Abstract
Immune checkpoint inhibitors (ICIs) are being used in patients with various advanced malignancies, and patient outcomes have improved considerably. Although ICIs can effectively treat tumors, 30–60% of patients experience immune-related adverse events (irAEs). Autoimmune encephalitis (AE) is a rare irAE that has become a novel topic in neuroimmunology and has received increasing attention in recent years. Herein, we report a rare case of GAD65-antibody–associated AE after metastatic small cell lung cancer treatment with pembrolizumab. The patient received IVIg therapy for AE and continuous pembrolizumab therapy without suspension of tumor treatment. At 1 year follow-up, both the patient’s AE symptoms and tumors were stable. We consider that the treatment of ICI-associated AE should be more individualized with prudent decision-making and should balance the tumor progression and AE treatment. In addition, we have also comprehensively reviewed the literature of ICI-associated AE, and summarized the clinical features, treatment, and prognosis of AE caused by ICI, thus broadening our understanding of the neurological complications caused by ICI.
1. Introduction
Immune checkpoint inhibitors (ICIs) are a class of monoclonal antibodies that target regulatory immune checkpoint molecules that inhibit T cell activation. ICIs can enhance T cell-mediated anti-tumor immunity and promote immune-mediated tumor cell clearance by blocking co-inhibitory signaling pathways [1,2,3]. Their antitumor effects have been recognized in clinical trials and have been approved by the FDA for the treatment of malignant tumors, such as melanoma, non-small cell lung cancer, colorectal cancer, and hepatocellular carcinoma [4].
ICIs can be divided into monoclonal antibodies against CTLA-4 and monoclonal antibodies against PD-1/PD-L1 depending on the pathways they act on [5]. Monoclonal antibodies against PD-1/PD-L1 are the favorable method for modern immunotherapy of solid tumors [6,7,8]. ICIs can enhance the anti-tumor immune response by removing the inhibitory effect of PD-1 or PD-L1 immune checkpoints on the activation and proliferation of T cells, and reduce the number and/or inhibitory activity of Treg cells [9]. By doing so, the killing function of T cells against the tumor is restored, leading to the inhibition of tumor development [1]. However, when ICIs induce activation of the immune system, they also nonspecifically lead to the destruction of the immune homeostasis of non-tumor tissues, resulting in severe immune and inflammatory reactions. These include clinical immune-related adverse events (irAEs) [10,11]. At present, the specific mechanism of irAE generation is not clear, and it is generally believed to be related to the disorder of immune homeostasis caused by ICI treatment [12,13]. There are large amounts of ICs on the surface of non-tumor cells; ICI combined with them can lead to the activation of complement in the body and increase the level of inflammation, thereby disrupting immune homeostasis [14].
Common irAEs include skin rash, itching, colitis, hepatitis, and all types of endocrine diseases [15]. Nervous system irAEs are relatively rare, which include central nervous system (CNS) irAEs and peripheral nervous system (PNS) irAEs. PNS irAEs mainly include myasthenia gravis, Guillain–Barre syndrome, and peripheral sensory motor neuropathy [16]. CNS irAEs are much rarer than PNS irAEs. ICI-associated autoimmune encephalitis (AE) is an uncommon complication that has rarely been reported [17,18], and thus not completely understood. Herein, we report the first case of pembrolizumab-associated GAD65 antibody AE with a favorable short-term prognosis after treatment. Furthermore, in order to better understand the CNS complications caused by ICI, we comprehensively reviewed ICI-associated AE.
2. Materials and Methods
References for the review were identified by searching English literature in the PubMed database published from 2016 to 2022, using the search terms (alone or in logical combinations): “immune checkpoint inhibitor”, “autoimmune encephalitis”, “immune-related adverse events”, “anti PD1”, “anti PDL1”, and “anti CTLA4.” The inclusion criteria are as follows: patients with (1) encephalitis symptoms identical to classical AE, regardless of whether neuronal autoantibodies were detected; (2) any AE symptoms associated with classic tumor neuronal autoantibodies; or (3) symptoms associated with autoantibodies against synaptic receptors or other neuron cell surface proteins after ICI treatment. Patients were excluded if neuronal autoantibodies were detected before ICI treatment [19].
