The Influence of Cross-Fostering on Alcohol Consumption and Depressive-Like Behaviors in HA and LA Mice: The Role of the Endogenous Opioid System
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Experiment Design
2.3. Drugs
2.4. Assessment of Depressive-Like Behaviors
2.5. Statistics
3. Results
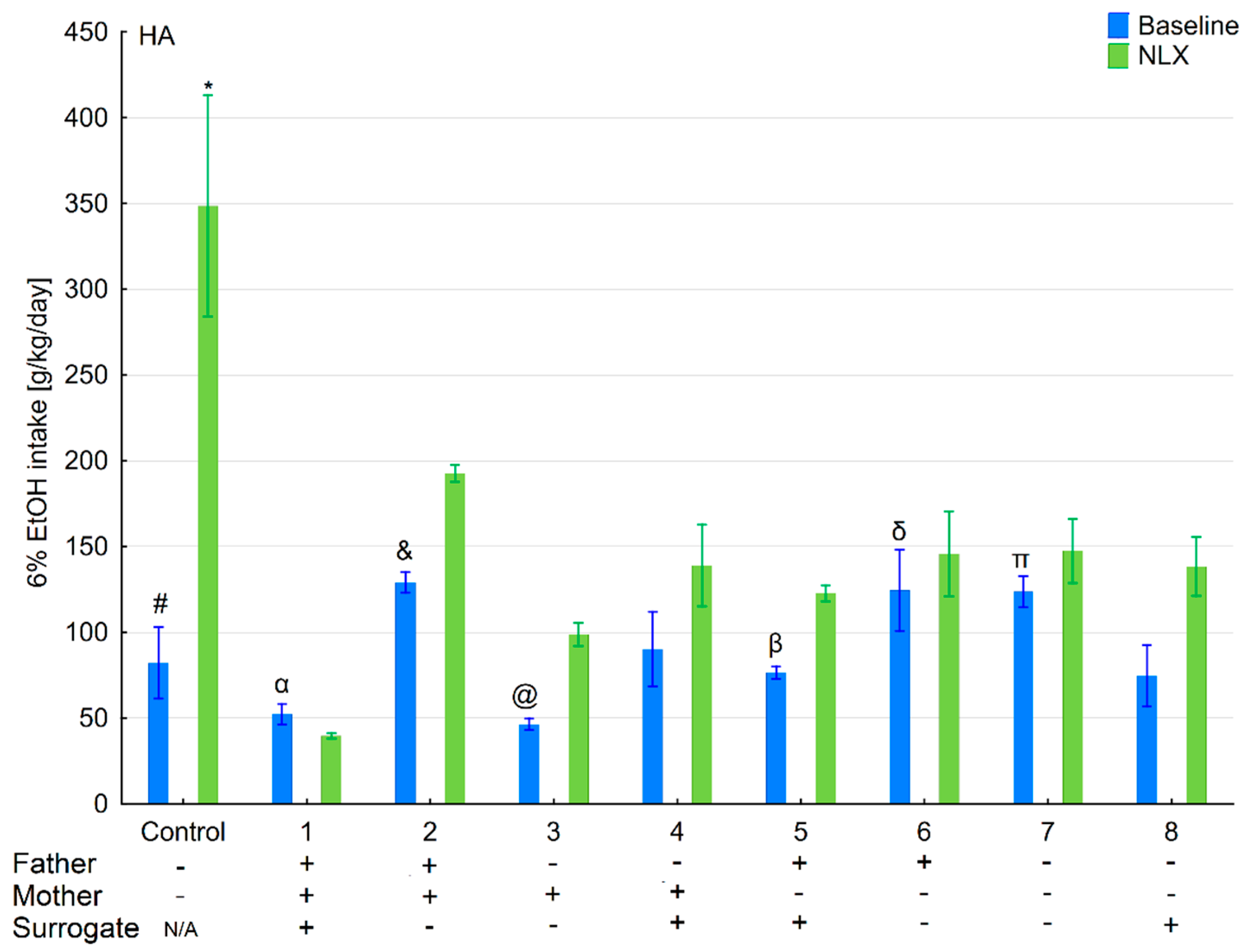
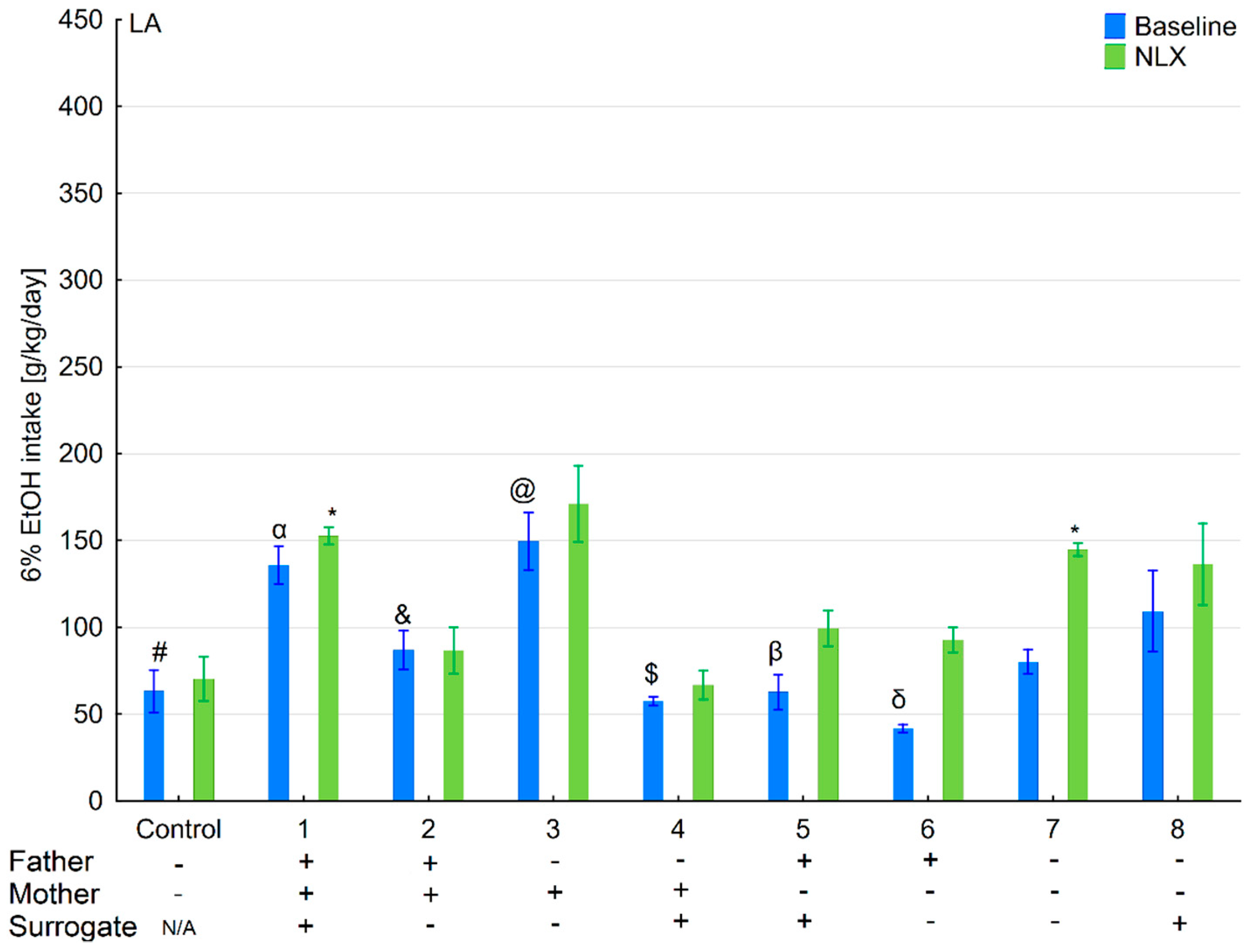
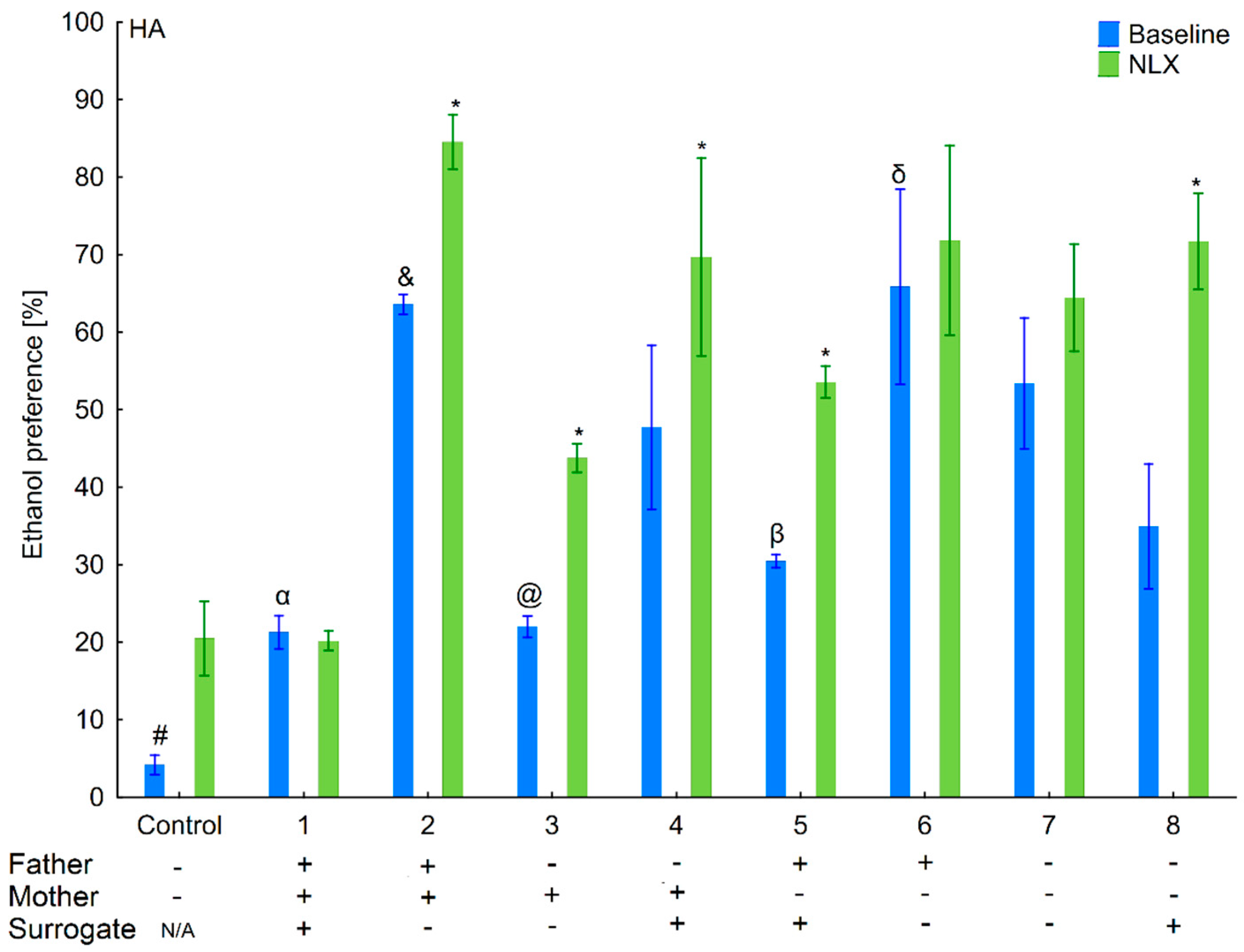
3.1. Changes in Ethanol Intake and Preference in the HA and LA Mice Reared by Surrogates of the Same Line
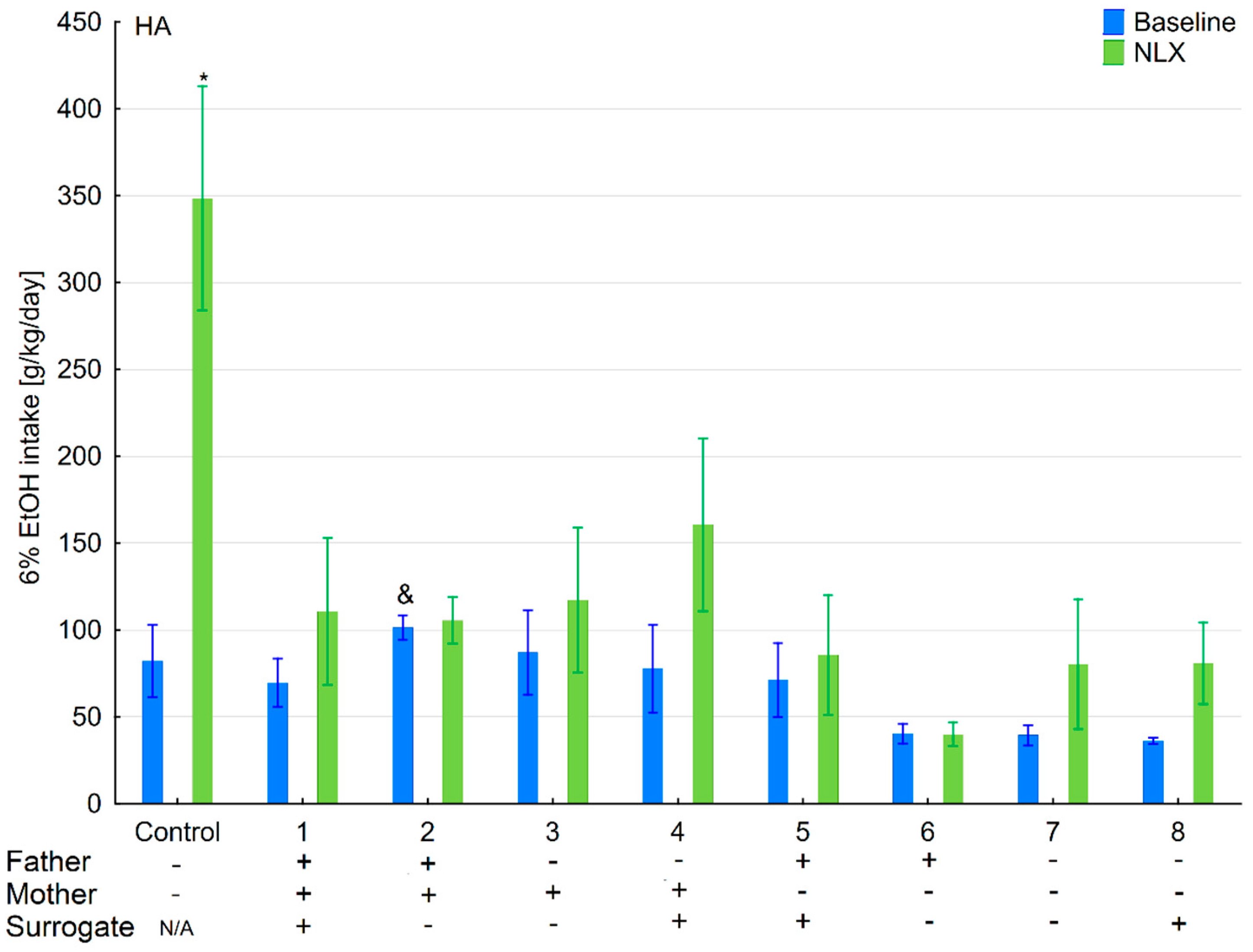
3.2. Effects of Naloxone on Ethanol Intake and Preference in the HA and LA Mice Reared by Surrogates of the Same Line
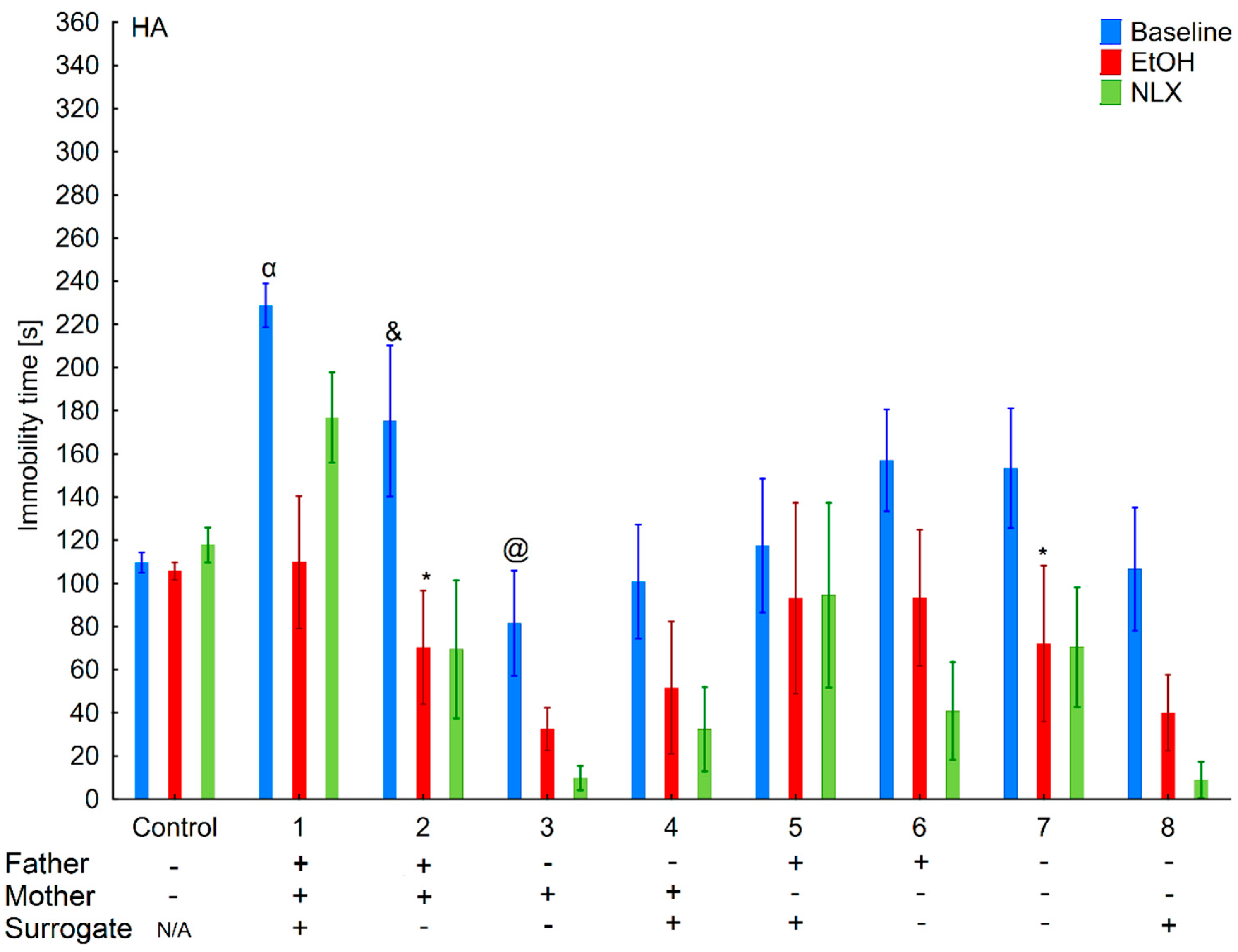
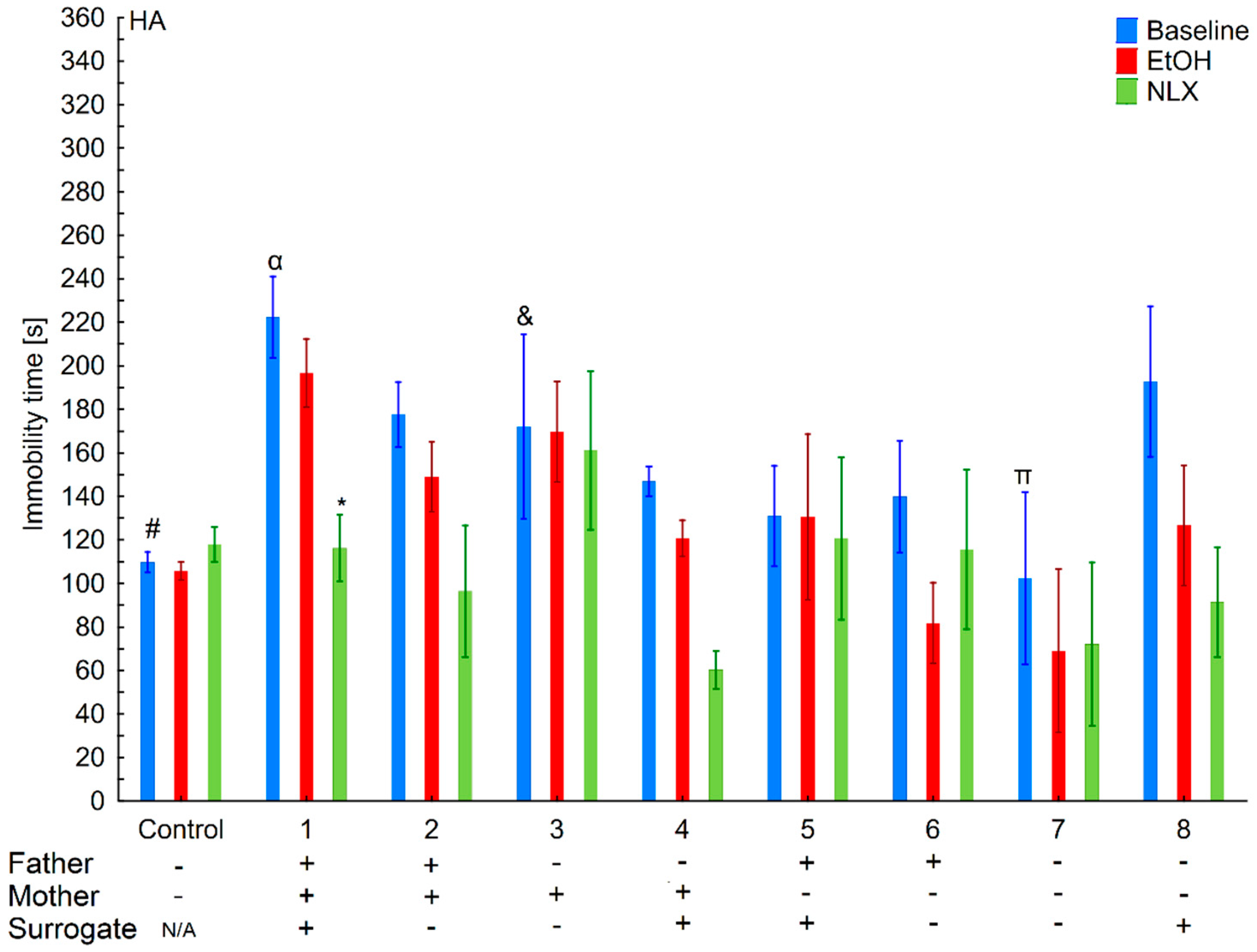
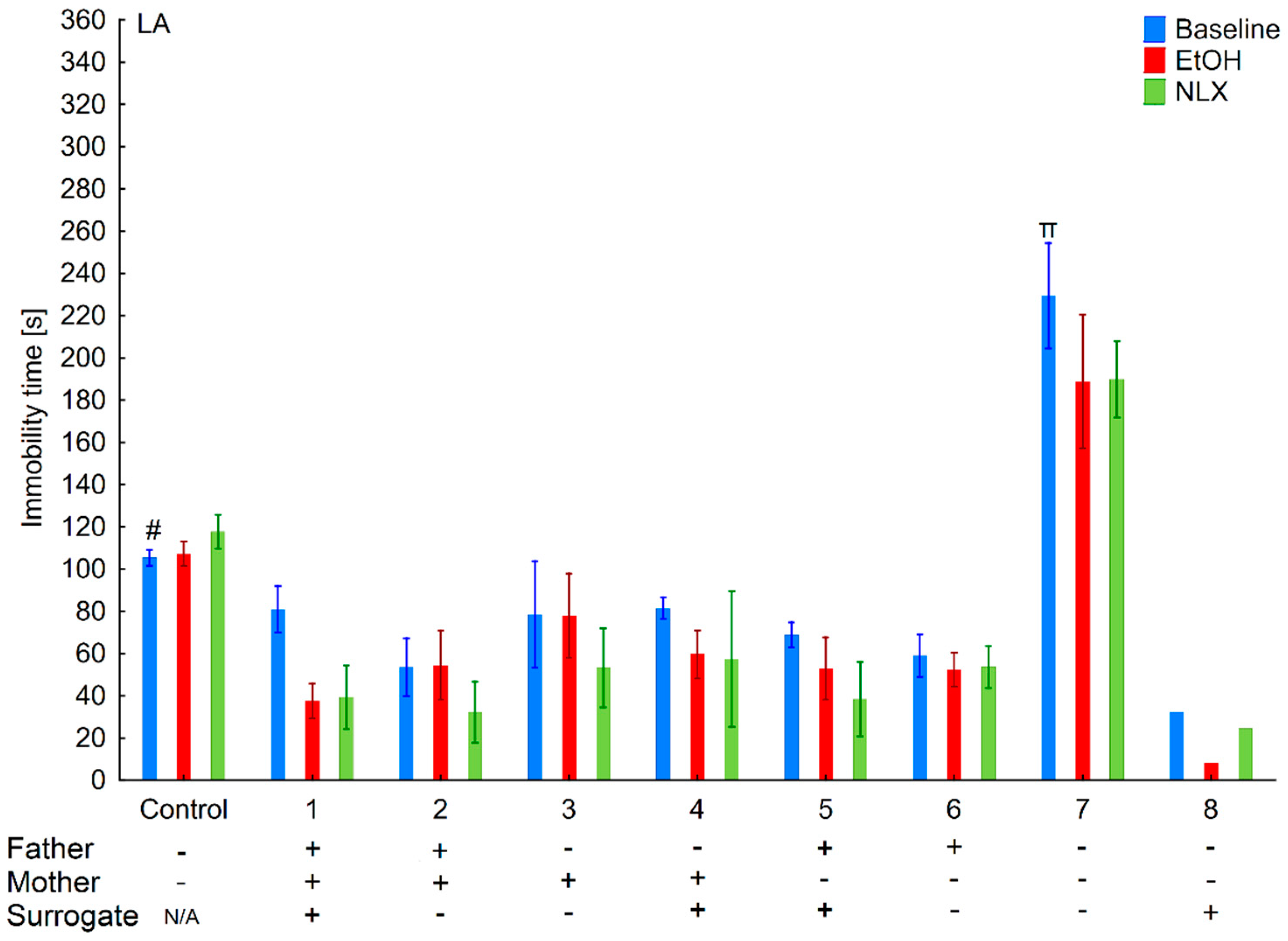
3.3. Changes in the Level of Depressive-Like Behaviors in the HA and LA Mice Reared by a Surrogate of the Same Line
3.4. Effects of Ethanol Consumption on Depressive-Like Behaviors in the HA and LA Mice Reared by a Surrogate of the Same Line
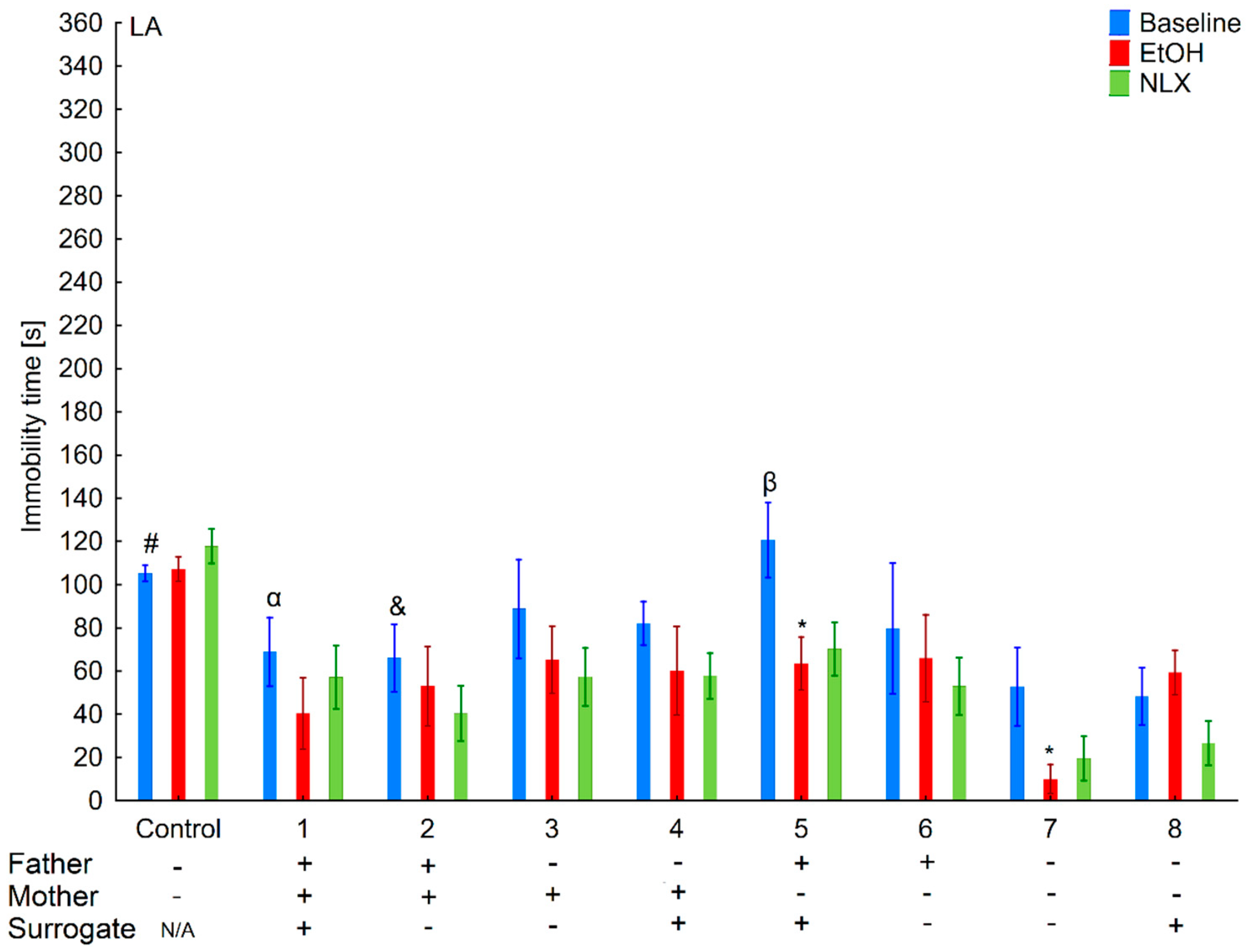
3.5. Effects of Naloxone on Depression-Like Behaviors in the LA and HA Mice Reared by a Surrogate of the Same Line
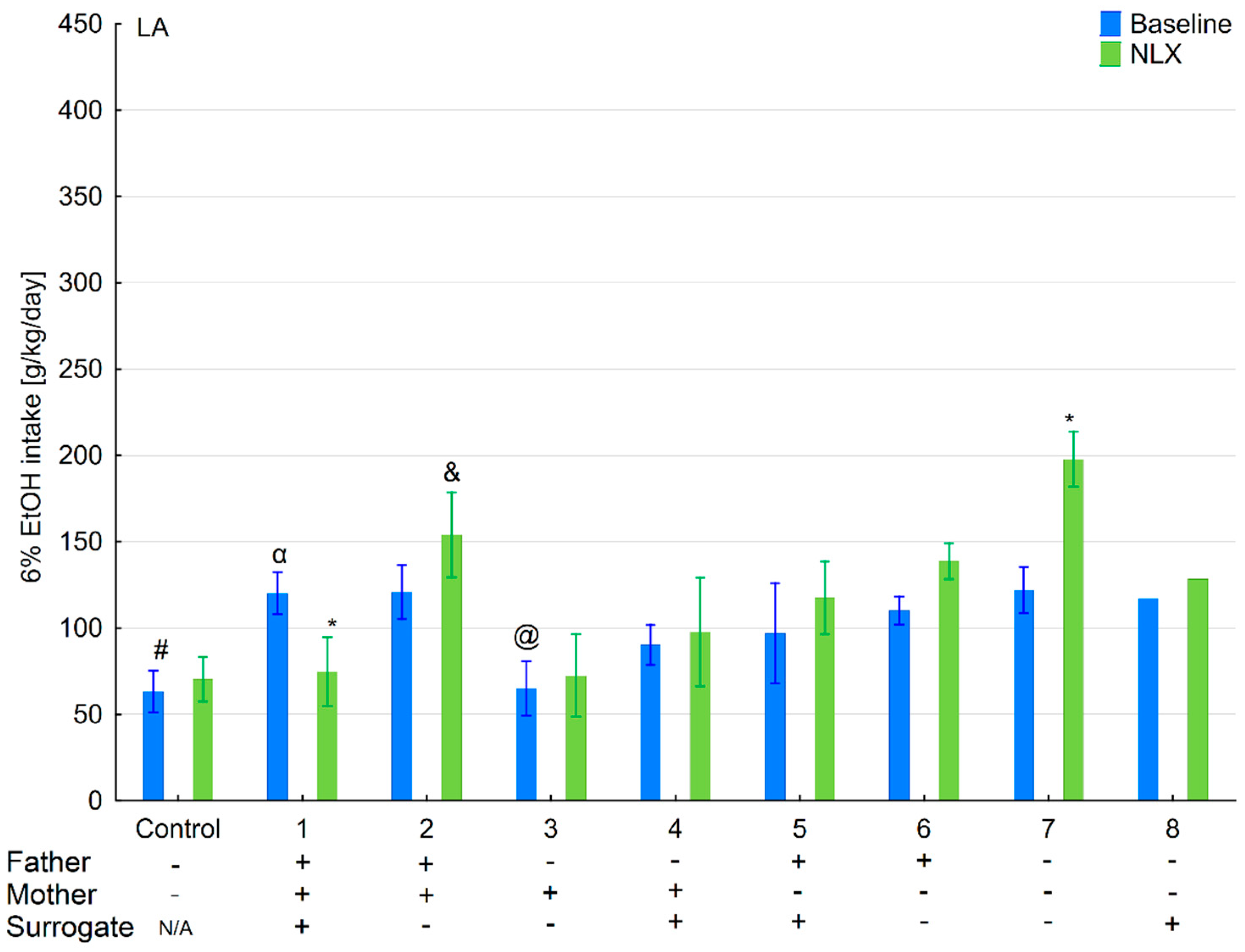
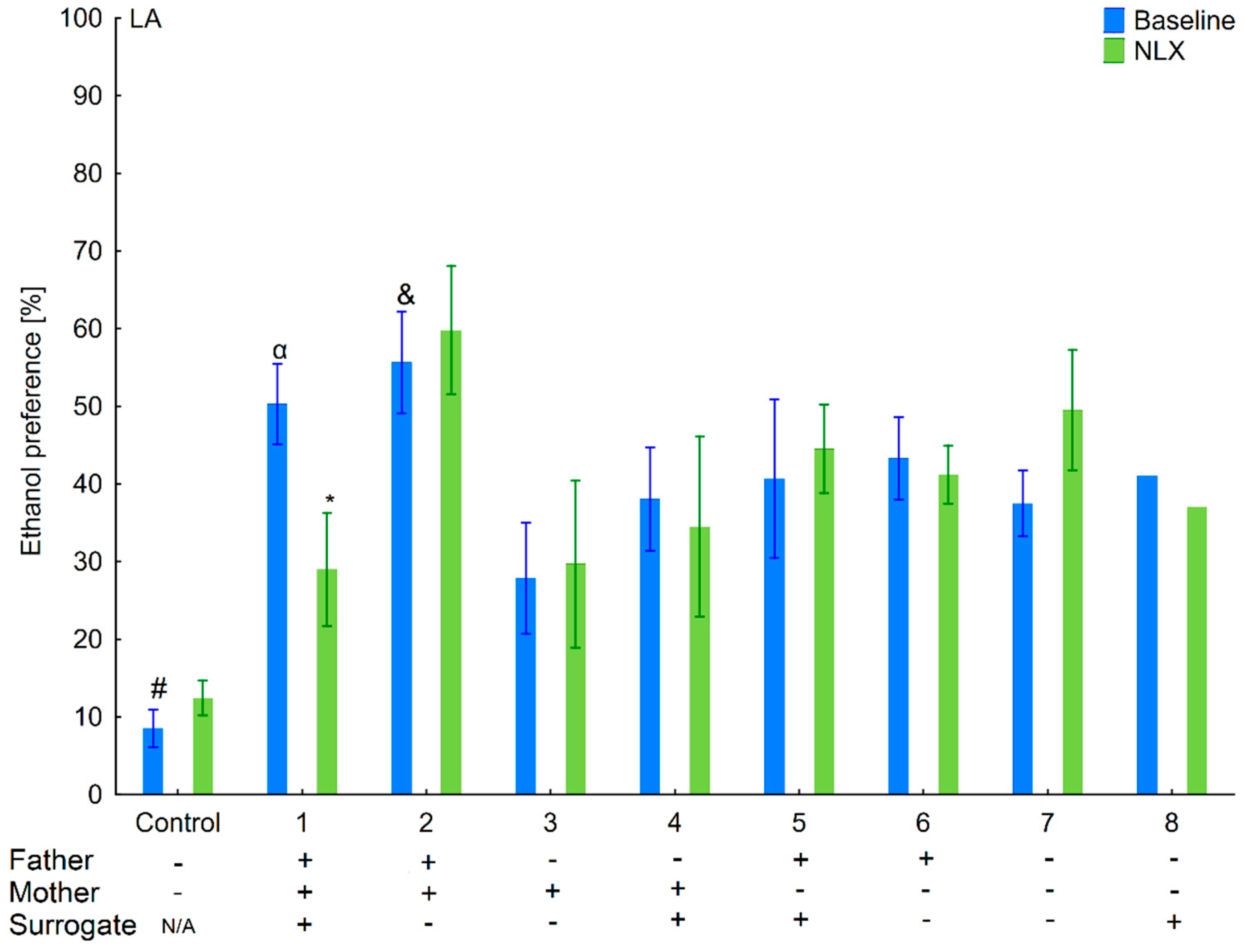
3.6. Changes in Ethanol Intake and Preference in the HA and LA Mice Reared by Surrogates of the Opposite Line
3.7. Effects of Naloxone on Ethanol Consumption and Preference in the HA and LA Mice Reared by Surrogates of the Opposite Line
3.8. Changes in the Level of Depressive-Like Behaviors of the HA and LA Mice Reared by a Surrogate of the Opposite Line
3.9. Effects of Ethanol Consumption on Depressive-Like Behaviors in the HA and LA Mice Reared by a Surrogate of the Same Line
3.10. Effects of Naloxone on Depression-Like Behaviors in the La and Ha Mice Rearing by A Surrogate of the Opposite Line
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Topiwala, A.; Allan, C.L.; Valkanova, V.; Zsoldos, E.; Filippini, N.; Sexton, C.; Mahmood, A.; Fooks, P.; Singh-Manoux, A.; Mackay, C.E.; et al. Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: Longitudinal cohort study. BMJ 2017, 357, j2353. [Google Scholar] [CrossRef] [PubMed]
