Positive Psychology Interventions in Early-Stage Cognitive Decline Related to Dementia: A Systematic Review of Cognitive and Brain Functioning Outcomes of Mindfulness Interventions
Abstract
1. Introduction
2. Materials and Methods
2.1. Compliance with PRISMA Guidelines
2.2. Eligibility Criteria
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Search Strategy
2.4. Selection Process
2.5. Data Items
2.5.1. Outcomes
2.5.2. Other Variables
- -
- Geographic location.
- -
- Participant characteristics: mean age and age range, proportion of male and female participants, total number of participants and group sizes, cognitive status of participants (SCD, MCI, mild AD dementia).
- -
- Intervention characteristics: specific PPI (e.g., mindfulness intervention), duration (number of weeks and sessions), frequency (e.g., 1 h per week), delivery method (online, in-person, group).
- -
- Study characteristics: type of study (RCT, pre–post intervention study), control group (e.g., active control, waitlist, treatment as usual), follow-up period.
2.6. Synthesis Methods
- Tabulating study characteristics: A table (Table 3) was created to summarize the key points of each study. Specifically, this table includes information about geographic location, design of the study (e.g., RCT, pre–post intervention study), population (e.g., SCD, MCI, mild AD demented), control group (no/active control group, waitlist, treatment as usual), PPI (e.g., mindfulness intervention), the setting where participants were recruited from (community or hospital).
- Tabulating the effectiveness of PPI interventions: A table (Table 4) was created to summarize the effectiveness of each study. This table contains assessment time (pre, post, follow-up), outcome measures and results.
- Comparing study characteristics against planned groups: studies were grouped based on type of PPI.
- Reviewer 1 and Reviewer 2 worked independently to group the results, and then they compared and discussed the findings.
3. Results
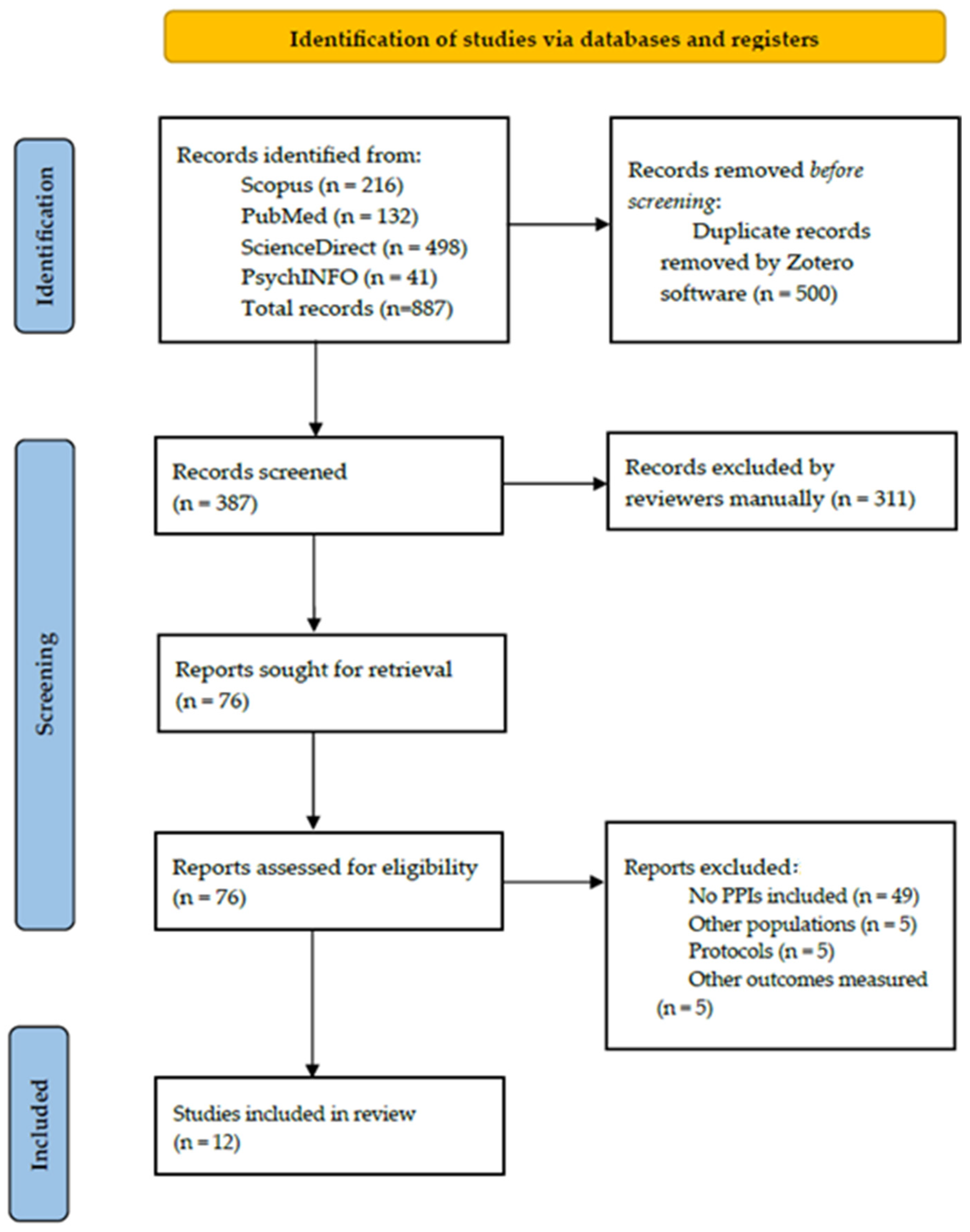
3.1. Study Selection
3.2. Excluded Studies
3.3. Study Characteristics
3.4. Participants
3.5. Interventions
3.6. Results of Synthesis
3.6.1. Mindful Awareness Practice (MAP)
3.6.2. Mindfulness-Based Training (MBT)
3.6.3. Mindfulness Training (MT)
3.6.4. Mindfulness Training Program (MTP)
3.6.5. Mindfulness-Based Intervention (MBI)
3.6.6. Mindfulness-Based Alzheimer’s Stimulation (MBAS)
3.6.7. Caring Mindfulness-Based Approach for Seniors (CMBAS)
3.7. Quality Appraisal
4. Discussion
4.1. Implication for Future Research
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SCD | Subjective Cognitive Decline |
| MCI | Mild cognitive impairment |
| AD | Alzheimer’s disease |
| CS | Character strengths |
| PPIs | Positive psychology interventions |
| RCT | Randomized controlled trials |
| aMCI | amnestic Mild Cognitive Impairment |
| MAP | Mindfulness Awareness Practice/Program |
| HEP | Health Education Program |
| MBT | Mindfulness-based Training |
| CRT | Cognitive rehabilitation therapy |
| TAU | Treatment as usual |
| MT | Mindfulness Training (MT) |
| PE | Psychoeducation |
| Pe | Error Positivity |
| MTP | Mindfulness Training Program |
| MBI | Mindfulness-based intervention |
| PBI | Psychoeducation-based intervention |
| MBAS | Mindfulness-based Alzheimer’s stimulation |
| CST | Cognitive Stimulation Therapy |
| PMR | Progressive Muscle Relaxation |
| CMBAS | Caring Mindfulness-Based Approach for Seniors |
| HSMP | Health Self-Management Program |
| PACC5 | Preclinical Alzheimer’s Cognitive Composite 5 |
| TMT-A | Trail-Making Test Part A |
| WAIS-IV | Wechsler Adult Intelligence Scale—Fourth Edition |
| TMT-B | Trail-Making Test Part B |
| CEQ | Credibility/Expectancy Questionnaire |
| ERPs | Event-Related Potentials |
| EEG | Electroencephalography |
| ERN | Error-Related Negativity |
| AMAS-E | Abbreviated Math Anxiety Scale—Elementary |
| NMR | Need for Mental Regulation |
| FFMQ | Five-Facet Mindfulness Questionnaire |
| RBANS | Repeatable Battery for the Assessment of Neuropsychological Status |
| MMSE | Mini-Mental State Examination |
| MoCA | Montreal Cognitive Assessment |
| MAAS | Mindful Attention and Awareness Scale |
| hs-CRP | High-sensitivity C-Reactive Protein |
| BDNF | Brain-Derived Neurotrophic Factor |
| DHEA-S | Dehydroepiandrosterone Sulfate |
| IL-6 | Interleukin 6 |
| IL-1β | Interleukin 1 beta |
| RAVLT | Rey Auditory Verbal Learning Test |
| Block Design Test | from WAIS, no abbreviation |
| fMRI | Functional Magnetic Resonance Imaging |
| CDR | Clinical Dementia Rating |
| WAIS-III | Wechsler Adult Intelligence Scale—Third Edition |
| MRI | Magnetic Resonance Imaging |
