Abstract
Susac Syndrome (SuS) is a rare autoimmune neurovascular disorder characterized by sudden visual loss, hearing disturbances, and encephalopathy. Pathology affects the small vessels of the brain, retina, and inner ear. Diagnosing SuS is challenging due to its rarity, complexity, and nonspecific symptoms. This single-case study presents a proteomic analysis of tear fluid from a patient with SuS, revealing upregulated proteins involved in immune dysregulation, cytoskeletal remodeling, and cellular repair. The activation of inflammatory proteins (e.g., S100), cytoskeletal and motility-related proteins (e.g., ezrin, radixin), and membrane transport proteins (e.g., aquaporin-5, chloride intracellular channel protein), together with activation of MAPK and NF-κB signaling pathways, highlights immune dysregulation and neurovascular damage in SuS. Hyperactivation of MAPK and NF-κB pathways leads to chronic neuroinflammation and decreased expression of neutrophil defensin 1, indicating a shift from a protective to a chronic inflammatory response. These findings from the personalized proteomic pattern of SuS support the potential of tear fluid proteomics for diagnosing SuS and offer valuable insights into its underlying molecular mechanisms.
1. Introduction
SuS is an orphan microangiopathic disease first described in 1979 and belongs to the class of systemic connective tissue diseases. In 2021, more than 450 cases were published, predominantly in women (80% of cases) []. Although SuS is primarily a disease of young adults, sporadic cases have also been reported in children [,]. The pathogenesis of SuS syndrome has not been fully elucidated; the studies conducted to date suggest an autoimmune background. Migraine-like headaches are the heralding symptom in over 80% of patients at disease onset. Over 75% of patients exhibit behavioral disturbances, which may range from apathy to aggressive behavior. John O. Susac completed the description of SuS by adding a note about hearing loss []. The clinical manifestation of the syndrome consists of a triad of symptoms, including encephalopathy, impaired thinking, short-term memory loss, slow thought processing, and reduced ability to solve problems. It is typically characterized by visual disturbances due to branch retinal artery occlusions and sensorineural hearing loss []. SuS is often under-recognized and difficult to diagnose as its uncommon presentation overlaps with other systemic autoimmune diseases []. Nowadays, the diagnosis of SuS is based on the clinical triad of encephalopathy, hearing loss, and branch retinal artery occlusions []. In many cases, the full clinical triad is either incomplete or not yet symptomatic at diagnosis. The clinical course of SuS is usually self-limiting, fluctuating, and monophasic, typically lasting 2 to 4 years. Cases with an incomplete triad of symptoms, nonspecific symptoms, make this diagnosis challenging. Simultaneous experience of headaches and vision disturbances may frequently be misinterpreted as migraine with aura. Brain magnetic resonance imaging (MRI), pure tone audiometry, and retinal fluorescein angiography (FAG) have high diagnostic value; however, biomarkers are still lacking in the diagnosis of SuS []. Early initiation of treatment is crucial in slowing the progression of the disease and reducing the likelihood of serious and irreversible complications. For example, in most cases, hearing loss. Extreme cases can even end in death []. Treatment is empirical, based on the hypothesis of inflammatory microvasculopathy, which supports the use of immunosuppressive and immunomodulatory drugs. High-dose corticosteroids are the mainstay of first-line treatment [].
The tear film maintains direct contact with the ocular surface and cornea, making it readily accessible for non-invasive sample collection. It is a complex mixture of proteins, including enzymes, mucins, hormones, growth factors, neuropeptides, and cytokines, as well as lipids, salts, and carbohydrates. Ocular surface diseases significantly alter the composition of tear fluid, positioning it as a promising source of candidate biomarkers for diagnostic and therapeutic monitoring purposes. Thus, tear proteomics can serve as a valuable analytical approach for elucidating protein functions involved in maintaining homeostasis and in the pathogenesis of diseases, as well as for identifying potential biomarkers. This case study of a male patient highlights the potential of tear fluid proteomic analysis as an innovative supplementary tool that may complement existing methods in personalized clinical diagnostics. Our results could also contribute to an understanding of the molecular mechanism of SuS.
- Case Presentation
A 31-year-old male patient was hospitalized in the neurological department with sudden disorientation and memory impairment that appeared after a 3-day headache. Neurological examination revealed prominent cognitive difficulties, apraxia, disconnection syndrome, mild horizontal nystagmus, and absence of overt ataxia. These findings were initially attributed to alternative aetiologies, which delayed the consideration of SuS. This highlights the diagnostic challenge of the condition, especially given the broad range of differential diagnoses, including multiple sclerosis (MS), systemic lupus erythematosus, and other vasculitic syndromes.
- Clinical Findings Supporting the Diagnosis of SuS Syndrome
The diagnosis of SuS often involves a combination of clinical suspicion, neuroimaging, and laboratory findings. The diagnosis of this case was confirmed through the following diagnostic methods. Computer tomography (CT) of the brain was negative for acute changes. MRI showed demyelinating white matter lesions of unclear etiology, predominantly in the corpus callosum. Elevated protein levels, proteinorachia were present in the cerebrospinal fluid (CSF), without evidence of autoimmunity or neuroinfection. Panfundoscopic examination, Optical Coherence Tomography (OCT), OCT angio, and FAG identified bilateral retinal artery occlusions and the characteristic “candle-wax drippings” appearance in the retinal arteries. These findings support the diagnosis of SuS. A transcranial ultrasound examination revealed an insignificant right-to-left shunt. An audiometric examination showed a normal audiogram bilaterally.
Serological tests revealed predominantly negative autoantibody results, consistent with typical findings in SuS. The white blood cell count was slightly elevated to 10.52 × 109/L. The microbiology test results showed that common viral infections, such as herpes viruses and hepatitis viruses, as well as other known viruses, were negative. The albumin level in the CSF was elevated to 899 mg/L (normal < 320 mg/L), which may indicate inflammation within the nervous system []. Additionally, the WBC count in the CSF was elevated to 12 cells/µL, within the expected inflammatory range for SuS. A slightly elevated mean platelet volume of 10.5 fl was observed.