3. Case Report
A 51-year-old man was admitted with an unsteady gait and slurred speech, which persisted for 20 days. He had undergone pulmonary nodule resection in April 2019. Frozen section pathology revealed small-cell carcinoma in the anterior segment of the right upper lobe. He received multiple doses of VP-16, carboplatin, chemotherapy, and radiotherapy from May 2019 to November 2020. In January 2021, positron emission tomography-computed tomography (PET-CT) revealed two round and low-density lesions with increased fluorodeoxyglucose (FDG) metabolism on the right liver lobe, enlarged lymph nodes with FDG hypermetabolism in the retroperitoneal area and the space between the right kidney and pancreas. The size of lesions on the right lobe of the liver were 1.3 × 1.1 cm and 1.34 × 1.44 cm. Considering the liver and lymph node metastases, on the 3rd and 25th of February 2021, the patient received pembrolizumab therapy. On 15 April 2021, he developed slurred speech and an unsteady gait. The patient was again treated with pembrolizumab on the 29 of April 2021, and his symptoms progressed significantly and his modified Rankin Scale (mRS) score deteriorated to 4. Neurological examination revealed dysmetria on the finger-to-nose test, positive Romberg’s sign, ataxic gait, and nystagmus. The cranial nerves were intact and there was no nuchal rigidity. The patient had normal muscle strength, muscle tone, and tendon reflexes. Cerebrospinal fluid (CSF) analysis showed normal opening pressure (120 mmH2O), nucleated cell count 2 × 106/L, protein 514.45 mg/L, chloride 128 mmol/L, and sugar 2.96 mmol/L. Cranial magnetic resonance imaging (MRI) showed evidence of mild cerebellar atrophy (Figure 1A,B). 18F-FDG-PET-CT indicated decreased metabolism in both cerebellar hemispheres.
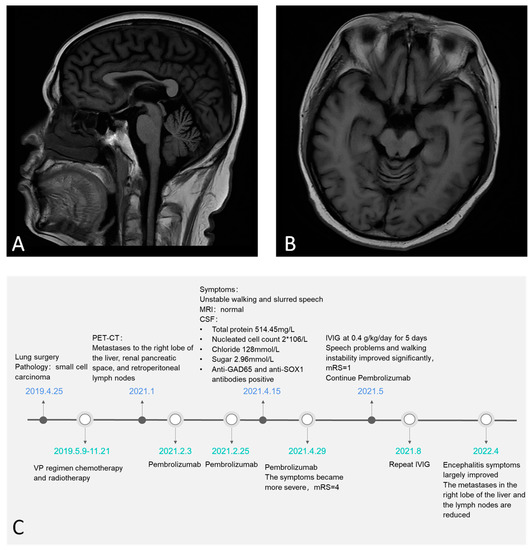
Figure 1.
Mild cerebella atrophy evidence on sagittal and axial brain MRI (A,B). The timeline of the patient (C).
The autoimmune encephalitis antibody panel revealed that anti-GAD65 and anti-SOX1 antibodies were present in both serum and CSF, while other antibodies were absent. The titers of GAD65 and SOX1 antibodies in the serum were 1:30 and 1:10, respectively; and while they were 1:100 and 1:10, respectively, in the CSF. Therefore, the patient was diagnosed with pembrolizumab-associated AE. The irAE grade was 3 according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0 (CTCAE-5.0) [20]. The patient was given intravenous immunoglobulin (IVIg) at 0.4 g/kg/day for 5 days, and his speech and gait improved significantly. His mRS improved to 1. Considering that excessive immunosuppressive therapy might lead to tumor recurrence, this patient did not receive glucocorticoid or immunosuppressive therapy. Furthermore, pembrolizumab treatment was continued for the tumor. He received IVIg therapy 3 months later to prevent the recurrence of AE symptoms. At the 1-year follow-up, his AE symptoms had largely recovered, and the tumor was stable. The titer of GAD65 antibodies in serum decreased to 1:1. CT showed that the size of the metastases in the right lobe of the liver were 0.3 × 0.51 cm and 0.67 × 0.88 cm. The retroperitoneal lymph nodes and lymph nodes in the right renal space between the pancreas were also reduced (Figure 1C, timeline for this patient).
