Abstract
This article presents the findings of a laboratory study investigating the stimulation and conditioning of seeds with biochar and the effects observed in the germination and emergence of Virginia mallow (Sida hermaphrodita (L.) Rusby) seedlings. The study shows that biochar, applied as a conditioner added to water in the process of seed hydration, improves their germination capacity. When the processed plant material was added to water at a rate of 5 g (approx. 1250 seeds) per 100 mL, the rate of germination increased to 45.3%, and was 23.3% higher when compared to the control group, and 7.3% higher than in the seeds hydrated without biochar. The beneficial effects of biochar application were also reflected in the increased mass of Virginia mallow seedlings. The mass of seedlings increased by 73.5% compared to the control sample and by 25.9% compared to the seeds hydrated without biochar. Given the low cost of charcoal applied during the hydro-conditioning process, the material can be recommended as a conditioner in large-scale production of Virginia mallow.
1. Introduction
Crop yield and seed quality are among the basic parameters reflecting the effectiveness of plant production. They are determined by a number of factors, such as genotype, environmental conditions during crop growth and maturation, as well as the storage, selection, germination and enhancement of seed material. Given the processes of climate change, various stress-inducing factors influence seeds during their growth and maturation in significant and various ways. By selecting plants as well as breeding new cultivars to achieve enhanced qualities, it is possible to acquire a high yield and fine quality of seeds resistant to disease and stress. Irrespective of the effects of such breeding projects, the yield and quality of seeds may be improved by applying biological methods during plant growth or maturation and in the processes of storing and preparing the seeds for sowing [1].
Virginia mallow (Sida hermaphrodita L. Rusby) is a plants successfully grown for its biomass, which is used as a raw material in the production of renewable energy. It is a fast-growing perennial plant cultivated for its ornamental, melliferous and medicinal properties and as a fibre and fodder crop; it also produces a large quantity of biomass, comparable to or greater than common osier. However, the poor germination rate, frequently amounting to only 30–50%, poses a challenge in the cultivation of the crop. Virginia mallow production is also limited due to the difficulties in establishing plantations. The problems related to Virginia mallow seed germination and the disproportions between germination rate and seedling emergence in the field make it impossible to plan crop density [2]. In order to eliminate this obstacle, the seeds can be subjected to a pre-sowing treatment. The method is designed to stimulate the germination of seeds and the emergence of seedlings in the shortest possible time under a wide range of environmental conditions. Most importantly, the processes of germination and emergence to a large degree determine seedling vigour, their susceptibility to disease, their capacity for further growth and development and, ultimately, crop productivity [3].
Poor germination rate due to dormancy, linked with imperviousness of seed coats to water and gases, may be improved with such treatments as scarification, i.e., the mechanical alteration of seed coats, or short-term refrigeration of seeds at low temperature, periodical chilling (vernalisation) as well as growth stimulation treatments, variability to drought and saline stress through [4,5]. Seed germination and plant growth may also be improved with a number of operations, e.g., the decontamination and screening of seeds for their physical properties and treatments based on such factors as temperature or magnetic fields, as well as their irradiation with light of varying wave length and intensity [1,6]. Enhanced germination rate may also be achieved if, prior to sowing, the seeds are soaked in aqueous monocultures of algae or Cyanobacteria [7]; on the other hand the exposure of adequately wetted seeds to high or low temperatures for a specific length of time results in the seedlings having higher resistance to cold weather and the plants having greater metabolic activity and growth [8].
The germination potential of seeds can also be effectively improved by the controlled wetting of seeds up to a specified level, followed by incubating for some time at an appropriate temperature. The related methods include hydro-conditioning, osmo-conditioning and matri-conditioning. They differ in terms of the wetting techniques applied:
- -
- hydro-conditioning involves direct seed humidification with water, without a medium;
- -
- matri-conditioning involves water carried by a solid inorganic substance with high negative water potential; and
- -
- osmo-conditioning involves water carried by osmotically active substances with low osmotic potential.
A specific amount of water imbibed by the seeds initiates most of the metabolic processes preceding germination, but it is insufficient for initiating the growth and emergence of radicles. The seeds are primed this way, at a temperature of 15 or 20 °C as a rule, for a time ranging from a few hours to several days. Prior to planting, adequately conditioned seeds may be dried and stored, preferably at low temperatures and mild relative air humidity (40%). The priming treatment leads to a faster germination and emergence of seedlings, and decreased vulnerability to environmental conditions during initial plant growth. The effectiveness of the conditioning may be increased by adding growth regulators to the water [1,9,10].
The quality of the seeds is also greatly dependent on the mother plants. Under field conditions the quality of the seeds may be increased by the biological fertilisation of mother plants with substances improving the environmental conditions, keeping mother plants a suitable distance away from each other, introducing microorganisms, algae or non-toxic Cyanobacteria that produce allelopathic interactions within the soil, and finally by treating mother plants with a bioformula mainly stimulating metabolic processes [11,12,13,14,15,16,17].
