Peripheral BDNF Levels in Individuals at Ultra-High Risk for Psychosis: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
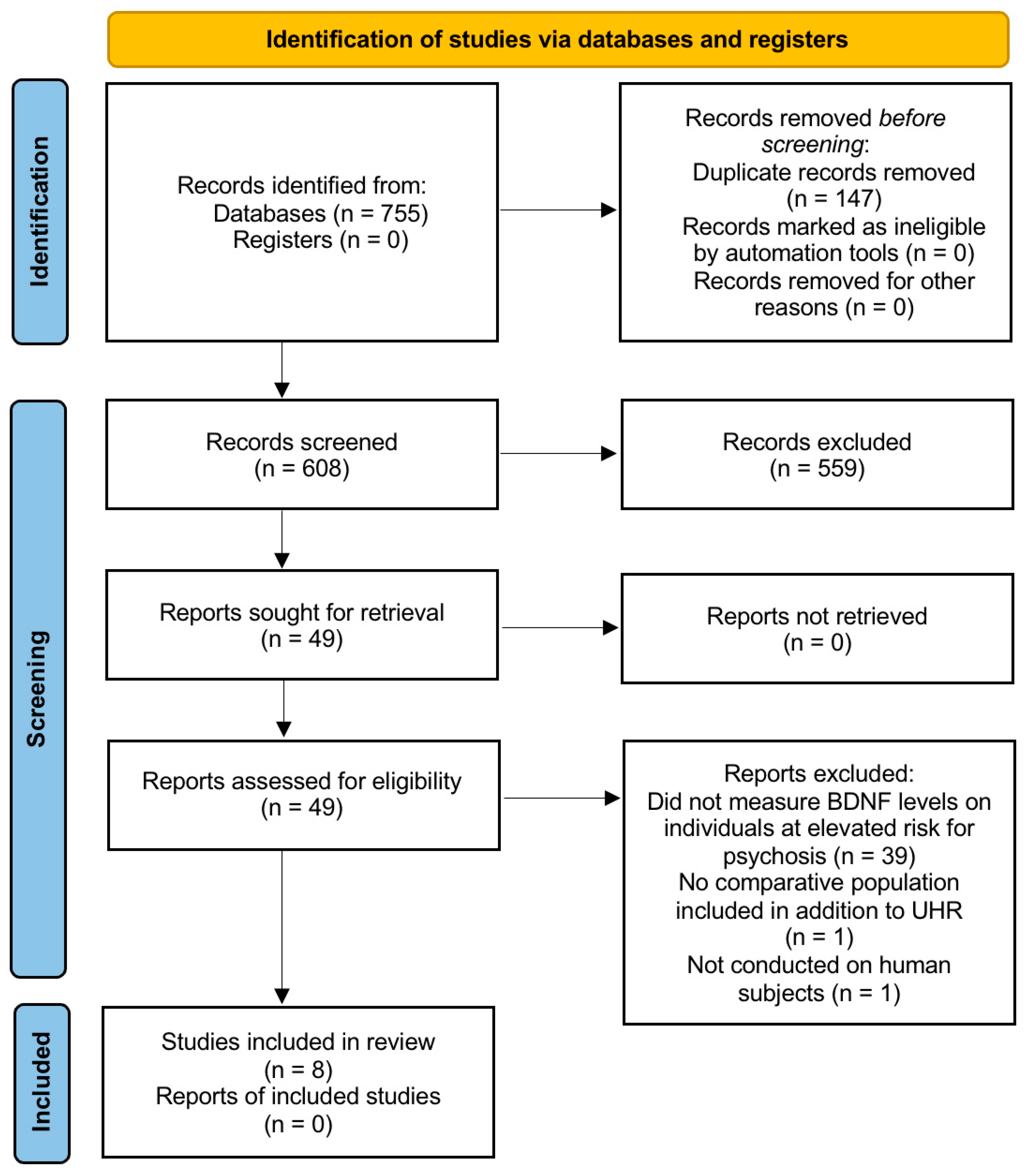
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias
3.4. Results of Individual Studies
3.5. Synthesis of Results
3.5.1. Comparison Between UHR and HC
3.5.2. Comparison Between UHR and FEP/CS
3.5.3. Longitudinal Associations Between BDNF Levels and Clinical Outcomes in UHR Individuals
4. Discussion
4.1. General Interpretation of Findings
4.2. Diagnostic Criteria and Clinical Subgroups
4.3. Genetic Factors and Variability in BDNF Expression
4.4. Pharmacological Influences on BDNF Expression
4.5. Lifestyle and Metabolic Influences on BDNF Expression
4.6. Potential Pathophysiological Mechanisms Underlying BDNF Variability in UHR
4.7. Limitations of the Evidence
4.8. Limitations of This Review
4.9. Implications for Practice and Future Research
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BDNF | Brain-Derived Neurotrophic Factor |
| UHR | Ultra-high risk |
| HC | Healthy controls |
| FEP | First-episode psychosis |
| CS | Chronic schizophrenia |
| ARMS | At-risk mental state |
| APS | Attenuated positive symptoms |
| BLIPS | Brief limited intermittent psychotic symptoms |
| BMI | Body mass index |
| JBI | Joanna Briggs Institute |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| CAARMS | Comprehensive Assessment of At-Risk Mental States |
| SIPS | Structured Interview for Prodromal Syndromes |
| LYRIKS | Longitudinal Youth-At-Risk Study |
References
- Charlson, F.J.; Ferrari, A.J.; Santomauro, D.F.; Diminic, S.; Stockings, E.; Scott, J.G.; McGrath, J.J.; Whiteford, H.A. Global Epidemiology and Burden of Schizophrenia: Findings From the Global Burden of Disease Study 2016. Schizophr. Bull. 2018, 44, 1195–1203. [Google Scholar] [CrossRef]
- GBD 2019 Mental Disorders Collaborators. Global, Regional, and National Burden of 12 Mental Disorders in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022, 9, 137–150. [Google Scholar] [CrossRef]
- Sponheim, S.R.; Jung, R.E.; Seidman, L.J.; Mesholam-Gately, R.I.; Manoach, D.S.; O’Leary, D.S.; Ho, B.C.; Andreasen, N.C.; Lauriello, J.; Schulz, S.C. Cognitive Deficits in Recent-Onset and Chronic Schizophrenia. J. Psychiatr. Res. 2010, 44, 421–428. [Google Scholar] [CrossRef]
- McCutcheon, R.A.; Keefe, R.S.E.; McGuire, P.K. Cognitive Impairment in Schizophrenia: Aetiology, Pathophysiology, and Treatment. Mol. Psychiatry 2023, 28, 1902–1918. [Google Scholar] [CrossRef] [PubMed]
- Heinrichs, R.W.; Zakzanis, K.K. Neurocognitive Deficit in Schizophrenia: A Quantitative Review of the Evidence. Neuropsychology 1998, 12, 426–445. [Google Scholar] [CrossRef]
