Abstract
Background: The aim of the study was to identify and critically evaluate the best available evidence on the impact of physical exercise on patients with primary Sjögren’s syndrome. Methods: Studies were searched in four electronic databases (PubMed, Web of Science, Scopus, and SportDiscus) from their inception up to September 2024. The methodological quality of the included studies was assessed using the 10-point Physiotherapy Evidence Database (PEDro) scale and the Methodological Index for Non-Randomized Studies (MINORS). Results: A total of four randomized controlled trials and one comparative study were analyzed. The training programs evaluated varied in duration, ranging from 12 to 28 weeks. Exercise was found to have a positive intra-group impact on fatigue, quality of life, and functional capacity. However, exercise does not demonstrate superior effects compared to standard treatment for improving quality of life and disease impact. Conclusions: It is essential to increase the number of studies involving individuals with primary Sjögren’s syndrome across various exercise conditions to more comprehensively evaluate the potential benefits.
Keywords:
fatigue; exercise; quality of life; metabolic syndrome; physical activity; sleep; anxiety; depression 1. Introduction
Sjögren’s syndrome is considered one of the most common systemic autoimmune diseases with a pooled prevalence of 60–82 cases per 100,000 inhabitants; and a female-male ratio of 10:1 [1]. Sjögren’s syndrome is identified by symptoms such as keratoconjunctivitis sicca (dry eyes) [2] and xerostomia (dry mouth) [3], often accompanied by swelling of the salivary glands and lacrimal glands [4]. Other common symptoms include fatigue, sleep disturbances [5], musculoskeletal pain [6], and gastrointestinal disturbances [7]. Primary Sjögren’s syndrome (pSS) occurs as an isolated autoimmune condition [8,9], while secondary Sjögren’s syndrome (sSS) is associated with other autoimmune diseases, such as rheumatoid arthritis [10], systemic sclerosis [11], systemic lupus erythematosus [12,13], or dermatomyositis [14].
This pathology negatively affects the joints, skin, lungs, and peripheral nervous system [15], with disease activity often coupled with fatigue, depression, and pain, leading to a reduced capacity for performing everyday activities and, consequently, an impaired health-related quality of life [16].
Currently, there is no cure for pSS, and the available treatments are primarily symptomatic with limited efficacy [17]. In this context, patients often turn to non-pharmacological therapies (i.e., oral lubricant, acupuncture, lacrimal punctum plugs psychodynamic group therapy) [18], hoping that their use can reduce the impact of symptoms on functionality and lead to an improvement in quality of life [18]. In this regard, physical exercise is widely recognized as a low-cost, non-pharmacological treatment capable of reducing symptom severity in several chronic diseases [19], and it could be considered a therapy of interest for patients with pSS for several reasons.
Firstly, in patients with pSS, fatigue is a disabling symptom that is strongly associated with declining physical function, yet it remains an unmet health need [20,21]. Fatigue, often regarded as the most challenging symptom by pSS patients, is believed to result from genetic and inflammatory factors. It is also thought to be linked to reduced fitness levels and lower physical activity [22]. In this regard, exercise training has consistently demonstrated positive effects in improving both fatigue and physical function in individuals with other autoimmune conditions [23,24,25]. Therefore, current clinical guidelines strongly recommend the incorporation of exercise into the management strategies for addressing fatigue in patients with pSS [26]. However, it is worth noting that only a limited number of studies have specifically investigated the direct effects of exercise interventions in individuals with pSS [27].
Secondly, patients with pSS often experience physical (e.g., myalgia, arthralgia) and mental (e.g., anxiety, depression) health issues, leading to a lower quality of life compared to the general population [28]. In this regard, exercise has been shown to have a beneficial impact on the quality of life of individuals with autoimmune conditions, positively affecting both their physical and mental health [29,30,31]. Finally, pSS is associated with a markedly increased risk for the development of non-Hodgkin lymphoma [32], a type of malignancy that has been found to be inversely related to physical activity levels, suggesting that higher levels of physical activity may help reduce this risk [33]. In this regard, previous research has indicated that physical activity levels are lower in individuals with pSS [34], while some authors have emphasized the need to increase these levels beyond the recommended minimum to mitigate the comorbidities associated with the condition [35].
In this context, research focusing on exercise interventions tailored for pSS is crucial, as exercise represents a promising non-pharmacological strategy for managing the diverse symptoms of this condition. Before recommending exercise as a therapeutic approach for patients with pSS, rehabilitation professionals must rely on accurate and current data regarding its potential benefits and side effects to ensure its safe and effective application. One way to achieve this is by conducting systematic reviews that compile and critically analyze the available scientific evidence on the topic. To the best of the authors’ knowledge, no systematic review has been published specifically addressing the impact of exercise on patients with pSS. The only identified work on the topic was a narrative review [36]. However, it had several limitations. First, it lacked a quality assessment of the included studies. Second, it did not exclusively focus on patients with pSS. Lastly, it included only a single non-randomized comparative study on pSS. Therefore, the aim of the study was to identify and critically evaluate the best available evidence on the impact of physical exercise on this population.
2. Materials and Methods
This systematic review was conducted in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [37]. Furthermore, the protocol for this review was registered with the Open Science Framework (OSF, https://doi.org/10.17605/OSF.IO/C238N (accessed on 29 October 2024)).
