Featured Application
This paper demonstrates that a combination of sodium butyrate and selected probiotic strains represents a supportive option for managing persistent symptoms in patients with symptomatic diverticular disease, especially following antibiotic treatment. It also contributes to restoring gut microbiota balance during recovery.
Abstract
Background: Symptomatic uncomplicated diverticular disease (SUDD) is a common condition in older adults, primarily managed through symptom control. Emerging evidence highlights the role of gut microbiota in symptom modulation and disease progression. Butyrate supplementation offers anti-inflammatory benefits and supports gut barrier integrity; when combined with specific probiotic strains, it may further promote microbiota balance. Objectives: To evaluate the clinical and microbiological effects of an oral formulation combining microencapsulated sodium butyrate with probiotic strains from four probiotic strains (Lacticaseibacillus rhamnosus, Lactiplantibacillus plantarum, Limosilactobacillus reuteri, and Bifidobacterium longum subsp. infantis) in patients with SUDD. Methods: This prospective, preliminary observation enrolled 23 patients. To control for high interindividual variability in microbiota composition, each participant served as their own control. The intervention lasted 12 weeks and included five scheduled visits, incorporating a 3-week washout period. Symptom severity and quality of life were assessed using validated questionnaires. Faecal microbiota composition was evaluated using 16S rRNA sequencing and strain-specific colonisation was monitored with qPCR. Results: Significant improvements were observed in seven out of nine reported symptoms, including reductions in abdominal pain, bloating, and discomfort. Overall symptom burden decreased, especially symptoms related to gas and stool consistency. Quality of life scores improved notably. qPCR confirmed colonisation by the administered probiotic strains. Microbiome analysis demonstrated individualized but meaningful improvements in microbial profiles. Conclusions: The combined use of microencapsulated sodium butyrate and selected probiotic strains led to measurable clinical improvements and the positive modulation of gut microbiota in patients with SUDD. This formulation was well tolerated and may represent a promising adjunct or standalone approach in the dietary management of SUDD.
1. Introduction
Colonic diverticulitis is a common gastrointestinal condition, particularly affecting older adults, and represents a growing public health challenge in Western countries. It arises from the formation of colonic diverticula, sac-like protrusions of the mucosa and submucosa through the muscular layer of the colon wall. While most diverticula remain asymptomatic (diverticulosis), a subset of individuals progress to symptomatic and inflammatory stages of disease. One such condition is Symptomatic Uncomplicated Diverticular Disease (SUDD), characterized by persistent abdominal pain or discomfort in the absence of overt inflammation. This distinguishes SUDD from acute diverticulitis or Segmental Colitis Associated with Diverticulosis (SCAD), which involve mucosal damage and systemic symptoms [1,2]. Despite extensive epidemiological and clinical research, the pathophysiology of SUDD remains incompletely understood. Various contributing factors have been proposed, including dietary habits, altered colonic motility, and visceral hypersensitivity—yet no unifying mechanism has been firmly established [3]. Current treatment strategies aim to alleviate symptoms and prevent complications, typically involving a high-fibre diet, locally acting antibiotics such as rifaximin, mesalazine, and sometimes long-term probiotic supplementation. However, the recommendation level for probiotics remains low due to limited and inconsistent clinical evidence. Surgical intervention may be considered in selected cases with persistent symptoms and impaired quality of life [4,5,6].
Over the past decade, the gut microbiota has emerged as a critical factor potentially influencing the transition from asymptomatic diverticulosis to symptomatic or inflammatory phenotypes. Several studies have documented distinct alterations in both faecal and mucosal microbiota composition among patients with SUDD. For instance, Barbara et al., 2017 reported reduced levels of Clostridium clusters IV and IX (including Faecalibacterium prausnitzii) and Lactobacillaceae, alongside shifts in Enterobacteriaceae and Bacteroides/Prevotella ratios [7,8,9,10]. Akkermansia, a mucin-degrading genus with anti-inflammatory properties, has shown variable abundance depending on the sampling site [7,9]. Other researchers have documented changes in Proteobacteria diversity, Bifidobacterium levels, and even fungal profiles between inflamed and non-inflamed mucosa [11,12,13]. Kvasnovsky et al., 2018 further linked specific bacterial patterns with symptom severity in SUDD, reinforcing the role of microbiome in clinical expression [14,15].
While the 2019 International Consensus on Diverticulosis and Diverticular Disease acknowledged that probiotics may reduce symptoms in SUDD [16], other guidelines remain cautious or do not recommend their use [17,18]. Earlier symptomatic reviews failed to confirm their efficacy due to study heterogeneity and methodological limitations [19]. However, more recent assessments suggest that certain probiotics may offer benefits by modulating inflammation and restoring microbial balance [20,21]. These insights have renewed interest in investigating targeted microbial interventions.
Among such interventions, sodium butyrate, a short-chain fatty acid naturally produced through microbial fermentation of dietary fibre, has gained attention. It plays a crucial role in maintaining intestinal barrier integrity, modulating inflammation, and promoting epithelial repair [22,23]. Preclinical and clinical studies have demonstrated its therapeutic potential in inflammatory bowel conditions [24,25]. Advances in microencapsulation technologies now allow for the targeted colonic release of sodium butyrate, thereby enhancing its clinical utility [26,27]. In parallel, interactions between butyrate-producing bacteria and Bifidobacterium species suggest that co-administration may exert synergistic effects in restoring microbial homeostasis and reducing inflammation.