| CAT12 | Computational Anatomy Toolbox version 12 |
| FMI | Freiburg Mindfulness Inventory |
| MAQ | Mindfulness Adherence Questionnaire |
| B-ADL | Bayer Activities of Daily Living Scale |
| DHL | Demographic, Health, and Lifestyle questionnaire |
| WHOQOL-BREF | World Health Organization Quality of Life—Brief Version |
| WHOQOL-OLD | World Health Organization Quality of Life—Older Adults Version |
| RRS | Ruminative Response Scale |
| CAMDEX-R | Cambridge Examination for Mental Disorders of the Elderly—Revised |
| CAMCOG | Cambridge Cognitive Examination |
| DAVID | Database for Annotation, Visualization and Integrated Discovery |
| OMIM | Online Mendelian Inheritance in Man |
References
- Randolph, J.J. Positive neuropsychology: The science and practice of promoting cognitive health. Appl. Neuropsychol. Adult 2018, 25, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Bell, G.; Singham, T.; Saunders, R.; John, A.; Stott, J. Positive psychological constructs and association with reduced risk of mild cognitive impairment and dementia in older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2022, 77, 101594. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia#:~:text=Key%20facts,injuries%20that%20affect%20the%20brain (accessed on 15 December 2024).
- Li, Y.-Q.; Yin, Z.-H.; Zhang, X.-Y.; Chen, Z.-H.; Xia, M.-Z.; Ji, L.-X.; Liang, F.-R. Non-pharmacological interventions for behavioral and psychological symptoms of dementia: A systematic review and network meta-analysis protocol. Front. Psychiatry 2022, 13, 1039752. [Google Scholar] [CrossRef] [PubMed]
- Papaliagkas, V.; Papantoniou, G.; Tsolaki, M.; Moraitou, D. Self-report Instruments of Cognitive Failures as screening tools for subjective cognitive impairment in older adults. Hell. J. Nucl. Med. 2017, 20, 58–70. [Google Scholar]
- Van Harten, A.C.; Mielke, M.M.; Swenson-Dravis, D.M.; Hagen, C.E.; Edwards, K.K.; Roberts, R.O.; Geda, Y.E.; Knopman, D.S.; Petersen, R.C. Subjective cognitive decline and risk of MCI. Neurology 2018, 91, e300–e312. [Google Scholar] [CrossRef]
- Li, W.; Yue, L.; Xiao, S. Subjective cognitive decline is associated with a higher risk of objective cognitive decline: A cross-sectional and longitudinal study. Front. Psychiatry 2022, 13, 950270. [Google Scholar] [CrossRef]
- Si, T.; Xing, G.; Han, Y. Subjective cognitive decline and related cognitive deficits. Front. Neurol. 2020, 11, 247. [Google Scholar] [CrossRef]
- Malekganou, M.; Frantzi, N.; Emmanouilidou, E.; Moraitou, D. The Relationship between mild behavioral disorder and subjective cognitive Decline in Older Adults: A Systematic review. Hell. J. Psychol. 2025, 22, 1–37. (In Greek) [Google Scholar]
- Lazarou, I.; Moraitou, D.; Papatheodorou, M.; Vavouras, I.; Lokantidou, C.; Agogiatou, C.; Gialaoutzis, M.; Nikolopoulos, S.; Stavropoulos, T.G.; Kompatsiaris, I.; et al. Adaptation and Validation of the Memory Alteration Test (M@T) in Greek Middle-Aged, Older, and Older-Old Population with Subjective Cognitive Decline and Mild Cognitive Impairment. J. Alzheimer Dis. 2021, 84, 1219–1232. [Google Scholar] [CrossRef]
- Rivas-Fernández, M.Á.; Lindín, M.; Zurrón, M.; Díaz, F.; Lojo-Seoane, C.; Pereiro, A.X.; Galdo-Álvarez, S. Neuroanatomical and neurocognitive changes associated with subjective cognitive decline. Front. Med. 2023, 10, 1094799. [Google Scholar] [CrossRef]
- Poptsi, E.; Tsardoulias, E.; Moraitou, D.; Symeonidis, A.L.; Tsolaki, M. REMEDES for Alzheimer-R4ALZ Battery: Design and development of a new tool of Cognitive Control assessment for the diagnosis of minor and major neurocognitive disorders. J. Alzheimer Dis. 2019, 72, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Poptsi, E.; Moraitou, D.; Tsardoulias, E.; Symeonidis, A.L.; Tsolaki, M. R4Alz-R: A cutting-edge tool for spotting the very first and subtle signs of aging-related cognitive impairment with high accuracy. GeroScience 2024. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Csukly, G.; Sirály, E.; Fodor, Z.; Horváth, A.; Salacz, P.; Hidasi, Z.; Csibri, É.; Rudas, G.; Szabó, Á. The Differentiation of Amnestic Type MCI from the Non-Amnestic Types by Structural MRI. Front. Aging Neurosci. 2016, 8, 52. [Google Scholar] [CrossRef]
- Gillis, C.; Mirzaei, F.; Potashman, M.; Ikram, M.A.; Maserejian, N. The incidence of mild cognitive impairment: A systematic review and data synthesis. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2019, 11, 248–256. [Google Scholar] [CrossRef]
- Flavell, L. Mild (Early-Stage) Alzheimer’s Disease—Alzheimer’s News Today; Alzheimer’s News Today. Available online: https://alzheimersnewstoday.com/mild-alzheimers-early-stage/ (accessed on 10 January 2025).
