Featured Application
Open Field is no longer recommended. There is not a clear “gold-standard” technique. A worldwide framework should be developed.
Abstract
Adjuvant radiotherapy improves local cancer control and the overall survival of women with breast cancer. However, it is unclear what the ideal radiotherapy (RT) planning technique is for these patients. The aim was to perform a synopsis of the literature comparing RT techniques to treat early-stage left breast tumours. A PRISMA guideline was used on this systematic review and registered in PROSPERO (CRD420251168901). For all the investigations, the Open-field technique (OF) showed worse results for the Planning Target Volume (PTV), lung and heart. Field-in-Field stood out in low doses and mean doses in OARs. IMRT distinguished itself in Homogeneity Index. VMAT provides higher Conformity Index results and thus an advantage in high and mean doses of OARs. Hybrid-IMRT and Hybrid-VMAT combine the advantages of two techniques; however, few studies have included them in their research. There is not a clear “gold-standard” technique, and the results depend heavily on many factors which affect the quality of the plans and the priorities of the departments. However, OF is no longer recommended. An international framework should be developed to allow for a standardisation of the plans, improving inter-departmental comparisons. And each department should perform their own comparison between the techniques available to them.
1. Introduction
In 2020, approximately 2.6 million new cases of breast cancer were diagnosed worldwide and almost 700,000 deaths were attributed to this pathology according to the World Health Organization (WHO) [1]. The benefits of adjuvant radiotherapy (RT) in the local control and overall survival for women with early breast cancer have been confirmed by the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), as part of a multidisciplinary approach [2,3].
RT planning for breast cancer is challenging and with the emergence of new techniques, the consensus on their use is not yet achieved. Three-Dimensional Conformal Radiation Therapy (3D-CRT), Intensity-Modulated Radiation Therapy (IMRT) and Volumetric Modulated Arc Therapy (VMAT) are the most common techniques currently used in the treatment of these patients [4,5,6,7].
In the past, the use of two opposed tangential Open-Fields (OF) represented the standard technique in 2D planning. Over time, the same beam arrangement was incorporated into 3D-CRT planning, which subsequently evolved into the Field-in-Field (FiF) technique, providing acceptable coverage of the breast tissue while minimising the dose to adjacent critical structures [4,5,7].
IMRT and VMAT have become preponderant techniques for various treatment sites. The steep dose gradients achieved with such techniques are accomplished through multiple leaf collimators (MLC), dose rate, and gantry speed modulation at the expense of the inherent complexity of these dosimetric plans [8,9]. However, there is no consensus on their advantages in breast cancer treatment.
IMRT combines multiple beam angles and MLC, allowing beam intensity modulation, thus creating complex and highly conformal dose profiles [8,9]. Likewise, VMAT delivers intensity-modulated beams but also performs full or partial, single, or multiple rotational arcs, resulting in treatments of less than two minutes [10,11,12,13]. Both these techniques have also been used to treat breast cancer.
Globally, there is no consensus on which RT technique is most beneficial for the treatment of breast tumours. Mostly because each technique seems to have pros and cons, resulting in conflicting evidence about the use of different techniques (evidence gap). Additionally, there was never a compilation and comparison of the existing evidence comparing the techniques (knowledge gap). This study will contribute to closing these gaps.
It is acknowledged that dose distribution does not depend only on the RT technique, i.e., there are other confounding factors. In addition to new RT-delivery techniques, patients have benefitted from introducing respiratory motion control methods such as deep-inspiration breath hold (DIBH), which impacts the dose distribution [14,15]. The introduction of new imaging verification modalities increased the accuracy of the treatment delivery, allowing to reduce CTV-PTV margins which also affects dose distribution [16]. As such, comparison between techniques must account for these and other confounding factors. This may be one of the reasons why this comparison is challenging, and, to our knowledge, there are no published systematic comparisons of the RT techniques for breast cancer. Lack of investment in new devices [17,18], funding per treatment [19,20,21] or lack and diversity of training [22,23,24] could also be some of the reasons for choosing one technique or another in individual RT centres, rather than evidence of better outcomes.
Although clinical guidelines for early-stage breast cancer describe recommended dose constraints and planning parameters for different radiotherapy techniques, they do not establish which technique provides the best overall dosimetric performance.
The aim of this study was to perform a synopsis of the evidence existing in the literature to perform a dosimetric comparison of RT techniques for the treatment of early-stage left breast tumours (pT1-2 N0) using the linear accelerator, assessing the advantages and disadvantages of each one in terms of dose constraints/objectives for target volumes and Organs-At-Risk (OARs).
To improve comparability of the studies, this study will focus only on left-sided early-stage breast cancer. This choice takes into account the challenge of having the heart close to the target volume.
2. Materials and Methods
2.1. Data Collection
This systematic review was performed according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) 2020 guidelines [25], and adhered to a pre-registered protocol in PROSPERO (registration number: CRD420251168901). The records were searched in the last 13 years from PubMed, ScienceDirect, Cochrane Library and PROSPERO until 31 July 2025. The search strategy was developed iteratively to achieve an optimal balance between sensitivity and specificity, combining terms related to dosimetry and early-stage breast cancer. Exclusion criteria were applied to restrict the results to photon-based, linear accelerator techniques. This approach ensured comprehensive coverage of relevant comparative dosimetric studies while maintaining a clear and reproducible methodology. This methodology allowed to collect and analyse numerous publications related to the topic. These databases were chosen since they include all high impact journals in radiotherapy planning.
2.2. The Inclusion and Exclusion Criteria
The primary inclusion criteria were (1) any study involving RT in early breast cancer using the linear accelerator; (2) comparing at least two techniques using photons; (3) the plan was performed in the whole left breast only, and (4) it contains relevant information on planned doses for target volumes and OARs.
The study was to be excluded if (1) it is not in English; (2) involves the treatment of the right-side breast only or both; (3) contains only one dosimetric planning technique or (4) planning was performed in a non-clinical Treatment Planning System (TPS).
2.3. Review Process and Quality Assessment
The review process was conducted following the PRISMA 2020 reporting guidelines for new systematic reviews. The duplicated studies from different databases were excluded using EndNote®. After reading the titles and abstracts, potentially related papers were collected and further assessed based on the inclusion and exclusion criteria. The final included studies of this systematic review were then selected and presented in the results section. Figure 1 outlines all these steps.