In this context, we designed a preliminary, exploratory study to assess the clinical and microbiological effects of an oral formulation combining microencapsulated sodium butyrate with selected probiotic strains (L. rhamnosus L. plantarum, L. reuteri, and B. longum subsp. infantis) in patients with SUDD following an episode of acute diverticulitis. The study evaluated patient-reported symptoms, stool consistency, quality of life, faecal microbiota composition, and the colonisation efficacy by the administered strains.
To account for the known high interindividual variability in gut microbiota, and in the absence of standardized dietary controls, we employed a within-subject design: each patient served as their own control. This approach allowed for personalized comparison over time and increased sensitivity in detecting individual responses to the intervention under real-life conditions. Although the study lacked a parallel control group—consistent with its pilot and feasibility-focused nature—it provided valuable first-line evidence to inform the design of larger, more structured trials. It also offered insights into refining inclusion criteria, outcome metrics, and methodological strategies for future research on microbial-based therapies in SUDD.
2. Materials and Methods
2.1. Study Design
This was a prospective, single-arm, open-label pilot study designed to evaluate the effects of a combined probiotic–butyrate product on clinical symptoms and microbiota composition in patients with Symptomatic Uncomplicated Diverticular Disease (SUDD).
The study protocol was approved by the Ethics Committee at the District Medical Chamber in Warsaw, Poland (approval no. KB/1373/21; resolution no. 58/21), and conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent prior to enrolment.
Study Plan Description
The investigated product, a commercially available formulation (Divertix, Probiome R&D, ul. dr. Jana Muszyńskiego 2, 90-151, Lodz, Poland), was used as add-on therapy to standard care. The daily dose was 300 mg of microencapsulated sodium butyrate and the following probiotic strains: Limosilactobacillus rhamnosus ATCC 53103—5 × 109 cfu, Lacticaseibacillus plantarum LMG P-21021—5 × 109 cfu, Limosilactibacillus reuterii DSM 23878—2 × 108 cfu, and Bifidobacterium longum ssp. infantis DSM 24687—2 × 108 cfu.
Each patient participated in the study for 12 weeks with 5 scheduled visits (V0–V4), including clinical assessments, questionnaires, and microbiome sampling (Table 1). At visit V1, patients received a Patient Diary with instructions on recording specific symptoms, along with a 9-week supply of the investigational product (+6 reserve portions), Figure 1. They were instructed to fill in the diary consistently throughout the study.

Table 1.
Schedule of study visits and assessments.
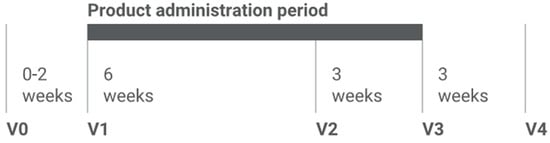
Figure 1.
Study design and intervention schedule. After the screening period (Visit 0), participants entered 9-week product administration phase (Visit 1 to Visit 3), with additional Visit 2 intended to control and increase patients` compliance, followed by a 3-week wash-out period (Visit 3 to Visit 4).
2.2. Study Population
Eligible participants were adults with SUDD experiencing persistent gastrointestinal symptoms despite standard management. All had endoscopically confirmed diverticular disease and a documented episode of diverticulitis within the past 6 months.
2.2.1. Inclusion Criteria
Participants had to meet all of the following conditions:
- Age 40–75 years;
- Endoscopic confirmation of diverticular disease;
- Episode of diverticulitis (left lower quadrant pain, fever, leukocytosis, antibiotic treatment) within the last 6 months;
- Presence of typical SUDD symptoms: abdominal pain, flatulence, and disturbed bowel movements;
- Stable background treatment with rifaximin (cyclically), antispasmodics (anticholinergics), painkillers, fiber, or mesalasine.
2.2.2. Exclusion Criteria
Subjects were excluded if they had the following:
- Any acute, uncontrolled disease;
- Chronic diseases not treated or not stable on treatment;
- Presence of inflammatory bowel diseases during exacerbation phase;
- Any treatment during last 12 months for severe, progressive, uncontrolled cardiological, pulmonary, nephrology, contagious, or psychiatric illness that could increase subject’s risk due to participation in the study;
- Present or suspected malignancy or previous oncological treatment in the last 5 years;
- Clinically significant cardiac arrhythmias observed in ECG examination or reported in history for the last 12 months;
- Any symptoms of COVID-19 infection;
- History of major colorectal surgery or severe trauma of rectum;
- Positive pregnancy test.
2.3. Assessment Parameters and Observational Objectives
Given the exploratory nature of this study, a structured set of assessment parameters was defined to guide the evaluation of potential changes in clinical symptoms and gut microbiome profiles following treatment. These parameters were categorized into primary, secondary, and exploratory objectives.
2.3.1. Primary Objective:
Change in clinical symptoms of diverticular disease assessed at Visit 3 (after 9 weeks of administration).
2.3.2. Secondary Objectives:
- Symptom changes at Visit 4 (3 weeks post treatment).
- Occurrence of diverticulitis recurrence at Visit 4.
- Change in patient-reported quality of life post intervention (Visit 3) and after washout (Visit 4).
- Detection of colonisation by at least one administered strain (L. rhamnosus, L. plantarum, L. reuteri or B. infantis) in SST2 (Visit 3).
- Microbiota composition changes (SST2 vs. SST1 and SST3 vs. SST1), including aerobe/anaerobe ratio.
2.3.3. Exploratory Objectives:
- Correlation between Wexner incontinence score and treatment.
- Correlation between symptom changes and microbiota profile (16S rRNA).
- Patient-specific correlation of microbiota changes, symptom improvement, and quality of life—toward future personalized interventions.
- Link between baseline microbiota and colonisation efficacy.
