Impact of Physician Height and Experience on Eye Lens Dose in Interventional Cardiology: An Initial Study
Abstract
1. Introduction
2. Materials and Methods
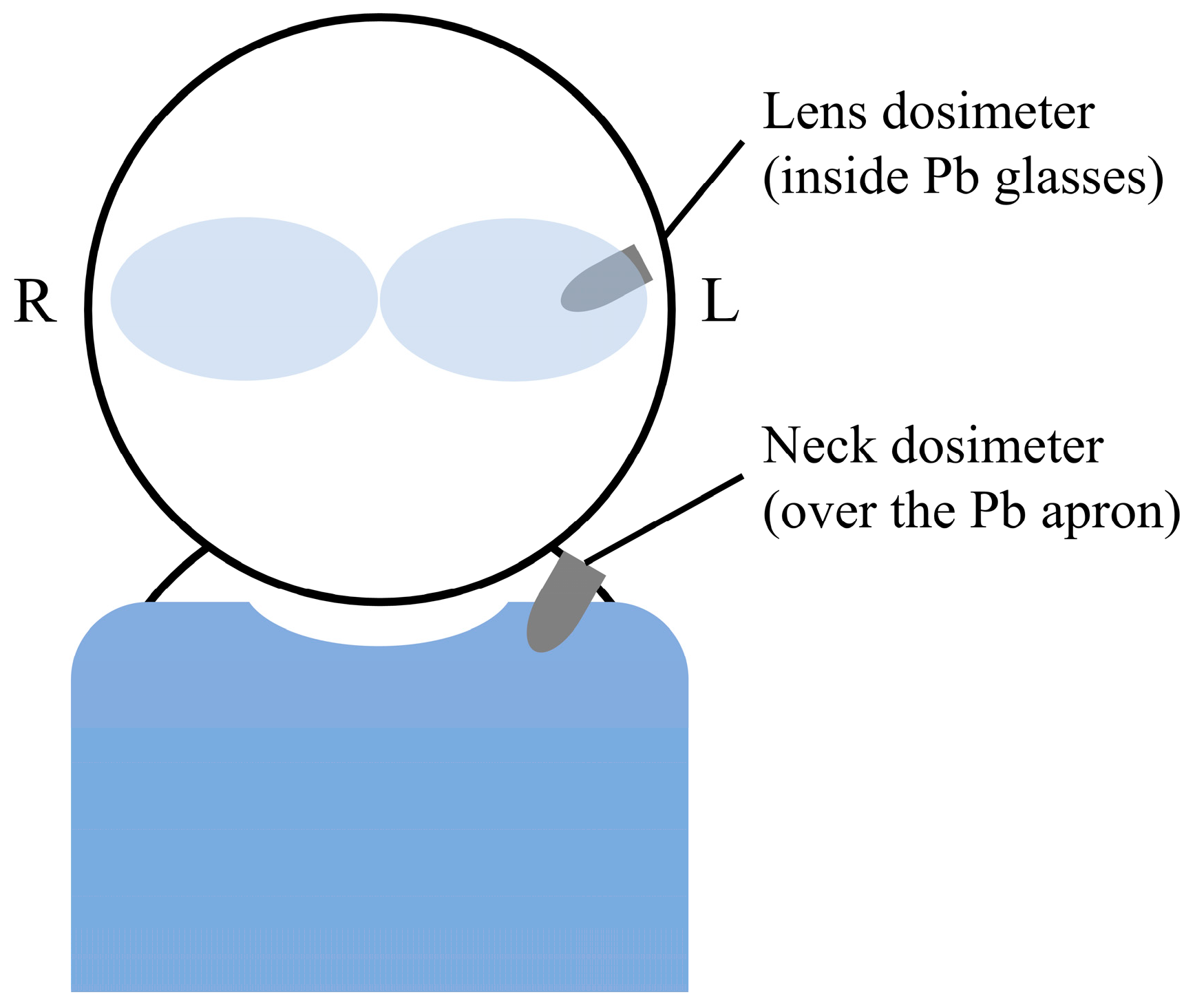
2.1. Equipment
2.2. Dose Evaluation
2.3. Statistics
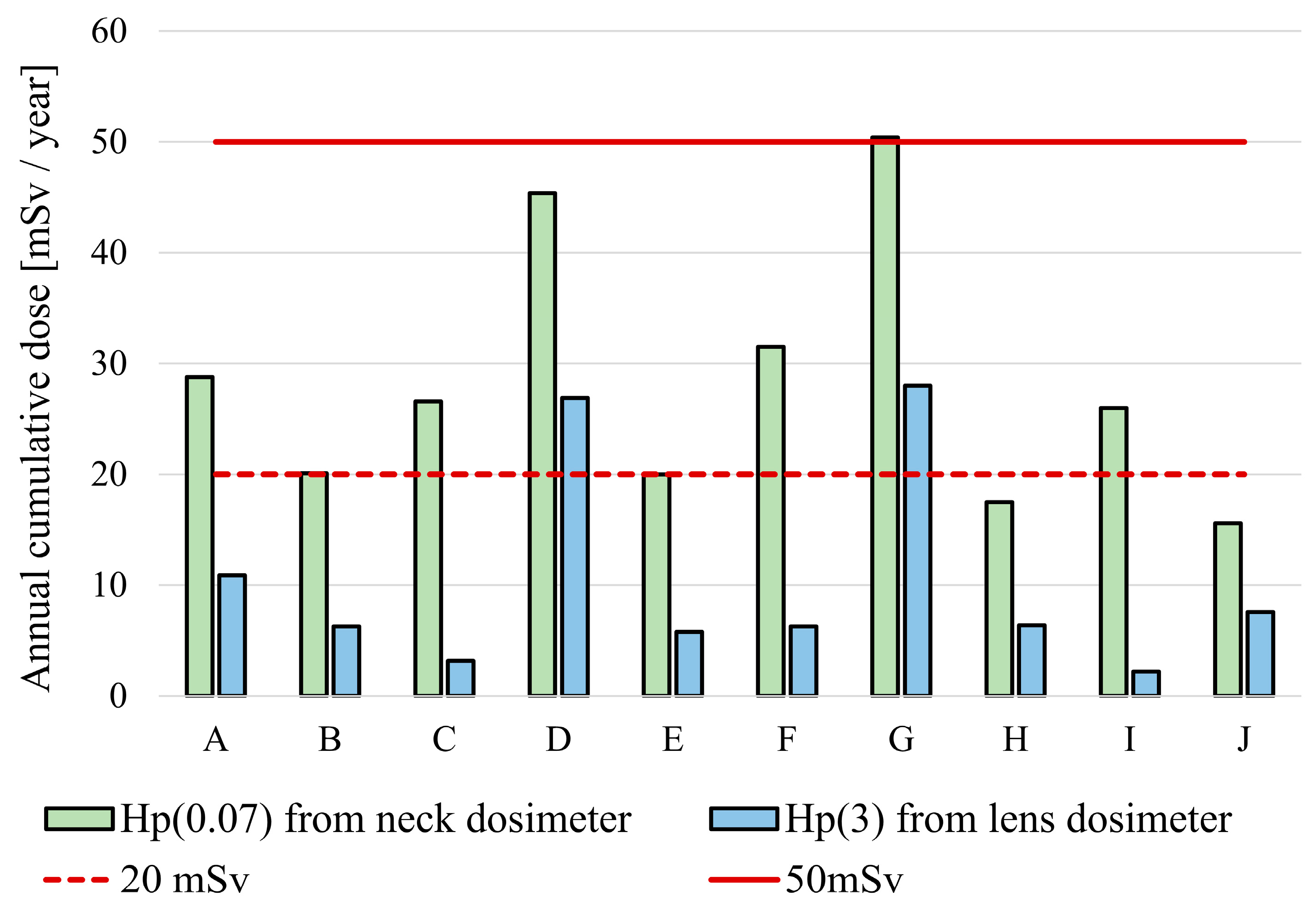
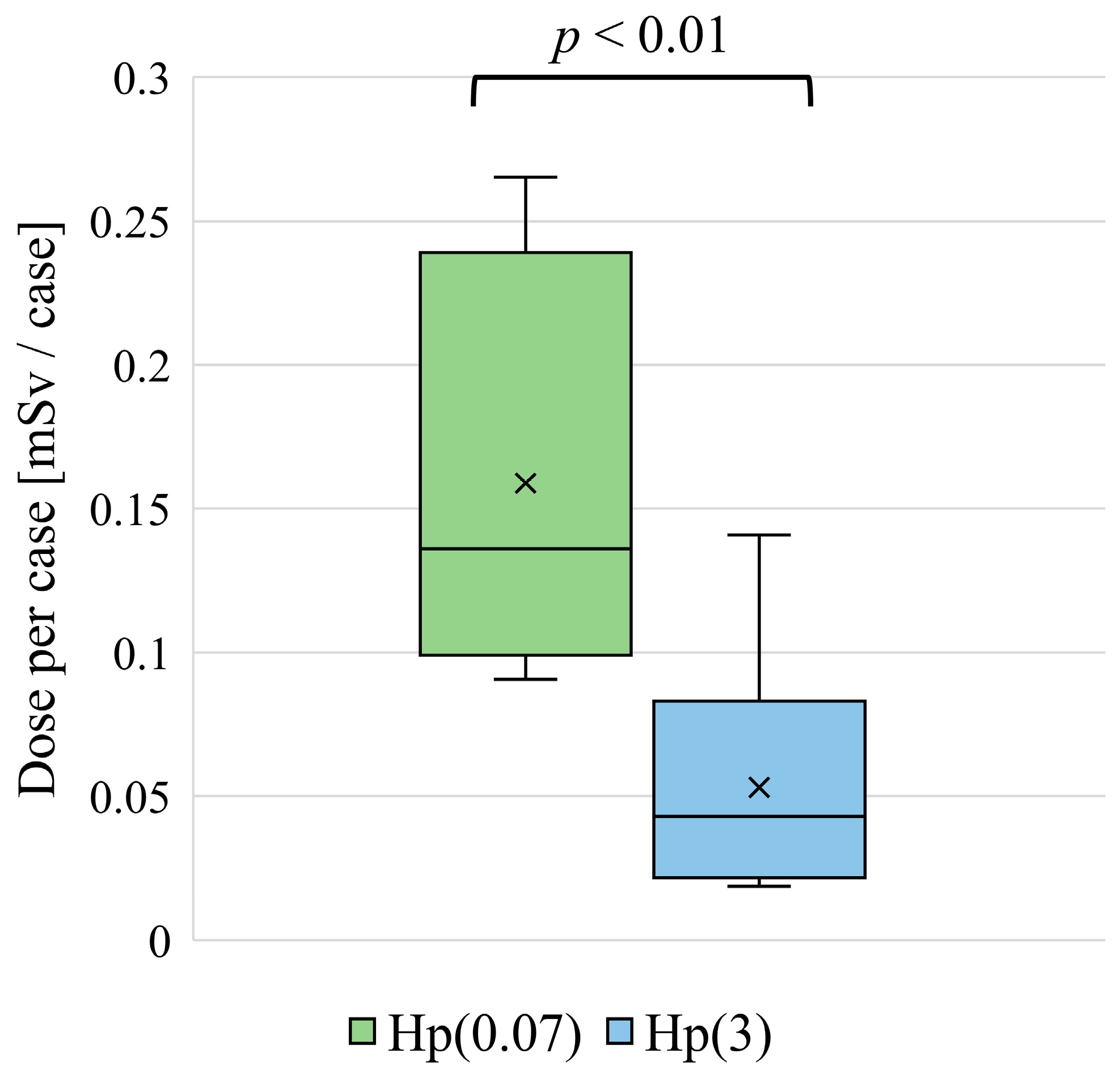
3. Results
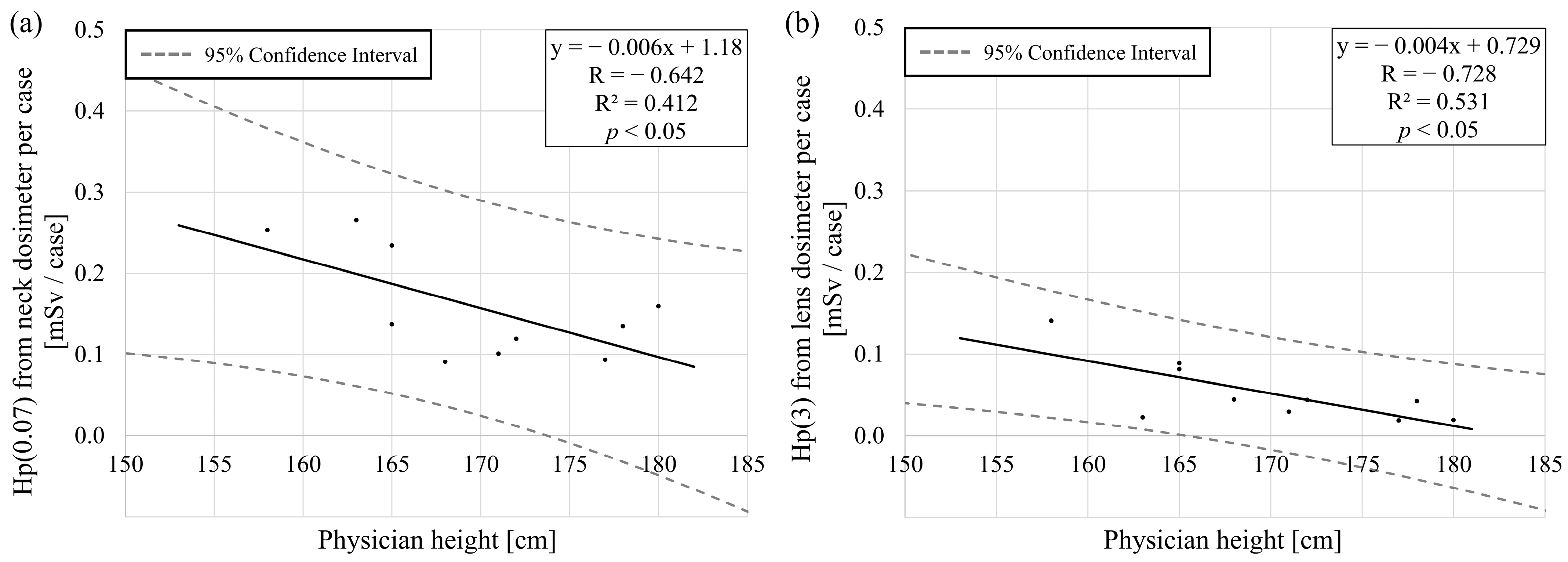
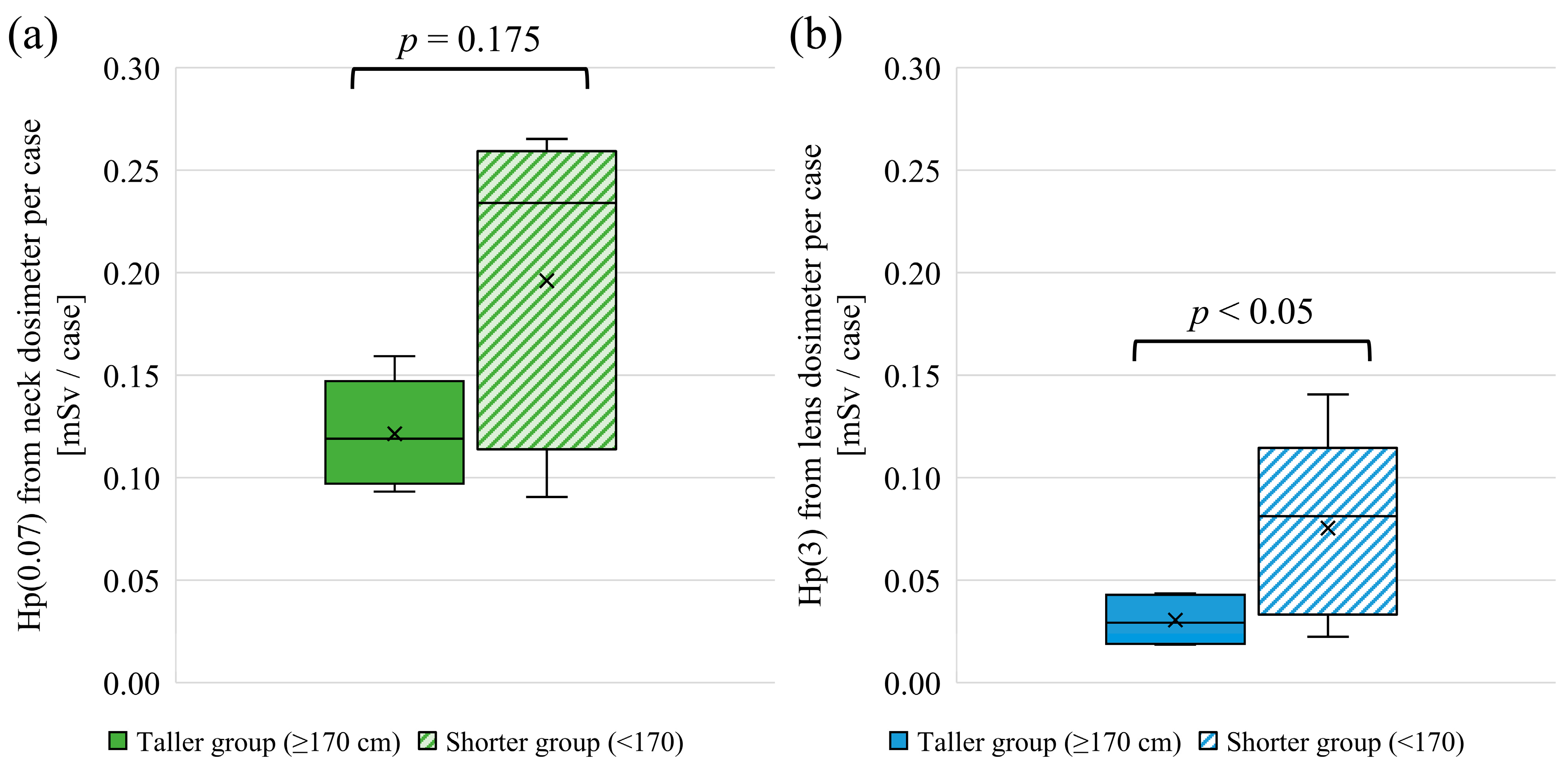
3.1. Height and Lens Dose
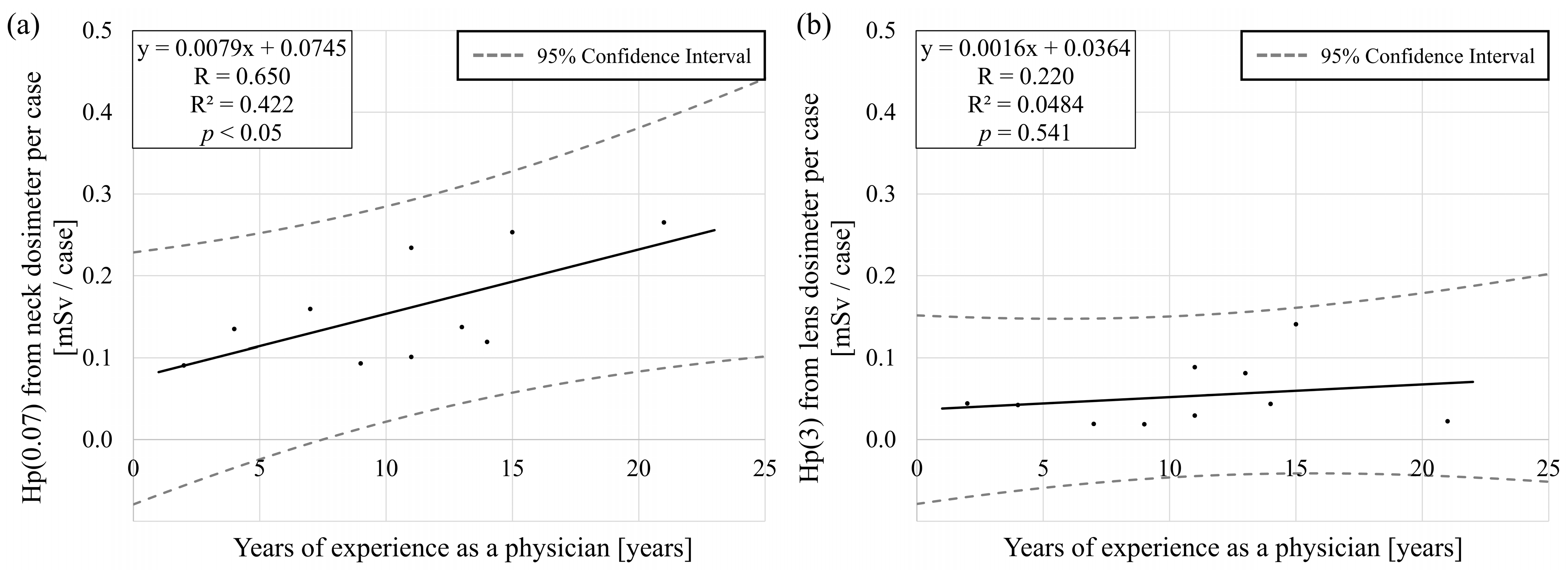
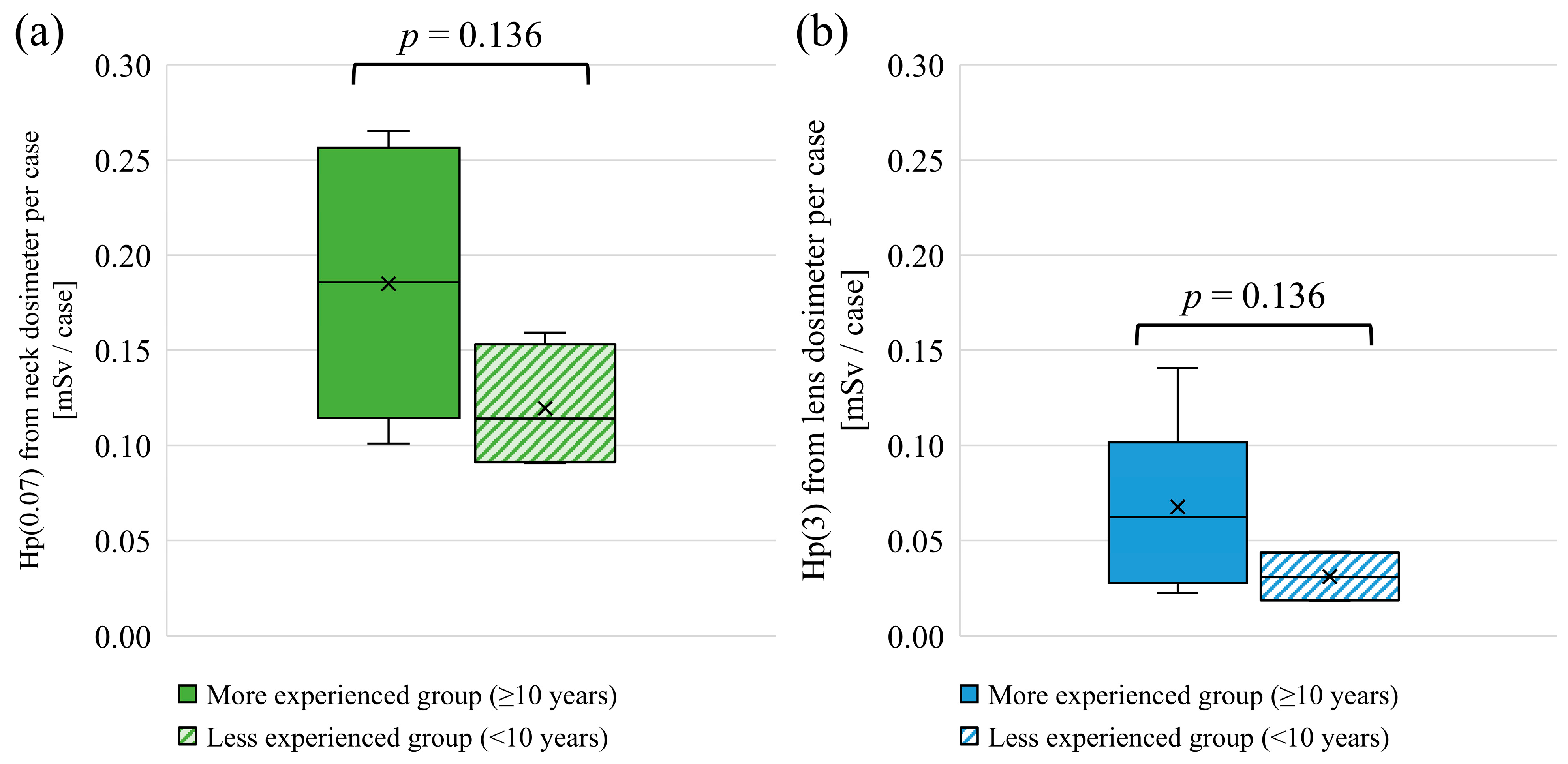
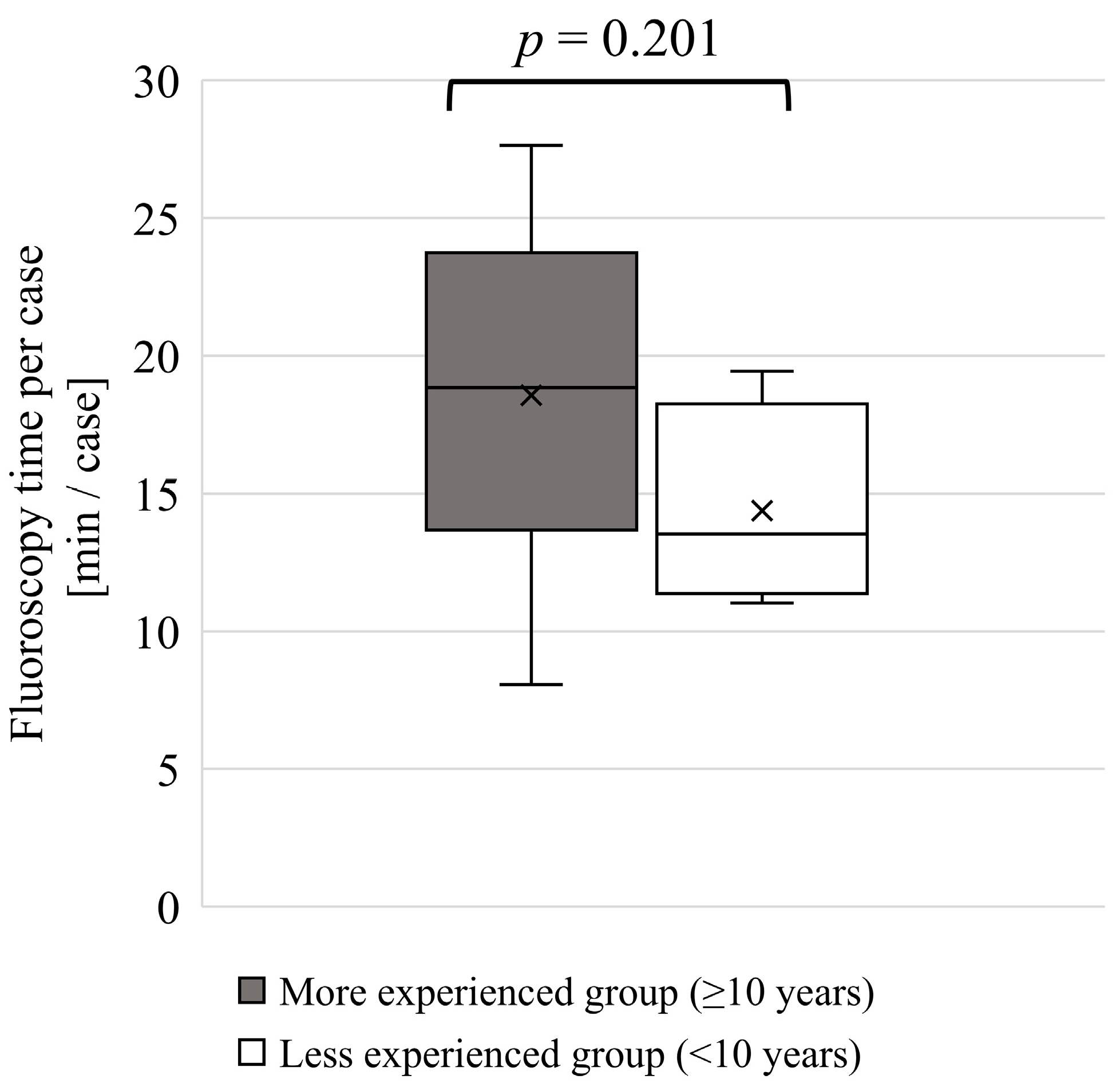
3.2. Experience as a Physician and Lens Dose
4. Discussion
4.1. Height and Lens Dose
4.2. Experience as a Physician and Lens Dose
4.3. Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ICRP | International Commission on Radiological Protection |
| IAEA | International Atomic Energy Agency |
| IR | Interventional Radiology |
| IC | Interventional Cardiology |
| Hp(10) | personal dose equivalent at 1 cm depth |
| Hp(0.07) | personal dose equivalent at 70 μm depth |
| Hp(3) | personal dose equivalent at 3 mm depth |
References
- ICRP. Radiological Protection in Cardiology. ICRP Publication 120; Ann. ICRP: Ottawa, CA, USA, 2013. [Google Scholar]
- Elfandi, A.; Safirstein, J.G. Transradial PCI and Same Day Discharge. Curr. Treat. Options Cardiovasc. Med. 2018, 20, 10. [Google Scholar] [CrossRef]
- Sandhu, K.; Nadar, S.K. Percutaneous Coronary Intervention in the Elderly. Int. J. Cardiol. 2015, 199, 342–355. [Google Scholar] [CrossRef]
- Gianoli, M.; de Jong, A.R.; Jacob, K.A.; Namba, H.F.; van der Kaaij, N.P.; van der Harst, P.; Suyker, W.J. Minimally Invasive Surgery or Stenting for Left Anterior Descending Artery Disease—Meta-Analysis. Int. J. Cardiol. Heart Vasc. 2022, 40, 101046. [Google Scholar] [CrossRef]
- Kato, M.; Chida, K.; Nakamura, M.; Toyoshima, H.; Terata, K.; Abe, Y. New Real-Time Patient Radiation Dosimeter for Use in Radiofrequency Catheter Ablation. J. Radiat. Res. 2019, 60, 215–220. [Google Scholar] [CrossRef]
- Chida, K.; Fuda, K.; Saito, H.; Takai, Y.; Takahashi, S.; Yamada, S.; Kohzuki, M.; Zuguchi, M. Patient Skin Dose in Cardiac Interventional Procedures: Conventional Fluoroscopy versus Pulsed Fluoroscopy. Catheter. Cardiovasc. Interv. 2007, 69, 115–121. [Google Scholar] [CrossRef]
- Srimahachota, S.; Udayachalerm, W.; Kupharang, T.; Sukwijit, K.; Krisanachinda, A.; Rehani, M. Radiation Skin Injury Caused by Percutaneous Coronary Intervention, Report of 3 Cases. Int. J. Cardiol. 2012, 154, e31–e33. [Google Scholar] [CrossRef]