2.1. Search Strategy
A comprehensive search was performed across four electronic databases: Scopus, Web of Science, SPORTDiscus, and MEDLINE/PubMed, covering the period from their inception to September 2024. Additionally, a manual search was conducted in the PEDro database [38], and the first 200 references retrieved from Google Scholar were also reviewed.
2.2. Eligibility Criteria
Eligible studies for inclusion in this review were randomized controlled trials (RCTs) or comparative studies that examined the effects of exercise on participants with primary Sjögren’s syndrome. The inclusion and exclusion of studies were guided by the Population, Intervention, Comparison, and Outcome (PICO) framework, as summarized in Table 1. Studies were eligible for the initial screening if they were published or accepted for publication in peer-reviewed journals. Abstracts from conference proceedings, books, theses, and dissertations were excluded. An accessible abstract was required for the screening process, and no language restrictions were applied.

Table 1.
Search Strategy and Inclusion/Exclusion Criteria Aligned with the PICO Framework (Population, Intervention, Comparison, and Outcome).
2.3. Study Selection
Duplicate references were removed using the Rayyan software version 1.5.0. (QCRI, Doha, Qatar) [39] prior to the screening process. The titles and abstracts of all identified studies were independently evaluated by both authors to determine their eligibility. After this initial screening, the selected studies were reviewed in detail by both researchers to confirm their inclusion. Any disagreements were resolved through discussion and mutual agreement. Full-text versions of studies deemed potentially relevant were retrieved for further assessment. Moreover, the reference lists of the included studies, as well as citations identified via Google Scholar, were examined to identify additional studies for potential inclusion in the review.
2.4. Data Extraction
A single researcher carefully extracted detailed information from the original studies, including data on sample characteristics (e.g., age, gender distribution, and diagnostic criteria) and details of the interventions, such as the type, duration, frequency, and intensity of the exercise protocols. Additional data included the assessed outcomes, main findings, reported adverse events, and participant drop-out rates. To ensure accuracy and reliability, a second investigator independently reviewed and verified all extracted data. The collected information was then summarized and presented in Table 2.

Table 2.
Descriptive characteristics of the included studies.
2.5. Quality Appraisal
The methodological quality of each RCT was obtained from the Physiotherapy Evidence Database (PEDro) [45], where such evaluations were available. For studies not assessed within the PEDro database, their methodological quality was independently evaluated by two researchers utilizing the PEDro scale [46]. The studies were subsequently categorized based on the following thresholds: excellent (scores of 9–10), good (scores of 6–8), fair (scores of 4–5), and poor (scores below 3) [47].
The Methodological Index for Non-Randomized Studies (MINORS) [48] was applied to assess the methodological quality of the comparative studies included. This tool comprises 12 items, each representing a quality criterion relevant to comparative studies. Each item is scored as follows: 0 (not reported), 1 (reported but inadequate), and 2 (reported and adequate). For comparative studies, the maximum achievable score is 24 points. Studies were categorized as high quality if they achieved a total score of 17 or higher, while those scoring below 17 were classified as low quality [49].
3. Results
3.1. Study Selection Results
A total of 541 records were identified through the database search. After the removal of duplicates, 363 records were screened based on their titles and abstracts, resulting in 18 articles being selected for full-text assessment. Ultimately, four RCTs and one comparative study fulfilled the inclusion criteria and were included in the systematic review (Figure 1).
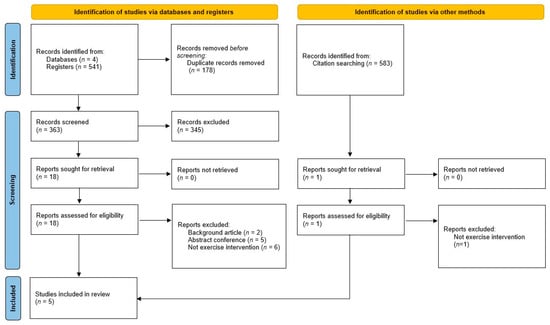
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) study flow diagram.
3.2. Design and Samples
The combined sample size across all included studies consisted of 244 women. Sample sizes were relatively small, ranging from 21 participants [44] to 60 participants [41]. All study participants were over 40 years old and had been diagnosed with primary pSS for a duration exceeding two years, except for Minali et al. [43], who did not report the time since diagnosis. The studies were published between 2007 and 2022. A summary of their main characteristics is presented in Table 2
3.3. Intervention Characteristics
Two of the interventions were based on resistance training [40,43], two on aerobic training [42,44], and one on both [41]. Exercise interventions lasted between 12 [40] and 28 [44] weeks. The studies reported frequencies ranging from 2 [46,47,48] to 3 [42,44] sessions per week. The duration of sessions varied from 30 to 60 min. The studies conducted by Minali et al. [43] and Dardin et al. [40] did not specify the duration of the sessions. Miyamoto et al. [42] and Strömbeck et al. [44] used heart rate to control exercise intensity, while Dardin et al. [40] and Minali et al. [43] used one repetition maximum. Garcia et al. [41] utilized maximum voluntary contraction and maximum oxygen uptake for this purpose.
