Abstract
A molecular network investigation of Psiloxylon mauritianum leaf extracts from five different specimens led to the detection of a diversity of flavonoids, triterpenes, and phloroglucinols. Some compounds from these molecular classes are reported to target fever-linked symptoms (antioxidant, antiviral, anti-inflammatory, and antibacterial activities) and may explain the plant’s success as a local traditional remedy. The phytochemical study of one extract allowed the isolation and characterization of an original seco-ring-A lupane structurally similar to the anti-inflammatory betulinic acid, 11 known triterpenes, 2 flavonoids, and a chalcone. Antiviral assays highlighted the in vitro anti-Zika activity of corosolic and betulinic acids found in the plant. Some interesting structure–activity relationships could be drawn between the new compound and the known active triterpenes.
1. Introduction
Psiloxylon mauritianum (Bouton ex Hook. fil.) Baill. is an evergreen dioecious tree belonging to the Myrtaceae family. It is the only species of the genus Psiloxylon, endemic to La Réunion and Mauritius islands, and is commonly found in mid-altitude forests ranging from 300 to 1000 m with a preference for wet conditions. The plant was registered in the French pharmacopoeia in 2013 for the traditional use of its leaves [1]. Indeed, previous ethnopharmacological studies have reported several traditional uses, such as treatment of amenorrhea, dysentery, general infections, gout, or diabetes [2,3]. Since then, P. mauritianum has been quite popular within the scientific community, with no less than six published papers dealing with the chemistry and biological activity of plant extracts or compounds [2,3,4,5,6,7]. It was previously demonstrated that the plant’s aqueous extract was rich in polyphenols and had in vitro antiviral activity against DENV and CHIKV [4]. The aqueous extract was also reported with in vitro antioxidative activity in red blood cells [6] and in vivo (zebra fish model) antidiabetic activity [7]. Although polyphenols mentioned in these studies were detected and annotated with modern UHPLC-HRMS/MS, no biological assay was conducted on isolated compounds. Other studies [3,5] focused their attention on bioguided isolation and characterization of antibacterial compounds from the non-polar extract of the plant. This led to the identification of triterpenes (corosolic, asiatic, ursolic, and oleanic acids) and two phloroglucinols with potent activity on methicillin-resistant Staphylococcus aureus. From our perspective, the plant has relatively few reported compounds compared to the numerous biological activities exhibited by its various extracts.
Therefore, as part of our ongoing investigation of traditional medicinal plant chemistry and search for new compounds, the fractionation of ethyl acetate (EtOAc) extract from the leaves of P. mauritianum was achieved. This extract showed a moderate cytotoxic activity against two cancer cell lines, as well as an antiviral activity against Zika virus. The goal of this study is twofold: to accurately map the chemical space of P. mauritanum and to investigate the activity of its isolated compounds.
2. Materials and Methods
2.1. Plant Collection
Fresh P. mauritianum leaves (575 g) were collected by Alexis Gorissen at coordinates 21°16′27.0″ S 55°28′13.10″ E (South of La Réunion, altitude: 375 m) in February 2021. A voucher specimen was submitted to the local herbarium (Conservatoire Botanique National de Mascarin) under the reference number REU025096. Fresh P. mauritianum leaves (10 g each) from four different specimens similar to the voucher were collected by Theo Ozga at coordinates 21°20′26.07″ S, 55°42′28.93″ E (South of La Réunion, altitude: 680 m) in April 2023.
2.2. Metabolite Extraction
Plant materials were dried with a dryer (ST3 model, Politech, Geispolsheim, France) at 40 °C and ground with a cutting mill (Fritsch, Idar-Oberstein, Germany). Extraction of metabolites was performed with the help of an accelerated solvent extractor (ASE 300, Dionex, Sunnyvale, CA, USA) with analytical grade EtOAc in 66 mL cells at 100 bar and 40 °C. EtOAc was evaporated with a rotatory evaporator at 40 °C (Büchi, Flawil, Switzerland) to yield dry extracts, which are stored at 4 °C. A total of 100 mg of each extract was resolubilised in a methanol:water mix (7:3, 100 mL) and partitioned two times in isohexane (100 mL) to remove most apolar compounds. The methanol-aqueous phase was dried and stored at 4 °C until analyses.
2.3. UPLC-HRMS/MS Analyses and Ion Identity Molecular Networking
P. mauritianum metabolite extracts were resolubilised in methanol at a 5 mg/mL concentration and were analysed on a WatersTM ACQUITY UPLC® I-class system (Waters®, Milford, CT, USA) coupled to a Thermo ScientificTM Orbitrap ExplorisTM 120 mass spectrometer (Thermo Scientific®, Bremen, Germany) using a Thermo Scientific OptaMax NG ion source with a heated electrospray ionization (HESI-II) probe, piloted by Xcalibur 3.1 (Thermo Scientific). The optimized HESI-II parameters were as follows in positive ionization mode: source voltage was set from 3.1 kV to 3.7 kV from 1 min to 6 min and maintained at 3.7 kV until 10 min; sheath gas flow rate (N2), 35 units; auxiliary gas flow rate, 10 units; sweep gas flow rate, 1.0. Ion transfer tube temperature and vaporizer temperature were both set to 320 °C. The mass analyser was calibrated using the Thermo Scientific EASY-IC ion source internal reference mass (fluoranthene). The mass spectrometer method was set to FullMS data-dependent MS2 (ddMS2) for a scan range between 100 to 1500 m/z. FullMS were acquired at a resolution of 30,000 for an expected peak width of 16 s, the normalized AGC target was set to 350%, and injection time was set to 120 ms. In ddMS2, the resolution was 15,000, the normalised AGC target 50%, the isolation window 1.5 m/z, and the stepped normalized collision energy 15/30/45. Injection time was set to 125 ms, and the parent ions were placed on the dynamic exclusion list for 2 s. The RF lens was set at 60%. Methanol was injected as blank. The chromatographic column was a 50 × 2.1 mm, 1.7 µm Waters™ ACQUITY UPLC® BEH C18 column (Waters). The injection volume was set to 1 µL. The mobile phases were (A) water and (B) acetonitrile, both with 0.1% of formic acid, and the gradient was set as follows: linear from 5 to 100% B over 7 min and 2 min of isocratic elution at 100% B, followed by 1 min at 5% B for column conditioning (ACN + 0.1% formic acid). The flow rate was set constant at 0.6 mL/min. Thermo raw MS data were converted in mzXML format using MS convert 3.0.24 software and processed with MZmine 3.2.8. software [8]. Intensity detection threshold for MS1 and MS2 was set to 7.5 × 105 and 0, respectively. ADAP Chromatogram Builder [9] was used to reconstruct the extracted ion chromatogram with a minimum scan group size of 5. Isotope features were removed using the 13C isotope filter module. Blank and P. mauritianum features were aligned (Join aligner), and the blank features were subtracted (feature list blank subtraction) from the feature table, ensuring only plant features remained in the final molecular network. The features IDs were reset (feature list row filter). Finally, features were metacorrelated, and adducts were searched using the module ion identity networking. The feature quantification table was imported into GNPS and analysed as a feature-based molecular network. Collapsed ion identity network was retrieved; IMN graph was visualized and annotated with Cytoscape Software version 3.10.0 [10].
