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Longo, M.; Tamayo, E.; Maner, W.; Al-Hendy, A.; Anderson, G.D.; Hankins, G.D.; Saade, G.R. The effect of over-expression of sFlt-1 on blood pressure and the occurrence of other manifestations of preeclampsia in unrestrained conscious pregnant mice. Am. J. Obstet. Gynecol. 2007, 196, e1–e7. [Google Scholar] [CrossRef]
- Maynard, S.E.; Min, J.-Y.; Merchan, J.; Lim, K.-H.; Li, J.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, F.W.; Stillman, I.E.; et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003, 111, 649–658. [Google Scholar] [CrossRef]
- Thadhani, R.; Kisner, T.; Hagmann, H.; Bossung, V.; Noack, S.; Schaarschmidt, W.; Jank, A.; Kribs, A.; Cornely, O.A.; Kreyssig, C.; et al. Pilot study of extracorporeal removal of soluble fms-like tyrosine kinase 1 in preeclampsia. Circulation 2011, 124, 940–950. [Google Scholar] [CrossRef]
- Thomas, C.P.; Andrews, J.I.; Liu, K.Z. Intronic polyadenylation signal sequences and alternate splicing generate human soluble Flt1 variants and regulate the abundance of soluble Flt1 in the placenta. FASEB J. 2007, 21, 3885–3895. [Google Scholar] [CrossRef]
- Matsubara, K.; Higaki, T.; Matsubara, Y.; Nawa, A. Nitric oxide and reactive oxygen ppecies in the pathogenesis of preeclampsia. Int. J. Mol. Sci. 2015, 16, 4600–4614. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.; Adhikari, S.; Gurusinghe, S.; Leaw, B.; Acharya, R.; Rahman, R.; Ciayadi, R.; Potdar, M.; Kelso, G.F.; Hearn, M.T.; et al. Inhibition of activin A signalling in a mouse model of pre-eclampsia. Placenta 2015, 36, 926–931. [Google Scholar] [CrossRef]
- Mandang, S.; Manuelpillai, U.; Wallace, E.M. Oxidative stress increases placental and endothelial cell activin A secretion. J. Endocrinol. 2007, 192, 485–493. [Google Scholar] [CrossRef]
- Manuelpillai, U.; Schneider-Kolsky, M.; Dole, A.; Wallace, E.M. Activin A and activin receptors in gestational tissue from preeclamptic pregnancies. J. Endocrinol. 2001, 171, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Brown, C.W.; Matzuk, M.M. Genetic analysis of the mammalian transforming growth factor-beta superfamily. Endocr. Rev. 2002, 23, 787–823. [Google Scholar] [CrossRef] [PubMed]
- Birukova, A.A.; Birukov, K.G.; Adyshev, D.; Usatyuk, P.; Natarajan, V.; Garcia, J.G.; Verin, A.D. Involvement of microtubules and Rho pathway in TGF-beta1-induced lung vascular barrier dysfunction. J. Cell. Physiol. 2005, 204, 934–947. [Google Scholar] [CrossRef] [PubMed]
- Birukova, A.A.; Adyshev, D.; Gorshkov, B.; Birukov, K.G.; Verin, A.D. ALK5 and Smad4 are involved in TGF-β1-induced pulmonary endothelial permeability. FEBS Lett. 2005, 579, 4031–4037. [Google Scholar] [CrossRef] [PubMed]
- Hinck, A.P. Structural studies of the TGF-βs and their receptors—Insights into evolution of the TGF-β superfamily. FEBS Lett. 2012, 586, 1860–1870. [Google Scholar] [CrossRef] [PubMed]
- Muttukrishna, S.; Knight, P.G.; Groome, N.P.; Redman, C.W.; Ledger, W.L. Activin A and inhibin A as possible endocrine markers for pre-eclampsia. Lancet 1997, 349, 1285–1288. [Google Scholar] [CrossRef]
- D’Antona, D.; Reis, F.M.; Benedetto, C.; Evans, L.W.; Groome, N.P.; de Kretser, D.M.; Wallace, E.M.; Petraglia, F. Increased maternal serum activin A but not follistatin levels in pregnant women with hypertensive disorders. J. Endocrinol. 2000, 165, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Hobson, S.R.; Acharya, R.; Lim, R.; Chan, S.T.; Mockler, J.; Wallace, E.M. Role of activin A in the pathogenesis of endothelial cell dysfunction in preeclampsia. Pregnancy Hypertens. 2016, 6, 130–133. [Google Scholar] [CrossRef]
- Schneider-Kolsky, M.E.; Manuelpillai, U.; Waldron, K.; Dole, A.; Wallace, E.M. The distribution of activin and activin receptors in gestational tissues across human pregnancy and during labour. Placenta 2002, 23, 294–302. [Google Scholar] [CrossRef]
- Schneider-Kolsky, M.; Manuelpillai, U.; Gargett, C.; Wallace, E.M. Activin betaA-subunit and activin receptors in human myometrium at term and during labour. Bjog 2001, 108, 869–874. [Google Scholar] [CrossRef]
