Exploration of the Common Gene Characteristics and Molecular Mechanism of Parkinson’s Disease and Crohn’s Disease from Transcriptome Data
Abstract
:1. Introduction
2. Materials and Methods
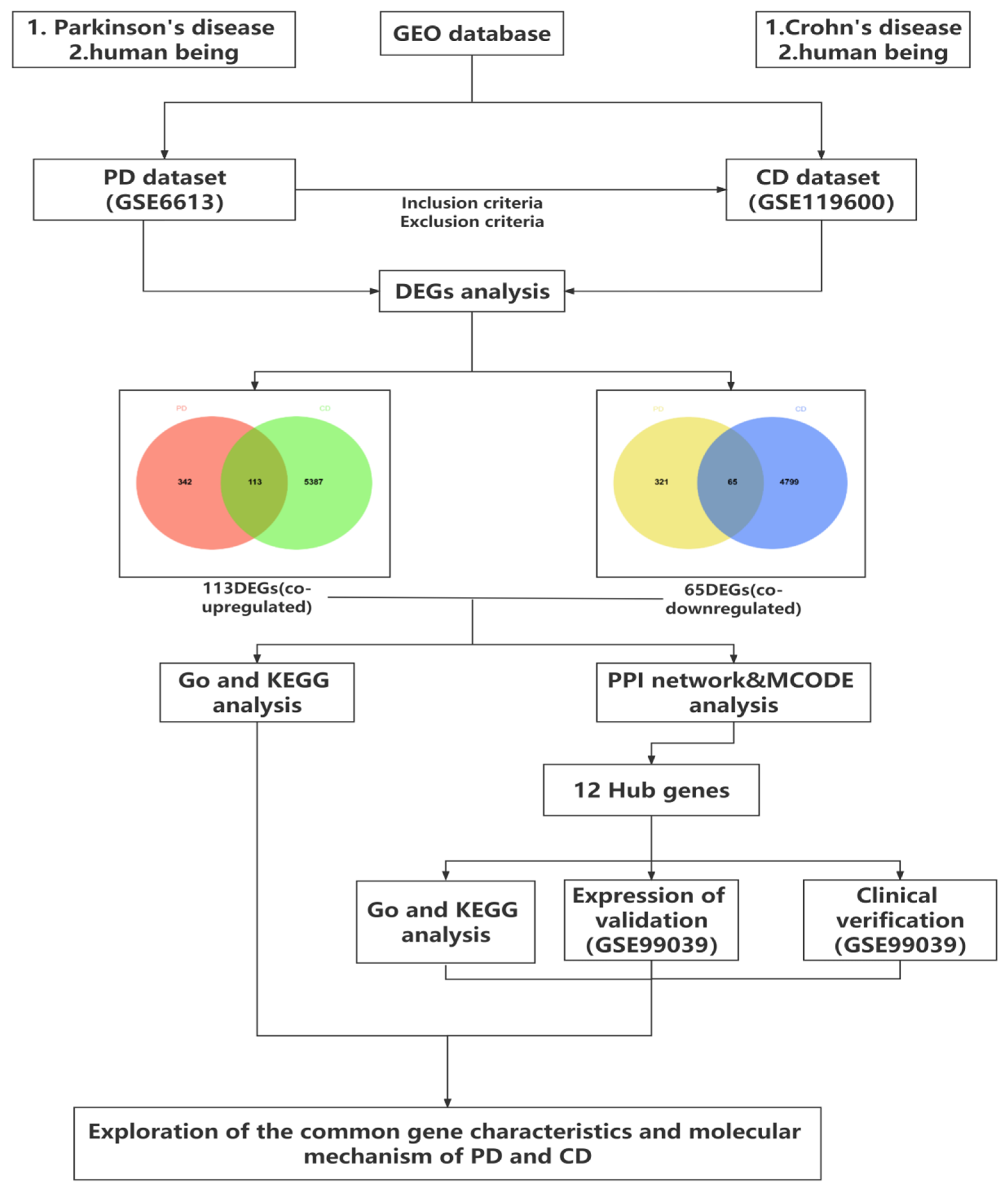
2.1. Data Collection and Processing
2.2. Identification of Differentially Expressed Genes
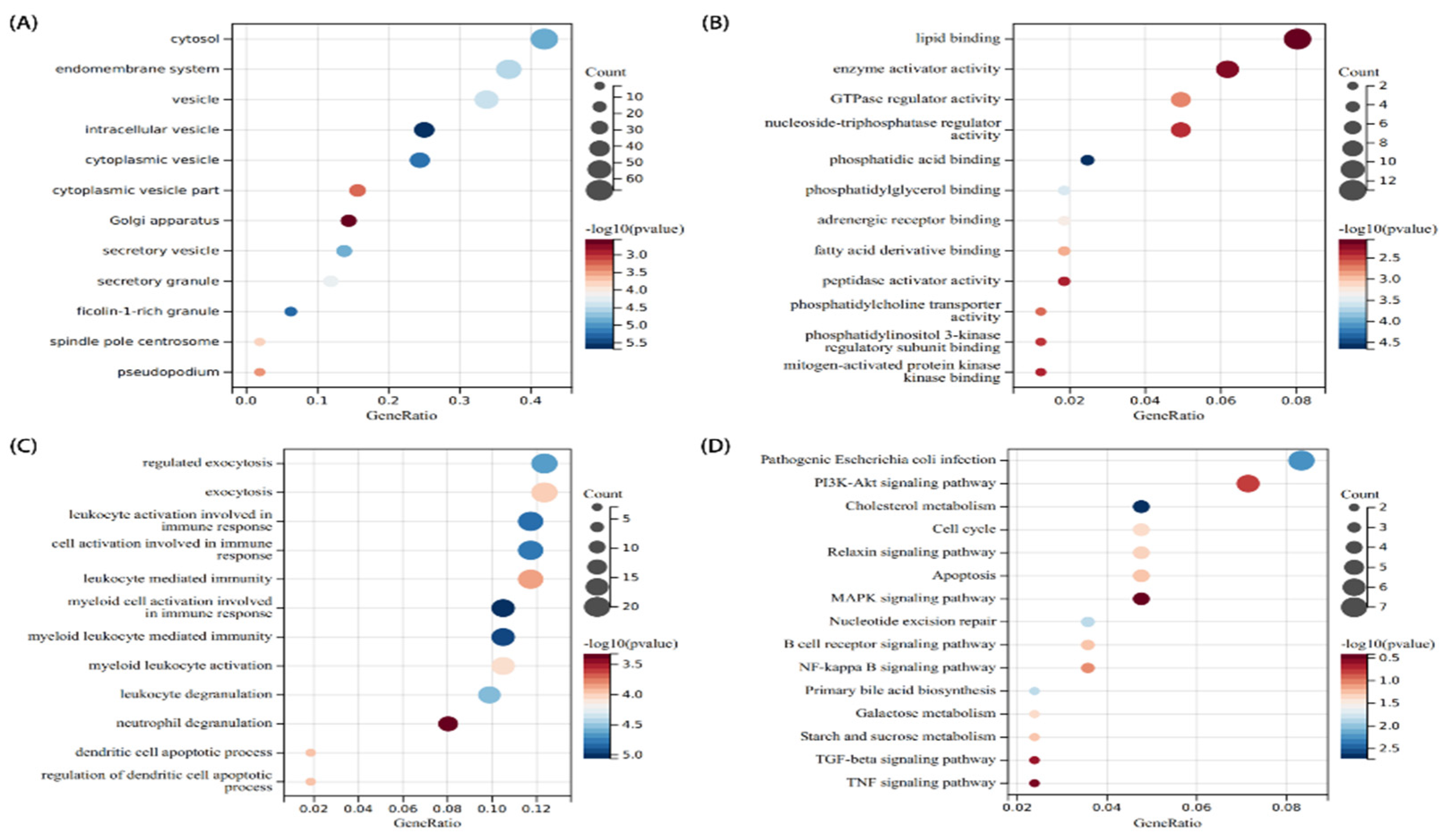
2.3. Functional Enrichment Analysis of Significant DEGs
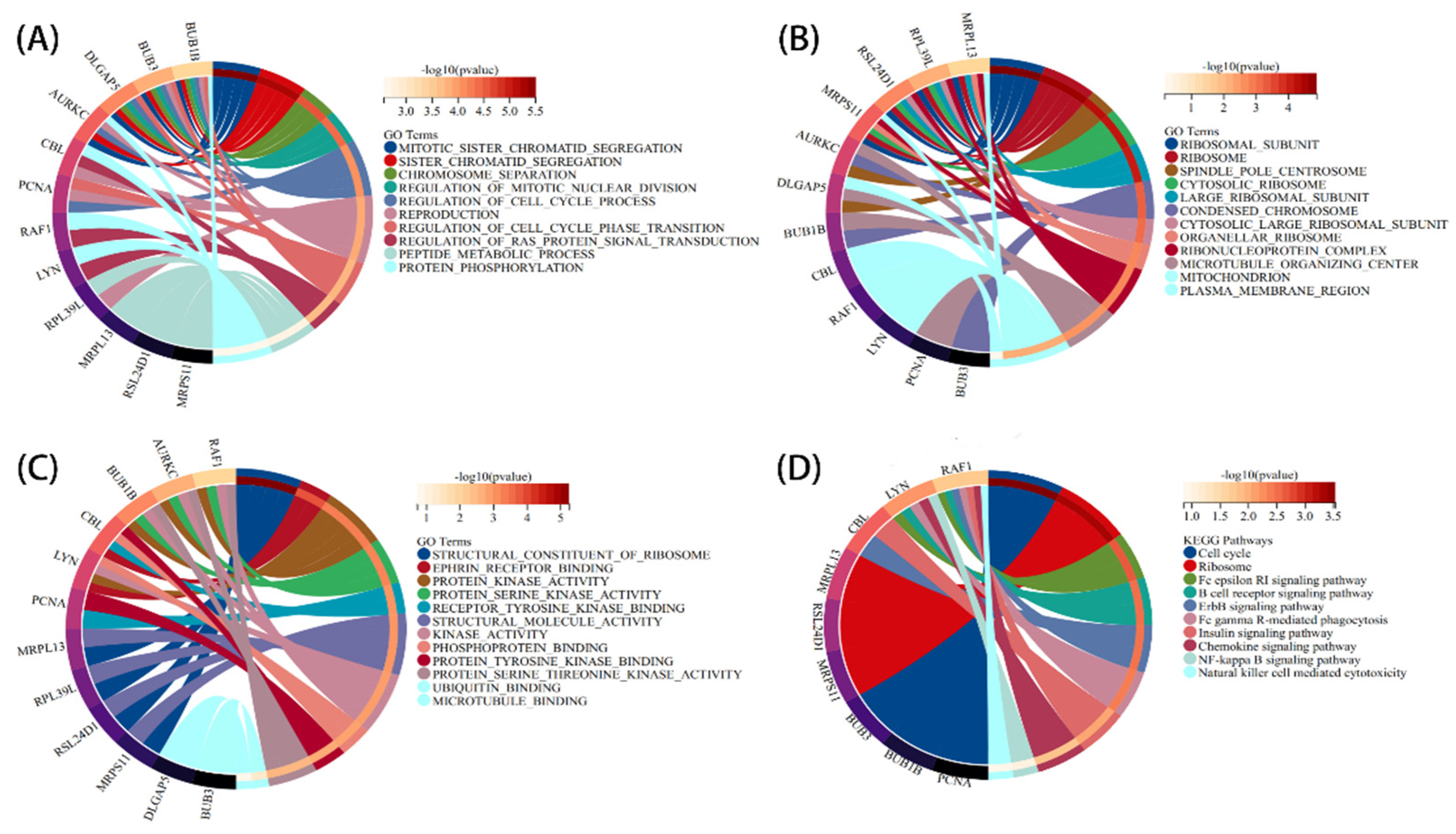
2.4. Protein–Protein Interaction (PPI) Network and Module Analysis
2.5. Statistical Analysis
3. Results
3.1. Identification of Common DEGs between PD and CD
3.2. Analysis of the Functional Characteristics of Common DEGs
3.3. PPI Network Analysis and Identification of Hub Gene
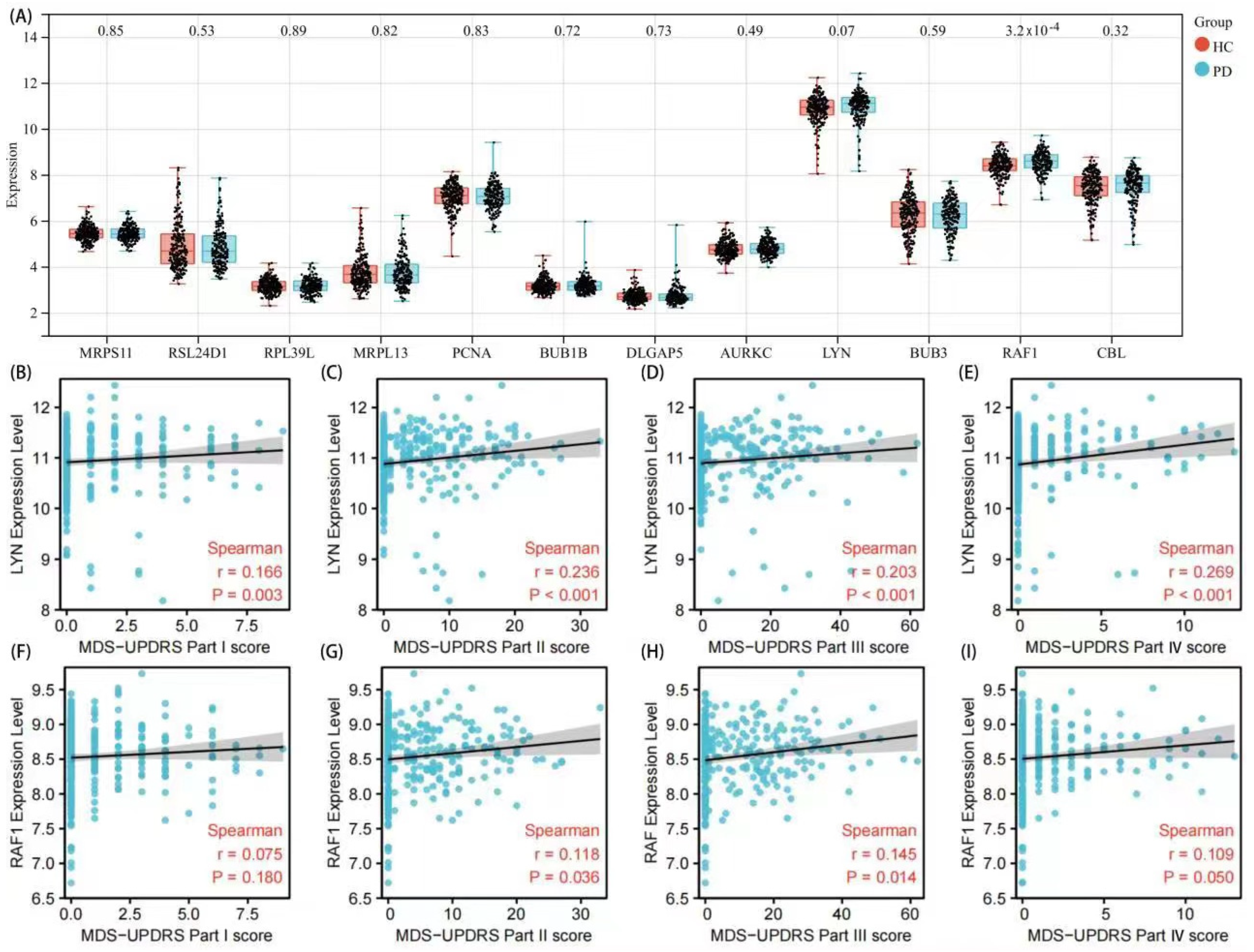
3.4. Validation of Hub Genes and Correlation Analysis with Clinical Features
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Surguchov, A. Biomarkers in Parkinson’s Disease. In Neurodegenerative Diseases Biomarkers; Peplow, P.V., Martinez, B., Gennarelli, T.A., Eds.; Neuromethods; Humana: New York, NY, USA, 2022; Volume 173, pp. 155–180. [Google Scholar]
- de Lau, L.M.; Breteler, M.M. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006, 5, 525–535. [Google Scholar] [CrossRef]
- Weimers, P.; Halfvarson, J.; Sachs, M.C.; Saunders-Pullman, R.; Ludvigsson, J.F.; Peter, I.; Burisch, J.; Olén, O. Inflammatory Bowel Disease and Parkinson’s Disease: A Nationwide Swedish Cohort Study. Inflamm. Bowel Dis. 2019, 25, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Gao, M.; Yung, K.K.L. Association of Intestinal Disorders with Parkinson’s Disease and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. ACS Chem. Neurosci. 