Abstract
This study investigated the effects of transcranial direct current stimulation (tDCS) over the dorsolateral prefrontal cortex (dlPFC) at three intensities (sham, 1 mA, 2 mA) on postural control, isokinetic strength, and cognitive performance in women with fibromyalgia (FM) and healthy controls (HCs). Using a double-blind, sham-controlled, crossover design, 26 participants (13 FM, 13 HC) completed sessions in randomized order, performing tasks under single- and dual-task conditions. Cognitive accuracy improved in both groups following 1 mA and 2 mA stimulation, particularly during single-task scenarios in static balance tasks. Notably, 2 mA tDCS reduced dual-task cost (DTC) in cognitive performance for the FM group, indicating decreased cognitive–motor interference. However, postural and strength outcomes showed no consistent intensity-dependent changes, with only selected nonlinear centers of pressure metrics (e.g., Lyapunov exponent, DFA) indicating possible modulation in FM. Isokinetic strength measures remained largely unaffected by tDCS across all intensities. Overall, the findings suggest that dlPFC-tDCS may selectively enhance cognitive function and reduce cognitive–motor interference in FM, especially under low-demand or higher-intensity stimulation conditions, while offering limited benefits for physical strength and balance.
1. Introduction
Fibromyalgia (FM) is a chronic syndrome affecting approximately 2.1% of the population (with a 4:1 female/male ratio) [1]. Epidemiological studies show that FM is more common in women and that its prevalence increases with age, with global estimates ranging from 0.2 percent to 6.6 percent and higher rates typically observed from mid-adulthood onward [2]. European and Spanish data report similar patterns, with the highest prevalence found in women between approximately 40 and 69 years of age [3,4]. FM is characterized by widespread musculoskeletal pain, fatigue, sleep disturbance, stiffness, anxiety, and depression [5]. In addition to these symptoms, women with FM often exhibit motor impairments—such as reduced mobility, muscle strength and balance—as well as cognitive deficits in domains like memory, attention, processing speed and executive function [6,7]. Together, these impairments substantially reduce quality of life and make daily activities (often involving multitasking) more difficult [1,8].
In daily life, motor and cognitive tasks frequently occur simultaneously (the dual-task paradigm), which can exacerbate FM deficits. Indeed, FM patients show greater dual-task interference (performance decline) than healthy controls [9]. The dorsolateral prefrontal cortex (dlPFC) is a key brain region for coordinating attention and executive control during dual-tasking [10], and it has been identified that people with FM show reduced volume in different subfields of the prefrontal cortex [11]. Transcranial direct current stimulation (tDCS) is a noninvasive technique that modulates cortical excitability by applying a weak electrical current through the scalp [12]. In healthy individuals, anodal tDCS targeting the left dlPFC has been shown to reduce dual-task costs and improve performance in both young and older adults [13,14]. By contrast, tDCS studies in FM have mostly focused on the primary motor cortex (M1) to alleviate pain [15,16,17]. Few trials have targeted the dlPFC in FM; those that did reported reductions in pain (but no improvements in sleep or quality of life). Given the dlPFC’s role in executive functions and its connections to pain-modulatory networks, stimulating this region with tDCS may simultaneously influence cognitive and motor symptoms in FM [17].
FM represents an ideal clinical model to investigate the functional effects of prefrontal tDCS because it simultaneously involves chronic pain, cognitive deficits, and altered cortical excitability within prefrontal and pain-related networks [11,18,19,20,21]. Neuroimaging studies have shown hypometabolism and gray matter reduction in the dorsolateral prefrontal cortex of FM patients, which correlate with deficits in executive control, attention, and pain modulation. Unlike other chronic pain conditions, FM integrates both sensory–discriminative and affective–cognitive alterations, making prefrontal neuromodulation particularly relevant [22,23,24]. Therefore, examining how different tDCS intensities over the dlPFC modulate cognitive–motor integration in FM can provide unique insights into the cortical mechanisms underlying both cognitive dysfunction and pain interference in this population.
Individuals with FM frequently exhibit impaired postural stability and an increased risk of falls [25]. Posturographic analyses have shown that FM patients display significantly greater center of pressure (CoP) sway area and higher CoP displacement velocities compared to healthy controls [26]. Moreover, they tend to exhibit less complex postural control patterns, as reflected by reduced sample entropy, suggesting a more rigid and less adaptable postural regulation [26]. Therefore, the combined use of traditional linear CoP parameters—such as total excursion, 95% confidence ellipse area, mean displacement velocities, and root mean square in both anteroposterior (AP) and mediolateral (ML) directions—and nonlinear variables—such as sample entropy, Lyapunov exponents, and detrended fluctuation analysis (DFA)—could provide complementary insights into the sensorimotor integration and neuromuscular control deficits [27] characteristic of FM. These metrics are clinically relevant and offer a comprehensive assessment of postural stability in this population.
Generalized muscle weakness and increased fatigability are commonly reported in individuals with FM [28,29]. Isokinetic dynamometry offers a valid and objective method to quantify muscle performance across the full range of motion. Variables such as maximal repetition work, total work, average power, and fatigue index provide detailed information on muscle force generation, endurance, and efficiency. Studies have consistently reported that FM patients exhibit lower values in these isokinetic strength parameters compared to healthy individuals [30,31]. This muscular weakness may further contribute to postural instability in FM [26,32]. Although no prior studies have specifically examined changes in posturography or isokinetic strength following tDCS in this population, evidence from other clinical groups suggests that anodal tDCS over motor-related regions can enhance isokinetic strength and balance performance [33]. These findings support the inclusion of such motor outcomes when evaluating the potential functional effects of tDCS in FM.
Despite its promise, the existing tDCS literature in FM has important limitations. A recent systematic review identified only about 14 randomized controlled trials of tDCS for FM pain/fatigue [34]. Most of these studies used 2 mA stimulation (in ~85.7% of trials) and applied multi-session protocols; only a small fraction (7.1%) used a lower intensity (1 mA). Such reports have noted that titration studies comparing different intensities are needed. Critically, no prior study has applied sham versus graded (1 mA and 2 mA) dlPFC-tDCS during dual-task motor–cognitive testing in FM. Furthermore, many existing trials have small samples and often lack rigorous double-blind sham controls or simultaneous assessment of both cognitive and motor outcomes. As a result, it remains unclear how tDCS intensity affects functional performance in FM and whether dlPFC stimulation can meaningfully reduce dual-task interference.
Accordingly, the present study aimed to fill these gaps by conducting a double-blind, sham-controlled, crossover trial of left dlPFC tDCS in women with FM and matched healthy controls. We will compare three conditions (sham, 1 mA, and 2 mA anodal tDCS) and measure their effects on motor performance (maximal muscle strength and postural balance) and cognitive accuracy under both single-task and dual-task conditions. By assessing performance in FM patients relative to healthy women, this design will determine whether and how tDCS intensity over the dlPFC modulates cognitive–motor function and dual-task interference in FM.
2. Materials and Methods
2.1. Trial Design
This study employed a double-blind, sham-controlled, crossover design. Each participant underwent the three tDCS intensities (sham, 1 mA, and 2 mA) in randomized order, with at least one week of washout between sessions. This crossover approach ensured that all participants experienced every experimental condition, allowing for repeated testing within subjects and thereby enhancing the reliability and internal validity of the findings. The order of tDCS intensity application was determined using a random number generator (Random Number Generator tool; Google, LLC, Mountain View, CA, USA).
The study procedures were approved by the Research Ethics Committee of the University of Extremadura (approval number: 192/2021) and adhered to the principles outlined in the Declaration of Helsinki [35]. The trial was prospectively registered in the Protocol Registration and Results System (NCT05266989). A comprehensive description of the methodology is available in the published study protocol [36]. Moreover, the study followed the Consolidated Standards of Reporting Trials (CONSORT) guidelines [37]. Findings related to the effects of this protocol on electroencephalographic activity and heart rate variability have been reported in a previous publication [38].
2.2. Participants and Setting
To be eligible for participation, individuals had to meet the following inclusion criteria: (a) be female and aged between 30 and 75 years; (b) have the ability to communicate effectively with the research team; and (c) in the case of the FM group, have a diagnosis confirmed by a rheumatologist in accordance with the criteria of the American College of Rheumatology [5]. Exclusion criteria included: (a) the presence of psychiatric disorders potentially associated with cognitive impairment, neurological conditions, or history of brain injury; (b) current pharmacological treatment for anxiety or depression; (c) inability to engage in physical exertion due to contraindications; (d) leg injury prior to six months, and (e) pregnancy.
A total of 26 women (mean age: 48.50 ± 7.92 years) participated in the study. Recruitment took place between January and February 2022, and participants were assigned to one of two groups: a fibromyalgia group (FM; n = 13), consisting of women recruited through the Fibromyalgia Association (AFIBROEX), and matched healthy controls (HC; n = 13) composed of women without any known pathologies. All experimental procedures and assessments were conducted at the Faculty of Sport Sciences, University of Extremadura (Cáceres, Spain), between 9:00 and 13:00 h. The study concluded in March 2022. All participants provided written informed consent prior to enrollment.
2.3. Intervention
tDCS was administered using an 8-channel wireless hybrid EEG neurostimulator (Starstim, Neuroelectrics, Barcelona, Spain). Stimulation was delivered through saline-soaked sponge electrodes (Sponstim®, 5.65 cm in diameter, 25 cm2 surface area).