Biochar is a fine-grained product of carbonisation, obtained through the pyrolysis of plant biomass and organic waste, and containing aromatic, aliphatic and oxidised compounds of carbon. The chemical composition of biochar is determined by the composition of the feedstocks and the way the process of pyrolysis is conducted. Biochars contain stable organic carbon, washed out carbon and ash. The mineral fraction of biochar comprises macro- and micro-elements which may act as a source of minerals for micro-organisms living in the soil. Biochar introduced into the soil is highly resistant to degradation and microbial decay, which means it preserves the chemical stability of the environment to which it is introduced [18,19,20,21,22]. Biochar may protect plants against infection [23]. Added to soil, it improves soil fertility and productivity and may lead to an increase in the land’s water capacity, as well as decreasing soil acidity. Biochar also has the capacity to retain and exchange nutrients, which results in a better availability of nutrients in plants [19,24,25,26].
In view of the growing body of evidence regarding the beneficial effects of biochar as a soil amendment, already successfully used in the production of various crops, the authors of this paper decided to expand the scope of this research and to apply biochar in seed conditioning. The purpose of the current study was to assess the usefulness of biochar in enhancing the viability of Virginia mallow seeds. It was designed as a preliminary laboratory study in order to verify whether further research would be justified, and if it would be practicable to apply the relevant method under field conditions.
2. Materials and Methods
2.1. Preparation and Characterisation of Biochars
Biochar used for the experiment was produced from barley straw. The material was examined for pH values, and contents of macro- and micro-elements. Biochar was refined in a mechanical lab mill, to obtain grains of a size a fraction below 5 mm. It was then sieved through a mesh with 1 mm openings, and rinsed with demineralised water several times to remove any contaminants. The biochar was then subjected to drying at 75 °C for 12 h.
The biochar used in the study was characterised by a pH value of 6.59 with total contents of carbon and nitrogen amounting to 74.35% and 0.93%, respectively. The tests also showed the water and ash content of the biochar being at a level of 9.11% and 11.57%, respectively. The biochar was characterised by a high content of volatile substances, i.e., 66.42% (Table 1).

Table 1.
The pH value of the biochar, with the content of the absorbable forms of macro-elements and the percent content of carbon and nitrogen in the biochar. x—mean, SD—standard deviation.
Table 2 presents the contents of selected macro- and micro-elements in the biochar generated from plant biomass. In view of the fact that many fertilisers used in agriculture today may contain toxic metals, the biochar used in the experiment was subjected to appropriate tests. The findings showed no aluminium, arsenic, cadmium or lead content in the material.

Table 2.
The contents of selected macro- and micro-elements in the biochar generated from biomass. x—mean, SD—standard deviation.
2.2. Plant Sample Collection
The study used commercially available Virginia mallow (Sida hermaphrodita R.) seeds, from a plantation located in the Warmia-Masuria Province, Poland.
2.3. Experimental Design
In the first stage of the experiment, 5 g of biochar was added to 100 mL of demineralised water and carefully mixed. Subsequently 0035 g of Virginia mallow seeds, with a moisture level of 5%, were introduced into the solution (1000 seeds of Virginia mallow weight approx. 4 g). The seeds were hydrated for four hours at 20 °C, after which they were transferred to hermetic containers and incubated at a temperature of 20 °C for two days. The seeds were also aerated every day. At the next stage, a new 5 g sample of Virginia mallow seeds was subjected to hydration in 100 mL of water, with no biochar added to the solution, following the same procedure as described above. All the seeds conditioned in the above way were then dried on filter paper in laboratory conditions, to achieve the initial moisture level, and their viability was assessed. For this purpose, 3 × 50 seeds were planted (representing all the variants, i.e., seeds hydrated with biochar, hydrated without biochar, and not hydrated) at a temperature of 20 °C, onto wet paper placed in Petri dishes. Each day the germinating specimens were counted, i.e., the seeds in which the radicle had emerged and reached a minimum length of 1 mm. After 14 days of the experiment, the plant material was collected for further testing. The seedlings (overground parts) collected for this purpose were weighed and rinsed several times with demineralised water, to remove any possible contamination.
2.4. Examination of Samples
The samples of biochar and the Virginia mallow seedlings were subjected to laboratory analyses using applicable analytical standards (Table 3).

Table 3.
The parameters analysed with the research methods used.
The pH of the biochar was determined by measuring the concentration of hydrogen ions, i.e., the activity of hydrogen ions (H+), with the use of the potentiometric method. The analysis was performed in a KCl solution with a concentration of 1 mol dm−3, assuming a mass of study sample to solution volume ratio of 1:2.5. All measurements were taken using the Nahita pH meter, model 907 (AUXILAB, Beriáin, Spain).
The total carbon and nitrogen content was tested using a TrueSpec CHN analyser from LECO (LECO Corporation, Saint Joseph, MI, USA).
The mineralisation of the material was repeated three times. The elemental content of the samples was determined using a method based on inductively-coupled plasma atomic emission spectroscopy (ICP-OES), using an iCAP Dual 6500 analyser from Thermo Scientific (Schaumburg, IL, USA). The mineralisation of the samples was performed in Teflon containers using a mixture of acids under specific conditions (Table 4). In each case, the sample obtained in the mineralisation process was supplemented with mineralised water to make up a volume of 50 mL. In the calibration step, standard solutions for all elements were prepared using a spectroscopic grade reagent (Thermo) with a three-step curve. The curve fit factor for all elements was over 0.99. The selection of a measuring line of appropriate length was validated by the method of standard additions. The recovery on selected lines was above 98.5% for each of the elements. Every time, the CRM 1515 (Certified Reference Material) was used and the selection of appropriate lines was carried out using the standard addition method. Every time we also used internal standards for matrix curve correction; these were yttrium (Y) and ytterbium (Yb), two elements not detected in the samples. The detection limit of the analytical method used for the elements studied was no worse than 0.01 mg kg−1.