2.4. Quality of Life Assessment
Quality of life was evaluated using the Polish version of the SF-36 questionnaire(adopted from the RAND 36-Item Health Survey; https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form.html, assessed on 5 September 2021). Assessments were performed at Visit 1 (baseline), Visit 3 (after 9 weeks), and Visit 4 (3 weeks post treatment). All 23 participants completed all the assessments. Changes were analysed using Friedman’s ANOVA (α = 0.05).
2.5. Microbiome Analysis
Three stool samples per subject were collected:
- –
- SST1 (baseline, Visit 1);
- –
- SST2 (end of supplementation, Visit 3);
- –
- SST3 (post washout, Visit 4).
Samples were preserved in DNA/RNA Shield (Zymo Research, Irvine, CA, USA). Genomic DNA was extracted using the ZymoBIOMICS DNA Miniprep Kit (Zymo Research, Irvine, CA, USA), following the manufacturer’s instructions. DNA samples were stored at −80 °C until library preparation, which was performed according to the “16S Metagenomic Sequencing Library Preparation Guide” (Illumina, San Diego, CA, USA). Sequencing was conducted on the Illumina MiSeq platform (Illumina, San Diego, CA, USA) in two batches. Bioinformatic analyses were performed separately for each pool using QIIME2 (v2022.2). Quality filtering and ASV table construction were conducted with DADA2. Taxonomic classification was performed using the SILVA 138.1 reference database of full-length 16S rRNA gene sequences [28,29,30,31]. Relative abundances were analysed at phylum, family, and genus levels. Timepoint comparisons (V1 vs. V3 vs. V4) were assessed using Friedman’s ANOVA.
2.6. Probiotic Strain Colonisation
The same stool samples (SST1, SST2, SST3) were analysed for probiotic colonisation. DNA was extracted as described above. Presence of individual probiotic strains was assessed by strain-specific real-time PCR. Two primer sets were designed for each strain, based on draft genomes sequences (NGS Illumina paired-end sequencing; SPAdes assembly [32]). Primer selectivity was tested using BLAST + 2.13.0 on NCBI Bacterial Genomes database (April–July 2022). qPCR was conducted on C1000 Touch PCR thermal cycler (BioRad, Feldkirchen, Germany) with Luna® Universal qPCR Master Mix (New England Biolabs, MA, USA). The presence, absence, or uncertainty of detection was assigned depending on number of positive reactions observed (product Tm verification completed with control reaction on particular strain’s genomic DNA template). Statistical significance was assessed using the Chi-square test or Fisher’s exact test (α = 0.05).
2.7. Statistical Analysis
The statistical analyses were performed using the software package StatSoft, Inc. Statistica (data analysis software system), version 13 (www.statsoft.com, assessed on 1 February 2023). The quantitative data were summarized using descriptive statistics (mean ± SD, median, and range). The distribution of each quantitative variable was checked for consistency against the normal distribution (Shapiro–Wilk test). The qualitative data were summarized in terms of percentages. To test the efficacy of probiotic composition in supporting the treatment was checked whether there were statistically significant differences in product evaluation between visits: V1 (before starting the administration of probiotic), V3 (9 weeks after starting the treatment) and V4 (after 3 weeks from the end of the treatment). For qualitative data a chi2 test or Fisher’s exact test was used; for quantitative data, a non-parametric Friedman’s ANOVA test. The assumed statistical significance level was α = 0.05. If statistically significant differences were found between consecutive visits, it was additionally checked whether there was a difference between visits V1 and V3 and V1 and V4. Due to multiple comparisons, Bonferroni correction was used; the assumed statistical significance level in this case was 0.025. Moreover, the non-parametric Mann–Whitney test was performed to evaluate the differences between subgroups according to division of study group in view of presence of clinical symptoms of diverticular disease. Correlation analysis was conducted using the Spearman or the Pearson correlation coefficient.
Safety data were assessed in whole (safety) population, summarised by the number and percentage of subjects in each subgroup (depending on the last completed visit) reporting at least one occurrence of an AE or serious AE (SAE). Safety laboratory was presented by descriptive statistics.
2.8. Data and Material Availability
All materials, datasets, and analysis pipelines associated with this study will be made available to qualified researchers upon reasonable request. Restrictions apply to the commercial composition (Divertix), which is protected under manufacturer licensing. No restrictions apply to the statistical or microbiome analysis code. The microbiome raw data will be publicly accessible in an appropriate database upon acceptance of the manuscript.
3. Results
3.1. Patients’ Characteristics
A total of 24 patients aged 40–84 years with endoscopically confirmed diverticular disease and a documented history of diverticulitis within the preceding 6 months were enrolled. All participants reported persistent gastrointestinal symptoms such as abdominal pain, bloating, or disturbed bowel habits despite stable, guideline-based therapy for at least six weeks prior to inclusion.
Of these, one participant discontinued after Visit 3 due to an unrelated surgical intervention and was excluded from the clinical efficacy analysis. The clinically evaluable (CE) population thus included 23 patients (eighteen females [78.3%], five males [21.7%]). The mean age of the CE group was 69.3 ± 7.2 years (median: 68.5 years; range: 50.4–84.9 years).
All patients received the investigational synbiotic formulation in addition to standard therapy. One protocol deviation occurred involving age eligibility: a participant above the 75-year upper limit was included due to clinical justification. This participant completed the study without adverse events and the deviation was pre-approved and documented.
Safety assessments were conducted for all 24 enrolled patients while clinical efficacy and microbiological objectives were analysed in the CE population (n = 23).