To target the prefrontal cortex (PFC), the anodal electrode was positioned over the left dlPFC, corresponding to the F3 site of the international 10–20 EEG system. The cathodal (return) electrode was placed over the contralateral supraorbital region at Fp2 [13,14,39,40,41,42,43,44,45]. Participants received anodal stimulation at either 1 mA or 2 mA, with 30 s ramp-up and ramp-down phases.
A stimulation duration of 20 min was selected to ensure that the acute effects would persist for at least one-hour post-stimulation [46]. In the sham condition, a double 30 s ramp was applied, a procedure implemented via the Neuroelectrics NIC1 software (v2.1.4.0). This approach was based on previous findings indicating that skin sensations typically subside within 30 s, thereby maintaining blinding effectiveness [47,48].
2.4. Instruments and Variables
Postural stability was assessed using two Kistler force platforms (Type 9286A; Kistler Instruments AG, Winterthur, Switzerland), which recorded AP and ML CoP displacements for both the left and right sides while participants maintained a quiet standing posture. Data were sampled at 200 Hz. To reduce high-frequency noise, a fourth-order low-pass infinite impulse response (IIR) filter with a cutoff frequency of 5 Hz was applied. This filter was designed using MATLAB’s R2024a (v24.1) designfilt function. These filtering parameters were selected to preserve the meaningful components of postural sway while minimizing sensor noise. Postural stability assessments were conducted under four distinct conditions: (1) standing 45 s with eyes open; (2) standing 45 s with eyes open while performing a concurrent cognitive task; (3) standing 45 s with eyes closed; and (4) standing 45 s with eyes closed while performing a concurrent cognitive task. Each trial lasted 45 s and was followed by a one-minute rest interval.
Isokinetic strength was assessed using the Multi-Joint 3 isokinetic dynamometer (Biodex Medical Systems, Inc., Shirley, NY, USA). The testing protocol consisted of five consecutive concentric contractions for both knee flexion and extension, forming a concentric/concentric assessment protocol [49,50]. These movements were performed continuously at an angular velocity of 60°/s across a range of motion from 0° (full knee extension) to 90° (knee flexion) [50]. Standardized setup procedures for knee flexion/extension, as outlined by the manufacturer, were strictly followed. Each participant was securely positioned in the dynamometer chair, ensuring alignment between the knee joint axis and the axis of rotation of the dynamometer. The dynamometer was maintained at a tilt angle of 0°, with the seat set at 90° and the seatback reclined to 85°. Prior to testing, the system was calibrated in accordance with the manufacturer’s guidelines, and the software was used to account for the weight of the tested limb to ensure measurement accuracy.
2.4.1. Primary Outcomes
Posturography
Traditional linear CoP variables were selected based on the recommendations of Duarte and Freitas [51]. These included: total excursion (TotEx), defined as the total length of the CoP trajectory; total displacement area (Area), represented by the 95% confidence ellipse of the CoP path; mean velocity of total CoP displacement (Total Vel CoP); mediolateral displacement (CoP ML); anteroposterior displacement (CoP AP); mean velocity in the mediolateral direction (Vel ML); mean velocity in the anteroposterior direction (Vel AP); and the root mean square (RMS) of CoP displacements in both the AP and ML directions (RMS AP and RMS ML).
Additionally, nonlinear CoP parameters were calculated following the guidelines of Stergiou [52]. These included: sample entropy in the mediolateral (SampEn ML) and anteroposterior (SampEn AP) directions; detrended fluctuation analysis (DFA) of the CoP time series in both directions (DFA Alpha ML and DFA Alpha AP); and the largest Lyapunov exponent derived from the CoP trajectories in the ML (LyE ML) and AP (LyE AP) directions. Sample entropy was computed using an embedding dimension of m = 2 and a tolerance value R = 0.2, which are commonly adopted parameters for physiological time series. The fluctuation function was calculated across box sizes ranging from 4 to N/10 (where N is the length of the signal), and the α exponent was derived from the slope of the log-log plot of fluctuation versus window size. Lyapunov exponent was calculated following the recommendations of [52]. The time delay (τ) between signals was determined using the Average Mutual Information (AMI) method, ensuring optimal reconstruction of the phase space in the appropriate embedding dimension (dim). The embedding dimension was estimated through mathematical procedures based on the algorithm proposed by Wolf et al. [53,54]. Separate nonlinear analyses were conducted for the AP and ML CoP trajectories.
Isokinetic Strength
The maximal repetition work (J), total work (J), average power (W) and fatigue index (%) were collected. The maximal repetition work (J) represents the maximum amount of work (force × distance) generated during a single repetition. This variable provides a more functionally relevant indicator of joint performance than peak torque, as it reflects the ability of the muscle to sustain force production throughout the entire range of motion, rather than at a single point. The total work (J) refers to the cumulative amount of work performed across all repetitions during the test. It provides an overall measure of muscular output over the entire effort bout. The average power (W) is calculated by dividing the total work by the time required to complete it. This parameter reflects muscular efficiency and the ability to produce work overtime, integrating both force and velocity components. The fatigue index (%) indicates the decline in performance over the duration of the test. It is calculated as the percentage decrease in work output from the first third to the last third of the repetitions, providing insight into muscular endurance and resistance to fatigue.
2.4.2. Secondary Outcomes
The Spanish version of the International Physical Activity Questionnaire (IPAQ) was utilized to evaluate both physical activity and sedentary behavior. This tool categorizes physical activity based on different domains, including occupational tasks, transportation, leisure activities, and household chores [55]. Cognitive function was assessed using the Montreal Cognitive Assessment (MoCA), a brief screening instrument designed to evaluate multiple cognitive domains, such as attention, concentration, executive functioning, memory, language, visuoconstructional skills, calculation, and orientation [56]. Previous research has shown that the MoCA is more sensitive than the Mini-Mental State Examination (MMSE) in detecting cognitive impairments in individuals with FM [57].
The EuroQol-5 Dimensions-5 Levels (EQ-5D-5L) instrument was employed to assess health-related quality of life (HRQoL) [58]. This questionnaire comprises five dimensions—mobility, self-care, usual activities, pain/discomfort, and anxiety/depression—each with five severity levels. Additionally, it includes a visual analog scale (VAS), allowing participants to rate their perceived overall health on a scale from 0 (the worst imaginable health state) to 100 (the best imaginable health state).
Height and weight were measured using a stadiometer (SECA 225, SECA, Hamburg, Germany).
2.5. Procedure
Each participant completed three sessions separated by at least one week, corresponding to the three stimulation conditions (sham, 1 mA, and 2 mA). All sessions followed the same experimental structure, divided into pre-tDCS, tDCS, and post-tDCS phases.
(1) Pre-tDCS phase. Participants completed the questionnaires EQ-5D-5L, IPAQ, and the MoCA. Subsequently, participants performed a series of physical (postural stability and isokinetic strength) and cognitive tasks under both single and dual-task conditions.
Postural stability. Participants performed four quiet standing trials on dual force platforms: (1) eyes open, (2) eyes closed, (3) eyes open with a concurrent cognitive task, and (4) eyes closed with a concurrent cognitive task. Each trial lasted 45 s [59], with the first 5 s excluded from analysis as adjustment time [60]. A 5 cm black dot was used as a fixation target, placed 1.5 m away at eye level. One-minute rest interval was provided between trials.
Strength assessment. Before testing, participants completed 10 squats as a warm-up, followed by a familiarization phase consisting of 10 knee flexion/extension repetitions at 120°/s. After one minute of rest, the isokinetic test was conducted using the Biodex system: five maximal concentric contractions for knee flexion and extension at 60°/s on the dominant leg.
The cognitive task consisted of counting backward by two, as quickly and accurately as possible, while simultaneously performing the standing and strength tasks. Each trial began with a randomly selected number greater than 100, which could be either odd or even [61].
(2) tDCS phase. tDCS was then applied for 20 min using one of the three randomly assigned conditions: sham, 1 mA, or 2 mA. Participants remained seated and relaxed during stimulation.
(3) Post-tDCS phase. The same battery of physical tests (balance and strength) was repeated in the same order as during the pre-tDCS phase.
A schematic overview of the participant flow, experimental procedures, and crossover design is presented in Figure 1 to enhance comprehension of the study.
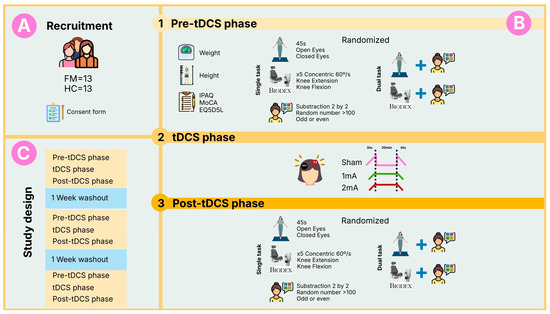
Figure 1.
Overview of the study design. (A) Recruitment and allocation of participants to the fibromyalgia (FM) and healthy control (HC) groups; (B) Experimental phases detailing the assessments, single and dual-tasks, and stimulation conditions performed by each participant; (C) Randomized crossover design illustrating the three performed sessions and the washout periods between sessions.
2.6. Statistical Analyses
The analysis of the impact of various tDCS intensities (sham, 1 mA, 2 mA) on balance and strength was conducted using IBM SPSS Statistics (version 24.0). Non-parametric tests, specifically the Wilcoxon rank test, were applied to evaluate differences between pre- and post-tDCS measurements for each intensity within both the fibromyalgia (FM) and healthy control (HC) groups. Additionally, the Friedman test was used to identify significant variations across the different tDCS intensities (sham, 1 mA, 2 mA), followed by pairwise comparisons using the Wilcoxon signed-rank test.
