Abstract
Developmental Language Disorder (DLD) is often associated with impairments in working memory (WM), executive functions (EF), and verbal fluency. Moreover, increasing evidence shows poorer performance of children with DLD on non-verbal intelligence tests relative to their typically developing (TD) peers. Yet, the degree and generality of relevant difficulties remain unclear. The present study aimed at investigating WM capacity, key EFs and verbal fluency in relation to non-verbal intelligence in Greek-speaking school-age children with DLD, compared to TD peers (8–9 years). To our knowledge, the present study is the first to attempt a systematic relevant assessment with Greek-speaking school-age children, complementing previous studies mostly involving English-speaking participants. The results showed that children with DLD scored lower than TD peers on the non-verbal intelligence measure. Groups did not differ in the inhibition measures obtained (tapping resistance to either distractor or proactive interference), but children with DLD were outperformed by TD peers in the WM capacity, updating, monitoring (mixing cost), and verbal fluency (phonological and semantic) measures. The effects showed limited (in the case of backward digit recall) or no dependence on non-verbal intelligence. Findings are discussed in terms of their theoretical and practical implications as well as in relation to future lines of research.
1. Introduction
Developmental Language Disorder (DLD) (previously known as Specific Language Impairment or Language Impairment) in children has been the focus of scientific attention for the last three decades. However, the mechanisms underlying this disorder remain unclear. There is growing evidence that besides linguistic factors, domain-general cognitive abilities may contribute to the difficulties associated with DLD [,]. According to Tomas and Vissers [], children with DLD seem to face multiple cognitive deficits which impede typical language acquisition and development. Fine-grained evaluations of cognitive abilities may thus contribute to accurate, early identification and diagnosis of DLD, as well as to a more comprehensive description of DLD profiles [,], that could, in turn, increase the effectiveness of relevant interventions, tailoring them to the linguistic as well as cognitive strengths and weaknesses of each child with DLD.
In such a context, the present study attempts a systematic exploration of cognitive capacities and functions in Greek-speaking school-age children with DLD. Specifically, we focus on working memory (WM) capacity, namely the ability to concurrently maintain and process information [,]). We also study key executive functions (EFs) in DLD, as defined by Miyake and colleagues []; that is, focusing on updating, set-shifting (otherwise, task switching), and inhibition. Such a tripartite structure has been confirmed in studies involving preschoolers [] as well as school-age children []. Moreover, we aim for a comprehensive approach to the study of inhibition. In doing so, we adopt the perspective of Friedman and Miyake [] and rely on their taxonomy of inhibition tasks, thus differentiating evidence with regard to prepotent response inhibition, resistance to distractor interference, and resistance to proactive interference. Moreover, given inconclusive evidence regarding verbal fluency in children with DLD—mostly stemming from studies with English-speaking children—we focus on two relevant indices; phonological and semantic fluency. Though the latter capacities have been related to language and executive control to different degrees, very few studies have discussed difficulties of children with DLD in both fluency aspects. The combined investigation of the aforementioned factors has scarcely been attempted, whereas there is no relevant evidence with Greek-speaking school-age children with DLD; the latter remains an under-examined population in the DLD literature. Finally, since more and more researchers emphasize the need to consider non-verbal intelligence as part of the DLD profile [,], the present study also investigates whether any differences observed between children with DLD and typically developing (TD) peers in WM, EFs and verbal fluency, are independent of non-verbal intelligence.
1.1. Developmental Language Disorder
Developmental Language Disorder is defined as difficulty with the acquisition of language skills (expressive and/or receptive) in the absence of any obvious cause, given normal non-verbal IQ and no primary physical disabilities, neurological disorder, or mental illness [,,]. DLD can be reliably diagnosed after the age of 4 years [,,] and is roughly characterized as a lag of about two years in the development of language skills []. It is observed in 5–10% of the population [,], which explains the significant research attention drawn to this disorder over the last 30 years [,,,].
Both genetic [,,,,,] and environmental risk factors (e.g., low socioeconomic status–SES—or fewer years of parental education) [,] have been identified for DLD. Moreover, both language-specific and domain-general accounts of the disorder have been proposed. Within the former, children with DLD are suggested to face difficulties in linguistic processing, mostly in grammatical processing, implying that other cognitive or neural processes remain largely intact as they develop [,,]. Such research focuses on establishing clinical markers of DLD in the language domain, which could be used for screening and intervention. On the other hand, several researchers claim that children with DLD have additional domain-general cognitive processing impairments. Specifically, DLD is approached as multidimensional, encompassing deficits in both the domain of language acquisition [,] as well as co-morbid problems in cognitive domains, including those of attention [], information processing [], simultaneous processing [], and verbal fluency [,,,]. For example, according to the Procedural Deficit Hypothesis (PDH), introduced by Ullman and Pierpont [], DLD can be largely explained by abnormal development of brain structures that support procedural memory; this, in turn, is assumed to result to grammatical impairments as well as deficits, however, in the development and execution of motor and cognitive processes. And although some researchers have suggested a single impaired cognitive mechanism underlying DLD [,], relying on comparisons of children with and without DLD on capacities such as sustained attention [,,] or auditory perception [,,,], others, have related the disorder to impairments in at least two cognitive processes [], emphasizing the relation of linguistic and higher-order processing (e.g., EF) in children with DLD.
In summary, the underlying causes for DLD are still not well understood. Although the diagnosis of the disorder is based on impaired language acquisition in the absence of cognitive impairment, more and more researchers are questioning such exclusionary criteria, suggesting the need for clearer insight to be obtained into the interaction of higher-order cognitive functioning with language development in children with DLD [,,].
1.2. Non-Verbal Intelligence in Children with DLD
Traditionally, the diagnosis of DLD has required non-verbal IQ within normal limits [,]. However, there are studies that have reported poorer non-verbal intellectual functioning in children with this disorder relative to TD peers [,,]. For example, in a recent meta-analysis, Gallinat and Spaulding [] found that children with DLD performed on average 10 points below the TD children on non-verbal intelligence []. The researchers actually suggest that poorer performance in non-verbal intelligence tests is part of the clinical profile of children with DLD. Moreover, in a recent project (CATALISE) which aimed for a consensus on terminology for unexplained language problems in children, it was overall claimed that discrepancy between verbal and non-verbal ability is not indicative of underlying etiology in DLD []. For example, identical twins with language problems often had very similar language profiles but differed in terms of non-verbal ability, with one twin meeting traditional discrepancy criteria for DLD and the other one not doing so []. Furthermore, for a child without an identified syndrome, the level of non-verbal IQ does not appear to determine responsiveness to therapy. Therefore, Bishop [] claimed that there is no justification for using verbal vs. non-verbal ability discrepancy as a diagnostic criterion, as it might exclude from diagnosis, and thus intervention, children with DLD that could have otherwise benefited. It seems that the non-verbal IQ diagnostic criterion of DLD has been at the center of an ongoing debate within the field [,].
1.3. Working-Memory Capacity in Children with DLD
Working-memory capacity regards an individual’s ability to concurrently process and store information, keeping it active and accessible as long as the task at hand necessitates that. There seems to be a growing consensus that the crucial element of WM is controlled attention and coordination of information flow through WM (attributed to the central executive of the Baddeley and Hitch 1974 model [,] and tapped by complex span tasks), rather than its storage capacity []. Such executive attentional control in WM constitutes a powerful predictor of language development [,,] and more generally, a strong determinant of learning capacity and scholastic attainment through the school years (regarding both literacy and numeracy) [,].
To no surprise, WM capacity has therefore received attention in the DLD literature as well. For example, besides performance in EF tasks (see discussion below), difficulties in WM (verbal and visuospatial; as tapped by cognitive measures as well as parental reports) differentiated preschool children with DLD and TD peers in the Vugs et al. study []. In a more recent study, Vugs and colleagues [] showed varying associations of WM with the linguistic skills of children with DLD (receptive and expressive vocabulary, oral comprehension, and syntactic development). Moreover, limitations in WM capacity (as tapped by verbal tasks) have been found to constrain language development of school-age children with DLD beyond any intelligence effects [,,] or general language ability effects (see also Archibald and Gathercole [], where relevant visuospatial STM/WM effects were less marked or insignificant). In one of the few systematic explorations of cognitive functioning in children with DLD (8- to 14-year-olds), Henry et al. [] reported smaller WM capacity, less efficient inhibition (prepotent response inhibition) and planning, and lower fluency among children with DLD, but no differences in task switching relative to TD peers. It is noted that the deficits observed were not constrained to verbal tasks and were independent of any IQ effects (verbal and non-verbal) []. Yet, such systematic explorations of cognitive functioning in children with DLD remain few. Given the relevant scope of the present study, the following section reviews evidence on key EFs in relation to DLD, focusing on updating in WM and task switching, and differentiating findings with regard to distinct inhibition functions; that is, response inhibition, resistance to distractor interference, and the least investigated in the DLD literature, resistance to proactive interference function [].
1.4. Executive Functions in Children with DLD
1.4.1. Updating in Children with DLD
Updating regards constant monitoring and rapid addition or deletion of WM contents in relation to the current task demands []. The specific function has been studied much less than WM capacity in relation to DLD. For example, in the aforementioned systematic investigation of EFs in children with DLD, Henry et al. [] did not include updating measures. Kapa and Plante [] did not discuss relevant findings in their review of EF difficulties of children with DLD, whereas only two studies (discussed below) are reported in the Montgomery et al. [] discussion of memory and language capacities in children with DLD [].
In one of the few relevant studies, Im-Bolter et al. [] assessed updating along with switching and attentional capacities in school-age children (7- to 12-year-olds). Researchers observed greater difficulties in the DLD group relative to the TD children, but only in the one-back condition of the dot-pattern recognition n-back task used. Let us note, however, that this condition sets minimum updating demands, requiring the child to compare each incoming stimulus with the one previously viewed (pressing the “yes” button when it is the same) and discard stimuli more than one-back. Yet, both children with DLD and TD peers performed at chance levels on the two-back condition of the task (where the “yes” button should be pressed when the stimulus is the same as the one viewed two trials back). This pattern limited further exploration of updating in children with DLD, since the two-back task version (also used in the present study) is considered a more valid measure of the specific function relative to its one-back version, and has, thus, more widely been used in relevant investigations. Specifically, the two-back version is assumed to set demands on the executive control system for maintaining accurate representations of information that changes over time while monitoring the process and resisting proactive interference []. In a recent study, Evans et al. [] did not observe significant differences between children with DLD and TD school-age peers (7- to 11-year-olds) in another updating measure employed (keep track task); neither were group differences observed on measures of attention switching and interference resolution. Null findings regarding updating were also obtained by Lukács et al. [] in a study involving 8-year-old children with DLD and TD peers in verbal and non-verbal n-back tasks. Overall, evidence regarding updating in children with DLD remains scarce and inconclusive.
