Abstract
Over the past few years, special attention has been paid to biomass valorization. The aim of this paper is to evaluate the effect of the phenolic rich extracts obtained from raw materials on the growth and development of lemon balm (Melissa officinalis L.). The extracts were obtained from the bark of spruce (Picea abies (L.) H. Karst) and beech (Fagus sylvatica L.) separated as waste product during wood processing. The growth and development of the plants was assessed by measuring elongation of vegetative organs, biomasses including root, stem, and leaf, and photosynthetic pigment content. In addition, the analysis of some histo-anatomic characteristics of the vegetative organs were made. Elongation biomasses and photosynthetic pigments concentration presented higher values in case of plants treated with beech bark phenolic extract compared to control plants. The spruce bark phenolic extract had a stimulatory effect on the germination but inhibited the growth and development of the plants. Both extracts increased the percentage of lignification in stems. These findings could contribute to the development of natural and eco-friendly substances that favor cultivation of lemon balm plants. Future research is needed in order to identify potential qualitative and quantitative changes in the essential oil of the aromatic plant treated with the tested solutions.
1. Introduction
Melissa officinalis L., which is also known as lemon balm, can be easily recognized due to its lemon scent. It is a perennial herbaceous plant belonging to the Lamiaceae family [1]. The cultivation of lemon balm requires a temperature between 15 °C and 35 °C, and 500 to 600 mm rainfall per year [2]. Like other plants from this family, lemon balm contains high concentrations of phenolic acids, which is mainly caffeic acid known as the principal compound in the synthesis of rosmarinic acid. These phenolic compounds contribute to the medicinal properties of lemon balm, which is associated to its antioxidant capacity [3]. The main active constituents of M. officinalis L. are volatile compounds (e.g., geranial, neral, citronellal, and geraniol), triterpenes (e.g., ursolic acid and oleanolic acid), and phenolics (e.g., caffeic acid derivates: luteolin, naringin, and hesperidin) [4]. Lemon balm herb (Melissae herba) and lemon balm leaves (Melissae folium) are the main parts of the plant used in phytotherapy as digestive antispasmodic and antiviral remedy [5]. M. officinalis L. has anti-inflammatory activity, which is used in traditional medicine to treat various diseases associated with inflammation and pain. Recent studies indicate the beneficial effects of the plant: anti-bacterial, spasmolytic, memory improvement, sedative, stress, and anxiety reductant [6].
Polyphenols are widely distributed products in the plant kingdom, which are found in fruits, vegetables, tea, and coffee [7]. They possess a wide variety of physiological effects such as anti-inflammatory and anti-allergenic but mostly antioxidant. These compounds can also be found in the bark of different tree species [8]. Among many other varieties of spruce, Picea abies (L.) H. Karst is specific to the Carpathian region (Romania). Spruce wood is commonly used in wood and the pulp industry while the bark is an important source of tannins, resins, and essential oils utilized in chemical, leathering, cosmetics, and pharmaceutical industries [9]. Likewise, beech (Fagus sylvatica L.) is a common and widely used material in the wood industry, but the bark of these species are still regarded as by-products [10]. Polyphenols have a significant role in the protection of living cells against biotic (fungi, infections, diseases) and abiotic (wounds, drought, and pollution) factors. Polyphenols are known for their antioxidant capacity [11,12]. The effects of polyphenolic extracts as antioxidant agents, plant growth regulators, or bioremediation agents have been successfully tested [12,13,14,15]. On the other hand, spruce bark can be used as natural fungicide, biodegradable, and less toxic than the synthetic fungicides [16], because of its rich content in tannins [17].
Taking into account all of this information, the aim of the study was to demonstrate the bioregulator effects of the two extracts (spruce bark polyphenolic extract-SBPE, and beech bark polyphenolic extract-BBPE), on the growth and development of Melissa officinalis L. Therefore, the physiological (germination, growth, and development) and structural modifications (vascular tissue, lignification, etc.) of the treated plants were analyzed and compared to the control plants. The objectives of the study were to obtain new bioregulator products that fit into nature and enter the natural cycle of the materials, to identify the influence of the natural bark extracts on the growth and development of lemon balm plants, and to orient the research toward an ecological agriculture for sustainable development.