3.4. Main Outcomes
3.4.1. Quality of Life
All of the studies included analyzed the effects of exercise on the quality of life of the participants using the SF-36 questionnaire and four of them reported significant intra-group improvements after the intervention. However, Strömbeck et al. [44] reported a significant reduction in the mental health score after the intervention
Notably, the effects of exercise were not found to be superior to control conditions in four studies. Only, Dardin et al. [40] showed significant improvements in the vitality and functional capacity subscales after 16 weeks of resistance training, compared to the control group, which was instructed not to engage in any regular exercise.
3.4.2. Disease Activity
Four studies analyzed the effects of exercise on disease activity, measured using the EULAR Sjögren’s Syndrome Disease Activity Index [40,41,42,43]. In this regard, Dardin et al. [40] observed a significant intra-group reduction after a 16-week resistance exercise intervention, but no inter-group differences were reported. The remaining studies did not report any changes in disease activity scores.
3.4.3. Functional Capacity
Four of the five studies reported information on the effects of exercise on functional capacity measurements [41,42,43,44], and all of them reported a significant positive effect derived from these practices compared to baseline measures.
When the inter-group analysis was performed, three studies showed a greater increase in maximum oxygen uptake after the exercise intervention compared to the control group, which did not follow a specific training program [41,42,44]. Additionally, Minali et al. [43] demonstrated that a 16-week resistance training intervention increased upper and lower limb strength more than in the control group.
3.4.4. Fatigue
Three studies aimed to identify the association between exercise practice and changes in fatigue-related parameters [40,42,44]. Significant intra-group improvements were observed in all of them after the exercise programs.
Inter-group analysis revealed that the exercise interventions led to a greater reduction in fatigue outcomes compared to the control group, which did not follow a specific training program [40,42,44].
3.4.5. Anxiety and/or Depression
Two studies evaluated the impact of exercise on anxiety and/or depression using standardized questionnaires. Miyamoto et al. [42] reported a significant reduction in the Beck Depression Inventory score following an aerobic intervention, although no significant differences were observed between the experimental and control groups. In contrast, Strömbeck et al. [44] found a significant decrease in depression scores, measured by the Hospital Anxiety and Depression Scale, within the exercise group compared to baseline. Additionally, the exercise group demonstrated a greater reduction in depression scores compared to the control group, which did not participate in a structured training program. However, no significant changes were observed in the anxiety scores.
3.4.6. Lipid Profile
Resistance and aerobic exercise programs did not have any significant impact on the lipid profile, according to the results reported in the only study investigating these outcomes [41].
3.4.7. Sleep Quality
One study explored the impact of resistance exercise training on sleep quality, assessed using actigraphy [40]. The results indicated that sleep latency and wake after sleep onset were reduced in the experimental group after 16 weeks. However, no significant differences between groups were found after the training period.
3.4.8. Glycemic Responses
According to the findings from the only study that examined this outcome, combined resistance and aerobic training resulted in a significant increase in HbA1c following a 28-week intervention [40]. Additionally, the improvement observed in the intergroup evaluation was statistically significant compared to the control group.
3.5. Dropouts and Adverse Events
Across all included studies, a total of 28 participants dropped out, with 14 of these occurring within the exercise groups. The main reasons for dropouts were health-related issues and/or discontinuation of the intervention. Adverse effects were reported in two of the five studies [42,43], which included one instance of chest pain during the first week of the intervention and four cases of joint pain.
3.6. Methodological Quality
The results of the methodological quality assessment of the RCTs were retrieved directly from PEDro for all of the RCTs [40,41,42,43]. The methodological quality was considered good in all of them. The only study evaluated using the MINORS scale was considered to be of high quality [44]. A full description of the quality analysis was also provided in Table 3.

Table 3.
Methodological quality appraisal of the included studies.
4. Discussion
This systematic review aimed to provide scientific evidence on the effectiveness of exercise as a therapeutic approach for patients with pSS. The results may be valuable to professionals looking for rehabilitation strategies to reduce the impact of this condition on patients’ overall health. After conducting an extensive and thorough search, only a small number of studies met the criteria for inclusion in this review. However, the majority of the studies analyzed were RCTs of good methodological quality. Considering the existing gap in the literature regarding the prescription of exercise for individuals with pSS, the findings of this review offer valuable and thought-provoking data that merit further discussion.
For instance, it is noteworthy that all the studies included reported on the effects of exercise on quality of life. This is an important topic to address, as treatment goals for patients with pSS should focus not only on extraglandular symptoms but also on improving quality of life outcomes [50]. Fatigue levels have also been considered as a predictor of quality of life in this population, given their detrimental impact on both physical and mental health [51].
Previous systematic reviews have demonstrated that exercise modalities, such as yoga and resistance training, result in significant improvements in the quality of life for patients with rheumatic diseases [52,53]. Interestingly, while intra-group analyses typically showed significant positive effects of exercise on this outcome, inter-group comparisons largely indicated that these effects were not superior to those achieved with standard treatment. This lack of effect of exercise on the usual treatment may be attributed to the limited impact that exercising has on several disease hallmarks that significantly influence the quality of life in this population, including dryness, autonomic responses, pruritus, and both oral and ocular disorders [54]. Additionally, it is important to note that exercise was not found to be superior to standard treatment in alleviating anxiety and depression, both of which are strongly linked to quality of life [55]. Similarly, other systematic reviews have noted that exercise could have a similar but not superior effect to standard treatment for depression [56] or anxiety [57]. Thus, it appears that the impact of exercise on the mental health of patients with pSS is limited.
