Mentha arvensis Essential Oil Exerts Anti-Inflammatory in LPS-Stimulated Inflammatory Responses via Inhibition of ERK/NF-κB Signaling Pathway and Anti-Atopic Dermatitis-like Effects in 2,4-Dinitrochlorobezene-Induced BALB/c Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Materials
2.3. Isolation of the Mentha Arvensis Essential Oil
2.4. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
2.5. Cell Culture
2.6. Cell Viability Analysis
2.7. Measurement of Nitric Oxide
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. RNA Isolation and Real-Time Polymerase Chain Reaction (RT-PCR)
2.10. Western Blot Analysis
2.11. DNCB-Induced AD Mice
2.12. Histological Observation
2.13. Statistical Analysis
3. Results
3.1. GC-MS Analysis of MAEO
3.2. The Effects of MAEO on LPS-Induced Inflammatory Mediators and Proinflammatory Cytokines in RAW 264.7 Macrophages
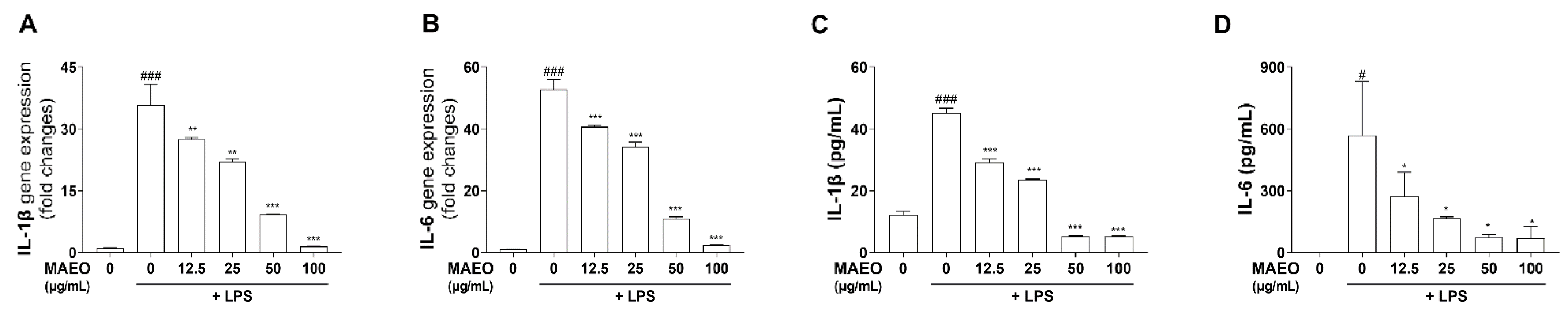
3.3. The Effects of MAEO on LPS-Induced Inflammatory Cytokines in RAW 264.7 Macrophages
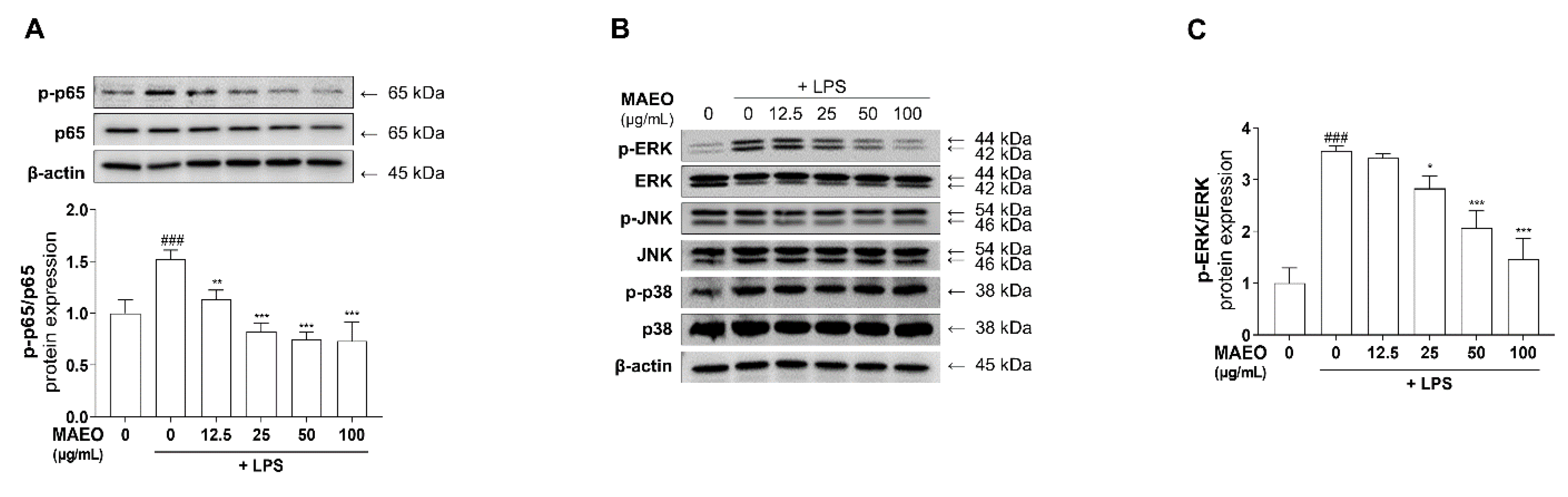
3.4. The Effects of MAEO on LPS-Induced ERK/NF-κB Activation in RAW 264.7 Macrophages
3.5. The Effects of MAEO on LPS-Induced Inflammatory Responses in HaCaT Human Keratinocytes
3.6. The Effects of MAEO on the DNCB-Induced AD Animal Model
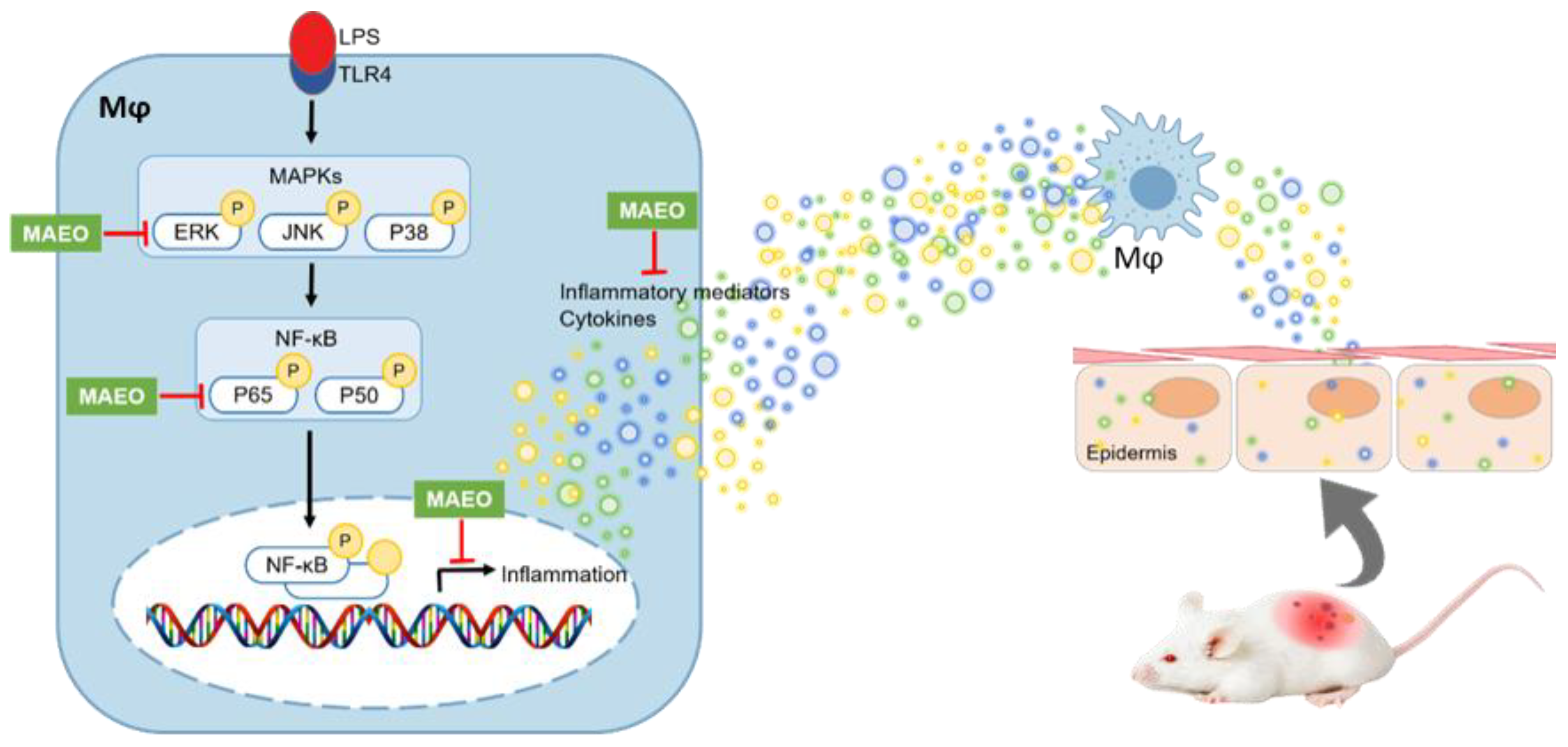
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bieber, T. Interleukin-13: Targeting an underestimated cytokine in atopic dermatitis. Allergy 2020, 75, 54–62. [Google Scholar] [CrossRef] [Green Version]
- Ha, J.; Lee, S.W.; Yon, D.K. Ten-Year trends and prevalence of asthma, allergic rhinitis, and atopic dermatitis among the Korean population, 2008-2017. Clin. Exp. Pediatr. 2020, 63, 278–283. [Google Scholar] [CrossRef]
- Song, S.; Lee, K.; Lee, Y.M.; Lee, J.H.; Lee, S.I.; Yu, S.D.; Paek, D. Acute health effects of urban fine and ultrafine particles on children with atopic dermatitis. Env. Res. 2011, 111, 394–399. [Google Scholar] [CrossRef]
- Oh, I.; Lee, J.; Ahn, K.; Kim, J.; Kim, Y.-M.; Sim, C.S.; Kim, Y. Association between particulate matter concentration and symptoms of atopic dermatitis in children living in an industrial urban area of South Korea. Environ. Res. 2018, 160, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Park, N.J.; Jegal, J.; Jo, B.G.; Choi, S.H.; Lee, S.W.; Uddin, M.S.; Kim, S.N.; Yang, M.H. Suppression of DNCB-Induced Atopic Skin Lesions in Mice by Wikstroemia indica Extract. Nutrients 2020, 12, 173. [Google Scholar] [CrossRef] [Green Version]