- Poptsi, E.; Tsatali, M.; Agogiatou, C.; Bakoglidou, E.; Batsila, G.; Dellaporta, D.; Kounti-Zafeiropoulou, F.; Liapi, D.; Lysitsas, K.; Markou, N.; et al. Longitudinal Cognitive and Physical Training Effectiveness in MCI, based on the experience of the Alzheimer’s Hellas Day Care Centre. J. Geriatr. Psychiatry Neurol. 2021, 35, 512–526. [Google Scholar] [CrossRef]
- Sharew, N.T. The effect of multimodal non-pharmacological interventions on cognitive function improvement for people with dementia: A systematic review. Front. Public Health 2022, 10, 894930. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Zhang, J.; Song, Z.; Wang, Y.; Wang, X.; Qu, H.; Wang, F.; Liu, C.; Gao, F. Effectiveness of non-pharmacological therapies on cognitive function in patients with dementia—A network meta-analysis of randomized controlled trials. Front. Aging Neurosci. 2023, 15, 1131744. [Google Scholar] [CrossRef]
- Seligman, M.E.P.; Csikszentmihalyi, M. Positive psychology: An introduction. Am. Psychol. 2000, 55, 5–14. [Google Scholar] [CrossRef]
- Brown, K.W.; Creswell, J.D.; Ryan, R.M. Handbook of Mindfulness: Theory, Research, and Practice; Guilford Publications: New York, NY, USA, 2015. [Google Scholar]
- Niemiec, R.M. Mindfulness and Character Strengths: A Practitioner’s Guide to MBSP; Hogrefe Publishing GmbH: Göttingen, Germany, 2023. [Google Scholar]
- Proyer, R.T.; Gander, F.; Wellenzohn, S.; Ruch, W. Positive psychology interventions in people aged 50–79 years: Long-term effects of placebo-controlled online interventions on well-being and depression. Aging Ment. Health 2014, 18, 997–1005. [Google Scholar] [CrossRef]
- Becker, N.B.; Del Rio, K.A.; Jesus, S.N.; Bonança, J.; Martins, R. Perspectives from Positive Psychology in Older Adults: Brief Literature review. J. Spat. Organ. Dyn. 2016, 4, 21–29. [Google Scholar]
- Zichnali, O.; Moraitou, D.; Pezirkianidis, C.; Stalikas, A. Examining the Effectiveness of Two Types of Forgiveness Intervention to Enhance Well-Being in Adults from Young to Older Adulthood. OBM Geriatr. 2018, 3, 1. [Google Scholar] [CrossRef]
- Giapraki, M.; Moraitou, D.; Pezirkianidis, C.; Stalikas, A. Humor in aging: Is it able to enhance wellbeing in community dwelling older adults? Psychol. J. Hell. Psychol. Soc. 2020, 25, 128. [Google Scholar] [CrossRef]
- Tsiflikioti, K.; Moraitou, D.; Pezirkianidis, C.; Papantoniou, G.; Sofologi, M.; Kougioumtzis, G.A.; Tsolaki, M. Enhancing Subjective Wellbeing in Older Individuals with Amnestic Mild Cognitive Impairment: A Randomized Trial of a Positive Psychology Intervention. Behav. Sci. 2023, 13, 838. [Google Scholar] [CrossRef]
- Positive Psychology Interventions to Improve Well-Being in Older Adults: A Systematic Review—University of Twente Student Theses. Available online: https://purl.utwente.nl/essays/82741 (accessed on 10 January 2025).
