Long-Term Effects of COVID-19 Pandemic on Migraine in Adolescents. A Retrospective Analysis of the Population Attending the Headache Center in Different Phases of the Pandemic
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection and Classification of the Patients
2.2. Instruments
2.2.1. Anxiety
2.2.2. Depression
2.3. Statistical Analysis
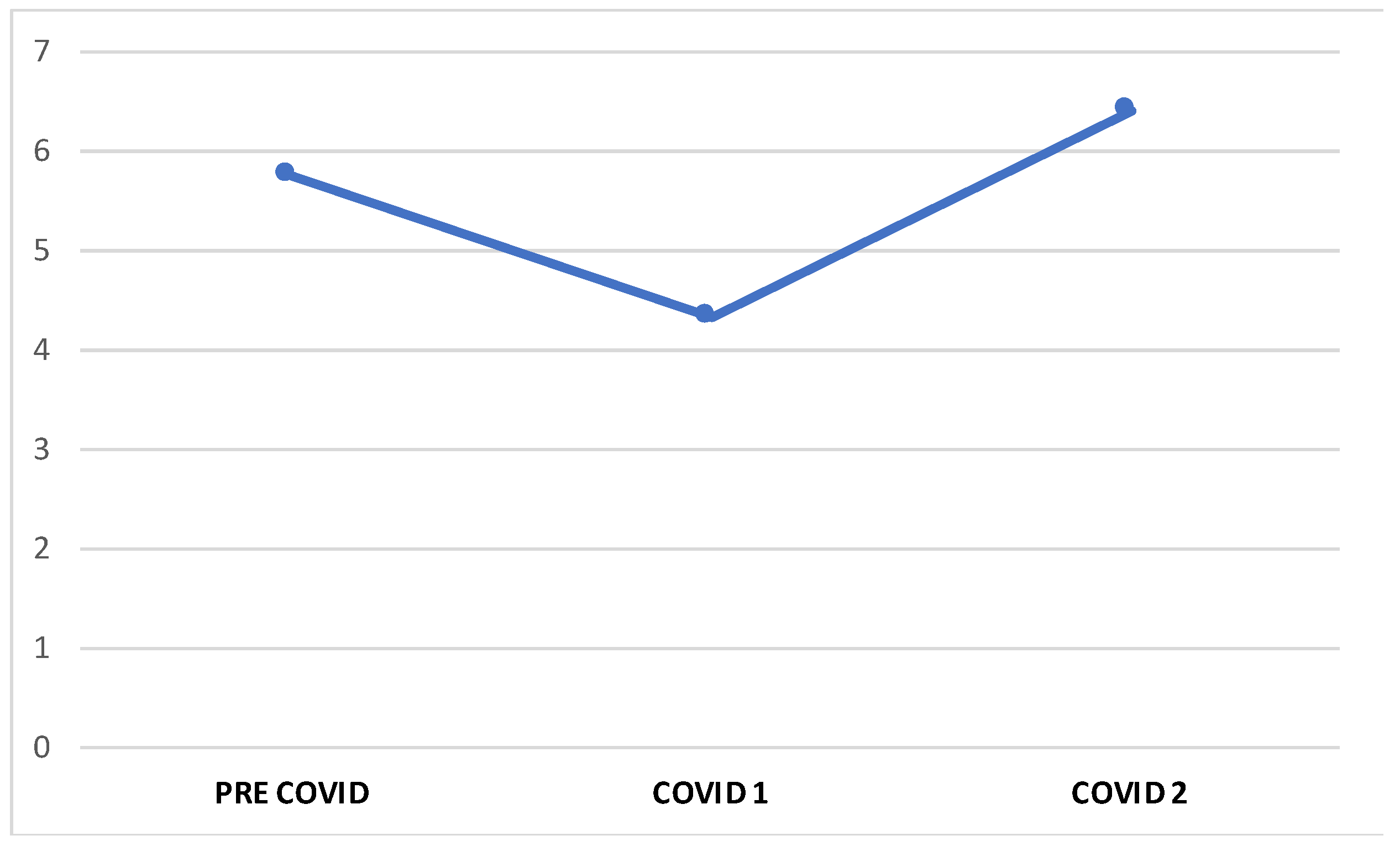
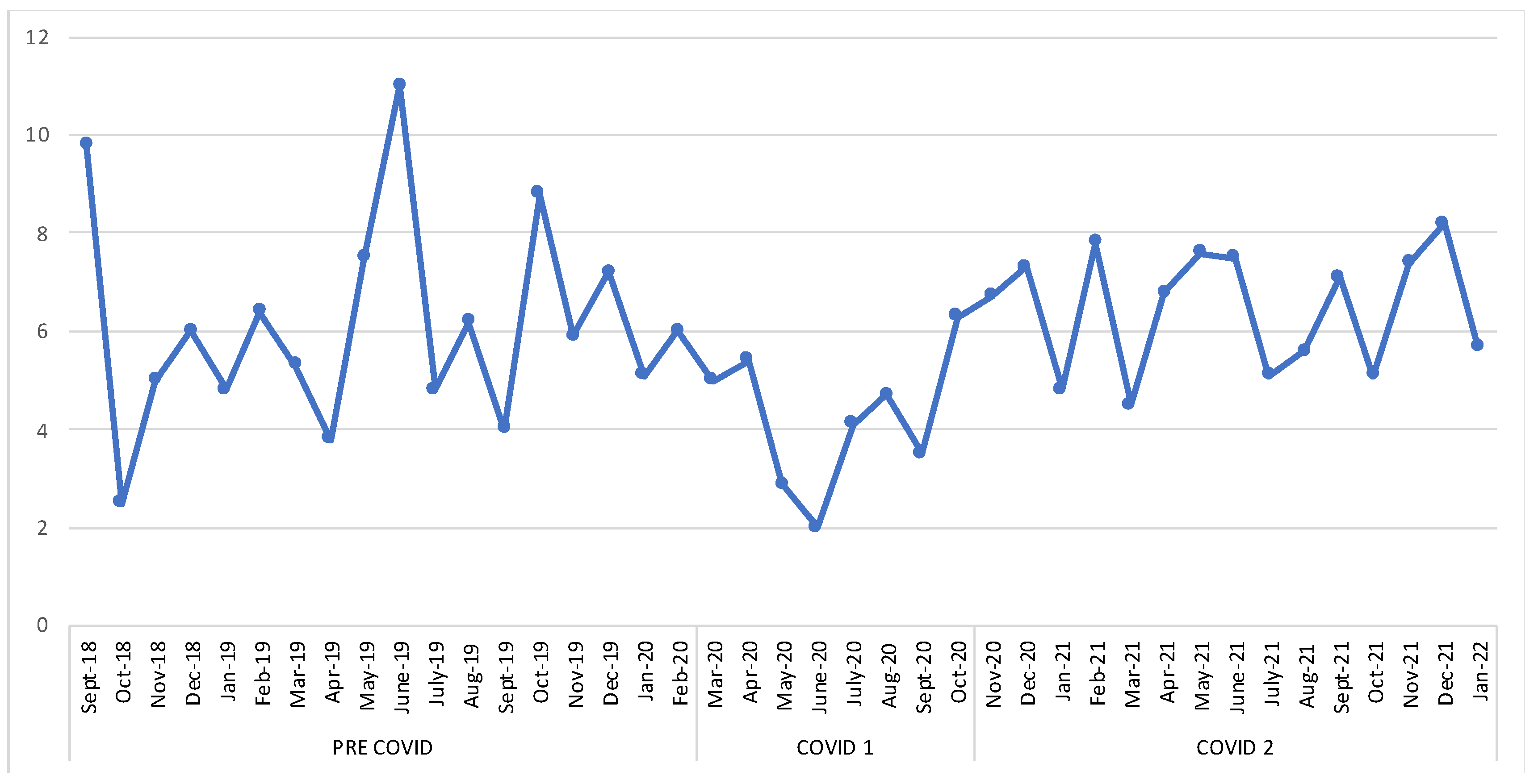
3. Results
3.1. Migraine Features
3.2. Psychological Findings
3.2.1. Anxiety
3.2.2. Depression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oosterhoff, B.; Palmer, C.A.; Wilson, J.; Shook, N. Adolescents’ Motivations to Engage in Social Distancing During the COVID-19 Pandemic: Associations With Mental and Social Health. J. Adolesc. Health 2020, 67, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Zhou, S.J.; Guo, Z.C.; Zhang, L.G.; Min, H.J.; Li, X.M.; Chen, J.X. The Effect of Social Support on Mental Health in Chinese Adolescents During the Outbreak of COVID-19. J. Adolesc. Health 2020, 67, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Bäuerle, A.; Teufel, M.; Musche, V.; Weismüller, B.; Kohler, H.; Hetkamp, M.; Dörrie, N.; Schweda, A.; Skoda, E.M. Increased generalized anxiety, depression and distress during the COVID-19 pandemic: A cross-sectional study in Germany. J. Public Health 2020, 42, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Shao, X.; Wang, Y.; Huang, Y.; Miao, J.; Yang, X.; Zhu, G. An investigation of mental health status of children and adolescents in china during the outbreak of COVID-19. J. Affect. Disord. 2020, 275, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Farooq, S.; Tunmore, J.; Wajid Ali, M.; Ayub, M. Suicide, self-harm and suicidal ideation during COVID-19: A systematic review. Psychiatry Res. 2021, 306, 114228. [Google Scholar] [CrossRef]