- Gianoulakis, C. Influence of the endogenous opioid system on high alcohol consumption and genetic predisposition to alcoholism. J. Psychiatry Neurosci. 2001, 26, 304–318. [Google Scholar]
- Craven, B.M.; Marlow, M.L.; Shiers, A.F. The Economics of Minimum Pricing for Alcohol. Econ. Aff. 2013, 33, 174–189. [Google Scholar] [CrossRef]
- Bierut, L.J. Genetic Vulnerability and Susceptibility to Substance Dependence. Neuron 2011, 69, 618–627. [Google Scholar] [CrossRef]
- Connor, J.P.; Haber, P.S.; Hall, W.D. Alcohol use disorders. Lancet 2016, 387, 988–998. [Google Scholar] [CrossRef]
- Roman, E.; Nylander, I. The impact of emotional stress early in life on adult voluntary ethanol intake-results of maternal separation in rats. Stress 2005, 8, 157–174. [Google Scholar] [CrossRef]
- Anacker, A.M.J.; Ryabinin, A.E. Biological contribution to social influences on alcohol drinking: Evidence from animal models. Int. J. Environ. Res. Public Health 2010, 7, 473–493. [Google Scholar] [CrossRef]
- Ieraci, A.; Mallei, A.; Popoli, M. Social Isolation Stress Induces Anxious-Depressive-Like Behavior and Alterations of Neuroplasticity-Related Genes in Adult Male Mice. Neural Plast 2016, 2016, 6212983. [Google Scholar] [CrossRef]
- Hall, F.S.; Huang, S.; Fong, G.W.; Pert, A.; Linnoila, M. Effects of isolation-rearing on voluntary consumption of ethanol, sucrose and saccharin solutions in Fawn Hooded and Wistar rats. Psychopharmacology 1998, 139, 210–216. [Google Scholar] [CrossRef]
- Nutt, D.J. The role of the opioid system in alcohol dependence. J. Psychopharmacol. 2014, 28, 8–22. [Google Scholar] [CrossRef]
- Sacharczuk, M.; Juszczak, G.; Sliwa, A.T.; Tymosiak-Zielinska, A.; Lisowski, P.; Jaszczak, K.; Pluta, R.; Lipkowski, A.; Sadowski, B.; Swiergiel, A.H. Differences in ethanol drinking between mice selected for high and low swim stress-induced analgesia. Alcohol 2008, 42, 487–492. [Google Scholar] [CrossRef]
- Sacharczuk, M.; Juszczak, G.; Swiergiel, A.H.; Jaszczak, K.; Lipkowski, A.W.; Sadowski, B. Alcohol reverses depressive and pronociceptive effects of chronic stress in mice with enhanced activity of the opioid system. Acta Neurobiol. Exp. (Wars) 2009, 69, 459–468. [Google Scholar]