- Tschentscher, N.; Woll, C.F.J.; Tafelmaier, J.C.; Kriesche, D.; Bucher, J.C.; Engel, R.R.; Karch, S. Neurocognitive Deficits in First-Episode and Chronic Psychotic Disorders: A Systematic Review from 2009 to 2022. Brain Sci. 2023, 13, 299. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, J.; Nili, M.; Xiang, P.; Nelson, J.K.; Pack, C.; Thompson, R.; Vasey, J.; Parks, J. Healthcare Resource Utilization Burden Associated with Cognitive Impairments Identified through Natural Language Processing among Patients with Schizophrenia in the United States. Schizophrenia 2025, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Yung, A.R.; McGorry, P.D.; McFarlane, C.A.; Jackson, H.J.; Patton, G.C.; Rakkar, A. Monitoring and Care of Young People at Incipient Risk of Psychosis. Schizophr. Bull. 1996, 22, 283–303. [Google Scholar] [CrossRef]
- McGorry, P.D.; Yung, A.R.; Phillips, L.J.; Yuen, H.P.; Francey, S.; Cosgrave, E.M.; Germano, D.; Bravin, J.; McDonald, T.; Blair, A.; et al. Randomized Controlled Trial of Interventions Designed to Reduce the Risk of Progression to First-Episode Psychosis in a Clinical Sample With Subthreshold Symptoms. Arch. Gen. Psychiatry 2002, 59, 921–928. [Google Scholar] [CrossRef]
- Yung, A.R.; Yung, A.R.; Pan Yuen, H.; Mcgorry, P.D.; Phillips, L.J.; Kelly, D.; Dell’olio, M.; Francey, S.M.; Cosgrave, E.M.; Killackey, E.; et al. Mapping the Onset of Psychosis: The Comprehensive Assessment of At-Risk Mental States. Aust. N. Z. J. Psychiatry 2005, 39, 964–971. [Google Scholar] [CrossRef]
- Fusar-Poli, P. Predicting Psychosis: Meta-Analysis of Transition Outcomes in Individuals at High Clinical Risk. Arch. Gen. Psychiatry 2012, 69, 220–229. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Borgwardt, S.; Bechdolf, A.; Addington, J.; Riecher-Rössler, A.; Schultze-Lutter, F.; Keshavan, M.; Wood, S.; Ruhrmann, S.; Seidman, L.J.; et al. The Psychosis High-Risk State: A Comprehensive State-of-the-Art Review. JAMA Psychiatry 2013, 70, 107–120. [Google Scholar] [CrossRef]
- Pinto, J.V.; Moulin, T.C.; Amaral, O.B. On the Transdiagnostic Nature of Peripheral Biomarkers in Major Psychiatric Disorders: A Systematic Review. Neurosci. Biobehav. Rev. 2017, 83, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Insel, T.R. Psychiatry as a Clinical Neuroscience Discipline. JAMA 2005, 294, 2221–2224. [Google Scholar] [CrossRef]
- Fuentes-Claramonte, P.; Estradé, A.; Solanes, A.; Ramella-Cravaro, V.; Garcia-Leon, M.A.; De Diego-Adeliño, J.; Molins, C.; Fung, E.; Valentí, M.; Anmella, G.; et al. Biomarkers for Psychosis: Are We There Yet? Umbrella Review of 1478 Biomarkers. Schizophr. Bull. Open 2024, 5, sgae018. [Google Scholar] [CrossRef]
- Park, H.; Poo, M. Neurotrophin Regulation of Neural Circuit Development and Function. Nat. Rev. Neurosci. 2013, 14, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell. Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [CrossRef]
- Howes, O.D.; Murray, R.M. Schizophrenia: An Integrated Sociodevelopmental-Cognitive Model. Lancet 2014, 383, 1677–1687. [Google Scholar] [CrossRef] [PubMed]