For balance and strength variables, the difference between post- and pre-tDCS values was computed. These differences were then compared between groups at each tDCS intensity using the Mann–Whitney U test. To minimize the risk of type I errors due to multiple comparisons, the Benjamini–Hochberg correction was applied [62]. Effect sizes (r) were also calculated and interpreted as follows: large (≥0.5), medium (≥0.3), and small (≥0.1) [63].
A sensitivity analysis performed in G*Power (v3.1.9.7) showed that, with n = 13 per group and α = 0.05, the study could detect within-group effects of dz = 0.87, between-group effects of d = 1.02, and across-intensity effects of f = 0.374, indicating adequate power for moderate to large effects but limited sensitivity for small effects.
3. Results
3.1. Descriptive Characteristics
Table 1 shows the descriptive characteristics of the participants. The FM group showed significant lower values of health-related quality of life when compared to the HC group (p = 0.001). No adverse effects were observed during the stimulation in any of the applied intensities (1 mA or 2 mA).

Table 1.
Descriptive characteristics of the participant.
3.2. Strength Under Dual and Single Tasks Conditions
Differences across the three stimulation intensities (sham, 1 mA, and 2 mA) were examined using the Friedman test, while pre-post effects within each intensity were assessed with the Wilcoxon signed-rank test. Between-group comparisons (FM vs. HC) were performed using the Mann–Whitney U test. The Benjamini–Hochberg correction was applied to control for multiple comparisons.
3.2.1. Single Motor Task Performance
Supplementary Tables S1 and S2 analyze single motor task performance during isokinetic flexion and extension. No significant differences were found in any of the variables or statistical tests performed.
3.2.2. Dual Motor Task Performance
Supplementary Tables S3 and S4 analyze dual motor task performance. No significant differences were found in any of the variables or statistical tests performed.
3.2.3. Dual-Task Cost Effects in Isokinetic Task
Supplementary Tables S5 and S6 analyze motor and cognitive dual-task costs during the extension and flexion phases. The FM group showed a significant increase in the fatigue index dual-task cost (DTC) after 2 mA stimulation (p = 0.012, r = 0.861) during the extension phase. Additionally, the HC group showed a greater reduction in dual-task cost compared to the FM group after 2 mA stimulation (p = 0.028, r = 0.544). No significant differences were found in any of the remaining DTCs calculated in this study for either the flexion or extension tasks.
3.2.4. Cognitive Performance Single and Dual-Task Conditions in Isokinetic Task
Supplementary Table S7 shows accuracy during the cognitive task. The FM group showed significant improvements in task accuracy following stimulation with 1 mA (p = 0.039, r = 0.57) and 2 mA (p = 0.013, r = 0.69) during the single cognitive task. The HC group showed improved accuracy under the sham condition (p = 0.018, r = 0.66) during the single cognitive task. No significant differences were observed in accuracy during the dual-task condition. These results are visually summarized in Figure 2, which illustrates pre- and post-tDCS accuracy values for both groups across the three stimulation intensities under single- and dual-task conditions. Regarding DTC, Supplementary Table S8 did not reveal any significant differences.
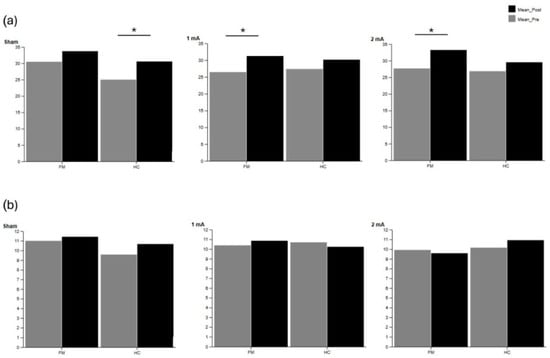
Figure 2.
Cognitive accuracy in the fibromyalgia (FM) and healthy control (HC) groups during (a) single-task and (b) dual-task conditions during isokinetic tasks. Pre- and post-tDCS values are shown for sham, 1 mA, and 2 mA intensities. Significant differences within groups are indicated with asterisks (p < 0.05).
3.3. Standing Balance Under Dual and Single Tasks Conditions
The same non-parametric approach was used for the balance outcomes. Within-condition changes (pre-post) were analyzed using the Wilcoxon test, differences among tDCS intensities with the Friedman test, and between-group comparisons with the Mann–Whitney U test. All p-values were adjusted using the Benjamini–Hochberg procedure.
3.3.1. Stability During Single Task with Open and Closed Eyes
Supplementary Table S9 shows linear and nonlinear CoP variables during the single task condition with eyes open. The HC group showed a significant increase in RMS AP after 1 mA (p = 0.012, r = 0.858).
Supplementary Table S10 shows linear and nonlinear CoP variables during the single task condition with eyes closed. Regarding pre-post differences, the HC group exhibited an increase in Area and RMS AP, as well as a significant reduction in LyE AP and SampEn after the sham condition (p < 0.05). In addition, RMS ML significantly increased after 2 mA in the HC group. The FM group exhibited an increase in LyE ML after 2 mA stimulation (p = 0.027, r = 0.727). These findings are illustrated in Figure 3, which displays the main linear (Area, RMS AP, RMS ML) and nonlinear (SampEn AP, LyE AP, LyE ML) postural sway variables under the eyes-closed single-task condition.
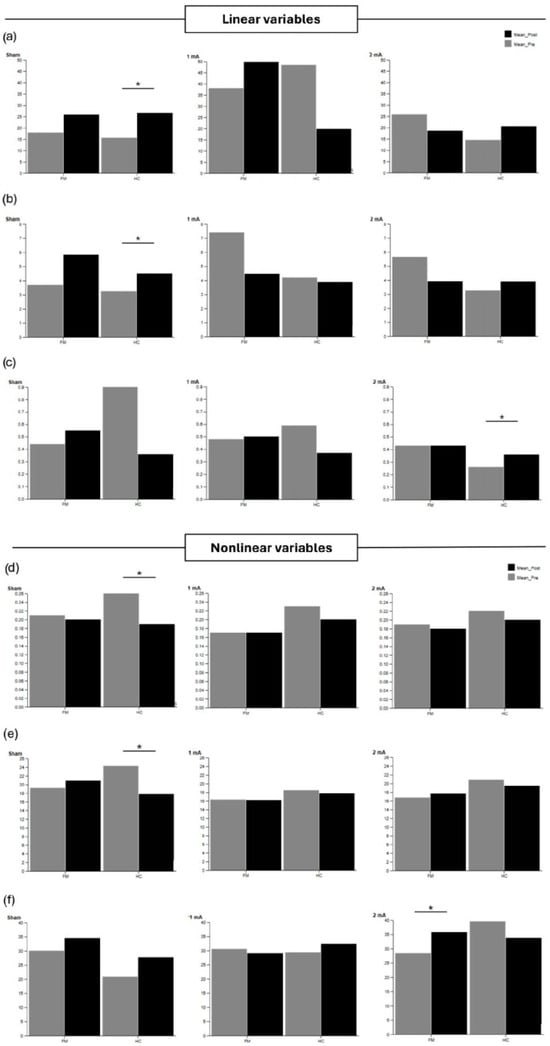
Figure 3.
Linear postural sway variables (a) Area, (b) RMS AP, (c) RMS ML, and nonlinear postural sway variables (d) SampEn AP, (e) LyE AP, (f) LyE ML, during the single-task eyes-closed condition in the fibromyalgia (FM) and healthy control (HC) groups. Pre-post values are shown for sham, 1 mA, and 2 mA intensities. Significant differences within groups are indicated with asterisks (p < 0.05).
Pairwise comparisons showed significant differences between sham, 1 mA, and 2 mA stimulations. In this regard, greater changes were observed after 1 mA compared to 2 mA and sham for LyE ML (p < 0.05) during the eyes-closed single balance task.
Between-group comparisons showed that the FM group obtained higher values of LyE AP and LyE ML after the sham condition, as well as higher LyE ML values after 1 mA stimulation.
3.3.2. Stability During Dual-Task with Open and Closed Eyes
Supplementary Table S11 shows linear and nonlinear CoP variables during the dual-task condition with eyes open. The FM group showed a significant reduction in LyE AP after 1 mA stimulation. For the HC group, a significant increase in CoP AP, TotEx, and total CoP velocity was observed after 1 mA stimulation (p > 0.05). In addition, LyE AP increased after 2 mA in the HC group. Between-group analyses showed that, after 2 mA stimulation, the HC group had higher LyE AP values than the FM group (p = 0.003, r = 0.639). These results are depicted in Figure 4, which presents the main linear (CoP AP, TotEx, total CoP velocity) and nonlinear (LyE AP) postural sway variables under the dual-task eyes-open condition.
