Sustainable Strategies for Sunburn Mitigation in Gala Apple Orchards: Effects on Yield, Fruit Quality, and Plant Physiology
Abstract
1. Introduction
2. Materials and Methods
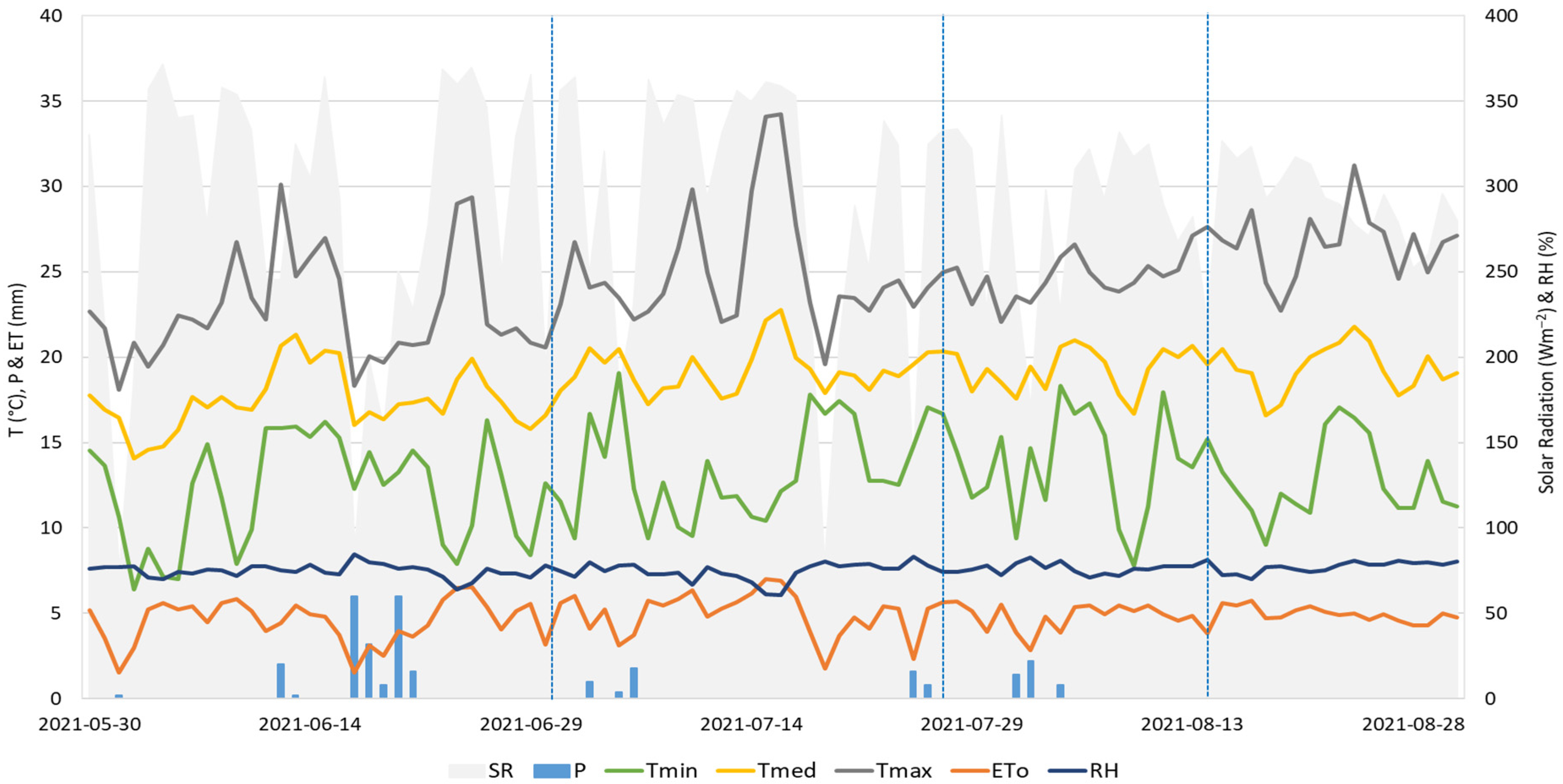
2.1. Experimental Site
| Date | DAFB | Treatment | Dose (Unit) |
|---|---|---|---|
| 28 May | 46 | FF: FG + AA + MKP + MAP; EK | 250 mL/100 L + 150 mL/100 L + 300 g/100 L + 300 g/100 L; 50 mL/100 L |
| 11 June | 60 | FF: CF + FL; EK | 2 kg/ha + 100 mL/100 L; 50 mL/100 L |
| 24 June | 73 | FF: NPK + BX; SS; SD; AW; VS; EK | 500 g/100 L + 150 g/100 L; 12 kg/ha; 2.5 kg/ha; 5 kg/ha; 2 L/100 L; 50 mL/100 L |
| 7 July | 86 | FF: RD + FG; EK | 2.5 kg/ha + 0.5 kg/ha; 50 mL/100 L |
| 21 July | 100 | FF: FT; EK | 3 kg/ha; 50 mL/100 L |
| 9 August | 119 | FF: ST + RD; EK | 2 L/ha + 2.5 kg/ha; 50 mL/100 L |
2.2. Agronomic Determinations
2.3. Physiological Determinations
2.4. Gene Expression Analysis
2.4.1. Ribonucleic Acid Extraction
2.4.2. Complementary DNA Synthesis by Reverse Transcription
2.4.3. Selection of Defense-Related Target Genes
2.4.4. Quantitative Real-Time PCR
2.4.5. Gene Expression Analysis Using the 2−ΔΔCq Method
2.5. Production and Fruit Quality Determinations
2.6. Statistical Analyses
3. Results
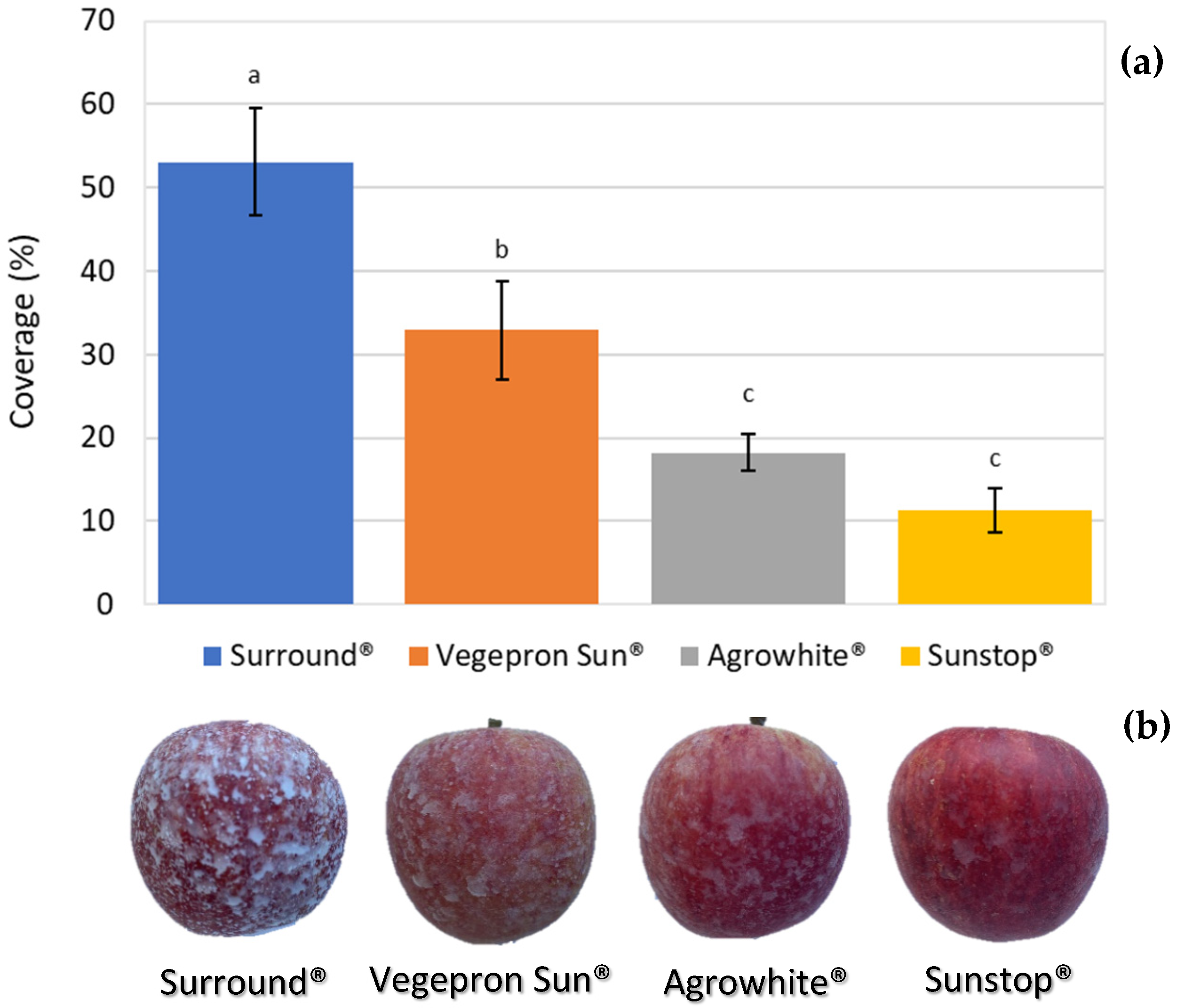
3.1. Percentage of Particle Films Coverage
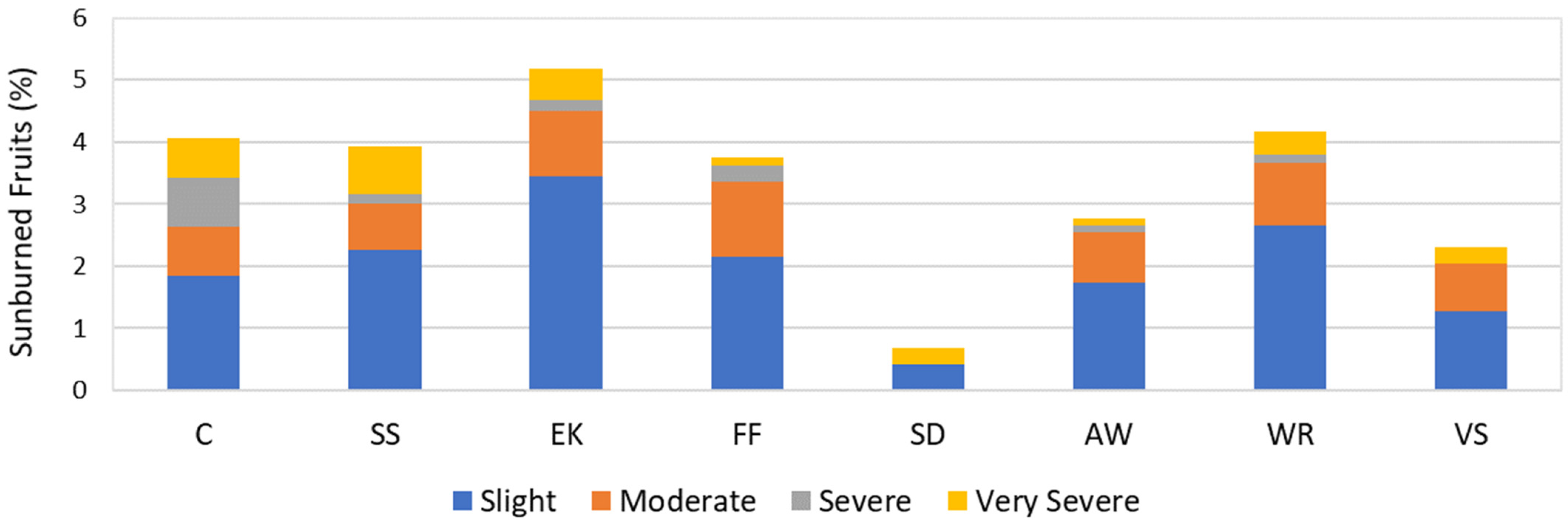
3.2. Agronomic Parameters
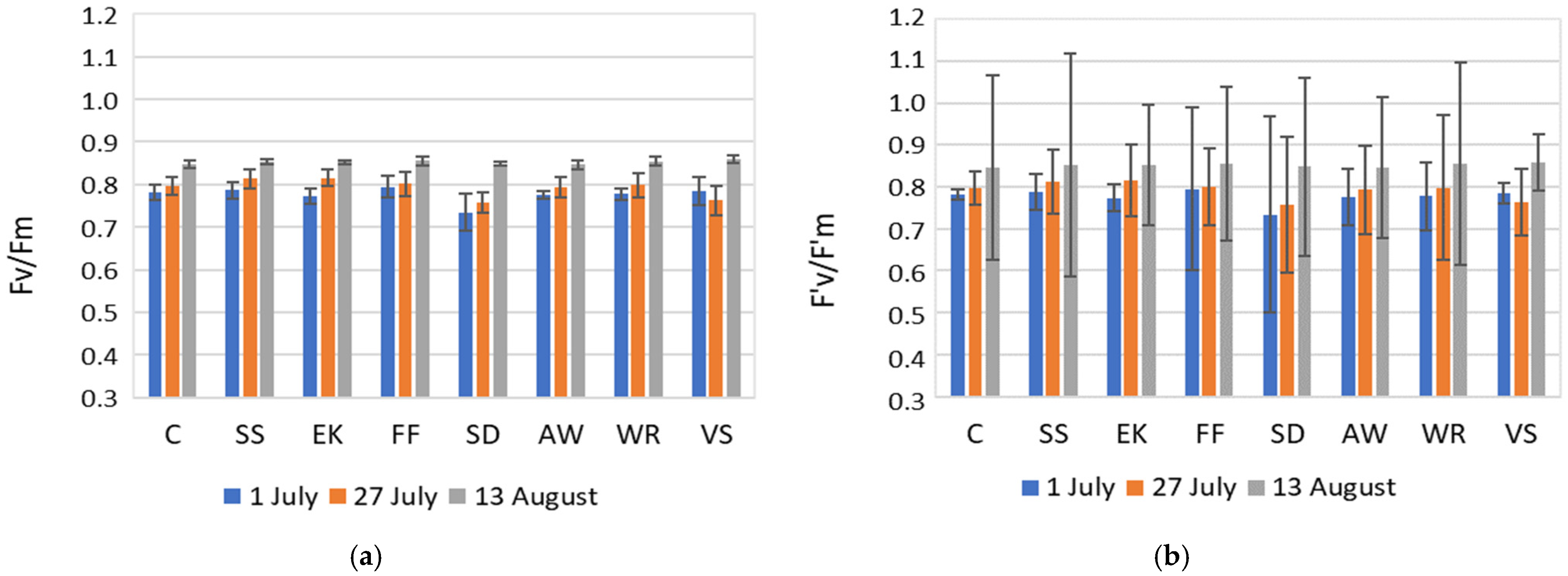
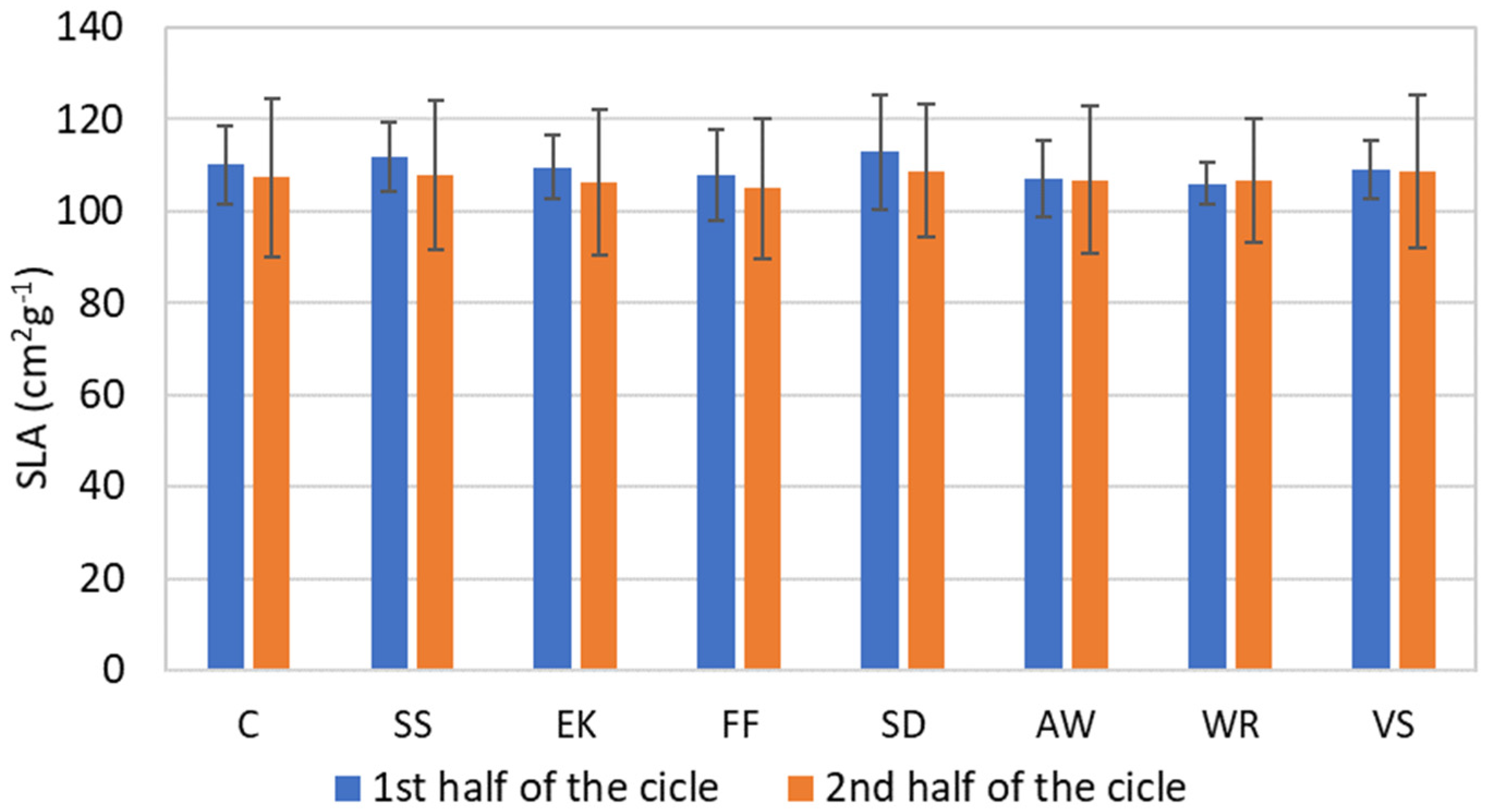
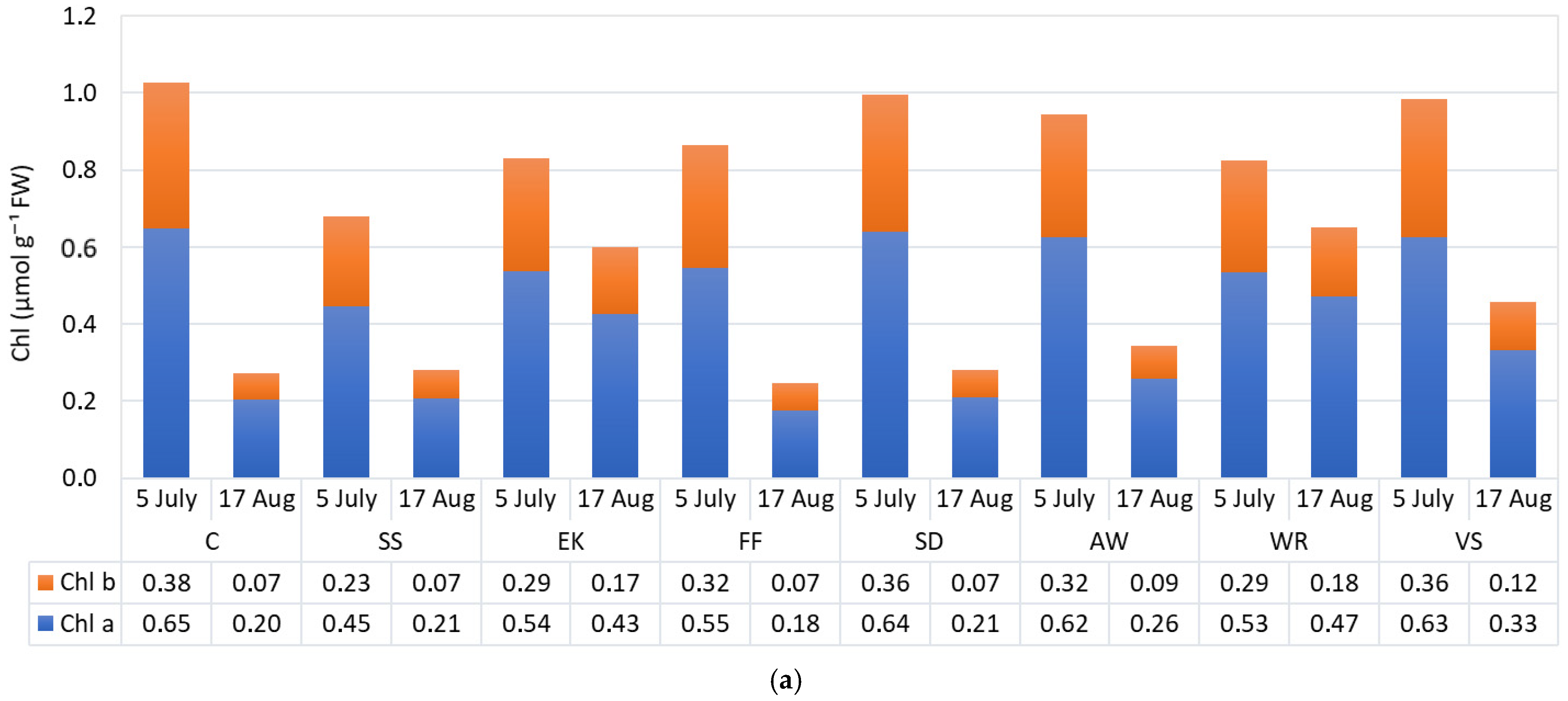
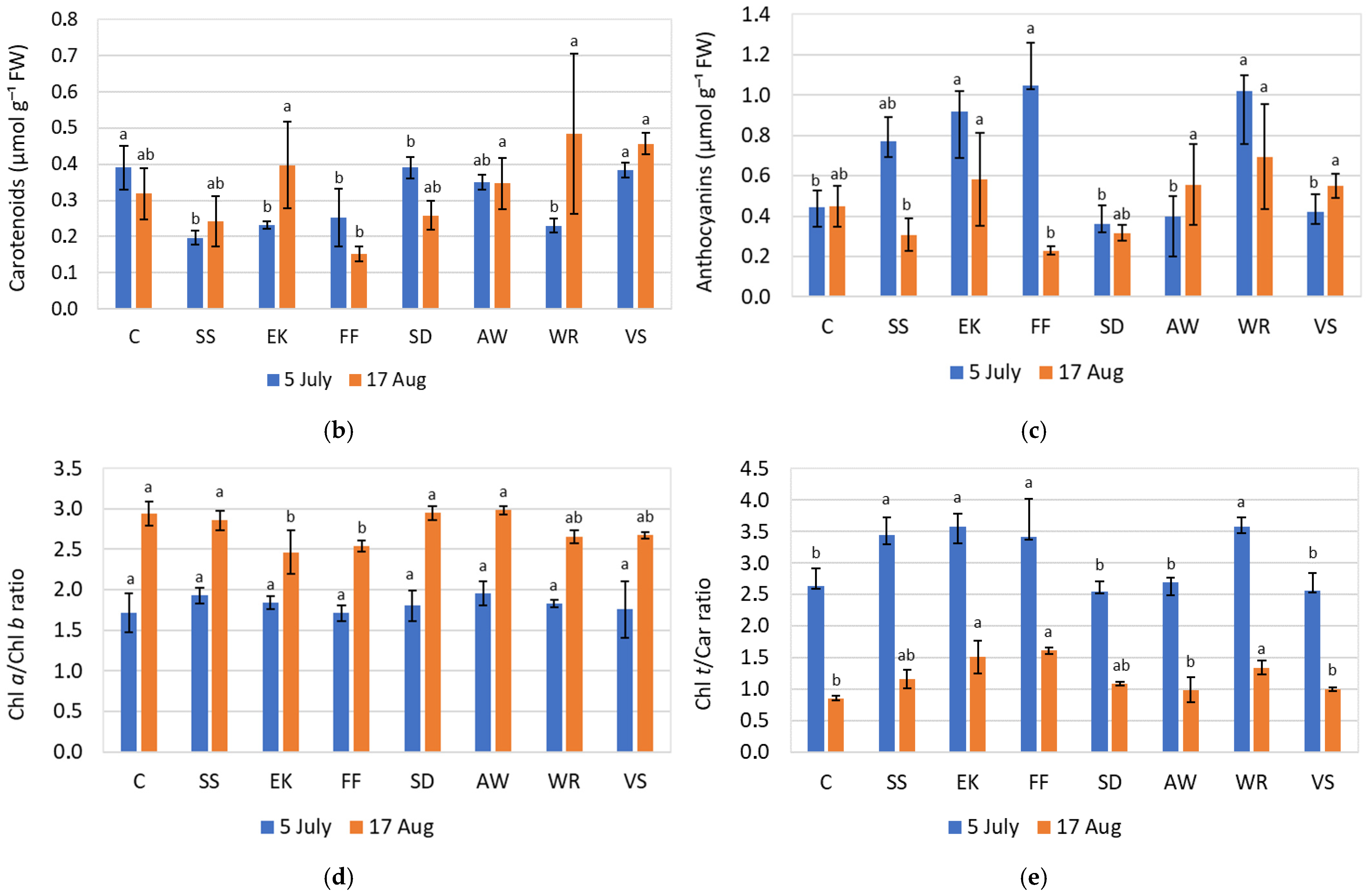
3.3. Physiological Parameters
4. Discussion
4.1. Agronomic Aspects
4.2. Plant Physiological Analyses
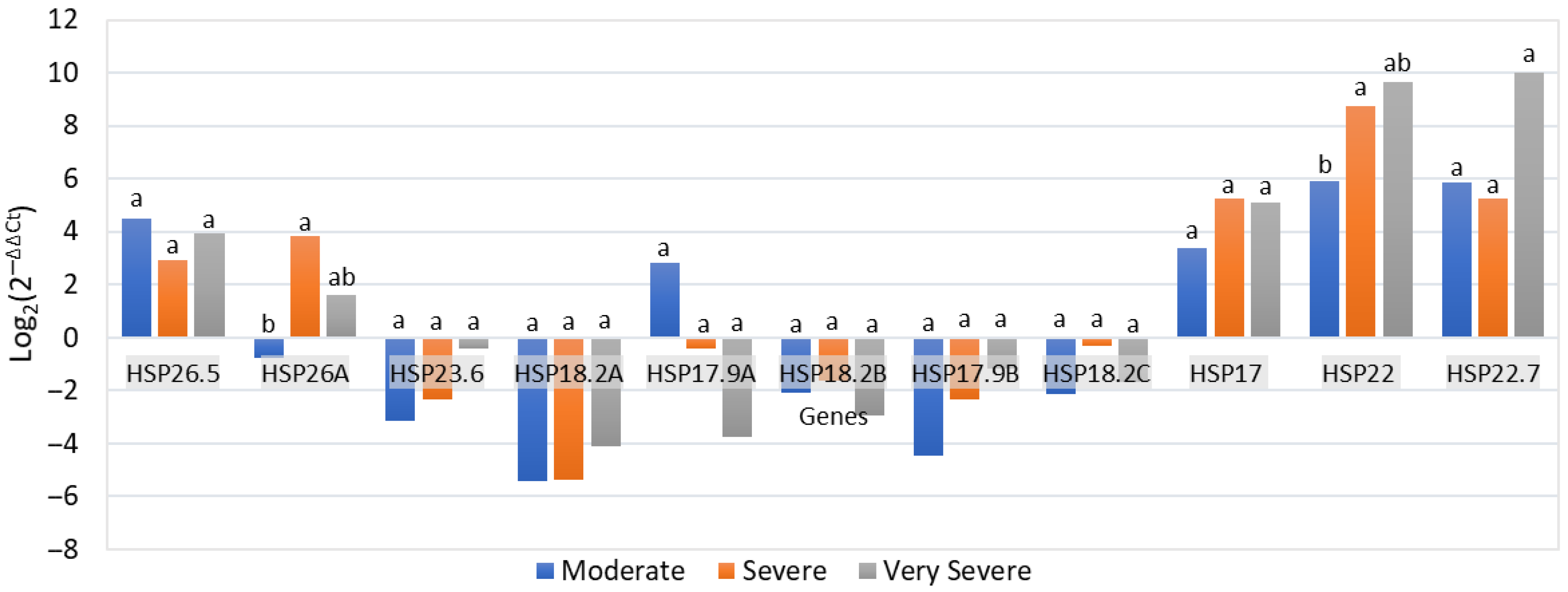
4.3. Expression of HSPs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| An | Net photosynthesis (µmol m−2 s−1) |
| AW | Agrowhite® (particle film treatment) |
