Polymorphisms in Glyoxalase I Gene Are Not Associated with Glyoxalase I Expression in Whole Blood or Markers of Methylglyoxal Stress: The CODAM Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
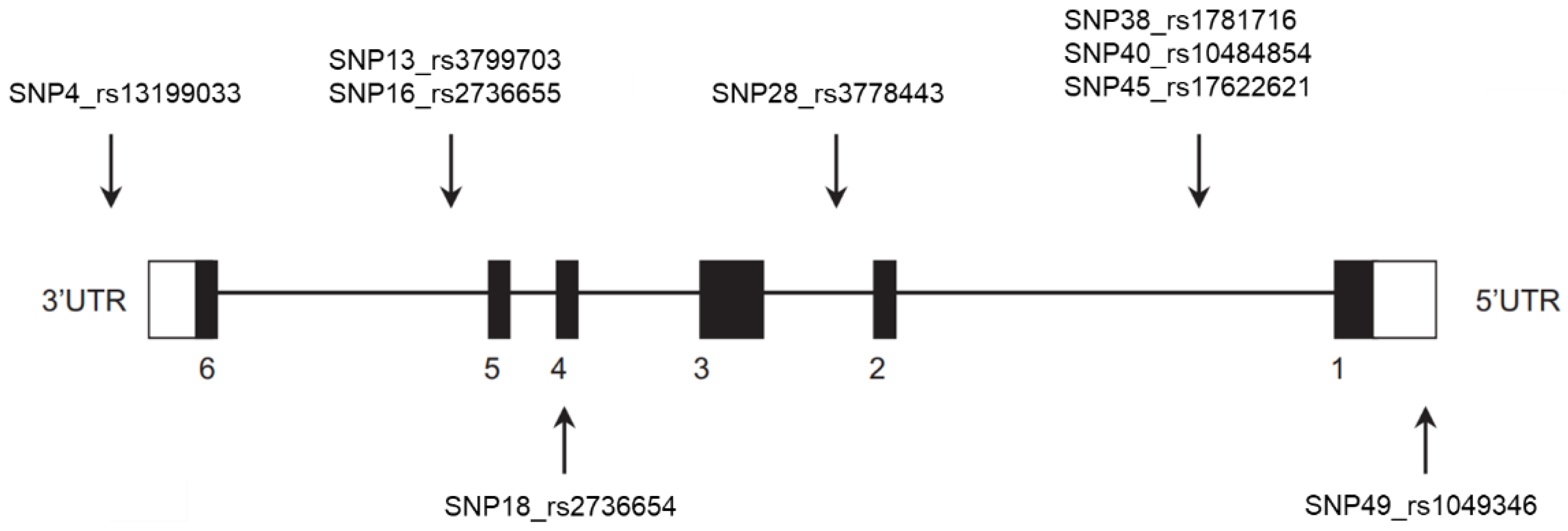
2.2. Single Nucleotide Polymorphism Selection and Genotyping
2.3. GLO1 mRNA Expression in Whole Blood
2.4. Markers of MGO Stress
2.5. iAUC of MGO after an OGTT
2.6. Definition of Glucose Metabolism Status
2.7. White Blood Cell Composition
2.8. Statistical Analysis
3. Results
3.1. Correlations between GLO1 mRNA Expression and Markers of MGO Stress
3.2. Association between GLO1 Polymorphisms and GLO1 mRNA Expression in Whole Blood
3.3. Association between GLO1 Polymorphisms and Markers of MGO Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thornalley, P.J. The glyoxalase system: New developments towards functional characterization of a metabolic pathway fundamental to biological life. Biochem. J. 1990, 269, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Maessen, D.E.M.; Stehouwer, C.D.A.; Schalkwijk, C.G. The role of methylglyoxal and the glyoxalase system in diabetes and other age-related diseases. Clin. Sci. 2015, 128, 839–861. [Google Scholar] [CrossRef] [PubMed]
- Schalkwijk, C.; Stehouwer, C.D.A. Methylglyoxal, a Highly Reactive Dicarbonyl Compound, in Diabetes, Its Vascular Complications, and Other Age-Related Diseases. Physiol. Rev. 2020, 100, 407–461. [Google Scholar] [CrossRef]
- Chaudhuri, J.; Bains, Y.; Guha, S.; Kahn, A.; Hall, D.; Bose, N.; Gugliucci, A.; Kapahi, P. The Role of Advanced Glycation End Products in Aging and Metabolic Diseases: Bridging Association and Causality. Cell Metab. 2018, 28, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nat. Cell Biol. 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Thornalley, P.J. Glyoxalase 1 Modulation in Obesity and Diabetes. Antioxid. Redox Signal 2019, 30, 354–374. [Google Scholar] [CrossRef]
- Shinohara, M.; Thornalley, P.J.; Giardino, I.; Beisswenger, P.; Thorpe, S.R.; Onorato, J.; Brownlee, M. Overexpression of glyoxalase-I in bovine endothelial cells inhibits intracellular advanced glycation endproduct formation and prevents hyperglycemia-induced increases in macromolecular endocytosis. J. Clin. Investig. 1998, 101, 1142–1147. [Google Scholar] [CrossRef]
- Kuhla, B.; Lüth, H.-J.; Haferburg, D.; Weick, M.; Reichenbach, A.; Arendt, T.; Münch, G. Pathological effects of glyoxalase I inhibition in SH-SY5Y neuroblastoma cells. J. Neurosci. Res. 2006, 83, 1591–1600. [Google Scholar] [CrossRef]