- Muttukrishna, S.; North, R.A.; Morris, J.; Schellenberg, J.C.; Taylor, R.S.; Asselin, J.; Ledger, W.; Groome, N.; Redman, C.W. Serum inhibin A and activin A are elevated prior to the onset of pre-eclampsia. Hum. Reprod. 2000, 15, 1640–1645. [Google Scholar] [CrossRef]
- Petraglia, F.; De Vita, D.; Gallinelli, A.; Aguzzoli, L.; Genazzani, A.R.; Romero, R.; Woodruff, T.K. Abnormal concentration of maternal serum activin-A in gestational diseases. J. Clin. Endocrinol. Metab. 1995, 80, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Makris, A.; Thornton, C.; Thompson, J.; Thomson, S.; Martin, R.; Ogle, R.; Waugh, R.; McKenzie, P.; Kirwan, P.; Hennessy, A. Uteroplacental ischemia results in proteinuric hypertension and elevated sFLT-1. Kidney Int. 2007, 71, 977–984. [Google Scholar] [CrossRef]
- Oujo, B.; Perez-Barriocanal, F.; Bernabeu, C.; Lopez-Novoa, J.M. Membrane and soluble forms of endoglin in preeclampsia. Curr. Mol. Med. 2013, 13, 1345–1357. [Google Scholar] [CrossRef] [PubMed]
- Hobson, S.R.; Gurusinghe, S.; Lim, R.; Alers, N.O.; Miller, S.L.; Kingdom, J.C.; Wallace, E.M. Melatonin improves endothelial function in vitro and prolongs pregnancy in women with early-onset preeclampsia. J. Pineal Res. 2018, 65, e12508. [Google Scholar] [CrossRef]
- Cox, A.G.; Gurusinghe, S.; Abd Rahman, R.; Leaw, B.; Chan, S.T.; Mockler, J.C.; Murthi, P.; Marshall, S.A.; Lim, R.; Wallace, E.M. Sulforaphane improves endothelial function and reduces placental oxidative stress in vitro. Pregnancy Hypertens. 2019, 16, 1–10. [Google Scholar] [CrossRef]
- Gurusinghe, S.; Cox, A.G.; Rahman, R.; Chan, S.T.; Muljadi, R.; Singh, H.; Leaw, B.; Mockler, J.C.; Marshall, S.A.; Murthi, P.; et al. Resveratrol mitigates trophoblast and endothelial dysfunction partly via activation of nuclear factor erythroid 2-related factor-2. Placenta 2017, 60, 74–85. [Google Scholar] [CrossRef]
- Miller, S.L.; Wallace, E.M.; Walker, D.W. Antioxidant therapies: A potential role in perinatal medicine. Neuroendocrinology 2012, 96, 13–23. [Google Scholar] [CrossRef]
- Lanoix, D.; Guerin, P.; Vaillancourt, C. Placental melatonin production and melatonin receptor expression are altered in preeclampsia: New insights into the role of this hormone in pregnancy. J. Pineal Res. 2012, 53, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Nakamura, Y.; Terron, M.P.; Flores, L.J.; Manchester, L.C.; Tan, D.X.; Sugino, N.; Reiter, R.J. Melatonin and pregnancy in the human. Reprod. Toxicol. 2008, 25, 291–303. [Google Scholar] [CrossRef]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolín, I.; Herrera, F.; Martín, V.; Reiter, R.J. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 2004, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Swarnamani, K.; Davies-Tuck, M.; Wallace, E.; Mol, B.W.; Mockler, J. A double-blind randomised placebo-controlled trial of melatonin as an adjuvant agent in induction of labour (MILO): A study protocol. BMJ Open 2020, 10, e032480. [Google Scholar] [CrossRef]
- Nakamura, Y.; Tamura, H.; Kashida, S.; Takayama, H.; Yamagata, Y.; Karube, A.; Sugino, N.; Kato, H. Changes of serum melatonin level and its relationship to feto-placental unit during pregnancy. J. Pineal Res. 2001, 30, 29–33. [Google Scholar] [CrossRef]
- Zeng, K.; Gao, Y.; Wan, J.; Tong, M.; Lee, A.C.; Zhao, M.; Chen, Q. The reduction in circulating levels of melatonin may be associated with the development of preeclampsia. J. Hum. Hypertens. 2016, 30, 666–671. [Google Scholar] [CrossRef]
- Paulis, L.; Simko, F. Blood pressure modulation and cardiovascular protection by melatonin: Potential mechanisms behind. Physiol. Res. 2007, 56, 671–684. [Google Scholar] [PubMed]
- Kansanen, E.; Kuosmanen, S.M.; Leinonen, H.; Levonen, A.L. The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox Biol. 2013, 1, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Vriend, J.; Reiter, R.J. The Keap1-Nrf2-antioxidant response element pathway: A review of its regulation by melatonin and the proteasome. Mol. Cell. Endocrinol. 2015, 401, 213–220. [Google Scholar] [CrossRef]
- Cudmore, M.; Ahmad, S.; Al-Ani, B.; Fujisawa, T.; Coxall, H.; Chudasama, K.; Devey, L.R.; Wigmore, S.J.; Abbas, A.; Hewett, P.W.; et al. Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation 2007, 115, 1789–1797. [Google Scholar] [CrossRef]
