Abstract
The study aimed to evaluate changes in buccal gingival contour following implant surgery using the simultaneous roll technique on Seibert class I defects, as determined by 3D intraoral scan data. Three patients requiring implant placement were recruited, and implants were placed using the roll flap technique. Digital impressions of the surgical site were obtained with a 3D intraoral scanner before surgery, after suture removal, and 4 months postoperatively. The results showed an overall increase in buccal gingival contour, with a maintained increase in soft tissue contour after 4 months. The study concluded that 3D scanning is a suitable method for assessing small changes in gingival contour, making it ideal for evaluating these changes. The roll flap technique was also found to effectively enhance the buccal gingival contour and soft tissue appearance around the implant collar.
1. Introduction
After the extraction of one or more teeth, volume reduction occurs both horizontally and vertically in the extraction socket during the tissue remodeling process. According to a systematic review of the literature, when assessing the magnitude of dimensional changes of both the hard and soft tissues of the alveolar ridge up to 12 months following tooth extraction in humans, the results showed an average reduction in the width of the alveolar ridge of 29 to 63% in 6 months, while for the height of the alveolar ridge the average reduction was 11 to 22% in 6 months [1]. For well-informed decision-making and thorough treatment planning that includes provisions for possible solutions, the extent of these dimensions alterations is crucial to anticipated issues during the rehabilitation of prosthetics. Furthermore, given the increased focus on aesthetics recently, a comprehensive understanding of the resorption pattern and changes in the bony and mucosal contours following extraction would significantly improve our capacity to reconstruct our patients to a level of optimal function and acceptable aesthetics [1].
Siebert and Allen classified the defects of the resorbed alveolar ridge based on horizontal and vertical absorption. In 1983, Siebert classified different types of alveolar ridge defects: Class I defects have a bucco-lingual loss of ridge contour with normal ridge height, Class II defects have a apico-coronal loss of ridge contour with normal ridge height, and Class III defects have a combined loss of the ridge contour [2]. In 1985, Allen classified apico-coronal loss of tissue as type A, buccolingual loss of tissue as type B, and combination loss of tissue as type C. Also, Allen classified the ridge defect according to the depth of the defect, mild as a depth of less than 3 mm, moderate as 3 to 6 mm, and severe as more than 6 mm [3].
Various soft tissue graft techniques have been proposed to increase the volume of the resorbed edentulous alveolar ridge, which include the roll technique, pouch procedure, onlay graft, and inlay graft [4,5,6,7,8,9]. The roll technique utilizes a pedicle flap graft and is indicated for Class I defects, according to Seibert’s classification [4,5,6]. The Pouch procedure employs a subepithelial connective tissue graft and is suitable for Class I and mild Class II defects [7,8]. The Onlay graft involves the use of either a free gingival graft or a connective tissue graft. It is recommended for severe Class II and Class III defects [9]. The Inlay graft also utilizes either a free gingival graft or a connective tissue graft and is applicable to Class I, mild Class II, and Class III defects [9].
Among them, the roll technique is effective for Seibert Class I defects. The roll technique can be divided into three methods, which is conventional roll technique, modified roll technique, and roll-in envelope technique. The conventional roll technique was designed to increase soft tissue volume around the pontic. The conventional roll technique includes a palatal de-epithelization procedure and the exposure of palatal bone, which leads to unfavorable healing on the palatal side. The pedicle is then rolled beneath the buccal mucosa to augment the soft tissue volume [5]. To avoid the unfavorable healing on the palatal side, the roll technique was modified using the trap-door technique. The epithelium over the palatal connective tissue is raised and preserved to cover the palatal bone in this technique. This modified roll technique induces primary healing on the palatal side [6]. The minimally invasive roll-in envelope technique was introduced. In the roll-in envelope technique, the palatal flap is not elevated, the crestal epithelium is de-epithelized, and a minimally invasive incision is made, followed by the insertion of the pedicle flap into the buccal pouch. This technique promotes better blood supply with less soft tissue manipulation [10].
