Abstract
Human dental pulp-derived stem cells (hDPSCs) are promising cellular sources for bone healing. The acceleration of their differentiation should be beneficial to their clinical application. Therefore, a conductive polypyrrole (PPy)-made electrical stimulation (ES) device was fabricated to provide direct-current electric field (DCEF) treatment, and its effect on osteo-differentiation of hDPSCs was investigated in this study. To determine the optimal treating time, electrical field of 0.33 V/cm was applied to hDPSCs once for 4 h on different days after the osteo-induction. The alizarin red S staining results suggested that ES accelerated the mineralization rates of hDPSCs. The quantification analysis results revealed a nearly threefold enhancement in calcium deposition by ES at day 0, 2, and 4, whereas the promotion effect in later stages was in vain. To determine the ES-mediated signaling pathway, the expression of genes in the bone morphogenetic protein (BMP) family and related receptors were quantified using qPCR. In the early stages of osteo-differentiation, the mRNA levels of BMP2, BMP3, BMP4, and BMP5 were increased significantly in the ES groups, indicating that these genes were involved in the specific signaling routes induced by ES. We are the first using DCEF to improve the osteo-differentiation of hDPSCs, and our results promise the therapeutic applications of hDPSCs on cell-based bone tissue engineering.
1. Introduction
Mesenchymal stem cells (MSCs) are one of the promising stem cell types due to their availability and relatively simple requirements for in vitro expansion and genetic manipulation [1]. In addition to the well-characterized MSCs derived from bone marrow, increasing evidence suggests that human dental pulp contains a substantial amount of stem cells, i.e., human dental pulp stem cells (hDPSCs) [2]. These cells demonstrate proliferation and differentiation properties similar to those of MSCs [3]. Unlike bone marrow stem cells, the harvest of hDPSCs does not require additional clinical procedures, making them a promising source of stem cells for tissue regeneration [4]. In addition to the application of generating dentin-like structures [5], hDPSCs also exhibit proliferative ability in vitro and can be induced to differentiate into numerous cell types, such as neurons, osteoblasts, and adipocytes [6,7,8]. Therefore, hDPSCs have been applied in several regenerative studies including ischemia [9], muscular dystrophy [10], neurological diseases [11], and diseases of bone and cartilages [12,13].
Bone is a specialized connective tissue that develops through the differentiation of osteo-progenitor cells, primarily osteoblasts, towards gradual ossification, i.e., osteogenesis [14]. Osteoblasts produce amorphously fibrous tissue that gradually becomes densely packed to form core bone matrix through adhesion between the secreted extracellular matrices (ECM) followed by calcium phosphate crystal deposition, which is known as bone mineralization [15]. When the stem cells were cultured in vitro, they could be osteo-induced by chemicals, including dexamethasone, 2-phospho-L-ascorbic acid, and β-glycerophosphate [16]. Because osteogenic growth factors, such as bone morphogenetic proteins (BMPs) and their receptors, can modulate the proliferation and differentiation of implanted osteogenic cells [17], another induction method is to transfect cells with certain kind of BMPs genes to increase their osteogenic capability [18]. Due to multiple functions of BMPs in postnatal bone growth and bone homeostasis [19], they are highly required for osteoblast differentiation and bone formation during embryonic development. Therefore, BMPs are broadly applied for bone regeneration to attract precursor cells from the host to invade scaffolds and induce osteoblastic differentiation.
In addition to chemical and biological inductions, physical cues are also applied for bone tissue engineering. Electrical stimulation (ES) has been proven to influence numerous cellular processes, including migration (via TORC2/PI3K), cell cycle, cell proliferation, and angiogenesis [20,21,22]. Therefore, different tissues, such as nerves, muscles, and cartilage, have been guided by ES to promote their development and regeneration [23]. Actually, hDPSCs have been administrated in vivo combing pulsed electrical magnetic field (PEMF) treatment for healing injured nerves, however, there was no difference when comparing to the PEMF only group [24]. In contrast, electrodes have been inserted to medium to directly stimulate hDPSCs, which significantly improved the expression of osteocalcin [25], suggesting that direct-current electric field (DCEF) may facilitate the differentiation of hDPSCs compared to the PEMF treatment. Regarding the bone repair, Wolff’s law indicates that bone regeneration always adapts to the loading. Because collagen in bone tissue demonstrates piezoelectricity, it has been hypothesized that mechanical signals delivered to cells may be mediated by electrical current generated by bone matrix [26]. Therefore, ES may be a potential treatment to promote differentiation of stem cells.
Although the insertion of electrodes to culture medium may easily provide DCEF treatment, this method may elicit unwanted redox reactions of the medium ingredients as well as the faradaic reaction and corrosion of the electrodes [27]. Therefore, we have previously fabricated a conductive polypyrrole (PPy) film to construct an ES device [28]. Different from 3D conductive scaffolds, 2D conductive films allow us to easily monitor cells [29,30]. These PPy films were applied for direct-current electric field (DCEF) treatment to rat bone marrow stromal cells (rBMSCs) [28]. Although these PPy films were not examined in vivo, rBMSCs demonstrated good adhesion and proliferation on these PPy films because of their good biocompatibility [31,32]. Our results revealed that the mineralization of rBMSCs can be highly promoted by DCEF treatment, and the improvement highly depended on the ES treating time [33].
Although our study indicated that DCEF may facilitate osteogenesis of rBMSCs, whether this ES provides similar effects on hDPSCs is still unclear. Therefore, ES devices fabricated using conductive PPy films were applied in this study to investigate the promotion effect of substrate-mediated ES treatment on osteo-differentiation of hDPSCs in vitro. Mineralization levels were illustrated by alizarin red S staining and quantified by the calcium-(ocresolphthalein complexone) (Ca-OCPC) complex method. The expression profiles of genes in the BMP family were also evaluated by qPCR. In addition, stimulations at different time points were performed to determine the temporal influences of ES on osteogenesis.
2. Materials and Methods
2.1. Materials
Fetal bovine serum (FBS) and trypsin-EDTA were obtained from Gibco/Thermo Fisher Scientific (Waltham, MA, USA). The DPSC BulletKit was obtained from Lonza (Basal, Switzerland). Pyrrole, ammonium persulfate, dexamethasone, 2-phospho-L-ascorbic acid trisodium salt, β-glycerophosphate disodium salt hydrate, Triton X-100, and glutaraldehyde were purchased from Sigma-Aldrich (St Louis, MO, USA).