- McElroy, E.; Patalay, P.; Moltrecht, B.; Shevlin, M.; Shum, A.; Creswell, C.; Waite, P. Demographic and health factors associated with pandemic anxiety in the context of COVID-19. Br. J. Health Psychol. 2020, 25, 934–944. [Google Scholar] [CrossRef]
- Ford, T.; Cross, L. Debate: Is there a true global children and young people’s mental health crisis, fact or fiction? Child Adolesc. Ment. Health 2021, 26, 272–273. [Google Scholar] [CrossRef]
- Heras, E.; Garibaldi, P.; Boix, M.; Valero, O.; Castillo, J.; Curbelo, Y.; Gonzalez, E.; Mendoza, O.; Anglada, M.; Miralles, J.C.; et al. COVID-19 mortality risk factors in older people in a long-term care center. Eur. Geriatr. Med. 2021, 12, 601–607. [Google Scholar] [CrossRef]
- Lahav, E.; Rosenboim, M.; Shahrabani, S.; Song, Y. Optimism and precautionary measures during the COVID-19 outbreak in China. Am. J. Health Behav. 2021, 45, 978–992. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Moore, E.; Robertson, C.; McMenamin, J.; Katikireddi, S.V.; Simpson, C.R.; Shi, T.; Agrawal, U.; McCowan, C.; Stock, S. Predicted COVID-19 positive cases, hospitalisations, and deaths associated with the Delta variant of concern, June-July, 2021. Lancet Digit. Health 2021, 3, e539–e541. [Google Scholar] [CrossRef] [PubMed]
- Godeau, D.; Petit, A.; Richard, I.; Roquelaure, Y.; Descatha, A. Return-to-work, disabilities and occupational health in the age of COVID-19. Scand J. Work. Env. Health 2021, 47, 408–409. [Google Scholar] [CrossRef]
- Bera, L.; Souchon, M.; Ladsous, A.; Colin, V.; Lopez-Castroman, J. Emotional and Behavioral Impact of the COVID-19 Epidemic in Adolescents. Curr. Psychiatry Rep. 2022, 24, 37–46. [Google Scholar] [CrossRef]