- Sutin, A.R.; Stephan, Y.; Terracciano, A. Psychological well-being and risk of dementia. Int. J. Geriatr. Psychiatry 2018, 33, 743–747. [Google Scholar] [CrossRef]
- Estrella, M.L.; Durazo-Arvizu, R.A.; Gallo, L.C.; Tarraf, W.; Isasi, C.R.; Perreira, K.M.; Zeng, D.; Marquine, M.J.; Lipton, R.B.; González, H.M.; et al. Psychosocial Factors Associated with Cognitive Function Among Middle-Aged and Older Hispanics/Latinos: The Hispanic Community Health Study/Study of Latinos and its Sociocultural Ancillary Study. J. Alzheimer Dis. 2020, 79, 433–449. [Google Scholar] [CrossRef]
- Lewis, N.A.; Hofer, S.M.; Bennett, D.A.; Hill, P.L. Sense of purpose in life and extending the cognitive healthspan: Evidence from multistate survival modeling. Aging Neuropsychol. Cogn. 2024, 32, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Friedman, E.M.; Thomas, P.A.; Sauerteig-Rolston, M.R.; Barnes, L.L.; Ferraro, K.F. Sustained purpose in life is associated with slower cognitive decline in older adults: A longitudinal analysis with a diverse national sample. J. Gerontol. Ser. B 2025, 80, gbaf021. [Google Scholar] [CrossRef]
- Tsormpatzoudi, S.O.; Moraitou, D.; Papaliagkas, V.; Pezirkianidis, C.; Tsolaki, M. Resilience in Mild Cognitive Impairment (MCI): Examining the Level and the Associations of Resilience with Subjective Wellbeing and Negative Affect in Early and Late-Stage MCI. Behav. Sci. 2023, 13, 792. [Google Scholar] [CrossRef]
- Gkintoni, E.; Vassilopoulos, S.P.; Nikolaou, G. Mindfulness-Based Cognitive Therapy in Clinical Practice: A Systematic Review of Neurocognitive Outcomes and Applications for Mental Health and Well-Being. J. Clin. Med. 2025, 14, 1703. [Google Scholar] [CrossRef]
- Antoniou, R.; Cryns, N.; Young, J.C.; Diaz, V.; Kramer, J.; Chiong, W. The Association between Trait Mindfulness and Metacognitive Efficiency in Healthy Older Adults. PsyArXiv 2024. [Google Scholar] [CrossRef]
- Greengross, G. Humor and Aging—A Mini-Review. Gerontology 2013, 59, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Tani, Y.; Koyama, Y.; Doi, S.; Sugihara, G.; Machida, M.; Amagasa, S.; Murayama, H.; Inoue, S.; Fujiwara, T.; Shobugawa, Y. Association between gratitude, the brain and cognitive function in older adults: Results from the NEIGE study. Arch. Gerontol. Geriatr. 2022, 100, 104645. [Google Scholar] [CrossRef] [PubMed]
- Morse, L.A.; Xiong, L.; Ramirez-Zohfeld, V.; Anne, S.; Barish, B.; Lindquist, L.A. Humor doesn’t retire: Improvisation as a health-promoting intervention for older adults. Arch. Gerontol. Geriatr. 2017, 75, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Moraitou, D.; Efklides, A. Wise thinking, hopeful thinking, and positive aging: Reciprocal relations of wisdom, hope, memory, and affect in Young, Middle-Aged, and older adults. In A Positive Psychology Perspective on Quality of Life; Social Indicators Research Series; Springer: Dordrecht, The Netherlands, 2012; pp. 189–218. [Google Scholar] [CrossRef]
- Chételat, G.; Mézenge, F.; Tomadesso, C.; Landeau, B.; Arenaza-Urquijo, E.; Rauchs, G.; André, C.; De Flores, R.; Egret, S.; Gonneaud, J.; et al. Reduced age-associated brain changes in expert meditators: A multimodal neuroimaging pilot study. Sci. Rep. 2017, 7, 10160. [Google Scholar] [CrossRef] [PubMed]
- Luders, E.; Cherbuin, N.; Gaser, C. Estimating brain age using high-resolution pattern recognition: Younger brains in long-term meditation practitioners. NeuroImage 2016, 134, 508–513. [Google Scholar] [CrossRef]
- Acevedo, B.P.; Pospos, S.; Lavretsky, H. The Neural Mechanisms of Meditative Practices: Novel Approaches for Healthy aging. Curr. Behav. Neurosci. Rep. 2016, 3, 328–339. [Google Scholar] [CrossRef]
- Lutz, A.; Chételat, G.; Collette, F.; Klimecki, O.M.; Marchant, N.L.; Gonneaud, J. The protective effect of mindfulness and compassion meditation practices on ageing: Hypotheses, models and experimental implementation. Ageing Res. Rev. 2021, 72, 101495. [Google Scholar] [CrossRef]
- Cotier, F.A.; Zhang, R.; Lee, T.M.C. A longitudinal study of the effect of short-term meditation training on functional network organization of the aging brain. Sci. Rep. 2017, 7, 598. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Whitfield, T.; Demnitz-King, H.; Schlosser, M.; Barnhofer, T.; Frison, E.; Coll-Padros, N.; Dautricourt, S.; Requier, F.; Delarue, M.; Gonneaud, J.; et al. Effects of a mindfulness-based versus a health self-management intervention on objective cognitive performance in older adults with subjective cognitive decline (SCD): A secondary analysis of the SCD-Well randomized controlled trial. Alzheimer Res. Ther. 2022, 14, 125. [Google Scholar] [CrossRef]
- Smart, C.M.; Segalowitz, S.J. Respond, don’t react: The influence of mindfulness training on performance monitoring in older adults. Cogn. Affect. Behav. Neurosci. 2017, 17, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Doshi, K.; Henderson, S.L.; Fan, Q.; Wong, K.F.; Lim, J. Mindfulness-Based training does not improve neuropsychological outcomes in mild cognitive impairment more than spontaneous reversion rates: A randomized controlled trial. J. Alzheimer Dis. 2021, 84, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.K.S.; Fam, J.; Feng, L.; Cheah, I.K.-M.; Tan, C.T.-Y.; Nur, F.; Wee, S.T.; Goh, L.G.; Chow, W.L.; Ho, R.C.-M.; et al. Mindfulness improves inflammatory biomarker levels in older adults with mild cognitive impairment: A randomized controlled trial. Transl. Psychiatry 2020, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.K.S.; Tan, X.R.; Todd, M.; Chen, A.C.-C.; Feng, L.; Lu, Y.; Yu, F.; Kua, E.H.; Mahendran, R. Effects of Mindful Awareness Practice (MAP) on Subclinical Depressive and Anxiety Symptoms and General Cognitive Function in Older Adults with Mild Cognitive Impairment: A 5-Year Follow-Up of the MAP-Randomized Controlled Trial. J. Alzheimer Dis. 2022, 90, 1677–1688. [Google Scholar] [CrossRef]