2.2. Cell Culture of Human Dental Pulp Stem Cells (hDPSCs) and Osteogenesis Induction
Human dental pulp stem cells (hDPSCs) were obtained from Lonza (Basel, Switzerland), which were isolated from adult third molars collected during the extraction of a donor’s “wisdom” teeth. These cells express CD105, CD166, CD29, CD90, and CD73, and they do not express CD34, CD45, and CD133 markers. After being thawed from cryopreserved tubes, the cells were maintained in DPSC BulletKit medium with 10% FBS. The osteogenesis of hDPSCs was induced by adding osteogenic supplements (100 μm ascorbic-2-phosphate, 10 mM β-glycerophosphate, and 100 nM dexamethasone) to the DPSC BulletKit medium.
2.3. The Preparation of Polypyrrole (PPy) Films and the Fabrication of the Electrical Stimulation Device
The fabrication of PPy films was performed following our previous publication [20] with slight modifications. Tissue culture polystyrene (TCPS) dishes with diameters of 10 cm were used as the substrates for PPy film deposition. First, 15 mL each of 0.1, 0.3, or 0.5 M of pyrrole and ammonium persulfate at 0.2 equivalent concentrations (i.e., 0.02, 0.06, and 0.1 M, respectively) were added to the dishes and gently mixed for 15 min at 4 °C to facilitate film formation. Afterward, the film was rinsed with deionized water and dried in an oven at 37 °C. The sheet resistances of these PPy films were examined using four-point probe (EverBeing, Hsinchu, Taiwan) analysis. To ensure coating uniformity, each film was examined at 20 different points in different regions. Afterward, the fabricated PPy films were trimmed to rectangles with dimensions of 60 mm × 58 mm. Polypropylene rings with diameters of 10 mm and heights of 8 mm were glued onto the PPy films to create wells for the cell culture (Figure 1). The opposite ends of the films were covered with tin foil paper as electrodes and fixed with stainless steel clips. The device was sterilized under UV light for 30 min. The culture areas were washed with phosphate-buffered saline (PBS) followed by culture medium. These ES devices were placed in polystyrene culture dishes with diameters of 15 cm for insulation, and the electrodes were connected in parallel to a DC power supply (Regulated DC power supply, Hola, Taiwan). In addition, these ES devices were examined by DCEF procedure for 12 h to ensure their electrical stability.
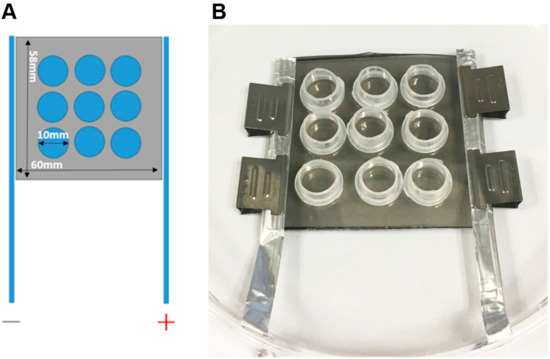
Figure 1.
The layout of the electrical stimulation (ES) device and the actual fabrication format. (A) The design of substrate-mediated ES device. The polypyrrole (PPy) films were deposited on the tissue culture polystyrene (TCPS) dishes and trimmed to rectangles with dimensions of 60 mm × 58 mm. Nine polypropylene rings with diameters of 10 mm were glued onto PPy films to constrain the area of cell culture. Two electrodes were placed at the opposite ends of the PPy films and were connected in parallel to an external DC power source. (B) The actual photo of the ES device.
2.4. Culture of hDPSCs on the Electrical Stimulation Devices and the Induction of Osteogenesis
For the DCEF treatment, the cells were seeded in regular culture medium on the PPy films at a density of 15,000 cells/cm2 for one day. Afterward, the medium was replaced with osteogenic medium, and the DCEF treatment with an electric field of 0.33 V/cm was immediately applied for 4 h, which was determined according to our previous study [28,33]. The medium was changed every three days.
2.5. Lactate Dehydrogenase (LDH) Assay
Cell numbers were quantified using the CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega, Madison, WI, USA) by measuring cytosolic lactate dehydrogenase (LDH) activity. Prior to the analysis, 100 μL of fresh medium was replaced to each well, and 15 μL of lysis buffer was added to release LDH from the live cells. After transferring 50 μL of LDH-releasing medium to 96-well microplates, 50 μL of LDH reagent was added and incubated for 30 min at room temperature. Finally, 50 μL of stop solution was added per well, and the absorbance at a wavelength of 490 nm was measured. A standard curve was generated using known cell amounts to calculate the cell numbers of the samples.
2.6. RNA Extraction and Real-Time Quantitative PCR (qPCR)
Cells in each experimental group were collected, and their mRNAs were extracted using TRIzol reagent (Life Technologies/Thermo Fisher Scientific, Waltham, MA, USA). The collected mRNA was reversely transcribed to cDNA using the SuperScript III First-Strand Synthesis System (Invitrogen/Thermo Fisher Scientific, Waltham, MA, USA). The relative mRNA levels were quantified using qPCR in the presence of a TaqMan probe and the TaqMan Master Mix (Roche Diagnostics GmbH, Mannheim, Germany), according to the manufacturer’s instructions. The primers used for the amplification of each gene are listed in Table 1. The expression levels of the target genes were normalized to that of GAPDH. The LightCycler Software (Version 4.05, Roche Diagnostics GmbH) was used to generate the quantitative data.

Table 1.
Primers for qPCR analysis.
2.7. Alizarin Red S Staining
The cells were washed with PBS, and then 70% ethanol was added to fix the cells at 4 °C for 1 h. Next, cells were washed with PBS, and the staining solution (40 mM alizarin red S, pH 4.2) was added at room temperature for 5 min. The staining solution was subsequently discarded, and then the cells were washed three times with distilled water. The stained images were visualized using a Nikon Eclipse 80i fluorescence microscope and captured using a cooled CCD apparatus (Nikon Instruments, Tokyo, Japan).
2.8. Quantitative and Qualitative Analyses of Calcium Deposition in the Extracellular Matrix (ECM)
The Ca-OCPC complex method was used to quantify the level of calcium deposition. Before the assay, the medium was removed from the well, which was washed twice with PBS. Next, 100 μL of 0.5 N acetic acid was added to release the calcium. Then, 10 μL of the calcium-released sample was added to 200 μL of calcium-binding reagent (0.1 mg/mL of o-cresolphthalein complexone (OCPC) and 1 mg/mL of 8-hydroxyquinoline) and 200 μL of buffer reagent (1.6 M 2-amino-2-methyl-1-propanol, pH 10.7). After 15 min incubation, 100 μL of purple-colored Ca-OCPC complex was collected for the measurement of absorbance at a wavelength of 575 nm, and these reads were compared to those of the CaCl2 standard solutions for quantification.