In this context, most of the analyzed studies indicated that disease severity was not influenced by participation in exercise training programs. The lack of efficacy of exercise in modifying disease activity has also been observed in patients with lupus [58]. This aligns with previous findings suggesting that, among patients with autoimmune diseases, the anticipated changes in inflammation biomarkers resulting from exercise are modest at best [59]. Hence, a reduced effect of exercise on disease activity is expected. Notably, prior research has shown that patient-reported symptoms, which relate to systemic activity, are associated with improved health-related quality of life in individuals with pSS [60]. This finding suggests that since exercise was ineffective in reducing disease severity, it likely also did not have a direct impact on the quality of life. The effects of current pharmacological therapies aimed at alleviating the symptoms of this disease are limited, primarily due to its unknown and complex etiology, with potential treatment targets for disease severity still requiring further research [61]. In this context, it appears that exercise should not be regarded as a more viable option than the existing prescribed drug therapies.
On the other hand, the reviewed studies highlighted a positive impact of exercise on physical function, which is particularly noteworthy given that functional status is often impaired in individuals with pSS [16]. This finding aligns with previous research suggesting that exercise can significantly benefit the physical functioning of patients with rheumatological diseases [62]. Based on the analyzed data, it can be hypothesized that this improvement may stem from increases in fitness levels associated with engaging in physical activity, as this factor is closely linked to the prevention of functional decline [63]. In this regard, it is worth noting that those who experienced a greater increase in their fitness levels compared to their peers undergoing standard treatment showed more pronounced improvements.
Similarly, exercise has also been shown to be effective in reducing the impact of fatigue. This is a significant finding, as patients with pSS often report that fatigue is their most challenging and burdensome symptom to manage [64]. Other published reviews on the effects of exercise in autoimmune and rheumatic diseases have also reported positive outcomes, particularly in reducing fatigue levels [23,65]. Fatigue in Sjögren’s disease is believed to be partly driven by pro-inflammatory cytokines. Therefore, it can be hypothesized that exercise may help alleviate fatigue by reducing the concentration levels of these pro-inflammatory cytokines, as has been suggested in other conditions [66]. The obtained results support the notion that, while pharmacological treatments for fatigue have not proven effective in managing this symptom, exercise appears to be a valuable, safer, and more cost-effective option [42].
The reviewed studies that provided data on outcomes related to sleep problems and metabolic health indicated that exercise had a similar effect to standard pharmacological treatment. Similarly, exercise has been shown to yield effects akin to pharmacological therapy in individuals with movement disorders (Franco, 2019), and to offer comparable benefits for metabolic health when evaluated alongside standard pharmacological approaches [67]. However, the limited number of investigations available restricts the ability to draw more definitive conclusions or engage in deeper discussion.
On a final note, it is important to highlight that dropouts due to health issues were reported in the exercise groups by the authors of the studies included in this review, with some cases being directly attributed to the exercise itself. This finding raises concerns about the feasibility of exercise interventions for individuals with pSS and suggests that caution should be exercised when recommending physical activity to this population.
The findings of this systematic review support the use of supervised physical exercise programs, conducted 2 to 3 days per week in sessions of 30 to 60 min, as a complementary, safe, and effective intervention for patients with pSS. Aerobic activities, such as supervised walking or Nordic walking, enhance cardiorespiratory capacity, while resistance training alleviates fatigue and pain, and improves vitality. Although no clear superiority over standard treatments was observed in areas such as mental health or inflammatory activity, these non-pharmacological interventions contribute significantly to improving physical functionality and daily quality of life, with high safety and adherence rates. Therefore, it is advisable for patients, trainers, and caregivers to consider incorporating this type of treatment.
This appears to be the first systematic review examining the efficacy of exercise for patients with pSS. Despite its originality, several limitations must be acknowledged, as they significantly impact the strength of the findings presented. Firstly, a reduced number of investigations were found. Thus, caution is required when interpreting the obtained results. Secondly, due to the variation in methods and interventions used across studies, it remains unclear which exercise protocol is most beneficial. Additionally, only one study directly compared the effectiveness of two exercise interventions, making it challenging to determine which type of exercise should be recommended. Finally, we could not eliminate the possibility that some studies used the same sample, which prevented the performance of a meta-analysis. Clearly, further RCTs with larger sample sizes are needed to determine whether exercise has superior effects compared to standard therapy on key disease hallmarks, such as disease activity, fatigue, and pain. The findings from these studies should help establish basic guidelines for safely prescribing exercise to individuals with pSS.
5. Conclusions
Exercise does not demonstrate superior effects compared to standard treatment for improving quality of life and disease impact in patients with pSS. However, it can still be recommended for enhancing physical function and reducing fatigue. Nonetheless, the evidence is insufficient to inform clinical decisions regarding exercise prescription for this population due to the limited number of studies identified.