- Homey, B.; Steinhoff, M.; Ruzicka, T.; Leung, D.Y. Cytokines and chemokines orchestrate atopic skin inflammation. J. Allergy Clin. Immunol 2006, 118, 178–189. [Google Scholar] [CrossRef]
- Park, S.O.; Park, B.S.; Ryu, C.M.; Ahn, Y.S. Effect of Herb Extracts Mixed with Houttuynia Cordata on Antiatopic Dermatitis in DNCB-Induced BALB/c Mouse. J. Korean Oil Chem. Soc. 2012, 29, 175–183. [Google Scholar]
- Coondoo, A.; Phiske, M.; Verma, S.; Lahiri, K. Side-effects of topical steroids: A long overdue revisit. Indian Derm. Online J. 2014, 5, 416–425. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target Ther. 2017, 2, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Han, J.H.; Ju, J.H.; Lee, Y.S.; Park, J.H.; Yeo, I.J.; Park, M.H.; Roh, Y.S.; Han, S.B.; Hong, J.T. Astaxanthin alleviated ethanol-induced liver injury by inhibition of oxidative stress and inflammatory responses via blocking of STAT3 activity. Sci. Rep. 2018, 8, 14090. [Google Scholar] [CrossRef] [PubMed]
- Won, J.H.; Kim, J.Y.; Yun, K.J.; Lee, J.H.; Back, N.I.; Chung, H.G.; Chung, S.A.; Jeong, T.S.; Choi, M.S.; Lee, K.T. Gigantol isolated from the whole plants of Cymbidium goeringii inhibits the LPS-induced iNOS and COX-2 expression via NF-kappaB inactivation in RAW 264.7 macrophages cells. Planta Med. 2006, 72, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Zamora, R.; Vodovotz, Y.; Billiar, T.R. Inducible nitric oxide synthase and inflammatory diseases. Mol. Med. 2000, 6, 347–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arter. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Aflatuni, A.; Uusitalo, J.; Ek, S.; Hohtola, A. Variation in the Amount of Yield and in the Extract Composition Between Conventionally Produced and Micropropagated Peppermint and Spearmint. J. Essent. Oil Res. 2005, 17, 66–70. [Google Scholar] [CrossRef]
- Zhao, B.T.; Kim, T.I.; Kim, Y.H.; Kang, J.S.; Min, B.S.; Son, J.K.; Woo, M.H. A comparative study of Mentha arvensis L. and Mentha haplocalyx Briq. by HPLC. Nat. Prod. Res. 2018, 32, 239–242. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.J.; Jang, G.Y.; Seo, K.H.; Kim, M.R.; Choi, Y.H.; Jung, J.W. Mentha arvensis attenuates cognitive and memory impairment in scopolamine-treated mice. Kor. J. Pharmacogn. 2020, 51, 70–77. [Google Scholar]