2.9. Statistical Analysis
The statistical analyses were performed using a two-tailed Student’s t-test to make comparison, and the errors were reported as standard deviations.
3. Results
3.1. Osteogenic Potential of Human Dental Pulp Stem Cells on Conductive PPy Films
For treatment of skeletal defects, osteo-conductive materials are critical to promote bone healing [34]. To determine whether PPy is a suitable substrate for cell culture in vitro, hDPSCs were seeded on PPy films. The morphology of the hDPSCs on PPy was maintained as spindle-like, which was similar to that of cells grown on TCPS (Figure 2A). The results of lactose dehydrogenase (LDH) assay revealed that there was nearly no difference in the proliferation rates of hDPSCs cultured on TCPS and PPy, suggesting the good biocompatibility of PPy (Figure 2B).
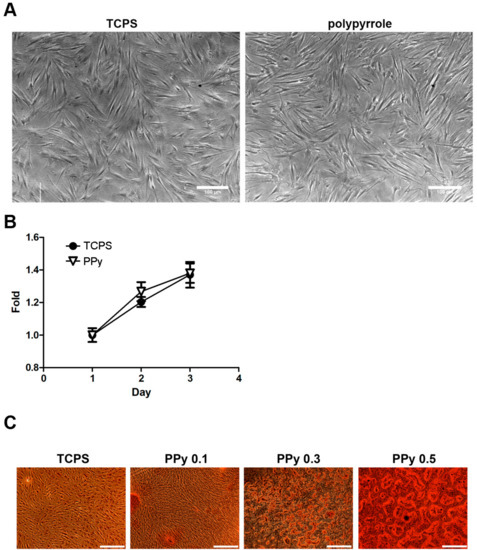
Figure 2.
(A) Phase contrast images of human dental pulp stem cells (hDPSCs) on TCPS (left) and PPy films (right). The hDPSCs were cultivated on TCPS or PPy for 3 days. The cells on both materials exhibited almost the same typical fibroblast-like morphology with comparable confluency, indicating that cell adhesion and extension were similar on these two surfaces (scale bar = 100 μm). (B) The LDH assays were applied to quantify the amounts of hDPSCs on TCPS or PPy films. All cell numbers were compared to those in day 1. The results showed comparable cell viability and proliferation between two surfaces, suggesting the good biocompatibility of PPy films (n = 3). (C) Alizarin red S staining was performed to evaluate the level of mineralization. The hDPSCs were seeded on PPy films prepared by pyrrole solutions in concentrations of 0.1, 0.3, and 0.5 M, respectively. The photos were taken 14 days after osteo-induction, which indicated that PPy films prepared in higher concentrations of pyrroles resulted in the better mineralization (scale bar = 500 μm).
To investigate the effects of conductivity of cell substrate on osteogenesis, differentiation concentrations of pyrrole were used to prepare PPy films. Four-point probe analysis was applied to measure sheet resistances of PPy films, and the results indicate that electrical resistances decreased with increasing pyrrole concentrations (Table 2), suggesting that the conductivity of PPy films can be easily manipulated. These PPy films were then applied as substrates to examine their effects on osteo-differentiation of hDPSCs. These seeded cells were induced by osteogenic medium, and alizarin red S staining was applied on day 14 to determine the level of calcium deposition (Figure 2C). The results showed that cells grown on PPy films with lower electrical resistances demonstrated higher levels of mineralization.

Table 2.
The sheet resistances of PPy films prepared by different concentrations of pyrrole.
3.2. Analysis of Gene Expression of the BMP Family and BMP Receptors in hDPSCs under Electrical Stimulation
Because PPy films were not only suitable but also beneficial to osteogenesis, these conductive substrates were further applied to investigate the potential of ES on facilitating hDPSCs differentiation. Bone morphogenetic proteins (BMPs) are well-known signal proteins in osteogenesis [35]; thus, the gene expression profiles of the BMP family and BMP receptors were evaluated in this study. After seeding hDPSCs on PPy films for one day, these cells were osteo-induced by replacing the culture medium with osteogenetic medium (day 0) and ES was immediately performed once for 4 h. The mRNAs were extracted from the hDPSCs with or without ES treatment on different days for qPCR analysis. Gene expressions were investigated from day 0 to day 6 because genes affected in early stage of differentiation may participate in the ES-driven signaling pathways. The qPCR results demonstrated increasing expression levels of BMP2, BMP3, BMP4, BMP5, and BMPR1B in the ES group (Figure 3). Among these up-regulated genes, the differences were significant for BMP2 on day 0 and 1, BMP3 on day 0 and day 4, BMP4 on day 0 and 1, BMP5 on day 0 and 2, and BMPR1B on day 0. It is worth noting that the expression of BMP2 on day 1 exhibited 7.7-fold increase, BMP3 on day 0 exhibited 3.9-fold increase, BMP4 on day 1 exhibited 2-fold increase, BMP5 on day 2 exhibited a nearly 2-fold increase, and BMPR1B on day 0 exhibited 2.2-fold increase by ES, compared to those of the control group. These significant changes suggested that these genes may be directly influenced by ES. The expression levels of BMP1, BMP6, and BMPR2 did not demonstrate significant difference between two groups. The gene expressions of BMP7 and BMPR1-A were also evaluated; however, the expressions of these two genes were undetectable in hDPSCs.
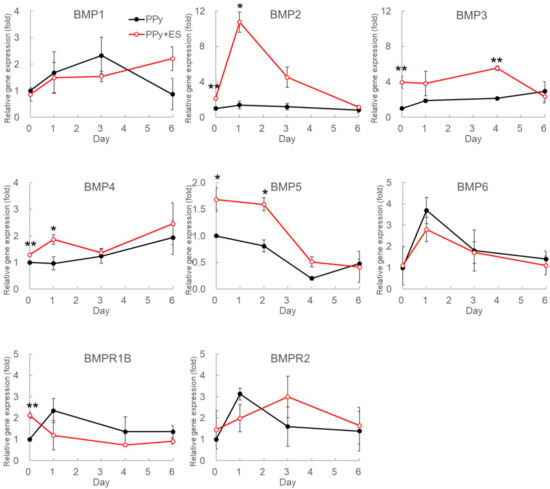
Figure 3.