| C | Control treatment |
| Chl a | Chlorophyll a |
| Chl b | Chlorophyll b |
| Chl t/Car | Total chlorophyll to carotenoid ratio |
| EK | Sunstop® (particle film treatment) |
| ETc | Cultural evapotranspiration (mm) |
| ETo | Reference evapotranspiration (mm) |
| FF | Fruit film treatment (as defined in the study, e.g., FF formulation) |
| Fv/Fm | Maximum photochemical efficiency of PSII in dark-adapted leaves |
| F′v/F′m | Maximum photochemical efficiency of PSII in light-adapted leaves |
| FW | Fresh weight |
| HSP | Heat shock protein |
| HSP18.2A/B/C | Small heat shock protein 18.2 variants |
| HSP17.9A/B | Small heat shock protein 17.9 variants |
| HSP17 | Small heat shock protein 17 |
| HSP22 | Small heat shock protein 22 |
| HSP22.7 | Small heat shock protein 22.7 |
| HSP23.6 | Small heat shock protein 23.6 |
| HSP26A | Small heat shock protein 26A |
| HSP26.5 | Small heat shock protein 26.5 |
| Kc | Crop coefficient |
| P | Precipitation (mm) |
| PSII | Photosystem II |
| SD | Surround® (particle film treatment) |
| sHSP | Small heat shock proteins |
| SLA | Specific leaf area (cm2 g−1) |
| SSC | Soluble solids content (°Brix) |
| SR | Solar radiation (W m−2) |
| SS | Sunstop® (particle film treatment, as used in tables) |
| TCSA | Trunk cross-sectional area (cm2) |
| Tmax | Maximum daily air temperature (°C) |
| Tmed | Mean daily air temperature (°C) |
| Tmin | Minimum daily air temperature (°C) |
| VS | Vegepron Sun® (particle film treatment) |
| WR | Another particle film treatment (as defined in the study; e.g., WR formulation) |
References
- Smit, A.; Steyn, W.J.; Wand, S.J.E. Effects of shade netting on gas exchange of blushed apple cultivars. Acta Hortic. 2008, 772, 73–80. [Google Scholar] [CrossRef]
