Featured Application
This paper describes a method for large-scale preparation of N-acetylneuraminic acid (NANA) reference materials (RMs) from fermentation products, which increases the yield of the RMs and reduces their cost. The prepared RMs can be used for quality control of related foods such as bird’s nest, health food, formula milk powder, etc.
Abstract
N-acetylneuraminic acid (NANA) is a key ingredient in bird’s nest, as well as in health products and infant formulas. However, the absence of suitable reference materials (RMs) makes it challenging to ensure the quality of these products. Currently, NANA is primarily extracted from bird’s nest, but this process is costly and difficult to scale up. In this study, we successfully prepared a large-scale yield of NANA RM from fermentation products, which increases the yield of the RM and reduces its cost. IR, MS, and NMR methods were used for the qualitative analysis of the NANA RM, and HPLC was used for homogeneity and stability testing. The F test showed that the NANA RM remained stable over a 12-month period. In collaboration with eight quantification laboratories in China, the certified value and uncertainty of the NANA RM were calculated. The certified value of the NANA RM is 98.26%, with an expanded uncertainty of 0.10% (k = 2). This prepared NANA RM can now be used for quality control in bird’s nest, health products, infant-formula-related food products, medicine, and cosmetics.
1. Introduction
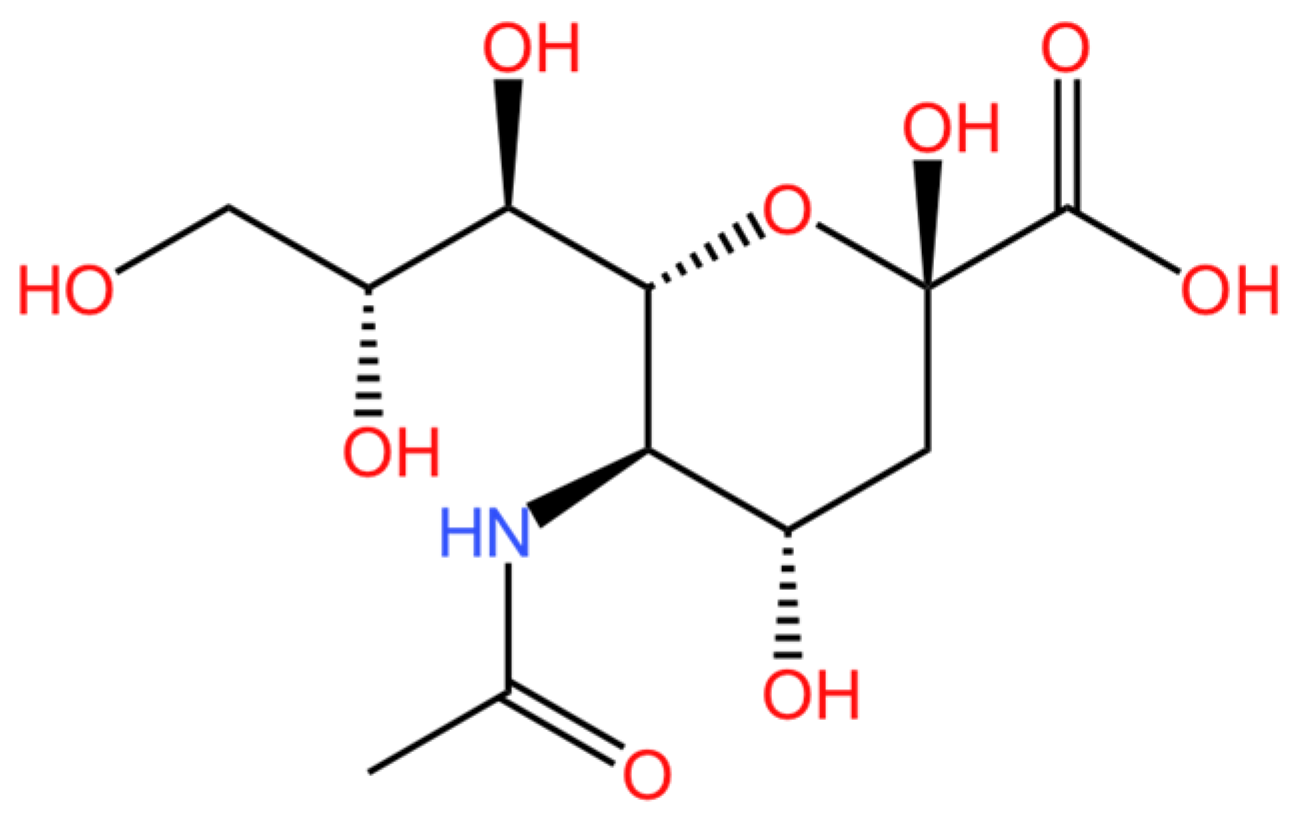
N-acetylneuraminic acid (NANA) is one of the most common sialic acids; its molecular structure is depicted in Figure 1 [1]. Sialic acids are a group of N- or O-substituted derivatives of neuraminic acids [2,3]. They are acidic amino sugars composed of nine carbon atoms and exhibit a pyranose structure [4]. Specifically, NANA is named 5-acetamido-3,5-dideoxy-d-glycerin-d-galactononosonic acid. The presence of different linkers on carbon 5 gives rise to various sialic acid derivatives. Among them, 5-acetamido-3,5-dideoxy-d-glycerin-d-galactononosonic acid and 3-deoxy-d-glycerin-d-galactononosonic acid (KDN) are two of the most significant. Other sialic acids are derived from these two primary forms [5,6].
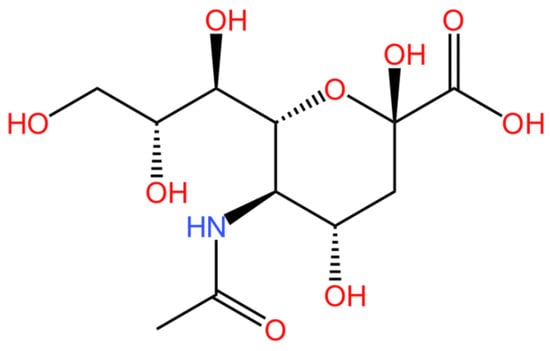
Figure 1.
The molecular structure of NANA.
Sialic acid is widely present in animal tissues and body fluids [7], and it is a crucial component of glycoproteins, oligosaccharides, and glycolipids [8]. NANA contributes to the smoothness of saliva, functions as an immune regulator [9], and plays a vital role in the development and maturation of the brain and nervous system [10]. Moreover, NANA is a natural nutrient found in bird’s nest [11], velvet antler [12], and eggs [13]. It is the primary functional ingredient in bird’s nest according to established standards. Renowned for its high nutritional value and unique texture, bird’s nest is considered a luxury food item, particularly in Asia, and is often used in gourmet cuisine and traditional medicinal soups. Its main components include proteins, carbohydrates, trace elements, and NANA.
Numerous countries regulate its use through legislation or by setting standards, permitting its addition to food as a dietary supplement [14], medicine, cosmetics, and so on. For instance, it was included in China’s list of new food resources in 2017 and has been incorporated into infant formula as a nutritional supplement in recent years [15,16]. Consequently, some manufacturers have started producing NANA products using fermentation-based methods [17].
However, certified reference materials for NANA are not readily available worldwide. As a result, the quality of NANA-related products cannot be assured. To meet analytical requirements and to ensure the accuracy, comparability, and traceability of test results, it is necessary to prepare certified reference materials for NANA.