Gene expressions of bone morphogenesis proteins (BMPs) and BMP receptors family in osteogenesis-induced hDPSCs under ES. To determine the ES effects on gene expressions during osteo-differentiation, hDPSCs were seeded to PPy films for 1 day and then were induced by osteogenic medium. In the same time, one-time DCEF treatment was performed for 4 h to stimulate cells immediately after medium replacement (day 0). The mRNAs were harvested from differentiated hDPSCs on different days, and the transcriptional levels of BMP family were determined using quantitative real-time PCR (qPCR). All relative results were compared to those from undifferentiated hDPSCs, and the red and black circles represent the relative gene expression levels of hDPSCs with or without DCEF treatment, respectively. Each value is the average ± SD of three independent experiments (n = 3; *: p < 0.05, **: p < 0.01).
3.3. Electrical Stimulation Enhanced the Calcium Deposition of hDPSCs on PPy Films Under Osteogenesis Induction
The qPCR results indicated that some of BMPs were up-regulated by ES treatment in the early osteogenesis stage. It is essential to evaluate whether these up-regulated BMPs indeed promoted osteo-differentiation and eventually improved bone matrix formation. In addition, the optimal ES treating time is still undetermined.
To investigate the temporal effects of ES on osteogenesis, hDPSCs were stimulated by DCEF once for 4 h at different time points after the induction with osteogenic medium. Mineralized matrix was stained by alizarin red S (Figure 4), and the deposited calcium was analyzed using Ca-OCPC complex method on day 12, day 14, and day 21 after osteo-induction (Figure 5).
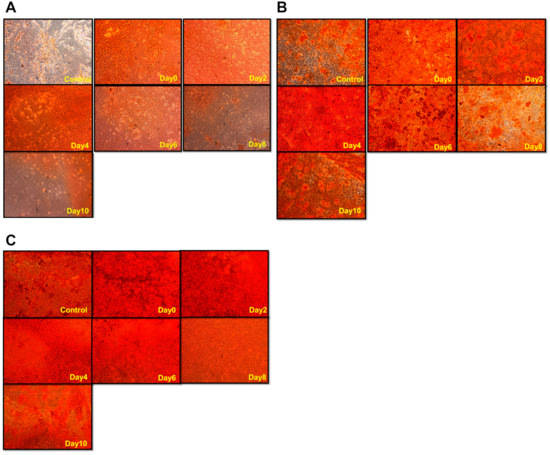
Figure 4.
The levels and distributions of mineralization of hDPSCs treated with direct-current electric field (DCEF) on different days during osteo-differentiation. After the induction of osteogenic medium, hDPSCs were stimulated by DCEF on different days (indicated by yellow words at the bottom-right corner of each image). Alizarin red S staining was performed on (A) day 12, (B) day 14, and (C) day 21 after osteo-induction to visualize the mineralization condition.
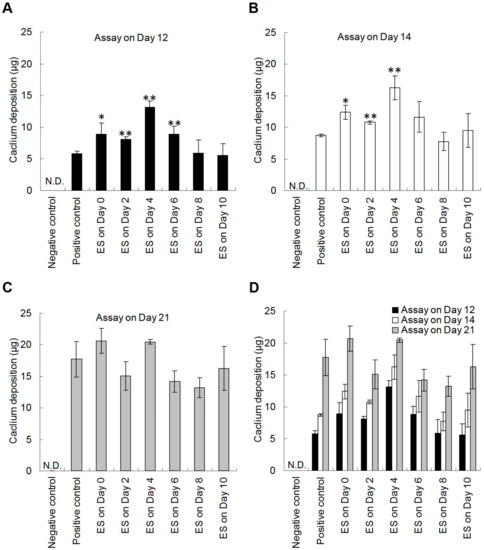
Figure 5.
The calcium deposition of hDPSCs under DCEF treatment on different days during osteo-differentiation. To determine the temporal effects of ES on mineralization, hDPSCs seeded on PPy films were treated with DCEF (0.33 V/cm) for 4 h on different days after the induction with osteogenic medium. The calcium deposition of cells was evaluated using calcium-(ocresolphthalein complexone) (Ca-OCPC) complex method on (A) day 12, (B) day 14, and (C) day 21 after osteo-induction. (D) The overall results were grouped to better understand the efficacy of ES. The negative and positive control groups were hDPSCs cultured on PPy films using normal or osteogenic media, respectively. These two control groups were not treated by DCEF. (n = 3; *: p < 0.05, **: p < 0.01 compared with the positive control group) (N.D.: Non-detectable).
The alizarin red S results demonstrated that DCEF highly improved mineralization (Figure 4). For the day 12 results, hDPSCs treated with DCEF on day 0, 2, and 4 all exhibited great enhancement in calcium deposition compared to that of the control group (no ES) (Figure 4A). However, the promotion effects were reduced when the ES was performed after day 6 or later. The results evaluated on day 14 demonstrated a similar trend (Figure 4B). These results indicate that the ES seemed to work mainly on the early stage of osteogenesis. However, when the alizarin red S staining was performed on day 21, there was almost no difference among groups (Figure 4C).
In addition to qualitative alizarin red S staining, Ca-OCPC complex method was also applied to measure the deposited calcium in ECM to quantitatively evaluate the level of mineralization (Figure 5). The results of day 12 and day 14 both indicated that hDPSCs treated with DCEF before day 6 exhibited a trend of gradual enhancement in calcium deposition compared to the control with statistical significance, and the optimum enhancement appeared on day 4 (Figure 5A,B). In addition, the calcium content reached a plateau by day 21 (Figure 5C). These results were consistent with the alizarin red S staining, suggesting that the ES-triggered pathways were likely involved in the early stages of the osteogenesis process, and the mineralization was therefore accelerated.