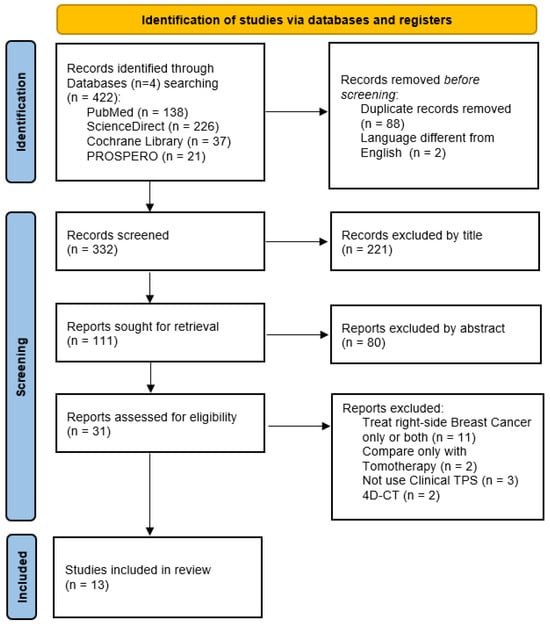
Figure 1.
Flowchart of the selection process in the systematic review, following the PRISMA 2020 flow diagram for new systematic reviews which included searches of databases and registers only.
Two independent reviewers adjudicated study quality and the assessment of study quality using The Joanna Briggs Institute’s (JBI) critical appraisal tools for Analytical Studies [26]. If any disagreements occurred, another independent researcher was consulted.
2.4. Data Extraction
For this review, the following information was extracted from each included study: first author, publication year, number of patients, age range, patient size range, RT treatment modality, target volume and OARs doses. These parameters were extracted and presented in a table for a quick evaluation of the existing literature.
To enable data extraction across studies, the values of the OAR parameters were approximated. This approach was required due to heterogeneity in fractionation dose regimens, contouring protocols, and reporting metrics, which hindered the direct standardisation of results. Although this may introduce some degree of bias, the methodology nonetheless enabled a meaningful synthesis of the existing evidence, contributing to the body of knowledge on radiotherapy techniques for left-sided breast cancer.
Due to the numerous variables that affect the quality of the plan in addition to the treatment technique (such as patient size, CTV-PTV margin, use of respiratory control, etc.), it is not possible to directly compare the studies with one another. Therefore, a meta-analysis could not be performed. The differences in sample sizes, inclusion criteria, and planning objectives make direct comparison between studies unfeasible.
Firstly, the analysis was carried out study-by-study, where techniques can be directly compared. Then a general comparison of the techniques was performed, grouping the evidence to discuss each technique’s positive aspects and disadvantages.
3. Results
3.1. Study Selection
Figure 1 shows 422 records were collected from 4 different databases. After excluding 88 duplicates and 2 non-English papers, and reading the titles, we obtained 111 papers for further assessment. A total of 31 reports were included for eligibility after full abstract reading, of which 11 were excluded for treating right-side breast cancer, 2 for having only one technique in a linear accelerator, 2 for not using a clinical TPS and 2 where the treatment plan was carried out in the 4DCT image. Finally, we included 13 studies in the current systematic review. The detailed information of each study is listed in Table 1 and Table 2.

Table 1.
Summary of systematic review data extraction.

Table 2.
Summary of systematic review data extraction. Most beneficial parameters from the intra-article analysis are highlighted.
3.2. Study Characteristics
For data evaluation, the techniques were divided into 6 groups:
- (1)
- Open-Fields (OF): a technique that contains only two parallel opposing tangential fields shaped by MLC, with or without wedges;
- (2)
- FiF: two parallel opposing tangential beams with subfields planned using forward planning;
- (3)
- IMRT: inverse planning technique with fields with modulated intensity with a fixed number of fields;
- (4)
- Hybrid-IMRT: a large percentage of dose prescribed with two parallel opposing tangential fields or FiF plus a small percentage with IMRT;
- (5)
- VMAT: inverse planning technique using partial or tangential arcs with modulated beam intensity;
- (6)
- Hybrid-VMAT: a large percentage of dose prescribed with two parallel opposing tangential fields or FiF plus a small percentage with VMAT using partial or tangential arcs;
Of the 13 articles, 11 (85%) addressed the VMAT [4,5,6,7,10,12,28,29,30,31,32], 9 (69%) IMRT techniques [4,5,6,7,10,27,31,32,33], 8 (62%) the FiF technique [4,5,6,27,28,29,30,32], 4 (31%) the OF [4,5,6,7], 4 (31%) the Hybrid-IMRT [6,28,31,33] and only 2 (15%) the Hybrid-VMAT technique [28,29]. Only 1 (9%) article compares at least 5 techniques [6]. One publication (9%), Mo et al. 2016 [7], only compared the VMAT technique using different configurations.
There is a similar distribution of TPSs across the papers evaluated: Eclipse [4,10,12,29,30] and Pinnacle [5,6,27,28,33] appeared in 5 (38%) articles and Monaco in 3 (23%) [7,30,31]. In 7 articles (54%) [4,5,6,7,12,32,33], conventional fractionation was used, with daily doses of 2 Gy over 25 fractions. Among the remaining 6 articles (46%), hypofractionation was adopted: 5 articles (27%) [10,27,28,29,31] used moderate hypofractionation (2.667–2.75 Gy per fraction over 15 or 16 fractions), and only 1 article (9%) [30] employed ultra-hypofractionation (5.2 Gy per fraction over 5 fractions). The sample size of the various articles ranges between 10 and 50 patients, with an average of 23 patients. All studies placed patients in the supine position on a breast support with both arms raised above the head. Regarding respiratory control, only 6 (46%) [6,27,28,31,32,33] studies explicitly reported the use of a specific breathing management technique. Among these, 4 were performed under free-breathing conditions [6,27,32,33], while two studies employed DIBH [28,31].
3.3. Dosimetric Data
The analysis included target volumes and OAR dose-volume parameters, and the values that appeared most frequently in the selected articles were evaluated.