4. Discussion
4.1. Pembrolizumab-Associated GAD65 Antibody AE, a Very Rare Complication of ICI
The incidence of irAEs in the nervous system after ICI treatment is 2% to 6% [21,22], of which the incidence of encephalitis is 0.05% [16]. As far as we know, ICI-associated AE is currently limited to a single case report or a few small case series [18,23]. Cases of ICI-associated AE with autoantibodies are very rare. This is the first case of pembrolizumab-associated GAD65 antibody-positive AE. We noticed that this patient experienced a reduction in tumor size and progression of ataxia with GAD65 and SOX1 autoantibodies after pembrolizumab treatment, which indicated a diagnosis of pembrolizumab-associated AE [19]. In this patient, pembrolizumab was very effective in the treatment of tumors; both the tumor size in the lung and tumor metastasis were well controlled after the treatment. In addition, after five days of IVIg treatment, the patient’s AE symptoms improved significantly, and we resumed the pembrolizumab treatment. At 1-year follow-up, both the patient’s AE symptoms and tumors were stable. In previous reports, steroids rather than IVIg were generally chosen for ICI-associated AE [24], and ICI treatment was usually discontinued. However, in our patient, timely and effective immunotherapy and continued treatment with pembrolizumab resulted in significant reduction of both AE and tumors. Our case may provide new directions for future treatment of ICI-associated AE.
4.2. Literature Review of ICI-Associated AE
By reviewing the literature, we found 50 cases of ICI-associated AE; of which, 28 and 22 cases were with and without autoantibodies, respectively. Patients’ age ranged from 19 to 81 years, with a mean age of 61.5 years, with a male-to-female ratio of 1:1. The patients had lung cancer (20), melanoma (12), renal cancer (6), pleural mesothelioma (3), Hodgkin’s lymphoma (2), ovarian cancer (1), breast cancer (1), endometrial cancer (1), uterine cancer (1), Merkel cell carcinoma (1), thymic carcinoma (1), and urothelial carcinoma (1). Thirty-nine patients were explicitly prescribed with ICI-specific drugs: Nivolumab (15), ipilimumab/Nivolumab (11), Pembrolizumab (7), Sintilimab (1), Durvalumab (2), Dostarlimab (1), and Atezolizumab (2). After the use of ICI, the patients presented with AE. The time from ICI use to AE symptom onset ranged from 4 days to 18 months (median, 3 months). Examination of CSF showed increased an cell number in 21 patients, increased protein in 35 patients, and a positive oligoclonal band (OCB) in 7 patients. Twenty-eight patients were found to have positive autoimmune antibodies in the cerebrospinal fluid or serum, including Ma2 Ab (10), GAD Ab (7), Hu Ab (3), NMDAR Ab (4), SOX1 Ab (2), Ri Ab (1), GABAbR Ab (1), and CASPR2 Ab (1) [25]. Hu Ab and NMDAR Ab were double-positive in one patient [26]. According to CTCAE-5.0, there were 3 Grade 2 cases, 29 Grade 3 cases, and 15 Grade 4 cases [20]. After treatment with steroids, IVIg, plasma exchange, and rituximab, the symptoms of encephalitis improved in 31 patients; 13 patients did not improve, and 6 patients died (Table 1).

Table 1.
ICI-associated AE.
This table summarized the reported cases of ICI-associated AE (abbreviations: NA = not available, FLAIR = fluid-attenuated inversion recovery, MTL = mesial temporal lobe, OCB = oligoclonal band). We noticed that among the reported cases, only seven cases were related to the GAD65 antibody [51], ranging in age from 33 to 64 years. All patients developed neuropsychiatric symptoms after using nivolumab alone or in combination with ipilimumab; none have been reported for pembrolizumab with the GAD65 antibody. Among the seven patients, four had poor prognosis or died. Six patients suspended ICI immediately after the onset of AE symptoms. Piepgras et al. reported a patient whose treatment with nivolumab was continued after controlling symptoms with steroids and infliximab but died after the encephalitis symptoms recurred [36]. In contrast to the previously reported patient, our patient had a good prognosis at the 1-year follow-up. The prognosis of ICI-associated AE varied after treatment.