Recently, the intraoral scan has emerged as a convenient imaging method that offers comprehensive 3D information. Intraoral scanners are devices for capturing direct optical impressions in dentistry. The integration of intraoral scanning with computer-aided design (CAD) software enables the comprehensive analysis and evaluation of surgical outcomes. By utilizing digital models, preoperative and postoperative intraoral scans can be superimposed to assess changes in oral structures. This method provides a valuable tool for clinicians to objectively evaluate treatment efficacy and track morphological alterations. This case report aims to investigate the application of intraoral scanning and CAD-based superimposition techniques for comparing the amount of buccal gingival contour changes following implant surgery with the simultaneous roll technique on Seibert class I defects. By measuring changes in the gingival contour, the results of the roll technique can be evaluated more objectively.
2. Materials and Methods
2.1. Case Selection and Description
The present retrospective case series study was performed in the Kyungpook Nation University Dental Hospital. Three patients, who met the inclusion criteria, were selected. A total of 3 implants were placed in Seibert Class I defects with the roll technique. The modified roll technique was used in two cases, and the roll-in-envelope technique was used in one case. The roll technique was used simultaneously with implant placement in two implants, and the roll technique was used during the second operation for the one implant (Figure 1).
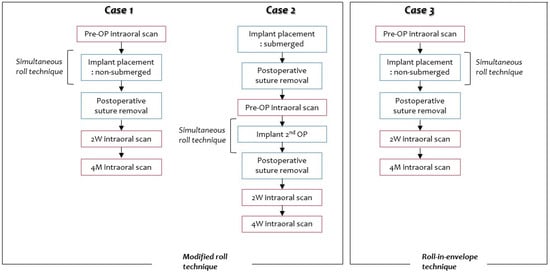
Figure 1.
Flow chart of Cases 1, 2, and 3. Intraoral scans were performed before surgery (pre-OP, pre-operation) and at 2 weeks and 4 months postoperatively.
For the 3 cases, the surgical procedures were performed by the same surgeon, and the data were also evaluated by one calibrated investigator. This study was approved by the Institutional Review Board of Kyungpook National University Dental Hospital (KNUDH-2025-01-01-00).
- Inclusion criteria
- -
- Patients with general good health;
- -
- Patients who received implant placement in posterior maxilla area;
- -
- Patients who received single implant placement.
- Exclusion criteria
- -
- Patients who received implant placement with guided bone regeneration.
2.2. Surgical Protocol
After local anesthesia, 2% lidocaine was used with 1:100,000 epinephrine. For the modified roll technique, a split thickness flap was elevated and inserted into the buccal side. An implant placement or second operation was performed, and healing abutment was connected. Suture was performed using 4-0 nylon. For the minimally invasive roll-in-envelope flap technique, the crestal epithelium is de-epithelialized, and a minimally invasive incision is made, followed by the insertion of the pedicle flap into the buccal pouch.
2.3. 3D Scan Protocol
- Pre-operation (pre-op) intraoral scan before surgery;
- Implant placement or second operation;
- 2-week, 4-week, and 4-month intraoral scan after the surgery (CS 3600; Carestream Dental, Stuttgart, Germany) (Figure 2);
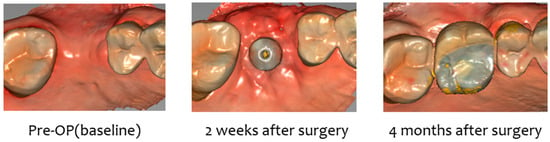 Figure 2. Image of 3D intaoral scan.
Figure 2. Image of 3D intaoral scan. - In Exocad Software (exocad GmbH, DentalCAD 3.0 Galway, Darmstadt, Germany, 2021), a mesh file was added and superimposed using the adjacent teeth as a reference. By overlapping the recorded scan data using Exocad Software, cross-sections were set, and the increase in buccal soft tissue at fixture level was measured at five sites (mesial, mesio-middle, mid, disto-middle, and distal) and averaged.
