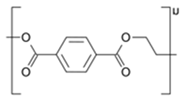
Abstract
Earthworms play a key role in maintaining a healthy soil ecosystem by providing organic matter cycling and influencing the soil’s structure and physicochemical properties. In addition, they have also become the subject of research in the context of soil contamination by plastic particles or microplastics. In this article, two species of earthworms, Dendrobaena veneta and Lumbricus terrestris, were subjected to the influence of a mixed fraction of microplastics at different concentrations and studied to determine the possible correlation in tissue accumulation and to illustrate the possible structural changes in plastics under the influence of earthworm digestive enzymes. Using FTIR spectrometry and plastic fluorescence, the polymer content of the earthworm tissues was determined, and significant differences in the accumulation of plastic particles in the cultured earthworm tissues at the micro- and macroscales were documented.
1. Introduction
Extensive research describes the effects of earthworms on soil properties, but increasingly, these animals are classified as being part of a much more complex environmental relationship, stimulating soil’s fertility and microbial biomass, which consequently affects the productivity of the ecosystem. Some of the most common activities of earthworms include changes in soil porosity, infiltration, compaction, hydraulic conductivity, water cycling, and oxygen availability [1]; increasing the availability of organic compounds for plants [2]; influencing the bacterial and fungal flora of the soil [3]; and participating in vermicomposting [4]. However, this does not mean that all the described species have the same impact on the environment, as there are described cases of negative effects on the soil, such as through soil erosion and reductions in nematode abundance and nutrient translocation and availability [5]. The classification of earthworms distinguishes more than 670 species of the family Lumbricidae and about 5000 species of the family Moniligastrida [6]. In addition, the ecological division into epigeic, endogeic, and anecic earthworms is described. Epigeic species are often found in mulches and composts, living above the soil surface in close proximity to areas with high mineral contents. They consume little or no soil and are usually small and darkly colored. Endogeic species are found in shallow soil layers, in the rhizosphere of plants, where they tunnel horizontally. They feed on soil that is more or less rich in mineral nutrients, reach a medium size, and are usually paler than epigenic ones. The largest representatives of the groups described in the ecological category are anecic, including L. terrestris. These earthworms are saprophages that dig vertical burrows up to a depth of 3 m. In addition, they show a strong attachment to their corridors, often returning to their cavities after leaving a trail of slime. These earthworms pull leaves and nutrients underground, thus significantly increasing the soil’s fertility and microbial abundance [7].
The presence of microplastics in soil is well documented, and despite the lack of a uniform analytical methodology, the effects on plants and animals are considered negative and often toxic. Studies conducted on earthworms have proven a decrease in their weight and fertility or an increase in stress markers, but increasingly, the discussion is directly about the fate of microplastic particles and the possibility of their degradation. To date, no study has confirmed the enzymatic decomposition of plastic into its elemental form [8], and those describing chemical degradation or structural change are subject to uncertainty regarding the apparatus used. Given the selective feeding of earthworms and their ability to discern (and avoid) certain foods, the determination of plastic content can only be achieved by tissue analysis. Microplastics that remain in the soil and are exposed to chemicals such as pesticides or herbicides, as well as water, wind, and friction, can change their properties without earthworm interference, which is erroneously described as earthworm aggregate. Many authors describe a controversy surrounding microplastic degradation in scientific articles, noting that the definition suggests any change in physical or chemical properties due to environmental (light, wind, water) and biological factors [9,10,11]. These authors also point out that for some polymers, their effect on earthworms is stimulatory and may not cause adverse side effects, and that the decrease in polymer mass may have been related to tissue accumulation [11]. A 2018 [12] study described fragmentation of plastic particles by L. terrestris, but as the author points out, there was no degradation, only a change in the size of low-density polyethylene particles. Lwagna further describes the role of microorganisms as one of the key steps in polyethylene fragmentation. An important factor is the species’ sensitivity to the presence of microplastic in the soil; under laboratory conditions, the post-exposure mortality can be easily determined, thereby excluding species that are unsuitable for testing [13]. Earthworm adaptations and their natural resistance have been described in detail by Lwanga [14], Alauzet [15], Wang [16], Chen [17], and Lithner [18]. In addition, Ding describes an individual mortality of 6–28% when feeding on polystyrene and polyethylene at higher concentrations reaching 2% [19]. The digestive system of earthworms is relatively simple and consists of the pharynx, esophagus, stomach, foregut, and hindgut. Digestive enzymes mix with the ingested soil and lead to the acquisition of nutrients, which is further aided by the intestinal membranes [20].
According to this study, earthworms freely swallow particles of the smallest size, effectively avoiding areas with increased plastic contents. Similarly, larger particles around 1 mm were absent from all tested intestinal contents, whereas particles from 1 to 44 μm were most commonly detected. These data are in accordance with numeral findings in the available literature, describing selective food intake by earthworms [21,22].
2. Purpose of the Study
In this study, the authors investigated the extent of plastic accumulation for the two best adapted species, D. veneta and L. terrestris, under outdoor environmental conditions (macroscale) and compared them to the results obtained under laboratory culture conditions (microscale). Exposing specimens to a mixed fraction of plastic polyethylene (PE) fluorescent green microspheres with sizes of 1–5 µm, 10–20 µm, 32–38 µm, 38–45 µm, and 53–63 µm and purple polyethylene microspheres with sizes of 38–45 µm and 75–90 µm. Polyethylene terephthalate (PET) in the form of irregular granule sizes of 10–5000 µm, polyamide (PA) in the form of fibers with sizes of 10–2000 µm, and polystyrene (PS) in the form of irregular film shreds with sizes of 10–5000 µm were also used. The plastics were supplied by Cospheric LLC Somis, CA 93066, USA.
3. Materials and Methods
3.1. Environmental Culture (Macro)
Juveniles of L. terrestris and D. veneta from in-house conservation breeding were transferred into 360 L containers, 665 mm × 880 mm × 1100 mm in size, made using high-density polyethylene (PE-HD) injection technology at a rate of 50 per container. The containers had a drainage system and ventilation holes in the underside. The feed was horse manure mixed with the topsoil at a ratio of 1/3 of the soil volume and was frozen for 48 h to avoid the presence of non-desirable individuals. Both the soil and manure were unevenly mixed with microplastic fractions. We used 0.2%, 0.4%, 0.6, 0.8%, and 1% fractions of the plastic mixture to the dry weight of the soil and manure, and the exposure time was 3 months. The containers were outdoors in a shady place during spring, with a daily temperature range of 5–25 °C.
After this period, 20 adult specimens from each sample were thoroughly cleaned with soil with running water and left for 2 min in distilled water to remove all soil particles from the body sepia. Individuals that were prepared in this way were transferred to dishes with a small amount of sterile agar for 24 h so that the earthworms would excrete the soil that had accumulated in their intestines and digestive system. The earthworms were then washed again with distilled water, placed in glass dishes, and treated with heat shock at −80 °C. In the next step, the earthworms were carefully stripped of all intestines, and the tissue was washed and left to dry at room temperature.
3.2. Laboratory Culture
In the laboratory culture, exactly the same culture conditions were used; however, the culture was carried out in 3 L containers, and the microplastic was evenly mixed with the entire volume of soil and manure.
3.3. Optical Analysis
The illustrations shown below were taken using an Olimpus BX 51 microscope, an Olimpus a300 objective (U-CMAD3), and an Olipmus U-TV1X converter (3701 Welsh Rd, Unit C, Willow Grove, Pennsylvania 19090, 1-866-570-3046) on Light (1) and UV (4) settings and then subjected to noise autocorrection without interfering with the color intensity and exposure. The method allows for the preliminary identification of these plastics in earthworm tissue, the determination of the exact location, and the use of the tissue for further FTIR analysis. In addition, it allows for a preliminary estimate of the amount of microplastics in the tissue. The obtained illustrations depict polyamide (Figure 1) and polyethylene terephthalate (Figure 2) and were taken as standards, with large fragments of the polymer and a clear fluorescence color (Figure 3).
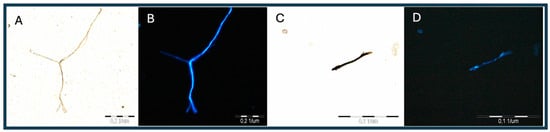
Figure 1.
(A–D) Images of PA fiber under visible and UV light (4).
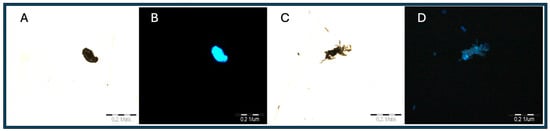
Figure 2.
(A,B) Images of PET molecule and (C,D) images of PS molecule under UV (4) light.
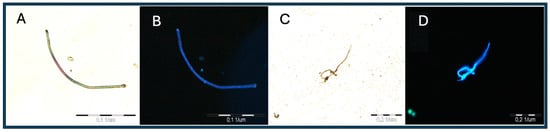
Figure 3.
Images (A–D) of the PA molecule under UV (3) light.
3.4. Fourier Transform Infrared Spectroscopy (FTIR Analysis)
To prepare the samples for FTIR analysis, a freeze-dryer with a vacuum pump, MRC AB Chem SM-450, was used, in which the earthworm tissues were placed in a tray and subjected to a drying process according to the manufacturer’s instructions for a period of 24 h. After this time, the tissues were placed in a mortar, crushed gently to a fine, uniform dust, and transferred to glass cuvettes.
The FTIR apparatus used in this study was a Jasco FTIR Model 6200, operating in the near-infrared (15,000 cm−1) and far-infrared (50 cm−1) regions. The resolution of the apparatus is 0.25 cm−1. Each complete, powdered tissue from one individual was divided into four smaller sections, each of which was subjected to FTIR analysis in 5 measurements; the results were summed, giving a total qualitative image for one individual. In this way, all tissues from both species were examined, identifying the plastics that were present. The characteristic peak values and polymer patterns are shown in Table 1.