- Lubbe, A.; Verpoorte, R. Cultivation of medicinal and aromatic plants for specialty industrial materials. Ind. Crop. Prod. 2011, 34, 785–801. [Google Scholar] [CrossRef]
- Souza, M.A.A.; Lemos, M.J.; Brito, D.M.C.; Fernandes, M.S.; Castro, R.N.; Souza, S.R. Production and Quality of Menthol Mint Essential Oil and Antifungal and Antigerminative Activity. Am. J. Plant Sci. 2014, 5, 3311–3318. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Chhoeun, T.B.; Kim, T.; Sowndhararajan, K.; Kim, S. The gender variation on the electroencephalographic activity in response to the exposure of black pepper essential oil from Kampong Cham, Cambodia. Flavour. Fragr. J. 2020, 35, 284–293. [Google Scholar] [CrossRef]
- Yang, Y.I.; Woo, J.H.; Seo, Y.J.; Lee, K.T.; Lim, Y.; Choi, J.H. Protective Effect of Brown Alga Phlorotannins against Hyper-inflammatory Responses in Lipopolysaccharide-Induced Sepsis Models. J. Agric. Food Chem. 2016, 64, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.I.; Shin, H.C.; Kim, S.H.; Park, W.Y.; Lee, K.T.; Choi, J.H. 6,6’-Bieckol, isolated from marine alga Ecklonia cava, suppressed LPS-induced nitric oxide and PGE(2) production and inflammatory cytokine expression in macrophages: The inhibition of NFkappaB. Int. Immunopharmacol. 2012, 12, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, A.; Gaire, B.P.; Kang, M.G.; Choi, J.W. S1P2 contributes to microglial activation and M1 polarization following cerebral ischemia through ERK1/2 and JNK. Sci. Rep. 2019, 9, 12106. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.S.; Park, S.J.; Ryu, S.; Kang, H.B.; Kim, T.W.; Choi, J.H.; Lee, J.Y.; Cho, Y.W.; Lee, K.T. Potent anti-inflammatory effect of a novel furan-2,5-dione derivative, BPD, mediated by dual suppression of COX-2 activity and LPS-induced inflammatory gene expression via NF-kappaB inactivation. Br. J. Pharmacol. 2012, 165, 1926–1940. [Google Scholar] [CrossRef] [Green Version]
- Kielkopf, C.L.; Bauer, W.; Urbatsch, I.L. Bradford Assay for Determining Protein Concentration. Cold Spring Harb. Protoc. 2020, 2020, 102269. [Google Scholar] [CrossRef] [PubMed]