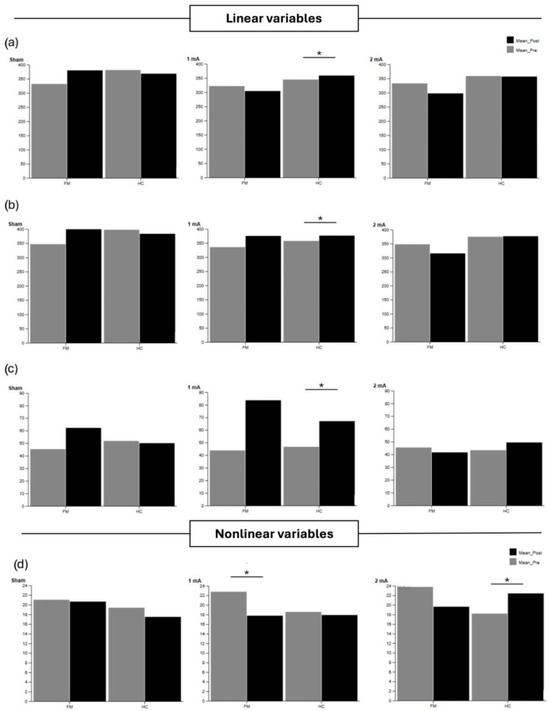
Figure 4.
Linear postural sway variables (a) CoP AP, (b) TotEx, (c) Total Vel CoP, and nonlinear postural sway variables (d) LyE AP, during the dual-task eyes-open condition in the fibromyalgia (FM) and healthy control (HC) groups. Pre-post differences are shown for sham, 1 mA, and 2 mA tDCS. Significant differences within and between groups are indicated with asterisks (p < 0.05).
Supplementary Table S12 shows linear and nonlinear CoP variables during the dual-task condition with eyes closed. In the FM group, pairwise comparisons showed a greater change after 1 mA compared to 2 mA in DFA Alpha AP (p = 0.036). Between-group comparisons showed higher values of RMS AP in the FM group compared to the HC group after the sham condition (p = 0.012, r = 0.595). Similarly, higher DFA Alpha AP values were detected in the FM group compared to the HC group after 1 mA stimulation (p = 0.021, r = 0.528).
3.3.3. Dual-Task Cost Effects in Standing Balance Tasks
Statistical analyses for cognitive accuracy followed the same procedure as above, with the Wilcoxon test used for within-condition effects and the Friedman test for across-intensity comparisons. Between-group analyses were performed with the Mann–Whitney U test.
Supplementary Table S13 shows the DTC of linear and nonlinear CoP variables during the eyes-open static balance task. No significant differences were observed in any of the comparisons.
Supplementary Table S14 shows the DTC of linear and nonlinear CoP variables during the eyes-closed static balance task. The FM group exhibited a significant decrease in RMS ML (p = 0.021, r = 0.697) after the sham condition, as well as a significant increase in Area after 1 mA stimulation (p = 0.041, r = 0.589). The HC group showed a significant reduction in DTC Area (p = 0.045, r = 0.702), along with a significant increase in SampEn AP (p = 0.016, r = 0.708) and LyE AP (p = 0.016, r = 0.708) after the sham condition. Between-group differences were observed in Area and RMS CoP AP during the sham condition, with higher values obtained in the FM group.
3.3.4. Cognitive Performance Single and Dual-Task Conditions in Standing Balance Tasks
Supplementary Table S15 shows cognitive accuracy during the single and dual-task conditions of the static balance tasks. During the eyes-open static balance task, the FM group showed significant improvements in task accuracy following stimulation with 1 mA (p = 0.017, r = 0.660) and 2 mA (p = 0.001, r = 0.884). The HC group also showed improved accuracy under the 2 mA condition (p = 0.001, r = 0.882) during the single cognitive task. During the eyes-closed static balance task, the FM group significantly increased accuracy after 1 mA (p = 0.014, r = 0.682) and 2 mA (p = 0.001, r = 0.883). The HC group also showed improved performance after the sham (p = 0.006, r = 0.762) and 2 mA (p = 0.001, r = 0.882) conditions. Additionally, between-group differences were detected in the 1 mA condition (with higher values for the FM group compared to the HC group in the eyes-open task), and in the sham condition (with higher values for the HC group compared to the FM group in the eyes-closed task). These findings are illustrated in Figure 5, which displays pre- and post-tDCS accuracy values for both groups under single- and dual-task static balance conditions across the three stimulation intensities (sham, 1 mA, 2 mA). Regarding DTC, Supplementary Table S16 shows cognitive performance during static balance tasks. In this context, the FM group exhibited a significant reduction in DTC after 2 mA stimulation (p = 0.003, r = 0.824).
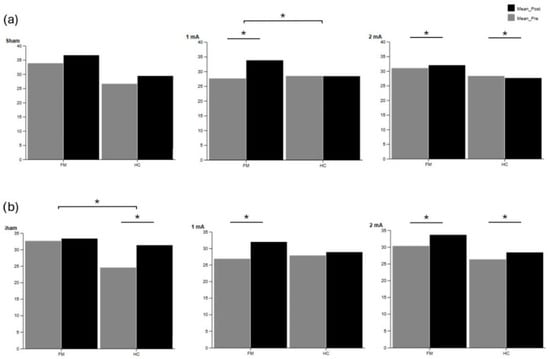
Figure 5.
Cognitive accuracy in the fibromyalgia (FM) and healthy control (HC) groups during (a) single-task and (b) dual-task conditions during static balance tasks. Pre- and post-tDCS values are shown for sham, 1 mA, and 2 mA intensities. Significant differences within groups are indicated with asterisks (p < 0.05).
4. Discussion
This study aimed to examine the dose-dependent effects of a single session of anodal tDCS over the dlPFC on postural control, isokinetic strength, and cognitive performance in FM and HC groups. The main findings indicate that 2 mA stimulation led to greater improvements in balance—particularly under more complex postural conditions—than 1 mA and sham, with effects observed in both groups but more pronounced in the FM group. Additionally, both 1 mA and 2 mA tDCS enhanced cognitive accuracy in the FM group, while only 2 mA reduced dual-task costs, suggesting a current intensity–dependent modulation of executive functioning under increased cognitive–motor demands. Additionally, tDCS was well tolerated, with no relevant adverse effects reported. This aligns with previous reviews that found no significant negative events in tDCS trials [64].
tDCS over the dlPFC demonstrated beneficial effects on postural stability and muscle strength in both cohorts, consistent with previous literature. A recent study [65] indicates that anodal tDCS over the dlPFC improves balance in both static and dynamic tasks. Masoudi, Ehsani, Hedayati, Ramezani and Jaberzadeh [65] observed significant reductions in CoP oscillations following anodal tDCS in patients with chronic low back pain. Our results suggest similar effects after 20 min of tDCS over the dlPFC at both 1 mA, and 2 mA. In this regard, we observed a trend toward decreased balance parameters (e.g., total path length and mean CoP velocity) compared to sham stimulation, particularly under dual-task cognitive conditions. These findings align with the notion that prefrontal neuromodulation enhances postural control [65,66]. These results are particularly relevant for the FM group (which exhibits balance deficits [67] and greater fear of falling [68]), as stimulation appeared to normalize performance, reducing the initially more pronounced instability indices.
Regarding isokinetic knee muscle strength, anodal tDCS over the dlPFC also provided benefits. Increases in total work and mean muscle power were recorded after active tDCS compared to sham. This aligns with the findings of Vieira et al. (2022) in trained men, where the same configuration (2 mA over dlPFC) significantly increased the total number of repetitions in weighted squats [69]. Furthermore, a systematic review concludes that tDCS enhances muscle strength across most evaluated parameters [64]. In this context, our data support the hypothesis that dlPFC tDCS may potentiate muscular work output [64,70]. However, some studies report contradictory results, such as Teymoori et al. [71], which found no improvement in maximal strength performance after M1 tDCS in healthy subjects. Future studies should further investigate tDCS effects on force production in athletes and special populations. Our study also revealed a trend toward reduced post-tDCS fatigue indices, suggesting that stimulation may moderate muscle fatigability—a plausible mechanism given the prefrontal cortex’s role in effort perception [72,73]. These results are particularly relevant for FM patients due to their documented struggles with strength and fatigue [74,75]. These ameliorations could also be partially attributable to a reduction in pain catastrophing and kinesiophobia and an improvement in cognitive function such as reduced attentional interference (increase in perception and attention after tDCS [76]. Also, recent evidence highlights that both cognitive decline and loss of muscle function—such as observed in sarcopenia—share common neural substrates involving prefrontal dysfunction and impaired executive control over motor actions [77,78,79]. These shared mechanisms suggest that neuromodulatory interventions like tDCS may have broader potential across conditions characterized by concurrent physical and cognitive impairments [80]. Therefore, exploring tDCS-induced changes in dual-task performance in fibromyalgia may contribute to a better understanding of how prefrontal modulation can mitigate functional decline in chronic conditions affecting both cognition and muscle performance.
The introduction of a concurrent cognitive task (counting backwards) degraded postural stability and muscle strength in the participants, reflecting the expected dual-task cost. However, anodal tDCS attenuated this dual-task interference, allowing participants to maintain better physical performance even while performing the cognitive task simultaneously. This effect aligns with prior studies in healthy and elderly populations [13,81]. For instance, Zhou et al. [42] reported that dlPFC tDCS significantly reduced the impact of cognitive loading on gait and postural control. Similarly, Concerto et al. [41] demonstrated that anodal prefrontal tDCS mitigated dual-task interference following exercise. Collectively, these findings suggest that dlPFC stimulation enhances executive function and divided attention, reducing cognitive–motor interference. Our results, showing reduced postural and strength deterioration under dual-task conditions after tDCS, support the idea that prefrontal neuromodulation facilitates the integration of cognitive and motor demands in complex scenarios. This is particularly critical for people with FM, who experience pronounced dual-task deficits [9,57,82] that directly impair activities of daily living.