- Wagner, L.K.; McNeese, M.D.; Marx, M.V.; Siegel, E.L. Severe Skin Reactions from Interventional Fluoroscopy: Case Report and Review of the Literature. Radiology 1999, 213, 773–776. [Google Scholar] [CrossRef]
- Koenig, T.R.; Wolff, D.; Mettler, F.A.; Wagner, L.K. Skin Injuries from Fluoroscopically Guided Procedures. Am. J. Roentgenol. 2001, 177, 3–11. [Google Scholar] [CrossRef]
- Inaba, Y.; Chida, K.; Murabayashi, Y.; Endo, M.; Otomo, K.; Zuguchi, M. An Initial Investigation of a Wireless Patient Radiation Dosimeter for Use in Interventional Radiology. Radiol. Phys. Technol. 2020, 13, 321–326. [Google Scholar] [CrossRef]
- Roguin, A.; Goldstein, J.; Bar, O.; Goldstein, J.A. Brain and Neck Tumors Among Physicians Performing Interventional Procedures. Am. J. Cardiol. 2013, 111, 1368–1372. [Google Scholar] [CrossRef]
- Eagan, J.T., Jr.; Jones, C.T. Cutaneous Cancers in an Interventional Cardiologist: A Cautionary Tale. J. Interv. Cardiol. 2011, 24, 49–55. [Google Scholar] [CrossRef]
- Vano, E.; Kleiman, N.J.; Duran, A.; Romano-Miller, M.; Rehani, M.M. Radiation-Associated Lens Opacities in Catheterization Personnel: Results of a Survey and Direct Assessments. J. Vasc. Interv. Radiol. 2013, 24, 197–204. [Google Scholar] [CrossRef]
- Kato, M.; Chida, K.; Ishida, T.; Sasaki, F.; Toyoshima, H.; Oosaka, H.; Terata, K.; Abe, Y.; Kinoshita, T. Occupational Radiation Exposure Dose of The Eye in Department of Cardiac Arrhythmia Physician. Radiat. Prot. Dosim. 2019, 187, 361–368. [Google Scholar] [CrossRef]
- Werner, N.; Nickenig, G.; Sinning, J.-M. Complex PCI Procedures: Challenges for the Interventional Cardiologist. Clin. Res. Cardiol. 2018, 107, 64–73. [Google Scholar] [CrossRef]
- Kheifets, M.; Vons, S.A.; Bental, T.; Vaknin-Assa, H.; Greenberg, G.; Samara, A.; Codner, P.; Wittberg, G.; Talmor Barkan, Y.; Perl, L.; et al. Temporal Trends in Complex Percutaneous Coronary Interventions. Front. Cardiovasc. Med. 2022, 9, 913588. [Google Scholar] [CrossRef]
- Ali, A.; Al-Nuaimi, M.; Khan, B.; Burki, S.; Nishtar, T.; Yaseen, M.; Shah, S.S.A.; Irfan, M. Assessment of Occupational Radiation Doses to Eye Lens during Interventional Radiology Procedures. Radiat. Phys. Chem. 2024, 225, 112131. [Google Scholar] [CrossRef]
- Bellamy, M.B.; Miodownik, D.; Quinn, B.; Dauer, L. Occupational Eye Lens Dose Over Six Years in The Staff of A Us High-Volume Cancer Center. Radiat. Prot. Dosim. 2020, 192, 321–327. [Google Scholar] [CrossRef]
- Merrachi, N.-A.; Bouchard-Bellavance, R.; Perreault, P.; Gilbert, P.; Soulez, G.; Bouchard, L.; Oliva, V.L.; Giroux, M.-F.; Normandeau, L.; Therasse, E. Eye Lens Dosimetry in Interventional Radiology: Assessment with Dedicated Hp(3) Dosimeters. Can. Assoc. Radiol. J. 2021, 72, 317–323. [Google Scholar] [CrossRef]