Table 1.
Characteristic peak values of polymeric materials for FTIR analysis.
3.5. Hydrogen Peroxide Digestion
The digestion of organic matter is a key step in the preparation of samples for the identification of plastic molecules, regardless of the original matrix, which may be soil, sediment, or tissue. The literature describes many protocols, taking into account both the type of reagent, digestion time, reaction temperature, and possible complications. Increasingly, one also encounters a division of reagents with respect to the hardness of the dissolved fraction into soft (delicate plant and animal tissues, among others) and hard (like muck, bone, and compacted soil) fractions [23]. In addition, the direct and indirect effects of the reagent on the contained microplastic (changes in its color, structure, and degradation) and the availability and harmfulness of reagents are important. The density and quantitative separation have been described in detail by Cole [24], Roch [25], Dehaut [26], Munno [27], and Lusher [28]. Among the most commonly used reagents, characterized by optimal efficiency and performance characteristics, are nitric acid, hydrochloric acid, potassium hydroxide, hydrogen peroxide, sodium hypochlorite, and sodium hydroxide. Recombinations of the temperature concentration and efficiency variants were described by Foekema [29], Karami [30], and Herrera [31].
In our study, a 30% H2O2 solution was used for tissues that were already highly fragmented and homogeneous and did not form aggregates, so it was possible to use it in smaller amounts and for a shorter period of 36 h. For digestion, glass cuvettes were used, in which the tissue material and H2O2 were placed. After this time, the hydrogen peroxide was removed from the vessel, and the remaining fraction was prepared for staining.
3.6. Microplastic Staining
Microplastic identification using dyes has become a popular method to quickly and efficiently identify plastic particles by providing specific complexes with which to differentiate them from organic and mineral matter. Individual dyes differ in their affinity for the plastic, fluorescence intensity, incubation time, and the type of polymers that they react with, and the most commonly described include oil red (Sudan-nitrogen dye), eosin (acid dye), Bengal pink, hostasol yellow, Nile Red (lipid dye) [32], alkali blue (bromothymol dye), crystal violet (aniline dye), neutral red (neutral red), and trifan blue [33]. The current literature indicates that the most widely used and effective dye is Nile Red, which provides high fluorescence in plastics, with a wide range of microplastics; therefore, it has found applications in translocation of microplastics in soil and living organisms (no toxicity to living cells, including human), in field monitoring of microplastics, and in many laboratory techniques [34]. According to the current knowledge, there is still no uniform methodology for dealing with all the stages of staining, and as the authors point out, this gap needs to be filled, especially with environmental studies [35].
Nile Red is a very stable photochemical dye, classified as a hydrophobic, heterocyclic, and metachromatic compound, and is almost insoluble in water [36]. Both its emission and quantum properties depend on the type of solvent used, and an increase in polarity most often leads to a red shift in fluorescence. The wide solvatochromic range and the effect of the environment on the color was described in detail by Martinez and Henary [37]. This study used acetone as a solvent at a concentration of 1 mg/mL, a volume ratio of 0.1–0.5 mL of dye solution per 100 mL of water sample, and an exposure time of 30 min.
3.7. Determination of Molecular Size
The plastics that were added to the soil were characterized by varying sizes and spatial shapes, making it important to determine the minimum and maximum particle sizes. For PE and PET, the authors relied on descriptions from the manufacturer (Cospheric LLS, Somis, CA, USA), while for the others, they used an Olimpus BX 51 microscope and Olimpus a300 objective (U-CMAD3) with CellB software with a scale function and variable microscope ruler, as well as a WITec ALPHA 300R and 300RSA spectrometers with a confocal microscope and True Surface attachment, and the image was taken at the 633 nm excitation line and 20× magnification (Figure 4).