Author Contributions
Conceptualization, D.G.-D. and C.A.-P.; data curation, R.B.-R. and R.C.-A.; formal analysis, R.B.-R. and R.C.-A.; methodology, C.A.-P., R.B.-R., R.C.-A., and D.G.-D.; project administration, D.G.-D. and C.A.-P.; supervision, D.G.-D. and C.A.-P.; writing—original draft preparation, C.A.-P.; writing—review and editing, C.A.-P., D.G.-D., and R.C.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Balint, G.; Watson Buchanan, W.; Kean, C.A.; Kean, W.; Rainsford, K.D. Sjögren’s Syndrome. Inflammopharmacology 2024, 32, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Roszkowska, A.M.; Oliverio, G.W.; Aragona, E.; Inferrera, L.; Severo, A.A.; Alessandrello, F.; Spinella, R.; Postorino, E.I.; Aragona, P. Ophthalmologic Manifestations of Primary Sjögren’s Syndrome. Genes 2021, 12, 365. [Google Scholar] [CrossRef] [PubMed]
- Błochowiak, K. Co-Existence of Dry Mouth, Xerostomia, and Focal Lymphocytic Sialadenitis in Patients with Sjögren’s Syndrome. Appl. Sci. 2024, 14, 5451. [Google Scholar] [CrossRef]
- Maurya, R.P.; Singh, V.; Manisha; Gupta, A.; Singh, V.P.; Kumar, A.; Yadav, A.; Singh, S. Dry Eye Disease Associated with Primary Sjogren Syndrome: An Update. Indian J. Clin. Exp. Ophthalmol. 2021, 7, 259–269. [Google Scholar] [CrossRef]
- Miyauchi, K.; Fujimoto, K.; Abe, T.; Takei, M.; Ogawa, K. Cross-Sectional Assessment of Sleep and Fatigue in Middle-Aged Japanese Women with Primary Sjogren Syndrome or Rheumatoid Arthritis Using Self-Reports and Wrist Actigraphy. Medicine 2021, 100, e27233. [Google Scholar] [CrossRef]
- Theander, L.; Strömbeck, B.; Mandl, T.; Theander, E. Sleepiness or Fatigue? Can We Detect Treatable Causes of Tiredness in Primary Sjögren’s Syndrome? Rheumatology 2010, 49, 1177–1183. [Google Scholar] [CrossRef][Green Version]
- Hedströma, A.; Kvarnström, M.; Lindberg, G.; Alsabeah, S.; Alsabeah, H.; Ndegwa, N.; Löhr, J.M.; Haas, S.L.; Vujasinovic, M. High Prevalence of Gastrointestinal Symptoms in Patients with Primary Sjögren’s Syndrome Cannot Be Attributed to Pancreatic Exocrine Insufficiency. Scand. J. Gastroenterol. 2022, 57, 1250–1256. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brito-Zerónn, P.; Sisó-Almirall, A.; Bosch, X. Primary Sjögren Syndrome. Br. Med. J. 2012, 344, e3821. [Google Scholar] [CrossRef]
- Bowman, S.J. Primary Sjögren’s Syndrome. Lupus 2018, 27, 32–35. [Google Scholar] [CrossRef]
- Alani, H.; Henty, J.R.; Thompson, N.L.; Jury, E.; Ciurtin, C. Systematic Review and Meta-Analysis of the Epidemiology of Polyautoimmunity in Sjögren’s Syndrome (Secondary Sjögren’s Syndrome) Focusing on Autoimmune Rheumatic Diseases. Scand. J. Rheumatol. 2018, 47, 141–154. [Google Scholar] [CrossRef]
- Tseng, C.C.; Yen, J.H.; Tsai, W.C.; Ou, T.T.; Wu, C.C.; Sung, W.Y.; Hsieh, M.C.; Chang, S.J. Increased Incidence of Sjogren’s Syndrome in Systemic Sclerosis: A Nationwide Population Study. Autoimmunity 2015, 48, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Ruacho, G.; Kvarnström, M.; Zickert, A.; Oke, V.; Rönnelid, J.; Eketjäll, S.; Elvin, K.; Gunnarsson, I.; Svenungsson, E. Sjögren’s Syndrome in Systemic Lupus Erythematosus—A Subset Characterized by a Systemic Inflammatory State. J. Rheumatol. 2010, 47, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Sieiro Santos, C.; Moriano Morales, C.; Álvarez Castro, C.; Díez Alvarez, E. Polyautoimmunity in Systemic Lupus Erythematosus: Secondary Sjogren Syndrome. Z. Rheumatol. 2023, 82, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.C.; Chang, S.J.; Tsai, W.C.; Ou, T.T.; Wu, C.C.; Sung, W.Y.; Hsieh, M.C.; Yen, J.H. Sex Differential Association of Dermatomyositis with Sjögren Syndrome. Can. Med. Assoc. J. 2017, 189, 187–193. [Google Scholar] [CrossRef]
- Rischmueller, M.; Tieu, J.; Lester, S. Primary Sjögren’s Syndrome. Best Pract. Res. Clin. Rheumatol. 2016, 30, 189–220. [Google Scholar] [CrossRef]
- Hackett, K.L.; Newton, J.L.; Frith, J.; Elliott, C.; Lendrem, D.; Foggo, H.; Edgar, S.; Mitchell, S.; Ng, W.F. Impaired Functional Status in Primary Sjögren’s Syndrome. Arthritis Care Res. 2012, 64, 1760–1764. [Google Scholar] [CrossRef]