- Ainsbury, E.A.; Barnard, S.G.R. Sensitivity and Latency of Ionising Radiation-Induced Cataract. Exp. Eye Res. 2021, 212, 108772. [Google Scholar] [CrossRef]
- Durchschlag, H.; Fochler, C.; Abraham, K.; Kulawik, B. Radiation Effects on Eye Components. Radiat. Phys. Chem. 1999, 55, 691–697. [Google Scholar] [CrossRef]
- Hamada, N.; Azizova, T.V.; Little, M.P. An Update on Effects of Ionizing Radiation Exposure on the Eye. Br. J. Radiol. 2020, 93, 20190829. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, K.; Lertsuwunseri, V.; Srimahachota, S.; Krisanachinda, A.; Tulvatana, W.; Khambhiphant, B.; Sudchai, W.; Rehani, M. Eye Lens Dosimetry and the Study on Radiation Cataract in Interventional Cardiologists. Phys. Medica 2017, 44, 232–235. [Google Scholar] [CrossRef]
- Vañó, E.; González, L.; Guibelalde, E.; Fernández, J.M.; Ten, J.I. Radiation Exposure to Medical Staff in Interventional and Cardiac Radiology. Br. J. Radiol. 1998, 71, 954–960. [Google Scholar] [CrossRef] [PubMed]
- ICRP. Avoidance of Radiation Injuries from Medical Interventional Procedures; ICRP Publication 85; Ann. ICRP: Ottawa, CA, USA, 2000. [Google Scholar]
- Nakashima, E.; Neriishi, K.; Minamoto, A. A reanalysis of atomic-bomb cataract data, 2000–2002: A threshold analysis. Health Phys. 2006, 90, 154. [Google Scholar] [CrossRef] [PubMed]
- Worgul, B.V.; Kundiyev, Y.I.; Sergiyenko, N.M.; Chumak, V.V.; Vitte, P.M.; Medvedovsky, C.; Bakhanova, E.V.; Junk, A.K.; Kyrychenko, O.Y.; Musijachenko, N.V.; et al. Cataracts among Chernobyl Clean-up Workers: Implications Regarding Permissible Eye Exposures. Radiat. Res. 2007, 167, 233–243. [Google Scholar] [CrossRef]
- Neriishi, K.; Nakashima, E.; Minamoto, A.; Fujiwara, S.; Akahoshi, M.; Mishima, H.K.; Kitaoka, T.; Shore, R.E. Postoperative Cataract Cases among Atomic Bomb Survivors: Radiation Dose Response and Threshold. Radiat. Res. 2007, 168, 404–408. [Google Scholar] [CrossRef]
- ICRP. ICRP Statement on Tissue Reactions/Early and Late Effects of Radiation in Normal Tissues and Organs—Threshold Doses for Tissue Reactions in a Radiation Protection Context; ICRP Publication 118; Ann. ICRP: Ottawa, CA, USA, 2012. [Google Scholar]
- Sagehashi, K.; Haga, Y.; Takahira, S.; Tanabe, M.; Nakamura, M.; Sota, M.; Kaga, Y.; Abe, M.; Tada, N.; Chida, K. Evaluation of Radiation Dose to the Lens in Interventional Cardiology Physicians before and after Dose Limit Regulation Changes. J. Radiol. Prot. 2024, 44, 031512. [Google Scholar] [CrossRef]