- Jann, M.W.; Slade, J.H. Antidepressant Agents for the Treatment of Chronic Pain and Depression. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2007, 27, 1571–1587. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, K.; Taniguchi, A.; Tokuyama, S. Changes in opioid receptors, opioid peptides and morphine antinociception in mice subjected to early life stress. Eur. J. Pharmacol. 2020, 881, 173173. [Google Scholar] [CrossRef]
- Granholm, L.; Todkar, A.; Bergman, S.; Nilsson, K.; Comasco, E.; Nylander, I. The expression of opioid genes in non-classical reward areas depends on early life conditions and ethanol intake. Brain Res. 2017, 1668, 36–45. [Google Scholar] [CrossRef]
- Moles, A.; Kieffer, B.L.; Amato, F.R. Deficit in Attachment Behavior in Mice Lacking the µ-Opioid Receptor Gene. Science 2004, 304, 1983. [Google Scholar] [CrossRef]
- Enoch, M.A. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology (Berl) 2011, 214, 17–31. [Google Scholar] [CrossRef]
- Poznański, P.; Leśniak, A.; Strzemecka, J.; Sacharczuk, M. Przegląd badań nad uzależnieniem od alkoholu w modelu myszy selekcjonowanych w kierunku wysokiej i niskiej analgezji postresowej. Health Probl. Civiliz. 2018, 12, 217–222. [Google Scholar] [CrossRef]
- Panocka, I.; Marek, P.; Sadowski, B. Differentiation of neurochemical basis of stress-induced analgesia in mice by selective breeding. Brain Res. 1986, 397, 156–160. [Google Scholar] [CrossRef]
- Poznanski, P.; Lesniak, A.; Korostynski, M.; Szklarczyk, K.; Lazarczyk, M.; Religa, P.; Bujalska-Zadrozny, M.; Sadowski, B.; Sacharczuk, M. Delta-opioid receptor antagonism leads to excessive ethanol consumption in mice with enhanced activity of the endogenous opioid system. Neuropharmacology 2017, 118, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology 1985, 85, 367–370. [Google Scholar] [CrossRef]
- Francis, D.D.; Szegda, K.; Campbell, G.; Martin, W.D.; Insel, T.R. Epigenetic sources of behavioral differences in mice. Nat. Neurosci. 2003, 6, 445–446. [Google Scholar] [CrossRef]
- White, J.M.; Legates, J.E.; Eisen, E.J. Maternal effects among lines of mice selected for body weight. Genetics 1968, 60, 395–408. [Google Scholar] [CrossRef]
- Moore, R.W.; Eisen, E.J.; Ulberg, L.C. Prenatal and postnatal maternal influences on growth in mice selected for body weight. Genetics 1970, 64, 59–68. [Google Scholar] [CrossRef]