3. Case Description and Results
3.1. Case 1
A 68-year-old female patient presented for the placement of an implant on area #16. The extraction of #16 was performed 5 months earlier due to crown-root fracture (Figure 3).
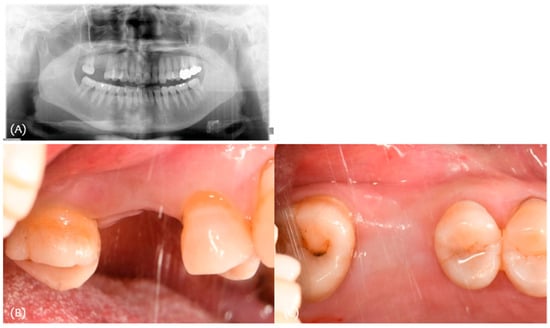
Figure 3.
(A) Initial panoramic radiograph; (B,C) initial clinical photograph.
As shown in Table 1, a Seibert Class I defect was observed with 3.5 mm horizontal defect. Also, 4–5 mm keratinized tissue width was observed (Table 1).

Table 1.
Clinical parameters for Case 1.
For procedure, a split thickness flap with two vertical incisions was made with the #15 blade (Figure 4A). A split thickness flap was elevated and inserted into the buccal side; the flap thickness was 2 mm (Figure 4B). An implant (Osstem TSIII 5.0 × 10) was placed, and healing abutment was connected (Figure 4C,D). A simple interrupted suture was performed using 4-0 nylon (Figure 4E).
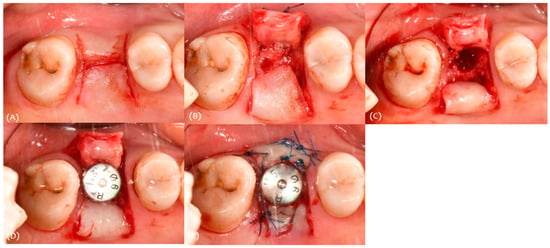
Figure 4.
Case 1. Procedure: (A) incision, (B) modified roll technique, (C) drilling for implant placement, (D) implant placement, and (E) suture.
Intraoral scan data were obtained for these stages (Figure 5). The soft tissue volume changes were evaluated by superimposing the scan data.
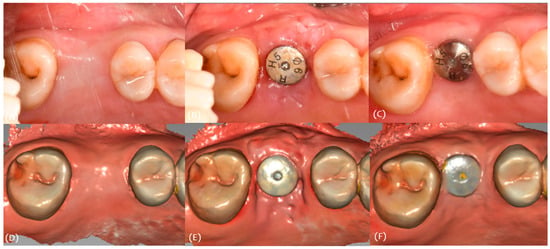
Figure 5.
Case 1. (A) Pre-op, (B) stich-out (2-week), (C) 4-month, (D) pre-op intraoral scan, (E) 2-week intraoral scan, and (F) 4-month intraoral scan.
Two weeks after surgery, the buccal soft tissue volume had increased by an average of 2.57 mm (Figure 6). Four months postoperatively; the average increase in buccal soft tissue volume was 1.31 mm (Figure 7).
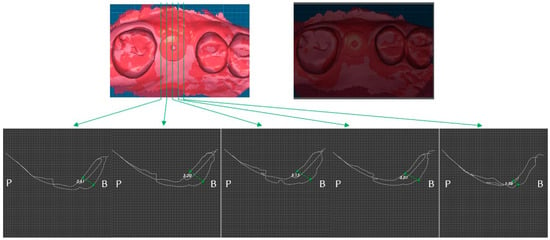
Figure 6.
Case 1. Measurement of increased soft tissue volume 2-week after surgery.
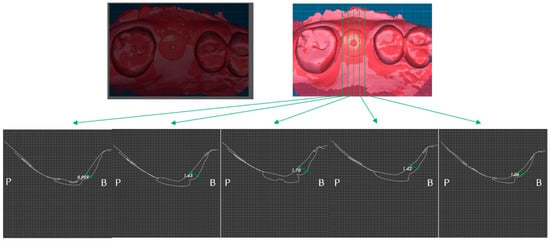
Figure 7.