2020, 11, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, D.; Kaufman, E.; Brundin, L.; Hall, S.; Surova, Y.; Hansson, O. Non-motor symptoms in patients with Parkinson’s disease—Correlations with inflammatory cytokines in serum. PLoS ONE 2012, 7, e47387. [Google Scholar] [CrossRef] [Green Version]
- Savica, R.; Carlin, J.M.; Grossardt, B.R.; Bower, J.H.; Ahlskog, J.E.; Maraganore, D.M.; Bharucha, A.E.; Rocca, W.A. Medical records documentation of constipation preceding Parkinson disease: A case-control study. Neurology 2009, 73, 1752–1758. [Google Scholar] [CrossRef] [Green Version]
- Tansey, M.G.; Wallings, R.L.; Houser, M.C.; Herrick, M.K.; Keating, C.E.; Joers, V. Inflammation and immune dysfunction in Parkinson disease. Nat. Rev. Immunol. 2022, 4, 1–17. [Google Scholar] [CrossRef]
- Yoo, B.B.; Mazmanian, S.K. The Enteric Network: Interactions between the Immune and Nervous Systems of the Gut. Immunity 2017, 46, 910–926. [Google Scholar] [CrossRef] [Green Version]
- Gracie, D.J.; Hamlin, P.J.; Ford, A.C. The influence of the brain–gut axis in inflammatory bowel disease and possible implications for treatment. Lancet Gastroenterol. Hepatol. 2019, 4, 632–642. [Google Scholar] [CrossRef]
- Houser, M.C.; Tansey, M.G. The gut-brain axis: Is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis? NPJ Parkinson’s Dis. 2017, 3, 3. [Google Scholar] [CrossRef]
- Bindas, A.J.; Kulkarni, S.; Koppes, R.A.; Koppes, A.N. Parkinson’s disease and the gut: Models of an emerging relationship. Acta Biomater. 2021, 132, 325–344. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Jiang, L.; Zhang, J.; Tong, X.; Chen, D.; Le, W. Intestinal Inflammation and Parkinson’s Disease. Aging Dis. 2021, 12, 2052–2068. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Lobbestael, E.; Vermeire, S.; Sabino, J.; Cleynen, I. Inflammatory bowel disease and Parkinson’s disease: Common pathophysiological links. Gut 2021, 70, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Houser, M.C.; Caudle, W.M.; Chang, J.; Kannarkat, G.T.; Yang, Y.; Kelly, S.D.; Oliver, D.; Joers, V.; Shannon, K.M.; Keshavarzian, A.; et al. Experimental colitis promotes sustained, sex-dependent, T-cell-associated neuroinflammation and parkinsonian neuropathology. Acta Neuropathol. Commun. 2021, 9, 139. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.C.; Lin, C.S.; Hsu, C.W.; Lin, C.L.; Kao, C.H. Association Between Parkinson’s Disease and Inflammatory Bowel Disease: A Nationwide Taiwanese Retrospective Cohort Study. Inflamm. Bowel Dis. 2016, 22, 1049–1055. [Google Scholar] [CrossRef] [Green Version]
- Villumsen, M.; Aznar, S.; Pakkenberg, B.; Jess, T.; Brudek, T. Inflammatory bowel disease increases the risk of Parkinson’s disease: A Danish nationwide cohort study 1977–2014. Gut 2019, 68, 18–24. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.; Chun, J.; Han, K.; Soh, H.; Kang, E.A.; Lee, H.J.; Im, J.P.; Kim, J.S. Patients with Inflammatory Bowel Disease Are at an Increased Risk of Parkinson’s Disease: A South Korean Nationwide Population-Based Study. J. Clin. Med. 2019, 8, 1191. [Google Scholar] [CrossRef] [Green Version]
- Qin, X.Y.; Zhang, S.P.; Cao, C.; Loh, Y.P.; Cheng, Y. Aberrations in Peripheral Inflammatory Cytokine Levels in Parkinson Disease A Systematic Review and Meta-analysis. JAMA Neurol. 2016, 73, 1316–1324. [Google Scholar] [CrossRef]