Regarding prefrontal tDCS and cognitive function in FM, Silva, Zortea, Carvalho, Leite, Torres, Fregni and Caumo [15] demonstrated that a single 20 min session of anodal tDCS applied over the dlPFC at 1 mA can enhance specific components of attention in patients with FM. In their study, stimulation led to significant improvements in orienting and executive control networks when compared to sham, while no relevant changes were observed in the alerting component of attention. These findings suggest that prefrontal tDCS may selectively modulate certain attentional subsystems rather than exerting a broad effect on all domains. This pattern aligns with the current study’s results, in which cognitive accuracy improved particularly in singler tasks. Taken together, the evidence indicates that dlPFC stimulation may preferentially support attentional mechanisms associated with executive function and goal-directed behavior, while having a limited impact on more basic alertness processes. Consistently with our results, a recent meta-analysis by Muñoz-Perete et al. [83] reported that tDCS lead to significant improvements in global cognitive function and selective attention, but not in mental flexibility or visual attention. Our results show more effects with closed eyes but limited effects with open eyes.
Beyond attention, several studies have reported benefits of repeated dlPFC tDCS on memory and executive tasks in FM. Santos et al. [84] found that eight daily sessions of 2 mA anodal dlPFC stimulation (combined with online working-memory training) significantly increased verbal episodic memory (Rey Auditory Verbal Learning Test immediate recall) compared to sham. The active group also improved verbal fluency and short-term memory (forward digit span) more than sham. Similarly, Serrano et al. [85] applied 20 sessions of home-based 2 mA left-dlPFC tDCS and observed a robust improvement in executive set-shifting (Trail Making Test B–A) and working memory/fluency tasks relative to sham. Together, these findings suggest that sustained anodal dlPFC stimulation can produce durable gains in episodic memory and executive function in FM, aligning with the present results that cognitive accuracy improved under both 1 mA and 2 mA stimulation in this population.
The present study provides novel insights into the intensity-dependent effects of prefrontal tDCS in individuals with FM, revealing a dissociation between physical and cognitive outcomes. While both 1 mA and 2 mA doses led to improvements in postural balance and muscle strength, there was no consistent advantage of the higher intensity. This absence of a linear dose–response effect aligns with neurophysiological evidence suggesting that increasing current does not always enhance efficacy. For instance, Batsikadze et al. [86] demonstrated that 2 mA of anodal tDCS over the motor cortex can paradoxically reverse the expected excitatory effects, and Esmaeilpour et al. [87] similarly highlighted that higher intensities do not necessarily produce stronger or more reliable outcomes. In line with these findings, our results suggest that low-intensity stimulation (1 mA) may be sufficient to elicit maximal or near-maximal benefits in motor-related functions, possibly due to compensatory mechanisms that become engaged at higher intensities. In contrast, cognitive outcomes displayed a different pattern. While both 1 mA and 2 mA improved accuracy on single tasks, only 2 mA produced a significant reduction in dual-task cost. This may reflect a dose-dependent recruitment of additional neural resources under higher cognitive load, consistent with the hypothesis that more demanding tasks require stronger neuromodulatory input to affect performance. Although most prior tDCS studies in FM have used 2 mA by default, few have directly compared different intensities on cognitive functioning. Our findings therefore fill a critical gap, suggesting that while low-intensity stimulation can yield measurable cognitive benefits, higher currents may be necessary to impact complex, integrative functions such as those required in dual-task scenarios. These results underscore the need for more systematic investigations into the dose–response relationships of tDCS across multiple functional domains in chronic pain populations.
From a clinical perspective, the reduction in DTC observed after dlPFC stimulation may have meaningful implications for daily functioning in fibromyalgia. Dual-task interference is a key factor contributing to falls, fatigue exacerbation, and difficulties in performing activities that require simultaneous motor and cognitive engagement, such as walking while conversing or performing household tasks [9,88,89]. By improving cognitive–motor integration, prefrontal tDCS could potentially enhance safety, autonomy, and perceived self-efficacy in daily life [90]. Moreover, combining tDCS with cognitive–motor training or exercise-based rehabilitation may help translate these acute effects into long-term functional improvements [91,92,93]. Future interventions could thus explore multimodal approaches integrating neuromodulation and dual-task training to better target the cognitive–motor impairments characteristic of FM.
Some limitations should be acknowledged. First, the sample size, although comparable to similar crossover tDCS studies in FM, may limit the generalizability of the findings. In addition, the sensitivity analysis indicated that the study was adequately powered to detect moderate to large effects but not small effects, which means that subtle changes may have gone undetected. Second, the short-term nature of the stimulation protocol precludes conclusions about long-term effects or clinical applicability. Thus, future research with larger and independent cohorts is needed to further confirm the generality of these findings. Third, the sample consisted of women, so the results cannot be extrapolated to men with FM. Furthermore, the motor outcomes assessed may be less sensitive to prefrontal stimulation than cognitive measures, and future studies should consider targeting alternative cortical sites (e.g., M1) when addressing strength or balance-related impairments in FM.
5. Conclusions
This study shows that dlPFC-tDCS can enhance cognitive accuracy in women with FM, particularly under single-task conditions, and reduce cognitive–motor interference at higher stimulation intensities. However, postural control and isokinetic strength were not consistently modulated by tDCS. These findings highlight the selective efficacy of prefrontal stimulation in improving cognitive function in FM, while suggesting limited effects on physical parameters. Future research should explore multimodal stimulation approaches and investigate long-term outcomes across broader FM phenotypes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app152212138/s1, Table S1: Motor performance during single task in the extension phase; Table S2: Motor performance during single task in the flexion phase; Table S3: Motor performance during dual-task in the extension phase; Table S4: Motor performance during dual task in the flexion phase; Table S5: Motor dual task cost during extension phase; Table S6: Motor dual task cost during flexion phase; Table S7: Cognitive accuracy during single and dual task conditions; Table S8: Cognitive Dual task cost; Table S9: Linear and non-linear CoP variables for single task condition during open eyes balance; Table S10: Linear and non-linear CoP variables for single task condition during closed eyes balance; Table S11: Linear and non-linear CoP variables for dual task condition during open eyes balance; Table S12: Linear and non-linear CoP variables for dual-task condition during closed eyes balance; Table S13: DTC of linear and non-linear CoP variables for open eyes balance; Table S14: DTC of linear and non-linear CoP variables for close eyes balance; Table S15: Cognitive accuracy during single and dual task conditions in the static balance tasks; Table S16: DTC of the cognitive accuracy during open and closed static balance tasks.
Author Contributions
Conceptualization, M.C.G.-A., N.G., R.C.-P., J.L.L.-L., F.J.D.-M. and S.V.; Data curation, M.C.G.-A. and M.M.-A.; Formal analysis, M.M.-A. and F.J.D.-M.; Investigation, M.C.G.-A., M.M.-A., N.G., R.C.-P., J.L.L.-L. and S.V.; Methodology, R.C.-P.; Resources, N.G.; Validation, R.C.-P., J.L.L.-L., F.J.D.-M. and S.V.; Writing—original draft, M.C.G.-A. and S.V.; Writing—review and editing, N.G., R.C.-P., J.L.L.-L., F.J.D.-M. and S.V. All authors have read and agreed to the published version of the manuscript.
Funding
In the framework of the Spanish National R + D + i Plan, the current project PID2019-107191RB-I00/AEI/10.13039/501100011033 was funded by the Spanish Agency of Research of the Spanish Ministry of Sciences and Innovation MICIU/AEI/10.13039/501100011033 and by “ERDF A way of making Europe”. This study was also co-funded by the Research Grant for Groups (GR21176), AFYCAV Group, co-funded in its part, at 85% by European Union, ERDF/EU and the Junta de Extremadura, “a way of doing Europe”. This study was supported by the Biomedical Research Excellence Networking Center on Frailty and Healthy Aging (CIBERFES) and ERDF/EU funds from the European Union (CB16/10/00477) “a way to make Europe”. The author M.C.G.-A. was supported by an internship contract (Resolution no. 552/2020) from the Junta de Extremadura (Regional Government of Extremadura), Extremadura Public Employment Service (SEXPE) and the European Social Fund (ESF/FSE) “a way of doing Europe”. The author M.M.-A. was supported by an internship contract (Resolution no. 586/2022) from the Spanish Ministry of Sciences and Innovation (reference PID2019-107191RB I00/AEI/10.13039/501100011033).
Institutional Review Board Statement
The study will be conducted in accordance with the Declaration of Helsinki, and is currently approved by the Research Ethics Committee of the University of Extremadura (approval reference: 192/2021, approval date: 15 December 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to the data of this research are confidential; the participants signed the informed consent form, assuring the confidentiality of their data.