Table 4.
The parameters of the mineralisation process.
2.5. Statistical Analyses
All parts of the experiment were independently repeated three times. The results obtained were subjected to statistical analyses using Statistica ver. 10.0. The results were statistically analysed with multiway ANOVA and differences between the means were assessed using the Duncan test.
3. Results and Discussion
Figure 1 shows the number of germinating seeds of Virginia mallow, at the relevant time, in the control sample and relative to the method of seed conditioning (hydro-conditioning alone, and hydro-conditioning in water with biochar added).
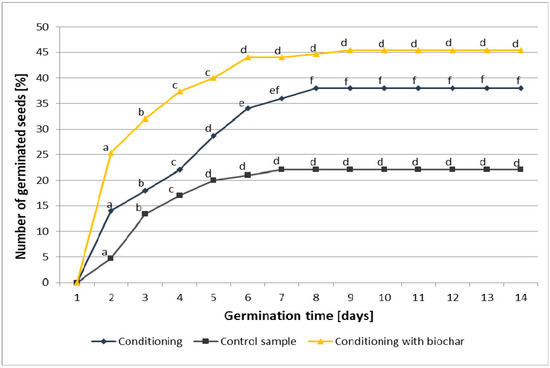
Figure 1.
Germination rate in Virginia mallow seeds in the control sample, the sample subjected to hydro-conditioning alone, and the sample subjected to hydro-conditioning with biochar added. According to the Duncan’s test, the means marked with the same letter are not significantly different at α ≤ 0.05 (differences between time germination inside each treatment).
The germination process in the three samples started at the same time—two days after the seeds were planted. The seeds in the control sample were found to have a germination rate of 22%. Poor germination rates observed in Virginia mallow are associated with the fact that the plant produces hard seeds, with a calcareous coat impervious to water. The impervious layer regulates seed dormancy, as well as the speed at which water penetrates the seeds to reach a sufficient hydration level [34]. The poor germination rate of the control sample could also have been affected by the climatic conditions occurring in the year the plants were harvested, the threshing method used and the duration of storage [35]. The seeds subjected to hydro-conditioning showed a better germination rate than the control sample, reaching 38%. Hydro-conditioning is rather commonly applied in order to improve the ability of hard seeds to germinate. The positive impact of this process on the germination and vigour of crops and garden plants has been reported by numerous researchers. Grzesik et al. [36] demonstrated the positive effects of hydration as a factor in stimulating growth and metabolic processes in Virginia mallow. The highest metabolic activity was observed in the seeds hydrated to achieve a 35–40% water content, and incubated at 20 °C for six days. Janas and Grzesik [37] showed that a pre-sowing hydro-conditioning applied to dill seeds can effectively enhance their viability and stimulate the faster emergence and growth of seedlings. The same authors determined the optimum conditions for hydro-conditioning applied to seedlings of root parsley. Parsley seeds hydrated to a hydration level of 45% and incubated at 20 °C for three to six days were found to germinate earlier and in larger numbers than non-hydrated seeds. The seedlings emerging from the former developed faster and contained more chlorophyll [38]. Similar findings were reported by Mendonça et al. [39] who found that seedlings from carrot seeds subjected to hydro-conditioning were faster to emerge and grow compared to the control sample. Xavier Fernanda da Motta et al. [40] established that hydro-conditioning is the most effective treatment to improve the germination and vigour of onion seeds. The positive effects of hydration, observed in germination, emergence and plant growth, are associated with metabolic activity, which is more rapidly initiated and increased in seeds before they are planted in soil, and in seedlings at the very beginning of their growth [36].
The addition of processed plant material (biochar from biomass) produced an increase in the number of germinating seeds. Biochar added at the rate of 5 g per 100 mL of water increased the speed of germination by 20.7%, and the germination capacity of the seeds increased to 45.3%, which was 23.3% higher than the control group; conversely, these values increased by 11.3% and 7.3%, respectively, when compared to the seeds subjected to hydration. The method applied may present several advantages over the methods of pre-sowing seed treatment and enhancement currently used. In the literature we can find numerous seed scarification methods, more or less effective, and varied in terms of their feasibility for use in mass production. A number of authors have investigated methods of breaking seed dormancywith the use of concentrated sulphuric acid [41,42,43,44,45,46,47]. Doliński et al. [47] observed an increase in the germination capacity of Virginia mallow seeds subjected to scarification with sulphuric acid. The germination capacity following the scarification varied depending on the year the seeds were harvested and on the duration of their storage [35,47]. Although it produces satisfying results, this method is hazardous to seeds (prolonged exposure to the acid leads to embryo death) and harmful to people carrying out the scarification process. Furthermore, scarification with sulphuric acid may lead to a decrease in seed vigour [41,43,47]. Mechanical processing is also frequently used to interrupt seed dormancy. However, such methods may prove troublesome for production on a large scale; examples include nicking lupin seed coats with a razor blade [48], manually rubbing burclover Medicago polymorpha seeds with sand paper, and shaking seeds of clover Trifolium subterraneum with sand in a laboratory shaker [49]. Germination rates may also be improved by a treatment involving the stimulation of seeds with a magnetic field, however, these techniques are rather costly and not as effective as other known methods of seed scarification [50,51]. Effective methods for breaking seed dormancy include thermal scarification. Dormancy was successfully broken in seeds of burclover, Medicago polimorpha, and clover, Trifolium subterraneum, following a short-term heat treatment with a blowlamp. As a result, seed germination rate increased from 10 to 79% for burclover, and from 46 to 95% for clover [49]. Thermal scarification may also be performed by immersing seeds in hot water or treating them with liquid nitrogen. Doliński et al. [35,47] observed a significant increase in the germination capacity of Virginia mallow seeds following a short treatment with hot water. The temperature required for interrupting the dormancy of a Virginia mallow seed depends on the length of time the seed has spent in storage. Sharma et al. [52] reported even a ten-fold increase in the viability of seeds of selected fast-growing tropical tree species, i.e., Albizzia lebbek, Albizzia procera, Peltophorum pterocarpum, Acacia auriculiformis and Leucaena leucocephala, following a short-term (1–10 min) treatment with hot water (100 °C). The dormancy of fodder galega seeds was interrupted following a treatment with liquid nitrogen (−196.5 °C) [53]. Notably, the duration and temperature of the treatments applied to break seed dormancy depends on the plant species.