2.4. Isolation and Characterization of Compounds
EtOAc leaf extract from the voucher specimen of P. mauritianum (3.3 g) was used to carry out a compound isolation. Solid phase extractions were carried out on 10 g reversed phase C18-E, 55 µm, 70 Å Strata cartridges (Phenomenex, Torrance, CA, USA). The dark-green extract (3.3 g) was subjected to three successive reverse phase SPE (1.1 g per cartridge) to yield eight fractions (F1–F8) of decreasing polarity: F1 (ACN/water, 3:7, 290.4 mg), F2 (ACN/water 1:1, 101.8 mg), F3 (ACN/water 65:35, 83.2 mg), F4 (ACN/water 75:25, 85.8 mg), F5 (ACN/water 85:15, 99.9 mg), F6 (ACN, 183.0 mg), F7 (MeOH, 302.3 mg), and F8 (DCM, 393.3 mg). Each fraction was evaporated. Preparative HPLC separations were performed using a 150 × 21.2 mm, 5 µm reversed phase C18 preparative column (Gemini, Phenomenex) on an Autopurif instrument (Waters, Milford, CT, USA) equipped with a PDA 2998 detector (Waters, Milford, CT, USA) coupled to a 2424 evaporative light scattering detector (Waters, Milford, CT, USA) piloted by MassLynx 4.2 software.
F2 was dissolved (ACN/water, 3:7, 10 mg/mL) and purified by repetitive preparative HPLC. 500 µL per injection, gradient of ACN/water, 2:8 to 7:3 over 26.31 min, followed by 5 min of isocratic elution (ACN). A constant flow rate was set at 20 mL/min. F3 was dissolved (ACN/water, 65:35, 10 mg/mL) and purified by repetitive preparative HPLC. 500 µL per injection, gradient of ACN/water, 1:1 to 8:2 over 23.43 min, followed by 6.57 min of isocratic elution (ACN). A constant flow rate was set at 20 mL/min. F5 was dissolved (ACN/water 85:15, 10 mg/mL) and purified by repetitive preparative HPLC. 500 µL per injection, gradient of ACN/water, 3:1 to 1:0 over 20.15 min, followed by 5 min of isocratic elution (ACN). A constant flow rate was set at 20 mL/min.
Fifteen compounds were retrieved in this process. They were characterized by extensive NMR and HRMS/MS. NMR spectra were performed on a 600 MHz Bruker Avance™ II+ spectrometer equipped with a TCI cryoprobe at 300 K. Compounds were solubilised in 80 µL of MeOD, introduced in a 2 mm o.d. Match NMR tube and centrifuged prior to analysis. Proton (1H) and carbon (13C) signals from the deuterated solvent (i.e., 49.00 ppm for 13C and 3.31 ppm for 1H for MeOD) were used as references. Optical rotation was measured on an MCP 200 Anton Paar modular circular polarimeter at 25 °C with a 589 nm wavelength in a 10 × 5 mm, i.e., 0.2 mL sample cell (Na D-line). HRMS spectra were acquired on a SYNAPT G2 HDMS (Waters, Guyancourt, France) equipped with an API oven and a TOF analyser. See supplementary information for NMR data of isolated known compounds. The characterization of one new compound will be further detailed in the results.
2.5. Cell, Virus, and Biological Reagents
Human cancer cell lines were obtained from the American Type Culture Collection (ATCC, Rockville, MD) and cultured according to the supplier’s instructions. K562 leukemia cells and A2780 ovarian carcinoma cells were grown in RPMI 1640 supplemented with 10% fetal calf serum and 1% glutamine. Cells were maintained at 37 °C in a humidified atmosphere containing 5% CO2.
Vero cells (ATCC, CCL-81) were cultured in Eagle’s minimum essential medium (MEM) supplemented with 5% of heat-inactivated fetal bovine serum (FBS), 2 mM L-glutamine, 1 mM sodium pyruvate, 100 U/mL of penicillin, 0.1 mg/mL of streptomycin, and 0.5 µg/mL of fungizone under a 5% CO2 atmosphere at 37 °C. ZIKV GFP, a molecular clone of the ancestral strain MR766 of ZIKV, is used for the assay [11]. Viruses were subsequently amplified in Vero E6 cells. ZIKV titration was carried out using a plaque-forming assay and expressed in PFU/mL.
2.6. Flow Cytometric Assay
Cells were harvested, fixed with 3.7% PFA in PBS for 10 min, and subjected to a flow cytometric analysis using CytoFLEX (Beckman Coulter, Brea, CA, USA). The percentage of GFP-positive cells was assessed using Cytexpert software (version 9.00, La Jolla, CA, USA).
2.7. Antiviral Assay
Vero cells were seeded and incubated (37 °C, 5% CO2 atmosphere) in a 96-well culture plate at a density of 2.0 × 104 cells per well. The cells were treated with three different concentrations of molecules (100, 25, and 6.25 µg/mL). Simultaneously, cells were infected with ZIKVGFP at a multiplicity of infection (MOI) of 1. After an incubation period of 24 h at 37 °C and 5% CO2, the culture medium was removed; the cells were rinsed with PBS (60 µL) and treated with 20 µL trypsin to detach them. Cells were fixed with culture medium MEM + 5% FBS mixed with paraformaldehyde (PFA) 3.7% for 10 min. Then they were submitted to a flow cytometry analysis using CytoFLEX (Beckman Coulter, Brea, CA, USA). The results were analysed using the Cytexpert software (Brea, CA, USA). The IC50 was determined using a nonlinear regression on the Graphpad prism software (version 9.5.0; La Jolla, CA, USA).
2.8. Cytotoxic Assay on Vero Cells
Twofold dilutions of plant extract ranging from 0.78 to 200 µg/mL were used to treat Vero cells at a density of 1.0 × 104 cells per well in 96-well culture plates. The cells were incubated for 48 h at 37 °C and 5% CO2 atmosphere. The cells were rinsed with PBS (60 µL), and 120 µL of the culture medium MEM containing 5% PBS mixed with 5 mg/mL MTT (3-[4-dimethylthiazol-2-yl]-2,5-diphenyltetrahzolium bromide) was added. After an incubation of 2 h, the MTT medium was removed, and the cells were suspended in 100 µL of DMSO. The absorbance of formazan crystals in viable cells was measured at 570 nm with a background subtraction at 690 nm. The CC50 was determined using a nonlinear regression on the Graphpad prism software (version 9.5.0; La Jolla, CA, USA).
2.9. Cell Viability Assay on Cancerous Cell Lines
Cell viability was determined by a luminescent assay according to the manufacturer’s instructions (Promega, Madison, WI, USA). Briefly, the cells were seeded in 96-well plates (2.5 × 103 cells/well) containing 90 μL of growth medium. After 24 h of culture, the cells were treated with the tested compounds at 1 and 10 μg/mL final concentrations. Control cells were treated with the vehicle. After 72 h of incubation, 100 μL of CellTiter Glo Reagent was added for 15 min before recording luminescence with a spectrophotometric plate reader PolarStar Omega (BMG LabTech, Ortenberg, Germany). The percent viability index was calculated from three experiments.