- Mana, L.; Schwartz-Pallejà, M.; Vila-Vidal, M.; Deco, G. Overview on Cognitive Impairment in Psychotic Disorders: From Impaired Microcircuits to Dysconnectivity. Schizophr. Res. 2024, 269, 132–143. [Google Scholar] [CrossRef]
- Green, M.J.; Matheson, S.L.; Shepherd, A.; Weickert, C.S.; Carr, V.J. Brain-Derived Neurotrophic Factor Levels in Schizophrenia: A Systematic Review with Meta-Analysis. Mol. Psychiatry 2011, 16, 960–972. [Google Scholar] [CrossRef]
- Nieto, R.R.; Carrasco, A.; Corral, S.; Castillo, R.; Gaspar, P.A.; Bustamante, M.L.; Silva, H. BDNF as a Biomarker of Cognition in Schizophrenia/Psychosis: An Updated Review. Front. Psychiatry 2021, 12, 662407. [Google Scholar] [CrossRef]
- Singh, S.; Roy, D.; Marzouk, T.; Zhang, J.-P. Peripheral Blood Levels of Brain-Derived Neurotrophic Factor in Patients with First Episode Psychosis: A Systematic Review and Meta-Analysis. Brain Sci. 2022, 12, 414. [Google Scholar] [CrossRef]
- Bora, E. Peripheral Inflammatory and Neurotrophic Biomarkers of Cognitive Impairment in Schizophrenia: A Meta-Analysis. Psychol. Med. 2019, 49, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, B.S.; Steiner, J.; Berk, M.; Molendijk, M.L.; Gonzalez-Pinto, A.; Turck, C.W.; Nardin, P.; Gonçalves, C.-A. Peripheral Brain-Derived Neurotrophic Factor in Schizophrenia and the Role of Antipsychotics: Meta-Analysis and Implications. Mol. Psychiatry 2015, 20, 1108–1119. [Google Scholar] [CrossRef]
- Sanada, K.; De Azúa, S.R.; Nakajima, S.; Alberich, S.; Ugarte, A.; Zugasti, J.; Vega, P.; Martínez-Cengotitabengoa, M.; González-Pinto, A. Correlates of Neurocognitive Functions in Individuals at Ultra-High Risk for Psychosis—A 6-Month Follow-up Study. Psychiatry Res. 2018, 268, 1–7. [Google Scholar] [CrossRef]
- Heitz, U.; Papmeyer, M.; Studerus, E.; Egloff, L.; Ittig, S.; Andreou, C.; Vogel, T.; Borgwardt, S.; Graf, M.; Eckert, A.; et al. Plasma and Serum Brain-Derived Neurotrophic Factor (BDNF) Levels and Their Association with Neurocognition in at-Risk Mental State, First Episode Psychosis and Chronic Schizophrenia Patients. World J. Biol. Psychiatry 2019, 20, 545–554. [Google Scholar] [CrossRef]
- Loch, A.A.; Pinto, M.T.C.; Andrade, J.C.; De Jesus, L.P.; De Medeiros, M.W.; Haddad, N.M.; Bilt, M.T.V.D.; Talib, L.L.; Gattaz, W.F. Plasma Levels of Neurotrophin 4/5, NGF and pro-BDNF Influence Transition to Mental Disorders in a Sample of Individuals at Ultra-High Risk for Psychosis. Psychiatry Res. 2023, 327, 115402. [Google Scholar] [CrossRef]