- Baldini, C.; Fulvio, G.; La Rocca, G.; Ferro, F. Update on the Pathophysiology and Treatment of Primary Sjögren Syndrome. Nat. Rev. 2024, 20, 473–491. [Google Scholar] [CrossRef]
- Hackett, K.L.; Deane, K.H.O.; Strassheim, V.; Deary, V.; Rapley, T.; Newton, J.L.; Ng, W.F. A Systematic Review of Non-Pharmacological Interventions for Primary Sjögren’s Syndrome. Rheumatology 2015, 54, 2025–2032. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Exercise as Medicine—Evidence for Prescribing Exercise as Therapy in 26 Different Chronic Diseases. Scand. J. Med. Sci. Sports 2015, 25, 1–72. [Google Scholar] [CrossRef]
- Segal, B.; Bowman, S.J.; Fox, P.C.; Vivino, F.B.; Murukutla, N.; Brodscholl, J.; Ogale, S.; McLean, L. Primary Sjögren’s Syndrome: Health Experiences and Predictors of Health Quality among Patients in the United States. Health Qual. Life Outcomes 2009, 7, 46. [Google Scholar] [CrossRef]
- Stack, R.J.; Southworth, S.; Fisher, B.A.; Barone, F.; Buckley, C.D.; Rauz, S.; Bowman, S.J. A Qualitative Exploration of Physical, Mental and Ocular Fatigue in Patients with Primary Sjögren’s Syndrome. PLoS ONE 2017, 12, e0187272. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.T.; Lendrem, D.W.; Ng, W.F.; Hackett, K.L.; Valim, V. Managing Fatigue in Patients with Primary Sjögren’s Syndrome: Challenges and Solutions. Open Access Rheumatol. Res. Rev. 2019, 11, 77–88. [Google Scholar] [CrossRef]
- Sharif, K.; Watad, A.; Bragazzi, N.L.; Lichtbroun, M.; Amital, H.; Shoenfeld, Y. Physical Activity and Autoimmune Diseases: Get Moving and Manage the Disease. Autoimmun. Rev. 2018, 17, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Razazian, N.; Kazeminia, M.; Moayedi, H.; Daneshkhah, A.; Shohaimi, S.; Mohammadi, M.; Jalali, R.; Salari, N. The Impact of Physical Exercise on the Fatigue Symptoms in Patients with Multiple Sclerosis: A Systematic Review and Meta-Analysis. BMC Neurol. 2020, 20, 93. [Google Scholar] [CrossRef]
- Hu, H.; Xu, A.; Gao, C.; Wang, Z.; Wu, X. The Effect of Physical Exercise on Rheumatoid Arthritis: An Overview of Systematic Reviews and Meta-Analysis. J. Adv. Nurs. 2021, 77, 506–522. [Google Scholar] [CrossRef]
- Price, E.J.; Rauz, S.; Tappuni, A.R.; Sutcliffe, N.; Hackett, K.L.; Barone, F.; Granata, G.; Ng, W.F.; Fisher, B.A.; Bombardieri, M.; et al. The British Society for Rheumatology Guideline for the Management of Adults with Primary Sjögren’s Syndrome. Rheumatology 2017, 56, 1828. [Google Scholar] [CrossRef]
- Strömbeck, B.; Ekdahl, C.; Manthorpe, R.; Jacobsson, L.T.H. Physical Capacity in Women with Primary Sjögren’s Syndrome: A Controlled Study. Arthritis Care Res. 2003, 49, 681–688. [Google Scholar] [CrossRef]
- Rojas-Alcayaga, G.; Herrera, A.; Espinoza, I.; Rios-Erazo, M.; Aguilar, J.; Leiva, L.; Shakhtur, N.; Wurmann, P.; Geenen, R. Illness Experience and Quality of Life in Sjögren Syndrome Patients. Int. J. Environ. Res. Public Health 2022, 19, 10969. [Google Scholar] [CrossRef]
- Najafi, P.; Hadizadeh, M.; Cheong, J.P.G.; Motl, R.W.; Abdullah, S.; Mohafez, H.; Poursadeghfard, M. Effects of Tele-Exercise Training on Physical and Mental Health and Quality of Life in Multiple Sclerosis: Do the Effects Differ by Modality and Clinical Disease Course? Mult. Scler. Relat. Disord. 2023, 80, 105129. [Google Scholar] [CrossRef]
- Yentür, S.B.; Ataş, N.; Öztürk, M.A.; Oskay, D. Comparison of the Effectiveness of Pilates Exercises, Aerobic Exercises, and Pilates with Aerobic Exercises in Patients with Rheumatoid Arthritis. Ir. J. Med. Sci. 2021, 190, 1027–1034. [Google Scholar] [CrossRef]
- Youssef, M.K. Effect of Training on Health Outcome Including Fatigue, Depression and Quality of Life in Patients with Systemic Lupus Erythromatosus. Beni-Suef Univ. J. Basic Appl. Sci. 2021, 10, 90. [Google Scholar] [CrossRef]