1.4.2. Inhibition in Children with DLD
Inhibition encompasses a set of processes that are important for language development, as well as learning more generally. In their taxonomy, Friedman and Miyake [] (see also Miyake and Friedman []) distinguish among three related functions: (a) prepotent response inhibition, which regards the deliberate overriding of dominant, automatic, or prepotent responses (e.g., tapped by stop-signal or Stroop tasks), (b) resistance to distractor interference, which regards the capacity to resolve conflict created by information that is irrelevant to the task at hand e.g., tapped by flanker tasks), and (c) resistance to proactive interference, that is, capacity to resist intrusions from information that was previously relevant but has since become irrelevant (e.g., tapped by tasks requiring recall of word lists drawn from the same category; see a relevant task in the methods section). Let us note that distractibility and inhibition scores have been found to correlate with standardized language measures in children with DLD, in line with the roles attributed to attention and inhibition in language processing more generally [,]. Overall, however, evidence regarding specific inhibition functions in children with DLD remains scarce and controversial.
Specifically, with regard to prepotent response inhibition, in one of the few systematic investigations of EFs in school-age children with DLD, Im-Bolter, Johnson, and Pascual-Leone [] documented impairment in an antisaccade task. This is consistent with evidence regarding the preschool years: for example, Spaulding [] found that preschoolers with DLD were less efficient in suppressing a prepotent, conflicting response in a stop-signal paradigm, whereas Roello et al. [] reported similar findings in a study using a Stroop-like task (Day-Night). Yet, Noterdaeme et al. [] found no differences between school-age children with DLD (elementary and high school) relative to TD children in a go/no go task. In line, differences were not observed in the Marton et al. [] study, where the stop-signal task was used with 10- to14-year-old children with DLD (see, however, their finding regarding sensitivity to interference below). Nor were differences reported between children with DLD and TD children (mean age: 7.8 years) in the Lukács et al. [] study, relying on accuracy and RT measures of Stroop task versions (verbal and non-verbal). Finally, in one of the few systematic explorations of EFs in children with DLD (8- to 14-year-olds), Henry et al. [] found that children with DLD had difficulties in only one among the inhibition measures obtained (that is, in a non-verbal motor inhibition measure) in comparison to TD children.
With regard to a second inhibition function, Spaulding [] reported decreased resistance to distractor interference, regardless of distractor modality (i.e., non-verbal auditory, linguistic, or visual), in preschoolers with DLD relative to TD peers. In line, Finneran et al. [] found significant accuracy but not also speed differences in favour of TD preschoolers in a task tapping monitoring of the target stimulus and inhibition of distractors. In consistence, with regard to the elementary-school years, children with DLD were found more easily distracted by interfering items relative to TD peers [], regardless of distractor type (e.g., words or pictures in the Victorino and Schwartz study [] with 9- to 12-year-olds). Yet, Evans et al. [] did not observe significant differences in interference inhibition between children with DLD and TD peers (7- to 11-year-olds).
Finally, we lack evidence on a third inhibition function in relation to DLD: that of resistance to proactive interference. As Marton et al. [] noted, the difficulties observed in children with DLD in verbal complex span tasks (such as the ones employed in the present study) might actually reflect poor resistance to both distractor and proactive interference (i.e., inhibiting lexical items that had been the focus of previous searches). Accordigly, with this suggestion, elementary school-age children with DLD were found more susceptible to proactive interference than children in the control group, in the verbal experimental task used by Marton et al. [].
1.4.3. Task Switching in Children with DLD
Task switching regards the ability to shift flexibly between mental sets [,]. Relevant findings for children with DLD are not characterized by consistency []. Task switching difficulties have been observed in studies with preschoolers [,]. On the other hand, though Im-Bolter and colleagues [] reported lower updating and attentional control-inhibition capacities in school-age children with DLD relative to TD peers, they found no differences between groups in task switching (see also Dibbets et al. []; Evans et al. []; Henry et al. [] for similar findings with verbal and non-verbal switching measures). In their recent meta-analysis, however, Pauls and Archibald [] did report poorer cognitive flexibility in children with DLD as tapped by switching tasks, the Wisconsin Card Sorting Test, as well as fluency tasks; however, this effect was smaller than the one regarding inhibition.
1.5. Verbal Fluency in Children with DLD
Verbal fluency tasks measure strategic search and retrieval of information stored in the mental lexicon and, thus, semantic memory []. They require participants to generate as many words as possible within one minute according to simple rules that target sounds (phonological fluency; e.g., items starting with particular letters, such as “f”, “a”, “s”) or semantic categories (semantic fluency; e.g., “animals” or “foods”) [,].
Verbal fluency tasks have been described as measures of EF [,], requiring goal-directed behaviors such as flexibility of thought, strategic planning, non-habitual responses, and error-monitoring, as well as tapping on search and retrieval of information from long-term memory. As noted above, for example, in their meta-analysis on EFs in DLD, Pauls and Archibald [] viewed the verbal fluency tasks as reflective of a subtype of cognitive flexibility that is related to the notion of generativity or creative fluency []. On the other hand, verbal fluency has also been viewed as a language measure [], indicating proficiency [] as a function of vocabulary size [,]. Finally, there are researchers acknowledging the mixed demands set by verbal fluency tasks, thus, approaching them as executive language tasks []. Within this context, there have also been attempts to disentangle the demands set by phonological versus semantic fluency in relevant tests. For example, performance on semantic fluency tests has been suggested to largely depend on lexical-retrieval speed as well as on the employment of visualization strategies to support controlled retrieval; whereas performance on phonological fluency tests has been suggested to heavily rely on vocabulary knowledge (see discussion in Gordon et al. []).
Despite such links to both language and EF, there are surprisingly few studies regarding verbal fluency in children with DLD, with findings remaining inconclusive so far []. Weckerly et al. [], for example, showed both semantic and phonological fluency difficulties in school-age children with DLD []. Rodríguez et al. [] reported problems with verbal fluency in the expressive DLD group of their study (not also in the expressive-receptive group). Verbal fluency difficulties were also observed in the DLD groups of the Lukács et al. [] and the Henry et al. [] (coupled with difficulties in non-verbal fluency) studies with school-age children. On the other hand, there have been studies reporting insignificant semantic fluency differences between children with DLD and TD peers [,]. Besides, controversial findings remain limited in languages other than English. A recently published study in the language of the present sample (one of the very few in Greek; see Mengisidou et al. []) found poorer semantic fluency performance in school-age children with DLD relative to TD peers; yet, researchers have not also used a phonological fluency measure.
1.6. Rationale of the Present Study
According to the literature reviewed, although several studies have focused on the performance of children with DLD in different cognitive tasks, it is not easy to draw a clear overall picture of relevant strengths and weaknesses. Actually, very few studies have employed measures tapping both WM capacity and different EFs [,,,], whereas even fewer have involved populations other than English-speaking children. In the previous sections, we have attempted a review of findings regarding WM capacity (as tapped by complex span tasks) as well as key EFs [] and verbal fluency in children with DLD. With regard to EFs, we have discussed evidence regarding updating, task switching, and inhibition, differentiating, however, among inhibition-related functions (response inhibition, resistance to distractor interference, and resistance to proactive interference) []. Let us note that these functions do not develop at the same rate through childhood (see discussion in Best and Miller [,]) and might not be equally vulnerable in children with developmental disorders. As Marton et al. [] noted, for example, even if school-age children with DLD perform similarly to TD peers in response inhibition, as some studies have shown, this might not be the case with regard to resistance to interference, which develops later.
Within this context, the present study aimed at investigating WM capacity, key EFs, and verbal fluency in Greek-speaking children with DLD and TD peers in relation to non-verbal intelligence. Even though these cognitive capacities play a key role in learning and thus, school performance as well as daily life, relevant systematic assessments in children with DLD remain limited in number; whereas, to our knowledge, this is the first study attempting a comprehensive relevant investigation in Greek-speaking school-age children with DLD. More generally, few studies have so far focused on DLD populations speaking a language other than English, despite evidence on possible modulation of the language-cognition interplay by the characteristics of the developing language []. It is noted that our parallel interest in non-verbal intelligence stems from recent evidence [,,,], showing significant differences in non-verbal intelligence measures between groups of children with DLD and TD peers even if, by definition, DLD diagnosis implies typical non-verbal intelligence. As noted in the relevant section of the introduction, however, the non-verbal IQ diagnostic criterion of DLD is actually at the center of an ongoing debate [,]. Thus, non-verbal intelligence was assessed in our study not only to obtain insight into the intellectual functioning of participants and inform group formation (ensuring inclusion of participants with average intelligence in both groups), but also to consider its possible role in the demonstration of group differences in WM capacity, the different EFs studied, and verbal fluency [].
We stated the following research questions:
- Do Greek-speaking school-age children with DLD have lower scores than TD peers on non-verbal intelligence?
- Are Greek-speaking school-age children with DLD outperformed by TD peers in tasks tapping (a) WM capacity, (b) key EFs; that is updating, the resistance to distractor interference and resistance to proactive interference functions of inhibition, and task switching, and (c) verbal (phonological or semantic) fluency (executive-language measures)?
- Are any group differences in WM capacity, EFs, and verbal fluency independent of non-verbal intelligence?
We stated the following hypotheses:
We expected children with DLD to be outperformed by TD peers on the non-verbal intelligence measure (even if both groups are of average intelligence), based on increasing evidence showing such group differences. With regard to the second and third research questions, we opted for a general hypothesis, given inconclusive findings in the literature as well as scarce evidence with Greek-speaking school-age children with DLD. We expected differences in favor of the TD children in the cognitive measures obtained that would be independent of non-verbal intelligence.
2. Materials and Methods
2.1. Participants
Participants were 58 monolingual Greek-speaking children attending the third grade of elementary state schools in Athens: 29 children with DLD and 29 TD peers (mean age 8.6 and 8.9 years, respectively). All participants had average intelligence (with a standard score of 85 or above in the Raven’s Educational CPM/CVS (Raven []; standardized for use with Greek children by Sideridis et al. []). Children with a diagnosed hearing impairment, neurological disorder, ADD/ADHD, or autism spectrum disorder were excluded from the sample. We recruited children with DLD based on an existing relevant diagnosis from a speech and language pathologist, which was further confirmed based on our assessments. Specifically, children in this group were found to perform approximately 1.25 SDs or more below the mean on two standardized language measures, following Tomblin []. The language measures included the vocabulary subtest from WISC-III in Greek [] and the Sentence completion subtest from the Athena test for the Diagnosis of Learning Difficulties []. All TD children performed within normal ranges in both standardized language tests.
The two groups were matched in terms of gender, χ2 (1, N = 58) = 0.624, p = 0.599 (DLD group: 15 boys, 14 girls; TD peers: 12 boys, 17 girls). Finally, given significant correlations between SES and cognitive development [,], a mean parental SES score was calculated for each participant, based on the sum of ratings regarding his/her mother’s and father’s (a) education level (from 0—did not finish elementary school, to 6—Master’s/PhD recipient), (b) type of occupation (0—unemployed, 1—blue collar, 2—white collar), and (c) position in occupation (0—unemployed to 6—big business owner or executive member of the private or public sector) [,]. The groups of children with DLD and TD peers were found matched on parental SES, F(1, 56) = 3.347, p = 0.073, ηp2 = 0.056 (MDLD = 6.05, MTD = 7.10).