2. Materials and Methods
2.1. Materials
Spruce (Picea abies (L.) H. Karst) and beech (Fagus sylvatica L.) bark waste-products were obtained from a wood processing company (Vatra Dornei, Romania). Prior to extraction, the bark was air dried at room temperature (10.4% humidity) and milled in a GRINDOMIX GM 200 (Retsch, GmbH, Haan, Germany) mill up to a mean particle diameter of 0.6 mm. The biomass was directly used without any pre-treatments.
2.2. Extraction Method
Crude extracts were obtained by applying a conventional extraction method (batch water extraction). This method was chosen because it is the most economical. Since the extract was applied directly to the plant, water instead of alcohol was used as a solvent. The extraction was done using 10 g of spruce bark. The vegetal material was added in an Erlenmeyer flask by adding it over using 125 mL of distilled water. The mixture was heated up to 70–80 °C in a water bath, for 45 min. This process was repeated four times until fully spruce bark was exhausted (colorless extract). The extracts were put together in a 1000-mL volumetric flask and completed to volume with distilled water [18]. The same method was applied for beech bark extraction. For further investigation, aqueous extracts were used in four working solutions with different concentrations and polyphenolic contents.
2.3. Characterization of the Extracts
Spruce and beech bark aqueous extracts were analyzed previously in terms of total polyphenols and tannins [19]. The total polyphenolic content (TPC) was determined using the Folin-Ciocalteu method. Results were expressed as milligrams of gallic acid equivalents (GAE) per bark gram (mg GAE/g).
2.4. Working Protocol
The experiment was conducted in the greenhouse of the Botanical Garden at the University of Medicine, Pharmacy, Sciences and Technology from Târgu Mureș. The seeding was conducted in 20 vegetation vessels/variant, filled with peat soil in different experimental variants (C—control, SBPE 1—spruce bark polyphenolic extract at a concentration of 0.25 g bark/100 mL water, SBPE 2—spruce bark polyphenolic extract at a concentration of 0.5 g bark/100 mL water, BBPE 1—beech bark polyphenolic extract at a concentration of 0.25 g bark/100 mL water, BBPE 2—beech bark polyphenolic extract at a concentration of 0.5 g bark/100 mL water). Five seeds/vegetation vessels were sown, totaling 100 seeds/variant.
Right after seeding, each experimental variant was treated with 10 mL of specific aqueous extract and with 10 mL of water for the control sample. This process was repeated after one, two, and four weeks from the moment of seeding. Meanwhile we determined germination capacity. Two months after seeding, the quantity of photosynthetic pigments (chlorophyll a, chlorophyll b, and carotenes) was measured, repeating the process after a month. Three and a half months after sowing elongation and biomass accumulation have been determined.
2.5. Biological Tests on Lemon Balm Seeds
A series of tests were applied on Melissa officinalis plants, such as the determination of germination capacity for the seeds, respectively, biomass accumulation, vegetative organ’s elongation (after their separation into roots, stems, and leaves), and photosynthetic pigments content as biometrical and physiological tests.
Germination counts were performed daily for 30 days. Mean daily germination was calculated by dividing cumulative germination percentage by the number of days in the test. Germination capacity (GC) was calculated as GC (%) = (Total germinated seeds + total un-germinated sound seeds/Total seeds tested) × 100 [20]. To evaluate the influence of polyphenolic extracts on plant growth and development, the lemon balm plants were separated into roots, stems, and leaves, which was followed by biometric measurements of plant elongation and quantitative determinations of biomass.
2.6. Determination of Photosynthetic Pigment Concentrations
For this part of the study, 0.1 g of fresh vegetal material was milled with quartz sand and extracted with acetone (80%). The results were recorded with the Analytik Jena Specord 210 Plus190 (UV/Vis)-1100 nm Spectrophotometer using synthetic quartz spectrophotometer cells (190–2500 nm, path length of 10 mm, and a volume of 1.75 mL). The following formulas [21] were used for the determination of carotenoid and chlorophyll contents at wavelengths of 470, 646, and 663 nm.
Chlorophyll a (µg/mL) = 12.21*A663 − 2.81*A646
Chlorophyll b (µg/mL) = 20.31*A646 − 5.03*A663
Carotenes (µg/mL) = (100*A470 − 3.27*[chl a] − 104*[chl b])/22
2.7. Histo-Anatomical Analysis
Cross sections (five sections for every sample) through the vegetative organs (root, stem, and leaves) of the plants were made. The obtained sections were double stained using iodine green and ruthenium red [7]. The stained sections were analyzed with a Motic Microscope and photographed with a Nikon Coolpix L22 camera, Tokyo, Japan. Analysis of microscopic images was performed with ImageJ Image Processing and Analysis in Java Version 1.51j8 (National Institute of Mental Health, Bethesda, MD, USA).