3.4. Enhanced Potential Derived from ES in the Process of Osteogenesis
Although our results demonstrated that the DCEF treatment effectively promoted mineralization, it was unclear whether the augment in calcium deposition was due to enhanced osteogenesis or an increase in cell number. To address this question, we quantified the cells by the LDH assay to determine the osteo-differentiation potential as Ca2+ content normalized with cell number. The level of mineralization in the early stage was analyzed on day 12, and the results showed that ES treatment before day 8 increased the differentiation potential twofold compared with the positive control group (Figure 6A). The results of assay on day 14 also showed the same trends (Figure 6B). However, when the analysis was performed on day 21, i.e., the late stage of mineralization, there was no difference between the experimental and positive control groups, indicating that ES plays a role in accelerating the rate of osteogenesis rather than in increasing the numbers of differentiated cells in our study model (Figure 6C). Again, there was no observed effect on the rate of osteogenesis when ES was applied after day 6, suggesting that the effect of ES on accelerating osteo-differentiation should mainly trigger the early pathways in the osteogenesis progress.
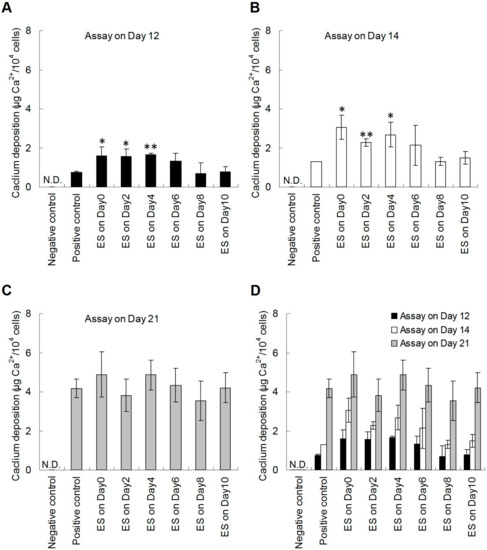
Figure 6.
The normalized quantification of calcium deposition of hDPSCs under DCEF treatment at different stages of osteo-differentiation. To distinguish whether the calcium deposition results were affected by cell proliferation, the quantification results in Figure 5 were divided by cell numbers for normalization. Cells were lysed and the released LDH were evaluated to determine cell numbers. The normalized results were evaluated on (A) day 12, (B) day 14, and (C) day 21 after osteo-induction. (D) The results from all experimental groups were grouped to better understand the efficacy of ES. The negative and positive control groups were hDPSCs cultured on PPy films using normal or osteogenic media, respectively. These two control groups were not treated by DCEF. (n = 3; *: p < 0.05, **: p < 0.01 compared with the positive control group) (N.D.: Non-detectable).
4. Discussion
Human dental pulp stem cells (hDPSCs) are a kind of mesenchymal stem cells derived from the pulp of human tooth. Because hDPSCs demonstrate the capacity of self-renewal and multilineage differentiation, they have therapeutic potentials similar to those of bone marrow stem cells [36]. In addition, hDPSCs can be extracted from teeth recovered during routine dental procedures, making them a convenient source of stem cells for cell-based therapy. Furthermore, the multilineage differentiation of hDPSCs makes them an alternative strategy for treating various human diseases, rather than limiting to the treatment of dental-related problems [37].
Although the application of biochemical cues is the gold standard to induce cell differentiation, the promising promotion effects of physical stimulations, especially electrical stimulation (ES), have also been proven. For example, neural differentiation of PC12 cells in the presence of nerve growth factor (NGF) can be significantly enhanced by ES treatment [38]. Mobini et al. also demonstrated that ES improves osteogenic-related gene expression at specific time points with different gene expression patterns between bone marrow and adipose-derived MSCs [39]. These findings suggest that ES may regulate the physiology of the cell and the differentiation potential of stem cells.
To date, the promotion effect of ES on the hDPSCs differentiation is rare. Im et al. have inserted electrodes in culture medium to stimulate hDPSCs by electrical current, and their results showed that this fluid-mediated ES treatment seems to improve cell proliferation, and the expression of OCN is slightly enhanced [25]. However, whether ES may promote osteo-differentiation, especially the level of mineralization, is still unclear. In addition, the exact role of electrical signals in regulating the biosynthetic activity and homeostasis of osteogenesis remains elusive.
In our previous study, conductive PPy films have been developed for ES to significantly improve the osteo-differentiation of rBMSCs [28]. These PPy films can be deposited onto various substrates, such as culture dishes, glass plates, and even metal devices. In addition, the electrical resistances of PPy films can be easily adjusted to meet specific requirements. These properties suggest that PPy-mediated ES treatment is a feasible approach to promote tissue regeneration [33].
Here, we demonstrated that hDPSCs could adhere on conductive PPy films with comparable proliferation rate to those on TCPS (Figure 2b). In addition, the lower resistances of PPy films resulted in the higher level of mineralization of hDPSCs (Figure 2c), which were in accordance with our previous finding of rBMSCs, suggesting that osteo-differentiation of hDPSCs can be improved by the conductivity of scaffolds [28].
Regarding the DCEF treatment, it can be either constant or in different waveforms, and the frequency of the electrical current may influence the biological effects [22]. Therefore, our previous study has treated rBMSCs using DCEF in different modes, including DCEF in constant and square waves in different frequency, offset, amplitude, and duty cycle [33]. Although these systematic examinations are helpful for optimization, we only applied 0.33 V/cm of continuous DCEF in this study because the goal of this study is to determine whether ES treatment may promote osteo-differentiation, and this constant electric field has been proven to stably improve osteo-differentiation of rBMSCs [28,33]. The DCEF treatment of hDPSCs not only enhanced osteogenic capacity but also promoted mineralization. In addition, only ES performed before day 6 resulted in increasing calcium deposition and mineralization. Therefore, we conclude that ES mainly triggers pathways in the early stages of osteo-differentiation.
Gene regulation plays an important role in osteogenesis. It has been shown that mesenchymal stem cells and osteo-progenitor cells can be differentiated into osteoblasts by certain key cytokines and functional proteins, including proteins in the BMP family, Runx2, and certain ECM proteins [40,41]. Bone morphogenesis proteins (BMPs) belong to the transforming growth factor-β (TGF-β) superfamily. Because BMPs comprise a group of proteins participating in bone formation [42], they are important in adult tissue homeostasis [43]. BMPs may initiate Smad-dependent or non-canonical pathways via binding to type I and type II heterotetrameric receptors [44]. According to a previous sequence alignment analysis, BMP2/4 and BMP5/6/7 are two groups of structurally related proteins; however, BMP1 and BMP3 are more distantly related [42]. BMP1 exhibits no sequence similarity to BMP2/4 or BMP5/6/7 because BMP1 is a metalloprotease that participates in collagen maturation and is therefore independent of BMP-mediated pathways [45]. In our study model, there was a 3.9-fold up-regulation in the expression of BMP3 on day 0. Although BMP3 has been shown to be a negative regulator of osteogenesis [46], it also has been reported that BMP3 expression in the perichondrium of chick limbs may regulate cartilage cell proliferation to ensure proper ossification [47]. Therefore, we speculate that the up-regulated expression of BMP3 by ES may play a role in modulating the levels of other BMP signaling, thereby enhancing mineralization. However, further experiments are needed to confirm this hypothesis.