Previously, high-purity NANA was typically obtained through the extraction and purification of bird’s nest extract [18]. Given that bird’s nest is an expensive food and the NANA content in it is not abundant, this led to a high cost for the corresponding reference materials. With advancements in the fermentation industry, sialic acid with a purity exceeding 95% can now be produced using synthetic biology and biological fermentation techniques [17]. High-purity NANA can be achieved by multiple recrystallizations of the crude NANA crystals, which significantly reduces the cost of certified reference materials for NANA. The prepared reference material of NANA can be utilized for quality control in bird’s nest, infant formula, and related products.
In this paper, a large-scale preparation method to obtain high-purity NANA from fermentation products with repeated recrystallization is introduced. Qualitative and quantitative analyses of this product are conducted. Following homogeneity tests, stability assessments, and constant value analyses, the corresponding certified reference materials for NANA are prepared.
2. Materials and Methods
2.1. Apparatus and Reagents
Instruments: 5100 high-performance liquid chromatography system (Dalian Elite Analytical Instrument Co., Ltd., Dalian, China); SK250H ultrasonic cleaner (Shanghai Kedao Ultrasonic Instrument Co., Ltd., Shanghai, China); VM200 vortex shaker (Thomson Biotechnology Co., Ltd., Beijing, China); YP3002 electronic balance (Shanghai Youke Instrument Co., Ltd., Shanghai, China); FreeZone 4.5 Plus freeze dryer (Labconco Corporation, Kansas City, MO, USA); Rotavapor® R-100 rotary evaporator (Buchi Laboratory Equipment Trading (Shanghai) Ltd., Shanghai, China); Spectrum 400 Fourier transform near-infrared spectrometer (Perkin Elmer Co., Ltd., Waltham, MA, USA); Bruker 400 MHz nuclear magnetic resonance spectrometer (Bruker Co., Ltd., Saarbrücken, Germany); ACCELA high-performance liquid chromatograph (Thermo Fisher Technology Co., Ltd., Shanghai, China); TSQ Quantum Access MAX Triple Quadrupole Mass Spectrometer (Thermo Fisher Technology Co., Ltd.); and Atlantis HILIC silica column (150 mm × 4.6 mm, 5 μm). Distilled water with a resistivity of 18.2 MΩ·cm−1 was obtained by Elga Centra R 200 water purification system (Elga Centra Co., Ltd., Buckinghamshire, UK).
Chemicals: raw material of NANA (>95.0%) was obtained from Wuhan Zhongke Optics Valley Green Biotechnology Co., Ltd. (Wuhan, China). The NANA reference standard (>98.0%) was purchased from Shanghai McLean Biochemical Technology Co., Ltd. (Shanghai, China). HPLC-grade methanol and acetonitrile were purchased from Merck Co., Ltd. (Eching, Germany). Analytical grade phosphoric acid was purchased from Sinopharm Chemical Reagent Beijing Co., Ltd. (Beijing, China).
2.2. Extraction and Purification of High Purity NANA
The raw material of NANA was made from food-grade glucose and corn syrup, fermented by Escherichia coli. This raw material of NANA from biological fermentation was dissolved in water to prepare a solution with a concentration of 400 g/L. The water temperature was controlled at 60 °C. The solution was quickly filtered with a 0.22 μm membrane and crystallized at 4 °C for 24 h. After solid–liquid separation, the crystal was washed with 75% ethanol aqueous solution, and vacuum dried at 60 °C for 24 h. Then, 5 times volume of anhydrous ethanol was added into the dried crystal; this was stirred at 60 °C for 5 h, and the crystal was filtered out. The crystal was washed with 75% ethanol aqueous solution and vacuum dried at 60 °C for 12 h. The bulk crystal was crushed into powder, and vacuum dried at 60 °C for 6 h to obtain the high-purity NANA. The purity of this product reaches to 98.5%. After qualitative analysis, the high-purity NANA was separately packed into clean and dry 2 mL brown sample bottles, each bottle containing 10 mg, and refrigerated at 4 °C.
2.3. Sample Packaging and Storage
Use 2 mL brown bottles for packaging. Divide the sample into 200 bottles in a relatively independent and clean space, with each bottle containing 10 mg. Weigh them on a one millionth scale, with a total of 200 bottles for each sample, numbered from 1 to 200. The packaged sample bottles are placed in a dryer and stored in a −20 °C freezer.
2.4. Qualitative Analysis
The high-purity NANA was qualitatively analyzed by IR, MS, and NMR.
The near-infrared spectrum was determined by Fourier transform near-infrared spectrometer, and the infrared spectrum was obtained by KBr compression method.
Ultra-high-resolution time-of-flight mass spectrometer was used, and determination was carried out in both positive and negative ion mode. The scanning range (m/z) was 100–1000.
The hydrogen and carbon NMR spectrums were measured with a 400 MHz NMR spectrometer. The deuterated reagent was DMSO-d6 (containing TMS) at 25 °C.
2.5. Quantitative Analysis
A 5100 high-performance liquid chromatography system (Dalian Elite Analytical Instrument Co., Ltd., Dalian, China) equipped with a Waters Atlantis HILIC silica column (150 mm × 4.6 mm, 5 μm) was used for quantitative analysis of the high-purity NANA. The temperature of the column was maintained at 25 °C, and the injection volume was 10 μL. Mobile phase was a solution of acetonitrile/0.1% phosphoric acid (95:5) with a flow rate of 1 mL/min. The detection wavelength was 203 nm. The purity of the sample was quantitatively analyzed by the normalization method for the sampled chromatographic peak area.
2.6. Determination of Moisture
We accurately weighed 20 mg of high-purity NANA, dissolved it in anhydrous methanol, and directly measured it using a V30S Karl Fischer moisture analyzer (METTLER TOLEDO, Zurich, Switzerland).
2.7. Determination of Ash
The determination of ash of NANA was carried out on a TGA 8000 (PE, Waltham, MA, USA) under an air atmosphere. The temperature ranged from 40 °C to 800 °C with a heating rate of 30 °C/min, and the temperature was held at 800 °C for 5 min. The determination was performed with parallel measurement 3 times.
2.8. Determination of Solvent Residue
The solvent residue in NANA was determined by a SHIMADZU, Nexis GC-2030 gas chromatography with headspace injection (Shimadzu Manufacturing Co., Ltd., Kyoto, Japan). The experimental conditions were as follows: chromatographic column: SHIMADZU Rtx-624 (30.0 m × 0.32 mm × 1.80 μm); forward sample port temperature: 200 °C; detector temperature: 220 °C; injection volume: 1 mL; the diversion ratio is 10:1; column flow rate: 2.0 mL/min; heating program: hold at 40 °C for 5 min, raise to 180 °C at a rate of 15 °C/min, and hold for 2 min; internal standard was n-propanol. The measurement was performed in three parallel replicates, and the average results were separately calculated for the contents of methanol and ethanol.