- Reale, M.; Iarlori, C.; Thomas, A.; Gambi, D.; Perfetti, B.; Di Nicola, M.; Onofrj, M. Peripheral cytokines profile in Parkinson’s disease. Brain Behav. Immun. 2009, 23, 55–63. [Google Scholar]
- de Almeida Titze, S.S.; Soto-Sánchez, C.; Fernandez, E.; Koprich, J.B.; Brotchie, J.M.; de Almeida Titze, R. The Promise and Challenges of Developing miRNA-Based Therapeutics for Parkinson’s Disease. Cells 2020, 9, 841. [Google Scholar] [CrossRef] [Green Version]
- Camacho-Soto, A.; Gross, A.; Searles Nielsen, S.; Dey, N.; Racette, B.A. Inflammatory bowel disease and risk of Parkinson’s disease in Medicare beneficiaries. Parkinsonism Relat. Disord. 2018, 50, 23–28. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Damian, S.; Andrea, F.; Stefan, W.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape string App: Network analysis and visualization of proteomics data. J. Proteome Res. 2019, 18, 623–632. [Google Scholar] [CrossRef]
- Mestre, T.A.; Bachoud-Lévi, A.C.; Marinus, J.; Stout, J.C.; Paulsen, J.S.; Como, P.; Duff, K.; Sampaio, C.; Goetz, C.G.; Cubo, E.; et al. Rating scales for cognition in Huntington’s disease: Critique and recommendations. Mov. Disord. 2018, 33, 187–195. [Google Scholar] [CrossRef]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, progression and mortality. Neurology 2001, 57 (Suppl. S3), S11–S26. [Google Scholar]
- Zhu, Y.; Yuan, M.; Liu, Y.; Yang, F.; Chen, W.Z.; Xu, Z.Z.; Xiang, Z.B.; Xu, R.S. Association between inflammatory bowel diseases and Parkinson’s disease: Systematic review and meta-analysis. Neural Regen Res. 2022, 17, 344–353. [Google Scholar]