- Zakkar, M.; Van der Heiden, K.; Luong Le, A.; Chaudhury, H.; Cuhlmann, S.; Hamdulay, S.S.; Krams, R.; Edirisinghe, I.; Rahman, I.; Carlsen, H.; et al. Activation of Nrf2 in endothelial cells protects arteries from exhibiting a proinflammatory state. Arterioscler. Thromb Vasc. Biol. 2009, 29, 1851–1857. [Google Scholar] [CrossRef]
- Kikuchi, M.; Ushida, Y.; Shiozawa, H.; Umeda, R.; Tsuruya, K.; Aoki, Y.; Suganuma, H.; Nishizaki, Y. Sulforaphane-rich broccoli sprout extract improves hepatic abnormalities in male subjects. World J. Gastroenerol. 2015, 21, 12457–12467. [Google Scholar] [CrossRef]
- Langston-Cox, A.; Leo, C.H.; Tare, M.; Wallace, E.M.; Marshall, S.A. Sulforaphane improves vascular reactivity in mouse and human arteries after “preeclamptic-like” injury. Placenta 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zuo, Q.; Huang, S.; Yu, X.; Jiang, Z.; Zou, S.; Fan, M.; Sun, L. Resveratrol inhibits trophoblast apoptosis through oxidative stress in preeclampsia-model rats. Molecules 2014, 19, 20570–20579. [Google Scholar] [CrossRef] [PubMed]
- Chapple, S.J.; Puszyk, W.M.; Mann, G.E. Keap1-Nrf2 regulated redox signaling in utero: Priming of disease susceptibility in offspring. Free Radic. Biol. Med. 2015, 88, 212–220. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, M.; Tong, M.; Xu, l.; Groom, K.; Stone, P.; Chamley, L. Melatonin supplementation prevents endothelial cell activation, possible relevance to preeclampsia: Endothelial dysfunction, anti-angiogenic factors. Pregnancy Hypertens. 2016, 6, 207–208. [Google Scholar] [CrossRef]
- Hannan, N.J.; Binder, N.K.; Beard, S.; Nguyen, T.V.; Kaitu’u-Lino, T.J.; Tong, S. Melatonin enhances antioxidant molecules in the placenta, reduces secretion of soluble fms-like tyrosine kinase 1 (sFLT) from primary trophoblast but does not rescue endothelial dysfunction: An evaluation of its potential to treat preeclampsia. PLoS ONE 2018, 13, e0187082. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, Y.; Xu, L.; Hickey, A.; Groom, K.; Stone, P.R.; Chamley, L.W.; Chen, Q. Melatonin prevents preeclamptic sera and antiphospholipid antibodies inducing the production of reactive nitrogen species and extrusion of toxic trophoblastic debris from first trimester placentae. Placenta 2017, 58, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.H.; Skepper, J.N.; Charnock-Jones, D.S.; Burton, G.J. Hypoxia-reoxygenation: A potent inducer of apoptotic changes in the human placenta and possible etiological factor in preeclampsia. Circ. Res. 2002, 90, 1274–1281. [Google Scholar] [CrossRef]
- Reiter, R.J.; Rosales-Corral, S.; Tan, D.X.; Jou, M.J.; Galano, A.; Xu, B. Melatonin as a mitochondria-targeted antioxidant: One of evolution’s best ideas. Cell. Mol. Life Sci. 2017, 74, 3863–3881. [Google Scholar] [CrossRef]
- Widschwendter, M.; Schröcksnadel, H.; Mörtl, M.G. Pre-eclampsia: A disorder of placental mitochondria? Mol. Med. Today 1998, 4, 286–291. [Google Scholar] [CrossRef]
- Lefebvre, T.; Roche, O.; Seegers, V.; Cherif, M.; Khiati, S.; Gueguen, N.; Desquiret-Dumas, V.; Geffroy, G.; Blanchet, O.; Reynier, P.; et al. Study of mitochondrial function in placental insufficiency. Placenta 2018, 67, 1–7. [Google Scholar] [CrossRef]
- Mandò, C.; De Palma, C.; Stampalija, T.; Anelli, G.M.; Figus, M.; Novielli, C.; Parisi, F.; Clementi, E.; Ferrazzi, E.; Cetin, I. Placental mitochondrial content and function in intrauterine growth restriction and preeclampsia. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E404–E413. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Han, T.L.; Chen, H.; Baker, P.N.; Qi, H.; Zhang, H. Impaired mitochondrial fusion, autophagy, biogenesis and dysregulated lipid metabolism is associated with preeclampsia. Exp. Cell. Res. 2017, 359, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Holland, O.; Dekker Nitert, M.; Gallo, L.A.; Vejzovic, M.; Fisher, J.J.; Perkins, A.V. Review: Placental mitochondrial function and structure in gestational disorders. Placenta 2017, 54, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef]