The mechanisms of ICI-associated AE may be due to the following reasons: (1) The immune response induced by ICI may cross-react with CNS autoantigens; (2) AE is closely associated with tumors [52]. ICI may simultaneously enhance the immune response of the tumor and the immune response to CNS; and (3) ICI may recognize innate immune molecules on the surface of neurons, and thus directly kill neurons through the complement system or cytotoxicity, leading to the release of intracellular antigens [53].
In addition to AE, ICI can also lead to other neurological complications. Neurological syndromes caused by ICI are mainly peripheral neuropathies, including myasthenia gravis [54], Guillain–Barre syndrome [55], chronic polyneuropathies [56], and mononeuropathies [57]. CNS complications include noninfectious encephalitis, demyelinating disease, and cerebral artery vasculitis [16]. Neurological complications associated with ICI treatment require medical attention.
4.3. Current Dilemma: The Balance between ICI Therapy, Tumor Progression, and AE Treatment
Immune-related adverse events are classified into five grades [20]. Due to the presence of pathogenic autoantibodies, the treatment of ICI-associated AE includes withdrawal of ICI, IVIg, plasma exchange, and immunosuppressive therapies. As discussed above, most scholars support stopping ICI therapy and initiating immunosuppressive therapy [58]. According to the principle of toxicity management, only Grade 1 irAEs could continue ICI. Grades 2–4 irAEs should withhold ICI and receive stronger immunosuppressive therapy [59]. However, discontinuation of ICI therapy may lead to tumor progression. In addition, strong immunosuppression is associated with tumor recurrence. Being able to balance the treatments may prove difficult. Based on our experience, the treatment of ICI-associated AE should be more individualized and prudent. We advocate that IVIg or plasma exchange may be the first choices for Grade 2 and Grade 3 patients, and ICI may not be discontinued at first. The patients need more close monitoring. If patients do not respond to first-line treatment or symptoms progression, ICI treatment needs to be suspended and stronger immunosuppressants can be used [60]. In the literature we reviewed, we found two Grade 2 patients who continued using ICI or were switched to another type of ICI, and had good prognosis [24]. However, because ICI-associated AE is a relatively new topic in neuroimmunology, we need more cases and multi-center research in the future.
5. Conclusions
This is the first case of pembrolizumab-associated AE with GAD65 and SOX1 antibodies, characterized by progressive cerebellar ataxia. It should be noted that this patient received IVIg therapy for AE and continuous pembrolizumab therapy without suspension of tumor treatment. At present, the treatment of ICI-associated AE needs to be more individualized with close monitoring, evaluation, and prudent decision-making. Finally, the mechanisms, clinical features, and treatment of ICI-associated AE are a new concept in the field of neuroimmunology, and further studies are needed.
Author Contributions
Conceptualization, Y.G. and J.P.; methodology, Y.G. and J.P.; validation, Y.G., J.P., D.S., L.P., Z.M., C.W. and H.M.; writing—original draft preparation, Y.G.; writing—review and editing, Y.G. and J.P.; visualization, Y.G. and J.P.; supervision, Q.Z. and S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Shanghai Shuguang Plan Project (18SG15), Shanghai outstanding young scholars Project, Shanghai talent development project (2019044), Clinical Research Plan of SHDC (SHDC 2020CR2027B) to S.C.
Institutional Review Board Statement
Ethical review and approval were waived for this study, as they are not required for retrospective case studies at the researcher’s institution.