The experiment described in this paper is the first to apply biochar as an additional conditioner in the process of seed hydration. Rogovska et al. [54] assessed the effects of biochars on seedling growth and absorption of allelochemicals present in corn (Zea mays L.) residues. Corn seeds were germinated in aqueous extracts of six varied biochars. Extracts from the six biochars had no effect on percent germination; however, extracts from three biochars produced at high conversion temperatures significantly inhibited shoot growth by an average of 16% relative to control (deionized water). In the literature we can find studies assessing the effects of biochar as a soil fertiliser on seed germination. Solaiman et al. [55] carried out a laboratory experiment which showed a positive association between biochar, obtained from various biological feedstocks, and increased germination rates in wheat seeds. Generally, the beneficial effects were produced by five types of biochar applied in doses <50 t h−1. Conversely, higher doses failed to stimulate the germination process, or even inhibited it. Shamim et al., [56] assessed the effects of biochars on seed germination, early growth of Oryza sativa L. In this study, the germination percentage of paddy increased in case of two of three biochar was above the control level but the difference was not significant. The third type of biochar shows decrease in germination percentage than control. Other reports on the related study found that there is no negative impact of biochar on the germination of paddy seeds [57]. The study based on forest seeds found that biochar application increases germination of seeds [58]. Saletnik et al. [19] showed that by supplementing soil with the most effective dose of biochar defined for that species, it is possible to achieve a 20% increase in the germination rate of Virginia mallow seeds. It was also observed that biochar from plant biomass, introduced to the soil, led to a significant increase in the mass obtained by plants in the early stages of their development [19].
Figure 2 presents the total mass of the seedlings harvested after 14 days of the experiment, relative to the sample type. The total mass of the plants collected from the control sample was 0.275 g. The mass of the plants collected from seeds subjected to conditioning with biochar was significantly greater than the mass of the seedlings from seeds subjected to hydroconditioning alone, or those from the control sample. The total mass of the seedlings increased by 73.1% compared to the control sample, and by 25.6% compared to the hydroconditioned sample.
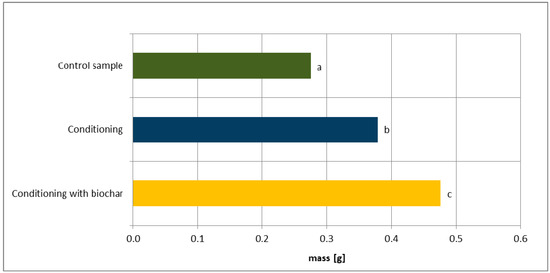
Figure 2.
The total masses of Virginia mallow seedlings in the experimental variants. According to Duncan’s test, means marked with the same letter are not significantly different at α ≤ 0.05.