2.8. Statistical Analysis
The statistical tests used were Kruskal-Wallis test for including a sample in a specific distribution and Mann-Whitney U test (non-parametric) for comparing two population means. The tests were applied in Past 2.17.
3. Results and Discussions
3.1. Extracts Characterization
The characteristics of polyphenolic extracts were summarized in a previous work [19]. The total phenolic content in the beech bark extract (determined by a classical method) was 36.66 mg GAE/g extract. Concerning spruce bark extract, the TPC value obtained was 113.56 mg GAE/g extract. Regarding tannin content, it was concluded that SBPE has 1.14% and BBPE has 1.47% [19]. The results showed that the beech bark extracts contained considerable quantities of aromatic compounds, such as (+)-catechin, (−)-epicatechin, quercetin-O-hexoside, taxifolin-O-hexosides, taxifolin-O-pentosides, B-type and C-type procyanidins, syringic acid, and coumaric acid-di-O-glycosides, coniferyl alcohol and sinapyl alcohol-glycosides, and (+) and (−) glucodistylin [11,18,19]. The compounds identified in spruce bark extract by HPLC were gallic acid (3.19 mg GAE/100 g), catechine (31 mg GAE/100 g), and vanillic acid (39.4 mg GAE/100 g) [13].
3.2. Seed Germination
Applying the tested solutions on seeds’ major differences were induced on the enzymatic level, which changed the germination capacity of the seeds. From Figure 1, it can be observed that the seeds treated with both extracts had a higher germination capacity in comparison with the control samples.
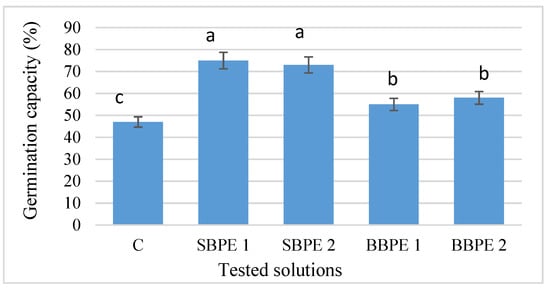
Figure 1.
Influence of bark extracts on Melissa officinalis L. seed germination capacity. Different letters indicate significant differences (p ≤ 0.05). Error bars represent the standard deviations of means. C—control. SBPE 1—spruce bark polyphenolic extract at a concentration of 0.25 g bark/100 mL water. SBPE 2—spruce bark polyphenolic extract at a concentration of 0.5 g bark/100 mL water. BBPE 1—beech bark polyphenolic extract at a concentration of 0.25 g bark/100 mL water. BBPE 2—beech bark polyphenolic extract at a concentration of 0.5 g bark/100 mL water.
3.3. Growth and Development of the Vegetative Organs
3.3.1. Biomass Accumulation in Vegetative Organs
For biomass accumulation, different results were obtained in case of tested solutions (Figure 2). Regarding the stem, spruce bark polyphenolic extract (SBPE) had a negative effect on biomasses, while beech bark polyphenolic extract (BBPE) increased biomasses. In case of roots, all the extracts presented an inhibitory action on biomass accumulation. Taking into account the leaves, plants treated with SBPE have shown a decrease in biomasses (28.8% for SBPE 1), while those treated with BBPE 1 had higher biomasses in comparison to the control samples (37% for BBPE 2). The most significant increase was linked to BBPE 2 (p = 0.0041), which confirmed the bioregulator effect of beech extracts on leaf biomass. These findings are important because the leaves are the main vegetative organ from which the essential oil is extracted.
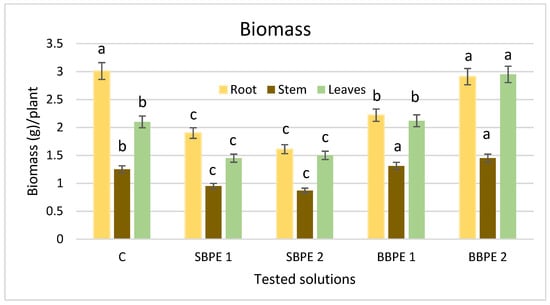
Figure 2.