The variables analysed related to the PTV are quite similar for the 6 techniques. The OF presents the lowest Conformity Index (CI) in all 4 (100%) articles in which it appears [4,5,6,7], while the VMAT has the highest CI in 9 (90% of the 10 articles where VMAT is compared with other techniques) [4,6,7,10,12,28,29,30,31]. IMRT has better results in 6 (75%) in the Homogeneity Index (HI) variable [4,5,6,7,10,27]. Only 7 articles (53%) describe the D95% parameter [10,12,27,28,29,30,32]. The dose parameters of all included studies are presented in Table 2. We encourage the readers to analyse this table carefully before continuing to read the results.
In the ipsilateral lung, three variables were analysed: mean dose [4,5,6,7,10,12,27,28,29,31,32,33] analysed in 12 articles (92%),V ≈ 40% [4,5,6,7,10,12,28,30,31,32,33] and V ≈ 10% in 11 articles (85%) [4,5,6,7,10,12,28,29,31,32,33]. The VMAT was presented as the technique with the lowest mean dose in 5 of the 10 articles (50%) [10,28,31,32], and the IMRT presented the highest result in 4 articles (44%) [4,27,32,33]. Regarding V ≈ 10%, the results varied greatly between the various studies, making it impossible to draw any general conclusions about this parameter; a certain technique may have the lowest results in one article and the highest in another study. OF showed the highest V ≈ 40% results in 3 articles (75%) [5,6,7] but curiously presents the lowest result in the article by Haciislamoglu et al. (2015) [4] compared to the other 3 techniques. The VMAT presents the lowest V ≈ 40% results in 6 (66%) articles [6,7,28,30,31,32].
When treating the left breast with RT, the heart is one of the most critical organs. In this review, the mean dose, V ≈ 10% and V ≈ 60% were analysed. Curiously, the VMAT has the lowest (44%) [6,7,28,31,32] and the highest results (44%) [4,5,10,29,30] of heart mean dose across different studies. Regarding V ≈ 10%, the FiF presented the lowest results in 3 articles (50%) [4,28,29,30], and the VMAT presented the highest results in 3 articles (57%) [4,10,29,30]. Regarding V ≈ 60%, OF showed the highest results in 3 (75%) articles [5,6,7] while the VMAT showed the lowest results in 5 (71%) articles [4,6,7,28,32].
The other two OARs evaluated are the contralateral breast and the contralateral lung, which reflect the distribution of the low doses: mean dose and V ≈ 10% were evaluated for both. The articles are very different from each other, with 2 articles in which all these values are lower for FiF [4,29], 1 for OF [7] and another for IMRT [10]. The OF and FiF techniques, when compared with the VMAT technique, presented lower values for all variables of these OARs, except in the article by Xie et al. (2020) [6].
Ramasubramanian et al. (2019) [12] compared the VMAT technique with three different configurations, as described in Table 1. The configuration of 4 tangential arcs (300–350° and reverse; 110–160° and reverse) had the lowest results in 8 of the 9 OAR parameters. However, the configuration of 4 partial arcs (300–50° and reverse; 50–160° and reverse) obtained better results for all parameters related to the PTV coverage, HI and CI.
4. Discussion
A substantial body of literature, comprising thirteen publications, was identified on this topic, indicating active research within the community. However, as previously noted, the use of disparate assessment parameters across research groups impedes direct comparison and the possibility of a meta-analysis.
Therefore, this is our main conclusion from this study. The lack of standardisation in reporting breast cancer planning parameters impedes the comparison of results between studies. Even parameters that are considered relatively standard in plan reporting (e.g., D95% PTV) were not consistently reported across the studies. This results in an inability to utilise the existing data for statistical analysis. We dare to conclude that this impedes healthcare professionals worldwide from confidently knowing whether there is (or not) an RT technique that is beneficial and should be the standard for all patients.
As a result, two recommendations are (i) that the standardisation of plan assessment parameters is urgent, as they do not exist, and (ii) to perform international large-scale research to compare RT techniques for treating breast cancer using these standardised parameters. Ideally, this would be complemented with a clinical outcome assessment.
Additionally, it became evident that a wide array of techniques is currently in use or under consideration for the treatment of left breast cancer, including OF, FiF, IMRT, VMAT, hybrid-IMRT, and hybrid-VMAT. This diversity is further compounded by variations within these techniques, such as the use of tangential or partial arcs in VMAT. The lack of a clear consensus on which technique is most beneficial suggests that each may offer distinct advantages.
Although this review sought to identify which technique achieves the most favourable dosimetric parameters, it is important to recognise that, from a clinical perspective, the optimal approach may not be the one that produces the best value for a single parameter. Instead, what is most relevant is the ability of a plan to achieve an overall balance between adequate PTV coverage and minimization of OAR doses. This concept of equilibrium may better reflect the complexity of clinical decision-making, where compromises are often necessary to optimise both tumour control and normal tissue protection.
The present review focused exclusively on dosimetric outcomes, which provide valuable insights into the relative quality of radiotherapy plans. However, the translation of these dosimetric findings into clinical outcomes such as toxicity reduction or survival improvement remains uncertain. The lack of standardised reporting of clinical endpoints across studies prevents a direct correlation between dosimetric and long-term clinical results. Therefore, the results of this review should be interpreted as an evaluation of planning quality rather than clinical effectiveness. Future research combining dosimetric analysis with clinical follow-up data is required to confirm whether the dosimetric advantages observed translate into improved patient outcomes.
The rapid evolution of fractionation schemes for early-stage breast cancer over the past decade also introduced a layer of complexity to this analysis. To address this, the authors were able to convert the different parameters, within a reasonable approximation, to facilitate data interpretation. This ability to compile and convert data from multiple sources represents a key advantage of this review, providing clinical settings with a unified, evidence-based resource to inform their practice.
4.1. Standard Techniques (OF, FiF, IMRT and VMAT)
The results showed that the OF technique shows worse results concerning the PTV, ipsilateral lung and heart, compared with other techniques. Advantages can only be demonstrated in low doses in the contralateral lung and in the contralateral breast, and only in some of the articles. It seems that, based on these studies, there are better options, which agrees with the worldwide tendency of using different techniques.
The FiF technique stood out compared to other techniques in terms of low doses and mean doses in OARs. For the ipsilateral lung and heart, in 4 of the 8 articles, a lower dose was observed with FiF [4,27,29,30]. The same trend is also seen in low and mean doses for the contralateral lung and contralateral breast; in 4 of the 5 articles [4,10,28,29] the lower doses were achieved with this technique. The differences observed in these studies may be due to the number, angulations, and weight of the fields and subfields, which may influence the final quality of the plane.