- Dobler, D.; Ahmed, N.; Song, L.; Eboigbodin, K.E.; Thornalley, P.J. Increased Dicarbonyl Metabolism in Endothelial Cells in Hyperglycemia Induces Anoikis and Impairs Angiogenesis by RGD and GFOGER Motif Modification. Diabetes 2006, 55, 1961–1969. [Google Scholar] [CrossRef]
- Brouwers, O.; Niessen, P.M.G.; Miyata, T.; Østergaard, J.A.; Flyvbjerg, A.; Peutz-Kootstra, C.J.; Sieber, J.; Mundel, P.H.; Brownlee, M.; Janssen, B.J.A.; et al. Glyoxalase-1 overexpression reduces endothelial dysfunction and attenuates early renal impairment in a rat model of diabetes. Diabetologia 2014, 57, 224–235. [Google Scholar] [CrossRef]
- Stratmann, B.; Engelbrecht, B.; Espelage, B.C.; Klusmeier, N.; Tiemann, J.; Gawlowski, T.; Mattern, Y.; Eisenacher, M.; Meyer, H.E.; Rabbani, N.; et al. Glyoxalase 1-knockdown in human aortic endothelial cells–effect on the proteome and endothelial function estimates. Sci. Rep. 2016, 6, 37737. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Thornalley, P.J. Glyoxalase in diabetes, obesity and related disorders. Semin. Cell Dev. Biol. 2011, 22, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, O.; Niessen, P.M.; Ferreira, I.; Miyata, T.; Scheffer, P.G.; Teerlink, T.; Schrauwen, P.; Brownlee, M.; Stehouwer, C.D.; Schalkwijk, C.G. Overexpression of Glyoxalase-I Reduces Hyperglycemia-induced Levels of Advanced Glycation End Products and Oxidative Stress in Diabetic Rats. J. Biol. Chem. 2011, 286, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
- Skapare, E.; Konrade, I.; Liepinsh, E.; Strele, I.; Makrecka, M.; Bierhaus, A.; Lejnieks, A.; Pirags, V.; Dambrova, M. Association of reduced glyoxalase 1 activity and painful peripheral diabetic neuropathy in type 1 and 2 diabetes mellitus patients. J. Diabetes Complicat. 2013, 27, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, N.M.; Stehouwer, C.D.; Schalkwijk, C.G. Methylglyoxal and glyoxalase I in atherosclerosis. Biochem. Soc. Trans. 2014, 42, 443–449. [Google Scholar] [CrossRef]
- Mäkinen, V.-P.; Civelek, M.; Meng, Q.; Zhang, B.; Zhu, J.; Levian, C.; Huan, T.; Segrè, A.V.; Ghosh, S.; Vivar, J.; et al. Integrative Genomics Reveals Novel Molecular Pathways and Gene Networks for Coronary Artery Disease. PLoS Genet. 2014, 10, e1004502. [Google Scholar] [CrossRef]
- Engelen, L.; Ferreira, I.; Brouwers, O.; Henry, R.M.A.; Dekker, J.M.; Nijpels, G.; Heine, R.J.; Van Greevenbroek, M.M.J.; Van Der Kallen, C.J.H.; Blaak, E.E.; et al. Polymorphisms in glyoxalase 1 gene are not associated with vascular complications: The Hoorn and CoDAM studies. J. Hypertens. 2009, 27, 1399–1403. [Google Scholar] [CrossRef]
- Kruijshoop, M.; Feskens, E.J.M.; Blaak, E.E.; De Bruin, T.W. Validation of capillary glucose measurements to detect glucose intolerance or type 2 diabetes mellitus in the general population. Clin. Chim. Acta 2004, 341, 33–40. [Google Scholar] [CrossRef]