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the patient privacy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dolladille, C.; Ederhy, S.; Sassier, M.; Cautela, J.; Thuny, F.; Cohen, A.A.; Fedrizzi, S.; Chrétien, B.; DA Silva, A.; Plane, A.-F.; et al. Immune Checkpoint Inhibitor Rechallenge After Immune-Related Adverse Events in Patients with Cancer. JAMA Oncol. 2020, 6, 865–871. [Google Scholar] [CrossRef]
- Gubin, M.M.; Zhang, X.; Schuster, H.; Caron, E.; Ward, J.P.; Noguchi, T.; Ivanova, Y.; Hundal, J.; Arthur, C.D.; Krebber, W.J.; et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature 2014, 515, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.C.; Levine, J.H.; Cogdill, A.P.; Zhao, Y.; Anang, N.-A.A.S.; Andrews, M.C.; Sharma, P.; Wang, J.; Wargo, J.A.; Pe’Er, D.; et al. Distinct Cellular Mechanisms Underlie Anti-CTLA-4 and Anti-PD-1 Checkpoint Blockade. Cell 2017, 170, 1120–1133.e17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, A.A.; Patel, V.G. The role of PD-L1 expression as a predictive biomarker: An analysis of all US Food and Drug Ad-ministration (FDA) approvals of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 278. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flaig, T.W. NCCN Guidelines Updates: Management of Muscle-Invasive Bladder Cancer. J. Natl. Compr. Cancer Netw. 2019, 17, 591–593. [Google Scholar]
- Messersmith, W.A. NCCN Guidelines Updates: Management of Metastatic Colorectal Cancer. J. Natl. Compr. Cancer Netw. 2019, 17, 599–601. [Google Scholar]
- Gubens, M.A.; Davies, M. NCCN Guidelines Updates: New Immunotherapy Strategies for Improving Outcomes in Non-Small Cell Lung Cancer. J. Natl. Compr. Cancer Netw. 2019, 17, 574–578. [Google Scholar]
- Dammeijer, F.; van Gulijk, M.; Mulder, E.E.; Lukkes, M.; Klaase, L.; van den Bosch, T.; van Nimwegen, M.; Lau, S.P.; Latupeirissa, K.; Schetters, S.; et al. The PD-1/PD-L1-Checkpoint Restrains T cell Immunity in Tumor-Draining Lymph Nodes. Cancer Cell 2020, 38, 685–700.e8. [Google Scholar] [CrossRef]
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. Mech. Dis. 2021, 16, 223–249. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brahmer, J.R.; Callahan, M.K.; Flores-Chavez, A.; Keegan, N.; Khamashta, M.A.; Lambotte, O.; Mariette, X.; Prat, A.; Suarez-Almazor, M.E. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Primers 2020, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.B.; Salama, A. A review of cancer immunotherapy toxicity. CA A Cancer J. Clin. 2020, 70, 86–104. [Google Scholar] [CrossRef] [Green Version]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef]
- Abdel-Wahab, N.; Safa, H.; Abudayyeh, A.; Johnson, D.H.; Trinh, V.A.; Zobniw, C.M.; Lin, H.; Wong, M.K.; Ab-delrahim, M.; Gaber, A.O.; et al. Checkpoint inhibitor therapy for cancer in solid organ transplantation recipients: An institutional experience and a systematic review of the literature. J. Immunother. Cancer 2019, 7, 106. [Google Scholar] [CrossRef]
- Williams, T.J.; Benavides, D.R.; Patrice, K.A.; Dalmau, J.O.; de Avila, A.L.; Le, D.T.; Lipson, E.J.; Probasco, J.C.; Mowry, E.M. Association of Autoimmune Encephalitis with Combined Immune Checkpoint Inhibitor Treatment for Metastatic Cancer. JAMA Neurol. 2016, 73, 928–933. [Google Scholar] [CrossRef]
- Chung, M.; Jaffer, M.; Verma, N.; Mokhtari, S.; Ramsakal, A.; Peguero, E. Immune checkpoint inhibitor induced anti-glutamic acid decarboxylase 65 (Anti-GAD 65) limbic encephalitis responsive to intravenous immunoglobulin and plasma exchange. J. Neurol. 2020, 267, 1023–1025. [Google Scholar] [CrossRef]
- Graus, F.; Dalmau, J. Paraneoplastic neurological syndromes in the era of immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2019, 16, 535–548. [Google Scholar] [CrossRef]
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE); Version 5.0; US Department of Health and Human Services: Washington, DC, USA, 2017.