Curiously, in three of these articles [4,5,27], FiF and IMRT with the same beam configuration are compared. In Jin et al. (2013) [5], IMRT shows a benefit (favourable results in 6 of the 8 parameters evaluated), while the Haciislamoglu et al. (2015) [4] article shows a slight benefit of the FiF technique (favourable results in 8 of the 12 parameters evaluated). In Carosi et al. (2020) [27], the two techniques are very similar and with very close values, with FIF benefiting the protection of OARs and IMRT benefiting the target volume objectives. Despite these three comparisons being quite similar, they achieved different results, showing that the difference between IMRT and FiF techniques is not extensive and that different plan details may influence the results. The IMRT technique distinguished itself in this systematic review regarding the HI in which it obtained better results in 75% of the articles [4,5,6,7,10,27].
The VMAT technique provides higher CI results in 90% of the articles [4,6,7,10,12,28,29,30,31]. This high level of CI also brings an advantage in high and mean doses of OARs. VMAT has the lowest lung mean dose [7,28,31,32] in 50% of the articles and the lowest lung V ≈ 40% in 63% of the studies [6,7,30,31,32]. The same happens in V ≈ 60% of the heart, where VMAT presents lower doses in 60% of the articles [4,6,7,28,32]. The VMAT weakness seems to be the low doses, since VMAT showed the highest heart V ≈ 10% in 63% of the articles [4,5,10,29,30], and the highest low-doses in the contralateral lung and contralateral breast, whereas other techniques presented lower results [4,7,10,28,29,31,32]. This is particularly important since trials have shown a correlation between RT and cardiac-related deaths, making cardiac side effects a limiting factor in improving breast radiotherapy [34,35]. In some patients where associated oncologic treatments mandate the lowest irradiation heart dose, VMAT with DIBH or isocentric lateral decubitus positioning are highly effective alternatives [36]. Due to the arc configuration and dose distribution method, the VMAT technique delivers low doses to a higher volume of the surrounding normal tissues, which is its main disadvantage, as it increases the risk of secondary cancers [37].
Still regarding the VMAT technique, one of the major limitations in comparing this technique is the different configuration setups that exist. In the 2 articles in which there is a comparison of this technique with more than one configuration (tangential arcs and partial arcs) [12,32], the results seem to be quite identical, not allowing us to conclude which will be the best configuration for this technique. Furthermore, as described in the results, the VMAT has the lowest results (44%) [6,7,31,32] and the highest results (44%) [4,5,10,30] regarding the heart mean dose, which can be due to these specific planning objectives (e.g., PTV coverage versus OAR sparing) that vary between institutions, but other potential factors could include patient size, dose calculation algorithms, and volume contouring protocols, among others.
4.2. Hybrid Techniques
Hybrid techniques have emerged as an alternative strategy, combining elements of tangential FiF fields with either IMRT or VMAT. The rationale for these strategies lies in the attempt to merge the strengths of each method while reducing their limitations.
Evidence suggests that Hybrid-IMRT, achieved by combining FiF tangential fields with IMRT beams, offers improved OAR sparing compared with IMRT alone. In the article by Zhang et al. (2018) [33], the hybrid-IMRT, with 70–80% of the prescription dose of the FiF technique and 20–30% with IMRT, presented lower doses in 7 of the 9 parameters observed in the OAR. Combining IMRT with the advantages inherent to the FiF technique described in this discussion, concerning low doses and mean doses in the OARs, stands out when compared with IMRT alone. However, when compared with the VMAT technique [6,28,31], VMAT seemed to outperform in the CI, HI, heart, and ipsilateral lung, with Hybrid-IMRT showing better results in doses to the D95%PTV, contralateral lung and contralateral breast.
Although Hybrid-IMRT does not consistently provide the most favourable results in individual parameters, it often demonstrates intermediate values that reflect balanced performance. Rather than excelling in a single aspect, this technique tends to offer a compromise between target coverage and OAR sparing, resulting in an overall equilibrium that may be advantageous in clinical practice, especially when both objectives are considered equally important. More comparative studies should be carried out using Hybrid-IMRT to understand the impact this technique has on the treatment of this pathology.
A similar rationale underlies the development of Hybrid-VMAT, which combines tangential FiF fields with VMAT arcs. In this case, the intention is likewise to mitigate the disadvantages of VMAT—particularly its tendency to increase low-dose exposure—while preserving its strengths in conformity and PTV coverage, which was compared in two studies included in this review [6,29].
The available evidence, however, remains mixed. Lamprecht et al. (2022) [28] reported that VMAT achieved better values in most parameters, with Hybrid-VMAT showing advantages in reducing doses to the contralateral breast and lung. In contrast, the study by Mishra et al. (2025) [29] found that VMAT offered the better values for PTV parameters, FiF provided the best values for OAR, and Hybrid-VMAT produced intermediate values for PTV and OARs. These findings suggest that Hybrid-VMAT, like Hybrid-IMRT, does not necessarily deliver the best outcomes in isolated metrics but may offer a more balanced distribution between PTV objectives and OAR constraints. Nevertheless, further studies are required to better understand the impact and potential clinical advantages of implementing Hybrid-VMAT in routine practice.
4.3. Broader Considerations
One of the issues that hinder this study from reaching clearer conclusions is that certain institutions give a greater emphasis on covering the target volume, while others give priority to lowering doses to adjacent OARs. This was particularly evident with the dose to the heart with the VMAT technique. The VMAT technique achieved the best heart results in some studies [6,7,31,32] and the worst results in other studies [4,5,10,30]—one possible explanation for this is that certain departments/dosimetrists give priority to the PTV parameters, while others give priority to the heart parameters. This inherent variability in institutional priorities highlights a key limitation of broad, multi-centre analyses. While this literature review provides a valuable, evidence-based foundation for decision-making, it does not substitute an individualised departmental assessment. Therefore, one of the recommendations from this study is that each clinical centre must conduct its own internal evaluation to determine the most beneficial technique accounting for a variety of factors, including local equipment, staff expertise, departmental protocols, and specific clinical priorities.