- Afonso, S.; Gonçalves, M.; Rodrigues, M.; Martinho, F.; Amado, V.; Rodrigues, S.; de Sousa, M.L. Conventional vs. Photoselective Nets: Impacts on Tree Physiology, Yield, Fruit Quality and Sunburn in “Gala” Apples Grown in Mediterranean Climate. Agronomy 2025, 15, 1812. [Google Scholar] [CrossRef]
- Barber, H.N.; Sharpe, P.J.H. Genetics and physiology of sunscald of fruits. Agric. Meteorol. 1971, 8, 175–191. [Google Scholar] [CrossRef]
- Reig, G.; Donahue, D.J.; Jentsch, P. The efficacy of four sunburn mitigation strategies and their effects on yield, fruit quality, and economic performance of Honeycrisp Cv. apples under Eastern New York (USA) climatic conditions. Int. J. Fruit Sci. 2019, 20, 541–561. [Google Scholar] [CrossRef]
- Felicetti, D.A.; Schrader, L.E. Changes in pigment concentrations associated with sunburn browning of five apple cultivars. I. Chlorophylls and carotenoids. Plant Sci. 2009, 176, 84–89. [Google Scholar] [CrossRef]
- Schrader, L.E.; Zhang, J.; Duplaga, W.K. Two types of sunburn in apple caused by high fruit surface (peel) temperature. Plant Health Prog. 2001, 2. [Google Scholar] [CrossRef]
- Felicetti, D.A.; Schrader, L.E. Photooxidative sunburn of apples: Characterization of a third type of apple sunburn. Int. J. Fruit Sci. 2008, 8, 160–172. [Google Scholar] [CrossRef]
- Xu, H.; Watanabe, Y.; Ediger, D.; Yang, X.; Iritani, D. Characteristics of Sunburn Browning Fruit and Rootstock-Dependent Damage-Free Yield of Ambrosia™ Apple after Sustained Summer Heat Events. Plants 2022, 11, 1201. [Google Scholar] [CrossRef]
- Brooks, C.; Fischer, D. Environmental stresses that cause sunburn of apple. Acta Hortic. 2003, 618, 397–405. [Google Scholar] [CrossRef]
- Wünsche, J.N.; Bowen, J.; Ferguson, I.; Woolf, A. Sunburn on apples—Causes and control mechanisms. Acta Hortic. 2004, 636, 631–636. [Google Scholar] [CrossRef]
- Lal, N.; Sahu, N. Management Strategies of Sunburn in Fruit Crops—A Review. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 1126–1138. [Google Scholar] [CrossRef]