Influence of bark extracts on biomass accumulation in lemon balm (Different letters indicate significant differences, p ≤ 0.05. Error bars represent the standard deviation of means. C—control. SBPE 1—spruce bark polyphenolic extract at a concentration of 0.25 g bark/100 mL water. SBPE 2—spruce bark polyphenolic extract at a concentration of 0.5 g bark/100 mL water. BBPE 1—beech bark polyphenolic extract at a concentration of 0.25 g bark/100 mL water. BBPE 2—beech bark polyphenolic extract at a concentration of 0.5 g bark/100 mL water.
3.3.2. Elongation of the Vegetative Organs
After three and a half months, the elongation of vegetative organs was measured (Figure 3). In case of stem, SBPE shortened the length (22% for SBPE 2) of the stem. On the other hand, plants treated with BBPE had longer stems than control plants (25% for BBPE 2). Regarding the root extension, no significant differences were observed in comparation with the control samples.
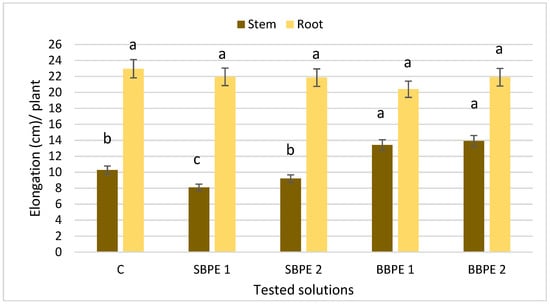
Figure 3.
Influence of bark extracts on vegetative organ elongation in lemon balm. Bars show that the same letter is not significantly different at p ≤ 0.05. Error bars represent the standard deviation of means. C—control. SBPE 1—spruce bark polyphenolic extract at a concentration of 0.25 g bark/100 mL water. SBPE 2—spruce bark polyphenolic extract at a concentration of 0.5 g bark/100 mL water. BBPE 1—beech bark polyphenolic extract at a concentration of 0.25 g bark/100 mL water. BBPE 2—beech bark polyphenolic extract at a concentration of 0.5 g bark/100 mL water.
3.3.3. Photo-Assimilating Pigment Content in Lemon Balm Primary Leaves
Table 1 demonstrates the effect of tested solutions on the chlorophyll content of the lemon balm plants, including chlorophyll a, b, carotenoids, and total chlorophyll. At BBPE2, it shows a stimulation of the quantity of photosynthetic pigments. On the other hand, SBPE 2 shows an inhibitory effect, which has the lowest value when compared with other experimental variants. Thus, an increase with 73% in chlorophyll a, 84.9% rise in chlorophyll b, and a 139.4% increase in carotenoids for BBPE 2 was observed, which proves the BBPE 2 is the most efficient extract for the biosynthesis of photo-assimilatory pigments in the leaves.

Table 1.
Content of photo-assimilatory pigments synthesized (mg/g) in lemon balm leaves.
3.4. Histo-Anatomical Aspects of the Lemon Balm
3.4.1. Internal Structure of the Root
Taking into consideration the internal structure of lemon balm’s (M. officinalis L.) root (Figure 4a), the rhizodermis exfoliates in the early stage of the development process. The cortex is separated into exoderma, cortical parenchyma, and endodermis. The vascular tissue’s origin is mostly cambial and forms two concentric rings, which includes a very narrow phloem and a very thick xylem with a high level of lignification and many vessels.
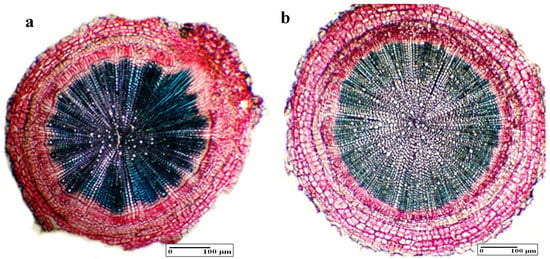
Figure 4.
The internal structure of the main root: (a) general aspect (control), (b) general aspect (BBPE 1—central cylinder, cortex, and rhizodermis).
Analyzing the internal structure of the roots, there are no significant differences between the samples. The only dissimilarity can be identified for BBPE 1 where the secondary xylem area is more developed compared with the control sample or other experimental samples (Table 2, Figure 4b).