Research has shown that there are a multitude of confounding factors that affect the plan quality besides the technique. These are the following:
- (1)
- Experience and capacity of the medical dosimetrist: greater experience, time dedicated to each plan, or better training in a given technique may bring about significant differences in the results of the plan [22,23,24];
- (2)
- Delineation of target volume and OARs: in several of the selected articles, the delineation protocols were not described, which can make comparisons between them difficult. Different delineation guidelines result in different outcomes in dose evaluation. The use of international protocols is very important, as numerous studies demonstrate better results when used [38,39,40];
- (3)
- Treatment Planning System and calculation algorithms: the execution of dosimetric plans with different Treatment Planning Systems, as well as the use of different calculation algorithms, may produce different results in a dosimetric plan. Allied to this, in advanced techniques such as IMRT and VMAT, inverse planning is performed in different ways. All of this may bring different results, not only in terms of dosimetry but also in terms of clinical results for the patient [41,42,43,44];
- (4)
- Economic impacts: greater funding for special techniques could lead to these being chosen in certain countries in order to combat problems with the rules for reimbursement (such as reimbursement for ultra- and hypofractionation in this case of the breast) [19,20,21];
- (5)
- Availability of equipment: the availability of devices capable of performing certain techniques is quite uneven globally, which could create greater dependence on the use of non-special techniques [17,18,21,45,46,47,48];
- (6)
- Culture: the belief that a certain technique has better results than another without carrying out a research study on the subject may lead to the choice of different techniques [18,21,46,48].
Other studies should consider these aspects that, in a more accentuated way or not, create barriers in choosing the technique across the world.
4.4. Limitations of the Study
Several studies have been carried out in which dosimetric techniques are compared for treating early-stage left breast tumours. However, comparison between the studies is impossible since they vary considerably regarding techniques used, dose prescription, dose-volume parameters reported, etc. This allowed us to compare techniques only within studies. Nevertheless, this evaluation allowed us to summarise the existing evidence, contributing to the body of knowledge and allows readers to perform their own comparison since all data was compiled into Table 2.
The sample size, as well as its description per study, does not seem to be sufficiently representative to draw stronger conclusions. In this selection of articles, 77% of the articles have less than 25 patients [4,5,6,7,10,27,28,29,30,32], which for the small differences observed in the dose parameters evaluated, may not be representative. The same happened with the average age of patients in which 6 articles do not present this information [4,5,6,28,31,33] and the average of the remaining articles varies considerably. Another relevant limitation is the lack of detailed information on patient status, tumour size and breast volume across the included studies. These factors may differ between patients and could influence the applicability or optimisation of each RT technique. A recommendation for further studies is to have a multi-centre, large scale, standardised comparison of the different RT techniques used for breast cancer.
Other information lacking in several articles is positioning techniques as well as the existence of breathing control mechanisms. The use or not of the DIBH technique, for example, will create different results, as already reported in other studies [14,15,49,50]. Once again, the comparison of techniques between articles is quite limited for these reasons and large-scale standardised studies would be beneficial.
The evaluation of the PTV is difficult to carry out in this selection of articles, since the evaluation parameters are quite different from article to article. The D95% parameter was the most used by the authors; however, it only appeared in 7 of the 13 (54%) selected articles [10,12,27,28,29,30,32]. The use of internationally standardised criteria could fill this gap in the evaluation of the PTV and OARs.
Another important limitation arises from the inconsistencies observed across studies: the same technique was sometimes reported as achieving both the lowest and the highest values for parameters such as, for example, the ipsilateral lung dose. These contradictions highlight that distribution is not determined solely by the treatment technique but also depends on other factors such as institutional planning priorities, contouring protocols, treatment planning systems, and planner experience [21,40,41,42,46,51,52]. This reinforces the need for comprehensive intra-departmental analyses to identify the most advantageous approach in each institutional context, while also recognising that the optimal technique is likely patient-specific, justifying individualised evaluation of planning strategies.
Finally, this review was limited to dosimetric outcomes, as most available studies did not report long-term clinical data. While dosimetric parameters are recognised surrogates for plan quality, they cannot alone predict clinical benefit. Future studies integrating both dosimetric and clinical outcome endpoints are warranted to better validate these planning techniques.
5. Conclusions
This is the first systematic literature review trying to summarise the existing evidence regarding the planning RT technique for early-stage left breast cancer.
Many authors compared different techniques to treat left early-stage breast cancer. From the literature analysis, there is not a clear “gold-standard” technique since different papers showed different benefits from different techniques.
However, it became clear that OF is no longer recommended, agreeing with the worldwide tendency to use other techniques. Further than that, it was not possible to clearly assess if FiF, IMRT, Hybrid-IMRT, VMAT, or Hybrid-VMAT are the preferable techniques since results varied between studies.
One of the main conclusions is that each technique has benefits and disadvantages and the preference may depend on many factors such as priorities of the department, equipment available, and human and time resources available, among others. These factors will themselves affect the quality of the plan (even when the same technique is used). The studies shown that some departments give preference to PTV coverage while others give preference to OAR sparing. This made the comparison between studies impossible. Therefore, the recommendation is that each clinical institution, according to its technological and human resources, carries out research or auditing to find out which is the most beneficial technique to apply for the treatment of this pathology in their local setting.
Another important conclusion was that different departments use different plan evaluation parameters, impeding a meta-analysis. Therefore, the recommendation is that an inter-department worldwide framework is developed to allow for a standardisation of these plans, improving inter-departmental comparisons.
Inter-departmental studies should be carried out with the same evaluative parameters/constraints to clarify if there is a “gold-standard”, opening opportunities for the creation of international guidelines. Allied with this, and perhaps most importantly, the dose-volume parameter comparisons should be supported by a careful assessment of the clinical impact when using different techniques, considering tumour control and the occurrence of side effects.