- Cecerska-Heryć, E.; Polikowska, A.; Serwin, N.; Michalczyk, A.; Stodolak, P.; Goszka, M.; Zoń, M.; Budkowska, M.; Tyburski, E.; Podwalski, P.; et al. The Importance of Oxidative Biomarkers in Diagnosis, Treatment, and Monitoring Schizophrenia Patients. Schizophr. Res. 2024, 270, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Yee, J.Y.; Lee, T.-S.; Lee, J. Levels of Serum Brain-Derived Neurotropic Factor in Individuals at Ultra-High Risk for Psychosis—Findings from the Longitudinal Youth at Risk Study (LYRIKS). Int. J. Neuropsychopharmacol. 2018, 21, 734–739. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Pruunsild, P.; Kazantseva, A.; Aid, T.; Palm, K.; Timmusk, T. Dissecting the Human BDNF Locus: Bidirectional Transcription, Complex Splicing, and Multiple Promoters. Genomics 2007, 90, 397–406. [Google Scholar] [CrossRef]
- Lu, B.; Nagappan, G.; Lu, Y. BDNF and Synaptic Plasticity, Cognitive Function, and Dysfunction. In Neurotrophic Factors. Handbook of Experimental Pharmacology; Lewin, G.R., Carter, B.D., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 220, pp. 223–250. ISBN 978-3-642-45105-8. [Google Scholar]
- Derksen, B.M.; Bruinsma, W.; Goslings, J.C.; Schep, N.W.L. The Kappa Paradox Explained. J. Hand Surg. 2024, 49, 482–485. [Google Scholar] [CrossRef]
- Aromataris, E.; Lockwood, C.; Porritt, K.; Pilla, B.; Jordan, Z. (Eds.) JBI Manual for Evidence Synthesis; JBI: Adelaide, Australia, 2024; ISBN 978-0-6488488-2-0. [Google Scholar]
- Kelsven, S.; De La Fuente-Sandoval, C.; Achim, C.L.; Reyes-Madrigal, F.; Mirzakhanian, H.; Domingues, I.; Cadenhead, K. Immuno-Inflammatory Changes across Phases of Early Psychosis: The Impact of Antipsychotic Medication and Stage of Illness. Schizophr. Res. 2020, 226, 13–23. [Google Scholar] [CrossRef]
- He, Y.; Yuan, L.; Li, Z.; Zhou, Y.; Ma, X.; Ouyang, L.; Chen, X. Plasma Protein Levels of Brain-Derived Neurotrophic Factor Pathways and Their Association with Cognitive Performance in Patients with Clinical High Risk for Psychosis and First Episode Psychosis. Schizophr. Res. 2019, 206, 460–461. [Google Scholar] [CrossRef]
- Counotte, J.; Bergink, V.; Pot-Kolder, R.; Drexhage, H.A.; Hoek, H.W.; Veling, W. Inflammatory Cytokines and Growth Factors Were Not Associated with Psychosis Liability or Childhood Trauma. PLoS ONE 2019, 14, e0219139. [Google Scholar] [CrossRef]
- Fernandes, B.S.; Berk, M.; Turck, C.W.; Steiner, J.; Gonçalves, C.-A. Decreased Peripheral Brain-Derived Neurotrophic Factor Levels Are a Biomarker of Disease Activity in Major Psychiatric Disorders: A Comparative Meta-Analysis. Mol. Psychiatry 2014, 19, 750–751. [Google Scholar] [CrossRef]
- Isayeva, U.; Manchia, M.; Collu, R.; Primavera, D.; Deriu, L.; Caboni, E.; Iaselli, N.; Sundas, D.; Tusconi, M.; Pinna, F.; et al. Exploring the Association between Brain-Derived Neurotrophic Factor Levels and Longitudinal Psychopathological and Cognitive Changes in Sardinian Psychotic Patients. Eur. Psychiatry 2022, 65, e71. [Google Scholar] [CrossRef] [PubMed]
- Brunoni, A.R.; Lopes, M.; Fregni, F. A Systematic Review and Meta-Analysis of Clinical Studies on Major Depression and BDNF Levels: Implications for the Role of Neuroplasticity in Depression. Int. J. Neuropsychopharmacol. 2008, 11, 1169–1180. [Google Scholar] [CrossRef]