2.2. Design
This study stems from a wider research project examining reading and writing skills in Greek-speaking school-age children, in relation to their language skills and cognitive functions, as well as their motivation, academic emotions, and psychological needs. In the present investigation, we adopted a quasi-experimental design comparing children with and without DLD (matched on gender and parental SES) on non-verbal intelligence, WM capacity (as tapped by complex span tasks), different EFs (updating, task switching and the inhibition-related functions of resistance to distractor interference and resistance to proactive interference), and verbal fluency (phonological and semantic fluency). The relevant tasks were given in random order in the context of four half-hour assessment sessions (to avoid fatigue) conducted within the same month.
2.3. Tasks
2.3.1. Screening Tools
Non-Verbal Intelligence
The Raven’s Coloured Progressive Matrices (CPM) test (Raven’s Educational CPM/CVS; Raven []; standardized for use with Greek children, Sideridis et al. []) was used to measure non-verbal intelligence (reasoning). Children are presented each time with a visual geometric design with a missing piece, and they are asked to pick among six choices the piece that should be filled in to complete the pattern. The test includes 36 items arranged in three sets. Items within a set become increasingly difficult, requiring greater capacity to encode and analyze information. A child’s score on the test is the sum of correct responses on all three sets.
Expressive Vocabulary
Participants were also given the vocabulary scale from the Wechsler Intelligence Scale for Children-WISC III, as standardized in Greek []. The test consists of 30 words and the child is asked to give an oral definition for each word presented (scored 0 to 2). The process is discontinued after four consecutive incorrect answers. A child’s score on the test is the sum of the scores received for all answers given until discontinuation/completion of the assessment.
Sentence Completion
Children were also assessed with the sentence completion subtest of the Athena test []. This test taps a child’s ability to exploit his/her experience with language as well as linguistic redundancies (mostly at the levels of grammar, semantics, as well as pragmatics) to fill in gaps in linguistic material. Specifically, children are orally presented with 32 simple sentences and they are asked to complete each one by producing the missing word or phrase (e.g., “Hives are the homes of…”, “He got angry, he got out of …”—“himself”, a metaphor-based Greek saying). A child’s total score is the sum of correct responses until completion of the assessment or its discontinuation after four consecutive incorrect responses.
2.3.2. Main Assessment Tools
Working-Memory Capacity
Participants’ WM capacity was assessed with the backward digit recall and the listening recall tests of the Working Memory Test Battery for Children (Pickering and Gathercole [], as adapted for use with Greek-speaking children by Chrysochoou []; see also [,,,]). These are two complex span tasks, requiring concurrent storage and processing of information; they were included in the sub-battery of the WMTB-C, assessing the central executive component of the Baddeley and Hitch [,] WM model. Besides their wide use in studies with TD children, such complex span tasks have been used in several studies involving children with DLD [,].
Specifically, in the backward digit recall test, children were asked to listen to a list of digits presented each time with a rate of one digit per second and recall it in reverse order. The test consists of a maximum of six blocks, each containing six lists of the same difficulty level (e.g., in the first block, the child is presented with lists of two digits, in the second block, with lists of three digits, etc.).
In the listening recall task, children listen to a set of short sentences (with the spoken duration of each kept up to 2 s). They are first asked to judge the veracity of each sentence in a set (e.g., “balls are square”—false, “scissors cut paper”—true) and then, to recall the final word of each sentence in the order it was presented (square, paper in the specific example). The test consists of a maximum of six blocks, including sets of increasing length (from one to six sentences per set). In both WM tests, testing ceases when children fail any three trials in a particular block. A child’s score on each test is the sum of his/her correct responses.
Updating
A 2-back version of the n-back task [,,] was used to assess updating (see relevant assessments in studies with children with DLD []. Children are presented with a series of digits on the computer screen, and they are asked to press a specific key (j) on the keyboard when the target digit matches the one presented two trials earlier. Each digit is presented in black font on a white background for 500 ms, and it is followed by a blank screen for 2500 ms. Order of presentation was pseudorandomized with the same digit, however, not presented twice in a row. The task starts with a practice block of 22 digits, including two dummy trials in the beginning (which were not analyzed further) as well as seven targets. The experimental block consists of 62 digits, among which the first two are dummy trials, and 20 are targets. The number of targets recognized is suggested to reflect updating capacity. A measure of response speed (reaction time—RT—in ms) for the correctly recognized targets is additionally obtained.
Inhibition: Resistance to Distractor Interference
To measure the ability to resist interference from distractors, namely from information in the environment that is not relevant to the task at hand (see Friedman and Miyake []), we used the Eriksen Flanker task [], as adapted for use with school-age children for the wider project that this study stems from (see design; []; the task was also used in []). Flanker-task versions have been used widely with TD children [,,,,] as well as children with disorders or learning disabilities [,,]. The task was developed and administered in E-Prime 2.0 software [].
In each trial, children are presented with a fixation cross followed by the presentation of the target letter, which is either A or Λ (specifically, the corresponding Greek letters A and L). Participants are asked to identify this target letter; a left or a right mouse click corresponds to the letters A and L, respectively. In the neutral condition trials, the target is flanked by asterisks (i.e., **A** or **L**), whereas in the compatible and the incompatible condition trials, the target letter is flanked by the same or the alternative letter, respectively, in uppercase or lowercase (i.e., AAAAA, ααAαα, ΛΛΛΛΛ, λλΛλλ in the compatible condition; ΛΛAΛΛ, λλAλλ, AAΛAA, λλΛλλ). Though the target is always presented in uppercase, the flanker-letter case is differentiated so as to avoid any effects of superficial, low-level (perceptual), target-flanker letter similarity effects [,]. Stimuli are presented in black font on white background. In each trial, the duration of fixation varied randomly across trials from 0 to 1200 ms. The target-flanker/asterisk arrays remain on screen until response [,].
Participants first complete a practice block of 24 trials with feedback (i.e., information was provided on whether the response was correct or not, along with the relevant RT in ms). The main test phase consists of three blocks of 24 trials each with no feedback provided. Within each block, trial order is pseudo-randomized, so that the trial type (neutral, compatible, incompatible) is not identical on more than two consecutive trials.
The interference control measure used in the analyses of the present study is calculated based on the difference between participants’ mean RT on the incompatible and the compatible condition trials. Incompatible trials are expected to elicit slower responses relative to compatible ones, due to competing action schemas.
Inhibition: Resistance to Proactive Interference
We adopted the task developed by Borella et al. [] to measure children’s capacity to resist proactive interference (a second core inhibition-related function according to Friedman and Miyake []). Specifically, in the practice block as well as in each of the four assessment task blocks, children are presented with three lists of words belonging to the same semantic category. Specifically, color words are used in the practice block and words referring to animals, fruits, professions, or body parts are included in the lists of the four assessment blocks. Word lists were translated from Italian to Greek and, as with the original task version, we placed emphasis on keeping the number of syllables in the words used similar across assessment lists in order to maintain memory demands equivalent.
Within each block, the words of each list are presented to the child on a computer screen with the use of PowerPoint presentation and at a rate of one word per 2.5 s. Children are asked to read each word in a list silently. They are then presented with a number on screen (different for each list) and are asked to count backward by two for 30 s (rehearsal prevention phase). A blank slide follows with a green tick symbol, and participants are required to produce orally as many words as they can recall from the list, regardless of presentation order. Block order was counterbalanced across participants.
Summing the number of correct responses for each list (first, second, and third) across the four (semantic category) blocks resulted in the calculation of two susceptibility to proactive interference indices per participant []. In each case, recall in list 1 was considered as a baseline in the assessment of the proactive interference build-up (given possible intrusions in lists 2 and 3 only). Susceptibility to PI for list 2 and list 3 was estimated using the formula (list 1–list 2) and (list 1–list 3). The average of the two was entered in the analyses as the overall susceptibility to proactive interference index. The smaller the average, the better the capacity to resist interference from information (words) that was relevant at some point, but should have been cleared afterwards so as to achieve task goals.
Task Switching
Participants’ ability to switch flexibly between tasks was assessed with the “How many—What number task” [] (see Im-Bolter et al. [] for use of the same task with children with DLD). Participants are presented with four types of stimuli cards consisting of either one (1 or 3) or three digits (1 1 1, 3 3 3). Two pure, non-switch blocks (each consisting of 4 practice and 24 assessment trials) are presented in the beginning in counterbalanced order across participants. Each pure block requires the application of one of two rules: in one block, participants are asked to identify the numeric value of the digits presented each time on the screen, pressing either 1 or 3 on the keyboard (What number block), whereas in the other pure block, they are asked to identify the number of digits presented on the screen using the same response keys (How many block). The rule that should be applied is presented in each trial at the top of the screen. In a second phase, two mixed blocks are presented (each consisting of 8 practice and 72 assessment trials). Participants are required to alternate between the aforementioned tasks (How many, What number). The rule that should be applied switch after every second trial. Trials are subject-paced and a variable response–stimulus interval of 0–300 ms is used. A 1000-Hz tone sounded for 300-ms on each error. Accuracy rates and mean RTs (on accurate responses) were obtained per condition: for pure blocks’ trials in the non-switch blocks and for switch and non-switch trials in the mixed blocks.
Verbal Fluency
Children were also given a verbal fluency task [], which consisted of two assessment sessions: a semantic fluency and a phonological fluency session (see relevant assessments with children with DLD [,,,]). In the semantic fluency test, participants are asked to produce as many different words as they can for each of three semantic categories (animals, fruit, objects). In the phonological fluency assessment, children are asked to produce as many different words as they can that begin with each of the three Greek letters: X (Chi), Σ (Sigma), and A (Alpha). Participants are given a minute for each category/letter session. Sessions and categories were administered in the aforementioned order. The sum of relevant words produced in each session, excluding repetitions and variations of previously given words, provided measures of semantic and phonological fluency, accordingly, for each participant.
2.4. Procedure
Following informed parental consent, as well as children’s assent, participants were individually tested in the school setting in one preliminary and three main assessment sessions. Each session lasted approximately 30–40 min and took place on a different weekday, with the whole assessment procedure completed within a fortnight. In the first, preliminary session, children were given the Raven’s and the language tests to ensure appropriate inclusion of children in the DLD and the TD groups. In the sessions that followed, the cognitive tasks were administered in pseudorandom order, taking care that no similar tasks (e.g., the verbal WM or updating measures, or the two inhibition-related measures) were administered within the same session.
3. Results
3.1. Do Greek-Speaking School-Age Children with DLD Have Lower Scores Than TD Peers on Non-Verbal Intelligence?
A comparison of the standard non-verbal intelligence scores of the two groups (measured with the Raven’s Colored Progressive Matrices) revealed a significant difference and a large effect size (p < 0.001, d = 1.21; see Table 1). Specifically, children with DLD scored significantly lower than TD peers on the non-verbal intelligence test (mean difference = 12.86).