The literature on this topic suggests that biochar beneficially affects crop yields, for instance Robertson et al. [58] planted lodgepole pine (Pinus contorta var. latifolia) or sitka alder (Alnus viridis ssp. sinuata) seeds in pots containing field-collected forest soils amended with 0.5, or 10% (dry mass basis) biochar with and without urea fertilizer (150 mg N kg−1). Pine had greater biomass in biochar fertilizer treatments compared to control and fertilizer-only treatments. Alder seedlings had greater shoot biomass when grown in biochar-amended soils compared with unamended control. According to Liu et al. [59] biochar amendments used in fields at a rate below 30 t h−1 resulted in increased productivity of these specific crops: legumes by 30%, vegetables—29%, grasses—13%, wheat—11%, maize—8%, rice—7% [59]. Liu et al. [60] demonstrated the positive effects of biochar made from wheat straw in the productivity of rapeseed and sweet potato. A biochar amendment applied at a rate of 40 t ha-1 resulted in a yield increase of 36.02% and 53.77%, respectively [60]. Hossain et al. [61] used wastewater sludge biochar as a fertiliser. With the amendment used at a rate of 10 t h−1 the yield of cherry tomato increased by 64% compared to control conditions. Chan et al. [62] reported the positive effects of poultry litter biochar, applied at a rate of 10 t h−1, in the increased mass of radish. Uzoma et al. [63] investigated the impact of cow manure biochar on maize yield. Biochar amendment applied at rates of 15 t and 20 t h−1 increased maize grain yield by 150% and 98%, respectively, compared to the control. The findings of the study suggested that cow manure biochar contained important nutrients which significantly contributed to increased maize productivity. Numerous authors point out that there is a threshold value for the application of biochar soil amendment when it comes to crop productivity. It was shown that the use of biochar at higher rates does not produce statistically significant effects on crop yields [64]. Saletnik et al. [19] observed a significant increase in the mass of Virginia mallow at an early stage of growth if biochar was added to soil. Furthermore, the study showed that a combination of biochar and ash from biomass, added at an optimum rate, improves the germination capacity of seeds, while increasing the potassium content of the overground parts of plants and reducing their concentrations of phosphorus and calcium [19]. Biochar has been also studied as method to reduce the greenhouse gas emissions from agriculture soils [65,66,67]. Cayuela et al. [66] found that biochar reduced soil N2O emissions by 54% in laboratory and field studies. The biochar feedstock, pyrolysis conditions and C/N ratio were shown to be key factors influencing emissions of N2O while a direct correlation was found between the biochar application rate and N2O emission reductions.
Table 5 presents the results reflecting the total contents of selected macro- and micro-elements in overground parts of Virginia mallow plants, in both the control and experimental samples, 14 days after the experimental culture was established.

Table 5.
Contents of selected macro- and micro-elements in overground parts of Virginia mallow plants in the control sample, in the hydro-conditioned sample, and the sample hydroconditioned with biochar added. X–mean, SD–standard deviation. According to Duncan’s test, means marked with the same letter are not significantly different at α ≤ 0.05.
In each case the samples analysed were characterised by having the highest potassium, calcium and phosphorus contents. The lowest levels of macro- and micro-elements were identified in plants grown from seeds conditioned with biochar. This may have been caused by the faster development and intensified metabolic processes, associated with a greater nutrient requirement, at the early stage of growth [36,68]. The samples differed most significantly in their potassium content. Potassium is absorbed extremely rapidly by plants, second only to nitrogen, though by young ones possessing the fast-growing meristematic tissue responsible for the development of roots and stems in particular. This element is an essential plant nutrient; in the case of a potassium deficiency it is sent to apical meristems and young leaves first of all. At the early stages of plant development, phosphorus is necessary for the normal growth of the root system, and calcium is an important factor in both the regulation of cellular metabolism and structural functions, as well as being a universal carrier of information [68,69,70,71].
4. Conclusions
The current study shows that by adding biochar to water in the process of hydro-conditioning it is possible to achieve an over 20% increase in the germination capacity of Virginia mallow seeds. It was noted that the hydro-conditioning of seeds with biochar added resulted in a significantly greater mass of the seedlings collected at the early stage of plant development.
These findings provide a justification for further research focusing on the use of biochar as a stimulator for seed germination. Subsequent laboratory tests should be expanded, e.g., to include analyses of the enzymes involved in the germination process. The method used should be developed further with respect to optimum temperatures, incubation time and biochar dosage. Research related to these factors should be continued under field conditions in order to confirm the beneficial effects of biochar in the development, growth and productivity of crops. The application of biochar as a biological factor stimulating seed germination, as presented in the experiment, seems to present advantages related to the practicality and simplicity of the treatment as well as to the availability of the conditioning material. This method may also prove to be a cost-efficient alternative to the existing methods of a pre-sowing scarification of seeds used in plant production.