3.2. Clinical Symptoms Assessment Based on Patient Diary
Clinical symptoms associated with diverticular disease were monitored during visits and via entries in the Patient Diary. Analysis focused on changes between baseline (Visit 1), post-treatment (Visit 3), and follow-up (Visit 4), using the Chi-square or Fisher’s exact test, with a significance threshold set at p < 0.05.
Statistically significant improvements were observed in several symptoms, including sudden rectal urgency, diarrhoea, itching/burning around the anus, abdominal discomfort, bloating, stomach ache, and palpation pain. The terms “abdominal discomfort” and “stomach ache” were intentionally recorded separately to reflect differences in how patients described pain location and character. Notably, both abdominal discomfort and stomach ache declined from nearly all patients at baseline to fewer than 30% at follow-up. In contrast, changes in constipation and intestinal peristalsis were not statistically significant. Detailed symptom trends are shown in Figure 2a–f and Figure 3a–c.
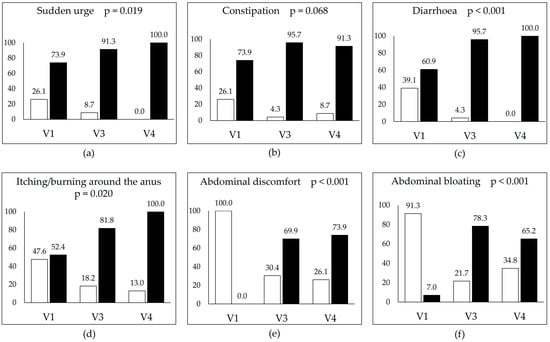
Figure 2.
Prevalence of selected gastrointestinal symptoms across the three study visits: baseline (V1), post-intervention (V3), and follow-up (V4). Each panel illustrates the percentage of participants reporting the presence (“Yes”, white bars) or absence (“No”, black bars) of the following symptoms: (a) sudden urge to defecate, (b) constipation, (c) diarrhoea, (d) itching/burning around the anus, (e) abdominal discomfort, and (f) abdominal bloating. Statistical significance (p-values) reflects the change over time. Improvements were observed in all symptoms, with the most pronounced and significant reductions being in diarrhoea, abdominal discomfort, and bloating.
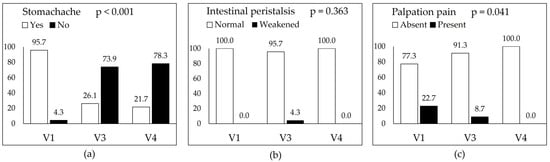
Figure 3.
Changes in selected physical examination parameters assessed during the three study visits: baseline (V1), post-intervention (V3), and follow-up (V4). Each panel presents the percentage of participants with a given clinical feature at each time point: (a) stomach ache (self-reported abdominal pain), (b) intestinal peristalsis (clinically assessed as normal or weakened), and (c) pain upon abdominal palpation. White bars indicate absence or normal status; black bars represent presence of the symptom or abnormal finding. Significant reductions over time were observed for stomach ache and palpation pain while changes in peristalsis were not statistically significant (p = 0.363).
At baseline, sudden rectal urgency was reported by six patients (26.1%). By Visit 3, only two individuals (8.7%) still experienced this symptom, and by Visit 4, it had resolved completely. The change was statistically significant (Figure 2a). Constipation was initially present in six patients (26.1%). At Visit 3, only one patient (4.3%) reported it, but by Visit 4, it returned in two cases (8.7%). Despite the favourable trend, this change was not statistically significant (Figure 2b). Diarrhoea affected 39.1% of patients at baseline. After nine weeks, only one case (4.3%) remained, and by Visit 4, the symptom had resolved entirely. This reduction was statistically significant (Figure 2c).
Itching or burning around the anus was reported by 47.6% of participants at baseline. By Visit 3, this decreased to four patients (18.2%), and at Visit 4, it further declined to three patients (13.0%). The reduction was statistically significant (Figure 2d). Abdominal discomfort was reported by all patients at baseline. By Visit 3, only seven patients (30.4%) still experienced it, and by Visit 4, six (26.1%) continued to report the symptom. The improvement was statistically significant (Figure 2e). Abdominal bloating was another common complaint, observed in 91.3% of participants at baseline. By V3, this dropped to five cases (21.7%), and at Visit 4, eight patients (34.8%) reported recurrence. Although higher than at Visit 3, this remained significantly improved compared to baseline (Figure 2f).
Stomach ache was reported by almost all patients at baseline (95.7%). By Visit 3, only six patients (26.1%), still reported it, and this further declined to five patients (21.7%) at Visit 4. This change was statistically significant (Figure 3a). Intestinal peristalsis was described as normal by all patients at baseline. At Visit 3, one participant (4.3%) reported reduced motility, but by Visit 4, normal peristalsis was again reported by all. The change was not statistically significant (Figure 3b). Pain upon palpation was noted in five patients (22.7%) at baseline. After 9 weeks (Visit 3), this decreased to two patients (8.7%), and by the follow-up visit (Visit 4), no patients reported this symptom. The improvement was statistically significant (Figure 3c).
To facilitate a broader visualisation of symptom dynamics throughout the study period, a heatmap was generated (Figure S1).
3.3. Quality of Life (SF-36)
Most of the SF-36 domains showed no statistically significant changes over the course of the study (see Supplementary Materials, Table S2a,b). The only domain with a significant difference was the item “change of general health perceptions”. This finding was supported by Friedman’s test, which indicated a statistically significant difference across visits. Post hoc analysis using the Wilcoxon paired test with Bonferroni correction (α = 0.025) confirmed this result. Median scores for general health perception increased from 50.0 at baseline (Visit 1) to 75.0 after 9 weeks (Visit 3) and subsequently returned to 50.0 at follow-up (Visit 4). The differences between V1 and V3, as well as between V1 and V4, were both statistically significant (p < 0.025; Figure 4). This suggests that participants experienced a meaningful improvement in their perceived general health during and after the intervention.