- Berman, S.B.; Pineda, F.J.; Hardwick, J.M. Mitochondrial fission and fusion dynamics: The long and short of it. Cell Death Differ. 2008, 15, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.M.; Williams, J.A.; Ding, W.X. Mitochondrial dynamics and mitochondrial quality control. Redox Biol. 2015, 4, 6–13. [Google Scholar] [CrossRef]
- Tilokani, L.; Nagashima, S.; Paupe, V.; Prudent, J. Mitochondrial dynamics: Overview of molecular mechanisms. Essays Biochem. 2018, 62, 341–360. [Google Scholar] [CrossRef]
- Colleoni, F.; Padmanabhan, N.; Yung, H.-W.; Watson, E.D.; Cetin, I.; Tissot van Patot, M.C.; Burton, G.J.; Murray, A.J. Suppression of mitochondrial electron transport chain function in the hypoxic human placenta: A role for miRNA-210 and protein synthesis inhibition. PLoS ONE 2013, 8, e55194. [Google Scholar] [CrossRef]
- Friedman, J.R.; Nunnari, J. Mitochondrial form and function. Nature 2014, 505, 335–343. [Google Scholar] [CrossRef]
- Casteilla, L.; Rigoulet, M.; Pénicaud, L. Mitochondrial ROS metabolism: Modulation by uncoupling proteins. IUBMB Life 2001, 52, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Holland, O.J.; Cuffe, J.S.M.; Dekker Nitert, M.; Callaway, L.; Kwan Cheung, K.A.; Radenkovic, F.; Perkins, A.V. Placental mitochondrial adaptations in preeclampsia associated with progression to term delivery. Cell. Death Dis. 2018, 9, 1150. [Google Scholar] [CrossRef]
- Jou, M.J.; Peng, T.I.; Reiter, R.J.; Jou, S.B.; Wu, H.Y.; Wen, S.T. Visualization of the antioxidative effects of melatonin at the mitochondrial level during oxidative stress-induced apoptosis of rat brain astrocytes. J. Pineal Res. 2004, 37, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Jou, M.J.; Peng, T.I.; Yu, P.Z.; Jou, S.B.; Reiter, R.J.; Chen, J.Y.; Wu, H.Y.; Chen, C.C.; Hsu, L.F. Melatonin protects against common deletion of mitochondrial DNA-augmented mitochondrial oxidative stress and apoptosis. J. Pineal Res. 2007, 43, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Okatani, Y.; Wakatsuki, A.; Shinohara, K.; Kaneda, C.; Fukaya, T. Melatonin stimulates glutathione peroxidase activity in human chorion. J. Pineal Res. 2001, 30, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Liu, X.; Rosales-Corral, S.A.; Acuna-Castroviejo, D.; Reiter, R.J. Mitochondria and chloroplasts as the original sites of melatonin synthesis: A hypothesis related to melatonin’s primary function and evolution in eukaryotes. J. Pineal Res. 2013, 54, 127–138. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Galano, A.; Jou, M.-J.; Acuna-Castroviejo, D. Melatonin Mitigates Mitochondrial Meltdown: Interactions with SIRT3. Int. J. Mol. Sci. 2018, 19, 2439. [Google Scholar] [CrossRef]
- López, A.; García, J.A.; Escames, G.; Venegas, C.; Ortiz, F.; López, L.C.; Acuña-Castroviejo, D. Melatonin protects the mitochondria from oxidative damage reducing oxygen consumption, membrane potential, and superoxide anion production. J. Pineal Res. 2009, 46, 188–198. [Google Scholar] [CrossRef]
- Andrabi, S.A.; Sayeed, I.; Siemen, D.; Wolf, G.; Horn, T.F.W. Direct inhibition of the mitochondrial permeability transition pore: A possible mechanism responsible for anti-apoptotic effects of melatonin. FASEB J. 2004, 18, 869–871. [Google Scholar] [CrossRef]
- Lanoix, D.; Lacasse, A.A.; Reiter, R.J.; Vaillancourt, C. Melatonin: The watchdog of villous trophoblast homeostasis against hypoxia/reoxygenation-induced oxidative stress and apoptosis. Mol. Cell. Endocrinol. 2013, 381, 35–45. [Google Scholar] [CrossRef]
- Nagai, R.; Watanabe, K.; Wakatsuki, A.; Hamada, F.; Shinohara, K.; Hayashi, Y.; Imamura, R.; Fukaya, T. Melatonin preserves fetal growth in rats by protecting against ischemia/reperfusion-induced oxidative/nitrosative mitochondrial damage in the placenta. J. Pineal Res. 2008, 45, 271–276. [Google Scholar] [CrossRef]
- Ireland, K.E.; Maloyan, A.; Myatt, L. Melatonin Improves Mitochondrial Respiration in Syncytiotrophoblasts From Placentas of Obese Women. Reprod. Sci. 2018, 25, 120–130. [Google Scholar] [CrossRef]