- Fam, J.; Sun, Y.; Qi, P.; Lau, R.C.; Feng, L.; Kua, E.H.; Mahendran, R. Mindfulness practice alters brain connectivity in community-living elders with mild cognitive impairment. Psychiatry Clin. Neurosci. 2019, 74, 257–262. [Google Scholar] [CrossRef]
- Klainin-Yobas, P.; Kowitlawakul, Y.; Lopez, V.; Tang, C.T.; Hoek, K.E.; Gan, G.L.; Lei, F.; Rawtaer, I.; Mahendran, R. The effects of mindfulness and health education programs on the emotional state and cognitive function of elderly individuals with mild cognitive impairment: A randomized controlled trial. J. Clin. Neurosci. 2019, 68, 211–217. [Google Scholar] [CrossRef]
- Yu, J.; Rawtaer, I.; Feng, L.; Fam, J.; Kumar, A.P.; Cheah, I.K.-M.; Honer, W.G.; Su, W.; Lee, Y.K.; Tan, E.C.; et al. Mindfulness intervention for mild cognitive impairment led to attention-related improvements and neuroplastic changes: Results from a 9-month randomized control trial. J. Psychiatr. Res. 2021, 135, 203–211. [Google Scholar] [CrossRef]
- Wong, W.P.; Coles, J.; Chambers, R.; Wu, D.B.-C.; Hassed, C. The Effects of Mindfulness on Older Adults with Mild Cognitive Impairment. J. Alzheimer Dis. Rep. 2017, 1, 181–193. [Google Scholar] [CrossRef]
- Larouche, E.; Hudon, C.; Goulet, S. Mindfulness mechanisms and psychological effects for aMCI patients: A comparison with psychoeducation. Complement. Ther. Clin. Pract. 2018, 34, 93–104. [Google Scholar] [CrossRef]
- Quintana-Hernández, D.J.; Miró-Barrachina, M.T.; Ibáñez-Fernández, I.J.; Pino, A.S.-D.; Quintana-Montesdeoca, M.P.; Vera, B.R.-D.; Morales-Casanova, D.; Del Carmen Pérez-Vieitez, M.; Rodríguez-García, J.; Bravo-Caraduje, N. Mindfulness in the maintenance of cognitive capacities in Alzheimer’s Disease: A randomized clinical trial. J. Alzheimer Dis. 2016, 50, 217–232. [Google Scholar] [CrossRef]
- Lim, H.-W.; Saw, W.-Y.; Feng, L.; Lee, Y.-K.; Mahendran, R.; Cheah, I.K.-M.; Rawtaer, I.; Kumar, A.P.; Kua, E.-H.; Mahendran, R.; et al. Dataset on gene expression in the elderly after Mindfulness Awareness Practice or Health Education Program. Data Brief 2018, 18, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Schlosser, M.; Demnitz-King, H.; Barnhofer, T.; Collette, F.; Gonneaud, J.; Chételat, G.; Jessen, F.; Kliegel, M.; Klimecki, O.M.; Lutz, A.; et al. Effects of a mindfulness-based intervention and a health self-management programme on psychological well-being in older adults with subjective cognitive decline: Secondary analyses from the SCD-Well randomised clinical trial. PLoS ONE 2023, 18, e0295175. [Google Scholar] [CrossRef] [PubMed]
- Namias, J.M.; Huff, M.J. Evaluating the effects of brief mindfulness practice on attentional control and episodic memory. J. Cogn. Psychol. 2024, 36, 720–741. [Google Scholar] [CrossRef]
- McBee, L. Mindfulness-Based Elder Care: A CAM Model for Frail Elders and Their Caregivers; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Kabat-Zinn, J. Full Catastrophe Living: Using the Wisdom of Your Body and Mind to Face Stress, Pain, and Illness; Delta: Atlanta, GE, USA, 1991. [Google Scholar]
- Segal, Z.V.; Williams, J.M.G.; Teasdale, J.D. Mindfulness-Based Cognitive Therapy for Depression, First Edition: A New Approach to Preventing Relapse; Guilford Press: New York, NY, USA, 2001. [Google Scholar]
- Hong, Q.N.; Fàbregues, S.; Bartlett, G.; Boardman, F.; Cargo, M.; Dagenais, P.; Gagnon, M.-P.; Griffiths, F.; Nicolau, B.; O’Cathain, A.; et al. The Mixed Methods Appraisal Tool (MMAT) version 2018 for information professionals and researchers. Educ. Inf. 2018, 34, 285–291. [Google Scholar] [CrossRef]
- Lazar, S.W.; Kerr, C.E.; Wasserman, R.H.; Gray, J.R.; Greve, D.N.; Treadway, M.T.; McGarvey, M.; Quinn, B.T.; Dusek, J.A.; Benson, H.; et al. Meditation experience is associated with increased cortical thickness. Neuroreport 2005, 16, 1893–1897. [Google Scholar] [CrossRef] [PubMed]
- Prakash, R.S.; De Leon, A.A.; Klatt, M.; Malarkey, W.; Patterson, B. Mindfulness disposition and default-mode network connectivity in older adults. Soc. Cogn. Affect. Neurosci. 2012, 8, 112–117. [Google Scholar] [CrossRef]
- Farb, N.a.S.; Segal, Z.V.; Anderson, A.K. Mindfulness meditation training alters cortical representations of interoceptive attention. Soc. Cogn. Affect. Neurosci. 2012, 8, 15–26. [Google Scholar] [CrossRef]
- Moore, A.; Gruber, T.; Derose, J.; Malinowski, P. Regular, brief mindfulness meditation practice improves electrophysiological markers of attentional control. Front. Hum. Neurosci. 2012, 6, 18. [Google Scholar] [CrossRef]
- Kabat-Zinn, J. Full Catastrophe Living (Revised Edition): Using the Wisdom of Your Body and Mind to Face Stress, Pain, and Illness; Bantam: Java, Indonesia, 2013. [Google Scholar]
- Strauss, C.; Cavanagh, K.; Oliver, A.; Pettman, D. Mindfulness-Based Interventions for People Diagnosed with a Current Episode of an Anxiety or Depressive Disorder: A Meta-Analysis of Randomised Controlled Trials. PLoS ONE 2014, 9, e96110. [Google Scholar] [CrossRef]
- Lindsay, E.K.; Creswell, J.D. Mechanisms of mindfulness training: Monitor and Acceptance Theory (MAT). Clin. Psychol. Rev. 2016, 51, 48–59. [Google Scholar] [CrossRef]