Acknowledgments
We are very grateful to the Extremadura Association of Fibromyalgia (AFIBROEX) in Cáceres for helping recruit the participants for this study.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Burckhardt, C.S.; Clark, S.R.; Bennett, R.M. Fibromyalgia and quality of life: A comparative analysis. J. Rheumatol. 1993, 20, 475–479. [Google Scholar]
- Marques, A.P.; do Espírito Santo, A.d.S.; Berssaneti, A.A.; Matsutani, L.A.; Yuan, S.L.K. Prevalence of fibromyalgia: Literature review update. Rev. Bras. Reumatol. 2017, 57, 356–363. [Google Scholar] [CrossRef]
- Gayà, T.F.; Ferrer, C.B.; Mas, A.J.; Seoane-Mato, D.; Reyes, F.Á.; Sánchez, M.D.; Dubois, C.M.; Sánchez-Fernández, S.A.; Vargas, L.M.R.; Morales, P.V.G. Prevalence of fibromyalgia and associated factors in Spain. Clin. Exp. Rheumatol. 2020, 123, 47–52. [Google Scholar]
- Branco, J.C.; Bannwarth, B.; Failde, I.; Carbonell, J.A.; Blotman, F.; Spaeth, M.; Saraiva, F.; Nacci, F.; Thomas, E.; Caubère, J.-P. Prevalence of fibromyalgia: A survey in five European countries. Semin. Arthritis Rheum. 2010, 39, 448–453. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.-A.; Goldenberg, D.L.; Häuser, W.; Katz, R.L.; Mease, P.J.; Russell, A.S.; Russell, I.J.; Walitt, B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin. Arthritis Rheum. 2016, 46, 319–329. [Google Scholar] [CrossRef]
- Glass, J.M. Review of cognitive dysfunction in fibromyalgia: A convergence on working memory and attentional control impairments. Rheum. Dis. Clin. 2009, 35, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Bell, T.; Trost, Z.; Buelow, M.T.; Clay, O.; Younger, J.; Moore, D.; Crowe, M. Meta-analysis of cognitive performance in fibromyalgia. J. Clin. Exp. Neuropsychol. 2018, 40, 698–714. [Google Scholar] [CrossRef] [PubMed]
- Sempere-Rubio, N.; Aguilar-Rodríguez, M.; Inglés, M.; Izquierdo-Alventosa, R.; Serra-Añó, P. Physical Condition Factors that Predict a Better Quality of Life in Women with Fibromyalgia. Int. J. Environ. Res. Public Health 2019, 16, 3173. [Google Scholar] [CrossRef]
- Villafaina, S.; Collado-Mateo, D.; Domínguez-Muñoz, F.J.; Fuentes-García, J.P.; Gusi, N. Impact of adding a cognitive task while performing physical fitness tests in women with fibromyalgia: A cross-sectional descriptive study. Medicine 2018, 97, e13791. [Google Scholar] [CrossRef]
- Friedman, N.P.; Robbins, T.W. The role of prefrontal cortex in cognitive control and executive function. Neuropsychopharmacology 2022, 47, 72–89. [Google Scholar] [CrossRef]
- Murillo-Garcia, A.; Leon-Llamas, J.L.; Villafaina, S.; Gusi, N. Fibromyalgia impact in the prefrontal cortex subfields: An assessment with MRI. Clin. Neurol. Neurosurg. 2022, 219, 107344. [Google Scholar] [CrossRef] [PubMed]
- Meinzer, M.; Lindenberg, R.; Darkow, R.; Ulm, L.; Copland, D.; Flöel, A. Transcranial direct current stimulation and simultaneous functional magnetic resonance imaging. J. Vis. Exp. JoVE 2014, 86, 51730. [Google Scholar] [CrossRef]
- Manor, B.; Zhou, J.; Jor’dan, A.; Zhang, J.; Fang, J.; Pascual-Leone, A. Reduction of Dual-task Costs by Noninvasive Modulation of Prefrontal Activity in Healthy Elders. J. Cogn. Neurosci. 2016, 28, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Manor, B.; Zhou, J.; Harrison, R.; Lo, O.Y.; Travison, T.G.; Hausdorff, J.M.; Pascual-Leone, A.; Lipsitz, L. Transcranial Direct Current Stimulation May Improve Cognitive-Motor Function in Functionally Limited Older Adults. Neurorehabilit. Neural Repair 2018, 32, 788–798. [Google Scholar] [CrossRef]
- Silva, A.F.; Zortea, M.; Carvalho, S.; Leite, J.; Torres, I.L.; Fregni, F.; Caumo, W. Anodal transcranial direct current stimulation over the left dorsolateral prefrontal cortex modulates attention and pain in fibromyalgia: Randomized clinical trial. Sci. Rep. 2017, 7, 135. [Google Scholar] [CrossRef]
- Caumo, W.; Lopes Ramos, R.; Vicuña Serrano, P.; da Silveira Alves, C.F.; Medeiros, L.; Ramalho, L.; Tomeddi, R.; Bruck, S.; Boher, L.; Sanches, P.R.S.; et al. Efficacy of Home-Based Transcranial Direct Current Stimulation over the Primary Motor Cortex and Dorsolateral Prefrontal Cortex in the Disability Due to Pain in Fibromyalgia: A Factorial Sham-Randomized Clinical Study. J. Pain 2024, 25, 376–392. [Google Scholar] [CrossRef]
- Valle, A.; Roizenblatt, S.; Botte, S.; Zaghi, S.; Riberto, M.; Tufik, S.; Boggio, P.S.; Fregni, F. Efficacy of anodal transcranial direct current stimulation (tDCS) for the treatment of fibromyalgia: Results of a randomized, sham-controlled longitudinal clinical trial. J. Pain Manag. 2009, 2, 353–361. [Google Scholar]
- Staud, R. Brain imaging in fibromyalgia syndrome. Clin. Exp. Rheumatol. 2011, 29, S109–S117. [Google Scholar]
- Sandström, A.; Ellerbrock, I.; Tour, J.; Kadetoff, D.; Jensen, K.; Kosek, E. Dysfunctional activation of the dorsolateral prefrontal cortex during pain anticipation is associated with altered subsequent pain experience in fibromyalgia patients. J. Pain 2023, 24, 1731–1743. [Google Scholar] [CrossRef]
- Luerding, R.; Weigand, T.; Bogdahn, U.; Schmidt-Wilcke, T. Working memory performance is correlated with local brain morphology in the medial frontal and anterior cingulate cortex in fibromyalgia patients: Structural correlates of pain–cognition interaction. Brain 2008, 131, 3222–3231. [Google Scholar] [CrossRef]
- Burgmer, M.; Gaubitz, M.; Konrad, C.; Wrenger, M.; Hilgart, S.; Heuft, G.; Pfleiderer, B. Decreased gray matter volumes in the cingulo-frontal cortex and the amygdala in patients with fibromyalgia. Psychosom. Med. 2009, 71, 566–573. [Google Scholar] [CrossRef]
- Napadow, V.; Harris, R.E. What has functional connectivity and chemical neuroimaging in fibromyalgia taught us about the mechanisms and management ofcentralized’pain? Arthritis Res. Ther. 2014, 16, 425. [Google Scholar] [CrossRef]
- Jensen, K.B.; Srinivasan, P.; Spaeth, R.; Tan, Y.; Kosek, E.; Petzke, F.; Carville, S.; Fransson, P.; Marcus, H.; Williams, S.C.R. Overlapping structural and functional brain changes in patients with long-term exposure to fibromyalgia pain. Arthritis Rheumatol. 2013, 65, 3293–3303. [Google Scholar] [CrossRef]
- Glass, J.M. Cognitive dysfunction in fibromyalgia and chronic fatigue syndrome: New trends and future directions. Curr. Rheumatol. Rep. 2006, 8, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Peinado-Rubia, A.; Osuna-Pérez, M.C.; Rodríguez-Almagro, D.; Zagalaz-Anula, N.; López-Ruiz, M.C.; Lomas-Vega, R. Impaired Balance in Patients with Fibromyalgia Syndrome: Predictors of the Impact of This Disorder and Balance Confidence. Int. J. Environ. Res. Public Health 2020, 17, 3160. [Google Scholar] [CrossRef] [PubMed]
- Sempere-Rubio, N.; López-Pascual, J.; Aguilar-Rodríguez, M.; Cortés-Amador, S.; Espí-López, G.; Villarrasa-Sapiña, I.; Serra-Añó, P. Characterization of postural control impairment in women with fibromyalgia. PLoS ONE 2018, 13, e0196575. [Google Scholar] [CrossRef] [PubMed]
- Stergiou, N.; Decker, L.M. Human movement variability, nonlinear dynamics, and pathology: Is there a connection? Hum. Mov. Sci. 2011, 30, 869–888. [Google Scholar] [CrossRef]
- Güler, H.; Yildizgören, M.T.; Üstün, N.; Paksoy, H.; Turhanoğlu, A.D. Isokinetic Assessment of the Wrist Muscles in Females with Fibromyalgia. Arch. Rheumatol. 2016, 31, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Yetişgin, A.; Tiftik, T.; Kara, M.; Karabay, İ.; Akkuş, S.; Ersöz, M. Isokinetic muscle performance of the hip and ankle muscles in women with fibromyalgia. Int. J. Rheum. Dis. 2016, 19, 551–556. [Google Scholar] [CrossRef]
- Tavares, L.F.; Germano Maciel, D.; da Silva, T.Y.P.B.; de Brito Vieira, W.H. Comparison of functional and isokinetic performance between healthy women and women with fibromyalgia. J. Bodyw. Mov. Ther. 2020, 24, 248–252. [Google Scholar] [CrossRef]
- Cubukcu, S.; Alimoglu, M.K.; Samanci, N.; Gurbuz, U. Isokinetic and isometric muscle strength of the knee flexors and extensors in patients with the fibromyalgia syndrome and chronic myofascial pain syndrome. J. Musculoskelet. Pain 2007, 15, 49–55. [Google Scholar] [CrossRef]