- Zhong, H.; Liu, S.; Wang, Y.; Xu, D.; Li, M.; Zhao, Y.; Zeng, X. Primary Sjögren’s Syndrome Is Associated with Increased Risk of Malignancies besides Lymphoma: A Systematic Review and Meta-Analysis. Autoimmun. Rev. 2022, 21, 103084. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.A.; Strader, C.; Chibbar, R.; Papatheodorou, S.; Dmytriw, A.A. The Relationship between Physical Activity and Lymphoma: A Systematic Review and Meta Analysis. BMC Cancer 2020, 20, 962. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.F.; Miller, A.; Bowman, S.J.; Price, E.; Kitas, G.; Pease, C.; Emery, P.; Lanyon, P.; Hunter, J.; Gupta, M.; et al. Physical Activity but Not Sedentary Activity Is Reduced in Primary Sjögren’s Syndrome. Rheumatol. Int. 2017, 37, 623–631. [Google Scholar] [CrossRef]
- Dassouki, T.; Benatti, F.B.; Pinto, A.J.; Roschel, H.; Lima, F.R.; Augusto, K.; Pasoto, S.; Pereira, R.M.R.; Gualano, B.; De Sá Pinto, A.L. Objectively Measured Physical Activity and Its Influence on Physical Capacity and Clinical Parameters in Patients with Primary Sjögren’s Syndrome. Lupus 2017, 26, 690–697. [Google Scholar] [CrossRef]
- Strömbeck, B.; Jacobsson, L.T.H. The Role of Exercise in the Rehabilitation of Patients with Systemic Lupus Erythematosus and Patients with Primary Sjögren’s Syndrome. Curr. Opin. Rheumatol. 2007, 19, 197–203. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Br. Med. J. 2021, 372, n71. [Google Scholar] [CrossRef]
- Shiwa, S.R.; Costa, L.O.P.; de Lima Moser, A.D.; de Carvalho Aguiar, I.; de Oliveira, L.V.F. PEDro: The Physiotherapy Evidence Database. Fisioter. Mov. 2011, 24, 523–533. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Sherrington, C.; Herbert, R.D.; Maher, C.G.; Moseley, A.M. PEDro. A Database of Randomized Trials and Systematic Reviews in Physiotherapy. Man. Ther. 2000, 5, 223–226. [Google Scholar] [CrossRef]
- Elkins, M.R.; Moseley, A.M.; Sherrington, C.; Herbert, R.D.; Maher, C.G. Growth in the Physiotherapy Evidence Database (PEDro) and Use of the PEDro Scale. Br. J. Sports Med. 2013, 47, 188–189. [Google Scholar] [CrossRef]
- Cashin, A.G.; McAuley, J.H. Clinimetrics: Physiotherapy Evidence Database (PEDro) Scale. J. Physiother. 2020, 66, 59. [Google Scholar] [CrossRef] [PubMed]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological Index for Non-Randomized Studies (Minors): Development and Validation of a New Instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Malgie, J.; Schoones, J.W.; Pijls, B.G. Decreased Mortality in COVID-19 Patients Treated with Tocilizumab: A Rapid Systematic Review and Meta-Analysis of Observational Studies. Clin. Infect. Dis. 2021, 72, e742–e749. [Google Scholar] [CrossRef] [PubMed]
- Strömbeck, B.E.; Theander, E.; Jacobsson, L.T.H. Effects of Exercise on Aerobic Capacity and Fatigue in Women with Primary Sjögren’s Syndrome. Rheumatology 2007, 46, 868–871. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.B.A.; Dardin, L.P.; Minali, P.A.; Trevisani, V.F.M. Cardiovascular Effect of Physical Exercise on Primary Sjogren’s Syndrome (PSS): Randomized Trial. Front. Med. 2021, 8, 719592. [Google Scholar] [CrossRef]
- Minali, P.A.; Pimentel, C.F.M.G.; de Mello, M.T.; Lima, G.H.O.; Dardin, L.P.; Garcia, A.B.A.; Goñi, T.C.S.; Trevisani, V.F.M. Effectiveness of Resistance Exercise in Functional Fitness in Women with Primary Sjögren’s Syndrome: Randomized Clinical Trial. Scand. J. Rheumatol. 2019, 49, 47–56. [Google Scholar] [CrossRef]
- Dardin, L.P.; Garcia, A.B.A.; Minali, P.A.; Pinto, A.C.P.N.; Trevisani, V.F.M. The Effects of Resistance Training in Patients with Primary Sjogren’s Syndrome. Clin. Rheumatol. 2022, 41, 1145–1152. [Google Scholar] [CrossRef]
- Miyamoto, S.T.; Valim, V.; Carletti, L.; Ng, W.F.; Perez, A.J.; Lendrem, D.W.; Trennel, M.; Giovelli, R.A.; Dias, L.H.; Serrano, É.V.; et al. Supervised Walking Improves Cardiorespiratory Fitness, Exercise Tolerance, and Fatigue in Women with Primary Sjögren’s Syndrome: A Randomized-Controlled Trial. Rheumatol. Int. 2019, 39, 227–238. [Google Scholar] [CrossRef]
- Devauchelle-Pensec, V.; Morvan, J.; Rat, A.C.; Jousse-Joulin, S.; Pennec, Y.; Pers, J.O.; Saraux, A. Effects of Rituximab Therapy on Quality of Life in Patients with Primary Sjögren’s Syndrome. Clin. Exp. Rheumatol. 2011, 29, 6. [Google Scholar]