Figure 4.
One method of measuring molecular size using Raman microscopy and confocal microscopy.
4. Results
4.1. Results of Microscopic Examination Analysis
The images below show tissue arranged on a basic slide and analyzed microscopically under UV (4) and green (3) light for the initial identification of microplastics and the estimation of their amount and position for further analysis (Figure 5). Scanning in the high resolution of the eyepiece makes it possible to identify fractions; however, the resolution of the objective lens does not allow for photography at this scale. In the figures below, PE (A, B), PS (O, P), PA (I, J), and PET (K, L) are confirmed.
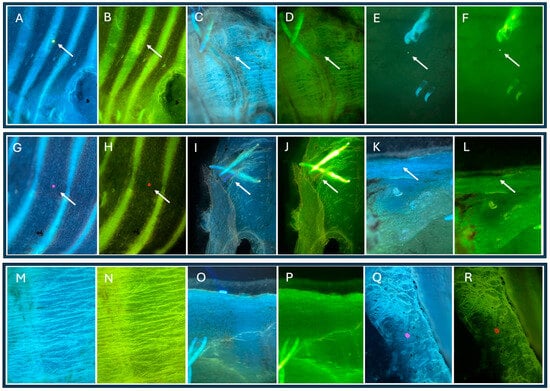
Figure 5.
Microplastics in optical microscope images using UV light and deep green to image microplastics in different color ranges. (A–R) are areas of the same fragment in different color ranges. The arrow indicates the location of the visible microplastic particle.
We also analyzed the results of the quantitative laboratory culture tests. Table 2, Table 3, Table 4 and Table 5 show the minimum and maximum number of particles that were detected in the tissues of one individual, averaged from 10 individual measurements. Table 2 and Table 3 show the results of the analysis of earthworm tissues that were cultured at the microscale. Table 4 and Table 5 show the results of the analysis of earthworm tissues that were cultured at the macroscale.

Table 2.
Plastic particles under visible, UV, deep green, and red light.

Table 3.
Plastic particles under visible, UV, deep green, and red light.

Table 4.
Plastic particles under visible, UV, deep green, and red light.

Table 5.
Plastic particles under visible, UV, deep green, and red light.
Regarding the environmental test results and quantitative laboratory culture results, Table 2, Table 3, Table 4 and Table 5 show the minimum and maximum amount of particles that were detected in the tissues of one individual (the average for 10 individuals is specified in parentheses
The differences in accumulation between micro- and macro-cultured individuals are clear, as the graphs below illustrate how macro-cultured individuals regress to soil microplastic concentrations above 0.6%, resulting in reduced tissue accumulation. The least pronounced changes occur for the smallest particle sizes. Figure 6, Figure 7, Figure 8 and Figure 9 present graphs showing how the accumulation of particles changes depending on the concentration of microplastic in the soil.

Figure 6.
Summary of the number of detected particles depending on their concentration in the soil—micro-culture.

Figure 7.
Summary of the number of detected particles depending on their concentration in the soil—micro-culture.