- Miklossy, J.; Arai, T.; Guo, J.; Klegeris, A.; Yu, S.; McGeer, E.G.; McGeer, P.L. LRRK2 expression in normal and pathologic human brain and in human cell lines. J. Neuropathol. Exp. Neurol. 2006, 65, 953–963. [Google Scholar] [CrossRef]
- Drouin-Ouellet, J.; St-Amour, I.; Saint-Pierre, M.; Lamontagne-Proulx, J.; Kriz, J.; Barker, R.A.; Cicchetti, F. Toll-Like Receptor Expression in the Blood and Brain of Patients and a Mouse Model of Parkinson’s Disease. Int. J. Neuropsychopharmacol. 2014, 18, pyu103. [Google Scholar] [CrossRef] [Green Version]
- Lewis, P.A. Leucine rich repeat kinase 2: A paradigm for pleiotropy. J. Physiol. 2019, 597, 3511–3521. [Google Scholar] [CrossRef]
- Hui, K.; Fernandez-Hernandez, H.; Hu, J.; Schaffner, A.; Pankratz, N.; Hsu, N.Y.; Chuang, L.S.; Carmi, S.; Villaverde, N.; Li, X.; et al. LRRK2Functional variants in the gene confer shared effects on risk for Crohn’s disease and Parkinson’s disease. Sci. Transl. Med. 2018, 10, eaai7795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadi Rastegar, D.; Hughes, L.; Perera, G.; Keshiya, S.; Zhong, S.; Gao, J.; Halliday, G.M.; Schüle, B.; Dzamko, N. Effect of LRRK2 protein and activity on stimulated cytokines in human monocytes and macrophages. NPJ Parkinson’s Dis. 2022, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Bartl, M.; Xylaki, M.; Bähr, M.; Weber, S.; Trenkwalder, C.; Mollenhauer, B. Evidence for immune system alterations in peripheral biological fluids in Parkinson’s disease. Neurobiol. Dis. 2022, 170, 105744. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Guo, X.; Liu, L.; Jin, Y.Y.; Lu, Y.Q.; Cao, Y.J.; Long, J.Y.; Wu, H.G.; Zhang, D.; et al. LncRNA-miRNA-mRNA Network Analysis Reveals the Potential Biomarkers in Crohn’s Disease Rats Treated with Herb-Partitioned Moxibustion. J. Inflamm. Res. 2022, 15, 1699–1716. [Google Scholar] [CrossRef]