Case 1. Measurement of increased soft tissue volume 4 months after surgery.
As shown in the Table 2, the buccal contour deficiency was improved. The average buccal contour deficiency of 2.58 mm improved by 1.31 mm (Table 2).

Table 2.
Increase buccal soft tissue volume for Case 1.
3.2. Case 2
A 74-year-old male patient presented for the placement of implant on #25 area. The extraction of #25 was performed 5 months earlier due to caries (Figure 8).
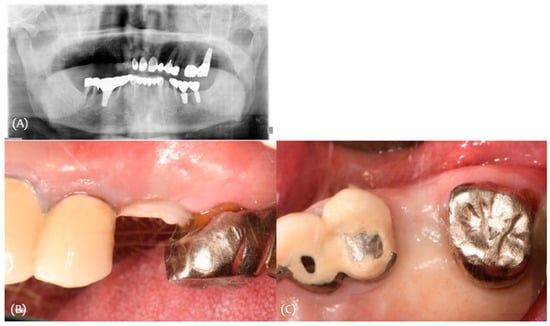
Figure 8.
Case 2. (A) Initial panoramic radiograph; (B,C) initial clinical photograph.
As shown in Table 3, a Seibert Class I defect was observed with 1.8 mm horizontal defect. Also, 4–5 mm keratinized tissue width was observed (Table 3).

Table 3.
Clinical parameters for Case 2.
For the procedure, an implant (Osstem TSIII 4.0 × 10) was placed before 3 months. A split thickness flap with two vertical incisions was made with blade #15 (Figure 9A). A split thickness flap was elevated and inserted into the buccal side; the flap thickness was 1.5 mm (Figure 9B,C). The holding suture was performed using 4-0 nylon (Figure 9D). Healing abutment was connected (Figure 9E).
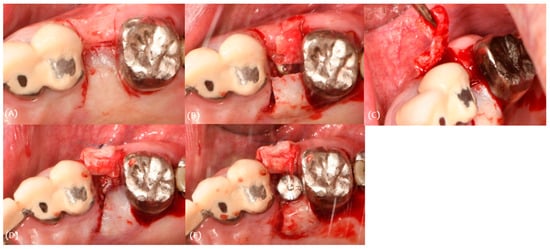
Figure 9.
Case 2. Procedure: (A) incision, (B,C) modified roll technique, (D) holding suture, and (E) healing abutment connection.
Intraoral scan data were obtained for these stages (Figure 10). The soft tissue volume changes were evaluated by superimposing the scan data.
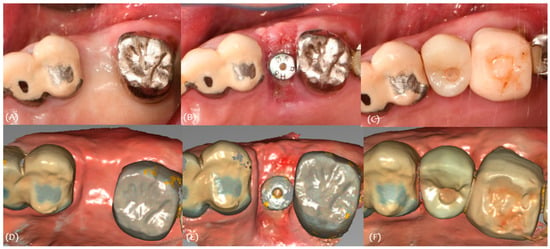
Figure 10.
Case 2. (A) Pre-op, (B) stich-out (2-week), (C) 4-month, (D) pre-op intraoral scan, (E) 2-week intraoral scan, and (F) 4-month intraoral scan.
Two weeks after surgery, the buccal soft tissue volume had increased by an average of 1.07 mm (Figure 11). Four months postoperatively, the average increase in buccal soft tissue volume was 0.53 mm (Figure 12).
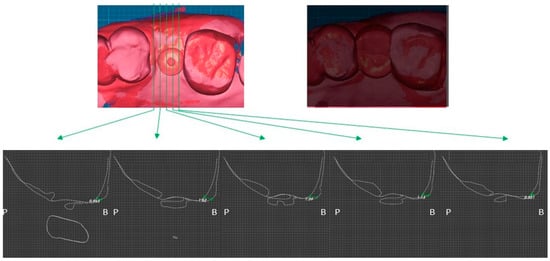
Figure 11.
Case 2. Measurement of increased soft tissue volume 2 weeks after surgery.