Positive immunoglobulin findings (IgG, IgM, and IgA) were identified in the CSF, indicating intrathecal immune activation. Immunoglobulin G (IgG) is typically associated with past infections or long-term immune responses, while Immunoglobulin M (IgM) is produced early in the course of an infection, reflecting an acute-phase response. Immunoglobulin A (IgA), although predominantly located on mucosal surfaces, when detected in the CSF, may indicate immune activation within the central nervous system. The presence of these immunoglobulins (IgG, IgM, IgA) in the CSF may reflect non-infectious inflammation or an underlying immunological process affecting the nervous system [].
To support the diagnosis of SuS, a multidisciplinary team of specialists in neurology, ophthalmology, and otolaryngology met to evaluate the patient. A detailed assessment of the clinical presentation and diagnostic findings was conducted to establish the diagnosis.
2. Materials and Methods
2.1. Collection of Tear Fluid
Tear fluid was collected from the patient and the control subjects using a glass microcapillary (Drummond, Broomall, PA, USA) at the Department of Ophthalmology, University Hospital Louis Pasteur in Košice, and immediately stored at −80 °C until analysis. The patient and 6 control subjects (males aged 24–31) who did not show any signs of illness were treated according to the Declaration of Helsinki. The control group consisted of participants who were informed of the study’s objectives and potential risks, and written informed consent was obtained following ethical guidelines. The study was approved by the ethical committee of the Louis Pasteur University Hospital in Košice (protocol code 2020/EK/06042 approved on 25 June 2020, and extended until January 2030 for studies involving human tear fluid.
2.2. Sample Preparation and Digestion
For proteomic analysis, 10 µL of tear fluid was taken, diluted with 10 µL 8 M urea in 0.05 M ammonium bicarbonate (ABC, Merck, Darmstadt, Germany). For the direct digestion process without any cleaning procedure, 5 µL of dithiothreitol (BioRad, Hercules, CA, USA), 0.025 M in 0.05 M ABC, was added to break disulfide cross-links in tear fluid proteins. The process was carried out in a thermomixer for 30 min at 37 °C. For alkylation, 5 µL of iodoacetamide (BioRad, Hercules, CA, USA), 0.084 M in 0.05 M ABC, was used, and the mixture was shaken in a thermomixer for 30 min at room temperature in the dark. Subsequently, the mixture was diluted with 30 µL of the solution of 70 µL 0.01 M CaCl2 in 10 mM ABC to lower the urea concentration and digested with 5 µL Trypsin Gold (Promega, Madison, WI, USA). The digestion was stopped by adding 20% formic acid (FA, Merck, Darmstadt, Germany) to obtain a pH ≤ 3. Subsequently, 3 µL of acetonitrile was added to the tear fluid and centrifuged at 14,000× g, 4 °C for 20 min.
2.3. LC-MS/MS Analysis
The supernatant was analyzed by an LC-MS/MS using an UltiMate™ 3000 UHPLC system coupled with a high-resolution, accurate-mass (HRAM), Orbitrap Eclipse™ Tribrid™ Mass Spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Peptides isolated from tear fluid were injected in a volume of 1 µL and trapped on PepMap™ Neo Trap Cartridge C 18 (5 mm, 5 μm particle), at a flow rate of 15 μL.min−1 and then separated on the Thermo Scientific™ EASY-Spray™ (Thermo Fisher Scientific, Waltham, MA, USA) analytical column (500 mm, 2 μm particle), at a temperature of 40 °C. The composition of mobile phase A consisted of 0.1% FA in water, while mobile phase B was a mixture of 0.1% FA in water and acetonitrile (20/80, v/v). Separation employed a 300 min linear gradient, from 2% to 40% solvent B, followed by 9 min increasing B up to 90%, and next a 10 min hold, all at a flow rate of 250 nl.min−1. The mass spectrometer was operated in positive data-dependent acquisition mode with a cycle time of 2 s between two MS1 scans. MS1 spectra were measured with a resolution of 120,000, with a maximum injection time of 100 ms, covering a scan range of 350–1700 Da. MS/MS data were acquired at a scan resolution of 30,000 with a maximum injection time of 50 ms, using HCD fragmentation at 30% collision energy, and an isolation window of m/z = 2 with a 10 ppm mass tolerance around the precursor. The dynamic exclusion was set to 60 s. Before each LC-MS run, a fluoranthene lock mass correction (RunStart EASY-IC™, Thermo Fisher Scientific, Waltham, MA, USA) was automatically performed. Each sample was measured twice as a technical duplicate.
2.4. Protein Identification and Label-Free Quantification
Acquired raw run files were analyzed using the Proteome Discoverer software, version 2.5.0.400. (Thermo Scientific™). Protein identification was performed using the combination of three search engines available in PD, MS Amanda 2.0, SequestHT, and Mascot, utilizing the Uniprot Homo sapiens database (release 2023_02, containing 182,026 sequences) for MS Amanda and SequestHT and the Swiss-Prot Homo Sapiens database for Mascot. Proteolytic enzyme specification during the search was trypsin, cleaving after lysine (K) and arginine (R) except when followed by proline (P). The fixed modification was set to Carbamidomethylation of cysteine, and variable modifications were set to oxidation of methionine, N-terminal acetylation, N-terminal methionine lost, and N-terminal methionine lost + acetylation. The mass tolerances for precursor and fragment ions were set at 10 ppm and 0.02 Da, respectively. The minimum cutoff for peptide length was set at six amino acids, and the maximum permissible missed cleavage was set at two. The identified spectra were rescored using a Percolator. For Label-Free Quantification (LFQ), both unique and razor peptides were used, with precursor abundance in the samples compared based on the integration of the identified peptide intensities. Protein groups were considered for peptide uniqueness, and shared quantitative results were used. The statistical significance of the LFQ results was determined using a background-based t-test with a predefined summed abundances setting, where protein abundances were calculated by summing the sample abundances of the connected peptide groups. The protein ratio calculation was performed using a pairwise ratio-based analysis. A non-nested design with no imputation method was applied. The extreme ratio values (100 and 0.01) were not considered for median selection.