Author Contributions
Conceptualization, F.M.C. and J.G.C.; methodology, F.M.C. and J.G.C.; software, I.F.; validation, M.M.P., A.M., P.M. and G.P.; formal analysis, G.P.; investigation, F.M.C., M.M.P., J.G.C. and R.O.-S.; resources, F.M.C.; data curation, F.M.C., M.M.P. and J.G.C.; writing—original draft preparation, F.M.C., R.O.-S. and J.G.C.; writing—review and editing, F.M.C. and J.G.C.; visualisation, I.F.; supervision, J.G.C.; project administration, M.M.P., A.M., P.M. and G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 3D-CRT | Three-Dimensional Conformal Radiation Therapy |
| CI | Conformity Index |
| CTV | Clinical Target Volume |
| D95% | Percentage of the prescribed dose that covered 95% of the target volume |
| DIBH | Deep-Inspiration Breath Hold |
| EBCTCG | Early Breast Cancer Trialists’ Collaborative Group |
| FiF | Field-in-Field |
| HI | Homogeneity Index |
| IMRT | Intensity-Modulated Radiation Therapy |
| JBI | Joanna Briggs Institute |
| MLC | Multiple Leaf Collimator |
| OARs | Organs-At-Risk |
| OF | Open-Fields |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PTV | Planning Target Volume |
| RT | Radiotherapy |
| TPS | Treatment Planning System |
| VMAT | Volumetric Modulated Arc Therapy |
| Vx | Percentage of organ receiving ≥ x (Gy or % of the prescribed dose) |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group; Darby, S.; McGale, P.; Correa, S.; Taylor, C.; Arriagada, R.; Clarke, M.; Cutter, D.; Davies, C.; Ewertz, M.; et al. Effect of Radiotherapy after Breast-Conserving Surgery on 10-Year Recurrence and 15-Year Breast Cancer Death: Meta-Analysis of Individual Patient Data for 10,801 Women in 17 Randomised Trials. Lancet 2011, 378, 1707–1716. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Favourable and Unfavourable Effects on Long-Term Survival of Radiotherapy for Early Breast Cancer: An Overview of the Randomised Trials. Lancet 2000, 355, 1757–1770. [Google Scholar] [CrossRef]
- Haciislamoglu, E.; Colak, F.; Canyilmaz, E.; Dirican, B.; Gurdalli, S.; Yilmaz, A.H.; Yoney, A.; Bahat, Z. Dosimetric Comparison of Left-Sided Whole-Breast Irradiation with 3DCRT, Forward-Planned IMRT, Inverse-Planned IMRT, Helical Tomotherapy, and Volumetric Arc Therapy. Phys. Med. 2015, 31, 360–367. [Google Scholar] [CrossRef]
- Jin, G.H.; Chen, L.X.; Deng, X.W.; Liu, X.W.; Huang, Y.; Huang, X.B. A Comparative Dosimetric Study for Treating Left-Sided Breast Cancer for Small Breast Size Using Five Different Radiotherapy Techniques: Conventional Tangential Field, Filed-in-Filed, Tangential-IMRT, Multi-Beam IMRT and VMAT. Radiat. Oncol. 2013, 8, 89. [Google Scholar] [CrossRef]
- Xie, Y.; Bourgeois, D.; Guo, B.; Zhang, R. Comparison of Conventional and Advanced Radiotherapy Techniques for Left-Sided Breast Cancer after Breast Conserving Surgery. Med. Dosim. 2020, 45, e9–e16. [Google Scholar] [CrossRef]
- Mo, J.C.; Huang, J.; Gu, W.D.; Gao, M.; Ning, Z.H.; Mu, J.M.; Li, Q.L.; Pei, H.L. A Dosimetric Comparison of Double-Arc Volumetric Arc Therapy, Step-Shoot Intensity Modulated Radiotherapy and 3D-CRT for Left-Sided Breast Cancer Radiotherapy after Breast-Conserving Surgery. Technol. Health Care 2017, 25, 851–858. [Google Scholar] [CrossRef]
- Kamperis, E.; Kodona, C.; Hatziioannou, K.; Giannouzakos, V. Complexity in Radiation Therapy: It’s Complicated. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 182–184. [Google Scholar] [CrossRef] [PubMed]
- Otto, K. Volumetric Modulated Arc Therapy: IMRT in a Single Gantry Arc. Med. Phys. 2008, 35, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Nithya, L.; Goel, V.; Sharma, D.; Vittal, K.; Marjara, N. Dosimetric Comparison of Different Planning Techniques in Left-Sided Whole-Breast Irradiation: A Planning Study. J. Med. Phys. 2020, 45, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.-J.; Chang, Z.; Horton, J.K.; Wu, Q.-R.J.; Yoo, S.; Yin, F.-F. Dosimetric Comparison of 3D Conformal, IMRT, and V-MAT Techniques for Accelerated Partial-Breast Irradiation (APBI). Med. Dosim. 2014, 39, 152–158. [Google Scholar] [CrossRef]
- Ramasubramanian, V.; Balaji, K.; Balaji Subramanian, S.; Sathiya, K.; Thirunavukarasu, M.; Radha, C.A. Hybrid Volumetric Modulated Arc Therapy for Whole Breast Irradiation: A Dosimetric Comparison of Different Arc Designs. Radiol. Med. 2019, 124, 546–554. [Google Scholar] [CrossRef]
- Liu, H.; Chen, X.; He, Z.; Li, J. Evaluation of 3D-CRT, IMRT and VMAT Radiotherapy Plans for Left Breast Cancer Based on Clinical Dosimetric Study. Comput. Med. Imaging Graph. 2016, 54, 1–5. [Google Scholar] [CrossRef]
- Chi, F.; Wu, S.; Zhou, J.; Li, F.; Sun, J.; Lin, Q.; Lin, H.; Guan, X.; He, Z. Dosimetric Comparison of Moderate Deep Inspiration Breath-Hold and Free-Breathing Intensity-Modulated Radiotherapy for Left-Sided Breast Cancer. Cancer Radiother. 2015, 19, 180–186. [Google Scholar] [CrossRef]
- Jarvis, L.A.; Loo, B.W.; Thorndyke, B.R.; Horst, K.C. Left Anterior Descending Coronary Artery Motion in Deep Inspiration Breath-Hold and Free Breathing Using 4D-CT Scanning: Potential Impact on Left-Sided Breast Cancer Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, S491. [Google Scholar] [CrossRef]