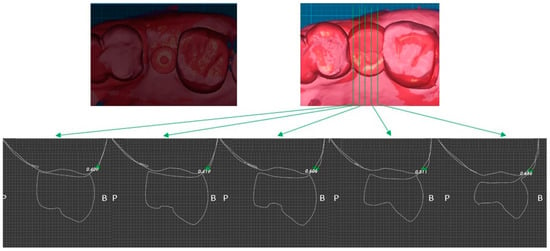
Figure 12.
Case 2. Measurement of increased soft tissue volume 4 months after surgery.
As shown in the Table 4, the buccal contour deficiency was improved. The average buccal contour deficiency of 1.44 mm improved by 0.52 mm (Table 4).

Table 4.
Increase buccal soft tissue volume for Case 2.
3.3. Case 3
A 66-year-old female patient presented for the placement of an implant in the #26,27 area. The extraction of #26 was performed 5 months earlier due to caries, and #27 was extracted 6 months earlier due to caries (Figure 13).
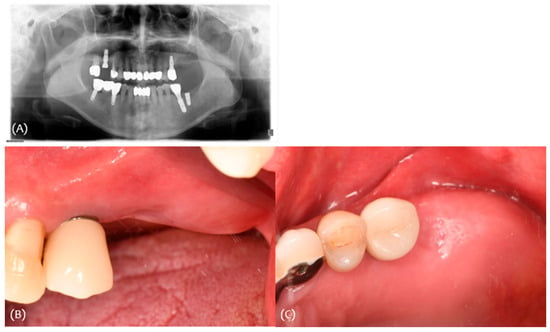
Figure 13.
Case 3. (A) Initial panoramic radiograph; (B,C) initial clinical photograph.
As shown in Table 5, a Seibert Class I defect was observed with 1.2 mm and 0.8 mm horizontal defect, respectively. Also, 5–6 mm keratinized tissue width was observed (Table 6).

Table 5.
Clinical parameters for Case 3.

Table 6.
Increase buccal soft tissue volume for Case 3.
For the procedure, a minimally invasive incision was made with blade #15 (Figure 14A); de-epithelization was performed (Figure 14B), followed by the insertion of the pedicle flap into the buccal pouch, and the pedicle flap thickness was 1.0 mm (Figure 14C). The implants (Osstem TSIII 4.5 × 10) were placed (Figure 14D), and healing abutments were connected (Figure 14E). A simple interrupted suture was performed using 4-0 nylon (Figure 14F).
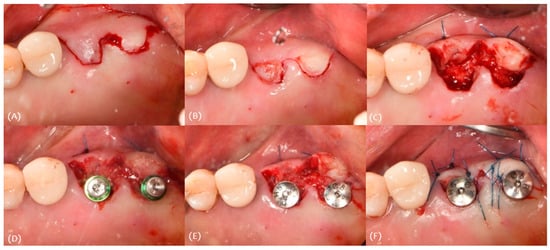
Figure 14.
Case 3. Procedure: (A) incision, (B) de-epithelization, (C) roll-in envelope technique, (D) implant placement, (E) healing abutment connection, and (F) suture.
Intraoral scan data were obtained for these stages (Figure 15). The soft tissue volume changes were evaluated by superimposing the scan data.
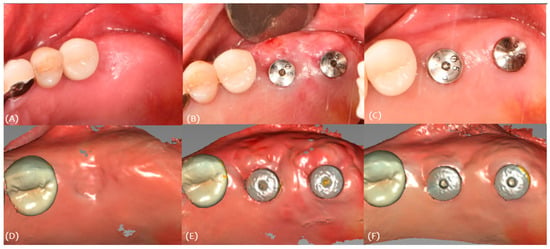
Figure 15.
Case 3. (A) Pre-op, (B) stich-out (2-week), (C) 4-month, (D) pre-op intraoral scan, (E) 2-week intraoral scan, and (F) 4-month intraoral scan.