Table 2.
The microscopic characteristics of vegetative organs in case of treated and control lemon balm plants.
3.4.2. Internal Structure of the Stem
The cross section is square shaped with four bulging ribs (Figure 5a). The epidermis is single-layered covered with a thin cuticle. On the surface of the stem, there are widely spread sector and secretory hairs. The cortex is collenchymatic especially toward the ribs. The vascular system consists of four-eight vascular bundles, with those near the ribs being very large, of a mostly secondary structure. At the stem level, it was observed that BBPE 1 and SPBE 1 have produced a more intense lignification process, when compared with the control (p ≤ 0.05, Table 2). Hence, SBPE 1 stimulates the formation of sclerenchymatic fibers covering the vascular tissue. Furthermore, an enhancement of the collenchyma genesis was observed. The BBPE 1 induced a better development of the vascular bundles especially the xylem (Figure 5b, Table 2). Considering the other experimental variants (SBPE 1, SBPE 2, and BBPE 2), two small vascular bundles had an opposite disposition. This proved a better development of the vascular system in comparison with the control sample.
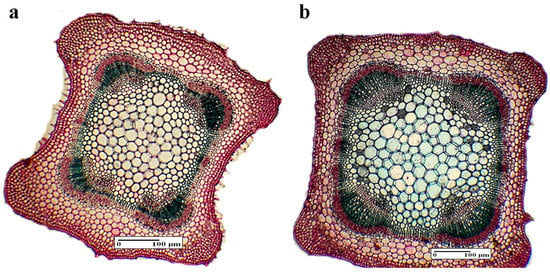
Figure 5.
The internal structure of the stem: (a) general aspect (control), (b) general aspect (BBPE 1).
3.4.3. Internal Structure of the Leaves
In the cross section, the main rib is highlighted on the inferior surface and contains a single vascular bundle (Figure 6a,b). The epidermal cells (from the upper and lower epidermis) are elongated tangentially, which is larger on the superior surface. The two types of hairs, which are tectorial and secretory (the latter containing a gland made out of single or multiple cells), had an irregular disposition. The mesophyll is split into palisade parenchyma and spongy tissue. Thus, the foliar blade has a bifacial hetero-facial structure. After examining the internal structure of the leaves, no significant differences were found in comparison with the control samples (Table 2). The only difference that can be observed is the BBPE 1 (Figure 7a,b), which is a less developed vascular bundle in the main string when compared to the control samples and to the other variants. Moreover, the lignification process is more intense such as in the stem.
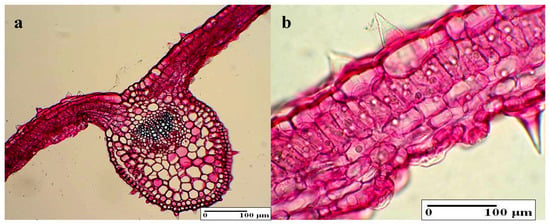
Figure 6.
The internal structure of the leaf: (a) general aspect (control), (b) mesophyl with secretory hairs (control).
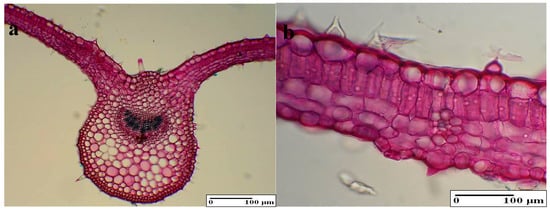
Figure 7.
The internal structure of the leaf: (a) general aspect (BBPE 1), (b) mesophyl with secretory hairs (BBPE 1).
Previous studies provide information on the stimulatory or inhibitory role of the polyphenolic compounds regarding the germination process and biomass accumulation. The authors conclude that global extracts induce similar effects to auxins and cytokinins on plant growth [13,15]. The results of this work confirm the previous findings. Thus, a higher number of grown plants for the variants treated with SBPE was observed. The final analysis shows a greater efficiency for the variants treated with BBPE in development except the roots, of which biomass and elongation were inhibited in the case of both extracts. BBPE is a good stimulant for photo-assimilating pigments, which correlates it to the quantity of chlorophyll a, b, and carotenoids obtained for Melissa officinalis’ leaves. At the stem level, beech and spruce extracts produced a more intense lignification process. It is known that the lignification process is associated with several consequences that occur in plants. They refer to the structural integrity of the cell wall, secondary growth of vascular tissues, strain and root resistance [22], fungal resistance [23], and prevention of autolytic cell wall degradation [24].