- Oranje, A.P.; Glazenburg, E.J.; Wolkerstorfer, A.; de Waard-van der Spek, F.B. Practical issues on interpretation of scoring atopic dermatitis: The SCORAD index, objective SCORAD and the three-item severity score. Br. J. Derm. 2007, 157, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Jeong, E.S.; Heo, S.H.; Seo, J.H.; Jeong, D.G.; Choi, Y.K. A Novel Model for Human Atopic Dermatitis: Application of Repeated DNCB Patch in BALB/c Mice, in Comparison with NC/Nga Mice. Korean Assoc. Lab. Anim. Sci. 2010, 26, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Jung, E.M.; Ahn, C.; Lee, G.S.; Lee, S.Y.; Kim, S.H.; Choi, I.G.; Park, M.J.; Lee, S.S.; Choi, D.H.; et al. Elemol from Chamaecyparis obtusa ameliorates 2,4-dinitrochlorobenzene-induced atopic dermatitis. Int. J. Mol. Med. 2015, 36, 463–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, J.Y.; Lee, J.H.; Lee, D.H.; Lee, J.H.; Kim, D.K. Umbelliferone reduces the expression of inflammatory chemokines in HaCaT cells and DNCB/DFE-induced atopic dermatitis symptoms in mice. Int. Immunopharmacol. 2019, 75, 105830. [Google Scholar] [CrossRef]
- Kasraie, S.; Werfel, T. Role of macrophages in the pathogenesis of atopic dermatitis. Mediat. Inflamm. 2013, 2013, 942375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richmond, J.M.; Harris, J.E. Immunology and skin in health and disease. Cold Spring Harb. Perspect. Med. 2014, 4, a015339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, W.; Ma, L.; Cai, C.; Gong, X. Caffeine Inhibits NLRP3 Inflammasome Activation by Suppressing MAPK/NF-kappaB and A2aR Signaling in LPS-Induced THP-1 Macrophages. Int. J. Biol. Sci. 2019, 15, 1571–1581. [Google Scholar] [CrossRef]
- Shi, Q.; Cao, J.; Fang, L.; Zhao, H.; Liu, Z.; Ran, J.; Zheng, X.; Li, X.; Zhou, Y.; Ge, D.; et al. Geniposide suppresses LPS-induced nitric oxide, PGE2 and inflammatory cytokine by downregulating NF-kappaB, MAPK and AP-1 signaling pathways in macrophages. Int. Immunopharmacol. 2014, 20, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Daham, K.; Song, W.L.; Lawson, J.A.; Kupczyk, M.; Gulich, A.; Dahlen, S.E.; FitzGerald, G.A.; Dahlen, B. Effects of celecoxib on major prostaglandins in asthma. Clin. Exp. Allergy 2011, 41, 36–45. [Google Scholar] [CrossRef] [Green Version]