Two weeks after surgery, the buccal soft tissue volume had increased by an average of 1.23 mm on #26 and 1.46 mm on #27 (Figure 16). Four months postoperatively, the average increase in buccal soft tissue volume was 0.82 mm on #26 and 0.47 mm on #47 (Figure 17).
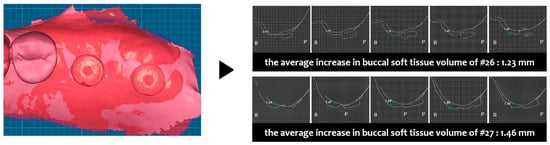
Figure 16.
Case 3. Measurement of increased soft tissue volume 2 weeks after surgery.
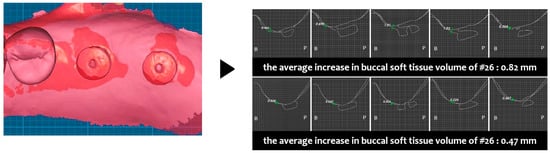
Figure 17.
Case 3. Measurement of increased soft tissue volume 4 months after surgery.
4. Discussion
These case series demonstrate that the soft tissues could be successfully augmented using the roll-flap technique. To achieve an increase in soft tissue volume, the thickness of the pedicle graft should be considered. In this case series, as the thickness of the pedicle graft increased, a greater gain in soft tissue volume was achieved.
The importance of mucosal thickness around implants has been demonstrated in many studies [11,12]. It is suggested that once the implant is exposed to the oral environment and function, a mucosal attachment of a certain minimum dimension (2 mm) is required to protect osseointegration [11]. According to Linkevicius et al., initially thin mucosal tissues can cause crestal bone loss after implant placement and 1 year in situ. If the initial tissue thickness is less than 2.5 mm, bone loss up to 1.45 mm can be expected within the first year of function [12]. Therefore, a soft tissue thickness of 2 mm or more prevents marginal bone loss around implants.
The role of peri-implant mucosal thickness is also important to prevent the exposure of the metal surface of prosthetics or abutments, leading to better aesthetic outcomes [13,14,15]. Differences in light reflection between ZrO2 and Ti implant abutments become imperceptible to the human eye when mucosal thickness exceeds 2 mm [13]. According to Lops et al., the thickness of the peri-implant soft tissue seemed to be a crucial factor for the impact of the abutment on the color of soft tissues with a thickness of ≤2 mm [14]. A mucosal thickness of 2 mm or more benefits implants, making the roll technique clinically significant. However, sufficient palatal flap thickness should be provided to avoid palatal flap thinning after the surgery.
The proper assessment of the soft tissue over time is important to evaluate the outcome of the roll flap technique. There are several methods to assess soft tissue and monitor changes over time. These include direct measurements, e.g., through a probe or a measurement on physical or digital dental models. Each method has its pros and cons. For example, it is crucial to avoid subjecting patients to unnecessary risks. Intraoral dental scans offer a convenient, risk-free imaging tool that offers detailed 3D information. According to Lee et al., digital measurements of the soft tissue using 3D scanned images can replace conventional clinical measurements using a periodontal probe since they are more accurate and reliable [16]. However, the intraoral scan has its limitations. There is difficulty scanning highly reflective or moist surfaces. Also, there is a learning curve, and there are purchasing and managing costs [17].
5. Conclusions
The use of the 3D scanner allows an assessment of relative changes in gingival contour. This method will measure changes in gingival tissues to within 1 micrometer in one plane, making it suitable for the assessment of small changes. Therefore, digital analysis would be an ideal method for the assessment of longitudinal changes in soft tissue contour.
Utilizing the simultaneous roll technique during implant surgery proves beneficial for enhancing buccal soft tissue volume. However, this study is a case report with a small sample size, which presents limitations and highlights the need for further research.
Author Contributions
Conceptualization, W.-S.D.; formal analysis, Y.-G.K.; investigation, W.-S.D.; resources, W.-S.D.; data curation, W.-S.D.; writing—original draft, Y.-J.S.; and writing—review and editing, S.-M.H. and J.-M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Kyungpook National University Dental Hospital (KNUDH-2025-01-01-00) on 22 January 2025.