- Molendijk, M.L.; Spinhoven, P.; Polak, M.; Bus, B.A.A.; Penninx, B.W.J.H.; Elzinga, B.M. Serum BDNF Concentrations as Peripheral Manifestations of Depression: Evidence from a Systematic Review and Meta-Analyses on 179 Associations (N = 9484). Mol. Psychiatry 2014, 19, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Cavaleri, D.; Moretti, F.; Bartoccetti, A.; Mauro, S.; Crocamo, C.; Carrà, G.; Bartoli, F. The Role of BDNF in Major Depressive Disorder, Related Clinical Features, and Antidepressant Treatment: Insight from Meta-Analyses. Neurosci. Biobehav. Rev. 2023, 149, 105159. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Cappucciati, M.; Rutigliano, G.; Lee, T.Y.; Beverly, Q.; Bonoldi, I.; Lelli, J.; Kaar, S.J.; Gago, E.; Rocchetti, M.; et al. Towards a Standard Psychometric Diagnostic Interview for Subjects at Ultra High Risk of Psychosis: CAARMS versus SIPS. Psychiatry J. 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Loch, A.A. Schizophrenia, Not a Psychotic Disorder: Bleuler Revisited. Front. Psychiatry 2019, 10, 328. [Google Scholar] [CrossRef]
- Hong, C.-J.; Liou, Y.-J.; Tsai, S.-J. Reprint of: Effects of BDNF Polymorphisms on Brain Function and Behavior in Health and Disease. Brain Res. Bull. 2012, 88, 406–417. [Google Scholar] [CrossRef]
- Egan, M.F.; Kojima, M.; Callicott, J.H.; Goldberg, T.E.; Kolachana, B.S.; Bertolino, A.; Zaitsev, E.; Gold, B.; Goldman, D.; Dean, M.; et al. The BDNF Val66met Polymorphism Affects Activity-Dependent Secretion of BDNF and Human Memory and Hippocampal Function. Cell 2003, 112, 257–269. [Google Scholar] [CrossRef]
- Moreno, I.; Stojanovic-Pérez, A.; Bulduk, B.; Sánchez-Gistau, V.; Algora, M.J.; Ortega, L.; Muntané, G.; Vilella, E.; Labad, J.; Martorell, L. High Blood Levels of Brain-Derived Neurotrophic Factor (BDNF) mRNA in Early Psychosis Are Associated with Inflammatory Markers. J. Psychiatr. Res. 2023, 164, 440–446. [Google Scholar] [CrossRef]
- Jin, P.; Andiappan, A.K.; Quek, J.M.; Lee, B.; Au, B.; Sio, Y.Y.; Irwanto, A.; Schurmann, C.; Grabe, H.J.; Suri, B.K.; et al. A Functional Brain-Derived Neurotrophic Factor (BDNF) Gene Variant Increases the Risk of Moderate-to-Severe Allergic Rhinitis. J. Allergy Clin. Immunol. 2015, 135, 1486–1493.e8. [Google Scholar] [CrossRef]
- Pivac, N.; Kim, B.; Nedić, G.; Joo, Y.H.; Kozarić-Kovačić, D.; Hong, J.P.; Muck-Seler, D. Ethnic Differences in Brain-Derived Neurotrophic Factor Val66Met Polymorphism in Croatian and Korean Healthy Participants. Croat. Med. J. 2009, 50, 43–48. [Google Scholar] [CrossRef]
- Shimizu, E.; Hashimoto, K.; Okamura, N.; Koike, K.; Komatsu, N.; Kumakiri, C.; Nakazato, M.; Watanabe, H.; Shinoda, N.; Okada, S.; et al. Alterations of Serum Levels of Brain-Derived Neurotrophic Factor (BDNF) in Depressed Patients with or without Antidepressants. Biol. Psychiatry 2003, 54, 70–75. [Google Scholar] [CrossRef]