- Haga, Y.; Chida, K.; Kaga, Y.; Sota, M.; Meguro, T.; Zuguchi, M. Occupational Eye Dose in Interventional Cardiology Procedures. Sci. Rep. 2017, 7, 569. [Google Scholar] [CrossRef]
- Ohno, S.; Konta, S.; Shindo, R.; Yamamoto, K.; Isobe, R.; Inaba, Y.; Suzuki, M.; Zuguchi, M.; Chida, K. Effect of Backscatter Radiation on the Occupational Eye-Lens Dose. J. Radiat. Res. 2024, 65, 450–458. [Google Scholar] [CrossRef]
- Yamada, A.; Haga, Y.; Sota, M.; Abe, M.; Kaga, Y.; Inaba, Y.; Suzuki, M.; Tada, N.; Zuguchi, M.; Chida, K. Eye Lens Radiation Dose to Nurses during Cardiac Interventional Radiology: An Initial Study. Diagnostics 2023, 13, 3003. [Google Scholar] [CrossRef]
- Hijikata, Y.; Yamashita, K.; Hatsusaka, N.; Nagata, T.; Kitamura, H.; Morota, K.; Matsuzaki, S.; Nakagami, K.; Hitomi, G.; Kuriyama, T.; et al. Prevalence of Cataractous Changes in the Eyes and Chronic Inflammatory Changes in the Hands Among Spine Surgeons. J. Bone Jt. Surg. 2025, 107, e25. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, T.; Moritake, T.; Nakagami, K.; Morota, K.; Hitomi, G.; Kitamura, H. Background Factors Affecting the Radiation Exposure of the Lens of the Eye among Nurses in Interventional Radiology: A Quantitative Observational Study. Nurs. Rep. 2024, 14, 413–427. [Google Scholar] [CrossRef] [PubMed]
- ICRP. The 2007 Recommendations of the International Commission on Radiological Protection; ICRP Publication 103; Ann. ICRP: Ottawa, CA, USA, 2007. [Google Scholar]
- IAEA. International Atomic Energy Agency, Implications for Occupational Radiation Protection of the New Dose Limit for the Lens of the Eye; IAEA TECDOC; IAEA: Vienna, Austria, 2013. [Google Scholar]
- Al-Haj, A.N.; Lobriguito, A.M.; Al-Gain, I. Staff Eye Doses in a Large Medical Centre in Saudi Arabia: Are They Meeting the New ICRP Recommendations? Radiat. Prot. Dosim. 2015, 165, 294–298. [Google Scholar] [CrossRef]
- Jacob, S.; Donadille, L.; Maccia, C.; Bar, O.; Boveda, S.; Laurier, D.; Bernier, M.-O. Eye Lens Radiation Exposure to Interventional Cardiologists: A Retrospective Assessment of Cumulative Doses. Radiat. Prot. Dosim. 2013, 153, 282–293. [Google Scholar] [CrossRef]
- Martin, C.J.; Magee, J.S. Assessment of Eye and Body Dose for Interventional Radiologists, Cardiologists, and Other Interventional Staff. J. Radiol. Prot. 2013, 33, 445. [Google Scholar] [CrossRef]
- Meijer, E.J.; van Zandvoort, D.W.H.; Loos, M.J.A.; Tseng, C.M.E.S.N.; van Pul, C. The Eye Lens Dose of the Interventionalist: Measurement in Practice. Phys. Medica 2022, 100, 1–5. [Google Scholar] [CrossRef]