3. Results and Discussion
3.1. Ion Identity Molecular Network Investigation of P. mauritianum Ethyl Acetate Extract
Ethyl acetate extract obtained from the leaf of the voucher P. mauritianum was analysed by UHPLC-HRMS/MS in a data-dependent acquisition mode and positive ionisation mode. The acquired MS1 and MS2 data were processed through the MZmine software pipeline [8], leading to the detection of 453 features. These features were structured and analysed through a feature-based ion identity molecular networking (IIMN) approach to gain a better understanding of their MS/MS spectral relationship and thus their chemical relatedness. MS/MS spectrum of features were compared and connected into a common cluster when exhibiting a cosine score over 0.7. Following the IIMN strategy, features having similar ion peak shapes, retention time, and characteristic ion mass differences (e.g., adducts) were clustered together. MS/MS spectra were also compared to the GNPS experimental mass spectra libraries, providing a spectral match for 61 features (13% of the set), which could be propagated within clusters. In parallel, MS1 and MS2 spectra of each feature were submitted to the SIRIUS pipeline [12] for an automated in silico annotation provided through the Sirius, Zodiac, CSI:FingerID, and Canopus modules. In this pipeline, each feature was attributed a molecular formula (based on its exact mass and isotopic pattern) by the Sirius and Zodiac modules, while a list of putative structure ranked according to a probability score was provided by the CSI:FingerID module. Finally, the Canopus module retrieved the ClassyFire chemical class for each candidate structure. The resulting dataset, visualized as an IIMN (Figure 1), was then manually curated by confronting the annotation provided by the GNPS and Sirius pipelines. The chemical annotations coming from Sirius and GNPS pipelines were compared and structured into a consensus annotation matching the metabolite annotation classification requirements [13], thus allowing to consolidate the confidence in the annotation level when the same annotation is provided by two different tools. This approach combined to the structural elucidation of compounds by NMR allowed the propogation of the annotation to poorly or unannotated features within each cluster, expanding the annotation coverage. Figure 2 and Figure 3 are zoomed views of selected clusters of this IIMN with structural annotations.

Figure 1.
Ion identity molecular network from P. mauritianum ethyl acetate extract.
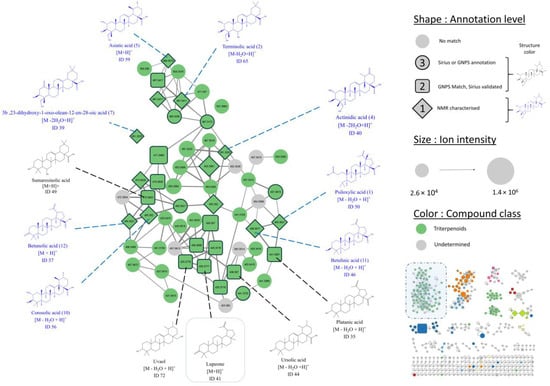
Figure 2.
Cluster 1 displaying features annotated as triterpenes. For readability, only one adduct per compound is displayed. The maximum number of edges per node is limited to five. (Edges with the lowest cosine are hidden.) Structures displayed in blue were isolated and characterized by NMR (level 1 annotation, 8 displayed out of 12), while structures in black are consensus provided by GNPS and Sirius (level 2 annotation, 5 displayed out of 11).
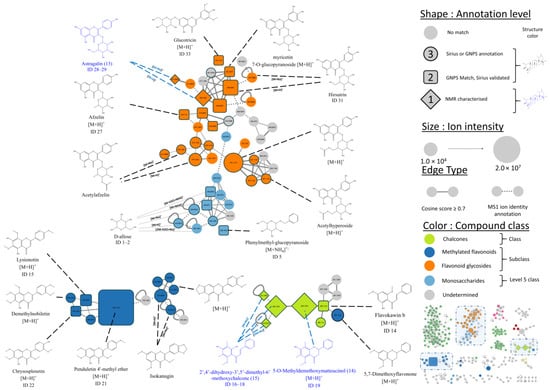
Figure 3.
IIMN zoomed view showing three clusters containing flavonoids. For readability purposes, the maximum number of edges is limited to five. (Edges with the lowest cosine are hidden). Structures displayed in blue were isolated and characterized by NMR (level 1 annotation, three displayed out of three). Structures in black are consensus results given by GNPS and Sirius annotation tools (level 2 annotation, square-shaped features, 11 displayed out of 14) or a Sirius match (level 3 annotation).
Following [13] metabolite annotation classification, three annotation levels were proposed for IIMN features. A feature with no consensus molecular formula provided by the two annotation tools was labelled with a level 3 low-confidence metabolomic annotation. Meanwhile, a feature with similar molecular formula and compound class given by GNPS and Sirius was labelled as a level 2 annotation. For instance, feature 41 (m/z = 425.3777, circled in Figure 2, bottom corner) was identified by GNPS as 17(21)-hopen-6-one ([M+H]+, C30H48O), while Sirius suggested lupeone ([M+H]+, C30H48O). Since both sources proposed the same molecular formula and compound class, the annotation is of level 2. A feature with no GNPS match could be manually assigned a level 3 annotation based on the similarity of its annotation with neighbor nodes, which may have a higher annotation level. For instance, seven nodes with no GNPS annotation were located in the same cluster (Figure 3, bottom left cluster) next to three nodes of level 2 annotation. Their structures suggested by Sirius were similar to the level 2 annotation structures of neighbor nodes. Therefore, they were attributed the level 2 annotation. Finally, compounds that were isolated and fully characterized by NMR during our study were pinpointed as level 1 annotations. Table S1, which summarises the information for level 1 and 2 annotations (73 features), can be found in the supplementary information.
The annotation results provided by Sirius combined with the GNPS spectral databases matches, and NMR structural elucidation of purified compounds were investigated in the context of the IIMN. This approach allowed the expansion of the annotation coverage and identified triterpenoids and flavonoids in their free, methylated, or glycosylated forms as the main classes of compounds of the P. mauritianum ethyl acetate extract.
The biggest cluster in terms of the number of nodes (Figure 2, cluster 1) harbours 79 features, 70 of which were labelled as triterpenoids, meaning the plant extract is rich in compounds from this class. The ion identity molecular network approach and a manual curation of the network allowed the identification of the different adducts generated during the ionisation process (mainly [M-XH2O+H]+, [M+NH4]+, and [M-CO+H]+) gathered in cluster 1. Finally, up to 21 different triterpenes (features with at least a level 2 annotation) were annotated in the extract, whereas only 4 were previously reported in P. mauritianum [3,5]. These later were also all identified in the present work: asiatic acid (ID 43), corosolic acid (ID 44), ursolic acid (ID 56), and oleanic acid (ID 59) (Figure 2, Table S1). In addition, several lupane triterpenes, which is a class never reported in the plant, such as lupeone (ID 41) or betunolic acid (ID 36), were identified for the first in P. mauritianum to our knowledge.
Free and glycosylated flavonoids were reported in previous plant investigations. For instance, kaempferol and quercetin, both free or linked to an unspecified sugar, were previously detected by HR-MS/MS analyses [4,6]. In the present cluster, one feature was identified as quercetin (ID 11, Table S1), one as kaempferol (ID 8), and three features as glycosylated kaempferol (ID 25, 27, and 28). Sugar of compound 13 was identified later by NMR as glucose.