Table 1.
Descriptive statistics and t-test results.
3.2. Are Greek-Speaking School-Age Children with DLD Outperformed by TD Peers in Tasks Tapping (a) WM Capacity; (b) Key EFs, That Is, Updating, the Resistance to Distractor Interference and Resistance to Proactive Interference Functions of Inhibition, and Task-Switching; as Well as (c) Verbal (Phonological and Semantic) Fluency (Executive Language Measures)?
Table 1 provides the descriptive statistics for the dependent variables of the study. A MANOVA was conducted to test the effect of group (TD vs. DLD) on participants’ WM capacity as tapped by the listening recall and the backward digit recall measures (r = 0.491, p < 0.001). Using Wilk’s lambda statistic, there was a significant effect of group on the measures, Λ = 0.63, F(2, 54) = 15.61, p < 0.001, ηp2 = 0.366. Similarly, separate univariate ANOVAs revealed significant effects of group on the listening recall test, F(1, 55) = 28.1, p < 0.001, ηp2 = 0.338, and the backward digit test, F(1, 55) = 12.59, p = 0.001, ηp2 = 0.186. In both cases, mean performance was higher in the TD relative to the DLD group (see Table 1).
We proceeded with t-tests for independent samples to compare the two groups (TD vs. DLD) on the updating, the inhibition, and the verbal fluency measures (see Table 1 for statistics). In line with the patterns observed for WM capacity, comparisons between the two groups on updating gave a statistically significant difference and a moderate effect size regarding the number of targets recognized in the n-back task; there was no effect of group on participants’ mean RT for targets recognized. On the other hand, the results showed that the groups did not differ on either inhibition measure obtained (the resistance to distractor interference and the resistance to proactive interference measures). Yet, significant group differences were found in verbal fluency. In both the phonological and the semantic fluency subtests, the TD children demonstrated higher mean scores than DLD peers (mean differences were 12.19 and 6.86, respectively).
Finally, a two-way, mixed-design ANOVA was performed on participants’ RTs in the task-switching paradigm employed, with Group (TD vs. DLD) as the between-subjects factor and trial type (pure blocks’ trials vs. switch trials vs. non-switch trials) as the within-subjects factor. Mauchly’s test indicated that the assumption of sphericity had been violated for the main effects of shifting, W = 0.447, p < 0.001, ε = 0.64. Therefore, degrees of freedom were corrected using Greenhouse–Geisser estimates of sphericity. Both main effects were significant. Specifically, there was a significant main effect of trial type on mean RTs [Fshifting(1.287, 72.098) = 113.86, p < 0.001, ηp2 = 0.67]. There was also a significant main effect of group [Fgroup(1, 56) = 12.24, p = 0.001, ηp2 = 0.179] indicating that, overall, the TD group was faster than the DLD group.
There was a significant interaction effect between trial type and group [Fshifting × group (1.287, 72.098) = 15.248, p < 0.001, ηp2 = 0.214], indicating that group had different effects on children’s RTs depending on trial type. The interaction graph (see Figure 1) reveals that whereas mean RTs in the pure blocks’ trials (i.e., the blocks where participants did not switch at all) were similar for both groups (mean difference = 28.8 ms, p = 0.753), there were significant speed differences between groups in the switch and the non-switch (repeat) trials of the mixed, task-switching blocks. Specifically, the TD group was faster in both conditions (mean difference = 780.9 ms, p < 0.001 and mean difference = 705.7 ms, p < 0.001, respectively). On the other hand, within each group, children were significantly slower in the mixed blocks’ switch trials relative to the pure blocks’ trials: mean differenceDLD = 1490.9 ms, p < 0.001 and mean differenceTD = 681.3 ms, p < 0.001. A significant difference was not observed between the mean RTs in the switch and the non-switch trials in either group: mean differenceDLD = 95 ms, p = 0.205 and mean differenceTD = 19.9 ms, p = 0.625. However, in both groups, children were much slower in the non-switch trials of the mixed blocks relative to the pure blocks’ trials: mean differenceDLD = 1395.9 ms, p < 0.001 and mean differenceTD = 661.4 ms, p < 0.001. This indicates a significant mixing cost in each group [,,], which, based on a t-test for independent samples, actually proved significantly smaller in magnitude in the TD group (661.43 ms) relative to the DLD group (1395.87 ms), t(56) = −4.03, p < 0.001, d = −1.06. The latter finding, however, could only be discussed with caution, due to the lack of a significant switching cost (the alternative cost of multitasking) in either group tested with the specific paradigm. Given the inability to cleanly separate the two cost types in the present study, we proceeded to a comparison of the general performance difference between pure and mixed blocks. It is noted that such comparisons have also been approached as indicative of mixing costs, reflecting general monitoring capacity and top-down processing [,] (see discussion in Philipp et al. []). The analysis showed that children were overall much slower in the mixed blocks’ relative to the pure blocks’ trials (mean differenceDLD = 1443.39 ms, p < 0.001 and mean differenceTD = 671.37 ms, p < 0.001), with the magnitude of the difference found significantly greater in the TD group (661.43 ms) relative to the DLD group (1395.87 ms), t(56) = −4.18, p < 0.001, d = −1.1.
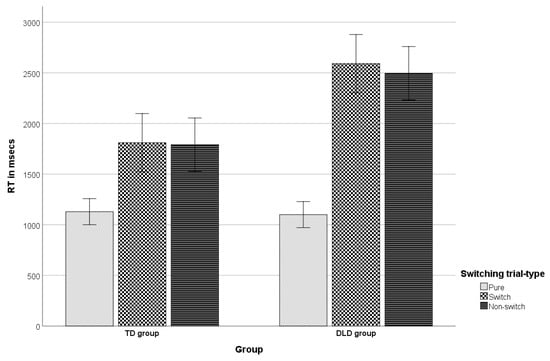
Figure 1.
The group by shifting interaction (RT-estimated marginal means and error bars in msecs).
3.3. Are Any Group Differences in WM Capacity, EFs, and Verbal Fluency Independent of Non-Verbal Intelligence?
Aiming to examine whether the observed group differences are independent of non-verbal intelligence, one-way analyses of covariance (ANCOVA), with the standard non-verbal intelligence score entered as a covariate, were additionally performed on all measures. We are not reporting these analyses for two reasons: (a) the homogeneity of regression slopes ANCOVA assumption was not met in several cases (specifically, in the case of the two working-memory measures, in the switch and the non-switch trials of the mixed blocks of the task-switching paradigm, and in phonological fluency), and (b) the results of the ANCOVAs did not differentiate any patterns observed in the t-tests, the MANOVA, and the mixed ANOVA reported above.
Thus, concerning the significant differences observed between groups in cognitive measures (see the previous section), we can conclude the following. Relying on the ANCOVAs that could be conducted, it seems that the group differences observed in the updating (targets recognized) and the semantic fluency measures can be approached as independent of non-verbal intelligence. On the other hand, in the cases that the ANCOVA results mentioned above (i.e., lack of patterns’ differentiation) cannot be reliably discussed, since the homogeneity of regression slope assumption was not met, we can only rely on the correlations between non-verbal intelligence and each cognitive measure to discuss possible dependence of group differences on non-verbal intelligence. Specifically, the latter showed a significant correlation with (a) the digit backwards measure in the DLD group only (r = 0.44, p = 0.016), (b) mean RT in the non-switch trials of the task-switching paradigm in the TD group only (r = −0.38, p = 0.044), and (c) the related mixing cost measure (pure vs. mixed blocks’ RTs) in the TD group only (r = −0.40, p = 0.031). There were no significant correlations between the non-verbal intelligence score and the listening recall, the phonological fluency, or speed (RT) of response in the switch trials of the relevant paradigm in either group tested (p > 0.05). Overall, our findings seem to suggest a limited possible interplay between non-verbal intelligence and differentiation of WM andEFs in children with DLD relative to TD peers. Effects seem limited to the backward digit recall (WM) measure and certain task-switching indices (non-switch trials’ RT and mixing cost), yet related to DLD only in the case of the specific WM measure.
4. Discussion
The aim of the present study was to investigate WM capacity, key EFs, and verbal fluency in relation to non-verbal intelligence in Greek-speaking school-age children with DLD in comparison to TD peers. To our knowledge, this is the first study that has attempted the combined assessment and comprehensive investigation of the aforementioned capacities in the specific population. Our first research question regarded possible differences between children with DLD and TD peers in non-verbal intelligence, despite both groups being of average intelligence (standard score of 85 or above in the Raven’s Educational CPM/CVS). In line with the hypothesis stated, children with DLD scored lower on the non-verbal intelligence test relative to TD peers. This is consistent with the meta-analysis of Gallinat and Spaulding [], in which children with DLD scoredon average 10 points below TD peers []. It seems that lower—even if average—non-verbal intelligence may be a part of the clinical profile of children with DLD. Future research is needed to gain a better understanding of the role of non-verbal intelligence in DLD and resolve the relevant, ongoing debate regarding DLD diagnosis.
Our second research question regarded the differentiation of children with DLD and TD peers in measures of WM capacity, key EFs, and verbal fluency. We opted for a general hypothesis, given inconclusive relevant findings as well as scarce evidence with the specific population (i.e., Greek-speaking school-age children with DLD). We expected differences in favor of the TD children in the measures obtained that would be independent of non-verbal intelligence (see relevant evidence in Kuusisto et al. []). Our hypothesis was confirmed in several cases, though there were some exceptions. That is, we did observe differences in favour of the TD group in several cognitive measures: specifically, in both WM capacity measures and the executive WM measure (updating, as tapped by the number of targets recognized), in mean RTs in the switch and the non-switch (mixed blocks’) trials of the task-switching paradigm, and the magnitude of the mixing cost in the same task (mixed vs. pure block’s RTs; though we did not observe a switching cost, see discussion below), as well as in both verbal fluency (phonological and semantic) measures obtained. There were no group differences though, in the inhibition measures (tapping resistance to distractor and resistance to proactive interference) or the speed at which targets were recognized in the updating task. Moreover, our findings seem to suggest limited possible interplay between non-verbal intelligence and differentiation of WM and EFs in children with DLD and TD peers. Relying on the ANCOVAs that were conducted, it seems that the aforementioned differences in updating (targets recognized) and semantic fluency persisted even when adjusting for any non-verbal intelligence effects. On the other hand, where ANCOVA results could not be reliably discussed (see Results), the examination of the correlation between non-verbal intelligence and each cognitive measure suggested a possible role of non-verbal intelligence in the differentiation of groups only in the cases of backward digit recall and certain task-switching indices (non-switch trials RT and mixing cost). However, the effect seemed related to DLD in the case of the specific WM measure only (only the latter correlated with non-verbal intelligence among children with DLD). The findings of this preliminary investigation with Greek-speaking school-age children—an under-examined population so far—are further discussed below in relation to the predominant results’ patterns in the literature and relevant theoretical suggestions, also directing the reader to future research.