- Scheijen, J.L.; Schalkwijk, C.G. Quantification of glyoxal, methylglyoxal and 3-deoxyglucosone in blood and plasma by ultra performance liquid chromatography tandem mass spectrometry: Evaluation of blood specimen. Clin. Chem. Lab. Med. 2014, 52, 85–91. [Google Scholar] [CrossRef]
- Hanssen, N.M.; Engelen, L.; Ferreira, I.; Scheijen, J.L.; Huijberts, M.S.; Van Greevenbroek, M.M.J.; Van Der Kallen, C.J.H.; Dekker, J.M.; Nijpels, G.; Stehouwer, C.D.; et al. Plasma Levels of Advanced Glycation Endproducts Nϵ-(carboxymethyl)lysine, Nϵ-(carboxyethyl)lysine, and Pentosidine Are not Independently Associated With Cardiovascular Disease in Individuals With or Without Type 2 Diabetes: The Hoorn and CODAM Studies. J. Clin. Endocrinol. Metab. 2013, 98, E1369–E1373. [Google Scholar] [CrossRef]
- Scheijen, J.L.; Hanssen, N.M.J.; Van De Waarenburg, M.P.H.; Jonkers, D.M.A.E.; Stehouwer, C.D.A.; Schalkwijk, C.G. L(+) and D(-) Lactate Are Increased in Plasma and Urine Samples of Type 2 Diabetes as Measured by a Simultaneous Quantification of L(+) and D(-) Lactate by Reversed-Phase Liquid Chromatography Tandem Mass Spectrometry. Exp. Diabetes Res. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Scheijen, J.L.; Hanssen, N.M.; Van Greevenbroek, M.M.J.; Van Der Kallen, C.J.H.; Feskens, E.J.M.; A Stehouwer, C.D.; Schalkwijk, C.G. Dietary intake of advanced glycation endproducts is associated with higher levels of advanced glycation endproducts in plasma and urine: The CODAM study. Clin. Nutr. 2018, 37, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Jaffé, M. Ueber den Niederschlag, welchen Pikrinsäure in normalem Harn erzeugt und über eine neue Reaction des Kreatinins. Z. Physiol. Chem. 1886, 10, 391–400. [Google Scholar]
- Allison, D.B.; Paultre, F.; Maggio, C.; Mezzitis, N.; Pi-Sunyer, F.X. The use of areas under curves in diabetes research. Diabetes Care 1995, 18, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate—A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Schumacher, D.; Morgenstern, J.; Oguchi, Y.; Volk, N.; Kopf, S.; Groener, J.B.; Nawroth, P.P.; Fleming, T.; Freichel, M. Compensatory mechanisms for methylglyoxal detoxification in experimental & clinical diabetes. Mol. Metab. 2018, 18, 143–152. [Google Scholar] [CrossRef]
- Jagt, D.L.V.; Hunsaker, L.A. Methylglyoxal metabolism and diabetic complications: Roles of aldose reductase, glyoxalase-I, betaine aldehyde dehydrogenase and 2-oxoaldehyde dehydrogenase. Chem. Interact. 2003, 143, 341–351. [Google Scholar] [CrossRef]