- Parchomchuk, P.; Meheriuk, M. Orchard cooling with pulsed overtree irrigation to prevent solar injury and improve fruit quality of ‘Jonagold’ apples. HortScience 1996, 31, 802–804. [Google Scholar] [CrossRef]
- Woolf, A.B.; Ferguson, I.B. Postharvest responses to high fruit temperatures in the field. Postharvest Biol. Technol. 2000, 21, 7–20. [Google Scholar] [CrossRef]
- Torres, C.; Yuri, J.; Neira, A.; Quilodrán, Á.; Razmilic, I.; Motomura, Y.; Palomo, I. Sunburn on Apples Is Associated with Increases in Phenolic Compounds and Antioxidant Activity as a Function of the Cultivar and Areas of the Fruit. J. Food Agric. Environ. 2010, 8, 920–925. [Google Scholar]
- Schrader, L.; Sun, J.; Zhang, J.; Seo, J.-H.; Jedlow, L.; Felicetti, D. Fruit Skin Disorders. In Proceedings of the Washington Tree Fruit Postharvest Conference, Yakima, WA, USA, 8 December 2004; WSU Tree Fruit Research & Extension Center: Wenatchee, WA, USA, 2004; pp. 1–4. Available online: https://www.researchgate.net/publication/266590374_FRUIT_SKIN_DISORDERS (accessed on 20 October 2025).
- Le Grange, M.; Wand, S.J.E.; Theron, K.I. Effect of kaolin applications on apple fruit quality and gas exchange of apple leaves. Acta Hortic. 2004, 636, 545–550. [Google Scholar] [CrossRef]
- Sarooghinia, S.; Esmaeilizadeh, M.; Dadpour, M.R. Foliar Application of Kaolin to Reduce Sunburn in ‘Red Delicious’ Apple. Eur. J. Hortic. Sci. 2020, 85, 9–16. [Google Scholar] [CrossRef]
- Leão de Sousa, M.; Gonçalves, M. Minimizing the Effects of Thermal Stress by Foliar Nutrition, Irrigation and Kaolin Applications in ‘Gala’ Apple Trees. In Proceedings of the IX International Symposium on Mineral Nutrition of Fruit Crops, Leuven, Belgium, 28–30 June 2021; ISHS: Leuven, Belgium, 2021; Volume 1333, pp. 185–194. [Google Scholar] [CrossRef]