4. Conclusions
To conclude, taking into account the results, spruce bark extract demonstrated great efficiency in the germination process. Beech bark extracts show a stimulation of the quantity of photosynthetic pigments and spruce bark extract shows an inhibitory effect.
At the internal structure, a better development of the secondary xylem area in root and vascular bundles (stem, leaf) of the plants treated with beech bark extract was observed. This was compared with the control sample or other experimental samples. On the other hand, spruce bark extract stimulates the formation of sclerenchymatic fibers covering the vascular tissue and it has been observed an enhancement of the collenchyma genesis.
This research can contribute to the ecological agriculture and stability of medicinal plant cultures. Beech and spruce bark aqueous extracts could be properly used as natural bioregulators in order to improve the efficiency and yield of lemon balm crops.
Further studies will take into account the enhancement of the quantity and quality of the active compounds with pharmaceutical use using the lemon balm plants with the beech bark extracts. Moreover, studies regarding the effect of the tannins from the studied extracts on different medicinal plants could be conducted in order to increase their efficiency.
Author Contributions
Conceptualization, C.T. Data curation, A.N., A.M. (Anca Mirica), A.M. (Andreea Milan), and I.B. Formal analysis, I.B. Funding acquisition, C.T. Methodology, C.T., and I.B. Project administration, C.T. Resources, C.T. and I.B. Visualization, C.T. and I.B. Writing—original draft, C.T., A.N., A.M. (Anca Mirica), and A.M. (Andreea Milan). Writing—review & editing, C.T. and I.B.
Funding
This work was supported by a grant of Ministery of Research and Innovation, CNCS—UEFISCDI, project number PN-III-P1-1.1-PD-2016-0892, within PNCDI III.
Acknowledgments
This work was supported by a grant of Ministery of Research and Innovation, CNCS—UEFISCDI, project number PN-III-P1-1.1-PD-2016-0892, within PNCDI III.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Javid, A.Z.; Haybar, H.; Dehghan, P.; Haghighizadeh, M.H.; Mohaghegh, S.M.; Ravanbakhsh, M.; Mohammadzadeh, A.; Bahrololumi, S.S. The effects of Melissa officinalis on echocardiography, exercise test, serum biomarkers, and blood pressure in patients with chronic stable angina. J. Herb. Med. 2018, 11, 24–29. [Google Scholar] [CrossRef]
- Saeb, K.; Gholamrezaee, S. Variation of essential oil composition of Melissa officinalis L. leaves during different stages of plant growth. Asian Pac. J. Trop. Biomed. 2012, 2 (Suppl. 2), S547–S549. [Google Scholar] [CrossRef]
- Boneza, M.M.; Niemeyer, E.D. Cultivar affects the phenolic composition and antioxidant properties of commercially available lemon balm (Melissa officinalis L.) varieties. Ind. Crops Prod. 2018, 112, 783–789. [Google Scholar] [CrossRef]
- Shakeri, A.; Sahebkar, A.; Javadi, B. Melissa officinalis L.—A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2016, 188, 204–228. [Google Scholar] [CrossRef] [PubMed]
- Seidler-Łożykowska, K.; Bocianowski, J.; Król, D. The evaluation of the variability of morphological and chemical traits of the selected lemon balm (Melissa officinalis L.) genotypes. Ind. Crops Prod. 2013, 49, 515–520. [Google Scholar] [CrossRef]
- Jalal, Z.; El Atki, Y.; Lyoussi, B.; Abdellaoui, A. Phytochemistry of the essential oil of Melissa officinalis L. growing wild in morocco: Preventive approach against nosocomial infections. Asian Pac. J. Trop. Biomed. 2015, 5, 458–461. [Google Scholar] [CrossRef]
- Tanase, C.; Boz, I.; Popa, V.I. Histo-anatomic aspects on Zea mays L. influenced by spruce bark polyphenolic extract. Romanian Biotechnol. Lett. 2016, 21, 11238–11245. [Google Scholar]