- Langston-Cox, A.; Muccini, A.M.; Marshall, S.A.; Yap, Y.; Palmer, K.R.; Wallace, E.M.; Ellery, S.J. Sulforaphane improves syncytiotrophoblast mitochondrial function after in vitro hypoxic and superoxide injury. Placenta 2020, 96, 44–54. [Google Scholar] [CrossRef]
- Son, G.W.; Kim, G.-D.; Yang, H.; Park, H.R.; Park, Y.S. Alteration of gene expression profile by melatonin in endothelial cells. Biochip J. 2014, 8, 91–101. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Y.; Zhang, X.; Zheng, L.; Zhu, B.; Yao, S.; Yang, L.; Du, J. Melatonin protects against hypoxia/reoxygenation-induced dysfunction of human umbilical vein endothelial cells through inhibiting reactive oxygen species generation. Acta Cardiol. Sin. 2018, 34, 424–431. [Google Scholar] [CrossRef]
- Cheng, J.; Yang, H.-L.; Gu, C.-J.; Liu, Y.-K.; Shao, J.; Zhu, R.; He, Y.-Y.; Zhu, X.-Y.; Li, M.-Q. Melatonin restricts the viability and angiogenesis of vascular endothelial cells by suppressing HIF-1α/ROS/VEGF. Int. J. Mol. Med. 2019, 43, 945–955. [Google Scholar] [CrossRef]
- Lemley, C.O.; Meyer, A.M.; Camacho, L.E.; Neville, T.L.; Newman, D.J.; Caton, J.S.; Vonnahme, K.A. Melatonin supplementation alters uteroplacental hemodynamics and fetal development in an ovine model of intrauterine growth restriction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R454–R467. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.L.; Yan, E.B.; Castillo-Meléndez, M.; Jenkin, G.; Walker, D.W. Melatonin provides neuroprotection in the late-gestation fetal sheep brain in response to umbilical cord occlusion. Dev. Neurosci. 2005, 27, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Richter, H.G.; Hansell, J.A.; Raut, S.; Giussani, D.A. Melatonin improves placental efficiency and birth weight and increases the placental expression of antioxidant enzymes in undernourished pregnancy. J. Pineal Res. 2009, 46, 357–364. [Google Scholar] [CrossRef]
- Welin, A.K.; Svedin, P.; Lapatto, R.; Sultan, B.; Hagberg, H.; Gressens, P.; Kjellmer, I.; Mallard, C. Melatonin reduces inflammation and cell death in white matter in the mid-gestation fetal sheep following umbilical cord occlusion. Pediatr. Res. 2007, 61, 153–158. [Google Scholar] [CrossRef]
- Miller, S.L.; Yawno, T.; Alers, N.O.; Castillo-Melendez, M.; Supramaniam, V.G.; VanZyl, N.; Sabaretnam, T.; Loose, J.M.; Drummond, G.R.; Walker, D.W.; et al. Antenatal antioxidant treatment with melatonin to decrease newborn neurodevelopmental deficits and brain injury caused by fetal growth restriction. J. Pineal Res. 2014, 56, 283–294. [Google Scholar] [CrossRef]
- Palmer, K.R.; Mockler, J.C.; Davies-Tuck, M.L.; Miller, S.L.; Goergen, S.K.; Fahey, M.C.; Anderson, P.J.; Groom, K.M.; Wallace, E.M. Protect-me: A parallel-group, triple blinded, placebo-controlled randomised clinical trial protocol assessing antenatal maternal melatonin supplementation for fetal neuroprotection in early-onset fetal growth restriction. BMJ Open 2019, 9, e028243. [Google Scholar] [CrossRef]
- Hardeland, R.; Madrid, J.A.; Tan, D.X.; Reiter, R.J. Melatonin, the circadian multioscillator system and health: The need for detailed analyses of peripheral melatonin signaling. J. Pineal Res. 2012, 52, 139–166. [Google Scholar] [CrossRef]
- Laflamme, K.; Wu, L.; Foucart, S.; de Champlain, J. Impaired basal sympathetic tone and alpha1-adrenergic responsiveness in association with the hypotensive effect of melatonin in spontaneously hypertensive rats. Am. J. Hypertens. 1998, 11, 219–229. [Google Scholar] [CrossRef]
- Simko, F.; Paulis, L. Melatonin as a potential antihypertensive treatment. J. Pineal Res. 2007, 42, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Zanoboni, A.; Zanoboni-Muciaccia, W. Experimental hypertension in pinealectomized rats. Life Sci. 1967, 6, 2327–2331. [Google Scholar] [CrossRef]
- Karppanen, H.; Lahovaara, S.; Mannisto, P.; Vapaatalo, H. Plasma renin activity and in vitro synthesis of aldosterone by the adrenal glands of rats with spontaneous, renal, or pinealectomy-induced hypertension. Acta Physiol. Scand. 1975, 94, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Holmes, S.W.; Sugden, D. Proceedings: The effect of melatonin on pinealectomy-induced hypertension in the rat. Br. J. Pharmacol. 1976, 56, 360P–361P. [Google Scholar]