Methoxylated flavonoids have never been isolated or reported from P. mauritianum. A cluster (Figure 3, bottom left, dark blue) contains 10 features identified as polymethoxylatedylated flavonoids. For instance, feature ID 21 was identified as gardenin B. Coelution of PMFs during the purification process made it difficult to obtain pure compounds, but NMR analysis of several fractions collected during the purification process confirmed the presence of several PMFs in the extract.
Finally, a cluster (Figure 3, bottom right, light green) contains seven features identified as chalcones, a class of flavonoids not reported in P. mauritianum yet. For instance, feature ID 14 was annotated as flavokawin b, a methylated chalcone. Chalcones are largely distributed in plants and are often reported for their biological activity [14]. Therefore, a chalcone was later isolated to confirm the cluster annotations and evaluate its activity.
3.2. Isolation and Characterization of Compounds by NMR
Fifteen compounds were identified through the purification strategy (Figure 4). The compounds 2–15 were known and characterized by comparison of their 13C and 1H NMR chemical shifts with literature references. A comparison table (Table S2) is available in the supplementary information, as well as all NMR analysis. Appendix A provides further details on yields and physical properties. Compounds 2 and 3 (namely terminolic acid and madecassic acid) and compounds 5 and 6 (namely asiatic acid and arjulonic acid) are in 1:1 mixtures, which could be characterized. All other compounds were retrieved pure. Compounds 13 (astragalin) and 14 (5-O-methyldemethoxymatteucinol) belong to the flavonoid family. Compound 15 (2′,4′-dihydroxy-3′,5′-dimethyl-6′-methoxychalcone) is a chalcone. Compounds 2–12 are triterpenes from lupane, oleanane, and ursane subfamilies. Compounds 5 and 10 were previously isolated and characterized in the plant species [5]. Compound 13 has been previously reported with HRMS data but never isolated in the plant [4]. All other 12 compounds (1–4, 6–9, and 11–15) have never been reported in P. mauritianum.
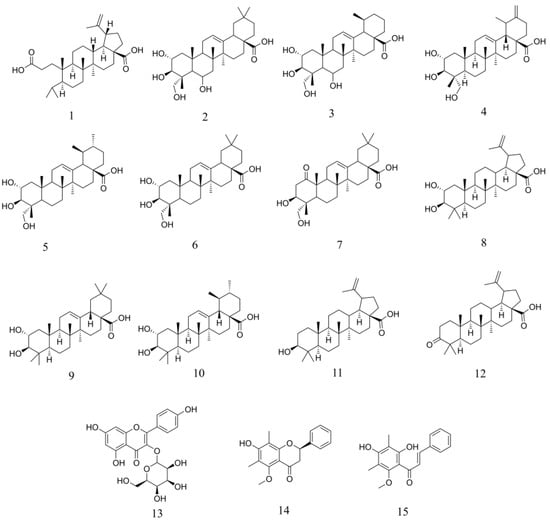
Figure 4.
Compounds isolated from P. mauritianum and characterized by NMR spectroscopy.
Chemical shifts of compound 1 have never been described in the literature. The HRESIMS analysis gave a molecular formula of C30H48O4 (m/z = 495.3447 [M+Na]+, calculated 495.3445), suggesting a triterpene structure. The 1H NMR spectrum enabled to show six methyl groups. The signal of diastereotopic methylene protons at δ1H 4.60 ppm and δ1H 4.72 ppm (diastereotopicity verified by edited HSQC) and the methyl group at δ1H 1.70 ppm are typical of isopropenyl moiety of a lupane triterpene. Moreover, compound 1 has many signals in common with compound 12 (betulinic acid, molecular formula C30H46O3). However, the C-3 signal at δ13C 221.0 ppm (corresponding to a ketone) present in compound 12 is not found in compound 1. Instead, a shielded signal at δ13C 179.2 ppm suggesting a carboxylic acid function is observed in compound 1 and not in compound 12. Presumably, compound 1 is an oxidized analog of compound 12, where the ketone in C-3 has been replaced by a carboxylic group with a ring opening. Furthermore, the signal of the protons of two methyl groups C-23, C-24 in compound 1 appeared as two duplets, whereas all methyl group signals in compound 12 show singlet signals. These two methyls at δ1H 0.92 and 0.81 ppm, respectively, correlate in HMBC spectrum with a carbone at δ13C 26.5 ppm and couple with the same unique proton at δ1H 1.92 ppm in COSY spectrum. Thus, it can be supposed that these two methyl groups are in positions 23 and 24, connected to the tertiary carbone C4. This validates the hypothesis the C3–C4 bond has been severed. Finally, the structure of compound 1 (Figure 5) has been unambiguously identified by extensive 1D and 2D NMR (COSY, HSQC, HMBC, and NOESY) analyses as (3S,4S,5R,8R,9R,10R,13S,14R,15R)-4-(2-carboxyethyl)-3-isopropyl-4,9,10-trimethyl-15-(prop-1-en-2yl)hexadecahydro-13H-cyclopenta[a]phenanthrene-13-carboxylic acid, named psiloxylic acid. The nine stereocenters have also been determined through NOE correlation. NMR data are reported in Table 1. Finally, the optical rotation was measured: = + 15.5 (c = 0.3257 g/100 mL, MeOH).
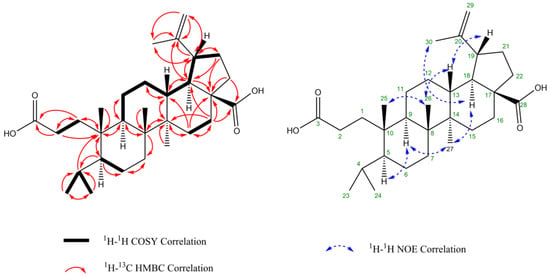
Figure 5.
Structure of compound 1 annotated with key HMBC, COSY, and NOESY correlations.

Table 1.
1H (600 MHz) and 13C NMR (150 MHz) data of compound 1 in MeOD at 300 K. Diastereotopic protons are marked with letters.
3.3. Biological Evaluations
The majority of the compounds isolated were obtained in quantities insufficient for concurrent assessment of antiviral and cytotoxic activities. Consequently, the decision regarding which activity to evaluate for each compound was informed by literature and preliminary findings from fraction testing. EtOAc extract of P. mauritianum showed moderate cytotoxic activity against the K562 leukemia cells and ovarian A2780 ovarian cancer cell line (Table 2).

Table 2.
Cell viability (% of the control) of cancerous cells in the presence of a given concentration of P. mauritianum crude extract.
The triterpenes (2–12) were generally reported for cytotoxic activity in the literature [15,16,17,18,19,20]. Since that field was already explored, it was preferred to test the isolated triterpenes in an antiviral assay. Compounds 1, 14, and 15 were tested and did not show significant cytotoxic activity. A fraction containing three methoxylated flavonoids previously mentioned was also tested and did not show significant activity. Thus, the cytotoxic potential of compounds was not further explored, and loss of activity was attributed to loss of synergy.