For further analysis with Proteome Discoverer, we required at least two unique peptides per protein identification. To ensure one representative per protein group, the ‘criterion master is equal to master’ setting was used. A high-confidence identification was set for the acceptance of proteins for comparative study. This means that the peptide-spectrum matches (PSMs) have been accepted at a False Discovery Rate (FDR) of ≤1% and validated using a target-decoy search strategy. To accept the protein fold change, the fold discovery rate was controlled to be ≤0.05, with probability score adjustments performed using the Benjamini–Hochberg correction method.
2.5. Pathway Enrichment Analysis
Pathway enrichment analysis was performed using the Reactome Pathway Analysis tool with the CAMERA algorithm (a gene set analysis algorithm similar to the classical GSEA algorithm as implemented in the limma package) (https://reactome.org, accessed on 4 August 2025). This analysis was based on a comparison of SuS case patient and control cases.
Gene set enrichment analysis (GSEA) was performed using the GSEA Preranked tool v. 4.2.3 (Broad Institute, Cambridge, MA, USA, https://www.gsea-msigdb.org/gsea, accessed on 3 February 2025). The analysis was based on a ranked gene list generated from the log2 fold change values between diseased and control samples and was performed using 1000 permutations. KEGG pathway gene sets from the MSigDB database were used for functional enrichment. To identify significantly enriched pathways, the FDR < 0.25 and nominal p-value < 0.05 criteria were determined using GSEA.
3. Results
Recent advances in proteomics have identified tear fluid as a promising non-invasive medium for biomarker discovery. In our case study, the tear fluid was analyzed by shotgun proteomic analysis to observe changes between the tear fluid of the patient with SuS and the healthy control. The integration of MS-based proteomic findings can provide novel insights into the pathogenesis of SuS. Mass spectrometry coupled to ultra-high-performance liquid chromatography (UHPLC-MS/MS) was used to analyze the proteomic composition of the tear fluid sample. The analysis resulted in the identification of 22,040 proteins, which were classified into 2880 distinct protein groups by setting the criteria that master is equal to master in the PD software version 2.5.0.400. (Supplementary Table S1). This approach ensures that only one representative (master) protein is reported per protein group, resulting in a final dataset comprising 2880 proteins. The volcano plot shown in Figure 1, based on a single SuS case compared with six healthy controls, illustrates proteins with marked changes in abundance, defined by a log2 fold change greater than 2 or less than −2, when comparing the SuS case to the control samples. The results show that the majority of significantly altered proteins are upregulated in the SuS sample.
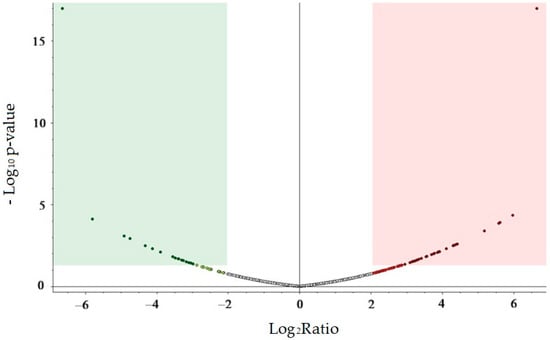
Figure 1.
Volcano plot, exploratory visualization of a single SuS case compared with six healthy controls, showing 2880 identified proteins with only master is equal to master criteria. The x-axis represents the log2 fold change ratio, and the y-axis represents the −log10 p-value. The green area indicates potentially significantly downregulated proteins with −log10 ≥ 1.3 (p-value ≤ 0.05) and log2 fold change ≤ −2, the red area indicates potentially significantly upregulated proteins, p-value ≤ 0.05 and log2 fold change ≥ 2. The central white region visualizes proteins with probably no significant expression change. Probability score adjustments were performed using the Benjamini–Hochberg correction method. Plot created with Thermo Proteome Discoverer 2.5.0.400 software.
The LFQ methods based on ion intensity was evaluated with stringent criteria of having a minimum of two unique peptides and high-confidence identification, with the limitation, that the protein must be identified in both SuS and at least in one subject in the control group, and the differences in the protein expression in the SuS patient and control case must be a log2 fold change ≥ 2, or log2 fold change ≤ −2. Twenty proteins meeting the established criteria were selected and are presented in Table 1. These 20 proteins are involved in various molecular functions. Although the direct links between these proteins and SuS are not yet well documented, their role in inflammation, oxidative stress, and endothelial function suggests that they may contribute to the disease’s pathophysiology.

Table 1.
A total of 20 proteins identified in both the SuS and control groups exhibited statistically significant changes in abundance (log2 fold change ≥ 2 or log2 fold change ≤ −2; p-value ≤ 0.05, unpaired t-test with Benjamini–Hochberg correction).
Proteome unsupervised hierarchical clustering, applied to these 20 proteins, using the Manhattan distance function applied to measured data, demonstrates, at the heat map, the power of proteomic analysis as a potential tool for identifying SuS in patients (Figure 2). This supports the strong potential utility of tear fluid, which may be potentially used to help the identification of SuS among other neuronal diseases.
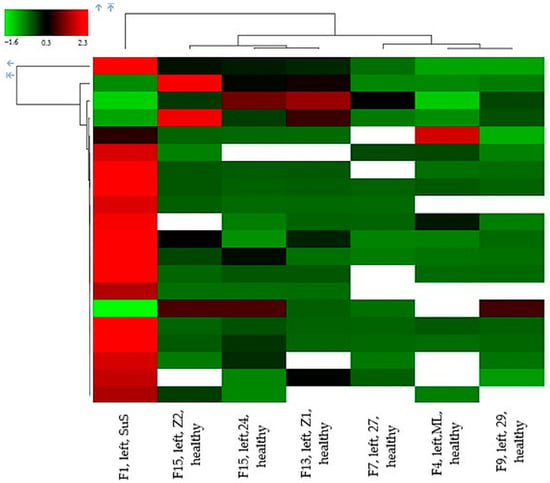
Figure 2.
Proteome unsupervised hierarchical clustering applied to 20 proteins with at least 2 unique peptides, high confidence identification, present in SuS and at least in one control, the differences in the protein expression in the SuS patient and control case must be a log2 fold change ≥ 2, and FDR ≤ 0.05. Data obtained with Proteome Discoverer 2.5.0.400.