- Wojdasiewicz, P.; Poniatowski, L.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesth. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Beyer, A.; Aebersold, R. On the Dependency of Cellular Protein Levels on mRNA Abundance. Cell 2016, 165, 535–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gourru-Lesimple, G.; Mathieu, C.; Thevenet, T.; Guillaume-Vasselin, V.; Jegou, J.F.; Boer, C.G.; Tomczak, K.; Bloyet, L.M.; Giraud, C.; Grande, S.; et al. Measles virus infection of human keratinocytes: Possible link between measles and atopic dermatitis. J. Derm. Sci. 2017, 86, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Lee, S. Immunomodulatory Effects of Eurya emarginata on NC/Nga Mice as Models for Atopic Dermatitis. J. Life Sci. 2014, 24, 447–453. [Google Scholar] [CrossRef]
- Nomura, T.; Kabashima, K. Advances in atopic dermatitis in 2015. J. Allergy Clin. Immunol. 2016, 138, 1548–1555. [Google Scholar] [CrossRef] [Green Version]
- Jekal, S.-J.; Park, M.-S.; Kim, D.-J. The Combined Effects of Curcumin Administration and 630 nm LED Phototherapy against DNCB-induced Atopic Dermatitis-like Skin Lesions in BALB/c Mice. Korean J. Clin. Lab. Sci. 2017, 49, 150–160. [Google Scholar] [CrossRef]
- Wu, Z.; Uchi, H.; Morino-Koga, S.; Shi, W.; Furue, M. Resveratrol inhibition of human keratinocyte proliferation via SIRT1/ARNT/ERK dependent downregulation of aquaporin 3. J. Derm. Sci. 2014, 75, 16–23. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Liu, J.; Kong, D.; Han, X.; Lei, P.; Xu, M.; Guan, H.; Hou, D. Ameliorative effects of sea buckthorn oil on DNCB induced atopic dermatitis model mice via regulation the balance of Th1/Th2. BMC Complement. Med. Ther. 2020, 20, 263. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, H.D.; Costa, J.G.; Lima, E.O.; Falcao-Silva, V.S.; Siqueira-Junior, J.P. Enhancement of the antibiotic activity against a multiresistant Escherichia coli by Mentha arvensis L. and chlorpromazine. Chemotherapy 2008, 54, 328–330. [Google Scholar] [CrossRef]
- Kurilov, D.V.; Kirichenko, E.B.; Bidyukova, G.F.; Olekhnovich, L.S.; Ku, L.D. Composition of the essential oil of introduced mint forms of Mentha piperita and Mentha arvensis species. Dokl. Biol. Sci. 2009, 429, 538–540. [Google Scholar] [CrossRef]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Nur, H.; Ismail, A.F.; Sharif, S.; RamaKrishna, S.; Berto, F. Antioxidant, Antimicrobial and Antiviral Properties of Herbal Materials. Antioxidants 2020, 9, 1309. [Google Scholar] [CrossRef]