- Canu, E.; Sarasso, E.; Filippi, M.; Agosta, F. Effects of pharmacological and nonpharmacological treatments on brain functional magnetic resonance imaging in Alzheimer’s disease and mild cognitive impairment: A critical review. Alzheimer’s Res. Ther. 2018, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Schutte, N.S.; Malouff, J.M. A meta-analytic investigation of the impact of curiosity-enhancing interventions. Curr. Psychol. 2022, 42, 20374–20384. [Google Scholar] [CrossRef]
- Mohanty, M.; Kumar, P. Multi-Component interventions in older adults having subjective Cognitive Decline (SCD)—A review article. Geriatrics 2022, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.; Cullen, K.; Keeney, C.; Canning, C.; Mooney, O.; Chinseallaigh, E.; O’Dowd, A. Effectiveness of positive psychology interventions: A systematic review and meta-analysis. J. Posit. Psychol. 2020, 16, 749–769. [Google Scholar] [CrossRef]
- Hendriks, T.; Schotanus-Dijkstra, M.; Hassankhan, A.; De Jong, J.; Bohlmeijer, E. The efficacy of multi-component positive psychology interventions: A systematic review and meta-analysis of randomized controlled trials. J. Happiness Stud. 2019, 21, 357–390. [Google Scholar] [CrossRef]
| Data Base | Search Term |
|---|---|
| Scopus | (TITLE-ABS-KEY (“positive psychology” OR “positive neuropsychology” OR “positive neuroscience” OR “positive psychology intervention” OR “psychological intervention” OR “mindfulness” OR “gratitude intervention” OR “meaning in life” OR “purpose in life” OR “well-being intervention” OR “hedonic well-being” OR “eudaimonic well-being” OR “character strengths” OR “virtues” OR “signature strengths” OR “strengths-based intervention”)) AND (TITLE-ABS-KEY (“mild cognitive impairment” OR “MCI” OR “subjective cognitive decline” OR “Alzheimer’s disease” OR “early-stage dementia” OR “mild dementia”)) AND (TITLE-ABS-KEY (“cognitive function” OR “cognitive decline” OR “memory” OR “executive function” OR “cognitive reserve” OR “brain function” OR “brain connectivity” OR “functional connectivity” OR “neural plasticity” OR “fMRI” OR “EEG” OR “fNIRS” OR “cortex” OR “prefrontal cortex” OR “anterior cingulate cortex” OR “temporal cortex” OR “neuroplasticity”)) AND PUBYEAR > 2014 AND PUBYEAR < 2026 AND (LIMIT-TO (DOCTYPE, “ar”) OR LIMIT-TO (DOCTYPE, “re”) OR LIMIT-TO (DOCTYPE, “ch”)) AND (LIMIT-TO (SUBJAREA, “MEDI”) OR LIMIT-TO (SUBJAREA, “NEUR”) OR LIMIT-TO (SUBJAREA, “PSYC”)) AND (LIMIT-TO (LANGUAGE, “English”)) Limited to: Title-Abstract-Keywords Areas of neuroscience, medicine and dentistry and psychology Publication Date: 2015–2025 |
| PubMed | (“positive psychology” OR “positive neuropsychology” OR “positive neuroscience” OR “positive psychology intervention” OR “positive psychological intervention” OR “mindfulness” OR “gratitude intervention” OR “meaning in life” OR “purpose in life” OR “well-being intervention” OR “hedonic well-being” OR “eudaimonic well-being” OR “character strengths” OR “virtues” OR “signature strengths” OR “strengths-based intervention”) AND (“mild cognitive impairment” OR “MCI” OR “subjective cognitive decline” OR “Alzheimer’s disease” OR “early-stage dementia” OR “mild dementia”) AND (“cognitive function” OR “cognitive decline” OR “memory” OR “executive function” OR “cognitive reserve” OR “brain function” OR “brain connectivity” OR “functional connectivity” OR “neural plasticity” OR “fMRI” OR “EEG” OR “fNIRS” OR “cortex” OR “prefrontal cortex” OR “anterior cingulate cortex” OR “temporal cortex” OR “neuroplasticity”) Areas of neuroscience, medicine and dentistry and psychology Publication Date: 2015–2025 |
| ScienceDirect | (“positive psychology” OR “character strengths” OR “mindfulness”) AND (“mild cognitive impairment” OR “subjective cognitive decline” OR “Alzheimer’s disease”) AND (“cognitive function” OR “brain function” OR “cortex”) Publication Date: 2015–2025 |
| PsychINFO | 1. “positive psychology” OR “positive neuropsychology” OR “positive neuroscience” OR “positive psychology intervention” OR “positive psychological intervention” OR “mindfulness” OR “gratitude intervention” OR “meaning in life” OR “purpose in life” OR “well-being intervention” OR “hedonic well-being” OR “eudaimonic well-being” OR “character strengths” OR “virtues” OR “signature strengths” OR “strengths-based intervention” |
| 2. “mild cognitive impairment” OR “MCI” OR “subjective cognitive decline” OR “Alzheimer’s disease” OR “early-stage dementia” OR “mild dementia”) | |
| 3. “cognitive function” OR “cognitive decline” OR “memory” OR “executive function” OR “cognitive reserve” OR “brain function” OR “brain connectivity” OR “functional connectivity” OR “neural plasticity” OR “fMRI” OR “EEG” OR “fNIRS” OR “cortex” OR “prefrontal cortex” OR “anterior cingulate cortex” OR “temporal cortex” OR “neuroplasticity” Limited to: Abstract Publication Date: 2015–2025 |
| Item Type | Publication Year | Author | DOI | Exclusion Reason |
|---|---|---|---|---|
| journalArticle | 2023 | Willroth et al. | 10.1177/09567976221119828 | No PPI * |
| journalArticle | 2024 | Namias et al. | 10.1080/20445911.2024.2381872 | Other population ** |
| journalArticle | 2024 | Ridder et al. | 10.1080/08098131.2024.2430789 | No PPI |
| journalArticle | 2022 | Ismail et al. | 10.1177/00914150211024185 | No PPI |
| journalArticle | 2022 | Ng et al. | 10.1111/appy.12518 | No PPI |
| journalArticle | 2024 | D’elia et al. | 10.1002/dad2.12558 | Other outcomes measured *** |
| bookSection | 2024 | Khalsa et al. | No PPI | |
| journalArticle | 2023 | Nicosia et al. | 10.1177/27536130231202989 | No PPI |
| journalArticle | 2023 | Sandison et al. | 10.3233/JAD-230004 | No PPI |
| journalArticle | 2025 | Šumec et al. | 10.1007/s12671-024-02500-9 | No PPI |
| journalArticle | 2021 | Galvin et al. | 10.3233/JAD-215077 | No PPI |