Considering the rarity of the condition under investigation, in the second analytical run, all identified proteins were subjected to pathway enrichment analysis using Reactome Pathway Analysis (Reactome.org), Camera analysis method, and GSEA, functional enrichment analysis to elucidate the biochemical mechanisms associated with SuS. Reactome confirmed upregulation of aquaporin-5, aldo-keto reductase, and glutamine gamma-glutamyl transferase 2 in accordance with Proteome Discoverer, as well as downregulation of neutrophil defensin 1. The GSEA revealed significant upregulation of the actin cytoskeleton pathway (NES = 1.460, p = 0.049, FDR = 0.046) in the SuS case compared to the healthy control (Figure 3). In this group, 11 core enrichment proteins (Radixin, Ezrin, Moesin, Myosin-14, Thymosin β-4, Alpha-Actinin 4, Actin-related protein 2/3 complex subunit 3, Transforming protein RhoA, Cell division control protein 42 homolog, Actin gamma 1, and Vinculin) were upregulated, with RDX (log2FC = 4.34) showing the strongest increase (Supplementary Table S2).
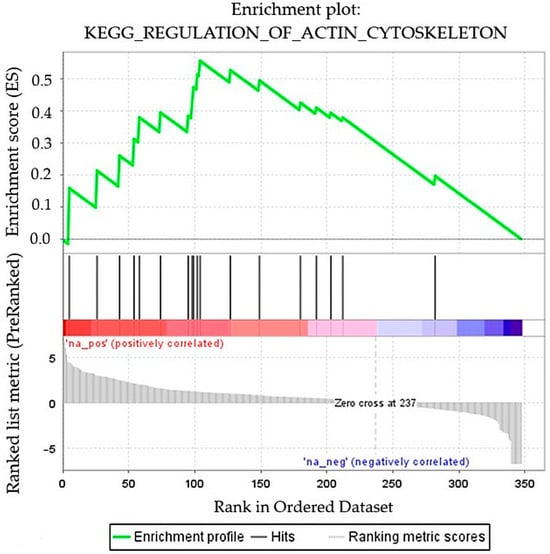
Figure 3.
GSEA of quantified proteins between the SuS patient and the healthy control groups. Enrichment plot: Regulation of actin cytoskeleton (NES = 1.460, p = 0.049, FDR = 0.046).
4. Discussion
SuS disease has a complex mechanism, with clinical manifestations that often overlap with other autoimmune and neurovascular disorders. Infections may trigger or exacerbate immune responses in SuS, leading to inflammation of blood vessels and disruption of the blood–brain barrier (BBB) in the brain, retina, and inner ear. These changes are reflected in body fluids. The tear fluid proteome reflects signaling processes on the ocular surface and in the central nervous system (CNS) through connections with the hematopoietic barrier and CSF.
Proteomic analysis of tear fluid from a single SuS patient identified upregulated proteins linked to BBB dysfunction and endothelial injury (aquaporin-5, chloride intracellular channel protein, neutrophil defensin 1, and S100 proteins), cytoskeletal remodeling (radixin and Na+/H+ Exchange Regulatory Cofactor 1), neuroinflammation and immune imbalance (lactotransferrin, immunoglobulin heavy chain variants, S100 proteins, dipeptidyl peptidase-4 and Thymosin β), and glandular secretion dysregulation (including aquaporin-5, reflecting altered fluid transport in glands, and lactotransferrin), highlighting immune imbalance and tissue repair processes in SuS. The up-regulated proteins identified by PD are listed in Table 2, proteins identified by GSEA, in addition to PD, are presented in Table 3. Additionally, the down-regulated proteins identified by PD are listed in Table 4. All tables include the proteins’ functions and potential interactions, which are annotated according to the UniProt database (Release 2025_04).

Table 2.
The up-regulated proteins identified in the male SuS case, compared to the control sample, identified with Proteome Discoverer 2.5.0.400.

Table 3.
The up-regulated proteins presented in the male SuS case, compared to the control sample, significantly identified by GSEA, in addition to Proteome Discoverer 2.5.0.400 software.

Table 4.
The down-regulated proteins identified in the male SuS case, compared to the control sample, identified with Proteome Discoverer 2.5.0.400 software.
This work aimed to investigate the correlation between changes in protein abundance in tear fluid and the characteristic manifestations of SuS, based on which SuS is clinically distinguished from other neurological diseases.
Immune cells, including neutrophils, macrophages, and lymphocytes, migrate in response to signals from inflammation, infections, or injuries, a process crucial for the immune response that requires dynamic changes in the cytoskeleton. Upregulated proteins confirmed by GSEA, as actin proteins like ARPC3 (component involved in the nucleation of branched actin filaments), ACTN4 (actin-binding protein), Tβ 4 (regulating actin dynamics), and MYH-14 (non-muscle myosin heavy chain involved in actin-based contractile processes), together with upregulated RHOA, CDC42, RDX, MSN, and EZR reorganize the cytoskeletal filaments. An active cytoskeleton enables immune cells to migrate to sites of inflammation or injury, traverse tissue barriers, perform phagocytosis, and support the inflammatory response [].
Elevated albumin level and WBC count in the patient’s CSF indicate inflammation within the nervous system. Proteomic analysis confirmed marked upregulation of proteins involved in inflammatory signaling and immune responses.
Tβ 4 is known to imply tissue repair efforts following inflammation []. Dipeptidyl peptidase 4 (DPP4), an enzyme involved in immune regulation and inflammation through modulation of cytokine activity, was observed to be ~11.7 times increased. Upregulation of DPP4 promotes degradation of regulatory peptides such as incretins, affecting glucose metabolism, immune responses, and cell signaling, while modulating MAPK and NF-κB pathways to regulate inflammation and immune cell activation []. Higher expression of these proteins was also observed in MS []. Their upregulation in SuS may reflect a shared protective response to microvascular injury and endothelial-glial stress. This theory is supported by strongly elevated values of LTF, which is a major innate-immune protein, and its level typically rises with ocular surface inflammation, infection, or neutrophil infiltration. Upregulation of LTF enhances iron-binding and antimicrobial activity, modulates immune responses and inflammation, and interacts with NF-κB signaling to regulate immune cell activation []. Many studies have shown that LTF is abnormally expressed in a variety of neurological diseases, especially neurodegenerative diseases such as AD and PD [].