- Hannam, K.D.; MacDonald, J.L. Tools for climate resilience in tree fruit II: A calcium carbonate-based foliar spray showed potential for protecting fruit quality during an unprecedented heat event. Can. J. Plant Sci. 2023, 103, 228–232. [Google Scholar] [CrossRef]
- Iglesias, I.; Alegre, S. The effects of reflective film on fruit color, quality, canopy light distribution, and profitability of ‘Mondial Gala’ apples. HortTechnology 2009, 19, 488–498. [Google Scholar] [CrossRef]
- Dayioglu, H.; Hepaksoy, S. Effects of Shading Nets on Sunburn and Quality of ‘Granny Smith’ Apple Fruits. Acta Hortic. 2016, 1139, 627–632. [Google Scholar] [CrossRef]
- Glenn, D.M.; Puterka, G.; Drake, S.; Unruh, T.; Knight, A.; Baherle, P.; Prado, E.; Baugher, T. Particle film application influences apple leaf physiology, fruit yield, and fruit quality. J. Am. Soc. Hortic. Sci. 2001, 126, 175–181. [Google Scholar] [CrossRef]
- Willsea, N.; Blanco, V.; Howe, O.; Campbell, T.; Casagrande Biasuz, E.; Kalcsits, L. Retractable netting and evaporative cooling for sunburn control and increasing red color for ‘Honeycrisp’ apple. HortScience 2023, 58, 1341–1347. [Google Scholar] [CrossRef]
- Direção-Geral de Agricultura e Desenvolvimento Rural (DGADR). Normas Técnicas para a Produção Integrada de Pomóideas; DGADR: Lisboa, Portugal, 2011. Available online: https://www.dgadr.gov.pt/pt/mediateca?catid=8&id=45%3Anormas-tecnicas-para-producao-integrada-de-pomoideas&task=download.send (accessed on 3 September 2025).
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration: Guidelines for Computing Crop Water Requirements; FAO Irrigation and Drainage Paper No. 56; Food and Agriculture Organization of the United Nations: Rome, Italy, 1998. [Google Scholar]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S.; Uhl, C. Photosynthesis-nitrogen relations in Amazonian tree species: I. Patterns among species and communities. Oecologia 1994, 97, 62–72. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Richardson, A.D.; Duigan, S.P.; Berlyn, G.P. An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol. 2002, 153, 185–194. [Google Scholar] [CrossRef]
- Anderson, M.D.; Prasad, T.K.; Stewart, C.R. Changes in isozyme profiles of catalase, peroxidase, and glutathione reductase during acclimation to chilling in mesocotyls of maize seedlings. Plant Physiol. 1995, 109, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Masato, O. An improved method for determination of L-ascorbic acid and L-dehydroascorbic acid in blood plasma. Clin. Chim. Acta 1980, 103, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.C.; Amâncio, S. Antioxidant defence system in plantlets transferred from in vitro to ex vitro: Effects of increasing light intensity and CO2 concentration. Plant Sci. 2002, 162, 33–40. [Google Scholar] [CrossRef]
- Conde, A.; Soares, F.; Breia, R.; Gerós, H. Postharvest dehydration induces variable changes in the primary metabolism of grape berries. Food Res. Int. 2018, 105, 261–270. [Google Scholar] [CrossRef]
- Coito, J.L.; Rocheta, M.; Carvalho, L.; Amâncio, S. Microarray-based uncovering reference genes for quantitative real time PCR in grapevine under abiotic stress. BMC Res. Notes 2012, 5, 220. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- CTIFL. Méthode de Détermination de L’indice D’amidon pour la Pomme et la Poire; Centre Technique Interprofessionnel des Fruits et Légumes: Paris, France, 1995. [Google Scholar]
- Cavaco, M. Normas Técnicas Para a Produção Integrada de Pomóideas; Série Divulgação n. 362; MADRP; DGADR: Lisboa, Portugal, 2012; Volume II, p. 254. Available online: https://www.dgadr.gov.pt/images/docs/prod_sust/normas_pi/i012008.pdf (accessed on 13 September 2025).