- Matrisciano, F.; Bonaccorso, S.; Ricciardi, A.; Scaccianoce, S.; Panaccione, I.; Wang, L.; Ruberto, A.; Tatarelli, R.; Nicoletti, F.; Girardi, P.; et al. Changes in BDNF Serum Levels in Patients with Major Depression Disorder (MDD) after 6 Months Treatment with Sertraline, Escitalopram, or Venlafaxine. J. Psychiatr. Res. 2009, 43, 247–254. [Google Scholar] [CrossRef]
- Madsen, C.A.; Navarro, M.L.; Elfving, B.; Kessing, L.V.; Castrén, E.; Mikkelsen, J.D.; Knudsen, G.M. The Effect of Antidepressant Treatment on Blood BDNF Levels in Depressed Patients: A Review and Methodological Recommendations for Assessment of BDNF in Blood. Eur. Neuropsychopharmacol. 2024, 87, 35–55. [Google Scholar] [CrossRef]
- Pillai, A.; Bruno, D.; Sarreal, A.S.; Hernando, R.T.; Saint-Louis, L.A.; Nierenberg, J.; Ginsberg, S.D.; Pomara, N.; Mehta, P.D.; Zetterberg, H.; et al. Plasma BDNF Levels Vary in Relation to Body Weight in Females. PLoS ONE 2012, 7, e39358. [Google Scholar] [CrossRef]
- Giese, M.; Unternaehrer, E.; Brand, S.; Calabrese, P.; Holsboer-Trachsler, E.; Eckert, A. The Interplay of Stress and Sleep Impacts BDNF Level. PLoS ONE 2013, 8, e76050. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Marin, R.; Ying, Z.; Suntsova, N.; Methippara, M.; Bashir, T.; Szymusiak, R.; Gomez-Pinilla, F.; McGinty, D. Suppression of Hippocampal Plasticity-related Gene Expression by Sleep Deprivation in Rats. J. Physiol. 2006, 575, 807–819. [Google Scholar] [CrossRef]
- Dinoff, A.; Herrmann, N.; Swardfager, W.; Lanctôt, K.L. The Effect of Acute Exercise on Blood Concentrations of Brain-derived Neurotrophic Factor in Healthy Adults: A Meta-analysis. Eur. J. Neurosci. 2017, 46, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, A.; Beiky, M.; Mohammadi, I.; Rajai, S.; Jafarabady, K.; Moradi, S.; Beikmohamadi, M.; Teixeira, A.L. Effect of Smoking on Brain-Derived Neurotrophic Factor (BDNF) Blood Levels: A Systematic Review and Meta-Analysis. J. Affect. Disord. 2024, 349, 525–533. [Google Scholar] [CrossRef]
| Article | Heitz et al. (2018) | Loch et al. (2023) | Sanada et al. (2018) | Yee et al. (2018) | ||||||||||||
| Group | UHR | FEP | CS | HC | UHR | HC | UHR | HC | UHR | |||||||
| Sample size (n) | 16 | 6 | 11 | 55 | 81 | 30 | 13 | 106 | 105 | |||||||
| Mean BDNF (ng/mL) | Serum | 19.11 (±4.67) | 24.48 (±2.40) | 28.08 (±3.99) | _ | _ | _ | _ | 3.3 (±1.5) | 3.7 (±1.4) | ||||||
| Plasma | 0.30 (±0.29) | 0.54 (±0.54) | 1.31 (±1.06) | 0.48 (±0.37) | 0.53 (±0.36) | 12.92 (±10.40) | 3.86 (±1.43) | _ | _ | |||||||
| p-value | Serum 0.015 * Plasma 0.09 | 0.596 | <0.001 * | 0.018 * | ||||||||||||
| UHR selection criteria | BSIP | SIPS | CAARMS | CAARMS | ||||||||||||
| Average age (years) | 24.6 | 29.4 | 38.4 | 25.1 | 24.8 | 24 | 22.2 | 22 | 21.8 | |||||||
| BMI (kg/m2) | Unstated | Unstated | Unstated | 22.3 | 22.5 | |||||||||||