Nearly 12-fold upregulate of S100A6 enhances calcium-dependent regulation of cell growth, differentiation, and motility, amplifying MAPK signaling and activating NF-κB to control inflammation and cell survival []. Upregulation of CMPK2, a mitochondrial enzyme critical for nucleotide metabolism and energy homeostasis, enhances RNA/DNA synthesis and activates MAPK and NF-κB pathways, promoting cellular metabolism, proliferation, and inflammation. Its dysregulation is linked to viral infections, neurodegenerative diseases, and autoimmune disorders []. Additionally, Aminopeptidase N upregulates peptide degradation, immune function, and cell adhesion, indirectly regulating inflammation and immune responses via NF-κB []. Upregulation of CRYAB, a small heat shock protein, protects cells from stress and prevents apoptosis []. Upregulated GGT2 enhances antioxidant defense and cell survival via glutathione metabolism, indirectly modulating NF-κB and MAPK pathways to control stress responses and inflammation []. Observed upregulation of IGH c2427_heavy_IGHV3-23_IGHD2-2_IGH14, an immunoglobulin gene rearrangement critical for antibody production, can promote immune cell activation and antibody response regulation []. More than 15 times upregulated PRDX4 enhances antioxidant defense by reducing hydrogen peroxide, protecting cells from oxidative stress, and indirectly modulating NF-κB and MAPK pathways to regulate inflammation and cellular protection []. Upregulation of NHE-RF1, over 17-fold in our measurements, enhances Na+/H+ exchange to maintain ion balance, pH, and cell volume, indirectly modulating MAPK and NF-κB pathways to influence responses to osmotic stress and inflammation []. It is often found to be elevated or dysregulated in neurodegenerative diseases, like Alzheimer’s disease (AD), Parkinson’s disease (PD), ischemic stroke, and brain injury [,].
Neutrophil defensin 1 (DEFA1), an α-defensin with antimicrobial and immunomodulatory roles, is regulated by immune and inflammatory pathways. In neurodegenerative conditions such as SuS, e.g., MAPK and NF-κB pathways are hyperactivated, leading to chronic neuroinflammation and a subsequent decrease in DEFA1 expression, marking a shift from acute protection to chronic neuroinflammation. DEFA1 in neurodegenerative conditions like AD was upregulated in both blood serum and CSF, reflecting active neuroinflammatory and innate immune processes [,]. Significant elevation of DEFA1 in CSF during bacterial and aseptic meningitis reflects acute innate immune activation []. In our SuS findings, DEFA1 was downregulated in tear fluid, similar to Sjögren’s syndrome (SS), suggesting a link to lacrimal gland dysfunction and impaired antimicrobial defense. Inflammation may be driven by cytotoxic T cells or anti-endothelial antibodies and is associated with cytoskeletal and cell adhesion alterations []. Similarly, the downregulation of ApoD, a lipocalin that regulates lipid metabolism and protects against oxidative stress, leads to increased oxidative damage and neurodegeneration, exacerbating vascular dysfunction in cells [,]. Nowadays, it is known that Apo D levels are altered in several neuropsychiatric disorders, especially in the plasma, and are suggested to be a potential biomarker for their diagnostics []. Additionally, the downregulation of the 60S Acidic Ribosomal Protein disrupts protein synthesis, impairing cellular processes like migration, proliferation, and repair []. Lastly, the cDNA FLJ41552 (Clone COLON2004478), hypothesized to encode a protein involved in cell signaling and growth regulation, likely indirectly influences the MAPK pathway, with its downregulation impairing immune responses and cell signaling, thereby contributing to autoimmune tissue damage [].
These proteins regulate immune cells and cytokines; their increase and decrease in SuS tear fluid reflect neurovascular injury and immune activation.
A water channel protein, AQP5, mediates tear secretion and ocular tissue hydration. In the SuS patient, its abundance was markedly increased (>63-fold), indicating inflammation and glandular dysfunction, and has been observed in some cancers []. AQP5 expression is often altered or elevated in salivary glands of patients with SS []. Elevated levels of AQP5 may indicate altered water transport in inflamed blood vessels and glands, which contributes to the dysregulation of ocular fluid. This mechanism is also observed in SuS, where AQP5 mislocalises and disrupts secretion in the lacrimal and salivary glands. These alterations occur alongside endothelial dysfunction and microvascular occlusions, which drive the cognitive, auditory, and visual deficits characteristic of the disease. Similarly, the upregulation of CLIC enhances chloride ion transport and cellular signaling, influencing cell volume regulation and apoptosis, while modulating MAPK and NF-κB pathways to affect cell survival and immune responses []. Given their critical importance, proteins such as CLIC4 may be implicated in the development of neurodegenerative diseases; their significant expression was observed in AD, and their blockage in mice with AD improves their cognitive functions []. The upregulation of these proteins is consistent with the analysis of CSF, which revealed elevated protein levels and minor pleocytosis. These findings support the diagnosis and differentiation of SuS [].
Some proteins act in multiple signaling pathways, indicating crosstalk between NF-κB, MAPK, and others, which regulate immunity, inflammation, cell proliferation, and apoptosis. Disruption of AQP5/ERM interactions may cause exocrine dysfunction and dry eye, while immune-driven endothelial injury underlies microvascular occlusions and neurological, visual, and auditory deficits in SuS, like SS []. Furthermore, similar ERM upregulation occurs in mild cognitive impairment and early AD, indicating cytoskeletal dysregulation, which may contribute to SuS associated cognitive symptoms []. Elevated levels of oxygen reactive species and tissue damage likely amplify these pathways [].