3. Results and Discussion
3.1. Qualitative Analysis
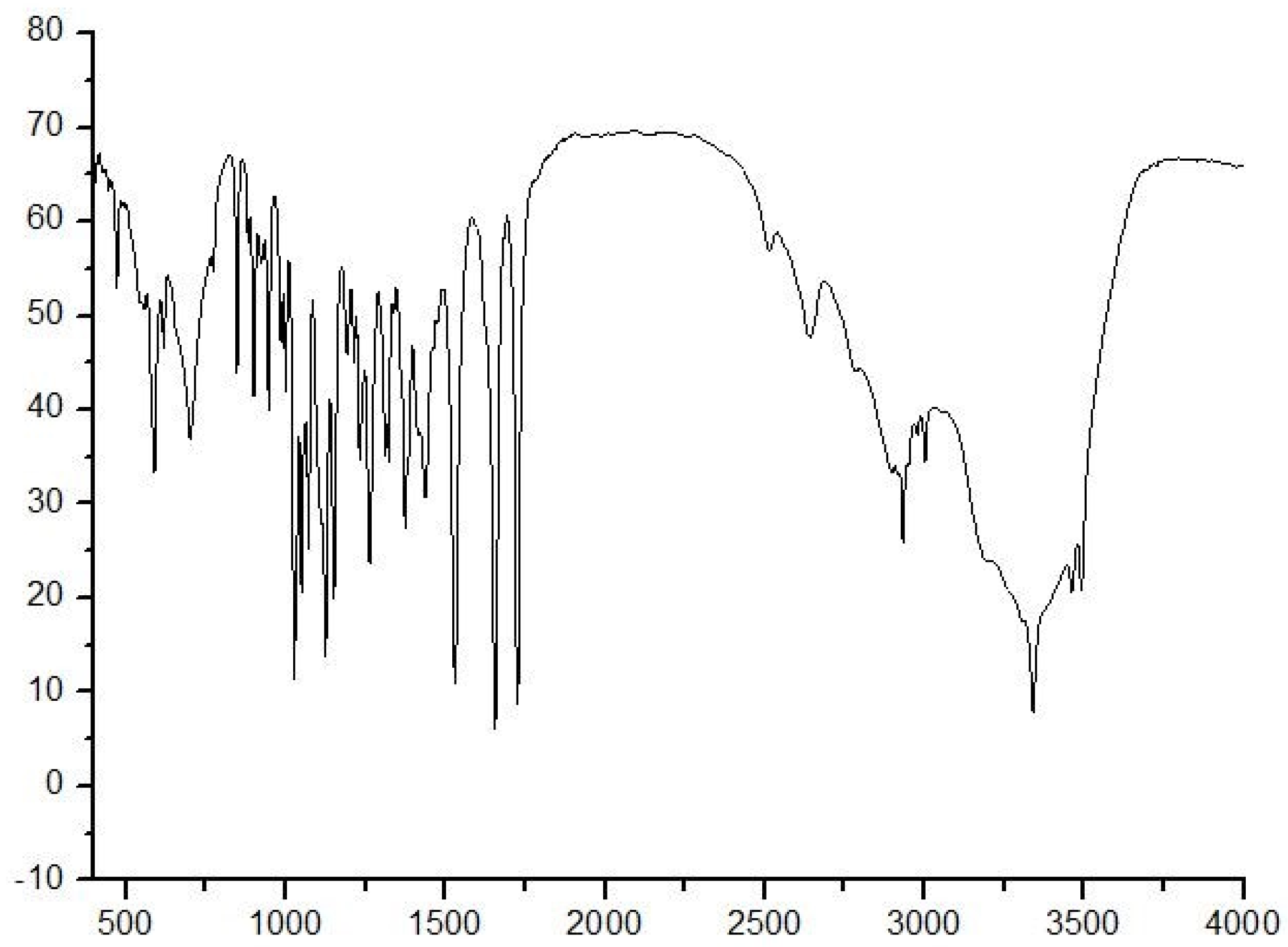
IR, MS, and NMR methods were used to characterize NANA. The IR spectrum of NANA is shown in Figure 2. For the IR of NANA, the peak at 3389.89 cm−1 represents the stretching vibration peak of the N–H bond, and the peak at 3600−3400 cm−1 is the stretching vibration peak of the hydroxyl group. The peak at 3300–2500 cm−1 is the stretching vibration of the O–H bond in the carboxyl group, which produces uneven and wide absorption peaks. The peak at 2933.72 cm−1 represents the stretching vibration of the C–H bond. The peak at 1722.95 cm−1 represents the stretching vibration of carbonyl group on carboxyl group. The peak at 1654.44 cm−1 represents the stretching vibration of carbonyl group on acylamino group. The peak at 1122 cm−1 represents the stretching vibration of the C–O–C bond. The peak at 1024 cm−1 represents the stretching vibration of the C–O bond, which is linked to the hydroxyl group.
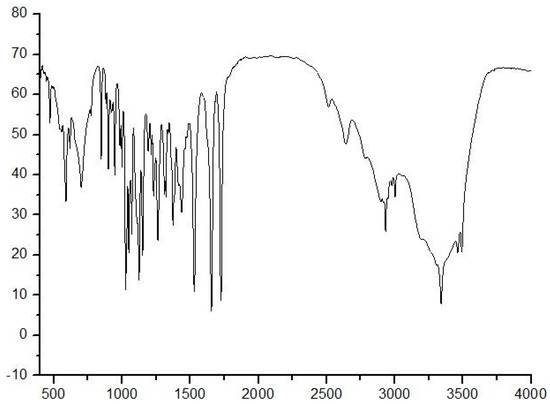
Figure 2.
The IR spectrum of NANA.
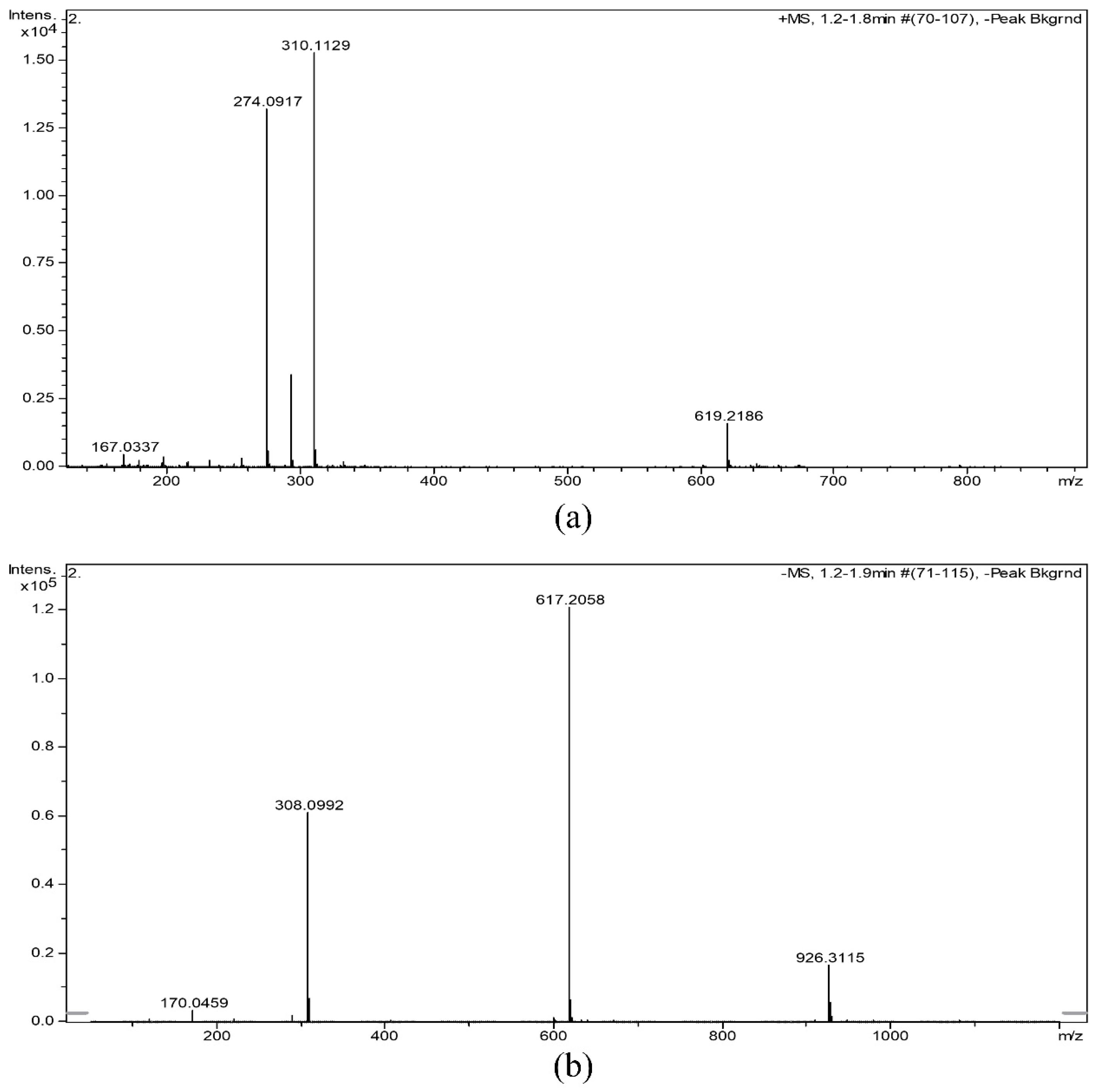
The molecular weight of NANA is 309.2691. The MS spectrum of NANA is displayed in Figure 3, showing that the m/z of [M + H]+ was 310.1129, the m/z of [M − H]+ was 308.0992, the m/z of [2M + H]+ was 619.2186, the m/z of [2M − H]+ was 617.2058, and the m/z of [3M − H]+ was 926.3115, which was consistent with the molecular weight of NANA. At the same time, there may be a tiny amount of dimer and trimer in the sample.