Author Contributions
Conceptualization, B.S.; Data curation, B.S., A.S. and G.Z.; Formal analysis, B.S., G.Z., M.B. and M.T.; Methodology, B.S., G.Z., M.B. and M.T.; Project administration, C.P.; Supervision, C.P.; Writing—original draft, B.S. and A.S.; Writing—review & editing, C.P. and A.S.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Grzesik, M.; Janas, R.; Górnik, K.; Romanowska-Duda, Z. Biological and physical methods of seed production and processing. J. Res. Appl. Agric. Eng. 2012, 57, 147–152. [Google Scholar]
- Tworkowski, J.; Szczukowski, S.; Stolarski, M.J.; Kwiatkowski, J.; Graban, Ł. Productivity and properties of virginia fanpetals biomass as fuel depending on the propagule and plant density. Fragm. Agron. 2014, 31, 115–125. [Google Scholar]
- Podleśny, J.; Sowiński, M. The effect of seeds stimulation by magnetic field on growth, development and dynamics of biomass accumulation in pea (Pisum sativum L.). Agric. Eng. 2005, 9, 103–110. [Google Scholar]
- Duczmal, K.; Tucholska, H. Nasiennictwo [Seed Production]; PWRiL: Poznań, Poland, 2000; Volume 1, pp. 205–234. [Google Scholar]
- Cavallaro, V.; Barbera, A.C.; Maucieri, C.; Gimma, G.; Scalisi, C.; Patanè, C. Evaluation of variability to drought and saline stress through the germination of different ecotypes of carob (Ceratonia siliqua L.) using a hydrotime model. Ecol. Eng. 2016, 95, 557–566. [Google Scholar] [CrossRef]
- Ciupak, A.; Szczurowska, I.; Gładyszewska, B.; Pietruszewski, S. Impact of laser light and magnetic field stimulation on the process of buckwheat seed germination. Tech. Sci. 2007, 10, 1–10. [Google Scholar] [CrossRef]
- Karthikeyanb, N.; Prasannaa, R.; Nainb, L.; Kaushik, B.D. Evaluating the potential of plant growth promoting Cyanobacteria as inoculants for wheat. Eur. J. Soil Biol. 2007, 43, 23–30. [Google Scholar] [CrossRef]
- Górnik, K. The effect of temperature treatments during ‘Wielkopolski’ sunflower seed imbibition and storage on plant tolerance to chilling. Folia Hortic. 2011, 23, 83–88. [Google Scholar] [CrossRef]
- Grzesik, M.; Janas, R. Effects of hydropriming on metabolic activity, seed germination and seedling emergence of carrot. J. Res. Appl. Agric. Eng. 2011, 56, 127–132. [Google Scholar]
- Badek, B.; van Duijn, B.; Grzesik, M. Effects of water supply methods and incubation on germination of China aster (Callistephus chinensis) seeds. Seed Sci. Technol. 2007, 35, 569–576. [Google Scholar] [CrossRef]
- Nahm, M.; Morhart, C. Virginia mallow (Sida hermaphrodita (L.) Rusby) as perennial multipurpose crop: Biomass yields, energetic valorization, utilization potentials, and management perspectives. GCB Bioenergy 2018, 10, 393–404. [Google Scholar] [CrossRef]
- Błażewicz-Woźniak, M. Effect of no-tillage and mulching with cover crops on yield of parsley. Folia Hortic. 2005, 17, 3–10. [Google Scholar]
- Górnik, K.; Grzesik, M. Effect of Asahi SL on China aster ‘Aleksandra’ seed yield, germination and some metabolic events. Acta Physiol. Plant. 2002, 24, 378–383. [Google Scholar] [CrossRef]
- Janas, R. Effect of tytanit on yield and quality of onion seeds. Postępy Nauk Rol. 2009, 541, 133–139. [Google Scholar]
- Janas, R.; Grzesik, M. Pro-ecological methods of improving horticultural plant seeds quality. Adv. Agric. Sci. Probl. Issues 2006, 510, 213–221. [Google Scholar]
- Grzesik, M.; Romanowska-Duda, Z.B. Technologia hydrokondycjonowania nasion ślazowca pensylwańskiego (Sida hermaphrodita) w aspekcie zmian klimatycznych [Technology for hydro-conditioning of Virginia mallow (Sida hermaphrodita) seeds in view of climate changes]. Prod. Biomasy Wybrane Probl. 2009, VII, 63–69. [Google Scholar]
- Grzesik, M.; Romanowska-Duda, Z.B. New Technologies of the energy plant production in the predicted climate changed conditions. Bjuleten Djerżawnowo Nikitsk. Bot. Sada. Ukr. Akad. Agrar. Nauk 2009, 99, 65–68. [Google Scholar]
- Saletnik, B.; Zaguła, G.; Bajcar, M.; Tarapatskyy, M.; Bobula, B.; Puchalski, C. Biochar as a Multifunctional Component of the Environment—A Review. Appl. Sci. 2019, 9, 1139. [Google Scholar] [CrossRef]
- Saletnik, B.; Bajcar, M.; Zaguła, G.; Czernicka, M.; Puchalski, C. Influence of biochar and biomass ash applied as soil amendment on germination rate of Virginia mallow seeds (Sida hermaphrodita R.). Econtechmod Int. Q. J. 2016, 5, 71–76. [Google Scholar]
- Lehman, J. Bio-energy in the black. Front. Ecol. Environ. 2007, 5, 381–387. [Google Scholar] [CrossRef]
- Malińska, K. Biochar - a response to current environmental issues. Eng. Prot. Environ. 2012, 15, 387–403. [Google Scholar]
- Lehmann, J.; Rilling, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biotechnol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Nigussie, A.; Kissi, E.; Misganaw, M.; Ambaw, G. Effect of biochar application on soil properties and nutrient uptake of lettuces (Lactuca sativa) grown in chromium polluted soils. Am. Eur. J. Agric. Environ. Sci. 2012, 12, 369–376. [Google Scholar]
- Karhu, K.; Mattila, T.; Bergstrom, I.; Regina, K. Biochar addition to agricultural soil increased CH4 uptake and water holding capacity—Results from a short-term pilot field study. Agric. Ecosyst. Environ. 2011, 140, 309–313. [Google Scholar] [CrossRef]
- Hossain, M.K.; Strezov, V.; Chan, K.Y.; Ziolkowski, A.; Nelson, P.F. Influence of pyrolysis temperature on production and nutrient properties of wastewater sludge biochar. J. Environ. Manag. 2011, 92, 223–228. [Google Scholar] [CrossRef]
- Lehman, J.; Joseph, S. Biochar for Environmental Management: Science and Technology; Earthscan: London, UK, 2009; Volume 2, pp. 13–30. [Google Scholar]
- Polish Committee for Standardization. Soil Quality—Determination of Ph; Polish Committee for Standardization: Warsaw, Poland, 1997.