SuS is primarily driven by autoantibodies and cytotoxic CD8+ T cell attacks on endothelial cells, triggering complement activation (especially of C3 and C5), which enhances inflammation and attracts immune cells. C5a-neutrophil crosstalk exacerbates this injury by enhancing neutrophil chemotaxis, adhesion, ROS release, and NET (neutrophil extracellular traps) formation []. These abnormal T cell-endothelial interactions further sustain endothelial injury. Further endothelial disruption in microangiopathy promotes cellular aggregation, increases inflammation, and reduces neuronal survival, ultimately leading to microvascular occlusion in the brain, retina, and cochlea.
The core pathological process in SuS is autoimmune-mediated endotheliopathy. Some researchers have proposed a possible viral etiology, though no clear causative agent has been identified []. Although the exact genetic causes remain unclear, individuals with a family history of autoimmune diseases and certain HLA alleles are at higher risk for SuS, with environmental factors like Epstein–Barr virus infections, hormonal influences, and chronic stress potentially triggering or exacerbating the disease [,]. This process can result in the formation of autoantibodies that target endothelial cells, contributing to vascular damage and the progression of disease. Minor contributing factors to SuS include hormonal influences, environmental triggers, chronic stress, and comorbid autoimmune diseases. SuS is significantly more common in women, which suggests that hormonal factors may affect both disease onset and severity. Psychological stress plays a compounding role by disrupting immune regulation and promoting inflammation, thereby exacerbating autoimmune activity. Additionally, the presence of other autoimmune disorders, such as systemic lupus erythematosus or MS, increases the risk of developing SuS, indicating a shared immunogenetic predisposition among these conditions []. Therefore, the clinical presentation of SuS and the tear fluid proteome may be influenced by factors such as sex, age, lifestyle, comorbidities, and environmental factors.
5. Conclusions
SuS is a rare autoimmune endotheliopathy characterized by encephalopathy, branch retinal artery occlusions, and sensorineural hearing loss. However, due to its diverse course and wide spectrum of symptoms, proper diagnosis is frequently challenging and can lead to misdiagnosis, such as with “atypical” MS. Differentiating SuS from MS at an early stage is key to achieving a favorable prognosis. Our case study demonstrates the potential of tear fluid proteomics as a supplementary diagnostic tool for SuS, providing valuable insights into the biochemical pathways associated with the disease. Proteomic analysis of tear fluid has detected more than 2880 distinct protein groups. The proteomic findings are consistent with serological and CSF profiles characteristic of SuS. Cluster analysis suggests the possibility of using proteomic analysis to help identify SuS from other neurological diagnoses.
However, it is important to note that this study is based on a single case involving an extremely rare disease. For this reason, no inferential statistics could be performed and all plots illustrate patterns of relative abundance, rather than statistically significant differences. Therefore, further research, such as a study on a larger set of patients with SuS, is necessary to confirm the importance of monitoring the levels of selected proteins and to generalize these findings. However, this study offers promising evidence that the expression levels of specific proteins, differentially regulated across various neurological disorders, may eventually serve as a supporting tool in establishing the final diagnosis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app152312446/s1, Table S1: 2880 distinct protein groups identified by Thermo Proteome Discoverer 2.5.0.400 software, classified by setting the criteria that master is equal to master; Table S2: The up-regulation of the actin cytoskeleton pathway with 11 enrichment proteins identified by the GSEA Preranked tool v. 4.2.3.
Author Contributions
Conceptualization, S.T., V.T., M.T., S.K. and T.I.; methodology, S.T., I.T., M.M., P.B., M.T. and V.T.; software, M.M. and P.B.; validation, M.M.; formal analysis, S.T., V.T., A.R., V.G., M.T., S.K. and T.I.; investigation, S.T., I.T. and V.T.; resources, T.I., S.K., M.T., A.R., V.G. and V.T.; data curation, S.T., I.T., M.M. and P.B.; writing—original draft preparation, S.T. and V.T.; visualization, S.T.; supervision, V.T.; project administration, V.T.; funding acquisition, V.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Slovak Research and Development Agency, Ministry of Education, Research, Development, and Youth of the SR, under project APVV-19-0476.
Institutional Review Board Statement
The study was conducted under the Declaration of Helsinki and approved by the ethical committee of Louis Pasteur University Hospital in Košice (protocol code 2020/EK/06042 and 25 June 2020 date of approval, extended until January 2030) for studies involving the tear fluid of humans.
Data Availability Statement
The data presented in this study are available on the PRIDE platform [http://www.ebi.ac.uk/pride], project accession: PXD070890 and 10.6019/PXD070890.
Acknowledgments
We would like to thank Monika Moravská and Marek Horňák from the Department of Ophthalmology, Pavol Jozef Šafárik University in Košice Faculty of Medicine for approving the research of patient with SuS and healthy subject who provided us with tear fluid for the tear fluid research. We thank Marlies Gijs for her support via COST Action CA24112 and the Tear Research Network.