- Thakor, A.S.; Herrera, E.A.; Seron-Ferre, M.; Giussani, D.A. Melatonin and vitamin C increase umbilical blood flow via nitric oxide-dependent mechanisms. J. Pineal Res. 2010, 49, 399–406. [Google Scholar] [CrossRef] [PubMed]
- McCarty, K.J.; Owen, M.P.T.; Hart, C.G.; Thompson, R.C.; Burnett, D.D.; King, E.H.; Hopper, R.M.; Lemley, C.O. Effect of chronic melatonin supplementation during mid to late gestation on maternal uterine artery blood flow and subsequent development of male offspring in beef cattle. J. Anim. Sci. 2018, 96, 5100–5111. [Google Scholar] [CrossRef]
- Capsoni, S.; Stankov, B.M.; Fraschini, F. Reduction of regional cerebral blood flow by melatonin in young rats. Neuroreport 1995, 6, 1346–1348. [Google Scholar] [CrossRef]
- Deniz, E.; Sahna, E.; Aksulu, H.E. Nitric oxide synthase inhibition in rats: Melatonin reduces blood pressure and ischemia/reperfusion-induced infarct size. Scand. Cardiovasc. J. SCJ 2006, 40, 248–252. [Google Scholar] [CrossRef]
- Hung, M.W.; Kravtsov, G.M.; Lau, C.F.; Poon, A.M.; Tipoe, G.L.; Fung, M.L. Melatonin ameliorates endothelial dysfunction, vascular inflammation, and systemic hypertension in rats with chronic intermittent hypoxia. J. Pineal Res. 2013, 55, 247–256. [Google Scholar] [CrossRef]
- Doolen, S.; Krause, D.N.; Dubocovich, M.L.; Duckles, S.P. Melatonin mediates two distinct responses in vascular smooth muscle. Eur. J. Pharmacol. 1998, 345, 67–69. [Google Scholar] [CrossRef]
- Weekley, L.B. Effects of melatonin on pulmonary and coronary vessels are exerted through perivascular nerves. Clin. Auton. Res. 1993, 3, 45–47. [Google Scholar] [CrossRef]
- Satake, N.; Oe, H.; Sawada, T.; Shibata, S. The mode of vasorelaxing action of melatonin in rabbit aorta. Gen. Pharmacol. 1991, 22, 219–221. [Google Scholar] [CrossRef]
- Satake, N.; Shibata, S.; Takagi, T. The inhibitory action of melatonin on the contractile response to 5-hydroxytryptamine in various isolated vascular smooth muscles. Gen. Pharmacol. 1986, 17, 553–558. [Google Scholar] [CrossRef]
- Satake, N.; Oe, H.; Shibata, S. Vasorelaxing action of melatonin in rat isolated aorta; possible endothelium dependent relaxation. Gen. Pharmacol. 1991, 22, 1127–1133. [Google Scholar] [CrossRef]
- Yang, Q.; Scalbert, E.; Delagrange, P.; Vanhoutte, P.M.; O’Rourke, S.T. Melatonin potentiates contractile responses to serotonin in isolated porcine coronary arteries. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H76–H82. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.S.; Sauder, C.L.; Ray, C.A. Melatonin differentially affects vascular blood flow in humans. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H670–H674. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; BaHammam, A.S.; Ojike, N.I.; Akinseye, O.A.; Kendzerska, T.; Buttoo, K.; Dhandapany, P.S.; Brown, G.M.; Cardinali, D.P. Melatonin and human cardiovascular disease. J. Cardiovasc. Pharmacol. Ther. 2017, 22, 122–132. [Google Scholar] [CrossRef]
- Jiki, Z.; Lecour, S.; Nduhirabandi, F. Cardiovascular benefits of dietary melatonin: A myth or a reality? Front. Physiol. 2018, 9, 528. [Google Scholar] [CrossRef]
- Tunstall, R.R.; Shukla, P.; Grazul-Bilska, A.; Sun, C.; O’Rourke, S.T. MT2 receptors mediate the inhibitory effects of melatonin on nitric oxide-induced relaxation of porcine isolated coronary arteries. J. Pharmacol. Exp. Ther. 2011, 336, 127–133. [Google Scholar] [CrossRef]
- Okatani, Y.; Wakatsuki, A.; Kaneda, C. Melatonin increases activities of glutathione peroxidase and superoxide dismutase in fetal rat brain. J. Pineal Res. 2000, 28, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Terron, M.P.; Flores, L.J.; Czarnocki, Z. Melatonin and its metabolites: New findings regarding their production and their radical scavenging actions. Acta Biochim. Pol. 2007, 54, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wu, Y.; Zhang, Y.; Zhang, H.; Shi, L. Melatonin activates BK(Ca) channels in cerebral artery myocytes via both direct and MT receptor/PKC-mediated pathway. Eur. J. Pharmacol. 2019, 842, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Zhang, H.; Jin, C.; Qiu, F.; Wu, Y.; Shi, L. Melatonin mediates vasodilation through both direct and indirect activation of BKCa channels. J. Mol. Endocrinol. 