Informed Consent Statement
Patient consent was waived due to it is prospective study.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tan, W.L.; Wong, T.L.; Wong, M.C.; Lang, N.P. A systematic review of post-extractional alveolar hard and soft tissue dimensional changes in humans. Clin. Oral Implants Res. 2012, 23 (Suppl. S5), 1–21. [Google Scholar] [CrossRef] [PubMed]
- Js, S. Reconstruction of deformed, partially edentulous ridges, using full thickness onlay grafts. Part I. Technique and wound healing. Compend. Contin. Educ. Dent. 1983, 4, 437. [Google Scholar]
- Allen, E.P.; Gainza, C.S.; Farthing, G.G.; Newbold, D.A. Improved technique for localized ridge augmentation. A report of 21 cases. J. Periodontol. 1985, 56, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Marzadori, M.; Stefanini, M.; Sangiorgi, M.; Mounssif, I.; Monaco, C.; Zucchelli, G. Crown lengthening and restorative procedures in the esthetic zone. Periodontology 2000 2018, 77, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Abrams, L. Augmentation of the deformed residual edentulous ridge for fixed prosthesis. Compend. Contin. Educ. Gen. Dent. 1980, 1, 205–213. [Google Scholar] [PubMed]
- Scharf, D.R.; Tarnow, D.P. Modified roll technique for localized alveolar ridge augmentation. Int. J. Periodontics Restor. Dent. 1992, 12, 415–425. [Google Scholar]
- Langer, B.; Calagna, L. The subepithelial connective tissue graft. J. Prosthet. Dent. 1980, 44, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Garber, D.A.; Rosenberg, E.S. The edentulous ridge in fixed prosthodontics. Compend. Contin. Educ. Dent. 1981, 2, 212–223. [Google Scholar] [PubMed]
- Seibert, J.; Nyman, S. Localized ridge augmentation in dogs: A pilot study using membranes and hydroxyapatite. J. Periodontol. 1990, 61, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Shakibaie, B.; Sabri, H.; Blatz, M.B.; Barootchi, S. Comparison of the minimally-invasive roll-in envelope flap technique to the holding suture technique in implant surgery: A prospective case series. J. Esthet. Restor. Dent. 2023, 35, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, I.; Berglundh, T.; Wennström, J.; Lindhe, J. The peri-implant hard and soft tissues at different implant systems. A comparative study in the dog. Clin. Oral Implants Res. 1996, 7, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Linkevicius, T.; Apse, P.; Grybauskas, S.; Puisys, A. The influence of soft tissue thickness on crestal bone changes around implants: A 1-year prospective controlled clinical trial. Int. J. Oral Maxillofac. Implants 2009, 24, 712–719. [Google Scholar] [PubMed]
- van Brakel, R.; Noordmans, H.J.; Frenken, J.; de Roode, R.; de Wit, G.C.; Cune, M.S. The effect of zirconia and titanium implant abutments on light reflection of the supporting soft tissues. Clin. Oral Implants Res. 2011, 22, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Lops, D.; Stellini, E.; Sbricoli, L.; Cea, N.; Romeo, E.; Bressan, E. Influence of abutment material on peri-implant soft tissues in anterior areas with thin gingival biotype: A multicentric prospective study. Clin. Oral Implants Res. 2017, 28, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Avila-Ortiz, G.; Gonzalez-Martin, O.; Couso-Queiruga, E.; Wang, H.L. The peri-implant phenotype. J. Periodontol. 2020, 91, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Jeon, Y.-S.; Strauss, F.-J.; Jung, H.-I.; Gruber, R. Digital scanning is more accurate than using a periodontal probe to measure the keratinized tissue width. Sci. Rep. 2020, 10, 3665. [Google Scholar] [CrossRef] [PubMed]
- Mangano, F.; Gandolfi, A.; Luongo, G.; Logozzo, S. Intraoral scanners in dentistry: A review of the current literature. BMC Oral Health 2017, 17, 149. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).