Specifically, children with DLD were outperformed by TD peers in (a) WM capacity (complex span tasks performance), in line with studies involving children that speak other languages (mostly English [,,]); and (b) executive WM, that is, updating (as tapped by targets recognized in the 2-back task), for which evidence remains scarce and inconclusive in relation to DLD (e.g., school-age children performed at chance in a 2-back task in the Im-Bolter et al. [], e.g., null findings were obtained in the Lukács et al. study []). There were no group differences, though, in the speed measure regarding recognition of targets in the updating task. Though the latter was not our primary updating index, it helped to clarify that participants with DLD were outperformed on accuracy rather than response speed for targets correctly identified. One limitation of the present study is that we did not also employ visuospatial WM tasks (that is, complex span or updating tasks), given assessment duration limitations. Yet, results’ patterns seemed aligned for both the listening recall measure (setting linguistic processing demands) as well as the backward digit recall measure and the 2-back task with digits used to measure updating (which set minimum demands for manipulation or processing of linguistic information). It should also be noted that recent studies have pointed to domain-general WM capacity constraints when using verbal and non-verbal complex span tasks to assess children with DLD [,]. Moreover, the nature of the stimuli presented (verbal vs. non-verbal) in n-back tasks did not differentiate the patterns of findings reported by Lukács et al. []; it is noted, however, that null findings were obtained in the latter study, in contrast to the present study. Future studies can further explore whether WM difficulties in DLD are related to WM capacity (executive control in WM as tapped by complex-span tasks) and/or the executive WM updating function. Such exploration is of value as it can inform the theoretical as well as clinical characterization of the WM—linguistic limitations association in children with DLD (see [] for a discussion). Future research can also guide the refinement of diagnostic tools and treatment methods to enhance WM functioning in this population. As Archibald [] recently proposed, every child with DLD may not have WM deficits, but when he/she does face severe relevant deficits, these might constitute an underlying cause of linguistic impairment (e.g., lexical and grammatical difficulties in []). On this basis, future research, as well as clinical practice should consider the employment of cognitive measures as well, in accurately interpreting the linguistic performance of children with DLD.
On the other hand, the two groups of children did not differ in the two inhibition-related measures employed in the present study, despite earlier suggestions for WM deficits in DLD co-occuring or even resulting from inefficient inhibition []; although, as Marton et al. [] noted, the direction of causality remains unclear. Specifically, our findings regarding resistance to distractor interference are aligned with those of certain studies [], but not others, showing significant group differences in favour of TD children [,]. Based on the findings of the latter, it has been suggested that deficient interference resolution hinders the ability of children with DLD to resolve conflict among semantic representations that are activated during language processing []. Such a suggestion, however, is not supported by the present study; whereas our conflicting results regarding WM capacity (significant group differences in favour of the TD group) and inhibition (null findings) are not supportive of suggestions that the difficulties of children with DLD in verbal WM tasks reflect poor inhibition (resistance to distractor and proactive interference) [,]. Future studies including children of different ages can show whether lack of group differences in the inhibition measures employed in the present study is related to our participants’ age (8.9 years; 3rd graders) and prioritization of interference resolution capacities in later phases of development. There is evidence to suggest, for example, that interference resolution develops later than response inhibition (not measured in the present study though) in TD children [] and that sustained attention and inhibition remain immature until 8 years of age [,]. Given the importance of the inhibition-related functions for language processing and learning more generally [,], age-related changes in inhibition merits future exploration in children with DLD as well.
With regard to task switching, although the two groups did not differ in the baseline condition (pure blocks), children with DLD had slower mean RTs than TD peers in both complex blocks’ conditions (switch and non-switch trials). Therefore, their performance seemed more greatly influenced by the complexity and demands of this mixed block, necessitating strategic engagement to deal with maintenance of multiple task sets. Moreover, both groups responded significantly slower in the trials of the complex blocks relative to the pure blocks’ trials. Notably, the magnitude of this mixing cost was significantly larger in the DLD relative to the TD group, indicating difficulties in time-sensitive shifting of mental sets, as well as worse general monitoring capacity and top-down processing in children with DLD [,]. However, as noted above, the present finding is interpreted with caution, and merely as a tendency that merits further investigation, given the lack of significant switch costs in the task []. Whether the latter resulted from the significant demands placed on very young children by the specific paradigm could be examined in future studies involving children of different ages in different switching tasks, besides the present. Further investigation is, in any case, necessitated to more cleanly separate mixing from switching costs in the study of the specific EF as a function of DLD.
Finally, TD children outperformed peers with DLD in both the phonological and semantic fluency measures obtained, with differences between the groups not proving dependent on non-verbal intelligence. As noted, the ANCOVA conducted for the semantic fluency measure with non-verbal intelligence as a covariate, did not change the results’ pattern; whereas the phonological fluency measure was not related to non-verbal intelligence in either group tested. In children with DLD, poorer phonological fluency has been attributed to impaired explicit (vs implicit) access to phonological representations (Deficient Phonological Access Hypothesis) [], whereas poorer semantic fluency has been related to a general slowing of retrieval processes in contrast to intact lexical-semantic representations (Retrieval Slowing Model) []. However, existing studies have mostly relied on overall verbal fluency measures [,]. Moreover, findings remain limited to languages other than English, although language characteristics can affect performance in tasks tapping on lexical access and vocabulary development []. In line with our results on semantic fluency (yet, contrast to findings in studies with English-speaking children [,]), a recently published study [] found poorer semantic fluency in Greek-speaking school-age children with DLD relative to TD peers. However, researchers did not additionally obtain a phonological fluency measure.
Future research could also consider including different DLD subtypes, that is, groups identified on the basis of expressive difficulties (like the present sample) as well as receptive-expressive groups, to help disentangle the language-cognitive interplay in DLD. For example, in one of the few relevant studies, Rodríguez et al. [] demonstrated problems with verbal fluency and WM (verbal and spatial) in the expressive DLD group, yet, poorer neuropsychological performance more generally, in the receptive-expressive DLD group.
5. Conclusions
The present study aimed at investigating WM capacity, key EFs, and verbal fluency indices in relation to non-verbal intelligence in children with DLD, relative to TD peers; following the limited in number comprehensive investigations of cognitive capacities in this population, and attempting a relevant exploration with Greek-speaking, school-age children with DLD, for the first time. In line with increasing relevant findings (see meta-analysis of Gallinat and Spaulding [], Kuusisto et al. []) and our first hypothesis, children with DLD scored lower than TD peers on non-verbal intelligence. Moreover, we expected differences in favor of the TD children on the WM capacity, EF, and verbal fluency measures, that would be independent of non-verbal intelligence. Our second hypothesis was partially confirmed. Our findings suggest lack of group differences in the inhibition-related functions studied (resistance to distractor interference and resistance to proactive interference). However, we found differences in favour of the TD group in the WM capacity (both complex span tasks), updating (targets recognized in the 2-back task), monitoring (mixing cost in the switching paradigm), and verbal fluency (phonological and semantic) measures, which did not seem dependent on non-verbal intelligence. The only group difference that showed possible dependence on non-verbal intelligence as a function of DLD, was that on the backward digit recall (WM) measure.
Future studies should further investigate the generality of the differences observed between groups in high-order cognitive capacities, employing verbal as well as non-verbal tasks [], and extending assessment to other capacities, such as planning. Moreover, longitudinal research can investigate the developmental trajectories of higher-order cognitive processes and language skills in children with DLD [], and help disentangle whether language and cognitive development is underlined by common mechanisms (e.g., WM and attentional processes) in children with DLD [,]. Finally, cross-linguistic research can explore universal, as well as possibly, language-specific features, in DLD profiles []. Such investigations can further inform clinical practice for children with DLD, contributing to more accurate mappings of WM, EFs, and verbal fluency capacities to language profiles, thus, enhancing screening procedures and the effectiveness of intervention programmes [].
Author Contributions
Conceptualization, A.M.R. and E.C.; methodology, A.M.R. and E.C.; formal analysis, P.R.; investigation, A.M.R., K.D., P.D. and D.F.; resources, A.M.R. and E.C.; data curation, E.C.; writing—original draft preparation, A.M.R., E.C. and P.R.; writing—review and editing, A.M.R., E.C., P.R., K.D., P.D. and D.F.; project administration, A.M.R., E.C., P.R., K.D., P.D. and D.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and the American Psychological Association’s ethical principles.
Informed Consent Statement
Informed consent was obtained from the parents of all children participating in the study; informed assent was additionally provided by participants themselves.