- Kaczynski, K.J.; Chang, C.Y.H.; Chimoff, J.; Koike, C.; Berde, C.B.; Logan, D.E.; Nelson, S.; Kossowsky, J. Initial Adjustment to the COVID-19 Pandemic and the Associated Shutdown in Children and Adolescents With Chronic Pain and Their Families. Front. Pain Res. 2021, 2, 713430. [Google Scholar] [CrossRef]
- Amiri, P.; Kazeminasab, S.; Nejadghaderi, S.A.; Mohammadinasab, R.; Pourfathi, H.; Araj-Khodaei, M.; Sullman, M.J.M.; Kolahi, A.A.; Safiri, S. Migraine: A Review on Its History, Global Epidemiology, Risk Factors, and Comorbidities. Front. Neurol. 2022, 12, 800605. [Google Scholar] [CrossRef]
- Lipton, R.B.; Bigal, M.E. Migraine: Epidemiology, impact, and risk factors for progression. Headache 2005, 45 (Suppl. 1), S3–S13. [Google Scholar] [CrossRef]
- Russo, A.; Bruno, A.; Trojsi, F.; Tessitore, A.; Tedeschi, G. Lifestyle Factors and Migraine in Childhood. Curr. Pain Headache Rep. 2016, 20, 9. [Google Scholar] [CrossRef]
- Tarantino, S.; Papetti, L.; De Ranieri, C.; Boldrini, F.; Rocco, A.M.; D’Ambrosio, M.; Valeriano, V.; Battan, B.; Vigevano, F.; Valeriani, M. Maternal Alexithymia and Attachment Style: Which Relationship with Their Children’s Headache Features and Psychological Profile? Front. Neurol. 2018, 8, 751. [Google Scholar] [CrossRef]
- Tarantino, S.; De Ranieri, C.; Dionisi, C.; Gagliardi, V.; Paniccia, M.F.; Capuano, A.; Frusciante, R.; Balestri, M.; Vigevano, F.; Valeriani, M. Role of the Attachment Style in Determining the Association Between Headache Features and Psychological Symptoms in Migraine Children and Adolescents. An Analytical Observational Case-Control Study. Headache 2017, 57, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Papetti, L.; Alaimo Di Loro, P.; Tarantino, S.; Grazzi, L.; Guidetti, V.; Parisi, P.; Raieli, V.; Sciruicchio, V.; Termine, C.; Toldo, I.; et al. I stay at home with headache. A survey to investigate how the lockdown for COVID-19 impacted on headache in Italian children. Cephalalgia 2020, 40, 1459–1473. [Google Scholar] [CrossRef] [PubMed]