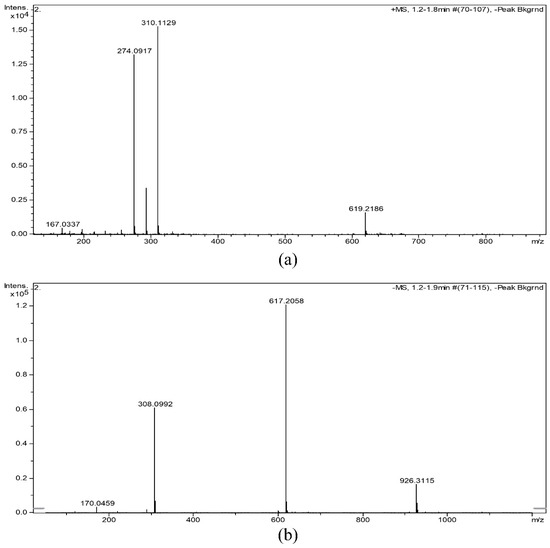
Figure 3.
(a) [M + H]+ and (b) [M − H]+ MS spectrum of NANA.
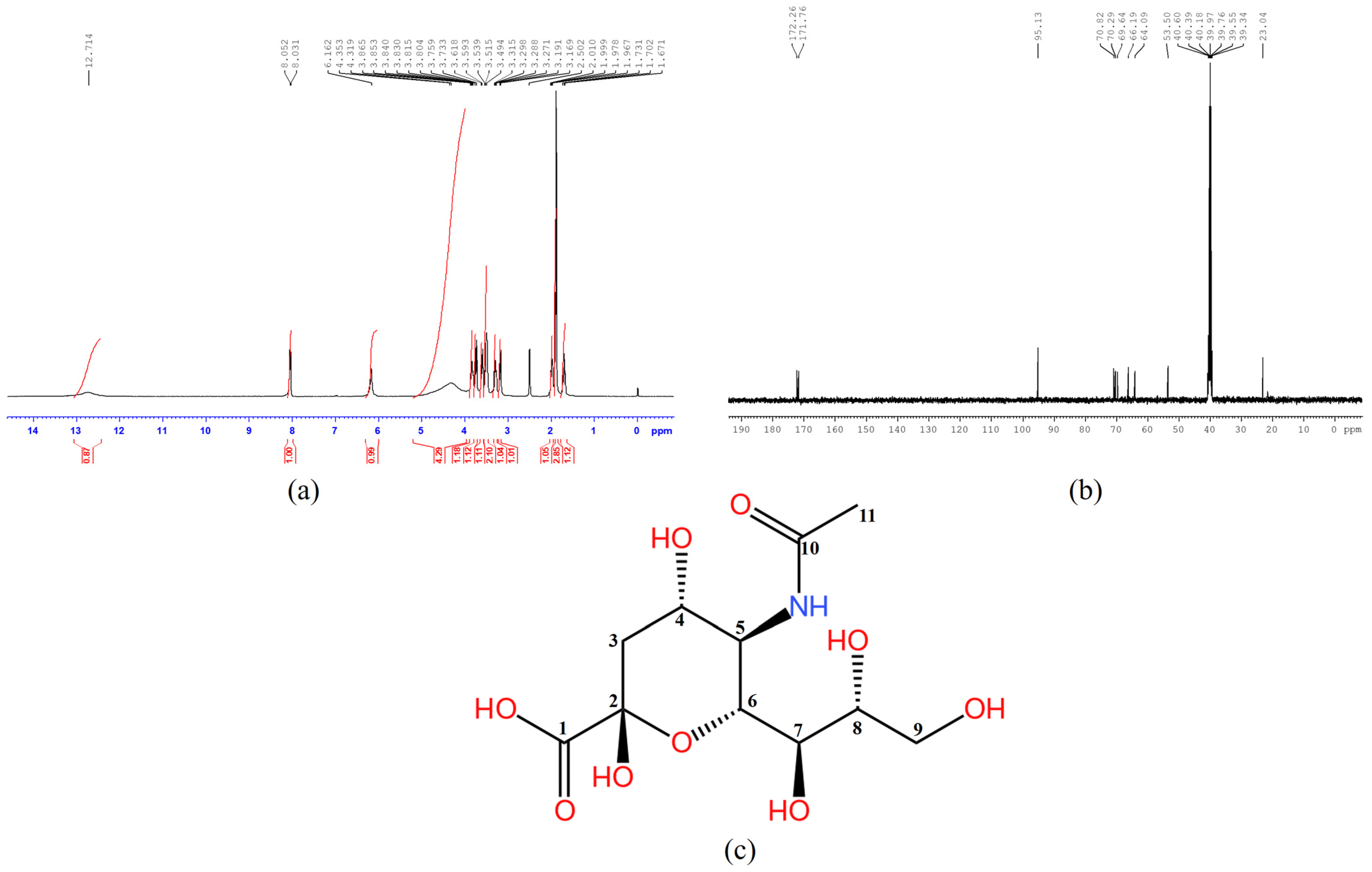
1HNMR (400 MHz, DMSO-d6) of NANA is shown in Figure 4a. 13CNMR (400 MHz, DMSO-d6) of NANA is shown in Figure 4b. The structure of NANA with atom numbering is shown in Figure 4c. The analysis of NMR data is shown in Table 1.