- Li, T.; Ning, B.; Kong, L.; Dai, B.; He, X.; Thomas, J.M.; Sawa, A.; Ross, C.A.; Smith, W.W. A LRRK2 GTP Binding Inhibitor, 68, Reduces LPS-Induced Signaling Events and TNF-α Release in Human Lymphoblasts. Cells 2021, 10, 480. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.G.; Yamashita, H.; Nakamura, T.; Fukuba, H.; Takahashi, T.; Hiji, M.; Kohriyama, T.; Matsumoto, M. Synphilin-1 transgenic mice exhibit mild motor impairments. Neurosci. Lett. 2008, 445, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Ingley, E. Functions of the Lyn tyrosine kinase in health and disease. Cell Commun. Signal. 2012, 10, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Chu, C.; Stewart, T.; Ginghina, C.; Wang, Y.; Nie, H.; Guo, M.; Wilson, B.; Hong, J.S.; Zhang, J. α-Synuclein, a chemoattractant, directs microglial migration via H2O2-dependent Lyn phosphorylation. Proc. Natl. Acad. Sci. USA 2015, 112, E1926–E1935. [Google Scholar]
- Portugal, C.C.; Almeida, T.O.; Socodato, R.; Relvas, J.B. Src family kinases (SFKs): Critical regulators of microglial homeostatic functions and neurodegeneration in Parkinson’s and Alzheimer’s diseases. FEBS J. 2021; early view. [Google Scholar]
- Li, J.; Fan, Y.; Zhang, Y.; Sun, D.J.; Fu, S.B.; Ma, L.; Jiang, L.H.; Cui, C.; Ding, H.F.; Yang, J. The Raf-1 inhibitor GW5074 and the ERK1/2 pathway inhibitor U0126 ameliorate PC12 cells apoptosis induced by 6-hydroxydopamine. Pharmazie 2012, 67, 718–724. [Google Scholar]
- Mei, M.; Su, B.; Harrison, K.; Chao, M.; Siedlak, S.L.; Previll, L.A.; Jackson, L.; Cai, D.X.; Zhu, X. Distribution, levels and phosphorylation of Raf-1 in Alzheimer’s disease. J. Neurochem. 2006, 99, 1377–1388. [Google Scholar] [CrossRef]