Figure 8.
Summary of the number of detected particles depending on their concentration in the soil—macro-culture.
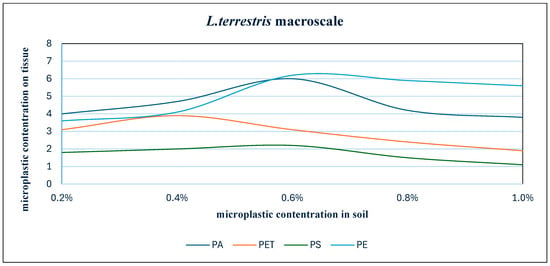
Figure 9.
Summary of the number of detected particles depending on their concentration in the soil—macro-culture.
Microplastic particles were detected in longitudinal and transverse muscles, along the metanephridia and nephrostome and in segmental ganglia. The most common type of plastic in optical microscopy were microgranules (PE) (Figure 1B and Figure 3C) and fibers (PA) with sizes of 1–43 um (other images). The green light spectrum allowed for deeper penetration and better imaging of fractions that were inaccessible to ultraviolet and visible light. Further analysis made it possible to accurately determine the type of plastic that was accumulated in the muscles. The selective and total particle counts are shown in Table 2 and Table 4 (Dendrobaena veneta) and Table 3 and Table 5. (Lumbricus terrestris) for the average individual from 10 samples.
4.2. Particle Size Analysis
To the authors’ knowledge, this is the first study of its kind in which the authors determined the sizes of particles that were accumulated in the bodies of living organisms, relative to any that were intentionally added to the soil (Figure 10). The data shown in the diagram (diagram x) represent the minimum and maximum size of each plastic and the size range of the particles that were isolated from the tissues. This study proved what particle sizes accumulate in tissues and what the reasons for this may be.
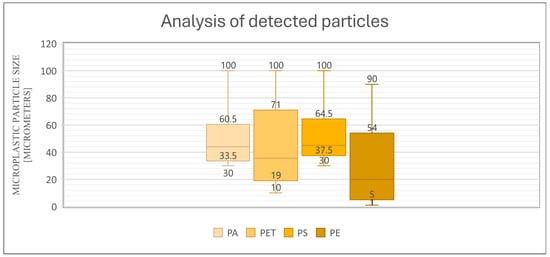
Figure 10.
Relationship between the particles added to the soil and the particles detected in the tissues for D. veneta and L. terrestris.
This study proved that on a macroscale, earthworms that are exposed to different concentrations of particles of different polyamides have the ability to accumulate tissue in that particular range. In the case of polyamide, the molecular size in the soil was in the range of 30 µm to 1000 µm ( in the graph, for simplicity, the maximum value is 100 µm) but in the tissues, particles were only detected in the range of 33.5 µm to 60.5 µm. In the case of PET, heterogeneous particles of 10 µm to 1000 µm were found in the soil, but in the tissues, only sizes in the range of 19–71 µm were detected. In the case of polystyrene, the polymer size in the soil was 30 µm to 1000 µm and detected in the range of 37.5 µm to 64.5 µm, and in the case of polyethylene, particles from 1 µm to 90 µm were added to the soil and detected in tissues from 5 µm to 54 µm, with the detection of particles below 10 µm only being possible due to the strong fluorescence of the particles.
4.3. FTIR Analysis Results
Spectral peaks for individual plastics were compared based on a database (https://spectra.chem.ut.ee/paint/binders/polyethylene-wax, accessed on 15 December 2024) and available pilot publications for plastics [38,39,40,41,42] and are presented in Table 1.
The graphs (Figure 11, Figure 12, Figure 13 and Figure 14) depict selected analyses of the plastics that were contained in the tissues, with characteristic peaks marked to identify the plastic. The red line indicates the peaks whose values were assigned to a particular polymer. In addition, the graphs show other peaks with different values, resulting from the analysis of the powdered tissue and the compounds that were contained in it.
Pectrum Obtained from Tissue Analysis, Showing Strong Tissue Background and Peaks Characteristic of Polyethylene.
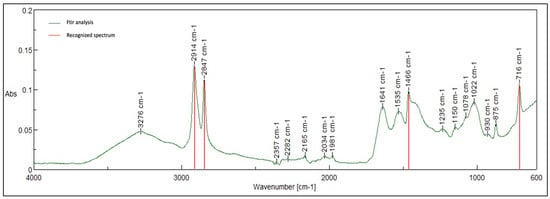
Figure 11.
Characteristic peaks for polyethylene-2914, 2847, 1466, and 716.
Spectrum Obtained from Tissue Analysis, Showing Strong Tissue Background and Peaks Characteristic of Polyamide.
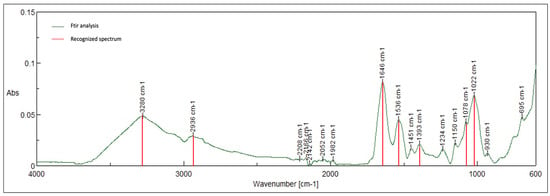
Figure 12.
Characteristic peaks for polyamide- 3280, 2936, 1646, 1536, 1393, 1078, and 1022.
Spectrum Obtained from Tissue Analysis, Showing Strong Tissue Background and Peaks Characteristic of Polyethylene Terephthalate.

Figure 13.
Characteristic peaks for pet 1713,1409, 1241, 1094, 1016, and 872.
Spectrum Obtained from Tissue Analysis, Showing Strong Tissue Background and Peaks Characteristic of Polyethylene Terephthalate.

Figure 14.
Characteristic peaks for polystyrene 3060, 3026, 2913, 2844,1601, 1492, 1450, 1027, 751, and 694.
4.4. Coefficient of Determination
The coefficient of determination, R2, was used as a measure of the fit of the mathematical model with the data obtained from Table 1, Table 2, Table 3, Table 4 and Table 5 for both species and in both breeding variants. This coefficient indicates what proportion of the variance in the data coincides with the correlation of the variables that are described in the model. Thus, we can say that it is a determinant of the fit of the sample to the model, described in the range of values between 0 and 1, with values close to unity corresponding to a higher model fit.
where
(SSres) is the sum of the squares of the residuals, that is, the sum of the squares of the differences between the actual values and the values predicted by the model:
(SStot) is the total sum of squares, that is, the sum of the squares of the differences between the actual values and the average value of the actual data:
The value of R2 ranges from 0 to 1, where a value closer to 1 indicates a better fit of the model to the data.
Using all the data, which are the variables of concentrations and types of plastics, we initially performed a determination analysis for the linear function and quadratic function, but with such a number of variables, the function was not sufficient, and the determination index at each analysis indicated 1. For a better and more accurate depiction of the statistical fit of the model, a third-degree power polynomial and fourth-degree polynomial analysis were performed and are presented in the tables (Table 6, Table 7, Table 8 and Table 9) and figures below (Figure 15, Figure 16, Figure 17 and Figure 18). To increase the clarity of the results, the authors used the abbreviation of the third-degree polynomial as (R2)3 and the fourth-degree polynomial as (R2)4.

Table 6.
Values of the third-degree (R2)3 and fourth-degree (R2)4 polynomial coefficients of D. veneta—micro-culture.

Table 7.
Values of the third-degree (R2)3 and fourth-degree (R2)4 polynomial coefficients of L. terrestris—micro-culture.

Table 8.
Values of the third-degree (R2)3 and fourth-degree (R2)4 polynomial coefficients of D. veneta—macro-culture.