- Bohari, A.; Hashim, S.; Ahmad, N.E.; Ghoshal, S.K.; Mohd Mustafa, S.N. Fluoroscopy-Guided Intervention Procedure Norms for Occupational Eye Radiation Dose: An Overall Evaluation. Radiat. Phys. Chem. 2021, 178, 108909. [Google Scholar] [CrossRef]
- Domienik, J.; Bissinger, A.; Grabowicz, W.; Jankowski, Ł.; Kręcki, R.; Makowski, M.; Masiarek, K.; Plewka, M.; Lubiński, A.; Peruga, J.Z. The Impact of Various Protective Tools on the Dose Reduction in the Eye Lens in an Interventional Cardiology—Clinical Study. J. Radiol. Prot. 2016, 36, 309. [Google Scholar] [CrossRef]
- Moriarty, H.K.; Clements, W.; Phan, T.; Wang, S.; Goh, G.S. Occupational Radiation Exposure to the Lens of the Eye in Interventional Radiology. J. Med. Imaging Radiat. Oncol. 2022, 66, 34–40. [Google Scholar] [CrossRef]
- Shindo, R.; Ohno, S.; Yamamoto, K.; Konta, S.; Inaba, Y.; Suzuki, M.; Zuguchi, M.; Chida, K. Comparison of Shielding Effects of Over-Glasses-Type and Regular Eyewear in Terms of Occupational Eye Dose Reduction. J. Radiol. Prot. 2024, 44, 023501. [Google Scholar] [CrossRef] [PubMed]
- van Rooijen, B.D.; de Haan, M.W.; Das, M.; Arnoldussen, C.W.K.P.; de Graaf, R.; van Zwam, W.H.; Backes, W.H.; Jeukens, C.R.L.P.N. Efficacy of Radiation Safety Glasses in Interventional Radiology. Cardiovasc. Interv. Radiol. 2014, 37, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- ICRU Report 60. Fundamental Quantities and Units for Ionizing Radiation. J. ICRU 1998. Available online: https://www.icru.org/report/fundamental-quantities-and-units-for-ionizing-radiation-report-60/ (accessed on 1 October 2025).
- Ishii, H.; Haga, Y.; Sota, M.; Inaba, Y.; Chida, K. Performance of the DOSIRISTM Eye Lens Dosimeter. J. Radiol. Prot. 2019, 39, N19. [Google Scholar] [CrossRef]
- Mori, Y.; Isobe, T.; Takei, H.; Miyazaki, S.; Kamizawa, S.; Tomita, T.; Kobayashi, D.; Sakurai, H.; Sakae, T. Evaluation of Basic Characteristics of 3-Mm Dose Equivalent Measuring Instrument for Evaluating Lens Exposure Dose in Radiotherapy. J. Med. Radiat. Sci. 2023, 70, 154–160. [Google Scholar] [CrossRef]
- Suliman, I.I. Review of Various Methods for Occupational Eye Lens Dosimetry in X-Ray Interventional Procedures. Radiat. Phys. Chem. 2025, 236, 112936. [Google Scholar] [CrossRef]
- Gangl, A.; Deutschmann, H.A.; Portugaller, R.H.; Stücklschweiger, G. Influence of Safety Glasses, Body Height and Magnification on the Occupational Eye Lens Dose during Pelvic Vascular Interventions: A Phantom Study. Eur. Radiol. 2022, 32, 1688–1696. [Google Scholar] [CrossRef]