- Bruna, J.; Argyriou, A.A.; Anastopoulou, G.G.; Alemany, M.; Nadal, E.; Kalofonou, F.; Piulats, J.M.; Simó, M.; Velasco, R.; Kalofonos, H.P. Incidence and characteristics of neurotoxicity in immune checkpoint inhibitors with focus on neuromuscular events: Experience beyond the clinical trials. J. Peripher. Nerv. Syst. 2020, 25, 171–177. [Google Scholar] [CrossRef]
- Xu, M.; Nie, Y.; Yang, Y.; Lu, Y.T.; Su, Q. Risk of Neurological Toxicities Following the Use of Different Immune Checkpoint Inhibitor Regimens in Solid Tumors: A Systematic Review and Meta-analysis. Neurologist 2019, 24, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Vogrig, A.; Fouret, M.; Joubert, B.; Picard, G.; Rogemond, V.; Pinto, A.-L.; Muñiz-Castrillo, S.; Roger, M.; Raimbourg, J.; Dayen, C.; et al. Increased frequency of anti-Ma2 encephalitis associated with immune checkpoint inhibitors. Neurol. Neuroimmunol. Neuroinflamm. 2019, 6, e604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taliansky, A.; Furman, O.; Gadot, M.; Urban, D.; Bar, J.; Shapira-Frumer, R.; Kaufman, B.; Asher, N.; Leibowitz-Amit, R.; Itay, A. Immune checkpoint inhibitors–related encephalitis in melanoma and non-melanoma cancer patients: A single center experience. Support. Care Cancer 2021, 29, 7563–7568. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Chen, S.; Liu, J. Sleep Disturbances in Autoimmune Neurologic Diseases: Manifestation and Pathophysiology. Front. Neurosci. 2021, 15, 687536. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.; Perez, M.A.; Perrone, C.M.; Bae, C.J.; Pruitt, A.A.; Lancaster, E. A case series of PD-1 inhibitor-associated paraneoplastic neurologic syndromes. J. Neuroimmunol. 2019, 334, 576980. [Google Scholar] [CrossRef]
- Brown, M.P.; Hissaria, P.; Hsieh, A.H.; Kneebone, C.; Vallat, W. Autoimmune limbic encephalitis with anti-contactin-associated protein-like 2 antibody secondary to pembrolizumab therapy. J. Neuroimmunol. 2017, 305, 16–18. [Google Scholar] [CrossRef]
- Kopecky, J.; Kubecek, O.; Geryk, T.; Slovackova, B.; Hoffmann, P.; Ziaran, M.; Priester, P. Nivolumab induced en-cephalopathy in a man with metastatic renal cell cancer: A case report. J. Med. Case Rep. 2018, 12, 262. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.; Dunn-Pirio, A.; Luedke, M.; Morgenlander, J.; Skeen, M.; Eckstein, C. Nivolumab-Induced Autoimmune En-cephalitis in Two Patients with Lung Adenocarcinoma. Case Rep. Neurol. Med. 2018, 2018, 2548528. [Google Scholar]
- Shibaki, R.; Murakami, S.; Oki, K.; Ohe, Y. Nivolumab-induced autoimmune encephalitis in an anti-neuronal autoanti-body-positive patient. Jpn. J. Clin. Oncol. 2019, 49, 793–794. [Google Scholar] [CrossRef]
- Mongay-Ochoa, N.; Vogrig, A.; Muñiz-Castrillo, S.; Honnorat, J. Anti-Hu-associated paraneoplastic syndromes triggered by immune-checkpoint inhibitor treatment. J. Neurol. 2020, 267, 2154–2156. [Google Scholar] [CrossRef]
- Shah, N.; Jacob, J.; Househ, Z.; Shiner, E.; Baird, L.; Soudy, H. Unchecked immunity: A unique case of sequential immune-related adverse events with Pembrolizumab. J. Immunother. Cancer 2019, 7, 247. [Google Scholar] [CrossRef] [Green Version]