Data Availability Statement
Data is available on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bishop, D.V. What causes specific language impairment in children? Curr. Dir. Psychol. Sci. 2006, 15, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, J.W.; Magimairaj, B.M.; Finney, M.C. Working Memory and Specific Language Impairment: An Update on the Relation and Perspectives on Assessment and Treatment. Am. J. Speech Lang. Pathol. 2010, 19, 78–94. [Google Scholar] [CrossRef]
- Tomas, E.; Vissers, C. Behind the scenes of developmental language disorder: Time to call neuropsychology back on stage. Front. Hum. Neurosci. 2019, 12, 517. [Google Scholar] [CrossRef] [PubMed]
- Conti-Ramsden, G.; Durkin, K. Language development and assessment in the preschool period. Neuropsychol. Rev. 2012, 22, 384–401. [Google Scholar] [CrossRef] [PubMed]
- Petruccelli, N.; Bavin, E.L.; Bretherton, L. Children with specific language impairment and resolved late talkers: Working memory profiles at 5 years. J. Speech Lang. Hear. Res. 2012, 55, 1690–1703. [Google Scholar] [CrossRef]
- Baddeley, A.D.; Hitch, G. Working memory. Psychol. Learn. Motiv. 1974, 8, 47–89. [Google Scholar]
- Baddeley, A. The episodic buffer: A new component of working memory? Trends Cogn. Sci. 2000, 4, 417–423. [Google Scholar] [CrossRef]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef]
- Garon, N.; Bryson, S.E.; Smith, I.M. Executive function in preschoolers: A review using an integrative framework. Psychol. Bull. 2008, 134, 31. [Google Scholar] [CrossRef]
- Lehto, J.E.; Juujärvi, P.; Kooistra, L.; Pulkkinen, L. Dimensions of executive functioning: Evidence from children. Br. J. Dev. Psychol. 2003, 21, 59–80. [Google Scholar] [CrossRef]
- Friedman, N.P.; Miyake, A. The relations among inhibition and interference control functions: A latent-variable analysis. J. Exp. Psychol. Gen. 2004, 133, 101. [Google Scholar] [CrossRef]
- Gallinat, E.; Spaulding, T.J. Differences in the performance of children with specific language impairment and their TD peers on non-verbal cognitive tests: A meta-analysis. J. Speech Lang. Hear. Res. 2014, 57, 1363–1382. [Google Scholar] [CrossRef] [PubMed]
- Bishop, D.V.; Snowling, M.J.; Thompson, P.A.; Greenhalgh, T. The CATALISE-2 consortium. Phase 2 of CATALISE: A multinational and multidisciplinary Delphi consensus study of problems with language development: Terminology. J. Child Psychol. Psychiatry 2017, 58, 1068–1080. [Google Scholar] [CrossRef]
- Leonard, L.B. Language learnability and specific language impairment in children. Appl. Psycholinguist. 2008, 10, 179–202. [Google Scholar] [CrossRef]
- Reilly, S.; Bishop, D.V.; Tomblin, B. Terminological debate over language impairment in children: Forward movement and sticking points. Int. J. Lang. Commun. Disord. 2014, 49, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Whitehurst, G.J.; Fischel, J.E. Practitioner Review: Early Developmental language Delay: What. If Anything. Should the Clinician Do About It? J. Child Psychol. Psychiatry 1994, 35, 613–648. [Google Scholar] [CrossRef] [PubMed]
- Paul, R. Clinical implications of the natural history of slow expressive language development. Am. J. Speech Lang. Pathol. 1996, 5, 5–21. [Google Scholar] [CrossRef]
- Rescorla, L.; Mirak, J.; Singh, L. Vocabulary growth in late talkers: Lexical development from 2; 0 to 3; 0. J. Child Lang. 2000, 27, 293–311. [Google Scholar] [CrossRef]
- Rice, M.L.; Redmond, S.M.; Hoffman, L. Mean Length of Utterance in Children with Specific Language Impairment and in Younger Control Children Shows Concurrent Validity and Stable and Parallel Growth Trajectories. J. Speech Lang. Hear. Res. 2006, 49, 793–808. [Google Scholar] [CrossRef]
- Tomblin, J.B.; Smith, E.; Zhang, X. Epidemiology of specific language impairment: Prenatal and perinatal risk factors. J. Commun. Disord. 1997, 30, 325–344. [Google Scholar] [CrossRef]
- Law, J.; Boyle, J.; Harris, F.; Harkness, A.; Nye, C. Prevalence and natural history of primary speech and language delay: Findings from a systematic review of the literature. Int. J. Lang. Commun. Disord. 2000, 35, 165–188. [Google Scholar]
- Leonard, L.B.; Eyer, J.A.; Bedore, L.M.; Grela, B.G. Three accounts of the grammatical morpheme difficulties of English-speaking children with specific language impairment. J. Speech Lang. Hear. Res. 1997, 40, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Tomas, E.; Demuth, K.; Petocz, P. The role of frequency in learning morphophonological alternations: Implications for children with specific language impairment. J. Speech Lang. Hear. Res. 2017, 60, 1316–1329. [Google Scholar] [CrossRef]
- Law, J.; Charlton, J.; Dockrell, J.; Gascoigne, M.; McKean, C.; Theakston, A. Early Language Development: Needs, Provision, and Intervention for Preschool Children from Socio-Economically Disadvantage Backgrounds; Education Endowment Foundation: London, UK, 2017; p. 203. [Google Scholar]
- Bishop, D.V.; North, T.; Donlan, C. Genetic basis of specific language impairment: Evidence from a twin study. Dev. Med. Child Neurol. 1995, 37, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Tomblin, J.B.; Buckwalter, P.R. Heritability of poor language achievement among twins. J. Speech Lang. Hear. Res. 1998, 41, 188–199. [Google Scholar] [CrossRef]
- Bishop, D.V. Specific language impairment as a language learning disability. Child Lang. Teach Ther. 2009, 25, 163–165. [Google Scholar] [CrossRef]
- Graham, S.A.; Fisher, S.E. Decoding the genetics of speech and language. Curr. Opin. Neurobiol. 2013, 23, 43–51. [Google Scholar] [CrossRef]
- Rice, M.L. Language growth and genetics of specific language impairment. Int. J. Speech Lang. Pathol. 2013, 15, 223–233. [Google Scholar] [CrossRef]
- Laws, G.; Bates, G.; Feuerstein, M.; Mason-Apps, E.; White, C. Peer acceptance of children with language and communication impairments in a mainstream primary school: Associations with type of language difficulty, problem behaviours and a change in placement organization. Child Lang. Teach Ther. 2012, 28, 73–86. [Google Scholar] [CrossRef]
- Van der Lely, H.K. Domain-specific cognitive systems: Insight from Grammatical-SLI. Trends Cogn. Sci. 2005, 9, 53–59. [Google Scholar] [CrossRef]
- Stavrakaki, S. Developmental perspectives on Specific Language Impairment: Evidence from the production of wh-questions by Greek SLI children over time. Adv. Speech Lang. Pathol. 2006, 8, 384–396. [Google Scholar] [CrossRef]
- Rothweiler, M.; Chilla, S.; Clahsen, H. Subject verb agreement in Specific Language Impairment: A study of monolingual and bilingual German-speaking children. Biling Lang. Cogn. 2012, 15, 39–57. [Google Scholar] [CrossRef]
- Bishop, D.V. Is specific language impairment a valid diagnostic category? Genetic and psycholinguistic evidence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1994, 346, 105–111. [Google Scholar] [PubMed]
- Conti-Ramsden, G.; Botting, N. Classification of children with specific language impairment: Longitudinal considerations. J. Speech Lang. Hear. Res. 1999, 42, 1195–1204. [Google Scholar] [CrossRef]
- Yew, S.G.K.; O’Kearney, R. Emotional and behavioural outcomes later in childhood and adolescence for children with specific language impairments: Meta-analyses of controlled prospective studies. J. Child Psychol. Psychiatry 2013, 54, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Marton, K. Visuo-spatial processing and executive functions in children with specific language impairment. Int. J. Lang. Commun. Disord. 2008, 43, 181–200. [Google Scholar] [CrossRef]
- Henry, L.A.; Messer, D.J.; Nash, G. Executive functioning and verbal fluency in children with language difficulties. Learn. Instr. 2015, 39, 137–147. [Google Scholar] [CrossRef]
- Lukács, Á.; Ladányi, E.; Fazekas, K.; Kemény, F. Executive functions and the contribution of short-term memory span in children with specific language impairment. Neuropsychology 2016, 30, 296. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, E.T.; Tamis-LeMonda, C.S. Trajectories of the home learning environment across the first 5 years: Associations with children’s vocabulary and literacy skills at prekindergarten. Child Dev. 2011, 82, 1058–1075. [Google Scholar] [CrossRef]
- Weckerly, J.; Wulfeck, B.; Reilly, J. Verbal fluency deficits in children with specific language impairment: Slow rapid naming or slow to name? Child Neuropsychol. 2001, 7, 142–152. [Google Scholar] [CrossRef]
- Ullman, M.T.; Pierpont, E.I. Specific language impairment is not specific to language: The procedural deficit hypothesis. Cortex 2005, 41, 399–433. [Google Scholar] [CrossRef]
- Archibald, L.M.; Gathercole, S.E. Short-term and working memory in specific language impairment. Int. J. Lang. Commun. Disord. 2006, 41, 675–693. [Google Scholar] [CrossRef] [PubMed]
- Falcaro, M.; Pickles, A.; Newbury, D.F.; Addis, L.; Banfield, E.; Fisher, S.E.; Monaco, A.P.; Simkin, Z.; Conti-Ramsden, G.; The SLI Consortium. Genetic and phenotypic effects of phonological short-term memory and grammatical morphology in specific language impairment. Genes Brain Behav. 2008, 7, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Spaulding, T.J.; Plante, E.; Vance, R.B. Sustained selective attention skills of preschool children with specific language impairment: Evidence for separate attentional capacities. J. Speech Lang. Hear. Res. 2008, 51, 16–34. [Google Scholar] [CrossRef]
- Finneran, D.A.; Francis, A.L.; Leonard, L.B. Sustained attention in children with Specific Language Impairment (SLI). J. Speech Lang. Hear. Res. 2009, 52, 915–929. [Google Scholar] [CrossRef]
- Ebert, K.D.; Kohnert, K. Sustained attention in children with primary language impairment: A meta-analysis. J. Speech Lang. Hear. Res. 2011, 54, 1372–1384. [Google Scholar] [CrossRef]
- Tallal, P.; Piercy, M. Developmental aphasia: Impaired rate of non-verbal processing as a function of sensory modality. Neuropsychologia 1973, 11, 389–398. [Google Scholar] [CrossRef]
- Bishop, D.V.; Carlyon, R.P.; Deeks, J.M.; Bishop, S.J. Auditory temporal processing impairment: Neither necessary nor sufficient for causing language impairment in children. J. Speech Lang. Hear. Res. 1999, 42, 1295–1310. [Google Scholar] [CrossRef]
- Wright, B.A.; Bowen, R.W.; Zecker, S.G. Nonlinguistic perceptual deficits associated with reading and language disorders. Curr. Opin. Neurobiol. 2000, 10, 482–486. [Google Scholar] [CrossRef]
- Ziegler, J.C.; Goswami, U. Reading acquisition, developmental dyslexia, and skilled reading across languages: A psycholinguistic grain size theory. Psychol. Bull. 2005, 131, 3. [Google Scholar] [CrossRef]