- Koenig, A.M.; Etzel, R.; Greger, W.; Viniol, S.; Fiebich, M.; Thomas, R.P.; Mahnken, A.H. Protective Efficacy of Different Ocular Radiation Protection Devices: A Phantom Study. Cardiovasc. Interv. Radiol. 2020, 43, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Liu, T.; Caracappa, P.F.; Lin, H.; Gao, Y.; Dauer, L.T.; Xu, X.G. Influences of Operator Head Posture and Protective Eyewear on Eye Lens Doses in Interventional Radiology: A Monte Carlo Study. Med. Phys. 2019, 46, 2744–2751. [Google Scholar] [CrossRef]
- Principi, S.; Farah, J.; Ferrari, P.; Carinou, E.; Clairand, I.; Ginjaume, M. The Influence of Operator Position, Height and Body Orientation on Eye Lens Dose in Interventional Radiology and Cardiology: Monte Carlo Simulations versus Realistic Clinical Measurements. Phys. Medica 2016, 32, 1111–1117. [Google Scholar] [CrossRef]
- ICRP. Education and Training in Radiological Protection for Diagnostic and Interventional Procedures; ICRP Publication 113; Ann. ICRP: Ottawa, CA, USA, 2009. [Google Scholar]
- Chida, K. What Are Useful Methods to Reduce Occupational Radiation Exposure among Radiological Medical Workers, Especially for Interventional Radiology Personnel? Radiol. Phys. Technol. 2022, 15, 101–115. [Google Scholar] [CrossRef]
- Cheriachan, D.; Hughes, A.M.; du Moulin, W.S.M.; Williams, C.; Molnar, R. Ionizing Radiation Doses Detected at the Eye Level of the Primary Surgeon During Orthopaedic Procedures. J. Orthop. Trauma 2016, 30, e230. [Google Scholar] [CrossRef]
- Nakamura, M.; Yaku, H.; Ako, J.; Arai, H.; Asai, T.; Chikamori, T.; Daida, H.; Doi, K.; Fukui, T.; Ito, T.; et al. JCS/JSCVS 2018 Guideline on Revascularization of Stable Coronary Artery Disease. Circ. J. 2022, 86, 477–588. [Google Scholar] [CrossRef]
- The Ministry of Health, Labour and Welfare. Ministry of Health, Labour and Welfare National Health and Nutrition Survey 2012—Report of the Physical Status Survey; Ministry of Health, Labour and Welfare: Tokyo, Japan, 2014. [Google Scholar]
- Dyrbye, L.N.; Varkey, P.; Boone, S.L.; Satele, D.V.; Sloan, J.A.; Shanafelt, T.D. Physician Satisfaction and Burnout at Different Career Stages. Mayo Clin. Proc. 2013, 88, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, M.; Haga, Y.; Sota, M.; Abe, M.; Kaga, Y.; Inaba, Y.; Suzuki, M.; Meguro, T.; Hosoi, Y.; Chida, K. Evaluation of Lens Doses among Medical Staff Involved in Nuclear Medicine: Current Eye Radiation Exposure among Nuclear-Medicine Staff. Appl. Sci. 2023, 13, 9182. [Google Scholar] [CrossRef]