| journalArticle | 2024 | Yang et al. | 10.1186/s12877-024-05090-2 | Protocol **** |
| journalArticle | 2023 | Wu et al. | 10.1186/s12883-023-03390-5 | No PPI |
| journalArticle | 2022 | Dewitte et al. | 10.1007/s10433-022-00689-z | No PPI |
| journalArticle | 2024 | O’Shea et al. | 10.3233/JAD-231346 | No PPI |
| journalArticle | 2023 | Strikwerda-Brown et al. | 10.1016/j.bpsgos.2022.01.001 | No PPI |
| journalArticle | 2025 | Lewis et al. | 10.1080/13825585.2024.2373846 | No PPI |
| journalArticle | 2024 | Shim et al. | 10.1080/13607863.2024.2364754 | Other outcomes measured |
| journalArticle | 2023 | Pluim et al. | 10.1177/07334648221139479 | No PPI |
| journalArticle | 2015 | Khalsa et al. | 10.3233/JAD-142766 | No PPI |
| journalArticle | 2022 | 10.1016/j.cct.2025.107811 | 10.1093/gerona/glac093 | No PPI |
| journalArticle | 2022 | Jopowicz et al. | 10.3390/brainsci12030345 | No PPI |
| journalArticle | 2022 | Stewart et al. | 10.1177/07334648221095514 | No PPI |
| journalArticle | 2023 | Quintana-Hernández et al. | 10.3233/JAD-220889 | Other outcomes measured |
| journalArticle | 2022 | Mace et al. | 10.1007/s10880-022-09843-2 | No PPI |
| journalArticle | 2023 | Abellaneda-Pérez et al. | 10.1186/s13195-023-01198-6 | No PPI |
| journalArticle | 2022 | Lenze et al. | 10.1001/jama.2022.21680 | No PPI |
| journalArticle | 2016 | Acevedo et al. | 10.1007/s40473-016-0098-x | No PPI |
| journalArticle | 2018 | Dos Santos et al. | 10.3389/fpsyg.2018.00371 | No PPI |
| journalArticle | 2021 | Lutz et al. | 10.1016/j.arr.2021.101495 | No PPI |
| journalArticle | 2022 | Vespa et al. | 10.1007/s40520-021-01907-x | Protocol |
| journalArticle | 2021 | Marchant et al. | 10.1159/000515669 | Other outcomes measured |
| journalArticle | 2017 | Nicholson et al. | 10.1177/0091415017709789 | No PPI |
| journalArticle | 2018 | Ismail et al. | 10.3233/JAD-180075 | No PPI |
| journalArticle | 2020 | Schlosser et al. | 10.1186/s12888-020-02884-7 | No PPI |
| journalArticle | 2021 | Khalsa et al. | 10.3233/JAD-201433 | No PPI |
| journalArticle | 2020 | McDonough et al. | 10.1037/neu0000606 | No PPI |
| journalArticle | 2020 | Chan et al. | 10.1080/14737175.2020.1810571 | No PPI |
| journalArticle | 2018 | Lardone et al. | 10.1155/2018/5340717 | No PPI |
| journalArticle | 2021 | Reynolds et al. | 10.1016/j.amjmed.2020.10.041 | No PPI |
| journalArticle | 2019 | Nakanishi et al. | 10.3233/JAD-190590 | No PPI |
| journalArticle | 2022 | MacAulay et al. | 10.1080/13607863.2021.1998352 | No PPI |
| journalArticle | 2018 | Chételat et al. | 10.1186/s13195-018-0388-5 | No PPI |
| journalArticle | 2018 | Zahodne et al. | 10.1017/S1355617717000935 | No PPI |
| journalArticle | 2021 | Sevinc et al. | 10.3389/fnagi.2021.702796 | Other population |
| journalArticle | 2018 | Marchant et al. | 10.1016/j.trci.2018.10.010 | Protocol |
| bookSection | 2017 | Crescentini et al. | No PPI | |
| journalArticle | 2016 | Trivedi et al. | 10.1177/0891988715598235 | No PPI |
| journalArticle | 2023 | Kawada et al. | 10.1007/s40520-023-02377-z | No PPI |
| journalArticle | 2023 | Schlosser et al. | 10.1371/journal.pone.0295175 | Other outcomes measured |
| journalArticle | 2022 | Wagner et al. | 10.1093/geroni/igac019 | No PPI |
| journalArticle | 2017 | Cook Maher et al. | 10.1371/journal.pone.0186413 | No PPI |
| journalArticle | 2015 | Yu et al. | 10.1161/STROKEAHA.114.008010 | No PPI |
| journalArticle | 2025 | Friedman et al. | 10.1093/geronb/gbaf021 | No PPI |
| journalArticle | 2017 | Morse et al. | 10.1016/j.archger.2017.10.013 | Other population |
| journalArticle | 2024 | Choukas et al. | 10.1016/j.archger.2023.105290 | No PPI |
| journalArticle | 2025 | Huang et al. | 10.1016/j.gerinurse.2025.01.011 | No PPI |
| journalArticle | 2023 | Liu et al. | 10.1016/j.jagp.2022.10.006 | No PPI |
| journalArticle | 2025 | Chao et al. | 10.1016/j.cct.2025.107811 | Protocol |
| journalArticle | 2023 | Kim et al. | 10.1016/j.pmedr.2023.102165 | No PPI |
| journalArticle | 2021 | Yang et al. | 10.1016/j.pmedr.2021.101490 | Other population |
| journalArticle | 2018 | Poisnel et al. | 10.1016/j.trci.2018.10.011 | Protocol |
| journalArticle | 2024 | Kaliman et al. | 10.1016/j.bpsgos.2024.100398 | Other population |
| bookChapter | 2019 | Deepak | 10.1016/bs.pbr.2018.10.030 | No PPI |
| S/N | Reference | Design | Population | Control Group | PPI | Recruited from |
|---|---|---|---|---|---|---|
| 1 | Whitfield et al., 2022 (Europe) [47] | Randomized controlled trial (RCT) | 147 older adults with SCD (Mean age: 72.7 years, SD: 6.9) from memory clinics:
| Active control group |
| Memory clinics |
| 2 | Smart and Segalowitz, 2017 (Canada) [48] | Randomized controlled trial (RCT) |
| Active control group |
| Community |
| 3 | Doshi et al., 2021 (Singapore) [49] | Randomized controlled trial (RCT) |
| Both active and passive control groups |
| Both a hospital memory clinic and the community |
| 4 | Ng et al., 2020 (Singapore) [50] | Randomized controlled trial (RCT) |
| Active control group |
| Community-based research center |
| 5 | Ng et al., 2022 (Singapore) [51] | Randomized controlled trial (RCT) |
| Active control group |
| Community-based research center |
| 6 | Fam et al., 2020 (Singapore) [52] | Randomized controlled trial (RCT) |
| Active control group |
| Community |
| 7 | Klainin-Yobas et al., 2019 (Singapore) [53] | Randomized controlled trial (RCT) |
| Active control group |
| Community |
| 8 | Yu et al., 2021 (Singapore) [54] | Randomized controlled trial (RCT) |
| Active control group |
| Community |
| 9 | Wong et al., 2017 (Australia) [55] | Longitudinal, mixed-methods observational study with a pre-/post-intervention design |