| Use of antipsychotics n° (%) | 1 (6.3) | 4 (66.7) | 11 (100) | Unstated | 0 (0) | 8 (61.5) | 0 (0) | 0 (0) | ||||||||
| Use of antidepressants n° (%) | Unstated | Unstated | Unstated | 0 (0) | 46 (43.8) | |||||||||||
| Use of nicotine or tobacco n° (%) | 11 (68.8) | 4 (66.7) | 11 (100) | 18 (32.7) | 26 (32.1) | 5 (16.7) | 8 (61.5) | 19 (18.1) | 31 (29.5) | |||||||
| Follow-up | No | 30 months | 6 months to UHR participants | 24 months | ||||||||||||
| Article | Kelsven et al. (2020) | Counotte et al. (2019) | He et al. (2019) | Cecerska-Heryć et al. (2024) | ||||||||||||
| Group | HC | UHR | FEP | HC | UHR | PS | HC | UHR | FEP | HC | UHR | FEP | CS | |||
| Sample size (n) | 7 | 11 | 50 | 39 | 11 | 38 | 29 | 30 | 30 | 34 | 13 | 31 | 72 | |||
| Mean BDNF (ng/mL) | Serum | _ | _ | _ | 18 a | 20 a | 19 a | _ | _ | _ | 35 a | 21 a | 21 a | 19 a | ||
| Plasma | 3.88 (±1.17) | 18.08 (±8.67) | 5.02 (±6.17) | _ | _ | _ | 11.56 (±1.06) | 11.43 (±1.27) | 12.59 (±1.25) | _ | _ | _ | _ | |||
| p-value | <0.001 * | 0.279 | 0.001 * | <0.01 * for HC and DS-FEP <0.05 * for HC and NDS | ||||||||||||
| UHR selection criteria | SIPS | CAARMS | SIPS | SIPS | ||||||||||||
| Average age (years) | 20.4 | 18.5 | 24.1 | 24 | 24 | 25.5 | 20.4 | 19.8 | 19.6 | 36 | 23 | 29 | 39 | |||
| BMI (kg/m2) | Unstated | 22.8 | 23.1 | 23 | Unstated | 25.6 | 22.7 | 24.3 | 28.1 | |||||||
| Use of antipsychotics n° (%) | 0 (0) | 0 (0) | 10 (20) | 0 (0) | 0 (0) | 0 (0) | Unstated | 0 (0) | 10 (76.9) | 28 (90.3) | 68 (94.4) | |||||
| Use of antidepressants n° (%) | Unstated | Unstated | Unstated | Unstated | ||||||||||||
| Use of nicotine or tobacco n° (%) | Unstated | 6 (15.4) | 9 (81.8) | 16 (42.1) | Unstated | 0 (0) | 0 (0) | 4 (12.9) | 23 (31.9) | |||||||
| Follow-up | No | No | No | No | ||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contreras, O.; Rivera, C.; Villaseca, C.; Mas, F.; Cartes, B.; Castillo-Passi, R.; Nieto, R.R. Peripheral BDNF Levels in Individuals at Ultra-High Risk for Psychosis: A Systematic Review. Brain Sci. 2025, 15, 928. https://doi.org/10.3390/brainsci15090928
Contreras O, Rivera C, Villaseca C, Mas F, Cartes B, Castillo-Passi R, Nieto RR. Peripheral BDNF Levels in Individuals at Ultra-High Risk for Psychosis: A Systematic Review. Brain Sciences. 2025; 15(9):928. https://doi.org/10.3390/brainsci15090928
Chicago/Turabian StyleContreras, Omar, Carla Rivera, Carolina Villaseca, Francisco Mas, Benjamín Cartes, Rolando Castillo-Passi, and Rodrigo R. Nieto. 2025. "Peripheral BDNF Levels in Individuals at Ultra-High Risk for Psychosis: A Systematic Review" Brain Sciences 15, no. 9: 928. https://doi.org/10.3390/brainsci15090928
APA StyleContreras, O., Rivera, C., Villaseca, C., Mas, F., Cartes, B., Castillo-Passi, R., & Nieto, R. R. (2025). Peripheral BDNF Levels in Individuals at Ultra-High Risk for Psychosis: A Systematic Review. Brain Sciences, 15(9), 928. https://doi.org/10.3390/brainsci15090928