- Stringaro, A.; Colone, M.; Angiolella, L. Antioxidant, Antifungal, Antibiofilm, and Cytotoxic Activities of Mentha spp. Essential Oils. Medicines 2018, 5, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, J.; Li, H.; Deng, X.; Ma, Z.; Fu, Q.; Ma, S. L-Menthone confers antidepressant-like effects in an unpredictable chronic mild stress mouse model via NLRP3 inflammasome-mediated inflammatory cytokines and central neurotransmitters. Pharm. Biochem. Behav. 2015, 134, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wang, H.; Wang, J.; Zhou, L.; Yang, P. Chemical Composition and Anti-Inflammatory, Cytotoxic and Antioxidant Activities of Essential Oil from Leaves of Mentha piperita Grown in China. PLoS ONE 2014, 9, e114767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brahmi, F.; Nury, T.; Debbabi, M.; Hadj-Ahmed, S.; Zarrouk, A.; Prost, M.; Madani, K.; Boulekbache-Makhlouf, L.; Lizard, G. Evaluation of Antioxidant, Anti-Inflammatory and Cytoprotective Properties of Ethanolic Mint Extracts from Algeria on 7-Ketocholesterol-Treated Murine RAW 264.7 Macrophages. Antioxidants 2018, 7, 184. [Google Scholar] [CrossRef] [Green Version]
- Guha, M.; Mackman, N. LPS induction of gene expression in human monocytes. Cell Signal. 2001, 13, 84–94. [Google Scholar] [CrossRef]
- Gkouveris, I.; Nikitakis, N.; Karanikou, M.; Rassidakis, G.; Sklavounou, A. Erk1/2 activation and modulation of STAT3 signaling in oral cancer. Oncol. Rep. 2014, 32, 2175–2182. [Google Scholar] [CrossRef] [Green Version]
- Spencer, S.; Kostel Bal, S.; Egner, W.; Lango Allen, H.; Raza, S.I.; Ma, C.A.; Gurel, M.; Zhang, Y.; Sun, G.; Sabroe, R.A.; et al. Loss of the interleukin-6 receptor causes immunodeficiency, atopy, and abnormal inflammatory responses. J. Exp. Med. 2019, 216, 1986–1998. [Google Scholar] [CrossRef]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.W.; Sun, Y.M. The IL-6/JAK/STAT3 pathway: Potential therapeutic strategies in treating colorectal cancer (Review). Int. J. Oncol. 2014, 44, 1032–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.E.; Kim, J.S.; Cho, D.H.; Park, H.J. Molecular Mechanisms of Cutaneous Inflammatory Disorder: Atopic Dermatitis. Int. J. Mol. Sci. 2016, 17, 1234. [Google Scholar] [CrossRef] [Green Version]