Previous studies have highlighted the antiviral activity of the EtOAc extract of P. mauritianum against emerging orthoflaviviruses, such as Zika and dengue [4]. Aiming to confirm that the chemical composition of the extract could inhibit Zika virus infection in Vero cells—a virus-permissive cell line—a molecular clone of the Zika virus expressing GFP was used for infection. The cells were treated with varying concentrations of the extract fractions simultaneously with infection. Monitoring of the infection was conducted through the assessment of fluorescence intensity via flow cytometry. From the dose-dependent curves obtained, a nonlinear regression was performed to determine the IC50, the concentration inhibiting 50% of the infection. Remarkably, the crude extract demonstrated an antiviral effect consistent with previous demonstrations [4], with fraction F5 showing the lowest IC50 at 17.57 µg/mL (Table 3). Fraction F5 exhibited consistent antiviral activity, with a slightly lower IC50 value than the EtOAc crude extract. Although the difference in activity is not substantial, the distinct chemical profile of F5, including known antiviral triterpenes such as betulinic acid and corosolic acid, justified further investigation into its isolated compounds. Based on these findings, we focused on the secondary metabolites in fraction F5 to further evaluate their antiviral potential.

Table 3.
Antiviral activity of P. mauritianum (EtOAc) crude extract and fractions against Zika virus.
Fraction F5 was identified as the most active in preliminary assays. Consequently, all terpenes (1, 9–12) from this fraction underwent initial antiviral testing against ZIKV (Table 4). Additionally, triterpenes 2–8 from the inactive fraction F3 served as negative controls. For positive control, quercetin-3-β-O-D-glucoside (Q3G), known for its anti-ZIKV efficacy [21,22], was used. The antiviral activity was evaluated as previously described for the fractions by infecting Vero cells infected with ZIKVGFP at an MOI of 1 and treating simultaneously with the compounds. The mitochondrial activity of the cells was assessed 48 h post-treatment using an MTT assay to determine cell viability. Both CC50 and IC50 values, showcased in Table 4, illustrate the compounds’ effectiveness. Compounds 2–4, 7–8, and 13, with IC50 values exceeding 100 µM, were not further explored. The selectivity index (SI)—the ratio of cell toxicity (CC50) to antiviral activity (IC50)—was calculated for compounds with notable antiviral effects. An SI of 1 or below is deemed unsatisfactory, suggesting greater toxicity than specific antiviral action.

Table 4.
Antiviral activity (ZIKV in Vero cells) and cell toxicity of isolated triterpenes. A higher SI means better suitability against the virus.
The positive control (isoquercitrin) exhibited strong antiviral activity (IC50 = 4.22 µM) and low toxicity (CC50 = 96.26 µM), hence a high SI. Notably, betulinic acid (11) and corosolic acid (10) from Fraction F5 displayed significant activity (IC50s of 20.21 µM and 26.80 µM, respectively). Although betulinic acid (11) has been previously reported as an antiviral agent [23,24], corosolic acid (10) showed a lower SI (1.16) compared to the positive control, suggesting its antiviral properties may be closely tied to its cytotoxicity.
Emerging structure–activity relationships reveal that corosolic acid (IC50 26.80) is more active than its oleanane counterpart, maslinic acid (IC50 82.97), hinting at steric factors affecting activity. However, this theory could not be fully investigated as the corresponding ursane–oleane pair (asiatic acid (5) and arjulonic acid (6)) were not isolated separately. Among the lupane triterpenes, betulinic (11), betunolic (12), and the novel psiloxylic acid (1) vary only at position 4, featuring alcohol, ketone, and carboxylic acid groups, respectively. Betulinic acid (11), with its alcohol group, shows higher antiviral activity and comparable cell toxicity to its ketone analogue (12). The new compound 1, an oxidized and ring-opened form of compound 12, exhibits lower cytotoxicity but also diminished antiviral activity. These initial findings lay the groundwork for optimizing a lupane derivative that could potentially be more active and less toxic [25].
3.4. Further Metabolite Investigations of Different P. mauritianum Specimens
The chemical composition of the P. mauritianum voucher specimen is in accordance with previous reported studies. However, phloroglucinols (namely aspidin VB and aspidin BB) [5], usually reported in the different published phytochemical investigation of the plant extracts, were missing. As these compounds have been reported to have potent antibacterial activity, which is one of the purposes of the traditional use of this plant species, we wanted to investigate if the extract chemical composition may vary according to the sample location. Four other specimens of P. mauritianum morphologically similar to the voucher specimen were collected at a different location (Basse Vallée, 25 km from the voucher’s site) on the same day and were all found within a radius of 10 m. The samples were prepared and analysed as previously, following the same extraction protocol and the same metabolomic profiling by UPLC-HRMS/MS to enable a proper comparison with the voucher specimen. To this end, MS2 data were analysed through a newly generated IIMN (Figure 6) to display the metabolomic differences between the different P. mauritianum specimens.
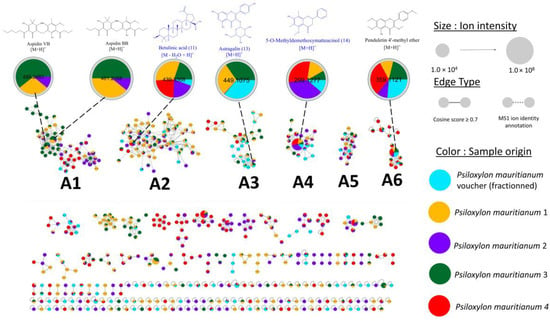
Figure 6.
IIMN of the fractioned P. mauritianum extract compared with four other specimens collected. Each color corresponds to a specimen. Blue structures are isolated and NMR-characterized.
The metabolite content of the five specimen extracts was rather heterogeneous. If methoxylated flavonoids (e.g., compound 14, cluster A4) and triterpenes (e.g., betulinic acid, cluster A2) previously described are present in all analysed samples, some compounds are found only in one or some of the specimens. For instance, psiloxylic acid (1), described for the first time in the current work, is specific to the voucher specimen. As all samples were processed and analysed the same way, psiloxylic acid is unlikely to be an extraction artefact derived from betulinic acid. Presumably, the voucher’s site, which has a lower altitude and is on a drier range of the mountain, favored the biosynthesis of psiloxylic acid. Interestingly, the phloroglucinols aspidin VB and aspidin BB were present in two samples analysed out of five. (Some traces could be found in a third sample). These two compounds are absent from the voucher’s sample but also from two specimens out of four having the same abiotic factors. This underlines the chemical phenotype of P. mauritianum does not depend solely on climate and location and hypothetically is affected by genetic variability [26], individual development, or endophytic relationship [27,28]. Little is known about these aspects for plants from La Réunion, and further work needs to be conducted to investigate P. mauritianum chemical variability.