- Bartold, M.; Kluczek, M. Estimating of chlorophyll fluorescence parameter Fv/Fm for plant stress detection at peatlands under Ramsar Convention with Sentinel-2 satellite imagery. Ecol. Inform. 2024, 81, 102603. [Google Scholar] [CrossRef]
- Glenn, D.M.; Puterka, G.J.; Vanderzwet, T.; Byers, R.E.; Feldhake, C. Hydrophobic particle films: A new paradigm for suppression of arthropod pests and plant diseases. J. Econ. Entomol. 1999, 92, 759–771. [Google Scholar] [CrossRef]
- Schupp, J.R.; Fallahi, E.; Chun, I. Effect of particle film on fruit sunburn, maturity and quality of ‘Fuji’ and ‘Honeycrisp’ apples. Compact. Fruit Tree 2002, 12, 87–90. [Google Scholar] [CrossRef]
- Gindaba, J.; Wand, S.J.E. Comparative effects of evaporative cooling, kaolin particle film, and shade net on sunburn and fruit quality in apples. HortScience 2005, 40, 592–596. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; da Silva, A.B.; Tavares, C.; Pastaneira, M.; Melo, J.; Ferro Rodrigues, C.; Machado, A.M.; Antunes, M.; Cruz, C.; da Silva, J.M.; et al. Influence of orchards fertilization management and post-harvest storage time on Malus domestica cv. ‘Gala’ fruit volatiles and quality parameters. J. Food Compos. Anal. 2025, 138, 107000. [Google Scholar] [CrossRef]
- IPMA. Normais Climatológicas 1981–2010. Available online: https://www.ipma.pt/pt/oclima/normais.clima/1981-2010/ (accessed on 10 March 2025).
- Glenn, D.M.; Erez, A.; Puterka, G.J.; Gundrum, P. Particle films affect carbon assimilation and yield in ‘Empire’ apple. J. Am. Soc. Hortic. Sci. 2003, 128, 356–362. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W. Photoprotection and other responses of plants to high light stress. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 599–626. [Google Scholar] [CrossRef]
- Demmig-Adams, B. Carotenoids and photoprotection in plants: A role for the xanthophyll cycle. Biochim. Biophys. Acta 1990, 1020, 1–24. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Liu, S.; Chen, Q. Plant stress response and adaptation via anthocyanins: A review. Sci. Prog. 2017, 100, 100230. [Google Scholar] [CrossRef]
- Teixeira, J.D.; Leão de Sousa, M.; Barros, S.C.; Parpot, P.; Almeida, C.; Sanches Silva, A. Impact of Photoselective Nets on Phenolic Composition and Antioxidant Capacity in Different Apple Cultivars Under the Same Edaphoclimatic Conditions. Molecules 2025, 30, 1995. [Google Scholar] [CrossRef]
- Vierling, E. The roles of heat shock proteins in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 579–620. [Google Scholar] [CrossRef]
- Barua, D.; Downs, C.A.; Heckathorn, S.A. Variation in chloroplast small heat-shock protein function is a major determinant of variation in thermotolerance of photosynthetic electron transport among ecotypes of Chenopodium album. Funct. Plant Biol. 2003, 30, 1071–1079. [Google Scholar] [CrossRef]
- Rampino, P.; Mita, G.; Pataleo, S.; De Pascali, M.; Di Fonzo, N.; Perrotta, C. Acquisition of thermotolerance and HSP gene expression in durum wheat (Triticum durum Desf.) cultivars. Environ. Exp. Bot. 2009, 66, 257–264. [Google Scholar] [CrossRef]
- Carvalho, L.C.; Coito, J.L.; Colaço, S.; Sangiogo, M.; Amâncio, S. Heat stress in grapevine: The pros and cons of acclimation. Plant Cell Environ. 2015, 38, 777–789. [Google Scholar] [CrossRef]
- Swindell, W.R.; Huebner, M.; Weber, A.P. Transcriptional profiling of Arabidopsis heat shock proteins and transcription factors reveals extensive overlap between heat and non-heat stress response pathways. BMC Genom. 2007, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Desikan, R.; Mackerness, S.A.-H.; Hancock, J.T.; Neill, S.J. Regulation of the Arabidopsis transcriptome by oxidative stress. Plant Physiol. 2001, 127, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Vinocur, B.; Shoseyov, O.; Altman, A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004, 9, 244–252. [Google Scholar] [CrossRef] [PubMed]
- UN. United Nations—Climate Change. Available online: https://www.un.org/en/climatechange/what-is-climate-change (accessed on 18 March 2025).