- Lyons, S.; Joyce, R.; Moynagh, P.; O’Donnell, L.; Blazkova, S.; Counihan, T.J. Autoimmune encephalitis associated with Ma2 antibodies and immune checkpoint inhibitor therapy. Pract. Neurol. 2020, 20, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Zheng, K.; Zhang, Y. Paraneoplastic Encephalitis and Enteric Neuropathy Associated with Anti-Hu Antibody in a Patient Following Immune-checkpoint Inhibitor Therapy. J. Immunother. 2020, 43, 165–168. [Google Scholar] [CrossRef]
- Hottinger, A.F.; de Micheli, R.; Guido, V.; Karampera, A.; Hagmann, P.; Du Pasquier, R. Natalizumab may control immune checkpoint inhibitor–induced limbic encephalitis. Neurol. Neuroimmunol. Neuroinflamm. 2018, 5, e439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piepgras, J.; Müller, A.; Steffen, F.; Lotz, J.; Loquai, C.; Zipp, F.; Dresel, C.; Bittner, S. Neurofilament light chain levels reflect outcome in a patient with glutamic acid decarboxylase 65 antibody–positive autoimmune encephalitis under immune checkpoint inhibitor therapy. Eur. J. Neurol. 2021, 28, 1086–1089. [Google Scholar] [CrossRef]
- Burke, M.; Hardesty, M.; Downs, W. A case of severe encephalitis while on PD-1 immunotherapy for recurrent clear cell ovarian cancer. Gynecol. Oncol. Rep. 2018, 24, 51–53. [Google Scholar] [CrossRef]
- Duong, S.L.; Barbiero, F.J.; Nowak, R.J.; Baehring, J.M. Neurotoxicities associated with immune checkpoint inhibitor therapy. J. Neuro-Oncol. 2021, 152, 265–277. [Google Scholar] [CrossRef]
- Ghous, G.; Shoukat, H.M.H.; Tarar, Z.I.; Zafar, M.U.; McGreevy, J.W. Encephalitis Associated with Hemophagocytic Lymphohistiocytosis Secondary to Immune Checkpoint Inhibitors: An Unfamiliar Spin-Off. Cureus 2021, 13, e16079. [Google Scholar] [CrossRef] [PubMed]
- Maniscalco, G.T.; Zekeridou, A.; Allegorico, L.; Ranieri, A.; Napolitano, M.; Pezzella, M.; Della Gatta, L.; Manzo, V.; Ferrari, S.; Mariotto, S. GAD65 autoimmunity after treatment with nivolumab: A multifocal presentation. Neurol. Sci. 2021, 42, 4289–4291. [Google Scholar] [CrossRef]
- Shechtman, Y.; Shalata, W.; Khoury, R.; Mahajna, A.; Weller, B.; Agbarya, A. Encephalitis Induced by Durvalumab During Treatment of Metastatic Small-Cell Lung Cancer: Illustrative Case and Review of the Literature. J. Immunother. 2021, 44, 243–247. [Google Scholar] [CrossRef]
- Yordduangjun, N.; Dishion, E.; McKnight, C.A.; Caplan, J.P. Immune Checkpoint Inhibitor–Associated Autoimmune Encephalitis. J. Acad. Consult. Psychiatry 2021, 62, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Nagasawa, H.; Katagiri, Y.; Wada, M. Atezolizumab-associated encephalitis in metastatic lung adenocarcinoma: A case report. J. Med. Case Rep. 2020, 14, 88. [Google Scholar] [CrossRef]
- Quach, H.T.; Robbins, C.J.; Balko, J.M.; Chiu, C.Y.; Miller, S.; Wilson, M.R.; Nelson, G.E.; Johnson, D.B. Severe Epididymo-Orchitis and Encephalitis Complicating Anti-PD-1 Therapy. Oncologist 2019, 24, 872–876. [Google Scholar] [CrossRef] [Green Version]
- Braden, J.; Lee, J.H. Immune Checkpoint Inhibitor Induced Pericarditis and Encephalitis in a Patient Treated with Ipilimumab and Nivolumab for Metastatic Melanoma: A Case Report and Review of the Literature. Front. Oncol. 2021, 11, 749834. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, H.; Suzuki, C.; Kon, T.; Nakamura, T.; Tanaka, H.; Sakamoto, Y.; Tomiyama, M. Bilateral Thalamic Lesions Associated with Atezolizumab-Induced Autoimmune Encephalitis. Neurology 2021, 96, 126–127. [Google Scholar] [CrossRef] [PubMed]