- Earle, F.S.; Gallinat, E.L.; Grela, B.G.; Lehto, A.; Spaulding, T.J. Empirical Implications of Matching Children With Specific Language Impairment to Children With Typical Development on Non-verbal IQ. J. Learn. Disabil. 2017, 50, 252–260. [Google Scholar] [CrossRef]
- Palikara, O.; Ralli, A.M. Developmental Language Disorder: Issues about terminology, prevalence, diagnostic criteria and heterogeneity. In Developmental Language Disorder in Children and Adolescents: Issues Regarding Orientation, Assessment and Intervention; Gutenberg: Athens, Greece, 2017; pp. 15–30. [Google Scholar]
- Bishop, D.V. Uncommon Understanding (Classic Edition): Development and Disorders of Language Comprehension in Children; Psychology Press, Taylor & Francis Group: London, UK, 2014. [Google Scholar]
- Botting, N. Non-verbal cognitive development and language impairment. J. Child Psychol. Psychiatry 2005, 46, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Kuusisto, M.A.; Nieminen, P.E.; Helminen, M.T.; Kleemola, L. Executive and intellectual functioning in school-aged children with specific language impairment. Int. J. Lang. Commun. Disord. 2017, 52, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Bishop, D.V.M. Pre-and perinatal hazards and family background in children with specific language impairments: A study of twins. Brain Lang. 1997, 56, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Ebbels, S. Introducing the SLI debate. Int. J. Lang. Commun. Disord. 2014, 49, 377–380. [Google Scholar] [CrossRef]
- Krassowski, E.; Plante, E. IQ variability in children with SLI: Implications for use of cognitive referencing in determining SLI. J. Commun. Disord. 1997, 30, 1–8. [Google Scholar] [CrossRef]
- Whitney, P.; Jameson, T.; Hinson, J.M. Impulsiveness and executive control of working memory. Pers. Individ. Dif. 2004, 37, 417–428. [Google Scholar] [CrossRef]
- Chrysochoou, E.; Bablekou, Z. Phonological loop and central executive contributions to oral comprehension skills of 5.5 to 9.5 years old children. Appl. Cogn. Psych. 2011, 25, 576–583. [Google Scholar] [CrossRef]
- Chrysochoou, E.; Bablekou, Z.; Tsigilis, N. Working Memory Contributions to Reading Comprehension Components in Middle Childhood Children. Am. J. Psychol. 2011, 124, 275–289. [Google Scholar] [CrossRef]
- Chrysochoou, E.; Bablekou, Z.; Masoura, E.; Tsigilis, N. Working memory and vocabulary development in Greek preschool and primary school children. Eur. J. Dev. Psychol. 2013, 10, 417–432. [Google Scholar] [CrossRef]
- Alloway, T.P. Working memory, but not IQ, predicts subsequent learning in children with learning difficulties. Eur. J. Psychol. Assess. 2009, 25, 92–98. [Google Scholar] [CrossRef]
- Maehler, C.; Schuchardt, K. Working memory in children with specific learning disorders and/or attention deficits. Learn. Individ. Differ. 2016, 49, 341–347. [Google Scholar] [CrossRef]
- Vugs, B.; Cuperus, J.; Hendriks, M.; Verhoeven, L. Visuospatial working memory in specific language impairment: A meta-analysis. Res. Dev. Disabil. 2013, 34, 2586–2597. [Google Scholar] [CrossRef] [PubMed]
- Vugs, B.; Knoors, H.; Cuperus, J.; Hendriks, M.; Verhoeven, L. Interactions between working memory and language in young children with specific language impairment (SLI). Child Neuropsychol. 2016, 22, 955–978. [Google Scholar] [CrossRef] [PubMed]
- Henry, L.A.; Messer, D.J.; Nash, G. Executive functioning in children with specific language impairment. J. Child Psychol. Psychiatry 2012, 53, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, E.; Bavin, E.; Efron, D.; Sciberras, E. A comparison of working memory profiles in school-aged children with specific language impairment, attention deficit/hyperactivity disorder, comorbid SLI and ADHD and their typically developing peers. Child Neuropsychol. 2012, 18, 190–207. [Google Scholar] [CrossRef]
- Marton, K.; Schwartz, R.G. Working memory capacity and language processes in children with specific language impairment. J. Speech Lang. Hear. Res. 2003, 46, 1138–1153. [Google Scholar] [CrossRef]
- Archibald, L.M.; Gathercole, S.E. Visuospatial immediate memory in specific language impairment. J. Speech Lang. Hear. Res. 2006, 49, 265–277. [Google Scholar] [CrossRef]
- Rodriguez, P.; Cucurull, G.; Gonzàlez, J.; Gonfaus, J.M.; Nasrollahi, K.; Moeslund, T.B.; Roca, F.X. Deep pain: Exploiting long short-term memory networks for facial expression classification. IEEE Trans. Cybern. 2017, 99, 1–11. [Google Scholar] [CrossRef]
- Miyake, A.; Friedman, N.P. The nature and organization of individual differences in executive functions: Four general conclusions. Curr. Dir. Psychol. Sci. 2012, 21, 8–14. [Google Scholar] [CrossRef]
- Kapa, L.L.; Plante, E. Executive function in SLI: Recent advances and future directions. Curr. Dev. Disord. Rep. 2015, 2, 245–252. [Google Scholar] [CrossRef]
- Montgomery, J.W.; Evans, J.L.; Fargo, J.D.; Schwartz, S.; Gillam, R.B. Structural relationship between cognitive processing and syntactic sentence comprehension in children with and without developmental language disorder. J. Speech Lang. Hear. Res. 2018, 61, 2950–2976. [Google Scholar] [CrossRef] [PubMed]
- Im-Bolter, N.; Johnson, J.; Pascual-Leone, J. Processing limitations in children with specific language impairment: The role of executive function. Child Dev. 2006, 77, 1822–1841. [Google Scholar] [CrossRef]
- Ecker, U.K.; Lewandowsky, S.; Oberauer, K.; Chee, A.E. The components of working memory updating: An experimental decomposition and individual differences. J. Exp. Psychol. Learn Mem. Cogn. 2010, 36, 170. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.L.; Gillam, R.B.; Montgomery, J.W. Cognitive predictors of spoken word recognition in children with and without developmental language disorders. J. Speech Lang. Hear. Res. 2018, 61, 1409–1425. [Google Scholar] [CrossRef] [PubMed]
- Spaulding, T.J. Investigating mechanisms of suppression in preschool children with specific language impairment. J. Speech Lang. Hear. Res. 2010, 53, 725–738. [Google Scholar] [CrossRef]
- Roello, M.; Ferretti, M.L.; Colonnello, V.; Levi, G. When words lead to solutions: Executive function deficits in preschool children with specific language impairment. Res. Dev. Disabil. 2015, 37, 216–222. [Google Scholar] [CrossRef]
- Noterdaeme, M.; Amorosa, H.; Mildenberger, K.; Sitter, S.; Minow, F. Evaluation of attention problems in children with autism and children with a specific language disorder. Eur. Child Adolesc. Psychiatry 2001, 10, 58–66. [Google Scholar] [CrossRef]
- Marton, K.; Campanelli, L.; Scheuer, J.; Yoon, J.; Eichorn, N. Executive function profiles in children with and without specific language impairment. Riv. Psicolinguist. Appl. 2012, 12, 57–73. [Google Scholar]
- Victorino, K.R.; Schwartz, R.G. Control of auditory attention in children with specific language impairment. J. Speech Lang. Hear. Res. 2015, 58, 1245–1257. [Google Scholar] [CrossRef]
- Marton, K.; Campanelli, L.; Eichorn, N.; Scheuer, J.; Yoon, J. Information processing and proactive interference in children with and without specific language impairment. J. Speech Lang. Hear. Res. 2014, 57, 106–119. [Google Scholar] [CrossRef]
- Dibbets, P.; Bakker, K.; Jolles, J. Functional MRI of task switching in children with specific language impairment (SLI). Neurocase 2006, 12, 71–79. [Google Scholar] [CrossRef]
- Pauls, L.J.; Archibald, L.M. Executive functions in children with specific language impairment: A meta-analysis. J. Speech Lang. Hear. Res. 2016, 59, 1074–1086. [Google Scholar] [CrossRef] [PubMed]
- Sauzéon, H.; Lestage, P.; Raboutet, C.; N’Kaoua, B.; Claverie, B. Verbal fluency output in children aged 7–16 as a function of the production criterion: Qualitative analysis of clustering, switching processes, and semantic network exploitation. Brain Lang. 2004, 89, 192–202. [Google Scholar] [CrossRef]
- Troyer, A.K. Normative data for clustering and switching on verbal fluency tasks. J. Clin. Exp. Neuropsychol. 2000, 22, 370–378. [Google Scholar] [CrossRef]
- Troyer, A.K.; Moscovitch, M.; Winocur, G. Clustering and switching as two components of verbal fluency: Evidence from younger and older healthy adults. Neuropsychology 1997, 11, 138–146. [Google Scholar] [CrossRef]
- Adams, A.M.; Bourke, L.; Willis, C. Working memory and spoken language comprehension in young children. Int. J. Psychol. 1999, 34, 364–373. [Google Scholar] [CrossRef]
- Pennington, B.F.; Ozonoff, S. Executive functions and developmental psychopathology. J. Child Psychol. Psychiatry 1999, 37, 51–87. [Google Scholar] [CrossRef]
- Diamond, A. Executive functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef]
- Whiteside, D.M.; Kealey, T.; Semla, M.; Luu, H.; Rice, L.; Basso, M.R.; Roper, B. Verbal fluency: Language or executive function measure? Appl. Neuropsychol. Adult. 2016, 23, 29–34. [Google Scholar] [CrossRef]
- Luo, L.; Luk, G.; Bialystok, E. Effect of language proficiency and executive control on verbal fluency performance in bilinguals. Cognition 2010, 114, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Prigatano, G.P.; Gray, J.A. Predictors of performance on three developmentally sensitive neuropsychological tests in children with and without traumatic brain injury. Brain Inj. 2008, 22, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Ruff, R.M.; Light, R.H.; Parker, S.B.; Levin, H.S. The psychological construct of word fluency. Brain Lang. 1997, 57, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Aita, S.L.; Beach, J.D.; Taylor, S.E.; Borgogna, N.C.; Harrell, M.N.; Hill, B.D. Executive, language, or both? An examination of the construct validity of verbal fluency measures. Appl. Neuropsychol. Adult 2018, 26, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.K.; Young, M.; Garcia, C. Why do older adults have difficulty with semantic fluency? Aging Neuropsychol. Cogn. 2018, 25, 803–828. [Google Scholar] [CrossRef]
- Rodríguez, V.A.; Santana, G.R.; Expósito, S.H. Executive functions and language in children with different subtypes of specific language impairment. Neurología 2017, 32, 355–362. [Google Scholar] [CrossRef]
- Kail, R.; Leonard, L.B. Word-finding abilities in language-impaired children. ASHA Monogr. 1986, 25, 1–39. [Google Scholar]
- Weyandt, L.L.; Willis, W.G. Executive functions in school-aged children: Potential efficacy of tasks in discriminating clinical groups. Dev. Neuropsychol. 1994, 10, 27–38. [Google Scholar] [CrossRef]
- Mengisidou, M.; Marshall, C.R.; Stavrakaki, S. Semantic fluency difficulties in developmental dyslexia and developmental language disorder (DLD): Poor semantic structure of the lexicon or slower retrieval processes? Int. J. Lang. Commun. Disord. 2020, 55, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Best, J.R.; Miller, P.H. A developmental perspective on executive function. Child Dev. 2010, 81, 1641–1660. [Google Scholar] [CrossRef]