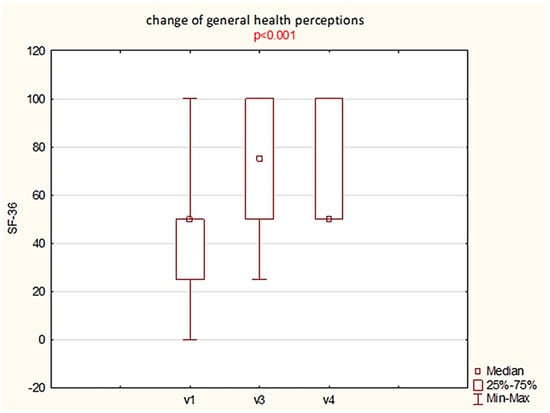
Figure 4.
Boxplot illustrating changes in the SF-36 domain “change of general health perceptions” across study visits (V1—baseline, V3—post-treatment, V4—follow-up). A statistically significant difference was observed (p < 0.001; Friedman test with Bonferroni correction).
No significant changes were observed in the remaining SF-36 domains, including physical functioning, emotional well-being, pain, social functioning, and energy/fatigue.
3.4. Microbiome Profiling
3.4.1. Relative Abundance and Microbiota Stability at Population Level
At the group level, no statistically significant differences were found in the abundances of phyla, families, or genera between Visit 1 (V1) and Visit 3 (V3), or between V1 and Visit 4 (V4) (p > 0.05 in all comparisons) (Supplementary Materials, Tables S3–S5). The overall microbiota composition changed in almost all cases (only for two patients, it remained with no change throughout whole study period; Table 2 and Table 3). Firmicutes and Bacteroidota abundances were generally better balanced after treatment. These two groups together accounted for over 90% of the total abundance in 74% of patients during Visit 1 (with four patients with B + F abundance below 83%), 82% during Visit 3 (with only one patient with B + F abundance below 87%), and again 74% of patients during Visit 4, but with only one below 87%. Generally, F/B was within the range of 0.38–5.6 during Visit 1 and tend to narrow during visit V3 and V4 (0.50–5.7 and 0.67–5.6 accordingly) but in most cases remained within the expected range (between 1.0 and 2.5). These phyla remained dominant throughout the study (Figure 5A,B). To complement the phylum-level overview, Supplementary Figure S2 provides individual graphs, separate for each patient with bar plots of the top 10 most abundant families across visits V1, V3, and V4. This figure illustrates the high interindividual variability.

Table 2.
Individual changes between visits V1 and V3 in patients’ microbiome profiles—summary. Symbols used: P—positive, N—negative, NC—no change; F + B—sum of Firmicutes and Bacteroidota abundances; F/B—ratio of Firmicutes and Bacteroidota abundances.

Table 3.
Individual changes between visits V1 and V4 in patients’ microbiome profiles—summary. Symbols used: P—positive, N—negative, NC—no change; F + B—sum of Firmicutes and Bacteroidota abundances; F/B—ratio of Firmicutes and Bacteroidota abundances.
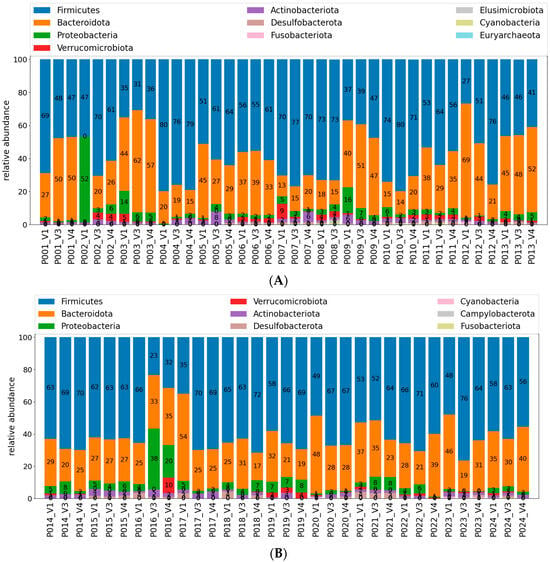
Figure 5.
(A) Relative abundance of bacterial phyla in stool samples collected across the study visits (V1, V3, and V4); patients 1–13. Each bar is depicted by patient number followed by visit number: P##_V#. Data are presented as stacked bar charts for individual participants, with each colour representing a different phylum out of 10 most frequent ones. Note patients with most manifested microbiota profiles changes: P002, P007, and P012. (B) Relative abundance of bacterial phyla in stool samples collected across the study visits (V1, V3, and V4); patients 14–24. Each bar is depicted by patient number followed by visit number: P##_V#. Data are presented as stacked bar charts for individual participants, with each colour representing a different phylum out of 9 most frequent ones. Note patients with most manifested microbiota profiles changes: P016, P021, and P23.
Individual patient profiles were further examined to assess (1) qualitative changes in microbiota composition when the four following aspects where considered: (2) increases in combined Firmicutes + Bacteroidota abundance, (3) normalisation or the F/B ratio, (4) reductions in potentially pathogenic taxa (e.g., Escherichia-Shigella, Enterobacteriaceae), and (5) increases in beneficial taxa (e.g., Bifidobacteriaceae, Lactobacillus, Akkermansiaceae).