2017, 59, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Girouard, H.; Chulak, C.; Lejossec, M.; Lamontagne, D.; de Champlain, J. Vasorelaxant effects of the chronic treatment with melatonin on mesenteric artery and aorta of spontaneously hypertensive rats. J. Hypertens. 2001, 19, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- van der Helm-van Mil, A.H.M.; van Someren, E.J.W.; van den Boom, R.; van Buchem, M.A.; de Craen, A.J.M.; Blauw, G.J. No influence of melatonin on cerebral blood flow in humans. J. Clin. Endocrinol. Metab. 2003, 88, 5989–5994. [Google Scholar] [CrossRef] [PubMed]
- Javanmard, S.H.; Heshmat-Ghahdarijani, K.; Mirmohammad-Sadeghi, M.; Sonbolestan, S.A.; Ziayi, A. The effect of melatonin on endothelial dysfunction in patient undergoing coronary artery bypass grafting surgery. Adv. Biomed. Res. 2016, 5, 174. [Google Scholar] [CrossRef]
- Kojsova, S.; Jendekova, L.; Zicha, J.; Kunes, J.; Andriantsitohaina, R.; Pechanova, O. The effect of different antioxidants on nitric oxide production in hypertensive rats. Physiol. Res. 2006, 55 (Suppl. 1), S3–S16. [Google Scholar] [PubMed]
- Pechánová, O.; Zicha, J.; Paulis, L.; Zenebe, W.; Dobesová, Z.; Kojsová, S.; Jendeková, L.; Sládková, M.; Dovinová, I.; Simko, F.; et al. The effect of N-acetylcysteine and melatonin in adult spontaneously hypertensive rats with established hypertension. Eur. J. Pharmacol. 2007, 561, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Hobson, S.R.; Mockler, J.C.; Lim, R.; Alers, N.O.; Miller, S.L.; Wallace, E.M. Melatonin for treating pre-eclampsia. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef]
- Okatani, Y.; Okamoto, K.; Hayashi, K.; Wakatsuki, A.; Tamura, S.; Sagara, Y. Maternal-fetal transfer of melatonin in pregnant women near term. J. Pineal Res. 1998, 25, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Hamada, F.; Wakatsuki, A.; Nagai, R.; Shinohara, K.; Hayashi, Y.; Imamura, R.; Fukaya, T. Prophylactic administration of melatonin to the mother throughout pregnancy can protect against oxidative cerebral damage in neonatal rats. J. Matern. Fetal Neonatal Med. 2012, 25, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Alers, N.O.; Jenkin, G.; Miller, S.L.; Wallace, E.M. Antenatal melatonin as an antioxidant in human pregnancies complicated by fetal growth restriction--a phase I pilot clinical trial: Study protocol. BMJ Open 2013, 3, e004141. [Google Scholar] [CrossRef]
- Fernando, S.; Wallace, E.M.; Vollenhoven, B.; Lolatgis, N.; Hope, N.; Wong, M.; Lawrence, M.; Lawrence, A.; Russell, C.; Leong, K.; et al. Melatonin in Assisted Reproductive Technology: A Pilot Double-Blind Randomized Placebo-Controlled Clinical Trial. Front Endocrinol 2018, 9, 545. [Google Scholar] [CrossRef] [PubMed]
- Silman, R.E. Melatonin: A contraceptive for the nineties. Eur. J. Obstet. Gynecol. Reprod. Biol. 1993, 49, 3–9. [Google Scholar] [CrossRef]
- Khezri, M.B.; Reihany, M.D.; Dabbaghi Ghaleh, T.; Mohammadi, N. Effect of Melatonin on Blood Loss After Cesarean Section: A Prospective Randomized Double-Blind Trial. J. Obstet. Gynaecol. India 2019, 69, 436–443. [Google Scholar] [CrossRef]
- Muñoz-Hoyos, A.; Sánchez-Forte, M.; Molina-Carballo, A.; Escames, G.; Martin-Medina, E.; Reiter, R.J.; Molina-Font, J.A.; Acuña-Castroviejo, D. Melatonin’s role as an anticonvulsant and neuronal protector: Experimental and clinical evidence. J. Child Neurol. 1998, 13, 501–509. [Google Scholar] [CrossRef]
- McCance, D.R.; Holmes, V.A.; Maresh, M.J.; Patterson, C.C.; Walker, J.D.; Pearson, D.W.; Young, I.S. Vitamins C and E for prevention of pre-eclampsia in women with type 1 diabetes (DAPIT): A randomised placebo-controlled trial. Lancet 2010, 376, 259–266. [Google Scholar] [CrossRef]
- Kalpdev, A.; Saha, S.C.; Dhawan, V. Vitamin C and E supplementation does not reduce the risk of superimposed PE in pregnancy. Hypertens. Pregnancy 2011, 30, 447–456. [Google Scholar] [CrossRef]