- Kurnianto, E.; Shinjo, A.; Suga, D. Prenatal and Postnatal Maternal Effects on Body Weight in Cross-fostering Experiment on Two Subspecies of Mice. Exp. Anim. 1998, 47, 97–103. [Google Scholar] [CrossRef][Green Version]
- Brandsch, H.; Kadry, A. The relative importance of prenatal and postnatal maternal influences on growth in mice. Theor. Appl. Genet. 1977, 51, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Matthews, P.A.; Samuelsson, A.-M.; Seed, P.; Pombo, J.; Oben, J.A.; Poston, L.; Taylor, P.D. Fostering in mice induces cardiovascular and metabolic dysfunction in adulthood. J. Physiol. 2011, 589, 3969–3981. [Google Scholar] [CrossRef]
- Anisman, H.; Zaharia, M.D.; Meaney, M.J.; Merali, Z. Do early-life events permanently alter behavioral and hormonal responses to stressors? Int. J. Dev. Neurosci. 1998, 16, 149–164. [Google Scholar] [CrossRef]
- Francis, D.; Diorio, J.; Liu, D.; Meaney, M.J. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science 1999, 286, 1155–1158. [Google Scholar] [CrossRef] [PubMed]
- Van Vugt, R.W.M.; Meyer, F.; van Hulten, J.A.; Vernooij, J.; Cools, A.R.; Verheij, M.M.M.; Martens, G.J.M. Maternal care affects the phenotype of a rat model for schizophrenia. Front. Behav. Neurosci. 2014, 8, 268. [Google Scholar] [CrossRef]
- Barrenha, G.D.; Chester, J. Effects of cross-fostering on alcohol preference and correlated responses to selection in high- and low-alcohol-preferring mice. Alcohol. Clin. Exp. Res. 2012, 36, 2065–2073. [Google Scholar] [CrossRef]
- King, S.M.; Keyes, M.; Malone, S.M.; Elkins, I.; Legrand, L.N.; Iacono, W.G.; McGue, M. Parental alcohol dependence and the transmission of adolescent behavioral disinhibition: A study of adoptive and non-adoptive families. Addiction 2009, 104, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.O.; Pickens, R.W. Familial Transmission of Alcoholism Among Nonalcoholics and Mild, Severe, and Dyssocial Subtypes of Alcoholism. Alcohol. Clin. Exp. Res. 2001, 25, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Bucholz, K.K.; McCutcheon, V.V.; Agrawal, A.; Dick, D.M.; Hesselbrock, V.M.; Kramer, J.R.; Kuperman, S.; Nurnberger, J.I., Jr.; Salvatore, J.E.; Schuckit, M.A.; et al. Comparison of Parent, Peer, Psychiatric, and Cannabis Use Influences Across Stages of Offspring Alcohol Involvement: Evidence from the COGA Prospective Study. Alcohol. Clin. Exp. Res. 2017, 41, 359–368. [Google Scholar] [CrossRef]