- Morgenstern, J.; Katz, S.; Krebs-Haupenthal, J.; Chen, J.; Saadatmand, A.; Cortizo, F.G.; Moraru, A.; Zemva, J.; Campos, M.C.; Teleman, A.A.; et al. Phosphorylation of T107 by CamKIIδ Regulates the Detoxification Efficiency and Proteomic Integrity of Glyoxalase 1. Cell Rep. 2020, 32, 108160. [Google Scholar] [CrossRef]
- Morgenstern, J.; Fleming, T.; Schumacher, D.; Eckstein, V.; Freichel, M.; Herzig, S.; Nawroth, P. Loss of Glyoxalase 1 Induces Compensatory Mechanism to Achieve Dicarbonyl Detoxification in Mammalian Schwann Cells. J. Biol. Chem. 2017, 292, 3224–3238. [Google Scholar] [CrossRef]
- Peculis, R.; Konrade, I.; Skapare, E.; Fridmanis, D.; Nikitina-Zake, L.; Lejnieks, A.; Pirags, V.; Dambrova, M.; Klovins, J. Identification of glyoxalase 1 polymorphisms associated with enzyme activity. Gene 2013, 515, 140–143. [Google Scholar] [CrossRef]
- Gabriele, S.; Lombardi, F.; Sacco, R.; Napolioni, V.; Altieri, L.; Tirindelli, M.C.; Gregorj, C.; Bravaccio, C.; Rousseau, F.; Persico, A.M. The GLO1 C332 (Ala111) allele confers autism vulnerability: Family-based genetic association and functional correlates. J. Psychiatr. Res. 2014, 59, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Maksoud, R.S.; Elsayed, W.S.; Elsayed, R.S. The influence of glyoxalase 1 gene polymorphism on its expression at different stages of breast cancer in Egyptian women. Genes Cancer 2017, 8, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Sakhi, A.K.; Berg, J.P.; Berg, T.J. Glyoxalase 1 enzyme activity in erythrocytes and Ala111Glu polymorphism in type 1-diabetes patients. Scand. J. Clin. Lab. Investig. 2012, 73, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Barua, M.; Jenkins, E.C.; Chen, W.; Kuizon, S.; Pullarkat, R.K.; Junaid, M.A. Glyoxalase I polymorphism rs2736654 causing the Ala111Glu substitution modulates enzyme activity-implications for autism. Autism Res. 2011, 4, 262–270. [Google Scholar] [CrossRef]
- Wu, J.C.; Li, X.H.; Peng, Y.D.; Wang, J.B.; Tang, J.F.; Wang, Y.F. Association of two glyoxalase I gene polymorphisms with nephropathy and retinopathy in Type 2 diabetes. J. Endocrinol. Investig. 2011, 34, e343–e348. [Google Scholar]
- Groener, J.B.; Reismann, P.; Fleming, T.; Kalscheuer, H.; Lehnhoff, D.; Hamann, A.; Roser, P.; Bierhaus, A.; Nawroth, P.P.; Rudofsky, G. C332C Genotype of Glyoxalase 1 and its Association with Late Diabetic Complications. Exp. Clin. Endocrinol. Diabetes 2013, 121, 436–439. [Google Scholar] [CrossRef]
- Xin, Y.; Hertle, E.; Van Der Kallen, C.J.H.; Schalkwijk, C.G.; Stehouwer, C.D.A.; Van Greevenbroek, M.M.J. Associations of dicarbonyl stress with complement activation: The CODAM study. Diabetologia 2020, 63, 1032–1042. [Google Scholar] [CrossRef]