The expression levels of BMP1 and BMP6 in our study model fluctuated in both control and ES-treated groups during the experimental time period, indicating that these BMPs may not be involved in ES-stimulated osteogenesis. BMP7 was reported to participate in eye and kidney development [48], but its expression is undetectable in hDPSCs. BMP2 has been studied extensively in osteogenesis [49], and numerous evidence indicates that BMP2 plays a crucial role in osteogenesis via its modulation of RUNX2 expression, especially in the early stages of osteogenesis [50]. In our study model, the expression of BMP2 was up-regulated 7.7-fold by ES on day 1. It was a significant change because no other BMPs exhibited such a profound up-regulation by ES in the early stages of osteogenesis. Therefore, we deduce that the ES-induced promotion of osteogenesis may be directly modulated via BMP2.
There are two types of BMP receptors, i.e., type I and type II, and these receptors participate in BMP-mediated signal transduction [51]. When these serine/threonine kinase receptors are triggered by a ligand, they form a heterotetrameric complex in which the type II receptor transphosphorylates the type I receptor, and the signal conducts though Smads to the nucleus [52]. In hDPSCs, the expression of BMPR1-A was undetectable with qPCR; therefore, we assume that BMPR1-B and BMPR2 are expressed in hDPSCs as heterotetramers to accept BMP protein-ligands.
In this study, we comprehensively investigated mRNA of BMPs and their receptors through qPCR analysis because BMPs are the most well-known growth factors to initiate osteo-differentiation. However, these qPCR results did not represent the corresponding protein expression levels. Further analysis such as Western blotting or ELISA should be performed to specifically determine ES effects on protein expressions. In addition, if BMPs induce osteo-differentiation of hDPSCs, relative outcome markers, such as Runx2, collagen I, alkaline phosphatase activity/expression, osteocalcin, and osteonectin, should thus be up-regulated [33]. Therefore, our future study will also focus on exploring the profiles of these outcome markers of osteogenic differentiation. As shown in Figure 4 and Figure 5, when the assay was performed on day 21, i.e., the late stage of osteo-differentiation, there was no difference between the experimental and positive control groups, suggesting that ES plays a role in accelerating but not increasing the level of mineralization. Similarly, it has been reported that the mineralization of rat bone marrow stromal cells may only be improved when ES is applied at early stage of osteo-differentiation [33]. However, Srirussamee et al. have applied ES to pre-osteoblasts (MC3T3-E1), and their results showed that the level of Runx2 expression remains unchanged during the early stage [27]. Because pre-osteoblasts are committed cells, their results implied that ES may mainly promote stem cell differentiation to therefore accelerate mineralization.
The promotion effect of ES treatment on osteogenesis has also been reported by Zhang et al. [53]. They seeded adipose-derived mesenchymal stem cells (AD-MSCs) to electrically conductive scaffold, and DCEF was applied to treat these seeded cells. Blockers of different voltage-gated ion channels were applied before ES treatment, and their results showed that the promotion effect of ES on AD-MSCs highly related to voltage-gated calcium channels. According to this study, we speculate that ES may promote the influx of calcium to bind calmodulin, by which CaM kinase is activated to regulate transcription factor of BMPs [54,55].
5. Conclusions
In this study, hDPSCs were successfully induced by osteo-differentiation, suggesting their potential use in bone regeneration. In addition, the differentiation levels were enhanced as hDPSCs were seeded on PPy films, indicating that the conductive substrates were favorable to osteogenesis. When these PPy films were applied to treat DCEF on hDPSCs, the mRNA levels of BMPs were significantly up-regulated. Therefore, the in vitro experiment showed that the calcium deposition of hDPSCs was effectively improved when DCEF was applied in the early stage of osteo-differentiation, which suggested that ES treatment can accelerate the mineralization of hDPSCs. To the best of our knowledge, this study is the first to use substrate-mediated ES treatment to enhance the osteo-differentiation and mineralization of hDPSCs, and our results should be beneficial for tissue engineering application.
Author Contributions
Conceptualization, W.-W.H.; Methodology, Y.-C.C.; Resources, C.-H.C.; Investigation, H.-W.K. and Y.-Y.M.; Formal analysis, H.-W.K. and T.-L.Y.; Writing—original draft preparation, C.-H.C. and Y.-C.C.; Writing—review and editing, W.-W.H.