- Dallavalle, G.; Pezzotti, E.; Provenzi, L.; Toni, F.; Carpani, A.; Borgatti, R. Migraine Symptoms Improvement During the COVID-19 Lockdown in a Cohort of Children and Adolescents. Front. Neurol. 2020, 11, 579047. [Google Scholar] [CrossRef] [PubMed]
- Parodi, I.C.; Poeta, M.G.; Assini, A.; Schirinzi, E.; Del Sette, P. Impact of quarantine due to COVID infection on migraine: A survey in Genova, Italy. Neurol Sci. 2020, 41, 2025–2027. [Google Scholar] [CrossRef]
- Aleyeidi, N.A.; Alqahtani, R.S.; Alotaibi, H.F.; Alotaibi, A.H.; Alotaibi, K.M.; Alnofiey, R.M. Exploring the Impact of the COVID-19 Quarantine on the Severity of Headache, Migraine, and Stress in Saudi Arabia. J. Pain Res. 2021, 14, 3827–3835. [Google Scholar] [CrossRef] [PubMed]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version); Cephalalgia: Pheonix, AZ, USA, 2013; Volume 33, pp. 629–808. [Google Scholar]
- Papetti, L.; Ursitti, F.; Moavero, R.; Ferilli, M.A.N.; Sforza, G.; Tarantino, S.; Vigevano, F.; Valeriani, M. Prophylactic Treatment of Pediatric Migraine: Is There Anything New in the Last Decade? Front. Neurol. 2019, 10, 771. [Google Scholar] [CrossRef] [PubMed]
- Oskoui, M.; Pringsheim, T.; Billinghurst, L.; Potrebic, S.; Gersz, E.M.; Gloss, D.; Holler-Managan, Y.; Leininger, E.; Licking, N.; Mack, K.; et al. Practice guideline update summary: Pharmacologic treatment for pediatric migraine prevention: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology 2019, 93, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.; Löwe, B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef]