- García-Pérez, M.-E.; Royer, M.; Herbette, G.; Desjardins, Y.; Pouliot, R.; Stevanovic, T. Picea mariana bark: A new source of trans-resveratrol and other bioactive polyphenols. Food Chem. 2012, 135, 1173–1182. [Google Scholar] [CrossRef]
- Lazar, L.; Talmaciu, A.I.; Volf, I.; Popa, V.I. Kinetic modeling of the ultrasound-assisted extraction of polyphenols from Picea abies bark. Ultrason. Sonochem. 2016, 32, 191–197. [Google Scholar] [CrossRef]
- Tanase, C.; Domokos, E.; Cosarca, S.; Miklos, A.; Imre, S.; Domokos, J.; Dehelean, C.A. Study of the ultrasound-assisted extraction of polyphenols from beech (Fagus sylvatica L.) bark. Bioresources 2018, 13, 2247–2267. [Google Scholar] [CrossRef]
- Gligorić, E.; Igić, R.; Suvajdžić, L.; Grujić-Letić, N. Species of the Genus Salix L.: Biochemical Screening and Molecular Docking Approach to Potential Acetylcholinesterase Inhibitors. Appl. Sci. 2019, 9, 1842. [Google Scholar] [CrossRef]
- Venturi, F.; Bartolini, S.; Sanmartin, C.; Orlando, M.; Taglieri, I.; Macaluso, M.; Lucchesini, M.; Trivellini, A.; Zinnai, A.; Mensuali, A. Potato Peels as a Source of Novel Green Extracts Suitable as Antioxidant Additives for Fresh-Cut Fruits. Appl. Sci. 2019, 9, 2431. [Google Scholar] [CrossRef]
- Tanase, C.; Boz, I.; Stingu, A.; Volf, I.; Popa, V.I. Physiological and biochemical responses induced by spruce bark aqueous extract and deuterium depleted water with synergistic action in sunflower (Helianthus annuus L.) plants. Ind. Crops Prod. 2014, 60, 160–167. [Google Scholar] [CrossRef]
- Kulbat, K. The role of phenolic compounds in plant resistance. Biotechnol. Food Sci. 2016, 80, 97–108. [Google Scholar]
- Popa, V.I.; Agache, C.; Beleca, C.; Popa, M. Polyphenols from spruce bark as plant growth regulator. Crop Res. 2002, 24, 398–406. [Google Scholar]
- Gabaston, J.; Richard, T.; Biais, B.; Waffo-Teguo, P.; Pedrot, E.; Jourdes, M.; Corio-Costet, M.-F.; Mérillon, J.-M. Stilbenes from common spruce (Picea abies) bark as natural antifungal agent against downy mildew (Plasmopara viticola). Ind. Crops Prod. 2017, 103, 267–273. [Google Scholar] [CrossRef]
- Kemppainen, K.; Siika-aho, M.; Pattathil, S.; Giovando, S.; Kruus, K. Spruce bark as an industrial source of condensed tannins and non-cellulosic sugars. Ind. Crops Prod. 2014, 52, 158–168. [Google Scholar] [CrossRef]
- Hofmann, T.; Nebehaj, E.; Stefanovits-Bányai, É.; Albert, L. Antioxidant capacity and total phenol content of beech (Fagus sylvatica L.) bark extracts. Ind. Crops Prod. 2015, 77, 375–381. [Google Scholar] [CrossRef]
- Cosarca, S.-L.; Moaca, E.-A.; Tanase, C.; Muntean, D.L.; Pavel, I.Z.; Dehelean, C.A. Spruce and beech bark aqueous extracts: Source of polyphenols, tannins and antioxidants correlated to in vitro antitumor potential on two different cell lines. Wood Sci. Technol. 2019, 53, 313–333. [Google Scholar] [CrossRef]
- Bargali, K.; Bargali, S.S. Germination capacity of seeds of leguminous plants under water deficit conditions: Implication for restoration of degraded lands in Kumaun Himalaya. Trop. Ecol. 2016, 57, 445–453. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, L.; Tu, L.; Liu, L.; Yuan, D.; Jin, L.; Long, L.; Zhang, X. Lignin metabolism has a central role in the resistance of cotton to the wilt fungus verticillium dahliae as revealed by RNA-seq-dependent transcriptional analysis and histochemistry. J. Exp. Bot. 2011, 62, 5607–5621. [Google Scholar] [CrossRef]
- O’Brien, T.P. Further observations on hydrolysis of the cell wall in the xylem. Protoplasma 1970, 69, 1–14. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).