- Duan, Z.; Chen, G.; Chen, L.; Stolzenberg-Solomon, R.; Weinstein, S.J.; Mannisto, S.; White, D.L.; Albanes, D.; Jiao, L. Determinants of concentrations of N(ε)-carboxymethyl-lysine and soluble receptor for advanced glycation end products and their associations with risk of pancreatic cancer. Int. J. Mol. Epidemiol. Genet. 2014, 5, 152–163. [Google Scholar]
- Bollong, M.J.; Lee, G.; Coukos, J.S.; Yun, H.; Zambaldo, C.; Chang, J.W.; Chin, E.N.; Ahmad, I.; Chatterjee, A.K.; Lairson, L.L.; et al. A metabolite-derived protein modification integrates glycolysis with KEAP1–NRF2 signalling. Nat. Cell Biol. 2018, 562, 600–604. [Google Scholar] [CrossRef]

| Glo1 Expr. | Plasma MGO and D-Lactate | Plasma AGEs | Urinary AGEs and D-Lactate | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| mRNA | MGO | D-lactate | iAUC MGO | Free CEL | Free MG-H1 | PB CEL | Free CEL | Free MG-H1 | D-lactate | ||
| Glo1 expr. | mRNA | mRNA | 0.15 ** | 0.17 ** | 0.10 * | 0.08 | 0.08 | −0.003 | 0.12 * | 0.11 * | 0.21 ** |
| Plasma MGO and D-lactate | MGO | MGO | 0.07 | −0.23 ** | 0.16 ** | 0.10 * | 0.06 | 0.09 * | −0.01 | 0.08 | |
| D-lactate | D-lactate | 0.15 ** | 0.17 ** | 0.09 * | 0.33 ** | 0.21 ** | 0.12 ** | 0.33 ** | |||
| iAUC MGO | iAUC MGO | 0.05 | −0.02 | −0.04 | 0.12* | 0.02 | 0.10 * | ||||
| Plasma AGEs | Free CEL | Free CEL | 0.69 ** | 0.10 * | 0.67 ** | 0.45 ** | 0.05 | ||||
| Free MG-H1 | Free MG-H1 | 0.07 | 0.45** | 0.75 ** | 0.01 | ||||||
| PB CEL | PB CEL | 0.04 | 0.02 | 0.05 | |||||||
| Urinary AGEs and D-lactate | Free CEL | Free CEL | 0.58 ** | 0.32 ** | |||||||
| Free MG-H1 | Free MG-H1 | 0.20 ** | |||||||||
| D-lactate | D-lactate | ||||||||||
| Glo1 Expression | Plasma | Urine | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GLO1 mRNA 2 | MGO | D-Lactate | MGO iAUC | D-Lactate | |||||||
| N | β | p | β | p | β | p | β | p | β | p | |
| SNP4 | |||||||||||
| AA | 423 | - | - | - | - | - | - | - | - | - | - |
| AT | 102 | −0.29 | 0.02 | 0.07 | 0.54 | 0.05 | 0.62 | 0.08 | 0.52 | 0.11 | 0.27 |
| TT | 7 | −0.39 | 0.30 | 0.64 | 0.08 | −0.08 | 0.83 | −0.37 | 0.38 | −0.04 | 0.91 |
| SNP13 | |||||||||||
| GG | 148 | - | - | - | - | - | - | - | - | - | - |
| AG | 249 | 0.17 | 0.14 | 0.02 | 0.85 | 0.29 | 0.004 | −0.01 | 0.91 | 0.02 | 0.85 |
| AA | 126 | 0.36 | 0.005 | 0.05 | 0.65 | 0.08 | 0.50 | 0.07 | 0.59 | −0.03 | 0.76 |
| SNP16 | |||||||||||
| GG | 397 | - | - | - | - | - | - | - | - | - | - |
| AG | 127 | 0.15 | 0.17 | −0.01 | 0.89 | 0.12 | 0.23 | 0.09 | 0.41 | 0.04 | 0.64 |
| AA | 11 | 0.21 | 0.46 | −0.11 | 0.71 | −0.17 | 0.57 | 0.04 | 0.91 | 0.06 | 0.85 |