Funding
This study was supported, in part, by research grants from the Ministry of Science and Technology in Taiwan (MOST 108-2628-E-008-002-MY3 and MOST 106-2314-B-281-001-MY3) and the Cathay General Hospital (CGH-MR-10210), and a joint grant from the National Central University and the Cathay General Hospital (104 CGH-NCU-A2), Taiwan.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Grayson, W.L.; Bunnell, B.A.; Martin, E.; Frazier, T.; Hung, B.P.; Gimble, J.M. Stromal cells and stem cells in clinical bone regeneration. Nat. Rev. Endocrinol. 2015, 11, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Campanella, V. Dental stem cells: Current research and future applications. Eur. J. Paediatr. Dent. 2018, 19, 257. [Google Scholar] [PubMed]
- Ballini, A.; De Frenza, G.; Cantore, S.; Papa, F.; Grano, M.; Mastrangelo, F.; Tete, S.; Grassi, F.R. In vitro stem cell cultures from human dental pulp and periodontal ligament: New prospects in dentistry. Int. J. Immunopathol. Pharmacol. 2007, 20, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Garzon, I.; Martin-Piedra, M.A.; Alaminos, M. Human dental pulp stem cells. A promising epithelial-like cell source. Med. Hypotheses 2015, 84, 516–517. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (dpscs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed]
- Karaoz, E.; Demircan, P.C.; Saglam, O.; Aksoy, A.; Kaymaz, F.; Duruksu, G. Human dental pulp stem cells demonstrate better neural and epithelial stem cell properties than bone marrow-derived mesenchymal stem cells. Histochem. Cell Biol. 2011, 136, 455–473. [Google Scholar] [CrossRef]
- Li, J.H.; Liu, D.Y.; Zhang, F.M.; Wang, F.; Zhang, W.K.; Zhang, Z.T. Human dental pulp stem cell is a promising autologous seed cell for bone tissue engineering. Chin. Med. J. (Engl.) 2011, 124, 4022–4028. [Google Scholar]
- Hilkens, P.; Gervois, P.; Fanton, Y.; Vanormelingen, J.; Martens, W.; Struys, T.; Politis, C.; Lambrichts, I.; Bronckaers, A. Effect of isolation methodology on stem cell properties and multilineage differentiation potential of human dental pulp stem cells. Cell Tissue Res. 2013, 353, 65–78. [Google Scholar] [CrossRef]
- Iohara, K.; Zheng, L.; Wake, H.; Ito, M.; Nabekura, J.; Wakita, H.; Nakamura, H.; Into, T.; Matsushita, K.; Nakashima, M. A novel stem cell source for vasculogenesis in ischemia: Subfraction of side population cells from dental pulp. Stem Cells 2008, 26, 2408–2418. [Google Scholar] [CrossRef]
- Yang, R.; Chen, M.; Lee, C.H.; Yoon, R.; Lal, S.; Mao, J.J. Clones of ectopic stem cells in the regeneration of muscle defects in vivo. PLoS ONE 2010, 5, e13547. [Google Scholar] [CrossRef]
- Apel, C.; Forlenza, O.V.; de Paula, V.J.; Talib, L.L.; Denecke, B.; Eduardo, C.P.; Gattaz, W.F. The neuroprotective effect of dental pulp cells in models of alzheimer’s and parkinson’s disease. J. Neural Transm. (Vienna) 2009, 116, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Machado, E.; Fernandes, M.H.; Gomes Pde, S. Dental stem cells for craniofacial tissue engineering. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.J.; Giannobile, W.V.; Helms, J.A.; Hollister, S.J.; Krebsbach, P.H.; Longaker, M.T.; Shi, S. Craniofacial tissue engineering by stem cells. J. Dent. Res. 2006, 85, 966–979. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Heng, B.C.; Lo, E.C.; Zhang, C. Current advance and future prospects of tissue engineering approach to dentin/pulp regenerative therapy. Stem Cells Int. 2016, 2016, 9204574. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.L.; Stanford, C.M.; Keller, J.C. Calcium and phosphate supplementation promotes bone cell mineralization: Implications for hydroxyapatite (ha)-enhanced bone formation. J. Biomed. Mater. Res. 2000, 52, 270–278. [Google Scholar] [CrossRef]
- Cheng, C.C.; Chung, C.A.; Su, L.C.; Chien, C.C.; Cheng, Y.C. Osteogenic differentiation of placenta-derived multipotent cells in vitro. Taiwan J. Obstet. Gynecol. 2014, 53, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Reddi, A.H.; Reddi, A. Bone morphogenetic proteins (bmps): From morphogens to metabologens. Cytokine Growth Factor Rev. 2009, 20, 341–342. [Google Scholar] [CrossRef]
- Rogers, M.B.; Shah, T.A.; Shaikh, N.N. Turning bone morphogenetic protein 2 (bmp2) on and off in mesenchymal cells. J. Cell. Biochem. 2015, 116, 2127–2138. [Google Scholar] [CrossRef]
- Xie, H.; Cui, Z.; Wang, L.; Xia, Z.; Hu, Y.; Xian, L.; Li, C.; Xie, L.; Crane, J.; Wan, M.; et al. Pdgf-bb secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat. Med. 2014, 20, 1270–1278. [Google Scholar] [CrossRef]
- McCaig, C.D.; Song, B.; Rajnicek, A.M. Electrical dimensions in cell science. J. Cell Sci. 2009, 122, 4267–4276. [Google Scholar] [CrossRef]
- Jeon, T.J.; Gao, R.; Kim, H.; Lee, A.; Jeon, P.; Devreotes, P.N.; Zhao, M. Cell migration directionality and speed are independently regulated by rasg and gbeta in dictyostelium cells in electrotaxis. Biol. Open 2019, 8, bio-042457. [Google Scholar] [CrossRef] [PubMed]
- Beugels, J.; Molin, D.G.M.; Ophelders, D.; Rutten, T.; Kessels, L.; Kloosterboer, N.; Grzymala, A.A.P.; Kramer, B.W.W.; van der Hulst, R.; Wolfs, T. Electrical stimulation promotes the angiogenic potential of adipose-derived stem cells. Sci. Rep. 2019, 9, 12076. [Google Scholar] [CrossRef] [PubMed]
- Peckham, P.H.; Knutson, J.S. Functional electrical stimulation for neuromuscular applications. Annu. Rev. Biomed. Eng. 2005, 7, 327–360. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.T.; Hei, W.H.; Kim, S.; Seo, Y.K.; Kim, S.M.; Jahng, J.W.; Lee, J.H. Co-treatment effect of pulsed electromagnetic field (pemf) with human dental pulp stromal cells and fk506 on the regeneration of crush injured rat sciatic nerve. Int. J. Neurosci. 2015, 125, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Im, A.-L.; Kim, J.; Lim, K.; Seonwoo, H.; Cho, W.; Choung, P.-H.; Chung, J.H. Effects of micro-electrical stimulation on regulation of behavior of electro-active stem cells. J. Biosyst. Eng. 2013, 38, 113–120. [Google Scholar] [CrossRef]
- Shamos, M.H.; Lavine, L.S.; Shamos, M.I. Piezoelectric effect in bone. Nature 1963, 197, 81. [Google Scholar] [CrossRef] [PubMed]