- Kaitu’u-Lino, T.J.; Brownfoot, F.C.; Beard, S.; Cannon, P.; Hastie, R.; Nguyen, T.V.; Binder, N.K.; Tong, S.; Hannan, N.J. Combining metformin and esomeprazole is additive in reducing sFlt-1 secretion and decreasing endothelial dysfunction—Implications for treating preeclampsia. PLoS ONE 2018, 13, e0188845. [Google Scholar] [CrossRef]
- Onda, K.; Tong, S.; Beard, S.; Binder, N.; Muto, M.; Senadheera, S.N.; Parry, L.; Dilworth, M.; Renshall, L.; Brownfoot, F.; et al. Proton pump inhibitors decrease soluble fms-like tyrosine kinase-1 and soluble endoglin secretion, decrease hypertension, and rescue endothelial dysfunction. Hypertension 2017, 69, 457–468. [Google Scholar] [CrossRef]
- Cluver, C.A.; Hannan, N.J.; van Papendorp, E.; Hiscock, R.; Beard, S.; Mol, B.W.; Theron, G.B.; Hall, D.R.; Decloedt, E.H.; Stander, M.; et al. Esomeprazole to treat women with preterm preeclampsia: A randomized placebo controlled trial. Am. J. Obstet. Gynecol. 2018, 219, 388.e1–388.e17. [Google Scholar] [CrossRef] [PubMed]
- Cudmore, M.J.; Ramma, W.; Cai, M.; Fujisawa, T.; Ahmad, S.; Al-Ani, B.; Ahmed, A. Resveratrol inhibits the release of soluble fms-like tyrosine kinase (sFlt-1) from human placenta. Am. J. Obstet. Gynecol. 2012, 206, 253.e10–253.e15. [Google Scholar] [CrossRef]
- Ding, J.; Kang, Y.; Fan, Y.; Chen, Q. Efficacy of resveratrol to supplement oral nifedipine treatment in pregnancy-induced preeclampsia. Endocr. Connect. 2017, 6, 595–600. [Google Scholar] [CrossRef]
- Langston-Cox, A.G.; Marshall, S.A.; Palmer, K.R.; Wallace, E.M. Prolong: A double-blind randomised placebo-controlled trial of broccoli sprout extract in women with early onset preeclampsia: A clinical trial protocol. BMJ Open 2019, 9, e027493. [Google Scholar] [CrossRef]
- Langston-Cox, A.G.; Anderson, D.; Creek, D.J.; Palmer, K.R.; Marshall, S.A.; Wallace, E.M. Sulforaphane bioavailability and effects on blood pressure in women with pregnancy hypertension. Reprod. Sci. 2021. [Google Scholar] [CrossRef]
| Number of Participants | Intervention | Primary Outcome | Study Type | |
|---|---|---|---|---|
| Swarnamani, K. et al., 2020 [54] | 774 women undergoing induction of labour | Four doses of 10 mg of melatonin or placebo | Requirement for caesarean section | Double-blind randomised placebo-controlled trial |
| Palmer K. R. et al., 2019 [103] | 336 women with FGR pregnancy between 23 + 0 and 31 + 6 weeks | 30 mg per day or placebo | Neurodevelopment (difference of 5 points in the cognitive domain of the Bayley-III) | Triple-blind, parallel, randomised placebo-controlled trial |
| Khezri, M. et al., 2019 [140] | One hundred and twenty women undergoing caesarean section | 3 or 6 mg of melatonin or placebo 20 min before caesarean section | Change in haemoglobin level | Double-blind randomised trial |
| Fernando, S. et al., 2018 [138] | One hundred and sixty women undergoing their first cycle of IVF or ICSI | 2, 4 or 8 mg of melatonin or placebo twice daily | Clinical pregnancy rate | Double-blind randomised placebo-controlled trial |
| Hobson S.R. et al., 2016 [47] | Twenty women with preeclampsia | 10 mg of melatonin three times daily | Diagnosis to delivery interval | Single-arm, open-label study |
| Alers N.O. et al., 2013 [137] | 12 women with FGR pregnancies < 34 wks | 4 mg melatonin twice daily | Biomarkers placental and circulating oxidative stress | Single-arm, open-label study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langston-Cox, A.; Marshall, S.A.; Lu, D.; Palmer, K.R.; Wallace, E.M. Melatonin for the Management of Preeclampsia: A Review. Antioxidants 2021, 10, 376. https://doi.org/10.3390/antiox10030376
Langston-Cox A, Marshall SA, Lu D, Palmer KR, Wallace EM. Melatonin for the Management of Preeclampsia: A Review. Antioxidants. 2021; 10(3):376. https://doi.org/10.3390/antiox10030376
Chicago/Turabian StyleLangston-Cox, Annie, Sarah A. Marshall, Daisy Lu, Kirsten R. Palmer, and Euan M. Wallace. 2021. "Melatonin for the Management of Preeclampsia: A Review" Antioxidants 10, no. 3: 376. https://doi.org/10.3390/antiox10030376
APA StyleLangston-Cox, A., Marshall, S. A., Lu, D., Palmer, K. R., & Wallace, E. M. (2021). Melatonin for the Management of Preeclampsia: A Review. Antioxidants, 10(3), 376. https://doi.org/10.3390/antiox10030376