| SNP18 | |||||||||||
| TT | 161 | - | - | - | - | - | - | - | - | - | - |
| GT | 277 | −0.07 | 0.49 | −0.20 | 0.04 | −0.03 | 0.78 | −0.03 | 0.77 | 0.03 | 0.76 |
| GG | 98 | −0.11 | 0.44 | −0.16 | 0.20 | −0.10 | 0.40 | −0.02 | 0.88 | −0.005 | 0.96 |
| SNP28 | |||||||||||
| GG | 458 | - | - | - | - | - | - | - | - | - | - |
| AG | 76 | 0.07 | 0.61 | 0.13 | 0.26 | 0.16 | 0.17 | −0.03 | 0.81 | −0.06 | 0.62 |
| AA | 4 | 0.16 | 0.75 | 0.26 | 0.59 | 0.34 | 0.49 | −0.10 | 0.84 | −0.69 | 0.12 |
| SNP38 | |||||||||||
| GG | 428 | - | - | - | - | - | - | - | - | - | - |
| CG | 96 | 0.05 | 0.71 | 0.11 | 0.31 | 0.16 | 0.67 | 0.01 | 0.94 | −0.02 | 0.86 |
| CC | 4 | 0.16 | 0.75 | 0.28 | 0.55 | 0.17 | 0.13 | −0.09 | 0.86 | −0.68 | 0.13 |
| SNP40 | |||||||||||
| CC | 273 | - | - | - | - | - | - | - | - | - | - |
| CT | 226 | −0.09 | 0.38 | −0.07 | 0.40 | −0.004 | 0.97 | −0.04 | 0.70 | −0.14 | 0.09 |
| TT | 35 | −0.03 | 0.88 | 0.12 | 0.48 | −0.08 | 0.65 | −0.17 | 0.36 | −0.28 | 0.09 |
| SNP45 | |||||||||||
| GG | 199 | - | - | - | - | - | - | - | - | - | - |
| AG | 252 | −0.16 | 0.11 | 0.01 | 0.91 | 0.001 | 0.10 | 0.000 | 0.99 | −0.09 | 0.32 |
| AA | 77 | −0.27 | 0.06 | 0.08 | 0.53 | 0.000 | 0.99 | −0.12 | 0.39 | −0.08 | 0.50 |
| SNP49 | |||||||||||
| AA | 135 | - | - | - | - | - | - | - | - | - | - |
| AG | 284 | 0.11 | 0.31 | 0.04 | 0.67 | 0.04 | 0.73 | 0.08 | 0.46 | 0.07 | 0.46 |
| GG | 117 | 0.26 | 0.06 | −0.07 | 0.57 | −0.10 | 0.41 | 0.08 | 0.58 | 0.20 | 0.08 |
| Plasma | Urine | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Free CEL | Free MG-H1 | PB CEL | Free CEL | Free MG-H1 | |||||||
| N | β | p | β | p | β | p | β | p | β | p | |
| SNP4 | |||||||||||
| AA | 423 | - | - | - | - | - | - | ||||
| AT | 102 | 0.16 | 0.14 | −0.07 | 0.50 | 0.17 | 0.13 | 0.15 | 0.19 | −0.06 | 0.60 |
| TT | 7 | 0.16 | 0.67 | −0.10 | 0.80 | −0.35 | 0.37 | 0.13 | 0.73 | −0.06 | 0.87 |
| SNP13 | |||||||||||
| GG | 148 | - | - | - | - | - | - | - | - | - | - |
| AG | 249 | 0.05 | 0.62 | 0.003 | 0.97 | −0.008 | 0.94 | −0.004 | 0.97 | −0.006 | 0.96 |
| AA | 126 | 0.10 | 0.39 | 0.07 | 0.53 | 0.003 | 0.98 | −0.09 | 0.49 | −0.10 | 0.40 |
| SNP16 | |||||||||||
| GG | 397 | - | - | - | - | - | - | - | - | - | - |
| AG | 127 | 0.11 | 0.27 | 0.12 | 0.24 | 0.01 | 0.90 | 0.06 | 0.57 | 0.03 | 0.77 |
| AA | 11 | 0.10 | 0.73 | 0.20 | 0.50 | 0.09 | 0.78 | −0.47 | 0.16 | −0.12 | 0.71 |
| SNP18 | |||||||||||
| TT | 161 | - | - | - | - | - | - | - | - | - | - |
| GT | 277 | −0.12 | 0.22 | −0.02 | 0.81 | −0.11 | 0.27 | 0.02 | 0.82 | 0.03 | 0.74 |
| GG | 98 | −0.12 | 0.33 | −0.02 | 0.87 | 0.008 | 0.95 | 0.08 | 0.57 | 0.10 | 0.44 |
| SNP28 | |||||||||||