- Srirussamee, K.; Mobini, S.; Cassidy, N.J.; Cartmell, S.H. Direct electrical stimulation enhances osteogenesis by inducing bmp2 and spp1 expressions from macrophages and pre-osteoblasts. Biotechnol. Bioeng. 2019. [Google Scholar] [CrossRef]
- Hu, W.W.; Hsu, Y.T.; Cheng, Y.C.; Li, C.; Ruaan, R.C.; Chien, C.C.; Chung, C.A.; Tsao, C.W. Electrical stimulation to promote osteogenesis using conductive polypyrrole films. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 37, 28–36. [Google Scholar] [CrossRef]
- Shi, Z.; Gao, X.; Ullah, M.W.; Li, S.; Wang, Q.; Yang, G. Electroconductive natural polymer-based hydrogels. Biomaterials 2016, 111, 40–54. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, N.; Ma, M. Electroconductive hydrogels for biomedical applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, e1568. [Google Scholar] [CrossRef]
- Mao, J.; Zhang, Z. Polypyrrole as electrically conductive biomaterials: Synthesis, biofunctionalization, potential applications and challenges. Adv. Exp. Med. Biol. 2018, 1078, 347–370. [Google Scholar] [PubMed]
- Li, C.; Hsu, Y.-T.; Hu, W.-W. The regulation of osteogenesis using electroactive polypyrrole films. Polymers 2016, 8, 258. [Google Scholar] [CrossRef]
- Hu, W.W.; Chen, T.C.; Tsao, C.W.; Cheng, Y.C. The effects of substrate-mediated electrical stimulation on the promotion of osteogenic differentiation and its optimization. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 1607–1619. [Google Scholar] [CrossRef]
- Dawson, E.R.; Suzuki, R.K.; Samano, M.A.; Murphy, M.B. Increased internal porosity and surface area of hydroxyapatite accelerates healing and compensates for low bone marrow mesenchymal stem cell concentrations in critically-sized bone defects. Appl. Sci. 2018, 8, 366. [Google Scholar] [CrossRef]
- Kugimiya, F.; Ohba, S.; Nakamura, K.; Kawaguchi, H.; Chung, U.I. Physiological role of bone morphogenetic proteins in osteogenesis. J. Bone Miner. Metab. 2006, 24, 95–99. [Google Scholar] [CrossRef]
- Struys, T.; Moreels, M.; Martens, W.; Donders, R.; Wolfs, E.; Lambrichts, I. Ultrastructural and immunocytochemical analysis of multilineage differentiated human dental pulp- and umbilical cord-derived mesenchymal stem cells. Cells Tissues Organs 2011, 193, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Potdar, P.D.; Jethmalani, Y.D. Human dental pulp stem cells: Applications in future regenerative medicine. World J. Stem Cells 2015, 7, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gilmore, K.J.; Moulton, S.E.; Wallace, G.G. Electrical stimulation promotes nerve cell differentiation on polypyrrole/poly (2-methoxy-5 aniline sulfonic acid) composites. J. Neural Eng. 2009, 6, 065002. [Google Scholar] [CrossRef]
- Mobini, S.; Leppik, L.; Thottakkattumana Parameswaran, V.; Barker, J.H. In vitro effect of direct current electrical stimulation on rat mesenchymal stem cells. PeerJ 2017, 5, e2821. [Google Scholar] [CrossRef]
- Fakhry, M.; Hamade, E.; Badran, B.; Buchet, R.; Magne, D. Molecular mechanisms of mesenchymal stem cell differentiation towards osteoblasts. World J. Stem Cells 2013, 5, 136–148. [Google Scholar] [CrossRef]
- Terhi, J.H.; Teuvo, A.H. Differentiation of osteoblasts and osteocytes from mesenchymal stem cells. Curr. Stem Cell Res. Ther. 2008, 3, 131–145. [Google Scholar]
- Wang, R.N.; Green, J.; Wang, Z.; Deng, Y.; Qiao, M.; Peabody, M.; Zhang, Q.; Ye, J.; Yan, Z.; Denduluri, S.; et al. Bone morphogenetic protein (bmp) signaling in development and human diseases. Genes Dis. 2014, 1, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Carreira, A.C.; Alves, G.G.; Zambuzzi, W.F.; Sogayar, M.C.; Granjeiro, J.M. Bone morphogenetic proteins: Structure, biological function and therapeutic applications. Arch. Biochem. Biophys. 2014, 561, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Akhtar, N.; Jamil, H.M.; Banik, R.S.; Asaduzzaman, S.M. Tgf-β/bmp signaling and other molecular events: Regulation of osteoblastogenesis and bone formation. Bone Res. 2015, 3, 15005. [Google Scholar] [CrossRef] [PubMed]
- Ge, G.; Greenspan, D.S. Developmental roles of the bmp1/tld metalloproteinases. Birth Defects Res. C Embryo Today 2006, 78, 47–68. [Google Scholar] [CrossRef] [PubMed]
- Daluiski, A.; Engstrand, T.; Bahamonde, M.E.; Gamer, L.W.; Agius, E.; Stevenson, S.L.; Cox, K.; Rosen, V.; Lyons, K.M. Bone morphogenetic protein-3 is a negative regulator of bone density. Nat. Genet. 2001, 27, 84. [Google Scholar] [CrossRef] [PubMed]
- Gamer, L.W.; Ho, V.; Cox, K.; Rosen, V. Expression and function of bmp3 during chick limb development. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2008, 237, 1691–1698. [Google Scholar] [CrossRef] [PubMed]
- Dudley, A.T.; Lyons, K.M.; Robertson, E.J. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 1995, 9, 2795–2807. [Google Scholar] [CrossRef]
- Nguyen, V.; Meyers, C.A.; Yan, N.; Agarwal, S.; Levi, B.; James, A.W. Bmp-2-induced bone formation and neural inflammation. J. Orthop. 2017, 14, 252–256. [Google Scholar] [CrossRef]
- Shahrul Hisham Zainal, A.; Thanaletchumi, M.; Intan Zarina Zainol, A.; Rohaya Megat Abdul, W.; Sahidan, S. A perspective on stem cells as biological systems that produce differentiated osteoblasts and odontoblasts. Curr. Stem Cell Res. Ther. 2017, 12, 247–259. [Google Scholar]
- Yadin, D.; Knaus, P.; Mueller, T.D. Structural insights into bmp receptors: Specificity, activation and inhibition. Cytokine Growth Factor Rev. 2016, 27, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, G.; Li, Y.P. Tgf-beta and bmp signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Hang, A.; Phan, E.; Wildsoet, C.F. Differential gene expression of bmp2 and bmp receptors in chick retina & choroid induced by imposed optical defocus. Vis. Neurosci. 2016, 33, E015. [Google Scholar] [PubMed]
- Siddappa, R.; Martens, A.; Doorn, J.; Leusink, A.; Olivo, C.; Licht, R.; van Rijn, L.; Gaspar, C.; Fodde, R.; Janssen, F.; et al. Camp/pka pathway activation in human mesenchymal stem cells in vitro results in robust bone formation in vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 7281–7286. [Google Scholar] [CrossRef] [PubMed]
- Zayzafoon, M. Calcium/calmodulin signaling controls osteoblast growth and differentiation. J. Cell. Biochem. 2006, 97, 56–70. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).