| No (pre-/post intervention design) |
| Community |
| 10 | Larouche et al., 2019 (Germany) [56] | Single-blind, preliminary randomized-controlled trial (RCT) |
| Active control group |
| Community |
| 11 | Quintana-Hernández et al., 2016 (Spain) [57] | Randomized clinical trial (RCT) |
| Pharmacological treatment-only control group |
| Community |
| 12 | Lim et al., 2018 (Singapore) [58] | Randomized Controlled Trial (RCT) |
| Active control group |
| Community |
| S/N | Reference | Assessment Time | Outcome Measures | Results |
|---|---|---|---|---|
| 1 | Whitfield et al., 2022 (Europe) [47] |
|
|
|
| 2 | Smart and Segalowitz, 2017 (Canada) [48] |
|
| Mindfulness Training:
|
| 3 | Doshi et al., 2021 (Singapore) [49] |
|
|
|
| 4 | Ng et al., 2020 (Singapore) [50] |
|
|
|
| 5 | Ng et al., 2022 (Singapore) [51] |
|
|
|
| 6 | Fam et al., 2020 (Singapore) [52] |
|
|
|
| 7 | Klainin-Yobas et al., 2019 (Singapore) [53] |
|
|
|
| 8 | Yu et al., 2021 (Singapore) [54] |
|
| Mindfulness Awareness Program (MAP) led to:
|
| 9 | Wong et al., 2017 (Australia) [55] |
|
|
|
| 10 | Larouche et al., 2019 (Germany) [56] |
|
|
|
| 11 | Quintana-Hernández et al., 2016 (Spain) [57] |
|
|
|
| 12 | Lim et al., 2018 (Singapore) [58] |
|
|
|
| Intervention | Cognitive Functions | Brain Functioning |
|---|---|---|
| MAP (Mindful Awareness Practice) |
|
|
| MBT (Mindfulness-Based Training) |
| Not reported. |
| MT (Mindfulness Training) |
|
|
| MTP (Mindfulness Training Program) |
| Not reported. |
| MBI (Mindfulness-Based Intervention) |
| Not reported. |
| MBAS (Mindfulness-Based Alzheimer’s Stimulation) |
| Not reported. |
| CMBAS (Caring Mindfulness-Based Approach for Seniors) |
| Not reported. |
| A. Quantitative Randomized Controlled Trials (n = 11) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | 2.1. Is Randomization Appropriately Performed? | 2.2. Are the Groups Comparable at the Baseline? | 2.3. Are There Complete Outcome Data? | 2.4. Are Outcome Assessors Blinded to the Intervention Provided? | 2.5 Did the Participants Adhere to the Assigned Intervention? | ||||||
| Yu et al. (2021) [54] | Yes | Yes | No | No | Yes | ||||||
| Klainin-Yobas et al. (2019) [53] | Yes | Yes | No | No | Can’t tell | ||||||
| Larouche et al. (2019) [56] | Yes | Yes | No | Yes | Yes | ||||||
| Doshi et al. (2021) [49] | Yes | Yes | No | Yes | Yes | ||||||
| Ng et al. (2022) [51] | Yes | Yes | No | Yes | Yes | ||||||
| Fam et al. (2020) [52] | Yes | Yes | No | Can’t tell | Yes | ||||||
| Quintana-Hernández et al. (2015) [57] | Yes | Yes | No | No | Yes | ||||||
| Whitfield et al. (2022) [47] | Yes | Yes | Yes | Yes | Yes | ||||||
| Smart and Segalowitz (2017) [48] | Yes | Yes | No | Yes | Yes | ||||||
| Ng et al. (2020) [50] | Yes | Yes | No | Yes | Can’t tell | ||||||
| Lim et al. (2018) [58] | Yes | Can’t tell | No | Can’t tell | Can’t tell | ||||||
| B. Quantitative Descriptive and Mixed Methods Study (n = 1) | |||||||||||
| Study | 4.1 | 4.2 | 4.3 | 4.4 | 4.5 | 5.1 | 5.2 | 5.3 | 5.4 | 5.5 | |
| Wong et al. (2017) [55] | Yes | No | Yes | No | Yes | Yes | Can’t tell | Can’t tell | Can’t tell | Yes | |
| Note. The criteria used are listed below due to space limitations in the table. | |||||||||||
| 4.1. Is the sampling strategy relevant to address the research question? 4.2. Is the sample representative of the target population? 4.3. Are the measurements appropriate? 4.4. Is the risk of nonresponse bias low? 4.5. Is the statistical analysis appropriate to answer the research question? 5.1. Is there an adequate rationale for using a mixed methods design to address the research question? 5.2. Are the different components of the study effectively integrated to answer the research question? 5.3. Are the outputs of the integration of qualitative and quantitative components adequately interpreted? 5.4. Are divergences and inconsistencies between quantitative and qualitative results adequately addressed? 5.5. Do the different components of the study adhere to the quality criteria of each tradition of the methods involved? | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasileiou, D.; Moraitou, D.; Diamantaras, K.; Papaliagkas, V.; Pezirkianidis, C.; Tsolaki, M. Positive Psychology Interventions in Early-Stage Cognitive Decline Related to Dementia: A Systematic Review of Cognitive and Brain Functioning Outcomes of Mindfulness Interventions. Brain Sci. 2025, 15, 580. https://doi.org/10.3390/brainsci15060580
Vasileiou D, Moraitou D, Diamantaras K, Papaliagkas V, Pezirkianidis C, Tsolaki M. Positive Psychology Interventions in Early-Stage Cognitive Decline Related to Dementia: A Systematic Review of Cognitive and Brain Functioning Outcomes of Mindfulness Interventions. Brain Sciences. 2025; 15(6):580. https://doi.org/10.3390/brainsci15060580
Chicago/Turabian StyleVasileiou, Dimitra, Despina Moraitou, Konstantinos Diamantaras, Vasileios Papaliagkas, Christos Pezirkianidis, and Magda Tsolaki. 2025. "Positive Psychology Interventions in Early-Stage Cognitive Decline Related to Dementia: A Systematic Review of Cognitive and Brain Functioning Outcomes of Mindfulness Interventions" Brain Sciences 15, no. 6: 580. https://doi.org/10.3390/brainsci15060580
APA StyleVasileiou, D., Moraitou, D., Diamantaras, K., Papaliagkas, V., Pezirkianidis, C., & Tsolaki, M. (2025). Positive Psychology Interventions in Early-Stage Cognitive Decline Related to Dementia: A Systematic Review of Cognitive and Brain Functioning Outcomes of Mindfulness Interventions. Brain Sciences, 15(6), 580. https://doi.org/10.3390/brainsci15060580