- Mouawad, M.; Lailey, O.; Poulsen, P.; O’Neil, M.; Brackstone, M.; Lock, M.; Yaremko, B.; Shmuilovich, O.; Kornecki, A.; Ben Nachum, I.; et al. Intrafraction Motion Monitoring to Determine PTV Margins in Early Stage Breast Cancer Patients Receiving Neoadjuvant Partial Breast SABR. Radiother. Oncol. 2021, 158, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Rosenblatt, E.; Izewska, J.; Anacak, Y.; Pynda, Y.; Scalliet, P.; Boniol, M.; Autier, P. Radiotherapy Capacity in European Countries: An Analysis of the Directory of Radiotherapy Centres (DIRAC) Database. Lancet Oncol. 2013, 14, e79–e86. [Google Scholar] [CrossRef]
- Yap, M.L.; Zubizarreta, E.; Bray, F.; Ferlay, J.; Barton, M. Global Access to Radiotherapy Services: Have We Made Progress During the Past Decade? J. Glob. Oncol. 2016, 2, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; MacNeill, F.; Penault-Llorca, F.; Eniu, A.; Sardanelli, F.; Nordström, E.B.; Poortmans, P. Why Is Appropriate Healthcare Inaccessible for Many European Breast Cancer Patients?—The EBCC 12 Manifesto. Breast 2021, 55, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Marta, G.N.; Ramiah, D.; Kaidar-Person, O.; Kirby, A.; Coles, C.; Jagsi, R.; Hijal, T.; Sancho, G.; Zissiadis, Y.; Pignol, J.-P.; et al. The Financial Impact on Reimbursement of Moderately Hypofractionated Postoperative Radiation Therapy for Breast Cancer: An International Consortium Report. Clin. Oncol. 2021, 33, 322–330. [Google Scholar] [CrossRef]
- Grover, S.; Xu, M.J.; Yeager, A.; Rosman, L.; Groen, R.S.; Chackungal, S.; Rodin, D.; Mangaali, M.; Nurkic, S.; Fernandes, A.; et al. A Systematic Review of Radiotherapy Capacity in Low- and Middle-Income Countries. Front. Oncol. 2015, 4, 380. [Google Scholar] [CrossRef]
- Dubois, N.; Nguyet Diep, A.; Ghuysen, A.; Declaye, J.; Donneau, A.F.; Vogin, G.; Fleckenstein, J.; Coucke, P.; Ben Mustapha, S. Training of Radiotherapy Professionals: Status, Content, Satisfaction and Improvement Suggestions in the Greater Region. BMC Med. Educ. 2022, 22, 485. [Google Scholar] [CrossRef]
- Oliveira, C.; Barbosa, B.; Couto, J.G.; Bravo, I.; Hughes, C.; McFadden, S.; Khine, R.; McNair, H.A. Advanced Practice Roles amongst Therapeutic Radiographers/Radiation Therapists: A European Survey. Radiography 2023, 29, 261–273. [Google Scholar] [CrossRef]
- Oliveira, C.; Barbosa, B.; Couto, J.G.; Bravo, I.; Khine, R.; McNair, H. Advanced Practice Roles of Therapeutic Radiographers/Radiation Therapists: A Systematic Literature Review. Radiography 2022, 28, 605–619. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological Quality of Case Series Studies: An Introduction to the JBI Critical Appraisal Tool. JBI Evid. Syst. 2020, 18, 2127–2133. [Google Scholar] [CrossRef] [PubMed]
- Carosi, A.; Ingrosso, G.; Turturici, I.; Valeri, S.; Barbarino, R.; Di Murro, L.; Bottero, M.; Lancia, A.; Ponti, E.; Bruni, A.; et al. Whole Breast External Beam Radiotherapy in Elderly Patients Affected by Left-Sided Early Breast Cancer: A Dosimetric Comparison between Two Simple Free-Breathing Techniques. Aging Clin. Exp. Res. 2020, 32, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, B.; Muscat, E.; Harding, A.; Howe, K.; Brown, E.; Barry, T.; Mai, G.T.; Lehman, M.; Bernard, A.; Hargrave, C.; et al. Comparison of Whole Breast Dosimetry Techniques—From 3DCRT to VMAT and the Impact on Heart and Surrounding Tissues. J. Med. Radiat. Sci. 2022, 69, 98–107. [Google Scholar] [CrossRef]
- Mishra, A.; Yadav, N.; Sharma, M.; Mittal, K.K.; Mishra, S.P.; Verma, T.R.; Tiwari, S. Dosimetric Evaluation of Three-Dimensional Conformal Radiotherapy, RapidArc, and Hybrid RapidArc Radiotherapy Techniques for Left-Sided Breast Cancer. J. Med. Phys. 2025, 50, 93–99. [Google Scholar] [CrossRef]
- Piras, A.; Menna, S.; D’Aviero, A.; Marazzi, F.; Mazzini, A.; Cusumano, D.; Massaccesi, M.; Mattiucci, G.C.; Daidone, A.; Valentini, V.; et al. New Fractionations in Breast Cancer: A Dosimetric Study of 3D-CRT versus VMAT. J. Med. Radiat. Sci. 2022, 69, 227–235. [Google Scholar] [CrossRef]
- Redapi, L.; Rossi, L.; Marrazzo, L.; Penninkhof, J.J.; Pallotta, S.; Heijmen, B. Comparison of Volumetric Modulated Arc Therapy and Intensity-Modulated Radiotherapy for Left-Sided Whole-Breast Irradiation Using Automated Planning. Strahlenther. Onkol. 2022, 198, 236–246. [Google Scholar] [CrossRef]
- Viren, T.; Heikkila, J.; Myllyoja, K.; Koskela, K.; Lahtinen, T.; Seppala, J. Tangential Volumetric Modulated Arc Therapy Technique for Left-Sided Breast Cancer Radiotherapy. Radiat. Oncol. 2015, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hu, B.; Xie, C.; Wang, Y. Dosimetric Comparison of Three Intensity--modulated Radiation Therapies for Left Breast Cancer after Breast--conserving Surgery. J. Appl. Clin. Med. Phys. 2018, 19, 79–86. [Google Scholar] [CrossRef]
- Cuzick, J.; Stewart, H.; Rutqvist, L.; Houghton, J.; Edwards, R.; Redmond, C.; Peto, R.; Baum, M.; Fisher, B.; Host, H. Cause-Specific Mortality in Long-Term Survivors of Breast Cancer Who Participated in Trials of Radiotherapy. J. Clin. Oncol. 1994, 12, 447–453. [Google Scholar] [CrossRef]