- Manea, L.; Gilbody, S.; McMillan, D. Optimal cut-off score for diagnosing depression with the Patient Health Questionnaire (PHQ-9): A meta-analysis. CMAJ 2012, 184, E191–E196. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Suzuki, K.; Takeshima, T.; Igarashi, H.; Imai, N.; Danno, D.; Yamamoto, T.; Nagata, E.; Haruyama, Y.; Mitsufuji, T.; Suzuki, S.; et al. Impact of the COVID-19 pandemic on migraine in Japan: A multicentre cross-sectional study. J. Headache Pain 2021, 22, 53. [Google Scholar] [CrossRef]
- Gentile, E.; Delussi, M.; Abagnale, C.; Caponnetto, V.; De Cesaris, F.; Frattale, I.; Guaschino, E.; Marcinnò, A.; Ornello, R.; Pistoia, F.; et al. Migraine during COVID-19: Data from Second Wave Pandemic in an Italian Cohort. Brain Sci. 2021, 11, 482. [Google Scholar] [CrossRef]
- Imran, N.; Aamer, I.; Sharif, M.I.; Bodla, Z.H.; Naveed, S. Psychological burden of quarantine in children and adolescents: A rapid systematic review and proposed solutions. Pak. J. Med. Sci. 2020, 36, 1106–1116. [Google Scholar] [CrossRef]
- DiSabella, M.; Pierce, E.; McCracken, E.; Ratnaseelan, A.; Vilardo, L.; Borner, K.; Langdon, R.; Fletcher, A.A. Pediatric Headache Experience During the COVID-19 Pandemic. J. Child Neurol. 2022, 37, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.K.; Webster, R.K.; Smith, L.E.; Woodland, L.; Wessely, S.; Greenberg, N.; Rubin, G.J. The psychological impact of quarantine and how to reduce it: Rapid review of the evidence. Lancet 2020, 395, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Delussi, M.; Gentile, E.; Coppola, G.; Prudenzano, A.M.P.; Rainero, I.; Sances, G.; Abagnale, C.; Caponnetto, V.; De Cesaris, F.; Frattale, I.; et al. Investigating the Effects of COVID-19 Quarantine in Migraine: An Observational Cross-Sectional Study From the Italian National Headache Registry (RICe). Front. Neurol. 2020, 11, 597881. [Google Scholar] [CrossRef] [PubMed]