Positive changes were observed in 13 of 23 patients both between V1 and V3, and between V1 and V4. In several cases, improvements involved reductions in potentially harmful genera alongside the appearance of beneficial groups. Otherwise, an increase in Bacteroides + Firmicutes abundance or gaining better balance in the B/F ratio were the rationale for judging the change as “positive”. Full summaries are presented in Table 2 and Table 3.
3.4.2. Intestinal Colonisation by Probiotic Strains
Detection rates for all four probiotic strains increased during supplementation, peaking at Visit 3 (Table 4 ). For L. rhamnosus ATCC 53103, its presence was confirmed in 95.7% of patients at Visit 3, compared to 26.1% at baseline. After discontinuation, levels dropped to 21.7%, while 43.5% had no detectable presence. L. plantarum LMG P-21021 was detected in all patients at V3, and persistent in 52.2% at V4, and L. reuteri DSM 23878—initially undetected—appeared in 43.5% of patients at V3 but persisted in only one individual (4.3%) at V4. In turn, B. infantis DSM 2,687 rose from 8.7% at baseline to 65.2% at V3, decreasing to 21.7% post treatment.

Table 4.
Detection rates of the four administered probiotic strains (L. rhamnosus ATCC 53103, L. plantarum LMG P-21021, L. reuteri DSM 23878, and B. infantis DSM 24687) in stool samples collected during the study. Data are presented as percentages of patients with confirmed presence, absence, or uncertain detection of each strain at baseline (V1), end of treatment (V3), and follow-up (V4).
These findings confirm temporary intestinal colonisation by all strains, with L. plantarum LMG P-21021 showing the highest persistence.
3.4.3. Association Between Microbiota and Clinical Symptoms
A statistically significant association was found between diarrhoea and higher F/B ratio at baseline (p = 0.030; Mann–Whitney U test). Patients with diarrhoea had notably elevated F/B ratios, suggesting a possible link between microbial imbalance and symptom expression (Figure 6).
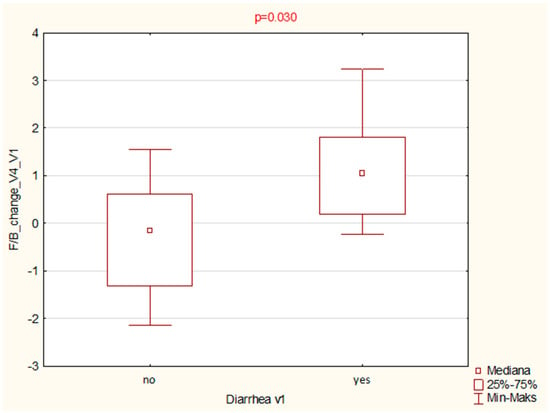
Figure 6.
Boxplot comparison of Firmicutes/Bacteroidota (F/B) ratios at baseline (V1) between patients reporting diarrhoea and those without this symptom. A statistically significant difference was observed (p = 0.030; Mann–Whitney U test).
3.4.4. Correlation Between Microbiota Changes, Symptoms, and Quality of Life
The analysis explored whether microbiota changes were associated with symptom severity or quality of life. Only one significant correlation was observed: a moderate negative relationship (Spearman’s rho = –0.554) between the increase in aerobic genera (V4 vs. V1) and pain intensity. Patients were also grouped by the presence or absence of diarrhoea, but no significant differences were found in SF-36 domains between these subgroups (Mann–Whitney U test).
3.4.5. Colonisation and Baseline Microbiota Composition
Using the Mann–Whitney U test, several associations were identified between successful colonisation and baseline taxa (Table 5). For example, successful colonisation with L. rhamnosus ATCC 53103 was significantly associated with the baseline abundance of Streptococcaceae and Roseburia at V1, as well as of Phascolarctobacterium and Acidaminococcaceae at V4. In the case of L. plantarum LMG P-21021, significant associations were observed with the families Veillonellaceae and [Ruminococcus]_torques_group. The presence of L. reuteri DSM 23878 at V3 correlated with the presence of Enterobacteriaceae, Clostridia_UCG-014, and Escherichia-Shigella. For Bifidobacterium infantis, relevant associations were found with several taxa from the Lachnospiraceae and Ruminococcaceae families, as well as with Acidaminococcaceae, Phascolarctobacterium, and Erysipelotrichaceae.

Table 5.
Associations between the presence or absence of specific probiotic strains at subsequent visits and the baseline gut microbiota composition (visit V1). Statistically significant relationships were identified using the Mann–Whitney U test (α = 0.05). Only bacterial taxa with p-values < 0.05 are reported.
3.5. Safety Evaluation
3.5.1. Adverse Events
All adverse events (AEs) were recorded from consent to the final visit (V4). Nine of twenty-four patients reported AEs, most of which were mild and did not require discontinuation. One patient withdrew due to surgery for pre-existing haemorrhoidal disease. No serious adverse events (SAEs) occurred. A detailed summary is provided in Supplementary Table S6. The product was well tolerated.
3.5.2. Blood Test
No statistically significant changes were observed in blood parameters across visits. Platelet counts approached significance (p = 0.051), suggesting a possible trend. Full results are shown in Supplementary Table S7.
3.5.3. Inflammatory Markers
No significant changes in CRP levels were observed during the study (p > 0.05).
4. Discussion
4.1. A Synergistic, Multi-Targeted Approach to SUDD
This study evaluated the impact of daily supplementation with microencapsulated sodium butyrate and four probiotic strains in patients with SUDD who remained symptomatic despite standard treatment. The intervention aimed to support microbiota balance and improve gut barrier function. Butyrate was microencapsulated for targeted colonic delivery [26] while probiotic strains were selected for their anti-inflammatory and microbiota-modulating properties, as recommended by the WGO guidelines (2017) [www.worldgastroenterology.org/UserFiles/file/guidelines/probiotics-and-prebiotics-english-2017.pdf, assessed on 1 September 2022)].