- Niebuhr, M.; Baumert, K.; Heratizadeh, A.; Satzger, I.; Werfel, T. Impaired NLRP3 inflammasome expression and function in atopic dermatitis due to Th2 milieu. Allergy 2014, 69, 1058–1067. [Google Scholar] [CrossRef]






| Target Gene | Primer Sequence | Accession Number | |
|---|---|---|---|
| iNOS | F | 5′-CAT GCT ACT GGA GGT GGG TG-3′ | NM_010927 |
| R | 5′-CAT TGA TCT CCG TGA CAG CC-3′ | ||
| COX-2 | F | 5′-TGC TGT ACA AGC AGT GGC AA-3′ | NM_011198 |
| R | 5′-GCA GCC ATT TCC TTC TCT CC-3′ | ||
| IL-6 | F | 5′-GAG GAT ACC ACT CCC AAC AGA CC-3′ | NM_031168 |
| R | 5′-AAG TGC ATC ATC GTT GTT CAT ACA-3′ | ||
| IL-1β | F | 5′-ACC TGC TGG TGT GTG ACG TT-3′ | NM_008361 |
| R | 5′-TCG TTG CTT GGT TCT CCT TG-3′ | ||
| β-Actin | F | 5′-ATC ACT ATT GGC AAC GAG CG-3′ | NM_007393 |
| R | 5′-TCA GCA ATG CCT GGG TAC AT-3′ | ||
| Compound Name | Retention Time | Area (%) | |
|---|---|---|---|
| 1 | Toluene | 3.285 | 0.04 |
| 2 | α-Pinene | 8.287 | 0.43 |
| 3 | β-Phellandrene | 9.733 | 0.28 |
| 4 | β-Pinene | 9.903 | 1.07 |
| 5 | 3-Octanol | 10.666 | 0.33 |
| 6 | Limonene | 11.748 | 3.06 |
| 7 | Eucalyptol (Cineole) | 11.846 | 0.06 |
| 8 | β-Ocimene | 11.985 | 0.32 |
| 9 | α-Pinene | 12.336 | 0.23 |
| 10 | Linalool oxide | 13.163 | 0.04 |
| 11 | Terpinolene | 13.634 | 0.04 |
| 12 | Linalool | 14.162 | 0.26 |
| 13 | Menthone | 16.091 | 25.71 |
| 14 | Menthol | 16.422 | 2.4 |
| 15 | isopulegone | 16.580 | 0.29 |
| 16 | Menthol | 16.873 | 36.27 |
| 17 | 2,3-Dimethyl-2-cyclopenten-1-one | 18.189 | 0.12 |
| 18 | Hexen-3-yl Valerate | 18.292 | 0.21 |
| 19 | Pulegone | 18.452 | 0.21 |
| 20 | Piperitone oxide | 18.827 | 0.44 |
| 21 | Piperitone | 19.009 | 9.29 |
| 22 | Menthol | 19.356 | 0.38 |
| 23 | Menthyl acetate racemic | 19.917 | 8.3 |
| 24 | Diosphenol | 20.141 | 0.1 |
| 25 | D-Verbenone | 21.258 | 0.12 |
| 26 | β-Bourbonene | 22.398 | 0.15 |
| 27 | β-Elemene | 22.529 | 0.05 |
| 28 | Jasmone | 22.670 | 0.04 |
| 29 | p-Menthane-1,2,3-triol | 23.064 | 1.94 |
| 30 | Caryophyllene | 23.350 | 0.88 |
| 31 | p-Menthane-1,2,3-triol | 23.516 | 0.12 |
| 32 | 1-Acetoxy-p-menth-3-one | 23.601 | 0.45 |
| 33 | 3,5,5-Trimethylbicyclo[4.1.0]heptan-2-one | 24.068 | 3.78 |
| 34 | α-Caryophyllene | 24.261 | 0.12 |
| 35 | Bicyclosesquiphellandrene | 24.420 | 0.31 |
| 36 | β-Cubebene | 24.902 | 0.84 |
| 37 | Rose butanoate | 24.990 | 0.03 |