- Mogil, J.S.; Sternberg, W.F.; Balian, H.; Liebeskind, J.C.; Sadowski, B. Opioid and Nonopioid Swim Stress-Induced Analgesia: A Parametric Analysis in Mice. Physiol. Behav. 1996, 59, 123–132. [Google Scholar] [CrossRef]
- Ceccanti, M.; Coccurello, R.; Carito, V.; Ciafrè, S.; Ferraguti, G.; Giacovazzo, G.; Mancinelli, R.; Tirassa, P.; Chaldakov, G.N.; Pascale, E.; et al. Paternal alcohol exposure in mice alters brain NGF and BDNF and increases ethanol-elicited preference in male offspring. Addict. Biol. 2016, 21, 776–787. [Google Scholar] [CrossRef]
- Rompala, G.R.; Finegersh, A.; Homanics, G.E. Paternal preconception ethanol exposure blunts hypothalamic-pituitary-adrenal axis responsivity and stress-induced excessive fluid intake in male mice. Alcohol 2016, 53, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Rompala, G.R.; Finegersh, A.; Slater, M.; Homanics, G.E. Paternal preconception alcohol exposure imparts intergenerational alcohol-related behaviors to male offspring on a pure C57BL/6J background. Alcohol 2017, 60, 169–177. [Google Scholar] [CrossRef]
- Rompala, G.R.; Homanics, G.E. Intergenerational Effects of Alcohol: A Review of Paternal Preconception Ethanol Exposure Studies and Epigenetic Mechanisms in the Male Germline. Alcohol. Clin. Exp. Res. 2019, 43, 1032–1045. [Google Scholar] [CrossRef]
- Sørensen, H.J.; Manzardo, A.M.; Knop, J.; Penick, E.C.; Madarasz, W.; Nickel, E.J.; Becker, U.; Mortensen, E.L. The Contribution of Parental Alcohol Use Disorders and Other Psychiatric Illness to the Risk of Alcohol Use Disorders in the Offspring. Alcohol. Clin. Exp. Res. 2011, 35, 1315–1320. [Google Scholar] [CrossRef]
- Mellentin, A.I.; Brink, M.; Andersen, L.; Erlangsen, A.; Stenager, E.; Bjerregaard, L.B.; Christiansen, E. The risk of offspring developing substance use disorders when exposed to one versus two parent(s) with alcohol use disorder: A nationwide, register-based cohort study. J. Psychiatr. Res. 2016, 80, 52–58. [Google Scholar] [CrossRef]