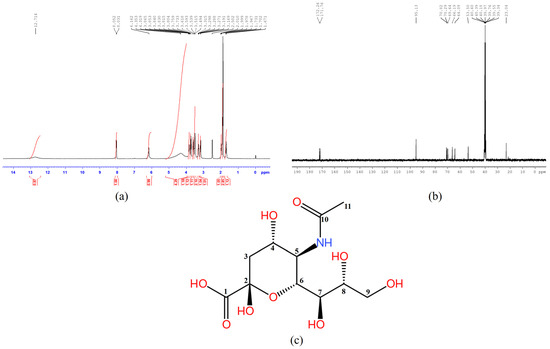
Figure 4.
The 1H (a) and 13C (b) NMR spectrum, and the structure of NANA with atom numbering (c).

Table 1.
NMR data of NANA (deuterated solvent was DMSO-d6).
Based on literature data and comparison with databases [19], the sample was identified as NANA.
3.2. Optimization of HPLC
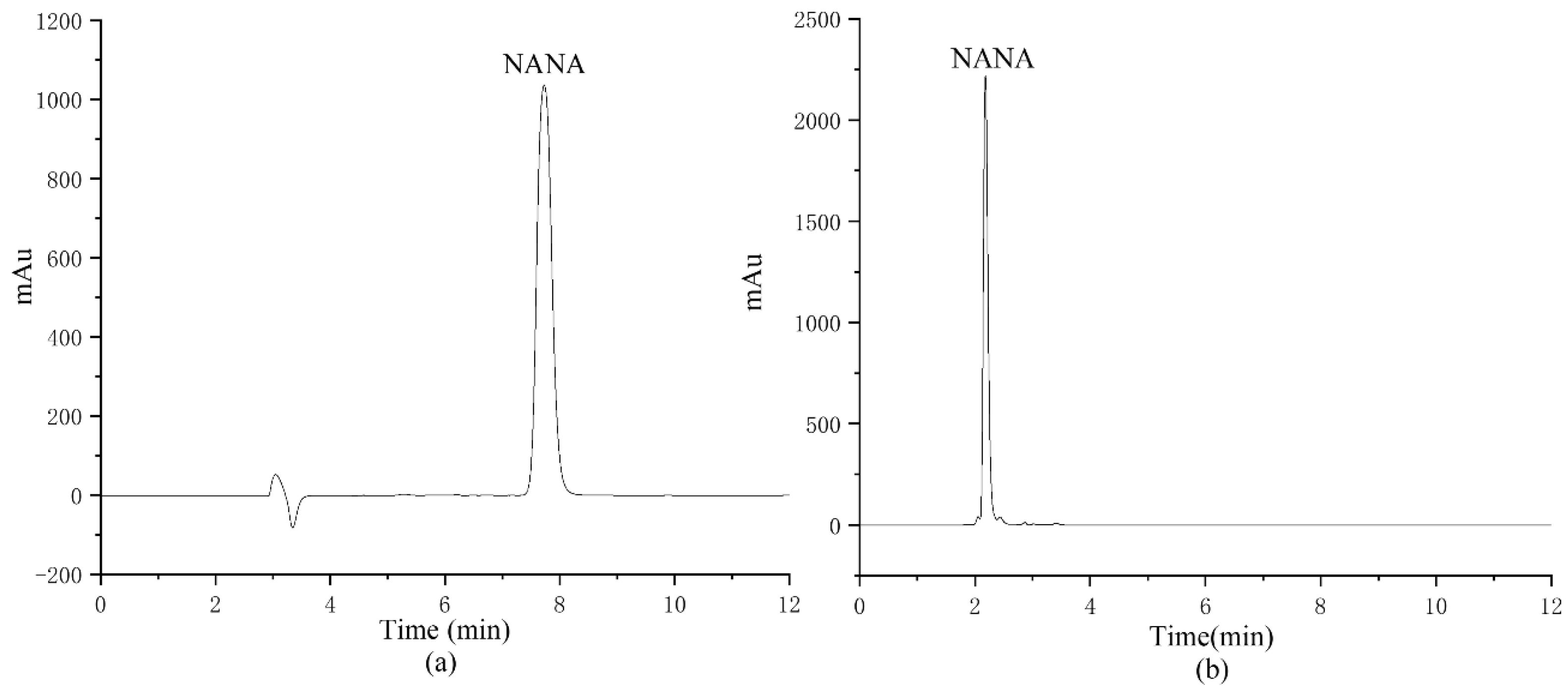
The column was optimized, and a Waters XTerra RP C18 column (150 mm × 4.6 mm, 5 μm) and a Waters Atlantis HILIC Silica column (150 mm × 4.6 mm, 5 μm) were chosen for comparison, respectively, as shown in Figure 5. Due to the high polarity of NANA, it has a very poor retention on a C18 column, while the HILIC column has a better retention effect on NANA. Therefore, the separation results of NANA on the C18 column cannot be quantified using the area normalization method. The HILIC column was selected for the quantitative analysis of NANA in the following experiments.
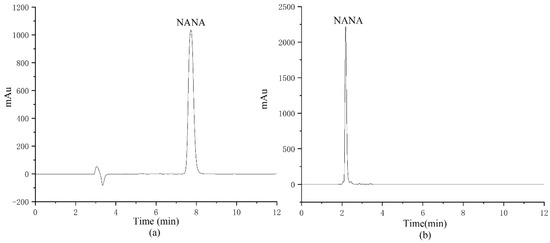
Figure 5.
The NANA chromatogram comparison of different columns: (a) the chromatogram of NANA by Waters Atlantis HILIC Silica column; (b) the chromatogram of NANA by Waters XTerra RP C18 column.
The mobile phase was also optimized. Methanol with 0.1% phosphoric acid (95:5) and acetonitrile with 0.1% phosphoric acid (95:5) were chosen as mobile phases for comparison. The results showed that the mobile phase of methanol with 0.1% phosphoric acid was less effective in the separation of N-acetylneuraminic acid. Acetonitrile with 0.1% phosphoric acid water mobile phase shows a better separation effect and ideal peak shape, so acetonitrile with 0.1% phosphoric acid (95:5) was selected as the mobile phase in the following experiments.
3.3. Determination of Moisture
This experiment used the Karl Fischer titration method to determine the moisture content of NANA samples. The experiment was conducted in parallel three times, and the measured moisture value of NANA was 0.36%. The results are shown in Table 2.

Table 2.
Moisture results of NANA.
The uncertainty caused by moisture mainly concerns the uncertainty generated by repeated measurements, which belongs to Class A standard uncertainty. It is calculated according to the following process:
The uncertainty calculation is as follows:
After measurement, the moisture content of NANA was 0.36% with an uncertainty of 0.01%.