| GG | 458 | - | - | - | - | - | - | - | - | - | - |
| AG | 76 | 0.12 | 0.32 | 0.14 | 0.26 | 0.04 | 0.76 | −0.01 | 0.97 | 0.03 | 0.81 |
| AA | 4 | 0.32 | 0.51 | 0.22 | 0.66 | 0.11 | 0.82 | −0.40 | 0.41 | −0.20 | 0.68 |
| SNP38 | |||||||||||
| GG | 428 | - | - | - | - | - | - | - | - | - | - |
| CG | 96 | 0.17 | 0.13 | 0.12 | 0.26 | 0.03 | 0.81 | 0.05 | 0.67 | 0.05 | 0.69 |
| CC | 4 | 0.32 | 0.49 | 0.22 | 0.65 | 0.12 | 0.81 | −0.38 | 0.43 | −0.20 | 0.69 |
| SNP40 | |||||||||||
| CC | 273 | - | - | - | - | - | - | - | - | - | - |
| CT | 226 | −0.06 | 0.50 | −0.03 | 0.77 | −0.06 | 0.48 | 0.02 | 0.83 | −0.03 | 0.76 |
| TT | 35 | 0.05 | 0.79 | 0.14 | 0.43 | −0.03 | 0.88 | 0.007 | 0.97 | 0.10 | 0.57 |
| SNP45 | |||||||||||
| GG | 199 | - | - | - | - | - | - | - | - | - | - |
| AG | 252 | −0.09 | 0.36 | −0.10 | 0.29 | 0.01 | 0.88 | −0.01 | 0.88 | −0.10 | 0.30 |
| AA | 77 | 0.17 | 0.21 | 0.003 | 0.98 | 0.11 | 0.42 | 0.13 | 0.33 | −0.05 | 0.71 |
| SNP49 | |||||||||||
| AA | 135 | - | - | - | - | - | - | - | - | - | - |
| AG | 284 | −0.24 | 0.02 | −0.11 | 0.26 | −0.03 | 0.78 | −0.12 | 0.27 | −0.03 | 0.80 |
| GG | 117 | −0.15 | 0.23 | −0.05 | 0.71 | −0.05 | 0.69 | −0.08 | 0.55 | 0.04 | 0.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maasen, K.; Hanssen, N.M.J.; van der Kallen, C.J.H.; Stehouwer, C.D.A.; van Greevenbroek, M.M.J.; Schalkwijk, C.G. Polymorphisms in Glyoxalase I Gene Are Not Associated with Glyoxalase I Expression in Whole Blood or Markers of Methylglyoxal Stress: The CODAM Study. Antioxidants 2021, 10, 219. https://doi.org/10.3390/antiox10020219
Maasen K, Hanssen NMJ, van der Kallen CJH, Stehouwer CDA, van Greevenbroek MMJ, Schalkwijk CG. Polymorphisms in Glyoxalase I Gene Are Not Associated with Glyoxalase I Expression in Whole Blood or Markers of Methylglyoxal Stress: The CODAM Study. Antioxidants. 2021; 10(2):219. https://doi.org/10.3390/antiox10020219
Chicago/Turabian StyleMaasen, Kim, Nordin M. J. Hanssen, Carla J. H. van der Kallen, Coen D. A. Stehouwer, Marleen M. J. van Greevenbroek, and Casper G. Schalkwijk. 2021. "Polymorphisms in Glyoxalase I Gene Are Not Associated with Glyoxalase I Expression in Whole Blood or Markers of Methylglyoxal Stress: The CODAM Study" Antioxidants 10, no. 2: 219. https://doi.org/10.3390/antiox10020219
APA StyleMaasen, K., Hanssen, N. M. J., van der Kallen, C. J. H., Stehouwer, C. D. A., van Greevenbroek, M. M. J., & Schalkwijk, C. G. (2021). Polymorphisms in Glyoxalase I Gene Are Not Associated with Glyoxalase I Expression in Whole Blood or Markers of Methylglyoxal Stress: The CODAM Study. Antioxidants, 10(2), 219. https://doi.org/10.3390/antiox10020219