Conflicts of Interest
Author Miriama Turoková was employed by the company Ophthalmology Clinic, ProCare Košice Polyclinic Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Dörr, J.; Krautwald, S.; Wildemann, B.; Jarius, S.; Ringelstein, M.; Duning, T.; Aktas, O.; Ringelstein, E.B.; Paul, F.; Kleffner, I. Characteristics of Susac syndrome: A review of all reported cases. Nat. Rev. Neurol. 2013, 9, 307–316. [Google Scholar] [CrossRef]
- David, C.; Sacré, K.; Henri-Feugeas, M.C.; Klein, I.; Doan, S.; Cohen, F.A.; Jouvent, E.; Papo, T. Susac syndrome: A scoping review. Autoimmun. Rev. 2022, 21, 103097. [Google Scholar] [CrossRef] [PubMed]
- Yalçınkaya, B.C.; Çetin, Ö.E.; Kılıç, H.; Demirci, O.; Çokyaman, T.; Uygunoğlu, U. A rare presentation of Susac syndrome: Report of three pediatric cases. Mult. Scler. Relat. Disord. 2021, 53, 103074. [Google Scholar] [CrossRef]
- Susac, J.O.; Hardman, J.M.; Selhorst, J.B. Microangiopathy of the brain and retina. Neurology 1979, 29, 313–316. [Google Scholar] [CrossRef]
- Sikorska, K.; Woźniak, M.; Dżaman, K. Susac syndrome—A review of current knowledge and own experience. Otolaryngol. Pol. 2023, 77, 20–25. [Google Scholar] [CrossRef]
- Kamieniecka, O. Susac Syndrome: A Multidisciplinary Approach to Diagnosis and Management with an Emphasis on Ophthalmic Involvement. Semin. Ophthalmol. 2024, 40, 727–732. [Google Scholar] [CrossRef]
- Alshehri, B.; Amir, A.; Alshehri, R.; Alturki, H.; Aljudi, T.; Alzuabi, A. Susac Syndrome with Classical Triad: A Case Report and Literature Review. Ann. Afr. Med. 2025, 24, 496–500, (In French and English). [Google Scholar] [CrossRef] [PubMed]
- Guttieres, L.; Vanneli, L.; Demortiere, S.; Perriguey, M.; Elziere, M.; Durozard, P.; Boutiere, C.; Rico, A.; Hilezian, F.; Stellmann, J.P.; et al. Fluorescein angiography as a surrogate marker of disease activity in Susac Syndrome. Neurol. Neuroimmunol. Neuroinflamm. 2025, 12, e200379. [Google Scholar] [CrossRef]
- Virhammar, J.; Nääs, A.; Fällmar, D.; Cunningham, J.L.; Klang, A.; Ashton, N.J.; Jackmann, S.; Westman, G.; Frithiof, R.; Blennow, K.; et al. Biomarkers for central nervous system injury in cerebrospinal fluid are elevated in COVID-19 and associated with neurological symptoms and disease severity. Eur. J. Neurol. 2021, 28, 3324–3331. [Google Scholar] [CrossRef]
- Patel, P.; Jamal, Z.; Ramphul, K. Immunoglobulin. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Guan, X.; Zhu, S.; Song, J.; Liu, K.; Liu, M.; Xie, L.; Wang, Y.; Wu, J.; Xu, X.; Pang, T. Microglial CMPK2 promotes neuroinflammation and brain injury after ischemic stroke. Cell Rep. Med. 2024, 5, 101522. [Google Scholar] [CrossRef]
- Kim, K.S.; Yang, H.I. Thymosin β4 in rheumatoid arthritis: Friend or foe. Biomed. Rep. 2017, 7, 205–208. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dhawan, P.; Richmond, A. Correction: A novel NF-κB-inducing kinase–MAPK signaling pathway up-regulates NF-κB activity in melanoma cells. J. Biol. Chem. 2022, 298, 102315. [Google Scholar] [CrossRef]
- Klemann, C.; Wagner, L.; Stephan, M.; von Hörsten, S. Cut to the chase: A review of CD26/dipeptidyl peptidase-4’s (DPP4) entanglement in the immune system. Clin. Exp. Immunol. 2016, 185, 1–21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosa, L.; Ianiro, G.; Cutone, A. Special issue “New insights into lactoferrin”. Int. J. Mol. Sci. 2025, 26, 9891. [Google Scholar] [CrossRef]
- Li, Y.Q.; Guo, C. A review on lactoferrin and central nervous system diseases. Cells 2021, 10, 1810. [Google Scholar] [CrossRef]
- Leśniak, W.; Filipek, A. S100A6 protein—Expression and function in norm and pathology. Int. J. Mol. Sci. 2023, 24, 1341. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yang, J.; Yang, Z.; Zhang, M.; Zhang, J.; Sun, C. Biological functions and clinical implications of the CMPK2 across multisystemic diseases. Cell Biosci. 2025, 15, 122. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Alvarez, L.; Martínez-Sánchez, M.E.; Gray, E.; Pérez-Figueroa, E.; Ortega, E. Aminopeptidase N/CD13 crosslinking promotes the activation and membrane expression of integrin CD11b/CD18. Biomolecules 2023, 13, 1488. [Google Scholar] [CrossRef]
- Becerra-Hernández, L.V.; Escobar-Betancourt, M.I.; Pimienta-Jiménez, H.J.; Buriticá, E. Crystallin alpha-B overexpression as a possible marker of reactive astrogliosis in human cerebral contusions. Front. Cell. Neurosci. 2022, 16, 838551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yao, C.; Zhou, X.; Liu, S.; Qi, L.; Zhu, S.; Zhao, C.; Hu, D.; Shen, W. Glutathione degrading enzymes in the complex landscape of tumors (review). Int. J. Oncol. 2024, 65, 72. [Google Scholar] [CrossRef]
- Rodriguez, I.; Carnevale, K.J.F. Systematic review: JAK-STAT regulation and its impact on inflammation response in ARDS from COVID-19. Immuno 2024, 4, 147–158. [Google Scholar] [CrossRef]
- Sun, H.; Xu, C.; Xiong, Z.; Liu, M.; Ning, X.; Zhuang, Y. Therapeutic Prospects and Potential Mechanisms of Prdx6: As a Novel Target in Musculoskeletal Disorders. Front. Physiol. 2025, 16, 1524100. [Google Scholar] [CrossRef]
- Nikolovska, K.; Seidler, U.E.; Stock, C. The role of plasma membrane sodium/hydrogen exchangers in gastrointestinal functions: Proliferation and differentiation, fluid/electrolyte transport and barrier integrity. Front. Physiol. 2022, 13, 899286. [Google Scholar] [CrossRef] [PubMed]