- Inaba, Y.; Jingu, K.; Fujisawa, M.; Otomo, K.; Ishii, H.; Kato, T.; Murabayashi, Y.; Suzuki, M.; Zuguchi, M.; Chida, K. Evaluation of Radiation Doses Received by Physicians during Permanent 198Au Grain Implant Brachytherapy for Oral Cancer. Appl. Sci. 2024, 14, 6010. [Google Scholar] [CrossRef]
- Inaba, Y.; Hitachi, S.; Watanuki, M.; Chida, K. Occupational Radiation Dose to Eye Lenses in CT-Guided Interventions Using MDCT-Fluoroscopy. Diagnostics 2021, 11, 646. [Google Scholar] [CrossRef]
- Fujibuchi, T.; Nakashima, M.; Arakawa, H.; Miyazaki, H.; Anam, C. Evaluation of Radiation Protection Effectivity in a Cardiac Angiography Room Using Visualized Scattered Radiation Distribution. J. Radiol. Prot. 2024, 44, 031510. [Google Scholar] [CrossRef]
- Fukuda, A.; Lin, P.-J.P. Covering the Patient’s Arm Support in Lead Reduced the Radiation Dose Rate to the Cardiologists During Percutaneous Coronary Interventions: A Phantom Study. Radiat. Prot. Dosim. 2020, 188, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Chida, K.; Munehisa, M.; Sato, T.; Inaba, Y.; Suzuki, M.; Zuguchi, M. Non-Lead Protective Aprons for the Protection of Interventional Radiology Physicians from Radiation Exposure in Clinical Settings: An Initial Study. Diagnostics 2021, 11, 1613. [Google Scholar] [CrossRef]
- Zanca, F.; Dabin, J.; Collard, C.; Alexandre, N.; De Groote, A.; Salembier, J.P.; Henry, M.; Rombaut, E.; Sghaier, S.; Massart, P.-E. Evaluation of a Suspended Radiation Protection System to Reduce Operator Exposure in Cardiology Interventional Procedures. Catheter. Cardiovasc. Interv. 2021, 98, E687–E694. [Google Scholar] [CrossRef]
- McCaffrey, J.P.; Tessier, F.; Shen, H. Radiation Shielding Materials and Radiation Scatter Effects for Interventional Radiology (IR) Physicians. Med. Phys. 2012, 39, 4537–4546. [Google Scholar] [CrossRef]
- Endo, M.; Haga, Y.; Sota, M.; Tanaka, A.; Otomo, K.; Murabayashi, Y.; Abe, M.; Kaga, Y.; Inaba, Y.; Suzuki, M.; et al. Evaluation of Novel X-Ray Protective Eyewear in Reducing the Eye Dose to Interventional Radiology Physicians. J. Radiat. Res. 2021, 62, 414–419. [Google Scholar] [CrossRef]
- ICRP. The Optimisation of Radiological Protection–Broadening the Process; ICRP Publication 101b; Ann. ICRP: Ottawa, CA, USA, 2006. [Google Scholar]
- Albayati, M.A.; Kelly, S.; Gallagher, D.; Dourado, R.; Patel, A.S.; Saha, P.; Bajwa, A.; El-Sayed, T.; Salter, R.; Gkoutzios, P.; et al. Editor’s Choice—Angulation of the C-Arm During Complex Endovascular Aortic Procedures Increases Radiation Exposure to the Head. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Eguchi, Y.; Yamazaki, C.; Hino, T.; Saida, T.; Chida, K. Development of a New Radiation Shield for the Face and Neck of IVR Physicians. Bioengineering 2022, 9, 354. [Google Scholar] [CrossRef] [PubMed]