Table 9.
Values of the third-degree (R2)3 and fourth-degree (R2)4 polynomial coefficients of L. terrestris—macro-culture.
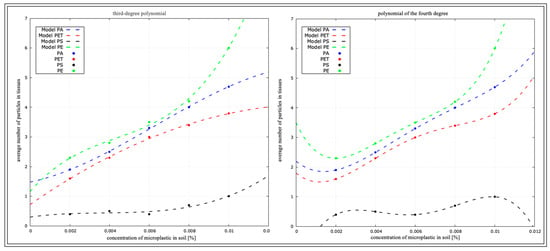
Figure 15.
Determination coefficient for D. veneta from micro-culture.
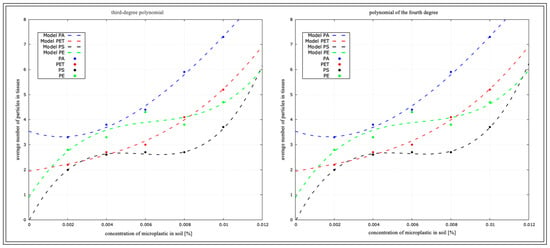
Figure 16.
Determination coefficient for L. terrestris from micro-culture.
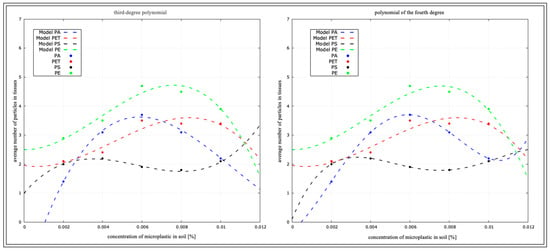
Figure 17.
Determination coefficient for D. veneta from macro-culture.
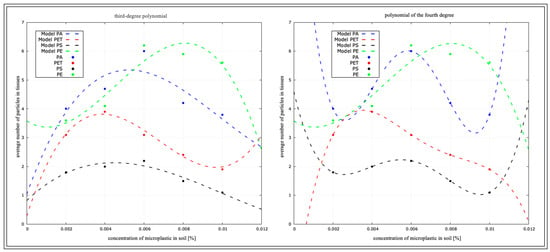
Figure 18.
Determination coefficient for L. terrestris from macro-culture.
Figure 15 rearranges the coefficient of determination for D. veneta in the micro-culture, based on the data in Table 2.
The function values for each plastic in the concentration correlation are shown in Table 6.
Figure 16 rearranges the coefficient of determination for L. terrestris in the micro-culture, based on the data presented in Table 3.
5. Discussion of the Results
This article addresses the extremely important issue of comparing experimental results from the laboratory to those of organisms living in the environment. Taking into account existing publications, which extensively describe the overestimation of the amount of microplastic in organisms, and the significant differences between internal and external studies, it is worthwhile to analyze the factors affecting these differences in results. An important aspect is the analysis of the factors influencing these discrepancies, which is important for the correct interpretation of the results and their application in assessing the actual impact of microplastics on organisms. Trakić et al. [43] emphasized that the toxicity of microplastics may be strongly dependent on the type of polymer and the presence of other contaminants, which further complicates the comparison of results from different environments. In turn, a review of [44] indicates that laboratory conditions may promote increased bioaccumulation of microplastics, because organisms do not have the ability to avoid them, as is the case in natural conditions. Such differences may lead to overestimates of the effects of microplastics on organisms, which have important implications for ecotoxicology.
The laboratory conditions in which the primary culture earthworms reproduced were an environment that was devoid of natural predators and rapid temperature changes and with a constant access to food. Placing earthworms in culture containers with microplastic contents that were evenly mixed with soil and food forced the earthworms to ingest particles as a consequence of starvation. In addition, the limited mobility and food selectivity meant that the microplastic exposure was consistently high. For this reason, the authors suggest that the obtained results should not only be interpreted as evidence of the ability of earthworms to accumulate microplastics, but also as a reliable estimate of their actual amount under environmental conditions. The rearing of earthworms in containers significantly affected the results of the study. The increased soil volume and depth of the containers made it possible for individuals to freely choose where to forage, thus contributing to the relocation of the microplastic. In turn, Trakić et al. [43] emphasized that differences in food mobility and availability are key factors influencing the degree of exposure of soil organisms to microplastics, which may significantly alter the results of ecotoxicological studies.
The identification of microparticles in soil and the determination of their concentration from soil analysis are methods that are subject to constant validation and qualitative and quantitative precision testing. Soil is an extremely complex structure, characterized by varying hydrological dynamics and organic contents. As indicated by studies by Wang [45], Qian [46], and Li [47], there are numerous obstacles to accurate concentration estimation, including the apparatus resolution, particle size, particle aggregation, bonding and adhesion to soil grains, and many others. The research conducted may be an indication of the possibility of using different types of apparatus to identify microplastics based on tissue analysis of earthworms. This method, while certainly requiring more measurements, can provide an open database in which each subsequent study will increase the pools of results that are available for comparison. Each of the aforementioned methods as a single tool is fraught with many possibilities for overestimation or underestimation, but combining them all in the right order offers a chance for effective plastic analysis. This approach is consistent with the conclusions of [43], where it was suggested that the effectiveness of microplastic detection methods depends on the size and shape of the particles and their interaction with body tissues, which means that the combination of several analytical methods can increase the reliability of the results. In turn, ref. [44], a literature review, indicates that the standardization of analytical procedures in microplastics research is crucial to obtain comparable results. Therefore, creating an open database based on various detection methods can be a valuable tool supporting the development of research on microplastics in terrestrial ecosystems.
5.1. Optical Analysis