- Polish Committee for Standardization. Chemical and Agricultural Analysis of the Soil—Determination of the Content of Absorbable Phosphorus in Mineral Soils; Polish Committee for Standardization: Warsaw, Poland, 1996.
- Polish Committee for Standardization. Chemical and Agricultural Analysis of the Soil—Determination of the Content of Potassium in Mineral Soils; Polish Committee for Standardization: Warsaw, Poland, 2002.
- Polish Committee for Standardization. Chemical and Agricultural Analysis of the Soil—Determination of the Content of Magnesium in Mineral Soils; Polish Committee for Standardization: Warsaw, Poland, 2004.
- British Standards Institution. Solid Biofuels—Determination of Total Carbon, Hydrogen and Nitrogen Content—Instrumental Methods; British Standards Institution: London, UK, 2011. [Google Scholar]
- Milestone. SK-10 High Pressure Rotor; HPR-PE-19 Carbon Black; Milestone: Shelton, CT, USA, 2019. [Google Scholar]
- Milestone. SK-10 High Pressure Rotor; HPR-AG-02 Dried Plant Tissue; Milestone: Shelton, CT, USA, 2019. [Google Scholar]
- Woodstock, L.W. Seed imbibition: A critical period for successful germination. J. Seed Technol. 1988, 12, 1–15. [Google Scholar]
- Doliński, R. Influence of treatment with hot water, chemical scarification and storage time on germination of Virginia fanpetals (Sida hermaphrodita (L.) Rusby) seeds. Biul. Inst. Hod. Aklim. Roślin 2009, 257, 293–303. [Google Scholar]
- Grzesik, M.; Janas, R.; Romanowska-Duda, Z. Stimulation of growth and metabolic processes in Virginia mallow (Sida hermaphrodita L. Rusby) by seed hydroconditioning. Probl. Agric. Eng. 2011, 4, 81–89. [Google Scholar]
- Grzesik, M.; Janas, R. Effect of conditioning on dill (Anethum graveolens L.) seed germination and plant emergence. J. Res. Appl. Agric. Eng. 2013, 58, 188–192. [Google Scholar]
- Grzesik, M.; Janas, R. Physiological method for improving seed germination and seedling emergence of root parsley in organic systems. J. Res. Appl. Agric. Eng. 2014, 59, 80–86. [Google Scholar]
- Mendonça, S.R.; Silva Pereira, J.C.; Teles da Cruz, A. Emergence of carrot seeds cv. Brasília submitted to hydro-conditioning. Ipê Agron. J. 2018, 2, 18–25. [Google Scholar]
- Xavier, F.M.; Brunes, A.P.; Cavalcante, J.A.; Meneghello, G.E.; Radke, A.K.; Noguez Martins, A.B.; Winke Dias, L.; Revers Meneguzzo, M.R. Germination of Allium cepa L. seeds subjected to physiological conditioning and drying. Rev. Ciênc. Agrár. 2017, 40, 1–10. [Google Scholar]
- Imani, A.F.; Salehi Sardoei, A.; Shahdadneghad, M. Effect of H2SO4 on Seed Germination and Viability of Canna indica L. Ornamental Plant. Int. J. Adv. Biol. Biomed. Res. 2014, 2, 223–229. [Google Scholar]
- Rostami, A.A.; Shasavar, A. Effects of Seed Scarification on Seed Germination and Early Growth of Olive Seedlings. J. Biol. Sci. 2009, 9, 825–828. [Google Scholar] [CrossRef][Green Version]
- Kheloufi, A.; Mansouri, L.M.; Boukhatem, F.Z. Application and use of sulphuric acid pretreatment to improve seed germination of three acacia species. Reforesta 2017, 3, 1–10. [Google Scholar] [CrossRef]
- Zare, S.; Tavili, A.; Darini, M.J. Effects of different treatments on seed germination and breaking seed dormancy of Prosopis koelziana and Prosopis Juliflora. J. For. Res. 2011, 22, 35–38. [Google Scholar] [CrossRef]
- Tanaka-Oda, A.; Kenzo, T.; Fukuda, K. Optimal germination condition by sulfuric acid pretreatment to improve seed germination of Sabina vulgaris. J. For. Res. 2009, 14, 251–256. [Google Scholar] [CrossRef]
- Saied, A.S.; Gebauer, J.; Buerkert, A. Effects of different scarification methods on germination of Ziziphus spina-christi seeds. Seed Sci. Technol. 2008, 36, 201–205. [Google Scholar] [CrossRef]
- Doliński, R.; Kociuba, W.; Kramek, A. Influence of short treatment with hot water, chemical scarification and gibberellic acid on germination of Virginia mallow (Sida hermaphrodita (L.) Rusby) seeds. Adv. Agric. Sci. Probl. Issues 2007, 517, 139–147. [Google Scholar]
- Mackay, W.A.; Davis, T.D.; Sankhla, D. Influence of scarification and temperature treatments on seed germination of Lupinus havardiiv. Seed Sci. Technol. 1995, 23, 815–821. [Google Scholar]
- Martin, I.; De la Caudra, C. Evaluation of different scarification methods to remove hard-seediness in Trifolium subterraneum and Medicago polimorpha accessions of the Spanish base genebank. Seed Sci. Technol. 2004, 32, 671–681. [Google Scholar] [CrossRef]