- Dias, L.H.; Miyamoto, S.T.; Giovelli, R.A.; de Magalhães, C.I.M.; Valim, V. Pain and Fatigue Are Predictors of Quality of Life in Primary Sjögren’s Syndrome. Adv. Rheumatol. 2021, 61, 28. [Google Scholar] [CrossRef] [PubMed]
- Sieczkowska, S.M.; de Orleans Casagrande, P.; Coimbra, D.R.; Vilarino, G.T.; Andreato, L.V.; Andrade, A. Effect of Yoga on the Quality of Life of Patients with Rheumatic Diseases: Systematic Review with Meta-Analysis. Complement. Ther. Med. 2019, 46, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Sieczkowska, S.M.; Coimbra, D.R.; Vilarino, G.T.; Andrade, A. Effects of Resistance Training on the Health-Related Quality of Life of Patients with Rheumatic Diseases: Systematic Review with Meta-Analysis and Meta-Regression. Semin. Arthritis Rheum. 2020, 50, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.T.; Valim, V.; Fisher, B.A. Health-Related Quality of Life and Costs in Sjögren’s Syndrome. Rheumatology 2021, 60, 2588–2601. [Google Scholar] [CrossRef]
- Cui, Y.; Xia, L.; Zhao, Q.; Chen, S.; Gu, Z. Anxiety and Depression in Primary Sjögren’s Syndrome: A Cross-Sectional Study. BMC Psychiatry 2018, 18, 131. [Google Scholar] [CrossRef]
- Kvam, S.; Kleppe, C.L.; Nordhus, I.H.; Hovland, A. Exercise as a Treatment for Depression: A Meta-Analysis. J. Affect. Disord. 2016, 202, 67–86. [Google Scholar] [CrossRef]
- Jayakody, K.; Gunadasa, S.; Hosker, C. Exercise for Anxiety Disorders: Systematic Review. Br. J. Sports Med. 2014, 48, 187–196. [Google Scholar] [CrossRef]
- Alexanderson, H.; Boström, C. Exercise Therapy in Patients with Idiopathic Inflammatory Myopathies and Systemic Lupus Erythematosus—A Systematic Literature Review. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101547. [Google Scholar] [CrossRef]
- Luo, B.; Xiang, D.; Ji, X.; Chen, X.; Li, R.; Zhang, S.; Meng, Y.; Nieman, D.C.; Chen, P. The Anti-Inflammatory Effects of Exercise on Autoimmune Diseases: A 20-Year Systematic Review. J. Sport Health Sci. 2024, 13, 353–367. [Google Scholar] [CrossRef]
- Cornec, D.; Devauchelle-Pensec, V.; Mariette, X.; Jousse-Joulin, S.; Berthelot, J.M.; Perdriger, A.; Puéchal, X.; Le Guern, V.; Sibilia, J.; Gottenberg, J.E.; et al. Severe Health-Related Quality of Life Impairment in Active Primary Sjögren’s Syndrome and Patient-Reported Outcomes: Data from a Large Therapeutic Trial. Arthritis Care Res. 2017, 69, 528–535. [Google Scholar] [CrossRef]
- Jonsson, R. Disease Mechanisms in Sjögren’s Syndrome: What Do We Know? Scand. J. Immunol. 2022, 95, e13145. [Google Scholar] [CrossRef] [PubMed]
- Fallon, K. The Role of Exercise in Management of Rheumatological Disease. Aust. J. Gen. Pract. 2021, 50, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Dugan, S.A.; Gabriel, K.P.; Lange-Maia, B.S.; Karvonen-Gutierrez, C. Physical Activity and Physical Function: Moving and Aging. Obstet. Gynecol. Clin. N. Am. 2018, 45, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Mengshoel, A.M.; Norheim, K.B.; Omdal, R. Primary Sjögren’s Syndrome: Fatigue Is an Ever-Present, Fluctuating, and Uncontrollable Lack of Energy. Arthritis Care Res. 2014, 66, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Perandini, L.A.; de Sá-Pinto, A.L.; Roschel, H.; Benatti, F.B.; Lima, F.R.; Bonfá, E.; Gualano, B. Exercise as a Therapeutic Tool to Counteract Inflammation and Clinical Symptoms in Autoimmune Rheumatic Diseases. Autoimmun. Rev. 2012, 12, 218–224. [Google Scholar] [CrossRef]
- Ayari, S.; Abellard, A.; Carayol, M.; Guedj, É.; Gavarry, O. A Systematic Review of Exercise Modalities That Reduce Pro-Inflammatory Cytokines in Humans and Animals’ Models with Mild Cognitive Impairment or Dementia. Exp. Gerontol. 2023, 175, 112141. [Google Scholar] [CrossRef]
- Türkel, İ.; Özerkliğ, B.; Atakan, M.; Aktitiz, S.; Koşar, Ş.; Yazgan, B. Exercise and Metabolic Health: The Emerging Roles of Novel Exerkines. Curr. Protein Pept. Sci. 2022, 23, 437–455. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).