During the tests and observations using the microscope described above, it was not possible to observe particles that were smaller than 10 um, excluding those with strong fluorescence, which does not mean that no such particles were accumulated in the tissues. Moreover, the authors are convinced that these plastics were contained, but the detection of such small particles is extremely difficult, and at any stage of processing, it is possible to miss them. Optical analysis works very well for identifying larger particles and tissue mapping, which makes it possible to mark all visible fragments for further analysis. And while Nile Red staining is a very promising method, there is still uncertainty about the level of precision of the described results below 10 µm. Similar limitations in the detection of small microplastic particles using optical microscopy and Nile Red staining have been reported in the literature. Research indicates that particle properties, such as morphology, color, and degree of degradation, may affect the detection efficiency after Nile Red staining. For example, black microplastics exhibit weak fluorescence, making them difficult to detect, and fibers are more difficult to identify compared to regularly shaped particles [48]. Therefore, although Nile Red staining is a useful tool for the rapid identification of microplastics, its limitations in the detection of small particles and the possibility of obtaining false positive results suggest the need to use complementary analytical methods, such as FTIR or Raman spectroscopy, to obtain more precise and reliable results.
5.2. Particle Concentration in Tissues
This study clearly shows significant differences in the tissue accumulation of earthworms that were reared under laboratory conditions and in outdoor containers with large soil volumes. The worms who were forced to forage in 3L containers showed a linear increase in microplastic particle concentration, which was triggered by the lack of mobility and stability of the plastics throughout the soil and food. These results further demonstrate the ability to accumulate microplastic in earthworm bodies to a high degree and the potential for further analysis for other polymer groups. Tests conducted on macro-cultured earthworms were variable with respect to the concentration, and it seems that earthworms avoided areas where microplastic accumulated at a concentration of ~0.6% and that the particle size also had a significant impact on the results. The authors draw the bold conclusion that earthworms are unable to sense plastic below 40 um, meaning that foraging in an area with a concentration of less than 0.6% and a particle size of less than 40 um occurred without the appearance of signs of stress or a desire to relocate. The size and concentration of microplastics in the soil have a significant impact on their accumulation in earthworms. Higher concentrations and smaller particle sizes increase the likelihood of their ingestion and accumulation in tissues [49]. In addition, particles with spherical shapes were most common in the tissue analysis, followed closely by irregular fragments of a similar size. The tissue images and optical analysis did not clearly prove signs of inflammation in the sites where the material was labeled, nor damage to the tissues themselves, as is often reported in articles. Therefore, it can be concluded that mechanical damage to the gastrointestinal tract or intestines can only be caused by larger fragments that damage tissues with their sharp edges during intestinal peristalsis. These conclusions are consistent with previous studies, although some aspects require further verification. In [50], which used Raman microscopy, the authors showed that spherical microplastics accumulate more often in earthworm tissues, which coincides with the results presented in the current study. However, some previous studies [43] have suggested that even small microplastic particles can cause subtle inflammatory reactions. Additionally, these results fit into the broader context of research on the ecotoxicity of microplastics in terrestrial ecosystems. Guo et al. [51] suggest that the ability of earthworms to avoid areas with high concentrations of microplastics may be a behavioral strategy to minimize its negative impact. Nevertheless, the lack of visible reactions to particles that are smaller than 40 µm requires further investigation, especially with regard to potential sublethal effects such as oxidative stress or metabolic disorders. The described particles are two orders of magnitude smaller, so there is a chance of internal tissue penetration. It is also possible that the smallest fragments of microplastic enter the body through the respiratory cavities along the body via the mucus produced. The available literature indicates that there are many problems associated with the detection of microplastic in soil and the whole procedure for its detection [52,53], pointing to the variability of the matrix that is soil, the number of samples taken, their size and spatial distribution, or the consideration of soil hydrodynamics.
5.3. Conclusions
The conducted research confirmed that there is an opportunity to use L. terrestris and D. veneta as indicator species for microplastic-contaminated soil, and that further studies may be helpful for a more accurate estimation of the correlation of soil and tissue concentrations, as well as for a broader understanding of the mechanisms in different soil types. In this case, earthworms become an interesting solution, because they actively move in the soil and are able to collect plastic from different depths. With a sufficiently large number of repetitions for a given region, concentration estimation can be a very helpful method. The most important issue remains the linearity of the results and the very fact that the concentration of plastics in the soil has a direct effect on the amount of plastics in the tissues. The authors suggest that it may be important to study individual internal organs and visualize the possible accumulation by a specific organ.
Author Contributions
Conceptualization, A.G.; validation, A.G.; investigation, M.K.; resources, A.K.; data curation, M.K.; writing—original draft, M.K.; writing—review & editing, A.G.; visualization, M.K.; supervision, A.G.; project administration, A.G.; funding acquisition, A.G. All authors have read and agreed to the published version of the manuscript.
Funding
The scientific research was funded by the statute subvention of the Czestochowa University of Technology (Faculty of Infrastructure and Environment).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ojha, R.B.; Devkota, D. Earthworms: “Soil and Ecosystem Engineers”—A Review. World J. Agric. Res. 2014, 2, 257–260. [Google Scholar]