| Total Patients N 418 | High Frequency N 206 (49%) | Low Frequency N 211 (51%) | Severe Intensity N 166 (40%) | Mild Intensity N 252 (60%) | Ongoing Prophylaxis Treatment N 169 (40%) | No Prophylaxis Treatment N 249 (60%) | |
|---|---|---|---|---|---|---|---|
| Demographic | |||||||
| features | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| Age in years | 14.2 (1.71) | 14.4 (1.74) | 13.9 (1.65) | 14.3 (1.75) | 14.1 (1.68) | 14.4 (1.75) | 14.1 (1.68) |
| Sex | Number (%) | Number (%) | Number (%) | Number (%) | Number (%) | Number (%) | Number (%) |
| Females | 308 (74) | 167 (81) | 141 (67) | 131 (43) | 177 (57) | 113 (37) | 195 (63) |
| Males | 110 (26) | 39 (19) | 71 (33) | 36 (33) | 74 (67) | 33 (30) | 77 (70) |
| Periods | Number (%) | Number (%) | Number (%) | Number (%) | Number (%) | Number (%) | Number (%) |
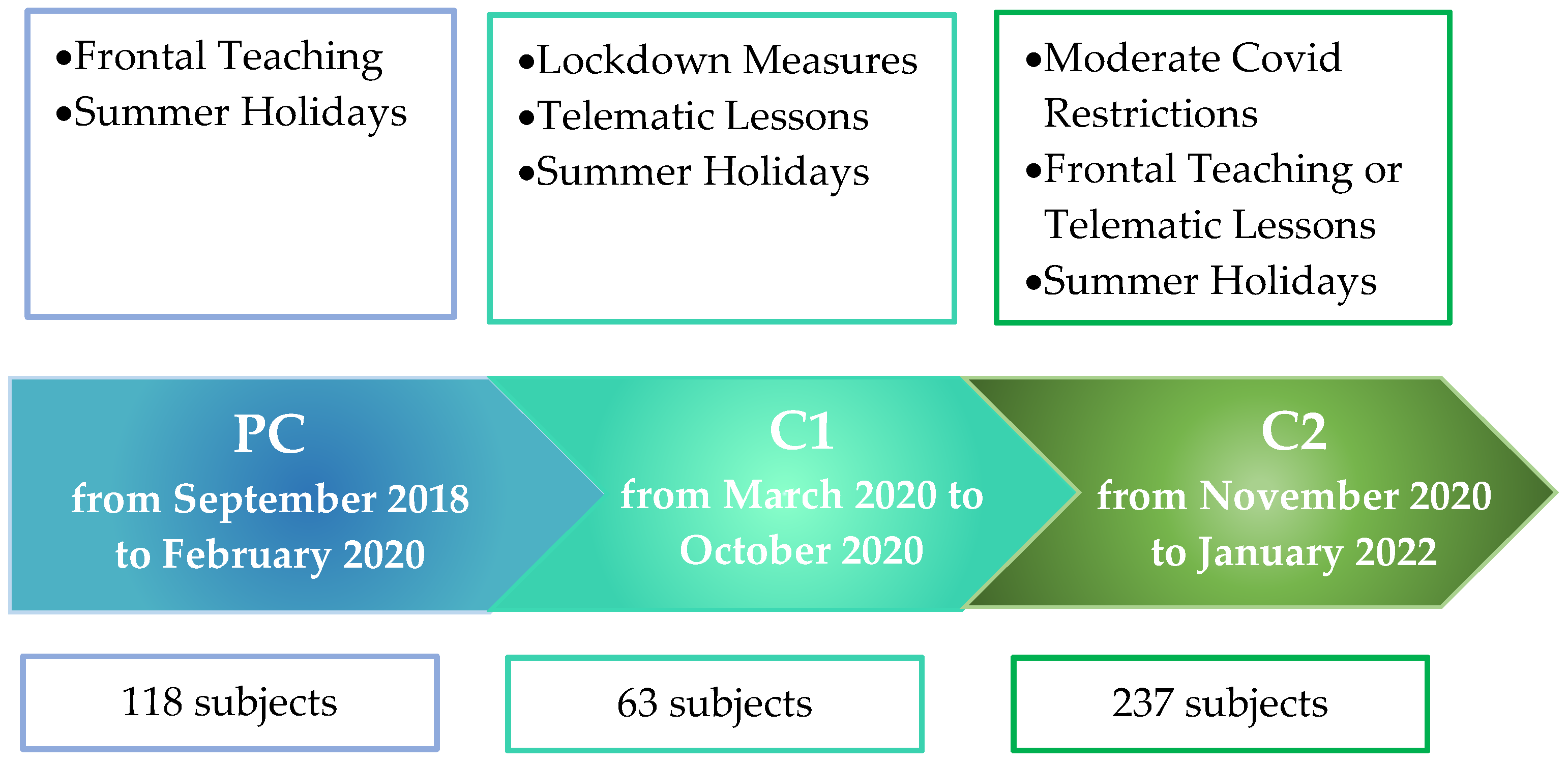
| Pre COVID | 118 | 53 (45) | 65 (55) | 46 (39) | 72 (61) | 31 (26) | 87 (74) |
| School period | 98 | 45 (46) | 53 (54) | 41 (42) | 57 (58) | 24 (24) | 74 (76) |
| Summer period | 20 | 8 (40) | 12 (60) | 5 (25) | 15 (75) | 7 (35) | 13 (65) |
| COVID | 300 | 153 (51) | 147 (49) | 121 (40) | 179 (60) | 115 (38) | 185 (62) |
| School period | 220 | 130 (56) | 130 (56) | 99 (42) | 134 (58) | 97 (42) | 136 (58) |
| Summer period | 80 | 23 (34) | 44 (66) | 22 (33) | 45 (67) | 18 (27) | 49 (73) |
| COVID 1 | 63 | 20 (32) | 43 (68) | 20 (32) | 43 (68) | 16 (25) | 47 (65) |
| School period | 36 | 11 (31) | 25 (69) | 13 (36) | 23 (64) | 13 (36) | 23 (64) |
| Summer period | 27 | 9 (33) | 18 (67) | 7 (26) | 20 (74) | 3 (11) | 24 (89) |
| COVID 2 | 237 | 133 (56) | 104 (44) | 100 (42) | 136 (58) | 99 (42) | 138 (58) |
| School period | 197 | 119 (60) | 78 (49) | 86 (44) | 111 (56) | 84 (43) | 113 (57) |