- Shionoya, Y.; Hattori, A.; Hanada, T.; Fujino, M. Case Report: Durvalumab-Associated Encephalitis in Extensive-Stage Small Cell Lung Carcinoma. Front. Oncol. 2021, 11, 693279. [Google Scholar] [CrossRef]
- Niki, M.; Nakaya, A.; Kurata, T.; Nakahama, K.; Yoshioka, H.; Kaneda, T.; Kibata, K.; Ogata, M.; Nomura, S. Pem-brolizumab-induced autoimmune encephalitis in a patient with advanced non-small cell lung cancer: A case report. Mol. Clin. Oncol. 2019, 10, 267–269. [Google Scholar]
- Nalbantoglu, M.; Altunrende, B.; Tuncer, O.G.; Akman, G. Autoimmune Encephalitis After Treatment of Hodgkin’s Lymphoma with the Immune Checkpoint Inhibitor Nivolumab. Noro Psikiyatr. Ars. 2021, 58, 163–165. [Google Scholar]
- Thouvenin, L.; Olivier, T.; Banna, G.; Addeo, A.; Friedlaender, A. Immune checkpoint inhibitor-induced aseptic menin-gitis and encephalitis: A case-series and narrative review. Ther. Adv. Drug Saf. 2021, 12, 20420986211004745. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Y.; Shen, D.; Zhou, Q. The pathogenicity of culprit antibody in autoimmune encephalitis. Chin. J. Contemp. Neurol. Neurosurg. 2022, 22, 1–7. [Google Scholar]
- Zhou, Q.; Zhu, X.; Meng, H.; Zhang, M.; Chen, S. Anti-dipeptidyl-peptidase-like protein 6 encephalitis, a rare cause of reversible rapid progressive dementia and insomnia. J. Neuroimmunol. 2020, 339, 577114. [Google Scholar] [CrossRef] [PubMed]
- Yshii, L.M.; Hohlfeld, R.; Liblau, R.S. Inflammatory CNS disease caused by immune checkpoint inhibitors: Status and perspectives. Nat. Rev. Neurol. 2017, 13, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-T.; Chen, Y.-P.; Lin, W.-C.; Su, W.-C.; Sun, Y.-T. Immune Checkpoint Inhibitor-Induced Myasthenia Gravis. Front. Neurol. 2020, 11, 634. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Hu, Y.; Wang, X.; Zhao, B. Guillain–Barré syndrome in patients treated with immune checkpoint inhibitors. J. Neurol. 2021, 268, 2169–2174. [Google Scholar] [CrossRef]
- Okada, K.; Seki, M.; Yaguchi, H.; Sakuta, K.; Mukai, T.; Yamada, S.; Oki, K.; Nakahara, J.; Suzuki, S. Polyradiculo-neuropathy induced by immune checkpoint inhibitors: A case series and review of the literature. J. Neurol. 2021, 268, 680–688. [Google Scholar] [CrossRef]
- Baldauf, M.C.; Kapauer, M.; Joerger, M.; Flatz, L.; Rodriguez, R.; Frank, S.; Felbecker, A.; Hartmann-Fussenegger, S.; Hundsberger, T. Pembrolizumab-Associated CD8+ Vasculitic Mononeuritis Multiplex in a Patient with Mesothelioma. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e993. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X. Immunotherapy for Refractory Autoimmune Encephalitis. Front. Immunol. 2021, 12, 790962. [Google Scholar] [CrossRef]
- Reid, P.D.; Cifu, A.S.; Bass, A.R. Management of Immunotherapy-Related Toxicities in Patients Treated with Immune Checkpoint Inhibitor Therapy. JAMA 2021, 325, 482–483. [Google Scholar] [CrossRef]
- Ni, Y.; Shen, D.; Zhang, Y.; Song, Y.; Gao, Y.; Zhou, Q.; He, L.; Yin, D.; Wang, Y.; Song, F.; et al. Expanding the clinical spectrum of anti-IgLON5 disease: A multicenter retrospective study. Eur. J. Neurol. 2022, 29, 267–276. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).