| 38 | Elixene | 25.258 | 0.05 |
| 39 | Butylated Hydroxytoluene (BHT) | 25.379 | 0.08 |
| 40 | γ-Cadinene | 25.675 | 0.05 |
| 41 | β-Cadinene | 25.779 | 0.07 |
| 42 | Calamenene | 25.854 | 0.09 |
| 43 | α-Murolene | 26.236 | 0.05 |
| 44 | 1,7-Dimethyl-4-propan-2-ylcyclodeca-2,7-dien-1-ol | 27.204 | 0.07 |
| 45 | Caryophyllene oxide | 27.355 | 0.03 |
| 46 | Cubenol | 28.110 | 0.11 |
| 47 | Benzeneacetic acid | 28.436 | 0.04 |
| 48 | α-Cadinol | 29.025 | 0.08 |
| 49 | Phytone | 32.856 | 0.04 |
| Total | 99.47 | ||
| Yield (v/w, %) | 1.06 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-Y.; Han, S.-D.; Kim, M.; Mony, T.J.; Lee, E.-S.; Kim, K.-M.; Choi, S.-H.; Hong, S.H.; Choi, J.W.; Park, S.J. Mentha arvensis Essential Oil Exerts Anti-Inflammatory in LPS-Stimulated Inflammatory Responses via Inhibition of ERK/NF-κB Signaling Pathway and Anti-Atopic Dermatitis-like Effects in 2,4-Dinitrochlorobezene-Induced BALB/c Mice. Antioxidants 2021, 10, 1941. https://doi.org/10.3390/antiox10121941
Kim S-Y, Han S-D, Kim M, Mony TJ, Lee E-S, Kim K-M, Choi S-H, Hong SH, Choi JW, Park SJ. Mentha arvensis Essential Oil Exerts Anti-Inflammatory in LPS-Stimulated Inflammatory Responses via Inhibition of ERK/NF-κB Signaling Pathway and Anti-Atopic Dermatitis-like Effects in 2,4-Dinitrochlorobezene-Induced BALB/c Mice. Antioxidants. 2021; 10(12):1941. https://doi.org/10.3390/antiox10121941
Chicago/Turabian StyleKim, So-Yeon, Sang-Deok Han, Minju Kim, Tamanna Jahan Mony, Eun-Seok Lee, Kyeong-Min Kim, Seung-Hyuk Choi, Sun Hee Hong, Ji Woong Choi, and Se Jin Park. 2021. "Mentha arvensis Essential Oil Exerts Anti-Inflammatory in LPS-Stimulated Inflammatory Responses via Inhibition of ERK/NF-κB Signaling Pathway and Anti-Atopic Dermatitis-like Effects in 2,4-Dinitrochlorobezene-Induced BALB/c Mice" Antioxidants 10, no. 12: 1941. https://doi.org/10.3390/antiox10121941
APA StyleKim, S.-Y., Han, S.-D., Kim, M., Mony, T. J., Lee, E.-S., Kim, K.-M., Choi, S.-H., Hong, S. H., Choi, J. W., & Park, S. J. (2021). Mentha arvensis Essential Oil Exerts Anti-Inflammatory in LPS-Stimulated Inflammatory Responses via Inhibition of ERK/NF-κB Signaling Pathway and Anti-Atopic Dermatitis-like Effects in 2,4-Dinitrochlorobezene-Induced BALB/c Mice. Antioxidants, 10(12), 1941. https://doi.org/10.3390/antiox10121941