After 9 weeks, significant improvement was observed in seven of nine core gastrointestinal symptoms, including pain, bloating, urgency, and anorectal discomfort. Some effects persisted or improved further during follow-up. Improved Wexner scores suggested the enhanced control of gas and liquid stool, pointing to added value in managing distal bowel dysfunction often overlooked in SUDD.
In order to maintain clarity, the discussion provides only a brief summary of earlier studies and concentrates primarily on the novel aspects of our results. While prior studies supported the potential of combining probiotics and butyrate, the results remained variable. Probiotics alone have shown inconsistent efficacy in SUDD. A 2016 systematic review of 11 trials concluded that the evidence base remains limited [19]. In a 2017 RCT of 143 SUDD patients, Kvasnovsky et al. found no significant difference in pain reduction between multi-strain probiotics and placebo [33]. High interindividual variability in symptoms and microbiota likely contributed to this result. Our within-subject design, in which each patient served as their own control, was chosen specifically to address this variability under real-life conditions.
In other studies, Tursi et al., 2013 reported improved outcomes with a combination of Lactobacillus casei DG and mesalazine versus monotherapy [34] while L. paracasei combined with a high-fibre diet outperformed fibre alone in reducing bloating and pain [35,36]. In acute uncomplicated diverticulitis, Ojetti et al., 2022 demonstrated significant pain and inflammatory marker reduction with L. reuteri ATCC PTA 4659 [37].
Butyrate also shows promise. In a randomised study, Krokowicz et al., 2014 found that 12-month supplementation with 300 mg/day microencapsulated sodium butyrate significantly reduced diverticulitis episodes and improved symptom relief [38]. These findings were reinforced by a retrospective study by Tursi et al., 2025, which demonstrated a significant reduction in abdominal pain intensity in SUDD patients receiving microencapsulated sodium butyrate [39]. Improvements were evident by day 45 and continued through day 90. Notably, pain reduction occurred without significant changes in stool form (Bristol score), indicating that the effect is likely independent of motility and more related to mucosal or anti-inflammatory mechanisms [39]. Preliminary data, including ours, suggest additive effects from combining probiotics and butyrate. This is mechanistically plausible: probiotics may improve bloating and bowel habits while butyrate targets inflammation and visceral hypersensitivity. Although no SUDD-specific trials exist to date, a randomized study by Gąsiorowska et al., 2024 in IBS patients found that a synbiotic containing microencapsulated sodium butyrate (300 mg) and multi-strain probiotics resulted in significantly greater global symptom relief compared to placebo (64.7% vs. 42.0%, p = 0.023) despite no difference in pain severity or quality of life [40]. Together, these data support the rationale for combined approaches in symptom modulation. Our findings extend these observations by documenting simultaneous symptom relief, the temporary colonisation of all administered strains, and individualized microbiota shifts.
Quality of life (QoL) is a key outcome in chronic diverticular disease, as persistent pain and bowel dysfunction markedly impair daily functioning [4,41,42]. In our study, the use of the 36-item SF-36 questionnaire proved partly suboptimal as elderly patients often found it exhausting, which may explain the lack of statistically significant findings. Still, general health perception improved significantly and in parallel with symptom relief. The available data suggest that QoL benefits are more consistently observed with butyrate than with probiotics. Krokowicz et al., 2014 reported that microencapsulated sodium butyrate reduced diverticulitis incidence and improved symptom relief, implying better quality of life [38]. In contrast, probiotic interventions in SUDD rarely show clear QoL gains, likely due to limited impact on pain. For example, the multi-strain probiotic used in a chronic SUDD trial did not improve pain or QoL vs. placebo [33]. Reviews and guidelines up to 2019 confirmed that evidence for QoL improvement with probiotics in diverticular disease remains insufficient [43]. Taken together, this pattern suggests that probiotics and butyrate may act through complementary mechanisms, with probiotics primarily improving bloating and bowel habits and butyrate targeting inflammation and visceral hypersensitivity. The combined intervention tested here may therefore offer broader clinical benefits than either component alone.