- Daring, D.L.; Sun, Z. Investigation of the Clinical Value of Three-Dimensional-Printed Personalised Vascular Models for the Education and Training of Clinicians When Performing Interventional Endovascular Procedures. Appl. Sci. 2025, 15, 5695. [Google Scholar] [CrossRef]
| Physician | Number of Procedures [Case] | Fluoroscopy Time Per Case [min] | Hp(0.07) from Neck Dosimeter [mSv] | Hp(3) from Lens Dosimeter [mSv] (Considering the Shielding Effect of Lead Glasses) | ||
|---|---|---|---|---|---|---|
| Annual Dose | Dose Per Case | Annual Dose | Dose Per Case | |||
| A | 123 | 21.4 | 28.8 | 0.234 | 10.9 | 0.0886 |
| B | 149 | 14.7 | 20.1 | 0.135 | 6.30 | 0.0423 |
| C | 167 | 19.4 | 26.6 | 0.159 | 3.20 | 0.0192 |
| D | 331 | 27.6 | 45.4 | 0.137 | 26.9 | 0.0813 |
| E | 198 | 8.07 | 20.0 | 0.101 | 5.80 | 0.0293 |
| F | 338 | 11.0 | 31.5 | 0.0932 | 6.30 | 0.0186 |
| G | 199 | 22.4 | 50.4 | 0.253 | 28.0 | 0.141 |
| H | 147 | 15.5 | 17.5 | 0.119 | 6.40 | 0.0435 |
| I | 98 | 16.3 | 26.0 | 0.265 | 2.20 | 0.0224 |
| J | 172 | 12.4 | 15.6 | 0.0907 | 7.60 | 0.0442 |
| Physician | Height [cm] | Experience [Years] |
|---|---|---|
| A | 165 | 11 |
| B | 178 | 4 |
| C | 180 | 7 |
| D | 165 | 13 |
| E | 171 | 11 |
| F | 177 | 9 |
| G | 158 | 15 |
| H | 172 | 14 |
| I | 163 | 21 |
| J | 168 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagehashi, K.; Haga, Y.; Kato, T.; Takahira, S.; Sota, M.; Kaga, Y.; Abe, M.; Tada, N.; Chida, K. Impact of Physician Height and Experience on Eye Lens Dose in Interventional Cardiology: An Initial Study. Appl. Sci. 2025, 15, 12137. https://doi.org/10.3390/app152212137
Sagehashi K, Haga Y, Kato T, Takahira S, Sota M, Kaga Y, Abe M, Tada N, Chida K. Impact of Physician Height and Experience on Eye Lens Dose in Interventional Cardiology: An Initial Study. Applied Sciences. 2025; 15(22):12137. https://doi.org/10.3390/app152212137
Chicago/Turabian StyleSagehashi, Kodai, Yoshihiro Haga, Toshiki Kato, Saki Takahira, Masahiro Sota, Yuji Kaga, Mitsuya Abe, Norio Tada, and Koichi Chida. 2025. "Impact of Physician Height and Experience on Eye Lens Dose in Interventional Cardiology: An Initial Study" Applied Sciences 15, no. 22: 12137. https://doi.org/10.3390/app152212137
APA StyleSagehashi, K., Haga, Y., Kato, T., Takahira, S., Sota, M., Kaga, Y., Abe, M., Tada, N., & Chida, K. (2025). Impact of Physician Height and Experience on Eye Lens Dose in Interventional Cardiology: An Initial Study. Applied Sciences, 15(22), 12137. https://doi.org/10.3390/app152212137