- Best, J.R.; Miller, P.H.; Jones, L.L. Executive functions after age 5: Changes and correlates. Dev. Rev. 2009, 29, 180–200. [Google Scholar] [CrossRef] [PubMed]
- Denckla, M.B. A theory and model of executive function: A neuropsychological perspective. In Attention, Memory, and Executive Function; Lyon, G.R., Krasnegor, N.A., Eds.; Paul H. Brookes: Baltimore, MD, USA, 1996; pp. 263–278. [Google Scholar]
- Raven, J. The Raven Progressive Matrices tests: Their theoretical basis and measurement model. In Uses and Abuses of Intelligence: Studies Advancing Spearman and Raven’s Quest for Non-Arbitrary Metrics; Royal Fireworks Press: Unionville, NY, USA, 2008; pp. 17–68. [Google Scholar]
- Sideridis, G.; Antoniou, F.; Mouzaki, A.; Simos, P. Raven’s Educational CPM/CVS; Motivo: Athens, Greece, 2015. [Google Scholar]
- Tomblin, J.B.; Records, N.L.; Zhang, X. A system for the diagnosis of specific language impairment in kindergarten children. J. Speech Lang. Hear. Res. 1996, 39, 1284–1294. [Google Scholar] [CrossRef]
- Georgas, J.; Paraskevopoulos, I.N.; Besevegis, E.; Giannitsas, N.D. The Hellenic WISC-III; Psychometric Laboratory, University of Athens: Athens, Greece, 1997. [Google Scholar]
- Paraskevopoulos, Ι.N.; Kalantzi-Aziz, A.; Giannitsas, N.D. Athena Test for the Diagnosis of Learning Difficulties; Ellinika Grammata: Athens, Greece, 1999. [Google Scholar]
- Bradley, R.H.; Corwyn, R.F. Socioeconomic status and child development. Annu. Rev. Psychol. 2002, 53, 371–399. [Google Scholar] [CrossRef] [PubMed]
- Burneo-Garcés, C.; Cruz-Quintana, F.; Pérez-García, M.; Fernández-Alcántara, M.; Fasfous, A.; Pérez-Marfil, M.N. Interaction between socioeconomic status and cognitive development in children aged 7, 9, and 11 years: A cross-sectional study. Dev. Neuropsychol. 2019, 44, 1–16. [Google Scholar] [CrossRef]
- Ladas, A.I.; Carroll, D.J.; Vivas, A.B. Attentional processes in low-socioeconomic status bilingual children: Are they modulated by the amount of bilingual experience? Child Dev. 2015, 86, 557–578. [Google Scholar] [CrossRef]
- Vivas, A.B.; Chrysochoou, E.; Ladas, A.I.; Salvari, V. The moderating effect of bilingualism on lifespan cognitive development. Cogn. Dev. 2020, 55, 100890. [Google Scholar] [CrossRef]
- Pickering, S.J.; Gathercole, S.E. Working Memory Test Battery for Children; Psychological Corporation: London, UK, 2001. [Google Scholar]
- Chrysochoou, Ε. Working Memory Contributions to Children’s Listening Comprehension in the Preschool and Elementary School Years. Ph.D. Thesis, Department of Early Childhood Education, Aristotle University of Thessaloniki, Thessaloniki, Greece, 2006. [Google Scholar]
- Chrysochoou, E.; Bablekou, Z.; Kazi, S.; Tsigilis, N. Differences in Vocabulary Knowledge as a Function of Children’s Oral Comprehension Performance in Greek: A Cross-Sectional Developmental Study. Am. J. Psychol. 2018, 131, 211–223. [Google Scholar] [CrossRef]
- Kirchner, W.K. Age differences in short-term retention of rapidly changing information. J. Exp. Psychol. 1958, 55, 352–358. [Google Scholar] [CrossRef]
- Jaeggi, S.M.; Buschkuehl, M.; Perrig, W.J.; Meier, B. The concurrent validity of the N-back task as a working memory measure. Memory 2010, 18, 394–412. [Google Scholar] [CrossRef]
- Pelegrina, S.; Lechuga, M.T.; García-Madruga, J.A.; Elosúa, M.R.; Macizo, P.; Carreiras, M.; Funtes, L.J.; Bajo, M.T. Normative data on the n-back task for children and young adolescents. Front. Psychol. 2015, 6, 1544. [Google Scholar] [CrossRef]
- Eriksen, B.A.; Eriksen, C.W. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept. Psychophys. 1974, 16, 143–149. [Google Scholar] [CrossRef]
- Chrysochoou, E.; Ralli, A.M.; Diakogiorgi, K.; Filippatou, D.; Roussos, P.; Dimitropoulou, P. Executive Functions and Reading Comprehension in School-Age Children. Conference Presentation Abstract. In Proceedings of the 5th Hellenic Conference on Developmental Psychology, Volos, Greece, 20–23 October 2016; Available online: http://developmental2016.uth.gr/ (accessed on 14 April 2021).
- Vicha, A. The Contribution of Working Memory and Motivation to Elementary Student’s Reading Comprehension. Master’s Thesis, Department of Primary Education, University of Ioannina, Ioannina, Greece, 2017. [Google Scholar]
- Hillman, C.H.; Pontifex, M.B.; Raine, L.B.; Castelli, D.M.; Hall, E.E.; Kramer, A.F. The effect of acute treadmill walking on cognitive control and academic achievement in preadolescent children. Neuroscience 2009, 159, 1044–1054. [Google Scholar] [CrossRef]
- Lonnemann, J.; Linkersdörfer, J.; Nagler, T.; Hasselhorn, M.; Lindberg, S. Spatial representations of numbers and letters in children. Front. Psychol. 2013, 4, 544. [Google Scholar] [CrossRef]
- McDermott, J.M.; Perez-Edgar, K.; Henderson, H.A.; Chronis-Tuscano, A.; Pine, D.S.; Fox, N.A. A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biol. Psychiatry 2009, 65, 445–448. [Google Scholar] [CrossRef]
- McDermott, J.M.; Perez-Edgar, K.; Fox, N.A. Variations of the flanker paradigm: Assessing selective attention in young children. Behav. Res. Methods 2007, 39, 62–70. [Google Scholar] [CrossRef]
- Chan, E.; Mattingley, J.B.; Huang-Pollock, C.; English, T.; Hester, R.; Vance, A.; Bellgrove, M.A. Abnormal spatial asymmetry of selective attention in ADHD. J. Child Psychol. Psychiatry 2009, 50, 1064–1072. [Google Scholar] [CrossRef]
- Censabella, S.; Noël, M.P. The inhibition of exogenous distracting information in children with learning disabilities. J. Learn. Disabil. 2005, 38, 400–410. [Google Scholar] [CrossRef]
- Censabella, S.; Noël, M.P. The inhibition capacities of children with mathematical disabilities. Child Neuropsychol. 2008, 14, 1–20. [Google Scholar] [CrossRef]
- Schneider, W.; Eschman, A.; Zuccolotto, A. E-Prime Reference Guide; Psychology Software Tools, Inc.: Pittsburgh, PA, USA, 2012. [Google Scholar]
- Bernard, J.B.; Chung, S.T. The dependence of crowding on flanker complexity and target–flanker similarity. J. Vis. 2011, 11, 1. [Google Scholar] [CrossRef]
- Denton, S.E.; Shiffrin, R.M. Primes and flankers: Source confusions and discounting. Atten. Percept. Psychophys. 2012, 74, 852–866. [Google Scholar] [CrossRef]
- Borella, E.; Carretti, B.; Pelegrina, S. The specific role of inhibition in reading comprehension in good and poor comprehenders. J. Learn. Disabil. 2010, 43, 541–552. [Google Scholar] [CrossRef]
- Cepeda, N.J.; Cepeda, M.L.; Kramer, A.F. Task switching and attention deficit hyperactivity disorder. J. Abnorm. Child Psychol. 2000, 28, 213–226. [Google Scholar] [CrossRef]
- Kosmidis, M.H.; Vlahou, C.H.; Panagiotaki, P.; Kiosseoglou, G. The verbal fluency task in the Greek population: Normative data, and clustering and switching strategies. J. Int. Neuropsychol. Soc. 2004, 10, 164. [Google Scholar] [CrossRef] [PubMed]
- Kray, J.; Lindenberger, U. Adult age differences in task switching. Psychol. Aging 2000, 15, 126–147. [Google Scholar] [CrossRef] [PubMed]
- Meiran, N. Modeling cognitive control in task-switching. Psychol. Res. 2000, 63, 234–249. [Google Scholar] [CrossRef]
- Rubin, O.; Meiran, N. On the origins of the task mixing cost in the cuing task-switching paradigm. J. Exp. Psychol. Learn. Mem. Cogn. 2005, 31, 1477–1491. [Google Scholar] [CrossRef] [PubMed]
- Koch, I.; Prinz, W.; Allport, A. Involuntary retrieval in alphabet-arithmetic tasks: Task-mixing and task-switching costs. Psychol. Res. 2005, 69, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Los, S.A. On the origin of mixing costs: Exporing information processing in pure and mixed blocks of trials. Acta Psychol. 1996, 94, 145–188. [Google Scholar] [CrossRef]
- Philipp, A.M.; Jolicoeur, P.; Falkenstein, M.; Koch, I. Response selection and response execution in task switching: Evidence from a go-signal paradigm. J. Exp. Psychol. Learn. Mem. Cogn. 2007, 33, 1062–1075. [Google Scholar] [CrossRef]
- Archibald, L.M. Working memory and language learning: A review. Child. Lang. Teach. Ther. 2017, 33, 5–17. [Google Scholar] [CrossRef]
- Marini, A.; Gentili, C.; Molteni, M.; Fabbro, F. Differential verbal working memory effects on linguistic production in children with Specific Language Impairment. Res. Dev. Disabil. 2014, 35, 3534–3542. [Google Scholar] [CrossRef] [PubMed]
- Bjorklund, D.F.; Harnishfeger, K.K. The resources construct in cognitive development: Diverse sources of evidence and a theory of inefficient inhibition. Dev. Rev. 1990, 10, 48–71. [Google Scholar] [CrossRef]
- Marton, K.; Kelmenson, L.; Pinkhasova, M. Inhibition control and working memory capacity in children with SLI. Psychologia 2007, 50, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Epstein, B.; Shafer, V.L.; Melara, R.D.; Schwartz, R.G. Can children with SLI detect cognitive conflict? Behavioral and electrophysiological evidence. J. Speech Lang. Hear. Res. 2014, 57, 1453–1467. [Google Scholar] [CrossRef] [PubMed]
- Ridderinkhof, K.R.; Band, G.P.; Logan, G.D. A study of adaptive behavior: Effects of age and irrelevant information on the ability to inhibit one’s actions. Acta Psychol. 1999, 101, 315–337. [Google Scholar] [CrossRef]
- De Luca, C.R.; Wood, S.J.; Anderson, V.; Buchanan, J.A.; Proffitt, T.M.; Mahony, K.; Pantelis, C. Normative data from the CANTAB. I: Development of executive function over the lifespan. J. Clin. Exp. Neuropsychol. 2003, 25, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Dempster, F.N.; Corkill, A.J. Interference and inhibition in cognition and behavior: Unifying themes for educational psychology. Educ. Psychol. Rev. 1999, 11, 1–88. [Google Scholar] [CrossRef]
- Schneider, D.W.; Logan, G.D. Task switching. Encycl. Neurosci. 2009, 9, 869–874. [Google Scholar]
- Ramus, F.; Szenkovits, G. What phonological deficit? Q. J. Exp. Psychol. 2008, 61, 129–141. [Google Scholar] [CrossRef]
- Lenio, S.; Lissemore, F.M.; Sajatovic, M.; Smyth, K.A.; Tatsuoka, C.; Woyczynski, W.A.; Lerner, A.J. Detrending changes the temporal dynamics of a semantic fluency task. Front. Aging Neurosci. 2016, 8, 252. [Google Scholar] [CrossRef]
- Marton, K.; Eichorn, N.; Campanelli, L.; Zakarias, L. Working memory and interference control in children with specific language impairment. Lang. Linguist. Compass 2016, 10, 211–224. [Google Scholar] [CrossRef]
- Ebert, K.D.; Pham, G. Including nonlinguistic processing tasks in the identification of developmental language disorder. Am. J. Speech Lang. Pathol. 2019, 28, 932–944. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).