4. Conclusions
With this study, we wanted to provide a state-of-the-art metabolite description of one of the most consumed plant remedies in La Réunion as a reference for future research works but also for the consumer. In the context of fighting viral infections, our anti-Zika bioguided investigation led us to isolate numerous triterpenes, which displayed some active compounds, like betulinic acid. Among them, psiloxylic acid, a new lupane triterpene structurally similar to betulinic acid, was reported. We suspect that some of these triterpenes may also have an anti-inflammatory activity through the COX-2 inhibitory pathway, which was demonstrated, for instance, for madecassic and betulic acids. We hope further work could be conducted on that matter, which would underline why P. mauritianum is so popular for treating fever-linked symptoms. Finally, we showed how variable the plant’s composition is, even on a restricted geographic and seasonal range. Further interdisciplinary investigations, such as characterizing the endophytic relationships on several specimens may uncover unsuspected interactions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15020496/s1, Figure S1: HRMS spectrum for psiloxylic acid (1) in positive mode featuring the sodium adduct; Figure S2: IR spectrum of psiloxylic acid (1); Figure S3: 1H NMR (600 MHz, CD3OD) spectrum for psiloxylic acid (1); Figure S4: 13C NMR (600 MHz, CD3OD) spectrum for psiloxylic acid (1); Figure S5: 1H-1H COSY NMR (600 MHz, CD3OD) spectrum for psiloxylic acid (1); Figure S6: 1H-13C HSQC NMR (600 MHz, CD3OD) spectrum for psiloxylic acid (1); Figure S7: 1H-13C HMBC NMR (600 MHz, CD3OD) spectrum for psiloxylic acid (1); Figure S8: 1H-1H NOESY NMR (600 MHz, CD3OD) spectrum for psiloxylic acid (1); Figure S9: 1H NMR (600 MHz, CD3OD) spectrum for terminolic acid (2) and madecassic acid (3); Figure S10: 1H-13C HSQC NMR (600 MHz, CD3OD) spectrum for terminolic acid (2) and madecassic acid (3); Figure S11: 1H-13C HMBC NMR (600 MHz, CD3OD) spectrum for madecassic acid (2) and terminolic acid (3); Figure S12: 1H NMR (600 MHz, CD3OD) spectrum for actinidic acid (4); Figure S13: 1H-13C HSQC NMR (600 MHz, CD3OD) spectrum for actinidic acid (4); Figure S14: 1H-13C HMBC NMR (600 MHz, CD3OD) spectrum for actinidic acid (4); Figure S15: 1H NMR (600 MHz, CD3OD) spectrum for asiatic acid (5) and arjunolic acid (6); Figure S16: 1H-13C HSQC NMR (600 MHz, CD3OD) spectrum for asiatic acid (5) and arjunolic acid (6); Figure S17: 1H-13C HMBC NMR (600 MHz, CD3OD) spectrum for asiatic acid (5) and arjunolic acid (6); Figure S18: 1H NMR (600 MHz, CD3OD) spectrum for 3b,23-dihydroxy-1-oxo-olean-12-en-28-oic acid (7); Figure S19: 1H-13C HSQC NMR (600 MHz, CD3OD) spectrum for 3b,23-dihydroxy-1-oxo-olean-12-en-28-oic acid (7); Figure S20: 1H-13C HMBC NMR (600 MHz, CD3OD) spectrum for 3b,23-dihydroxy-1-oxo-olean-12-en-28-oic acid (7); Figure S21: 1H NMR (600 MHz, CD3OD) spectrum for alphitolic acid (8); Figure S22: 1H-13C HSQC NMR (600 MHz, CD3OD) spectrum for alphitolic acid (8); Figure S23: 1H-13C HMBC NMR (600 MHz, CD3OD) spectrum for alphitolic acid (8); Figure S24: 1H NMR (600 MHz, CD3OD) spectrum for maslinic acid (9); Figure S25: 1H-13C HSQC NMR (600 MHz, CD3OD) spectrum for maslinic acid (9); Figure S26: 1H-13C HMBC NMR (600 MHz, CD3OD) spectrum for maslinic acid (9); Figure S27: 1H NMR (600 MHz, CD3OD) spectrum for corosolic acid (10); Figure S28: 1H-13C HSQC NMR (600 MHz, CD3OD) spectrum for corosolic acid (10); Figure S29: 1H-13C HMBC NMR (600 MHz, CD3OD) spectrum for corosolic acid (10); Figure S30: 1H NMR (600 MHz, CD3OD) spectrum for betulinic acid (11); Figure S31: 1H-13C HSQC NMR (600 MHz, CD3OD) spectrum for betulinic acid (11); Figure S32: 1H-13C HSQC NMR (600 MHz, CD3OD) spectrum for betulinic acid (11); Figure S33: 1H NMR (600 MHz, CD3OD) spectrum for betulonic acid (12); Figure S34: 1H-13C HSQC NMR (600 MHz, CD3OD) spectrum for betulonic acid (12); Figure S35: 1H-13C HMBC NMR (600 MHz, CD3OD) spectrum for betulonic acid (12); Figure S36: 1H NMR (600 MHz, CD3OD) spectrum for astragalin (13); Figure S37: 1H-13C HSQC NMR (600 MHz, CD3OD) spectrum for astragalin (13); Figure S38: 1H-13C HMBC NMR (600 MHz, CD3OD) spectrum for astragalin (13); Figure S39: 1H NMR (600 MHz, CD3OD) spectrum for 5-O-methyldemethoxymatteucinol (14); Figure S40: 1H-13C HSQC NMR (600 MHz, CD3OD) spectrum for 5-O-methyldemethoxymatteucinol (14); Figure S41: 1H-13C HMBC NMR (600 MHz, CD3OD) spectrum for 5-O-methyldemethoxymatteucinol (14); Figure S42: 1H NMR (600 MHz, CD3OD) spectrum for 2′,4′-dihydroxy-3′,5′-dimethyl-6′-methoxychalcone (15); Figure S43: 1H-13C HSQC NMR (600 MHz, CD3OD) spectrum for 2′,4′-dihydroxy-3′,5′-dimethyl-6′-methoxychalcone (15); Figure S44: 1H-13C HMBC NMR (600 MHz, CD3OD) spectrum for 2′,4′-dihydroxy-3′,5′-dimethyl-6′-methoxychalcone (15); Table S2: Comparison of chemical shifts (1H and 13C) with literature values; Table S1: Summary table of level 1 and 2 annotations from IIMN.
Author Contributions
Conceptualization, T.O., A.G.-B. and J.S.; formal analysis, T.O., G.H., P.C. and J.H.; investigation, T.O., G.H., P.C.R., J.H. and J.B.; resources, C.E.K., J.-L.W. and E.F.Q.; data curation, T.O., G.H. and R.M.-G.; writing—original draft preparation, T.O.; writing—review and editing, A.G.-B. and J.S.; supervision, A.G.-B.; project administration, A.G.-B. and J.S.; funding acquisition, A.G.-B., J.S. and C.E.K. All authors have read and agreed to the published version of the manuscript.
Funding
Plant collection, extraction, phytochemical investigation, and cytotoxic assays were funded by the European Regional Development Funds GURDTI 2018-1829-0002370 (FEDER PHAR, EU-Region Reunion-French State national counterpart). Antiviral assays were funded by European Regional Development Funds SYNERGIE RE0028005 (EU-Region Reunion-French State national counterpart).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All raw UHPLC-MS/MS data and the MZMine batch file used can be accessed on the MassIVE repository under the accession number MSV000095770. NMR raw data (1H, 13C, gCOSY, gHSQC, gHMBC, NOESY) of all compounds are made freely available at https://zenodo.org/doi/10.5281/zenodo.13963119 (accessed on 26 December 2024).