- Wang, X.; Lu, Y.; Wu, L.; Zhao, C.; Song, C.; Yu, S.; Zhao, B.; Zhao, T.; Liu, H.; Dou, C.; et al. Moxibustion Inhibits the ERK Signaling Pathway and Intestinal Fibrosis in Rats with Crohn’s Disease. Evid. Based Complement. Alternat. Med. 2013, 198282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, Z.; Shu, Y.; Gao, C.; Wang, X.; Qi, G.; Zhang, P.; Li, M.; Shi, J.; Tian, B. CDK5-mediated phosphorylation and autophagy of RKIP regulate neuronal death in Parkinson’s disease. Neurobiol. Aging 2014, 35, 2870–2880. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, H.; Qian, X.; Tian, W.; Cao, L. Exploration of the Common Gene Characteristics and Molecular Mechanism of Parkinson’s Disease and Crohn’s Disease from Transcriptome Data. Brain Sci. 2022, 12, 774. https://doi.org/10.3390/brainsci12060774
Zheng H, Qian X, Tian W, Cao L. Exploration of the Common Gene Characteristics and Molecular Mechanism of Parkinson’s Disease and Crohn’s Disease from Transcriptome Data. Brain Sciences. 2022; 12(6):774. https://doi.org/10.3390/brainsci12060774
Chicago/Turabian StyleZheng, Haoran, Xiaohang Qian, Wotu Tian, and Li Cao. 2022. "Exploration of the Common Gene Characteristics and Molecular Mechanism of Parkinson’s Disease and Crohn’s Disease from Transcriptome Data" Brain Sciences 12, no. 6: 774. https://doi.org/10.3390/brainsci12060774
APA StyleZheng, H., Qian, X., Tian, W., & Cao, L. (2022). Exploration of the Common Gene Characteristics and Molecular Mechanism of Parkinson’s Disease and Crohn’s Disease from Transcriptome Data. Brain Sciences, 12(6), 774. https://doi.org/10.3390/brainsci12060774