- Byers, J.E.; Cuddington, K.; Jones, C.G.; Talley, T.S.; Hastings, A.; Lambrinos, J.G.; Crooks, J.A.; Wilson, W.G. Using Ecosystem Engineers to Restore Ecological Systems. Trends Evol. 2006, 21, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Lwanga, E.; Mendoza-Vega, J.; Ribeiro, O.; Gertsen, H.; Peters, P.; Geissen, V. Is the Polylactic Acid Fiber in Green Compost a Risk for Lumbricus terrestris and Triticum aestivum? Polymers 2021, 13, 703. [Google Scholar] [CrossRef]
- Rorat, A.; Suleiman, H.; Grobelak, A.; Grosser, A.; Kacprzak, M.; Płytycz, B.; Vandenbulcke, F. Interactions between sewage sludge-amended soil and earthworms—Comparison between Eisenia fetida and Eisenia andrei composting species. Environ. Sci. Pollut. Res. 2016, 23, 3026–3035. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, P.; Spain, A.V. Soil Ecology; Kluwer Academic Publishers: New York, NY, USA, 2001. [Google Scholar]
- Blakemore, R.J. An Updated List of Valid, Invalid and Synonymous Names of Criodriloidea and Lumbricoidea (Annelida: Oligochaeta: Criodrilidae, Sparganophilidae, Ailoscolecidae, Hormogastridae, Lumbricidae, Lutodrilidae). A Series of Searchable Texts on Earthworm Biodiversity, Ecology and Systematics from Various Regions of the World-Supplemental. CD Publication Under ICZN (1999: Art. 8). 2008. Available online: https://www.researchgate.net/publication/292401504 (accessed on 14 October 2012).
- Geraskina, A.; Shevchenko, N. Spatial Distribution of the Anecic Species of Earthworms Dendrobaena nassonovi nassonovi (Oligochaeta: Lumbricidae) in the Forest Belt of the Northwestern Caucasus. Forests 2023, 14, 2367. [Google Scholar] [CrossRef]
- Tedesco, D.E.A.; Castrica, M.; Tava, A.; Panseri, S.; Balzaretti, C.M. From a Food Safety Prospective: The Role of Earthworms as Food and Feed in Assuring Food Security and in Valuing Food Waste. Insects 2020, 11, 293. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Ziersch, L.; Hempel, S. Microplastic Transport in Soil by Earthworms. Sci. Rep. 2017, 7, 1362. [Google Scholar] [CrossRef] [PubMed]
- Arab, M.; Yu, J.; Nayebi, B. Microplastics in Sludges and Soils: A Comprehensive Review on Distribution, Characteristics, and Effects. ChemEngineering 2024, 8, 86. [Google Scholar] [CrossRef]
- Kim, H. A Study on the Utilization of the Earthworms Eisenia Fetida and Eisenia Andrei for the Disposal of Polymers. Int. J. Environ. Sci. Dev. 2016, 7, 355–358. [Google Scholar] [CrossRef]
- Lwanga, E.H.; Thapa, B.; Yang, X.; Gertsen, H.; Salánki, T.; Geissen, V.; Garbeva, P. Decay of Low-Density Polyethylene by Bacteria Extracted from Earthworm’s Guts: A Potential for Soil Restoration. Sci. Total Environ. 2018, 624, 753–757. [Google Scholar] [CrossRef]
- Khaldoon, S.; Lalung, J.; Maheer, U.; Kamaruddin, M.A.; Yhaya, M.F.; Alsolami, E.S.; Alorfi, H.S.; Hussein, M.A.; Rafatullah, M. A Review on the Role of Earthworms in Plastics Degradation: Issues and Challenges. Polymers 2022, 14, 4770. [Google Scholar] [CrossRef] [PubMed]
- Lwanga, E.H.; Gertsen, H.; Gooren, H.; Peters, P.; Salánki, T.; van der Ploeg, M.; Besseling, E.; Koelmans, A.A.; Geissen, V. Incorporation of Microplastics from Litter into Burrows of Lumbricus Terrestris. Environ. Pollut. 2017, 220, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Alauzet, N.; Garreau, H.; Bouché, M.; Vert, M. Earthworms and the Degradation of Lactic Acid-Based Stereocopolymers. J. Polym. Environ. 2002, 10, 53–58. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, G.; Wang, Y.; Mu, H.; Shi, X.; Wang, C.; Wu, N. The Global Trend of Microplastic Research in Freshwater Ecosystems. Toxics 2023, 11, 539. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, X.; Leng, Y.; Wang, J. Defense Responses in Earthworms (Eisenia Fetida) Exposed to Low-Density Polyethylene Microplastics in Soils. Ecotoxicol. Environ. Saf. 2020, 187, 109788. [Google Scholar] [CrossRef] [PubMed]
- Lithner, D.; Larsson, A.; Dave, G. Environmental and Health Hazard Ranking and Assessment of Plastic Polymers Based on Chemical Composition. Sci. Total Environ. 2011, 409, 3309–3324. [Google Scholar] [CrossRef]
- Ju, H.; Yang, X.; Osman, R.; Geissen, V. Effects of microplastics and chlorpyrifos on earthworms (Lumbricus terrestris) and their biogenic transport in sandy soil. Environ. Pollut. 2023, 316, 120483. [Google Scholar] [CrossRef]
- Edwards, C.A.; Fletcher, K.E. Interactions between Earthworms and Microorganisms in Organic-Matter Breakdown. Agric. Ecosyst. Environ. 1988, 24, 235–247. [Google Scholar] [CrossRef]
- Karthikeyan, M.; Gajalakshmi, S.; Abbasi, S.A. Ingestion of Sand and Soil by Phytophagous Earthworm Eudrilus Eugeniae: A Finding of Relevance to Earthworm Ecology as Well as Vermitechnology. Arch. Agron. Soil Sci. 2014, 60, 1795–1805. [Google Scholar] [CrossRef]
- Sanchez-Hernandez, J.C.; Capowiez, Y.; Ro, K.S. Potential Use of Earthworms to Enhance Decaying of Biodegradable Plastics. ACS Sustain. Chem. Eng. 2020, 8, 4292–4316. [Google Scholar]
- Li, J.; Liu, Y.; Gao, Y.; Li, X.; Gong, Y. Study on the Extraction Method of Microplastic System in Textile Wastewater. Polymers 2023, 15, 1394. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Webb, H.; Lindeque, P.K.; Fileman, E.S.; Halsband, C.; Galloway, T.S. Isolation of microplastics in biota-rich seawater samples and marine organisms. Sci. Rep. 2015, 4, 4528. [Google Scholar] [CrossRef] [PubMed]
- Roch, S.; Brinker, A. Rapid and efficient method for the detection of microplastic in the gastrointestinal tract of fishes. Environ. Sci. Technol. 2017, 51, 4522–4530. [Google Scholar] [CrossRef]
- Dehaut, A.; Cassone, A.L.; Frère, L.; Hermabessiere, L.; Himber, C.; Rinnert, E.; Rivière, G.; Lambert, C.; Soudant, P.; Huvet, A.; et al. Microplastics in seafood: Benchmark protocol for their extraction and characterization. Environ. Pollut. 2016, 215, 223–233. [Google Scholar] [CrossRef]
- Munno, K.; Helm, P.A.; Jackson, D.A.; Rochman, C.; Sims, A. Impacts of temperature and selected chemical digestion methods on microplastic particles. Environ. Toxicol. Chem. 2018, 37, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.L.; Welden, N.A.; Sobral, P.; Cole, M. Sampling, isolating and identifying microplastics ingested by fish and invertebrates. Anal. Methods 2017, 9, 1346–1360. [Google Scholar] [CrossRef]