Acknowledgments
Many thanks to Laurent JANCI (ADPAPAM), who gave access to the piece of land in Mahavel, where the voucher was collected.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A. Yields, Chemical Characterization and References for Isolated Compounds
Psiloxylic acid (1): 4.0 mg white powder; = +15.5 (c = 0.3257 g/100mL, MeOH); HRESIMS m/z 495.3447 (calculated for C30H48O4Na+, 495.3445);
Terminolic acid + madecassic acid (2+3): 0.5 mg, white powder; ESIMS m/z 487.3 [M+H]+; [29,30]
Actinidic acid (4): 0.4 mg white powder. ESIMS m/z = 487.3 [M+H]+; [31]
Asiatic acid + arjulonic acid (5+6): 3.7 mg white powder. ESIMS m/z 489.3 [M+H]+; [32,33]
3b,23-dihydroxy-1-oxo-olean-12-en-28-oic acid (7): 0.4 mg white powder; ESIMS m/z 487.3 [M+H]+; [34]
Alphitolic acid (8): 1.0 mg white powder. ESIMS m/z 469.3 [M+H]+; [32]
Maslinic acid (9): 1.0 mg white powder; ESIMS m/z 473.3 [M+H]+; [33]
Corosolic acid (10): 1.6 mg white powder; ESIMS m/z 473.3 [M+H]+; [32]
Betulinic acid (11): 2.0 mg white powder; ESIMS m/z 439.3 [M-H2O+H]+; [32]
Betunolic acid (12): 0.5 mg white powder; ESIMS m/z 455.3 [M+H]+; [35]
Astragalin (13): 0.6 mg, white-yellowish powder; ESIMS m/z 449.1 [M+H]+; [36]
5-O-methyldemethoxymatteucinol (14): 0.4 mg yellow powder; ESIMS m/z 299.1 [M+H]+; [37]
2′,4′-dihydroxy-3′,5′-dimethyl-6′-methoxychalcone (15): 1.0 mg yellow powder; ESIMS m/z 299.1 [M+H]+; [38]
References
- Smadja, J.; Marodon, C. Le Grand Livre Des Plantes Médicinale de l’île de La Réunion Inscrites à La Pharmacopée Française (Tome 1); Editions Orphie: Saint-Denis, La Réunion, 2016; p. 232. ISBN 979-10-298-0092-4. [Google Scholar]
- Mahomoodally, M.; Korumtollee, H.; Chady, Z. Psiloxylon mauritianum (Bouton Ex Hook.f.) Baillon (Myrtaceae)-a Promising Traditional Medicinal Plant from the Mascarene Islands. J. Intercult. Ethnopharmacol. 2014, 3, 192. [Google Scholar] [CrossRef] [PubMed]
- Rangasamy, O.; Mahomoodally, F.M.; Gurib-Fakim, A.; Quetin-Leclercq, J. Two Anti-Staphylococcal Triterpenoid Acids Isolated from Psiloxylon mauritianum (Bouton Ex Hook.f.) Baillon, an Endemic Traditional Medicinal Plant of Mauritius. S. Afr. J. Bot. 2014, 93, 198–203. [Google Scholar] [CrossRef][Green Version]
- Clain, E.; Haddad, J.G.; Koishi, A.C.; Sinigaglia, L.; Rachidi, W.; Desprès, P.; NDuarte dos Santos, C.; Guiraud, P.; Jouvenet, N.; El Kalamouni, C. The Polyphenol-Rich Extract from Psiloxylon mauritianum, an Endemic Medicinal Plant from Reunion Island, Inhibits the Early Stages of Dengue and Zika Virus Infection. Int. J. Mol. Sci. 2019, 20, 1860. [Google Scholar] [CrossRef]
- Sorres, J.; André, A.; Elslande, E.V.; Stien, D.; Eparvier, V. Potent and Non-Cytotoxic Antibacterial Compounds Against Methicillin-Resistant Staphylococcus Aureus Isolated from Psiloxylon mauritianum, A Medicinal Plant from Reunion Island. Molecules 2020, 25, 3565. [Google Scholar] [CrossRef]
- Checkouri, E.; Reignier, F.; Robert-Da Silva, C.; Meilhac, O. Evaluation of Polyphenol Content and Antioxidant Capacity of Aqueous Extracts from Eight Medicinal Plants from Reunion Island: Protection against Oxidative Stress in Red Blood Cells and Preadipocytes. Antioxidants 2020, 9, 959. [Google Scholar] [CrossRef]
- Ghaddar, B.; Gence, L.; Veeren, B.; Bringart, M.; Bascands, J.-L.; Meilhac, O.; Diotel, N. Aqueous Extract of Psiloxylon mauritianum, Rich in Gallic Acid, Prevents Obesity and Associated Deleterious Effects in Zebrafish. Antioxidants 2022, 11, 1309. [Google Scholar] [CrossRef]
- Schmid, R.; Heuckeroth, S.; Korf, A.; Smirnov, A.; Myers, O.; Dyrlund, T.S.; Bushuiev, R.; Murray, K.J.; Hoffmann, N.; Lu, M.; et al. Integrative Analysis of Multimodal Mass Spectrometry Data in MZmine 3. Nat. Biotechnol. 2023, 41, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Myers, O.D.; Sumner, S.J.; Li, S.; Barnes, S.; Du, X. One Step Forward for Reducing False Positive and False Negative Compound Identifications from Mass Spectrometry Metabolomics Data: New Algorithms for Constructing Extracted Ion Chromatograms and Detecting Chromatographic Peaks. Anal. Chem. 2017, 89, 8696–8703. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Gadea, G. A Robust Method for the Rapid Generation of Recombinant Zika Virus Expressing the GFP Reporter Gene. Virology 2016, 497, 157–162. [Google Scholar] [CrossRef]
- Dührkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J.; Böcker, S. SIRIUS 4: A Rapid Tool for Turning Tandem Mass Spectra into Metabolite Structure Information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Goodacre, R.; Griffin, J.L.; Hankemeier, T.; Hardy, N.; et al. Proposed Minimum Reporting Standards for Chemical Analysis. Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Narwal, S. Exploring Chalcone Derivatives: Synthesis and Their Therapeutic Potential. J. Mol. Struct. 2024, 1303, 137554. [Google Scholar] [CrossRef]
- Huang, C.-F.; Hung, T.-W.; Yang, S.-F.; Tsai, Y.-L.; Yang, J.-T.; Lin, C.; Hsieh, Y.-H. Asiatic Acid from Centella Asiatica Exert Anti-Invasive Ability in Human Renal Cancer Cells by Modulation of ERK/P38MAPK-Mediated MMP15 Expression. Phytomedicine 2022, 100, 154036. [Google Scholar] [CrossRef]