- Pietruszewski, S.; Kania, K. Effect of magnetic field on germination and yield of wheat. Int. Agrophys. 2010, 24, 297–302. [Google Scholar]
- Komarzyński, K.; Pietruszewski, S. Influence of alternating magnetic field on the germination of seeds with low germination capacity. Acta Agrophys. 2008, 11, 429–435. [Google Scholar]
- Sharma, S.; Naithani, R.; Vargtrese, B.; Keshavkant, S.; Naithani, S.C. Effect of hot-water treatment on seed germination of some fast growing tropical tree species. J. Trop. For. 2008, 24, 49–53. [Google Scholar]
- Tworkowski, J.; Szczukowski, S.; Jakubiuk, P. Skaryfikacja a wartość siewna nasion rutwicy wschodniej (Galega orientalis Lam.) [Scarification versus viability of galega (Galega orientalis Lam.) seeds]. Adv. Agric. Sci. Probl. Issues 1999, 468, 233–240. [Google Scholar]
- Rogovska, N.; Laird, D.; Cruse, R.M.; Trabue, S.; Heaton, E. Germination Tests for Biochar Quality. J. Environ. Qual. 2012, 41, 1014–1022. [Google Scholar] [CrossRef]
- Solaiman, Z.M.; Murphy, D.V.; Abbot, L.K. Biochars influence seed germination and early growth of seedlings. Plant Soil 2012, 353, 273–287. [Google Scholar] [CrossRef]
- Shamim, M.; Saha, N.; Hye, F.B. Effect of biochar on seed germination, early growth of Oryza sativa L. and soil nutrients. Trop. Plant Res. 2018, 5, 336–342. [Google Scholar] [CrossRef]
- Kamara, A.; Kamara, A.; Mansaray, M.; Sawyerr, P. Effects of biochar derived from maize stover and rice straw on the germination of their seeds. Am. J. Agric. For. 2014, 2, 246–249. [Google Scholar] [CrossRef]
- Robertson, S.J.; Rutherford, P.M.; Lo’ pez-Gutie´rrez, J.C.; Massicotte, H.B. Biochar enhances seedling growth and alters root symbioses and properties of sub-boreal forest soils. Can. J. Soil Sci. 2012, 92, 329–340. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, A.; Ji, C.; Joseph, S.; Bian, R.; Li, L.; Pan, G.; Paz-Ferreiro, J. Biochar’s effect on crop productivity and the dependence on experimental conditions–A meta-analysis of literature data. Plant Soil. 2013, 373, 583–594. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, X.; Jing, Y.; Li, Q.; Zhang, J.; Huang, Q. Effects of biochar amendment on rapeseed and sweet potato yields and water stable aggregate in upland red soil. Catena 2013, 123, 45–51. [Google Scholar] [CrossRef]
- Hossain, M.K.; Strezov, V.; Chan, K.Y.; Nelson, P.F. Agronomic properties of wastewater sludge biochar and bioavailability of metals in production of cherry tomato. Chemosphere 2010, 78, 1167–1171. [Google Scholar] [CrossRef]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Using poultry litter biochars as soil amendments. Aust. J. Soil Res. 2008, 46, 437–444. [Google Scholar] [CrossRef]
- Uzoma, K.C.; Inoue, M.; Andry, H.; Fujimaki, H.; Zahoor, A.; Nishihara, E. Effect of cow manure biochar on maize productivity under sandy soil condition. Soil Use Manag. 2011, 27, 205–212. [Google Scholar] [CrossRef]
- Lehmann, J.; Gaunt, J.; Rondon, M. Biochar sequestration in terrestrial ecosystems–A review. Mitig. Adapt. Strateg. Glob. Chang. 2006, 11, 395–419. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Maucieri, C.; Liu, S.; Zou, J. Annual nitric and nitrous oxide emissions response to biochar amendment from an intensive greenhouse vegetable system in southeast China. Sci. Hortic. 2019, 246, 879–886. [Google Scholar] [CrossRef]
- Cayuela, M.L.; Van Zwieten, L.; Singh, B.P.; Jeffery, S.; Roig, A.; Sánchez-Monedero, M.A. Biochar’s role in mitigating soil nitrous oxide emissions: A review and meta-analysis. Agric. Ecosyst. Environ. 2014, 191, 5–16. [Google Scholar] [CrossRef]
- Maucieri, C.; Zhang, Y.; McDaniel, M.D.; Borin, M.; Adams, M.A. Short-term effects of biochar and salinity on soil greenhouse gas emissions from a semi-arid Australian soil after re-wetting. Geoderma 2017, 307, 267–276. [Google Scholar] [CrossRef]
- Szweykowska, A. Plant Physiology; Wydawnictwo Naukowe: Poznań, Poland, 1999; pp. 67–78. [Google Scholar]
- Krzywy, E. Fertilization of Soils and Plants; Akademia Rolnicza: Szczecin, Poland, 2000; p. 177. [Google Scholar]
- Wińska-Krysiak, M. Calcium transporting proteins in plants. Acta Agrophysica 2006, 7, 751–762. [Google Scholar]
- Bezak-Mazur, E.; Stoińska, R. The importance of phosphorus in the environment—Review article. Arch. Waste Manag. Environ. Prot. 2013, 15, 33–42. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).