- Muto, L.H.A.; Sauer, J.F.; Yuan, S.L.K.; Marques, A.P. FRI0472-HPR Assessment of postural control, strenght of lower limbs and pain in individuals with and without fibromyalgia. Ann. Rheum. Dis. 2012, 71, 748. [Google Scholar] [CrossRef]
- Sohn, M.K.; Jee, S.J.; Kim, Y.W. Effect of transcranial direct current stimulation on postural stability and lower extremity strength in hemiplegic stroke patients. Ann. Rehabil. Med. 2013, 37, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Azarkolah, A.; Noorbala, A.A.; Ansari, S.; Hallajian, A.H.; Salehinejad, M.A. Efficacy of Transcranial Direct Current Stimulation on Pain Level and Disability of Patients with Fibromyalgia: A Systematic Review of Randomized Controlled Trials with Parallel-Group Design. Brain Sci. 2023, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Gomez-Alvaro, M.C.; Villafaina, S.; Leon-Llamas, J.L.; Murillo-Garcia, A.; Melo-Alonso, M.; Sánchez-Gómez, J.; Molero, P.; Cano-Plasencia, R.; Gusi, N. Effects of Transcranial Direct Current Stimulation on Brain Electrical Activity, Heart Rate Variability, and Dual-Task Performance in Healthy and Fibromyalgia Women: A Study Protocol. Behav. Sci. 2022, 12, 37. [Google Scholar] [CrossRef]
- Dwan, K.; Li, T.; Altman, D.G.; Elbourne, D. CONSORT 2010 statement: Extension to randomised crossover trials. BMJ 2019, 366, l4378. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Alvaro, M.C.; Gusi, N.; Cano-Plasencia, R.; Leon-Llamas, J.L.; Murillo-Garcia, A.; Melo-Alonso, M.; Villafaina, S. Effects of Different Transcranial Direct Current Stimulation Intensities over Dorsolateral Prefrontal Cortex on Brain Electrical Activity and Heart Rate Variability in Healthy and Fibromyalgia Women: A Randomized Crossover Trial. J. Clin. Med. 2024, 13, 7526. [Google Scholar] [CrossRef]
- Lee, J.; Dong, S.; Jeong, J.; Yoon, B. Effects of Transcranial Direct Current Stimulation over the Dorsolateral Prefrontal Cortex (PFC) on Cognitive-Motor Dual Control Skills. Percept. Mot. Ski. 2020, 127, 803–822. [Google Scholar] [CrossRef]
- Wrightson, J.G.; Twomey, R.; Ross, E.Z.; Smeeton, N.J. The effect of transcranial direct current stimulation on task processing and prioritisation during dual-task gait. Exp. Brain Res. 2015, 233, 1575–1583. [Google Scholar] [CrossRef]
- Concerto, C.; Babayev, J.; Mahmoud, R.; Rafiq, B.; Chusid, E.; Aguglia, E.; Coira, D.; Battaglia, F. Modulation of prefrontal cortex with anodal tDCS prevents post-exercise facilitation interference during dual task. Somatosens. Mot. Res. 2017, 34, 80–84. [Google Scholar] [CrossRef]
- Zhou, J.; Hao, Y.; Wang, Y.; Jor’dan, A.; Pascual-Leone, A.; Zhang, J.; Fang, J.; Manor, B. Transcranial direct current stimulation reduces the cost of performing a cognitive task on gait and postural control. Eur. J. Neurosci. 2014, 39, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhou, J.; Chen, H.; Manor, B.; Lin, J.; Zhang, J. Effects of transcranial direct current stimulation (tDCS) on multiscale complexity of dual-task postural control in older adults. Exp. Brain Res. 2015, 233, 2401–2409. [Google Scholar] [CrossRef] [PubMed]
- Pineau, N.; Robin, A.; Bulteau, S.; Thomas-Ollivier, V.; Sauvaget, A.; Deschamps, T. Does the transcranial direct current stimulation improve dual-task postural control in young healthy adults? Cogn. Process. 2020, 22, 291–298. [Google Scholar] [CrossRef]
- Jor’dan, A.J.; Bernad-Elazari, H.; Mirelman, A.; Gouskova, N.A.; Lo, O.Y.; Hausdorff, J.M.; Manor, B. Transcranial Direct Current Stimulation May Reduce Prefrontal Recruitment During Dual Task Walking in Functionally Limited Older Adults—A Pilot Study. Front. Aging Neurosci. 2022, 14, 843122. [Google Scholar] [CrossRef] [PubMed]
- Priori, A. Brain polarization in humans: A reappraisal of an old tool for prolonged non-invasive modulation of brain excitability. Clin. Neurophysiol. 2003, 114, 589–595. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Liebetanz, D.; Antal, A.; Lang, N.; Tergau, F.; Paulus, W. Modulation of cortical excitability by weak direct current stimulation—Technical, safety and functional aspects. In Supplements to Clinical Neurophysiology; Elsevier: Amsterdam, The Netherlands, 2003; Volume 56, pp. 255–276. [Google Scholar] [CrossRef]
- Paulus, W. Transcranial direct current stimulation (tDCS). In Supplements to Clinical Neurophysiology; Elsevier: Amsterdam, The Netherlands, 2003; Volume 56, pp. 249–254. [Google Scholar] [CrossRef]
- Henriksen, M.; Lund, H.; Christensen, R.; Jespersen, A.; Dreyer, L.; Bennett, R.M.; Danneskiold-Samsøe, B.; Bliddal, H. Relationships between the fibromyalgia impact questionnaire, tender point count, and muscle strength in female patients with fibromyalgia: A cohort study. Arthritis Rheumatol. 2009, 61, 732–739. [Google Scholar] [CrossRef]
- Coban, O.; Yildirim, N.U.; Yasa, M.E.; Akinoglu, B.; Kocahan, T. Determining the number of repetitions to establish isokinetic knee evaluation protocols specific to angular velocities of 60°/second and 180°/second. J. Bodyw. Mov. Ther. 2021, 25, 255–260. [Google Scholar] [CrossRef]
- Duarte, M.; Freitas, S.M. Revision of posturography based on force plate for balance evaluation. Rev. Bras. Fisioter. 2010, 14, 183–192. [Google Scholar] [CrossRef]
- Stergiou, N. Nonlinear Analysis for Human Movement Variability; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Wolf, A.; Swift, J.B.; Swinney, H.L.; Vastano, J.A. Determining Lyapunov exponents from a time series. Phys. D Nonlinear Phenom. 1985, 16, 285–317. [Google Scholar] [CrossRef]
- Wurdeman, S.R. Lyapunov exponent. In Nonlinear Analysis for Human Movement Variability; CRC Press: Boca Raton, FL, USA, 2018; pp. 83–110. [Google Scholar]
- Roman-Viñas, B.; Serra-Majem, L.; Hagströmer, M.; Ribas-Barba, L.; Sjöström, M.; Segura-Cardona, R. International physical activity questionnaire: Reliability and validity in a Spanish population. Eur. J. Sport Sci. 2010, 10, 297–304. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Murillo-Garcia, A.; Leon-Llamas, J.L.; Villafaina, S.; Rohlfs-Dominguez, P.; Gusi, N. MoCA vs. MMSE of fibromyalgia patients: The possible role of dual-task tests in detecting cognitive impairment. J. Clin. Med. 2021, 10, 125. [Google Scholar] [CrossRef]
- Herdman, M.; Gudex, C.; Lloyd, A.; Janssen, M.F.; Kind, P.; Parkin, D.; Bonsel, G.; Badia, X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual. Life Res. 2011, 20, 1727–1736. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Liu, P.; Xiao, F.; Liu, Z.; Wang, Y. Review of the upright balance assessment based on the force plate. Int. J. Environ. Res. Public Health 2021, 18, 2696. [Google Scholar] [CrossRef]
- Scoppa, F.; Capra, R.; Gallamini, M.; Shiffer, R. Clinical stabilometry standardization: Basic definitions–acquisition interval–sampling frequency. Gait Posture 2013, 37, 290–292. [Google Scholar] [CrossRef]
- Gomez-Alvaro, M.C.; Leon-Llamas, J.L.; Melo-Alonso, M.; Villafaina, S.; Domínguez-Muñoz, F.J.; Gusi, N. Test-Retest Reliability of Isokinetic Strength in Lower Limbs under Single and Dual Task Conditions in Women with Fibromyalgia. J. Clin. Med. 2024, 13, 1288. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect size estimates: Current use, calculations, and interpretation. J. Exp. Psychol. Gen. 2012, 141, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Machado, S.; Jansen, P.; Almeida, V.; Veldema, J. Is tDCS an adjunct ergogenic resource for improving muscular strength and endurance performance? A systematic review. Front. Psychol. 2019, 10, 1127. [Google Scholar] [CrossRef] [PubMed]
- Masoudi, M.; Ehsani, F.; Hedayati, R.; Ramezani, M.; Jaberzadeh, S. Different montages of transcranial direct current stimulation on postural stability in chronic low back pain patients: A randomized sham-controlled study. J. Back Musculoskelet. Rehabil. 2024, 37, 1151–1161. [Google Scholar] [CrossRef]