| Abbreviation | Treatment | Commercial Entity | Composition/Description |
|---|---|---|---|
| C | Control | — | Without treatment |
| EK | Kiplant Eckosil® | AsfertGlobal | 4.1% Si |
| FF | Foliar Fertilization | Tradecorp | Fertilization strategy (see Table 2) |
| WR | Water Reinforcement | — | Increase in irrigation allocation |
| SD | Surround® | BASF | 95% Kaolin |
| AW | Agrowhite® | Codiagro | 33–46% CaO, 0.3–0.42% MgO |
| VS | Vegepron Sun® | UPL | 60% CaCO3 |
| SS | Sunstop® | Fitosistema | 20% CaCO3 |
| NCBI Ref | Gene | Forward Primer (5′→3′) | Reverse Primer (5′→3′) | bp |
|---|---|---|---|---|
| XM_002282480 | ACT1 | GCCTCCGATTCTCTCTGCTCTC | TCACCATTCCAGTTCCATTGTCAC | 158 |
| AF369525 | ACT2 | TGGATTCTGATGGTGTGAGTC | CAATTTCCCGTTCAGCAGTAGTGG | 167 |
| XM_002272382 | HSP20 | CCTCTGGCAACCCACAAAC | GGTCCATTGCGTCCATCAT | 292 |
| XM_002270560 | HSP23.6 | CCGCCTCCTCTCCTCTCC | TCTTCGCCATCATCGTAGTCG | 109 |
| XM_002263340 | HSP22 | TCTTCGCCATCATCGTAGTCG | GAGCACCCCATTCTCAAGC | 192 |
| XM_002280785 | HSP18.2A | GAGGTGAAGATAGAGGTGGACG | ACACCGTTCTCCATAGTAGCCT | 192 |
| XM_002280644 | HSP17.9A | GAAGGAGGAAGTGAAGGTTGAG | AACTTCCCCACCCTCCTCT | 177 |
| XM_002281249 | HSP18.2B | CGTCAAGGAGTACCCCAATTC | CTCCCTTCCTCAACCTCTACCT | 170 |
| XM_002280449 | HSP17.9B | CCGTTCCAAGACTTCCCATT | ACACGCCATCTTGACAAACC | 230 |
| XM_002281224 | HSP18.2C | TTCCTACGCCTTCATCATCG | CTCGGTGCCACTTGTCATTC | 235 |
| XM_002267889 | HSP26.5 | CCATTCCAGGACTTCCCATT | ATCAGTCGGAGTCCATGTATCG | 109 |
| Treatments | Average Number of Fruits per Tree | Production (kg per Tree) | Productivity (t/ha) | Average Weight per Fruit (kg) | TCSA (cm2) | Normalized Production (kg cm−2 of TCSA) |
|---|---|---|---|---|---|---|
| C | 144.80 ± 9.70 | 18.88 ± 2.22 | 35.00 ± 4.11 | 0.129 ± 0.007 | 30.24 ± 2.63 | 0.62 ± 0.04 |
| SS | 126.80 ± 22.72 | 16.85 ± 3.99 | 31.20 ± 7.39 | 0.130 ± 0.008 | 31.99 ± 3.32 | 0.50 ± 0.06 |
| EK | 110.80 ± 16.17 | 14.25 ± 1.92 | 26.40 ± 3.56 | 0.130 ± 0.004 | 28.86 ± 1.36 | 0.50 ± 0.07 |
| FF | 142.40 ± 9.29 | 19.59 ± 0.91 | 36.30 ± 1.69 | 0.139 ± 0.006 | 26.36 ± 1.92 | 0.75 ± 0.04 |
| SD | 142.00 ± 2.01 | 16.47 ± 2.01 | 30.50 ± 3.73 | 0.118 ± 0.005 | 30.86 ± 3.68 | 0.54 ± 0.06 |
| AW | 168.00 ± 18.55 | 21.65 ± 2.81 | 40.10 ± 5.21 | 0.128 ± 0.004 | 32.53 ± 2.02 | 0.66 ± 0.07 |
| WR | 152.60 ± 16.65 | 21.80 ± 2.70 | 40.40 ± 4.99 | 0.142 ± 0.007 | 33.98 ± 2.67 | 0.66 ± 0.10 |
| VS | 153.40 ± 20.97 | 18.98 ± 1.54 | 35.20 ± 2.86 | 0.128 ± 0.007 | 31.19 ± 2.83 | 0.61 ± 0.03 |
| Treatments | SSC (°Brix) | Firmness (kg cm−2) | Starch | °Hue | Dry Matter (%) |
|---|---|---|---|---|---|
| C | 13.20 ± 0.17 ab | 7.78 ± 0.14 ab | 6.75 ± 0.54 a | 55.19 ± 16.40 a | 15.83 ± 0.07 bc |
| SS | 13.36 ± 0.19 ab | 7.60 ± 0.12 ab | 5.92 ± 0.56 ab | 42.45 ± 11.56 b | 15.93 ± 0.09 bc |
| EK | 13.82 ± 0.24 ab | 7.59 ± 0.11 ab | 5.67 ± 0.66 ab | 55.88 ± 23.69 ab | 16.30 ± 0.27 ab |
| FF | 14.01 ± 0.17 a | 7.65 ± 0.09 ab | 6.00 ± 0.59 ab | 43.59 ± 12.40 b | 16.84 ± 0.10 a |
| SD | 12.83 ± 0.18 b | 8.02 ± 0.11 a | 4.75 ± 0.45 b | 62.02 ± 16.59 a | 15.43 ± 0.17 c |
| AW | 13.64 ± 0.20 ab | 7.35 ± 0.12 b | 5.67 ± 0.79 ab | 43.62 ± 15.66 b | 16.32 ± 0.22 ab |
| WR | 13.14 ± 0.19 ab | 7.34 ± 0.09 b | 6.58 ± 0.56 a | 47.52 ± 15.92 b | 15.63 ± 0.23 c |
| VS | 13.25 ± 0.15 ab | 7.39 ± 0.12 b | 5.42 ± 0.78 ab | 51.74 ± 16.14 ab | 16.20 ± 0.18 ab |
| Treatments | An (µmol m−2 s−1) | ||
|---|---|---|---|
| 1 July | 27 July | 13 August | |
| C | 12.49 ± 0.78 ab | 14.58 ± 2.25 ab | 16.42 ± 1.84 a |
| SS | 11.61 ± 1.04 ab | 13.80 ± 2.10 ab | 16.31 ± 1.89 a |
| EK | 11.10 ± 0.33 b | 14.12 ± 2.64 ab | 15.47 ± 3.16 ab |
| FF | 12.06 ± 0.76 ab | 16.28 ± 3.19 a | 16.60 ± 1.65 a |
| SD | 11.83 ± 1.43 ab | 12.76 ± 2.13 b | 13.94 ± 2.77 ab |
| AW | 11.94 ± 1.83 ab | 12.41 ± 2.94 b | 15.53 ± 2.83 ab |
| WR | 13.45 ± 1.27 a | 14.14 ± 3.10 ab | 16.55 ± 1.87 a |
| VS | 11.26 ± 0.74 b | 12.28 ± 3.51 b | 13.19 ± 2.54 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, M.; Carvalho, L.; Gonçalves, M.; Ferreira, S.; de Sousa, M.L. Sustainable Strategies for Sunburn Mitigation in Gala Apple Orchards: Effects on Yield, Fruit Quality, and Plant Physiology. Appl. Sci. 2025, 15, 11644. https://doi.org/10.3390/app152111644
Rodrigues M, Carvalho L, Gonçalves M, Ferreira S, de Sousa ML. Sustainable Strategies for Sunburn Mitigation in Gala Apple Orchards: Effects on Yield, Fruit Quality, and Plant Physiology. Applied Sciences. 2025; 15(21):11644. https://doi.org/10.3390/app152111644
Chicago/Turabian StyleRodrigues, Margarida, Luísa Carvalho, Marta Gonçalves, Susana Ferreira, and Miguel Leão de Sousa. 2025. "Sustainable Strategies for Sunburn Mitigation in Gala Apple Orchards: Effects on Yield, Fruit Quality, and Plant Physiology" Applied Sciences 15, no. 21: 11644. https://doi.org/10.3390/app152111644
APA StyleRodrigues, M., Carvalho, L., Gonçalves, M., Ferreira, S., & de Sousa, M. L. (2025). Sustainable Strategies for Sunburn Mitigation in Gala Apple Orchards: Effects on Yield, Fruit Quality, and Plant Physiology. Applied Sciences, 15(21), 11644. https://doi.org/10.3390/app152111644