3.4. Ash Content Determination
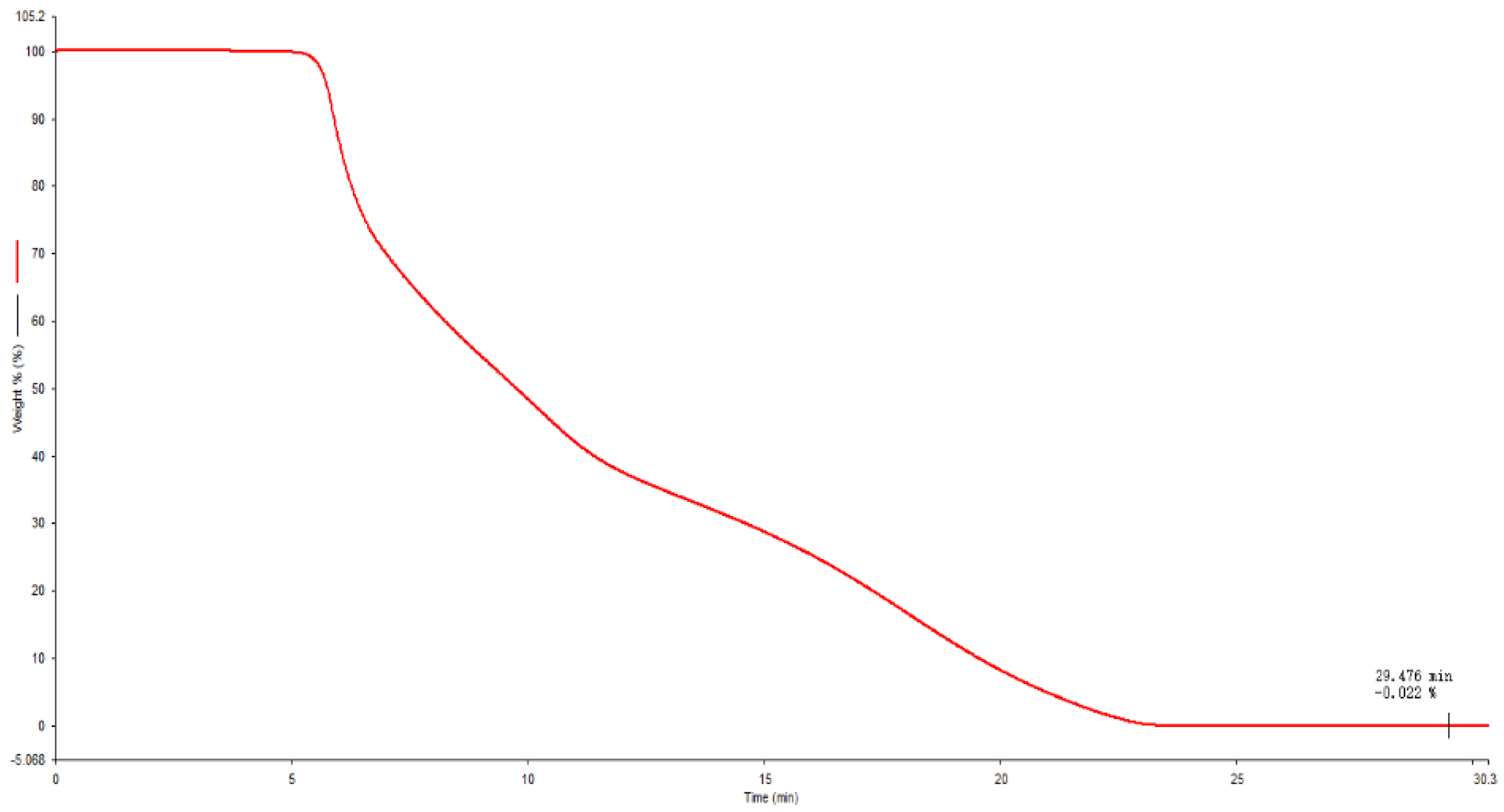
The experiment used a thermogravimetric analyzer TGA 8000 to measure the ash content of standard samples in an air atmosphere. The heating rate was 30 °C/min, the set temperature was raised from 40 °C to 800 °C, and the holding time was 5 min. Three parallel measurements were taken, and the ash content value obtained was 0.026%. The results are shown in Table 3 and Figure 6.

Table 3.
Ash results of NANA.
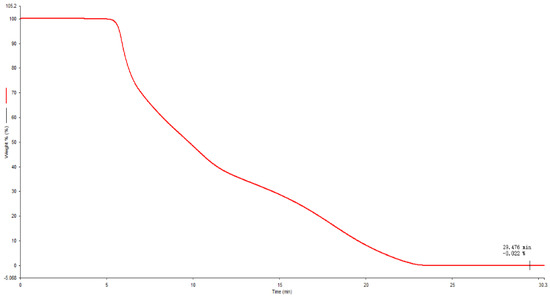
Figure 6.
TGA determination curve of NANA.
The uncertainty caused by ash content mainly concerns the uncertainty generated by repeated measurements, which belongs to Class A standard uncertainty. It is calculated according to the following process:
The uncertainty calculation is as follows:
The ash content of NANA was 0.026% with an uncertainty of 0.004%.
3.5. Determination of Residual Solvent Content
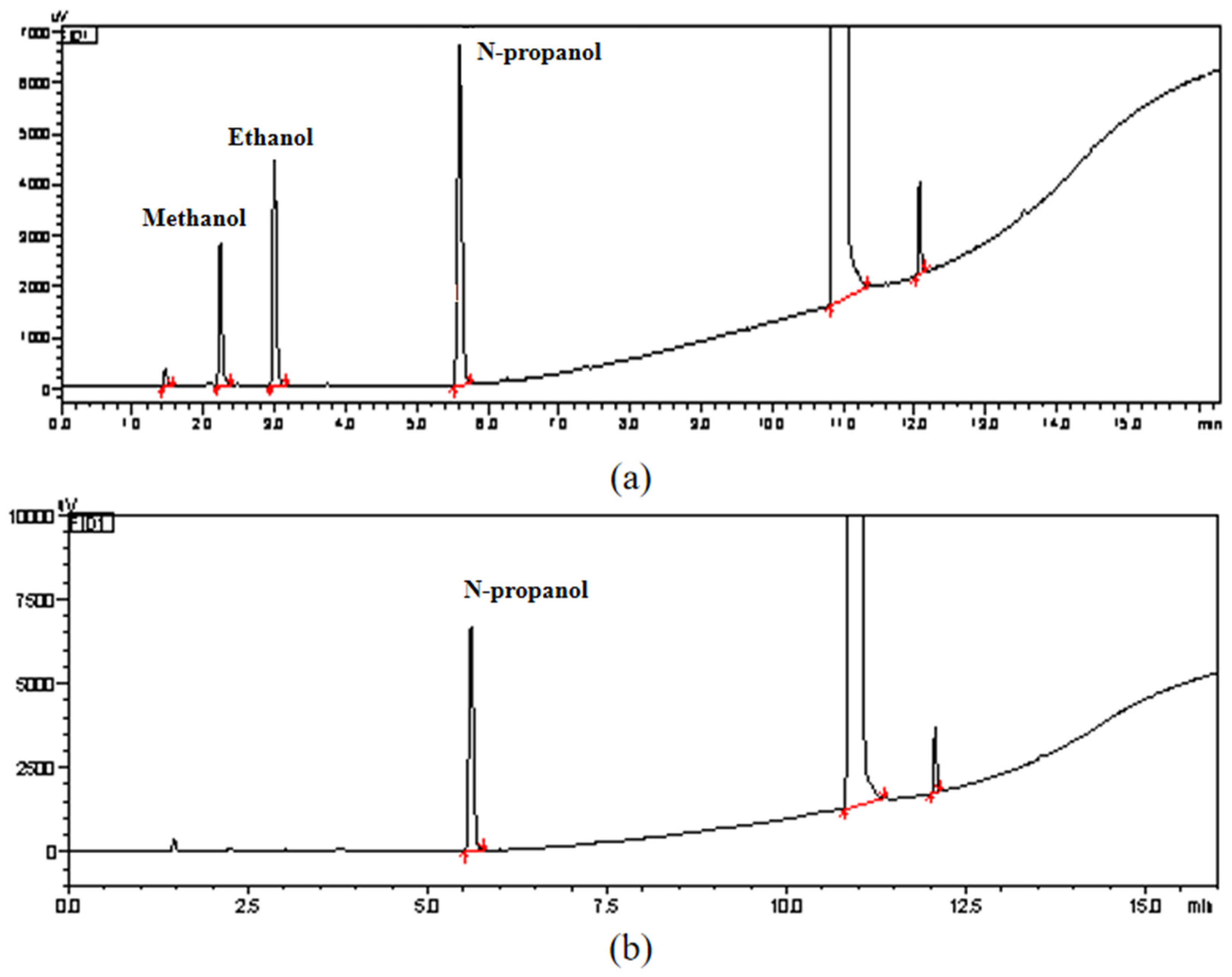
The solvent residue in the sample was determined by the solvent residue determination method in accordance with General Rule 0861 of the 2020 edition of the Pharmacopoeia of the People’s Republic of China. Figure 7 shows the chromatograms of methanol and ethanol standards and samples. It can be seen from the figure that no residual solvents of methanol and ethanol were detected in this sample of NANA. Therefore, the residual solvent content and uncertainty in this sample can be ignored.