- McCutcheon, V.V.; Agrawal, A.; Kuo, S.I.C.; Su, J.; Dick, D.M.; Meyers, J.L.; Edenberg, H.J.; Nurnberger, J.I.; Kramer, J.R.; Kuperman, S.; et al. Associations of parental alcohol use disorders and parental separation with offspring initiation of alcohol, cigarette and cannabis use and sexual debut in high-risk families. Addiction 2018, 113, 336–345. [Google Scholar] [CrossRef]
- Fava, M.; Kendler, K.S. Major depressive disorder. Neuron 2000, 28, 335–341. [Google Scholar] [CrossRef]
- Weissman, M.M.; Wickramaratne, P.; Nomura, Y.; Warner, V.; Pilowsky, D.; Verdeli, H. Offspring of depressed parents: 20 years later. Am. J. Psychiatry 2006, 163, 1001–1008. [Google Scholar] [CrossRef]
- Tully, E.C.; Iacono, W.G.; McGue, M. An adoption study of parental depression as an environmental liability for adolescent depression and childhood disruptive disorders. Am. J. Psychiatry 2008, 165, 1148–1154. [Google Scholar] [CrossRef] [PubMed]
- Malkesman, O.; Lavi-Avnon, Y.; Maayan, R.; Weizman, A. A cross-fostering study in a genetic animal model of depression: Maternal behavior and depression-like symptoms. Pharmacol. Biochem. Behav. 2008, 91, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lerch, S.; Brandwein, C.; Dormann, C.; Gass, P.; Chourbaji, S. What makes a good mother? Implication of inter-, and intrastrain strain “cross fostering” for emotional changes in mouse offspring. Behav. Brain Res. 2014, 274, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Barbazanges, A.; Vallee, M.; Mayo, W.; Day, J.; Simon, H.; Le Moal, M.; Maccari, S. Early and Later Adoptions Have Different Long-Term Effects on Male Rat Offspring. J. Neurosci. 1996, 16, 7783–7790. [Google Scholar] [CrossRef]
- Liang, F.; Diao, L.; Liu, J.; Jiang, N.; Zhang, J.; Wang, H.; Zhou, W.; Huang, G.; Ma, D. Paternal ethanol exposure and behavioral abnormities in offspring: Associated alterations in imprinted gene methylation. Neuropharmacology 2014, 81, 126–133. [Google Scholar] [CrossRef]
- Ridgeway, B. Depression, Alcohol Abuse, and Alcoholism in One versus Two Parents and the Implications for Child Attachment and Self-Regulation in Infancy through Adolescence. Int. Sch. Res. Not. 2015, 2015, 275649. [Google Scholar] [CrossRef]