- Collier, J.M.; Metwally, S.; McFarland, M.; Krishna, S.; Kurella, P.; Fiesler, V.; Stauffer, M.; Begum, G.; Kofler, J.; Sun, D. Upregulation of Na/H Exchanger in Astrogliosis and Early Alzheimer’s Disease Pathogenesis. Aging Dis. 2025, 16, 3546–3566. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Liu, X.; Tian, B.; Cheng, Y.; Li, L. Neuroprotective effect of Na+/H+ exchangers isoform-1 inactivation against 6-hydroxydopamine-induced mitochondrial dysfunction and neuronal apoptosis in Parkinson’s disease models. Drug Dev. Res. 2021, 82, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Williams, W.M.; Torres, S.; Siedlak, S.L.; Castellani, R.J.; Perry, G.; Smith, M.A.; Zhu, X. Antimicrobial peptide β-defensin-1 expression is upregulated in Alzheimer’s brain. J. Neuroinflamm. 2013, 10, 127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Szekeres, M.; Ivitz, E.; Datki, Z.; Kálmán, J.; Pákáski, M.; Várhelyi, Z.P.; Klivényi, P.; Zádori, D.; Somogyvári, F.; Szolnoki, Z.; et al. Relevance of defensin β-2 and α-defensins (HNP1–3) in Alzheimer’s disease. Psychiatry Res. 2016, 239, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Maffei, F.A.; Heine, R.P.; Whalen, M.J.; Mortimer, L.F.; Carcillo, J.A. Levels of antimicrobial molecules defensin and lactoferrin are elevated in the cerebrospinal fluid of children with meningitis. Pediatrics 1999, 103, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, F.; Nakamori, M. Blood-brain barrier disruption in neuroimmunological disease. Int. J. Mol. Sci. 2024, 25, 10625. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chandrasekaran, P.; Weiskirchen, S.; Weiskirchen, R. Structure, Functions, and Implications of Selected Lipocalins in Human Disease. Int. J. Mol. Sci. 2024, 25, 4290. [Google Scholar] [CrossRef]
- Kolanek, A.; Cemaga, R.; Maciejczyk, M. Role and Diagnostic Significance of Apolipoprotein D in Selected Neurodegenerative Disorders. Diagnostics 2024, 14, 2814. [Google Scholar] [CrossRef]
- Del Valle, E.; Rubio-Sardón, N.; Menéndez-Pérez, C.; Martínez-Pinilla, E.; Navarro, A. Apolipoprotein D as a potential biomarker in neuropsychiatric disorders. Int. J. Mol. Sci. 2023, 24, 15631. [Google Scholar] [CrossRef]
- Du, H.; Xu, T.; Yu, S.; Wu, S.; Zhang, J. Mitochondrial metabolism and cancer therapeutic innovation. Signal Transduct. Target. Ther. 2025, 10, 245. [Google Scholar] [CrossRef]
- Misra, R.; Rasmussen, J.; Sripadrao, S.; Sudhakar, H.; Gopu, A.; Ahmed, N.; Williams, Y.; Frasier, K. JAK/STAT Pathway in Psoriasis and Psoriatic Arthritis: Insights into Inflammation and Tissue Remodeling. Dermis 2025, 5, 38. [Google Scholar] [CrossRef]
- Kitchen, P.; Öberg, F.; Sjöhamn, J.; Hedfalk, K.; Bill, R.M.; Conner, A.C.; Conner, M.T.; Törnroth-Horsefield, S. Plasma membrane abundance of human aquaporin 5 is dynamically regulated by multiple pathways. PLoS ONE 2015, 10, e0143027. [Google Scholar] [CrossRef]
- Qi, W.; Tian, J.; Wang, G.; Yan, Y.; Wang, T.; Wei, Y.; Wang, Z.; Zhang, G.; Zhang, Y.; Wang, J. Advances in cellular and molecular pathways of salivary gland damage in Sjögren’s syndrome. Front. Immunol. 2024, 15, 1405126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zayani, Z.; Matinahmadi, A.; Tavakolpournegari, A.; Bidooki, S.H. Exploring Stressors: Impact on Cellular Organelles and Implications for Cellular Functions. Stresses 2025, 5, 26. [Google Scholar] [CrossRef]
- Chen, R.; Pan, C.; Mao, X.; Zhang, Y.; Chen, G.; Xu, M.; Nivar, J.; Tao, Y.; Cao, H.; Li, J. Chloride intracellular channel 4 blockade improves cognition in mice with Alzheimer’s disease: CLIC4 protein expression and tau protein hyperphosphorylation. Int. J. Biol. Macromol. 2024, 278, 134972. [Google Scholar] [CrossRef]
- Fonderska, P.; Tomalka-Kochanowska, J.; Kochanowski, J.; Domitrz, I. Susac’s syndrome diagnostic difficulties—The neurological point of view. Neurol. Neurochir. Pol. 2022, 56, 141–147. [Google Scholar] [CrossRef]
- D’Agostino, C.; Parisis, D.; Chivasso, C.; Hajiabbas, M.; Soyfoo, M.S.; Delporte, C. Aquaporin-5 Dynamic Regulation. Int. J. Mol. Sci. 2023, 24, 1889. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vega, I.E.; Umstead, A.; Wygant, C.M.; Beck, J.S.; Counts, S.E. Ezrin expression is increased during disease progression in a tauopathy mouse model and Alzheimer’s disease. Curr. Alzheimer Res. 2018, 15, 1086–1095. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marrodan, M.; Fiol, M.P.; Correale, J. Susac syndrome: Challenges in the diagnosis and treatment. Brain 2022, 145, 58–871. [Google Scholar] [CrossRef] [PubMed]
- Gross, C.C.; Meyer, C.; Bhatia, U.; Yshii, L.; Kleffner, I.; Bauer, J.; Tröscher, A.R.; Schulte-Mecklenbeck, A.; Herich, S.; Schneider-Hohendorf, T.; et al. CD8+ T cell-mediated endotheliopathy is a targetable mechanism of neuro-inflammation in Susac syndrome. Nat. Commun. 2019, 10, 5779. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; Vieira, B.; Maio, T.; Moreira, J.; Sampaio, F. Susac’s syndrome: An updated review. Neuroophthalmology 2020, 44, 355–360. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Draborg, A.H.; Duus, K.; Houen, G. Epstein-Barr virus in systemic autoimmune diseases. Clin. Dev. Immunol. 2013, 2013, 535738. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zeng, Y.; Suo, C.; Yao, S.; Lu, D.; Larsson, H.; D’Onofrio, B.M.; Lichtenstein, P.; Fang, F.; Valdimarsdóttir, U.A.; Song, H. Genetic associations between stress-related disorders and autoimmune disease. Am. J. Psychiatry 2023, 180, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, S.; Nannini, G.; Cianchi, F.; Coratti, F.; Amedei, A. The impact of microbiota–immunity–hormone interactions on autoimmune diseases and infection. Biomedicines 2024, 12, 616. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).