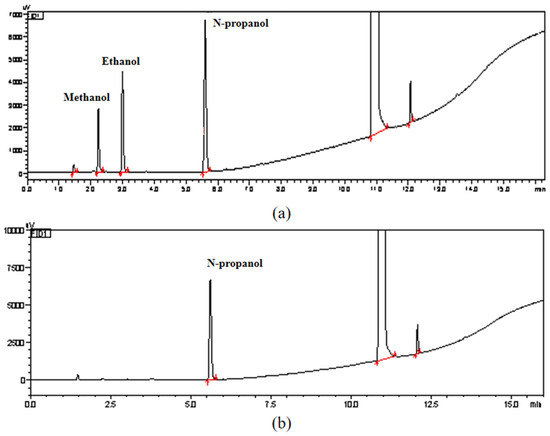
Figure 7.
(a) Chromatograms of methanol, ethanol, and n-propanol standards; (b) chromatogram for the determination of methanol and ethanol in NANA samples.
3.6. Homogeneity Test
The homogeneity test of NANA reference materials was carried out according to ISO Guide 35 [20]. From the 200 bottles of packaged samples, 20 bottles of samples were randomly taken out. The sample bottle numbers taken out were measured three times in a positive sequence, a reverse sequence, and a random sequence. Each bottle was detected three times. The results are shown in Table 4 and Table 5.

Table 4.
The homogeneity test measurement data of NANA (n = 3), %.

Table 5.
Analysis results of homogeneity study of NANA.
Check the F critical value table, obtain F0.05(19, 40) = 2.03, and F = MSintra-bottle/MSinter-bottle = 1.99 < F0.05(19, 40), indicating that the standard substance is homogeneous.
The variance between bottles, S2A, is calculated according to Equation (1) (n0 = 3):
S2A = (MSinterbottle − MSintrabottle)/n0
The standard deviation between bottles, Sbb, is the square root of the variance: Sbb = SA = 0.0155.
The repeatability standard deviation Sr is calculated from the square root of the MS bottle, Sr = 0.027.
3.7. Stability Investigation
The stability of the NANA reference material was checked at 0, 1, 3, 6 and 12 months, according to the principle of first density and then sparseness. The author conducted a stability test on the prepared NANA reference material under different time periods for a period of 12 months. The results are shown in Table 6.

Table 6.
Stability test results for NANA.
Analyzing the data in Table 4 and using a straight line as an empirical model to predict whether there is a significant change in the slope value, the stability change of a reference standard can be predicted. Slope β1 is calculated from Equation (2):
- —regression coefficient, the slope of the line;
- —point in time I;
- —average of all time points, = 1.49;
- —detected value at time point I;
- —mean of all test results, y = 98.58;
- —number of stability measurements, n = 8.
Intercept: β1 = − = 98.60.
Standard deviation of a point on a straight line:
Uncertainty associated with the slope :
A distribution factor with n − 2 degrees of freedom and p = 0.95 (95% confidence level) has a t = 2.441, and the slope is insignificant because |β1| < t0.95, n − 2 − s(β1); thus, no instability is observed for this sample. Uncertainty of long-term stability at 6 months (T = 6): .
The above results indicate that no significant instability of NANA standards was observed over a 12-month period. Longer stability periods will be determined based on continued testing.
3.8. Valuation Results
The constant value testing process of NANA reference material was according to ISO Guide 35 [20] standard requirements, through the collaboration of multiple laboratories to determine the value of the experiment. There were eight laboratories participating in the testing. Each laboratory measured two bottles, and each bottle was measured three times, providing six independent repeatable sets of measurement data. All laboratory selection methods were based on methods that have been tried and tested and on the individual laboratory. The experimental methods were consistent. The quantitative data and the results of their ANOVAs are shown in Table 7 and Table 8, respectively.

Table 7.
HPLC purity determination results of NANA.

Table 8.
ANOVA results for purity determination of NANA.
Total average :
Uncertainty associated with the overall mean:
- —intra-bottle variance for each measurement: ;
- m—number of participating laboratories;
- —variance between bottles measured m times;
- N—number of qualitative laboratory measurements.
3.9. Uncertainty Assessment Results
The uncertainty consists of three parts: the uncertainty introduced by the inhomogeneity of the standard substance; the uncertainty introduced by the instability of the standard substance; and the uncertainty introduced by the process of determining the value of the standard substance.
Uncertainty introduced by homogeneity 0.0155%.
Uncertainty introduced by stability: the stability study of this standard was conducted over a period of six months, so its uncertainty .
Uncertainty in the valuation results .
Synthetic standard uncertainty
Extended uncertainty U = ku = 0.10%, k = 2.
3.10. Expression of the Results of the Determinations
The moisture content of NANA was 0.36%, the ash content was 0.026%, and the residual solvent content can be ignored. Therefore, the purity of NANA reference material was 98.65 − 0.36 − 0.03% ≈ 98.26%, and the extended uncertainty is 0.10% (k = 2).
3.11. Economic Analysis
The high-purity NANA used in this study was a biological fermentation product with the advantages of high purity and high yield. The unit price can reach USD 0.5/g, and it can be made into a standard substance at a cost of about USD 1.5/g. Due to the high content of NANA in bird’s nest, ranging from 7% to 12% [21], the cost of extracting high-purity NANA is approximately USD 30/g. When used as a standard substance, the cost will be higher, making this method more economical.
4. Conclusions
A large amount of NANA reference material was developed from fermentation products with repeated recrystallization in this study. The quality of prepared high-purity NANA products was determined by IR, MS, and NMR, which confirmed that the prepared high-purity product was NANA. The homogeneity test and stability investigation were performed by HPLC. The results of the investigation were judged by statistical methods and met the requirements of homogeneity and stability of the standard. The uncertainty of the calibration results was evaluated to ensure the accuracy and reliability of the values and uncertainty when the user uses the standards in different packages at different times and in different spaces, which is important for the accurate determination of NANA. The prepared reference material can meet the requirements of analysis, testing, and quality control of foods, medicines, and cosmetics containing NANA, and provide technical support and guaranteed traceability for the accuracy, comparability, and traceability of the test results. Most importantly, through economic analysis, the cost of the NANA reference material prepared by this study was 60 times lower than that prepared by traditional methods, demonstrating the significant economic value of this method.