- Anda, R.F.; Whitfield, C.L.; Felitti, V.J.; Chapman, D.; Edwards, V.J.; Dube, S.R.; Williamson, D.F. Adverse childhood experiences, alcoholic parents, and later risk of alcoholism and depression. Psychiatr. Serv. 2002, 53, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Kelley, M.L.; Pearson, M.R.; Trinh, S.; Klostermann, K.; Krakowski, K. Maternal and paternal alcoholism and depressive mood in college students: Parental relationships as mediators of ACOA-depressive mood link. Addict. Behav. 2011, 36, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Marmorstein, N.R.; Iacono, W.G.; McGue, M. Associations between substance use disorders and major depression in parents and late adolescent–emerging adult offspring: An adoption study. Addiction 2012, 107, 1965–1973. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.H.; Powell, J.E.; Marshall, E.J.; Peters, T.J. Quality of life in alcohol-dependent subjects--a review. Qual. Life Res. 1999, 8, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Paljärvi, T.; Koskenvuo, M.; Poikolainen, K.; Kauhanen, J.; Sillanmäki, L.; Mäkelä, P. Binge drinking and depressive symptoms: A 5-year population-based cohort study. Addiction 2009, 104, 1168–1178. [Google Scholar] [CrossRef]
- Young-Wolff, K.C.; Kendler, K.S.; Sintov, N.D.; Prescott, C.A. Mood-Related Drinking Motives Mediate the Familial Association Between Major Depression and Alcohol Dependence. Alcohol. Clin. Exp. Res. 2009, 33, 1476–1486. [Google Scholar] [CrossRef]
- Lesniak, A.; Chmielewska, D.; Poznanski, P.; Bujalska-Zadrozny, M.; Strzemecka, J.; Sacharczuk, M. Divergent Response to Cannabinoid Receptor Stimulation in High and Low Stress-Induced Analgesia Mouse Lines Is Associated with Differential G-Protein Activation. Neuroscience 2019, 404, 246–258. [Google Scholar] [CrossRef]
- Ruda-Kucerova, J.; Babinska, Z.; Amchova, P.; Stark, T.; Drago, F.; Sulcova, A.; Micale, V. Reactivity to addictive drugs in the methylazoxymethanol (MAM) model of schizophrenia in male and female rats. World J. Biol. Psychiatry 2017, 18, 129–142. [Google Scholar] [CrossRef]
- Ruda-Kucerova, J.; Pistovcakova, J.; Amchova, P.; Sulcova, A.; Machalova, A. Prenatal exposure to modafinil alters behavioural response to methamphetamine in adult male mice. Int. J. Dev. Neurosci. 2018, 67, 37–45. [Google Scholar] [CrossRef]