- Foekema, E.M.; Gruijter, C.D.; Mergia, M.T.; van Franeker, J.A.; Murk, A.T.J.; Koelmans, A.A. Plastic in North Sea Fish. Environ. Sci. Technol. 2013, 47, 8818–8824. [Google Scholar] [CrossRef] [PubMed]
- Karami, A.; Golieskardi, A.; Choo, C.K.; Romano, N.; Ho, Y.B.; Salamatinia, B. A high-performance protocol for extraction of microplastics in fish. Sci. Total Environ. 2017, 578, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Herrera, A.; Garrido-Amador, P.; Martínez, I.; Samper, M.D.; López-Martínez, J.; Gómez, M.; Packard, T.T. Novel methodology to isolate microplastics from vegetal-rich samples. Mar. Pollut. Bull. 2018, 129, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Maes, T.; Jessop, R.; Wellner, N.; Haupt, K.; Mayes, A.G. A rapid-screening approach to detect and quantify microplastics based on fluorescent tagging with Nile Red. Sci. Rep. 2017, 7, 44501. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Girão, A.V.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Identifying a quick and efficient method of removing organic matter without damaging microplastic samples. Sci. Total Environ. 2019, 686, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Li, L.; Chen, Q.; Lee, J.S.; Gong, J.; Shi, H. Application of internal persistent fluorescent fibers in tracking microplastics in vivo processes in aquatic organisms. J. Hazard. Mater. 2021, 401, 12333. [Google Scholar] [CrossRef] [PubMed]
- Shruti, V.C.; Pérez-Guevara, F.; Elizalde-Martínez, I.; Kutralam-Muniasamy, G. First study of its kind on the microplastic contamination of soft drinks, cold tea and energy drinks—Future research and environmental considerations. Sci. Total Environ. 2020, 726, 138580. [Google Scholar] [CrossRef]
- Greenspan, P.; Fowler, S.D. Spectrofluorometric studies of the lipid probe, nile red. J. Lipid Res. 1985, 26, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Martinez, V.; Henary, M. Nile red and nile blue: Applications and syntheses of structural analogues. Chem. A Eur. J. 2016, 22, 13764–13782. [Google Scholar] [CrossRef]
- Löder, M.G.J.; Gerdts, G. Methodology Used for the Detection and Identification of Microplastics—A Critical Appraisal. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Pereira, T.; Freitas, C.; Costa, J.L.; Morgado, J.; Silva, F.; Negrão, E.; de Lima, B.F.; da Silva, M.C.; Madureira, A.J.; Ramos, I.; et al. Comprehensive Perspective for Lung Cancer Characterisation Based on AI Solutions Using CT Images. J. Clin. Med. 2021, 10, 118. [Google Scholar] [CrossRef]
- Primpke, S.; Christiansen, S.H.; Cowger, W.; De Frond, H.; Deshpande, A.; Fischer, M.; Holland, E.B.; Meyns, M.; O’Donnell, B.A.; Ossmann, B.E.; et al. Critical Assessment of Analytical Methods for the Harmonized and Cost-Efficient Analysis of Microplastics. Appl. Spectrosc. 2020, 74, 1012–1047. [Google Scholar] [CrossRef]
- Renner, K.O.; Foster, H.A.; Routledge, E.J.; Scrimshaw, M.D. A Comparison of Different Approaches for Characterizing Microplastics in Selected Personal Care Products. Environ. Toxicol. Chem. 2022, 41, 880–887. [Google Scholar] [CrossRef]
- Wander, L.; Lommel, L.; Meyer, K.; Braun, U.; Paul, A. Development of a low-cost method for quantifying microplastics in soils and compost using near-infrared spectroscopy. Meas. Sci. Technol. 2022, 33, 075801. [Google Scholar] [CrossRef]
- Trakić, T.; Popović, F.; Sekulić, J.; Hackenberger, D.K. Ecotoxicological Effects of Commercial Microplastics on Earthworm Eisenia fetida (Savigny, 1826) (Clitellata; Lumbricidae). Agriculture 2024, 14, 267. [Google Scholar] [CrossRef]
- Sanchez-Hernandez, J.C. Microplastic contamination and earthworms: Current trends and research needs. CABI Rev. 2024, 19, 1. [Google Scholar] [CrossRef]
- Wang, J.; Coffin, S.; Sun, C.; Schlenk, D.; Gan, J. Negligible Effects of Microplastics on Animal Fitness and HOC Bioaccumulation in Earthworm Eisenia Fetida in Soil. Environ. Pollut. 2019, 249, 776–784. [Google Scholar] [CrossRef]
- Qian, Z.; Zhang, H.; Yang, Z.; Yuan, L.; Yong, X.; Feng, C.; Chen, T.; Lu, Y. Separation of microplastics from a coastal soil and their surface microscopic features. Chin. Sci. Bull. 2016, 61, 1604–1611. [Google Scholar]
- Li, Q.; Wu, J.; Zhao, X.; Gu, X.; Ji, R. Separation and identification of microplastics from soil and sewage sludge. Environ. Pollut. 2019, 254, 113076. [Google Scholar] [CrossRef]
- Süssmann, J.; Fischer, E.K.; Hildebrandt, L.; Walz, E.; Greiner, R.; Rohn, S.; Fritsche, J. Nile red staining for rapid screening of plastic-suspect particles in edible seafood tissues. Anal. Bioanal. Chem. 2024, 416, 3459–3471. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wang, Q.; Li, Z.; Chen, Y.; Li, H.; Zhang, J.; Wang, X.; Liu, J.; Cao, B.; Zou, G.; et al. Ecological risk of microplastic toxicity to earthworms in soil: A bibliometric analysis. Front. Environ. Sci. 2023, 11, 1126847. [Google Scholar] [CrossRef]
- Klimasz, M.; Grobelak, A. Accumulation of Spherical Microplastics in Earthworms Tissues-Mapping Using Raman Microscopy. Appl. Sci. 2024, 14, 10117. [Google Scholar] [CrossRef]
- Liu, T.; Hou, B.; Zhang, Y.; Wang, Z. Determination of Biological and Molecular Attributes Related to Polystyrene Microplastic-Induced Reproductive Toxicity and Its Reversibility in Male Mice. Int. J. Environ. Res. Public Health 2022, 19, 14093. [Google Scholar] [CrossRef]
- Zhang, S.; Bao, A.; Lin, X.; Jia, G.; Zhang, Q. Microplastic Accumulation in Agricultural Soils with Different Mulching Htories in Xinjiang, China. Sustainability 2023, 15, 5438. [Google Scholar] [CrossRef]
- Salikova, N.S.; Rodrigo-Ilarri, J.; Rodrigo-Clavero, M.-E.; Urazbayeva, S.E.; Askarova, A.Z.; Magzhanov, K.M. Environmental Assessment of Microplastic Pollution Induced by Solid Waste Landfills in the Akmola Region (North Kazakhstan). Water 2023, 15, 2889. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).