- Cha, S.; Choi, J.; Moon, C.; Cho, K. Non-invasive brain stimulation contributing to postural control with and without stroke: A systematic review and meta-analysis. Sci. Rep. 2025, 15, 26020. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.D.; Horak, F.B.; Winters-Stone, K.; Irvine, J.M.; Bennett, R.M. Fibromyalgia is associated with impaired balance and falls. J. Clin. Rheumatol. Pract. Rep. Rheum. Musculoskelet. Dis. 2009, 15, 16–21. [Google Scholar] [CrossRef]
- Collado-Mateo, D.; Gallego-Diaz, J.M.; Adsuar, J.C.; Domínguez-Muñoz, F.J.; Olivares, P.R.; Gusi, N. Fear of Falling in Women with Fibromyalgia and Its Relation with Number of Falls and Balance Performance. BioMed Res. Int. 2015, 2015, 589014. [Google Scholar] [CrossRef][Green Version]
- Vieira, L.A.F.; Lattari, E.; de Jesus Abreu, M.A.; Rodrigues, G.M.; Viana, B.; Machado, S.; Oliveira, B.R.R.; Maranhão Neto, G.A. Transcranial Direct Current Stimulation (tDCS) Improves Back-Squat Performance in Intermediate Resistance-Training Men. Res. Q. Exerc. Sport 2022, 93, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Etemadi, M.; Amiri, E.; Tadibi, V.; Grospretre, S.; Valipour Dehnou, V.; Machado, D. Anodal tDCS over the left DLPFC but not M1 increases muscle activity and improves psychophysiological responses, cognitive function, and endurance performance in normobaric hypoxia: A randomized controlled trial. BMC Neurosci. 2023, 24, 25. [Google Scholar] [CrossRef]
- Teymoori, H.; Amiri, E.; Tahmasebi, W.; Hoseini, R.; Grospretre, S.; Machado, D.G.d.S. Effect of tDCS targeting the M1 or left DLPFC on physical performance, psychophysiological responses, and cognitive function in repeated all-out cycling: A randomized controlled trial. J. NeuroEng. Rehabil. 2023, 20, 97. [Google Scholar] [CrossRef]
- Soutschek, A.; Nadporozhskaia, L.; Christian, P. Brain stimulation over dorsomedial prefrontal cortex modulates effort-based decision making. Cogn. Affect. Behav. Neurosci. 2022, 22, 1264–1274. [Google Scholar] [CrossRef]
- Soutschek, A.; Tobler, P.N. Causal role of lateral prefrontal cortex in mental effort and fatigue. Hum. Brain Mapp. 2020, 41, 4630–4640. [Google Scholar] [CrossRef]
- Cerón-Lorente, L.; Valenza, M.C.; Pérez-Mármol, J.M.; del Carmen García-Ríos, M.; Castro-Sánchez, A.M.; Aguilar-Ferrándiz, M.E. The influence of balance, physical disability, strength, mechanosensitivity and spinal mobility on physical activity at home, work and leisure time in women with fibromyalgia. Clin. Biomech. 2018, 60, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.; Benzo, R.P.; Whipple, M.O.; McAllister, S.J.; Erwin, P.J.; Saligan, L.N. Beyond pain in fibromyalgia: Insights into the symptom of fatigue. Arthritis Res. Ther. 2013, 15, 221. [Google Scholar] [CrossRef]
- Alcon, C.; Margerison, S.; Kirse, H.; Zoch, C.; Laurienti, P.; Seminowicz, D.; Wang-Price, S. The Effect of Combining Transcranial Direct Current Stimulation and Pain Neuroscience Education in Patients with Chronic Low Back Pain and High Pain Catastrophizing: An Exploratory Clinical, Cognitive, and fMRI Study. Brain Behav. 2025, 15, e70543. [Google Scholar] [CrossRef]
- Trost, W.; Hars, M.; Fernandez, N.; Herrmann, F.; Chevalley, T.; Ferrari, S.; Gold, G.; Rizzoli, R.; Vuilleumier, P.; Trombetti, A. Functional brain changes in sarcopenia: Evidence for differential central neural mechanisms in dynapenic older women. Aging Clin. Exp. Res. 2023, 35, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jia, X.; Li, Y.; Li, H.; Yang, Q. The longitudinal bidirectional association between sarcopenia and cognitive function in community-dwelling older adults: Findings from the China Health and Retirement Longitudinal Study. J. Glob. Health 2023, 13, 04182. [Google Scholar] [CrossRef] [PubMed]
- Cipolli, G.C.; Yassuda, M.S.; Aprahamian, I. Sarcopenia is associated with cognitive impairment in older adults: A systematic review and meta-analysis. J. Nutr. Health Aging 2019, 23, 525–531. [Google Scholar] [CrossRef]
- Du, J.; Tao, X.; Zhu, L.; Wang, H.; Qi, W.; Min, X.; Wei, S.; Zhang, X.; Liu, Q. Development of a visualized risk prediction system for sarcopenia in older adults using machine learning: A cohort study based on CHARLS. Front. Public Health 2025, 13, 1544894. [Google Scholar] [CrossRef] [PubMed]
- Usman, J.S.; Wong, T.W.-L.; Ng, S.S.M. Effects of transcranial direct current stimulation on dual-task performance in older and young adults: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. Plus 2024, 1, 100047. [Google Scholar] [CrossRef]
- Beyrek, B.; Naz, İ.; Emuk, Y.; Köprülüoğlu, M.; Felekoğlu, E.; Uzun, E.; Nas, K. Investigation of the dual-task performance and affecting factors in female patients with fibromyalgia syndrome. Women Health 2023, 63, 277–284. [Google Scholar] [CrossRef]
- Muñoz-Perete, J.M.; Cano-Sánchez, J.; Castellote-Caballero, Y.; Vico-Rodríguez, P.; Cano-Orihuela, M.; Sánchez-Alcalá, M.; Carcelén-Fraile, M.d.C. Effectiveness of Transcranial Stimulation on Cognitive Abilities of Older Adults with Mild Cognitive Impairment. J. Clin. Med. 2025, 14, 2472. [Google Scholar] [CrossRef]
- Santos, V.; Zortea, M.; Alves, R.L.; Naziazeno, C.; Saldanha, J.S.; Carvalho, S.; Leite, A.; Torres, I.; Souza, A.; Calvetti, P.; et al. Cognitive effects of transcranial direct current stimulation combined with working memory training in fibromyalgia: A randomized clinical trial. Sci. Rep. 2018, 8, 12477. [Google Scholar] [CrossRef]
- Serrano, P.V.; Zortea, M.; Alves, R.L.; Beltrán, G.; Bavaresco, C.; Ramalho, L.; Alves, C.F.d.S.; Medeiros, L.; Sanches, P.R.S.; Silva, D.P., Jr. The effect of home-based transcranial direct current stimulation in cognitive performance in fibromyalgia: A randomized, double-blind sham-controlled trial. Front. Hum. Neurosci. 2022, 16, 992742. [Google Scholar] [CrossRef]
- Batsikadze, G.; Moliadze, V.; Paulus, W.; Kuo, M.F.; Nitsche, M.A. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J. Physiol. 2013, 591, 1987–2000. [Google Scholar] [CrossRef] [PubMed]
- Esmaeilpour, Z.; Marangolo, P.; Hampstead, B.M.; Bestmann, S.; Galletta, E.; Knotkova, H.; Bikson, M. Incomplete evidence that increasing current intensity of tDCS boosts outcomes. Brain Stimul. 2018, 11, 310–321. [Google Scholar] [CrossRef]
- Murillo-Garcia, A.; Villafaina, S.; Leon-Llamas, J.L.; Sánchez-Gómez, J.; Domínguez-Muñoz, F.J.; Collado-Mateo, D.; Gusi, N. Mobility Assessment under Dual Task Conditions in Women with Fibromyalgia: A Test-Retest Reliability Study. PM&R 2021, 13, 66–72. [Google Scholar]
- Khan, M.J.; Kannan, P.; Wong, T.W.-L.; Fong, K.N.K.; Winser, S.J. A systematic review exploring the theories underlying the improvement of balance and reduction in falls following dual-task training among older adults. Int. J. Environ. Res. Public Health 2022, 19, 16890. [Google Scholar] [CrossRef] [PubMed]
- Ljubisavljevic, M.R.; Oommen, J.; Filipovic, S.; Bjekic, J.; Szolics, M.; Nagelkerke, N. Effects of tDCS of dorsolateral prefrontal cortex on dual-task performance involving manual dexterity and cognitive task in healthy older adults. Front. Aging Neurosci. 2019, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Caumo, W.; Franca, B.R.; Orzechowski, R.; Bueno, G.; França, A.; da Silva, J.V.d.S.; Sanches, P.R.S.; Da Silva, D.P.; Torres, I.L.S.; Hirakata, V.N. Home-Based Transcranial Direct Current Stimulation vs Placebo for Fibromyalgia: A Randomized Clinical Trial. JAMA Netw. Open 2025, 8, e2514262. [Google Scholar] [CrossRef]
- Ben Izhak, S.; Jacoby, N.; Diedrich, L.; Antal, A.; Lavidor, M. Enhanced cognitive performance in older adults through combined cognitive training and transcranial direct current stimulation. Sci. Rep. 2025, 15, 24114. [Google Scholar] [CrossRef]
- Luo, N.; Zhao, B.; Wang, H.; Wu, J.; Luo, Y.; Yuan, M.; Xu, C. Effect of transcranial direct current stimulation combined with cognitive rehabilitation on cognitive function and activities of daily living in patients with post-stroke cognitive impairment: A systematic review and meta-analysis. Front. Neurol. 2025, 16, 1523001. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).