- Sung, B.; Kang, Y.J.; Kim, D.H.; Hwang, S.Y.; Lee, Y.; Kim, M.; Yoon, J.-H.; Kim, C.M.; Chung, H.Y.; Kim, N.D. Corosolic Acid Induces Apoptotic Cell Death in HCT116 Human Colon Cancer Cells through a Caspase-Dependent Pathway. Int. J. Mol. Med. 2014, 33, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Rzeski, W.; Stepulak, A.; Szymański, M.; Sifringer, M.; Kaczor, J.; Wejksza, K.; Zdzisińska, B.; Kandefer-Szerszeń, M. Betulinic Acid Decreases Expression of Bcl-2 and Cyclin D1, Inhibits Proliferation, Migration and Induces Apoptosis in Cancer Cells. Naunyn-Schmied Arch. Pharmacol. 2006, 374, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.-B.; Ma, S.-Y.; Liu, H.-X.; Huang, C.-S.; Liao, N. Cytotoxic Triterpenoids from Roots of Actinidia chinensis. Chem. Biodivers. 2018, 15, e1700454. [Google Scholar] [CrossRef]
- Reyes-Zurita, F.J.; Rufino-Palomares, E.E.; Lupiáñez, J.A.; Cascante, M. Maslinic Acid, a Natural Triterpene from Olea Europaea L., Induces Apoptosis in HT29 Human Colon-Cancer Cells via the Mitochondrial Apoptotic Pathway. Cancer Lett. 2009, 273, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, Z.-Q.; Zhou, X.-D.; Yao, Q.-Y.; Chen, Z.-L.; Chu, L.-L.; Yu, H.-H.; Yang, Y.-P.; Li, B.; Wang, W. New Terpenoids from Potentilla Freyniana Bornm. and Their Cytotoxic Activities. Molecules 2022, 27, 3665. [Google Scholar] [CrossRef]
- Gaudry, A.; Bos, S.; Viranaicken, W.; Roche, M.; Krejbich-Trotot, P.; Gadea, G.; Desprès, P.; El-Kalamouni, C. The Flavonoid Isoquercitrin Precludes Initiation of Zika Virus Infection in Human Cells. Int. J. Mol. Sci. 2018, 19, 1093. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.; He, S.; Siragam, V.; Bi, Y.; Mbikay, M.; Chretien, M.; Qiu, X. Antiviral Activity of Quercetin-3-β-O-D-Glucoside against Zika Virus Infection. Virol. Sin. 2017, 32, 545–547. [Google Scholar] [CrossRef]
- Peyrat, L.-A.; Eparvier, V.; Eydoux, C.; Guillemot, J.-C.; Stien, D.; Litaudon, M. Chemical Diversity and Antiviral Potential in the Pantropical Diospyros Genus. Fitoterapia 2016, 112, 9–15. [Google Scholar] [CrossRef][Green Version]
- Loe, M.W.C.; Hao, E.; Chen, M.; Li, C.; Lee, R.C.H.; Zhu, I.X.Y.; Teo, Z.Y.; Chin, W.-X.; Hou, X.; Deng, J.; et al. Betulinic Acid Exhibits Antiviral Effects against Dengue Virus Infection. Antivir. Res. 2020, 184, 104954. [Google Scholar] [CrossRef]
- Baltina, L.A.; Flekhter, O.B.; Nigmatullina, L.R.; Boreko, E.I.; Pavlova, N.I.; Nikolaeva, S.N.; Savinova, O.V.; Tolstikov, G.A. Lupane Triterpenes and Derivatives with Antiviral Activity. Bioorganic Med. Chem. Lett. 2003, 13, 3549–3552. [Google Scholar] [CrossRef]
- Katz, E.; Li, J.; Jaegle, B.; Ashkenazy, H.; Abrahams, S.R.; Bagaza, C.; Holden, S.; Pires, C.J.; Angelovici, R.; Kliebenstein, D.J. Genetic Variation, Environment and Demography Intersect to Shape Arabidopsis Defense Metabolite Variation across Europe. Plant Biol. 2021, 10, e67784. [Google Scholar]
- He, Y.; Xu, D.; Li, L. Biosynthetic Mechanisms of Secondary Metabolites Promoted by the Interaction Between Endophytes and Plant Hosts. Front. Microbiol. 2022, 13, 928967. [Google Scholar]
- Li, Z.; Xiong, K.; Wen, W.; Li, L.; Xu, D. Functional Endophytes Regulating Plant Secondary Metabolism: Current Status, Prospects and Applications. Int. J. Mol. Sci. 2023, 24, 1153. [Google Scholar] [CrossRef]
- Li, X.-C.; Joshi, A.S.; ElSohly, H.N.; Khan, S.I.; Jacob, M.R.; Zhang, Z.; Khan, I.A.; Ferreira, D.; Walker, L.A.; Broedel, S.E.; et al. Fatty Acid Synthase Inhibitors from Plants: Isolation, Structure Elucidation, and SAR Studies. J. Nat. Prod. 2002, 65, 1909–1914. [Google Scholar] [CrossRef]
- Du, Q.; Jerz, G.; Chen, P.; Winterhalter, P. Preparation of Ursane Triterpenoids from Centella Asiatica Using High Speed Countercurrent Chromatography with Step-Gradient Elution. J. Liq. Chromatogr. Relat. Technol. 2004, 27, 2201–2215. [Google Scholar] [CrossRef]
- Ma, J.T.; Li, D.W.; Liu, J.K.; He, J. Advances in Research on Chemical Constituents and Their Biological Activities of the Genus Actinidia. Nat. Prod. Bioprospecting 2021, 11, 573–609. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, M.C.; Delporte, C.; Backhouse, N.; Erazo, S.; Letelier, M.E.; Cassels, B.K.; Silva, X.; Alegría, S.; Negrete, R. Topical Anti-Inflammatory Activity of 2α-Hydroxy Pentacyclic Triterpene Acids from the Leaves of Ugni Molinae. Bioorganic Med. Chem. 2006, 14, 5673–5677. [Google Scholar] [CrossRef] [PubMed]
- Rudiyansyah; Garson, M.J. Secondary Metabolites from the Wood Bark of Durio z Ibethinus and Durio k Utejensis. J. Nat. Prod. 2006, 69, 1218–1221. [Google Scholar] [CrossRef]
- Okada, Y.; Omae, A.; Okuyama, T. A New Triterpenoid Isolated from Lagerstronemia Speciosa (L.) PERS. Chem. Pharm. Bull. 2003, 51, 452–454. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, Y.; Sakai, K.; Matsunaga, S.; Tokuda, H.; Tanaka, R. Cancer Chemopreventive Effect of Orally Administrated Lupane-Type Triterpenoid on Ultraviolet Light B Induced Photocarcinogenesis of Hairless Mouse. Cancer Lett. 2006, 240, 94–101. [Google Scholar] [CrossRef]
- Guo, X.; Wang, D.; Duan, W.; Du, J.; Wang, X. Preparative Isolation and Purification of Four Flavonoids from the Petals of Nelumbo Nucifera by High-Speed Counter-Current Chromatography. Phytochem. Anal. 2010, 21, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Hufford, C.D.; Oguntimein, B.O. New Dihydrochalcones and Flavanones From Uvaria Angolensis. J. Nat. Prod. 1982, 45, 337–342. [Google Scholar] [CrossRef]
- Pavan, F.R.; Leite, C.Q.F.; Coelho, R.G.; Coutinho, I.D.; Honda, N.K.; Cardoso, C.A.L.; Vilegas, W.; Leite, S.R.d.A.; Sato, D.N. Evaluation of Anti-Mycobacterium Tuberculosis Activity of Campomanesia Adamantium (Myrtaceae). Quím. Nova 2009, 32, 1222–1226. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).