Author Contributions
T.L. performed the data analysis of certification and wrote the draft of this paper; he also applied for financial support for the project. X.C. performed the purifying experiments of NANA and was involved in the valuation experiment. L.Y. was involved in the experiments of CBD certification and the data analysis of certification. W.W. performed the uniformity and stability inspection, and the data analysis of certification. J.Y. revised the manuscript. J.W. was involved in the data analysis of certification. D.X. revised the manuscript and provided guidance for this project. All authors have read and agreed to the published version of the manuscript.
Funding
This work was sponsored by the National Key Research and Development Program of China, Ministry of Science and Technology, China (2023YFF1104900), and the youth top talent project of the State Administration for Market Regulation, State Administration for Market Regulation, China (QNBJ201313).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are contained within this article.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| NANA | N-acetylneuraminic acid |
| RM | Reference material |
| IR | Infrared |
| MS | Mass spectrum |
| NMR | Nuclear magnetic resonance |
| UV | Ultraviolet |
| HPLC | High-performance liquid chromatography |
| ANOVA | Analysis of Variance |
References
- Comb, D.G.; Roseman, S. The sialic acids I. The structure and enzymatic synthesis of N-acetylneuraminic acid. J. Biol. Chem. 1960, 235, 2529–2537. [Google Scholar] [CrossRef]
- Zhao, M.; Zhu, Y.; Wang, H.; Zhang, W.; Mu, W. Recent advances on N-acetylneuraminic acid: Physiological roles, applications, and biosynthesis. Synth. Syst. Biotechnol. 2023, 8, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Cheeseman, J.; Kuhnle, G.; Spencer, D.I.R.; Osborn, H.M.I. Assays for the identification and quantification of sialic acids: Challenges, opportunities and future perspectives. Bioorganic Med. Chem. 2021, 30, 115882. [Google Scholar] [CrossRef]
- Otsuka, H.; Uchimura, E.; Koshino, H.; Okano, T.; Kataoka, K. Anomalous Binding Profile of Phenylboronic Acid with N-Acetylneuraminic Acid (Neu5Ac) in Aqueous Solution with Varying pH. J. Am. Chem. Soc. 2003, 125, 3493–3502. [Google Scholar] [CrossRef] [PubMed]
- Yeşilyurt, B.; Şahar, U.; Deveci, R. Determination of the type and quantity of sialic acid in the egg jelly coat of the sea urchin Paracentrotus lividus using capillary LC-ESI-MS/MS. Mol. Reprod. Dev. 2015, 82, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, B.; Ma, M.; Cai, Z. A Sensitive and Selective Fluorimetric Method of Quick Determination of Sialic Acids in Egg Products by Lectin-CdTe Quantum Dots as Nanoprobe. J. Food Sci. 2014, 79, C2434–C2440. [Google Scholar] [CrossRef]
- Lacomba, R.; Salcedo, J.; Alegria, A.; Lagarda, M.J.; Barbera, R.; Matencio, E. Determination of sialic acid and gangliosides in biological samples and dairy products: A review. J. Pharm. Biomed. Anal. 2010, 51, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, J.; Liu, Y.; Xu, D. Sialic acid metabolism as a potential therapeutic target of atherosclerosis. Lipids Health Dis. 2019, 18, 173. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Thuy-Boun, P.S.; Pfeiffer, W.; Vartabedian, V.F.; Torkamani, A.; Teijaro, J.R.; Wolan, D.W. Identifcation of an N-acetylneuraminic acid-presenting bacteria isolated from a human microbiome. Sci. Rep. 2021, 11, 4763. [Google Scholar]
- Galuska, C.E.; Rudloff, S.; Kuntz, S.; Borsch, C.; Reutzel, M.; Eckert, G.; Galuska, S.P.; Kunz, C. Metabolic fate and organ distribution of 13C-3′-sialyllactose and 13C-N-acetylneuraminic acid in wild-type mice—No evidence for direct incorporation into the brain. J. Funct. Foods 2020, 75, 104268. [Google Scholar] [CrossRef]
- Wong, Z.C.F.; Chan, G.K.L.; Wu, K.Q.Y.; Poon, K.K.M.; Chen, Y.; Dong, T.T.X.; Tsim, K.W.K. Complete digestion of edible bird’s nest releases free N-acetylneuraminic acid and small peptides: An efficient method to improve functional properties. Food Funct. 2018, 9, 5139–5149. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.H.; Chun, E.H.; Hyun, J.H.; Choi, S.W.; Su, S.T.; Kim, W.; Kim, D.O.; Kim, B.Y.; Baik, M.Y. Optimization of hot water extraction and ultra high pressure extraction for deer antler. Food Sci. Biotechnol. 2015, 24, 507–512. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Gou, X.L.; Lin, N.; Le, S.F.; Du, N.; Yan, H.; Zhang, J.H. Determination of N-acetylneuraminic acid in poultry eggs by ultra performance liquid chromatography–tandem mass spectrometry. J. Anal. Chem. 2017, 72, 886–889. [Google Scholar] [CrossRef]
- Zhang, Y.; Imam, M.U.; Ismail, M.; Ismail, N.; Ideris, A.; Abdullah, M.A. High fat diet-induced inflammation and oxidative stress are attenuated by N-acetylneuraminic acid in rats. J. Biomed. Sci. 2015, 22, 96. [Google Scholar]
- Wu, C.; Xia, L.; Liu, L.; Qu, F.; Sun, Z.; Zhao, X.; You, J. A sensitive and efficient method for determination of N-acetylhexosamines and N-acetylneuraminic acid in breast milk and milk-based products by high-performance liquid chromatography via UV detection and mass spectrometry identification. J. Chromatogr. B 2016, 1011, 14–23. [Google Scholar]
- Zhu, W.; Chen, X.; Yuan, L.; Wu, J.; Yao, J. Degradation Kinetics and Shelf Life of N-acetylneuraminic Acid at Different pH Values. Molecules 2020, 25, 5141. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.I.; Zhang, X.; Lv, X.; Basharat, S.; Shahbaz, U.; Li, J.; Du, G.; Liu, L.; Liu, Y. Enzymatic production of N-acetylneuraminic acid: Advances and perspectives. Syst. Microbiol. Biomanuf. 2022, 2, 130–146. [Google Scholar] [CrossRef]
- Ling, A.J.W.; Chang, L.S.; Babji, A.S.; Latip, J.; Koketsu, M.; Lim, S.J. Review of sialic acid’s biochemistry, sources, extraction and functions with special reference to edible bird’s nest. Food Chem. 2022, 367, 130755. [Google Scholar] [CrossRef] [PubMed]
- Klepach, T.; Zhang, W.; Carmichael, I.; Seianni, A.S. 13C−1H and 13C−13C NMR J-couplings in 13C-labeled N-acetyl-neuraminic acid: Correlations with molecular structure. J. Org. Chem. 2008, 73, 4376–4387. [Google Scholar] [CrossRef] [PubMed]
- ISO GUIDE 35:2017; Reference Materials-Guidance for Characterization and Assessment of Homogeneity and Stability. International Organization for Standardization: Geneva, Switzerland, 2017.
- Song, Y.; Lin, Y.; Zhang, N. Detection Status and Composition Difference of Nutritional Components in Different Types of Edible Bird’s Nests. Food Nutr. China 2024, 30, 42–51. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).