| Experimental Group | Parental Variant | ||
|---|---|---|---|
| Biological Father | Biological Mother | Surrogate Mother | |
| 1 | + | + | + |
| 2 | + | + | − |
| 3 | − | + | − |
| 4 | − | + | + |
| 5 | + | − | + |
| 6 | + | − | − |
| 7 | − | − | − |
| 8 | − | − | + |
| Control | − | − | No transfer |
| Experimental Group | HA Within | HA Between | LA Witihn | LA Between |
|---|---|---|---|---|
| 1 | 6♂ | 5♂ | 6♂ | 5♂ |
| 2 | 6♂ | 5♂ | 6♂ | 4♂ |
| 3 | 6♂ | 5♂ | 6♂ | 4♂ |
| 4 | 6♂ | 4♂ | 6♂ | 4♂ |
| 5 | 6♂ | 5♂ | 6♂ | 5♂ |
| 6 | 6♂ | 5♂ | 6♂ | 4♂ |
| 7 | 6♂ | 5♂ | 6♂ | 4♂ |
| 8 | 6♂ | 2♂ 2♀ | 6♂ | 4♂ |
| Control | 6♂ | 6♂ | 6♂ | 6♂ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawrocka, A.; Poznański, P.; Łazarczyk, M.; Gorzałczyński, M.; Skiba, D.; Wolińska, R.; Bujalska-Zadrożny, M.; Lutfy, K.; Sadowski, B.; Sacharczuk, M. The Influence of Cross-Fostering on Alcohol Consumption and Depressive-Like Behaviors in HA and LA Mice: The Role of the Endogenous Opioid System. Brain Sci. 2021, 11, 622. https://doi.org/10.3390/brainsci11050622
Nawrocka A, Poznański P, Łazarczyk M, Gorzałczyński M, Skiba D, Wolińska R, Bujalska-Zadrożny M, Lutfy K, Sadowski B, Sacharczuk M. The Influence of Cross-Fostering on Alcohol Consumption and Depressive-Like Behaviors in HA and LA Mice: The Role of the Endogenous Opioid System. Brain Sciences. 2021; 11(5):622. https://doi.org/10.3390/brainsci11050622
Chicago/Turabian StyleNawrocka, Agata, Piotr Poznański, Marzena Łazarczyk, Michał Gorzałczyński, Dominik Skiba, Renata Wolińska, Magdalena Bujalska-Zadrożny, Kabirullah Lutfy, Bogdan Sadowski, and Mariusz Sacharczuk. 2021. "The Influence of Cross-Fostering on Alcohol Consumption and Depressive-Like Behaviors in HA and LA Mice: The Role of the Endogenous Opioid System" Brain Sciences 11, no. 5: 622. https://doi.org/10.3390/brainsci11050622
APA StyleNawrocka, A., Poznański, P., Łazarczyk, M., Gorzałczyński, M., Skiba, D., Wolińska, R., Bujalska-Zadrożny, M., Lutfy, K., Sadowski, B., & Sacharczuk, M. (2021). The Influence of Cross-Fostering on Alcohol Consumption and Depressive-Like Behaviors in HA and LA Mice: The Role of the Endogenous Opioid System. Brain Sciences, 11(5), 622. https://doi.org/10.3390/brainsci11050622