- Konstantinou, E.; Varveris, A.; Solomou, G.; Antoniadis, C.; Tolia, M.; Mazonakis, M. Radiation Dose to Critical Cardiac Structures from Three-Dimensional Conformal Radiation Therapy (3D-CRT), Intensity-Modulated Radiation Therapy (IMRT) and Volumetric Modulated Arc Therapy (VMAT) Techniques for Left-Sided Breast Cancer. J. Pers. Med. 2024, 14, 63. [Google Scholar] [CrossRef]
- Cheptea, C.; Loap, P.; Allali, S.; Fourquet, A.; Cao, K.; Kirova, Y. Optimizing the Integration of Modern Systemic Therapies and Advanced Radiotherapy Techniques in Breast Cancer Management: An Expert Opinion from the Institut Curie Breast Radiotherapy Group. Int. J. Cancer 2025. early view. [Google Scholar] [CrossRef]
- Newhauser, W.D.; Durante, M. Assessing the Risk of Second Malignancies after Modern Radiotherapy. Nat. Rev. Cancer 2011, 11, 438–448. [Google Scholar] [CrossRef]
- Offersen, B.V.; Boersma, L.J.; Kirkove, C.; Hol, S.; Aznar, M.C.; Sola, A.B.; Kirova, Y.M.; Pignol, J.-P.; Remouchamps, V.; Verhoeven, K.; et al. ESTRO Consensus Guideline on Target Volume Delineation for Elective Radiation Therapy of Early Stage Breast Cancer. Radiother. Oncol. 2015, 114, 3–10. [Google Scholar] [CrossRef]
- Eldesoky, A.R.; Yates, E.S.; Nyeng, T.B.; Thomsen, M.S.; Nielsen, H.M.; Poortmans, P.; Kirkove, C.; Krause, M.; Kamby, C.; Mjaaland, I.; et al. Internal and External Validation of an ESTRO Delineation Guideline—Dependent Automated Segmentation Tool for Loco-Regional Radiation Therapy of Early Breast Cancer. Radiother. Oncol. 2016, 121, 424–430. [Google Scholar] [CrossRef]
- Mathew, T.; Foroudi, F. Consistency of ESTRO and RTOG Contouring Guidelines for Target Volume Delineation in Early Stage Breast Cancer. Int. J. Radiol. Radiat. Ther. 2020, 7, 133–140. [Google Scholar] [CrossRef]
- Khan, F.M.; Gibbons, J.P. Khan’s the Physics of Radiation Therapy, 6th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2020; ISBN 978-1-4963-9752-2. [Google Scholar]
- Perez, C.; Brady, L. Perez and Brady’s Principles and Practice of Radiation Oncology, 7th ed.; Halperin, E.C., Wazer, D.E., Perez, C.A., Brady, L.W., Eds.; Wolters Kluwer: Philadelphia, PA, USA; Baltimore, MD, USA; New York, NY, USA, 2019; ISBN 978-1-4963-8679-3. [Google Scholar]
- Basran, P.S.; Zavgorodni, S.; Berrang, T.; Olivotto, I.A.; Beckham, W. The Impact of Dose Calculation Algorithms on Partial and Whole Breast Radiation Treatment Plans. Radiat. Oncol. 2010, 5, 120. [Google Scholar] [CrossRef] [PubMed]
- Gaur, G.; Dangwal, V.K.; Banipal, R.P.S.; Singh, R.; Kaur, G.; Grover, R.; Sachdeva, S.; Kang, M.S.; Singh, S.; Garg, P.; et al. Dosimetric Comparison of Different Dose Calculation Algorithms in Postmastectomy Breast Cancer Patients Using Conformal Planning Techniques. J. Med. Phys. 2023, 48, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Chua, M.L.K.; Chitapanarux, I.; Kaidar-Person, O.; Mwaba, C.; Alghamdi, M.; Mignola, A.R.; Amrogowicz, N.; Yazici, G.; Bourhaleb, Z.; et al. Global Radiotherapy Demands and Corresponding Radiotherapy-Professional Workforce Requirements in 2022 and Predicted to 2050: A Population-Based Study. Lancet Glob. Health 2024, 12, e1945–e1953. [Google Scholar] [CrossRef]
- Chu, F. The Latest Developments and Applications in Radiation Therapy Equipment and Technology. Int. J. Public Health Med. Res. 2024, 2, 27–32. [Google Scholar] [CrossRef]
- Abdel-Wahab, M.; Gondhowiardjo, S.S.; Rosa, A.A.; Lievens, Y.; El-Haj, N.; Polo Rubio, J.A.; Prajogi, G.B.; Helgadottir, H.; Zubizarreta, E.; Meghzifene, A.; et al. Global Radiotherapy: Current Status and Future Directions—White Paper. JCO Glob. Oncol. 2021, 7, 827–842. [Google Scholar] [CrossRef]
- Christ, S.M.; Willmann, J. Measuring Global Inequity in Radiation Therapy: Resource Deficits in Low- and Middle-Income Countries Without Radiation Therapy Facilities. Adv. Radiat. Oncol. 2023, 8, 101175. [Google Scholar] [CrossRef] [PubMed]
- Ferdinand, S.; Mondal, M.; Mallik, S.; Goswami, J.; Das, S.; Manir, K.S.; Sen, A.; Palit, S.; Sarkar, P.; Mondal, S.; et al. Dosimetric Analysis of Deep Inspiratory Breath-Hold Technique (DIBH) in Left-Sided Breast Cancer Radiotherapy and Evaluation of Pre-Treatment Predictors of Cardiac Doses for Guiding Patient Selection for DIBH. Tech. Innov. Patient Support Radiat. Oncol. 2021, 17, 25–31. [Google Scholar] [CrossRef]
- Stowe, H.B.; Andruska, N.D.; Reynoso, F.; Thomas, M.; Bergom, C. Heart Sparing Radiotherapy Techniques in Breast Cancer: A Focus on Deep Inspiration Breath Hold. Breast Cancer Targets Ther. 2022, 14, 175–186. [Google Scholar] [CrossRef]
- Eckstein, J.; Taylor, P.; Zheng, R.; Lee, L.; Chen, W.; Potters, L.; Evans, C. Implementation of External Beam Five-Fraction Adjuvant Breast Irradiation in a US Center. Cancers 2022, 14, 1556. [Google Scholar] [CrossRef] [PubMed]
- Merten, R.; Fischer, M.; Kopytsia, G.; Wichmann, J.; Lange, T.; Knöchelmann, A.C.; Becker, J.-N.; Klapdor, R.; Hinrichs, J.; Bremer, M. Linac-Based Ultrahypofractionated Partial Breast Irradiation (APBI) in Low-Risk Breast Cancer: First Results of a Monoinstitutional Observational Analysis. Cancers 2023, 15, 1138. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).