| Summer period | 40 | 14 (35) | 26 (65) | 15 (37) | 25 (63) | 15 (37) | 25 (63) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Checchi, M.P.; Tarantino, S.; Ursitti, F.; Monte, G.; Moavero, R.; Sforza, G.; Ferilli, M.A.N.; Grimaldi Capitello, T.; Vigevano, F.; Valeriani, M.; et al. Long-Term Effects of COVID-19 Pandemic on Migraine in Adolescents. A Retrospective Analysis of the Population Attending the Headache Center in Different Phases of the Pandemic. Brain Sci. 2023, 13, 273. https://doi.org/10.3390/brainsci13020273
Checchi MP, Tarantino S, Ursitti F, Monte G, Moavero R, Sforza G, Ferilli MAN, Grimaldi Capitello T, Vigevano F, Valeriani M, et al. Long-Term Effects of COVID-19 Pandemic on Migraine in Adolescents. A Retrospective Analysis of the Population Attending the Headache Center in Different Phases of the Pandemic. Brain Sciences. 2023; 13(2):273. https://doi.org/10.3390/brainsci13020273
Chicago/Turabian StyleChecchi, Martina Proietti, Samuela Tarantino, Fabiana Ursitti, Gabriele Monte, Romina Moavero, Giorgia Sforza, Michela Ada Noris Ferilli, Teresa Grimaldi Capitello, Federico Vigevano, Massimiliano Valeriani, and et al. 2023. "Long-Term Effects of COVID-19 Pandemic on Migraine in Adolescents. A Retrospective Analysis of the Population Attending the Headache Center in Different Phases of the Pandemic" Brain Sciences 13, no. 2: 273. https://doi.org/10.3390/brainsci13020273
APA StyleChecchi, M. P., Tarantino, S., Ursitti, F., Monte, G., Moavero, R., Sforza, G., Ferilli, M. A. N., Grimaldi Capitello, T., Vigevano, F., Valeriani, M., & Papetti, L. (2023). Long-Term Effects of COVID-19 Pandemic on Migraine in Adolescents. A Retrospective Analysis of the Population Attending the Headache Center in Different Phases of the Pandemic. Brain Sciences, 13(2), 273. https://doi.org/10.3390/brainsci13020273