4.2. Gut Microbiota Modulation and Combined Therapeutic Strategy
Microbiota modulation is increasingly viewed as a valid strategy in SUDD. Probiotics can suppress pathogens and support beneficial taxa [44] while sodium butyrate nourishes colonocytes, lowers pH, and promotes anaerobes [45] in addition to inhibiting NF-κB-driven inflammation [46]. This study assessed microbiota changes via 16S rRNA sequencing at the phylum, family, and genus levels. No significant shifts in overall diversity were observed after 9 weeks or post washout (Tables S3–S5), likely due to the small sample size and individual variability. However, individual analysis revealed improvements in thirteen of twenty-three patients (Table 3), with seven maintaining changes after washout and six improving only afterward. Two patients showed no changes; in two, dysbiosis persisted (Table 4), suggesting potential for personalized microbiota remodelling despite absence of group-level significance. Few studies have examined microbiota composition in diverticular disease, most focusing on diagnostic profiles [7,13,14,47]. Our findings contribute new evidence on microbiota shifts after dietary intervention. We also observed a significant difference in the Firmicutes/Bacteroidetes ratio between patients with and without diarrhoea. Although this ratio is debated in diverticular disease [12,47,48], it has been linked to dysbiosis and GI disorders [8,33] and may reflect symptom-related microbial patterns. Our results are consistent with previous reports of reduced diversity and loss of beneficial taxa in diverticular disease [49], with earlier studies showing microbiota modulation by rifaximin or Lactobacillus strains [33], and normalisation following sodium butyrate treatment [39]. Prior data also connect higher Ruminococcus and lower Roseburia abundance with bloating [14], calprotectin levels with Lactobacillus, and pain with Cyanobacterium. Other studies noted the enrichment of Akkermansia, Barnesiella, and Coriobacteria in diverticulitis [15] and depletion of Clostridium clusters IV and IX with the loss of SCFA producers like propionate-producing taxa [7,50]. Although no consistent group-level effects were seen, individual shifts support a synergistic model: probiotics contribute functional traits while butyrate enhances the local environment. Interindividual variability highlights the complexity of host–microbiota dynamics and suggests potential for targeted microbiota-based therapies. In our study, more than half of the patients showed favourable microbiota changes, with several persisting beyond the supplementation period. This indicates that the intervention was able to trigger not only short-term compositional shifts but also more stable ecological effects in some individuals. These microbial changes occurred alongside clinical improvements, including reductions in pain, bloating, and urgency, and better self-reported health status. The parallel improvement in symptoms and microbiota supports the view that the observed effects were not merely coincidental. Although the sample size was limited, the within-subject design allowed us to capture the course of these changes and provides a proof of concept that combined butyrate–probiotic therapy can influence both symptoms and microbiota in SUDD. Further studies should explore whether baseline microbial profiles predict treatment response, which could guide a more personalized application of synbiotics in this setting.
4.3. Colonisation Capacity
The ability of probiotic strains to colonize the colon is therapeutically relevant. Most strains are transient—they survive gastrointestinal transit, exert effects during supplementation, and decline after discontinuation [51,52]. This transient presence still holds value in diverticular disease, where it may help suppress pathogenic bacteria in diverticula and support mucosal balance [52,53,54]. In our study, all four administered strains were detectable during supplementation, with L. plantarum showing the highest persistence—greater than typically reported in SUDD monotherapy [53,54]. This aligns with data showing that Lactobacillus and Bifidobacterium strains are present during intake but rarely persist afterward [52,55,56] and that sustained effects often require continued or repeated dosing [57,58,59,60]. Sodium butyrate may have contributed to this favourable colonisation profile. While direct evidence is limited, its effects on epithelial integrity, inflammatory tone, and mucin stability [45,46,60] may indirectly support probiotic engraftment. This fits the synbiotic concept, where postbiotics enhance probiotic efficacy. In an IBS trial, fructooligosaccharides promoted the colonisation of administered strains and native butyrate producers, improving outcomes [40]. We also observed 16 significant correlations between the presence of probiotic strains and baseline microbiota taxa, suggesting that the individual microbiota composition may affect colonisation success. This supports the link between microbial imbalance and mucosal immune activation described in recent studies on SUDD pathophysiology [7,55]. Beyond confirming the transient presence of the administered strains, our results suggest that colonisation capacity in SUDD is not uniform but depends on the existing microbial landscape. This individualized engraftment pattern has not been reported previously in this patient group and provides a novel perspective on why synbiotic interventions may produce heterogeneous outcomes. Importantly, the observation that certain strains persisted longer than expected, particularly L. plantarum, indicates that butyrate may play a supportive role in promoting colonisation. Together, these findings point to the possibility of stratifying patients by baseline microbiota to improve the predictability and clinical impact of future synbiotic therapies.
5. Conclusions
This exploratory study provided early evidence that a synbiotic formulation—combining microencapsulated sodium butyrate with four well-characterized probiotic strains (Lactobacillus rhamnosus GG, Lactobacillus plantarum, Lactobacillus reuteri, and Bifidobacterium infantis)—may offer a safe and effective option for managing SUDD.
Despite the small sample size and exploratory design, the intervention led to notable symptom improvements, especially in abdominal pain, bloating, and urgency. It was well tolerated. Post-treatment microbiological analysis confirmed transient but effective colonisation, and individual microbiota profiles showed meaningful, positive shifts. These findings justify further research, ideally through larger, controlled trials, to assess the long-term clinical utility of microbiome-targeted therapies in SUDD.
6. Patents
The product used in this study, Divertix, is the subject of a patent application: PCT/PL2024/050077.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15189942/s1, Table S1. Summary of selected laboratory parameters assessed in the study groups. The table includes results of the blood tests, haematological parameters, and key biochemical markers such as sodium, potassium, creatinine, urea, and C-reactive protein (CRP). Table S2. (a) Descriptive statistics (mean ± SD and median [min–max]) for six SF-36 domains related to physical functioning, emotional limitations, and fatigue across all study visits (V1, V3, and V4, n = 23). (b) Descriptive statistics (mean ± SD and median [min–max]) for additional SF-36 domains including emotional well-being, social functioning, pain, general health, and change in general health perception over the course of the study (n = 23, V1, V3, and V4). Table S3–S5. Significance testing for changes in the abundances of all represented phyla, selected families, and selected genera between visits V1 and V3 or V4. Table S6. Summary of adverse events reported during the study. Events are described by type, severity, relationship to the investigational product, and whether they affected study completion. No adverse events were assessed as related to the intervention. Table S7. Laboratory test results across study visits (V1—baseline, V3—post-treatment, V4—follow-up). Data are presented as mean ± SD and median (min–max). Parameters assessed include haematocrit, platelet count, haemoglobin, electrolytes, renal function markers, and C-reactive protein (CRP). Figure S1. Heatmap illustrating changes in symptom prevalence across study visits (V1—baseline, V3—post-treatment, V4—follow-up). Figure S2. Relative abundance of top 10 the most numerous group on the level of family for each patient individually. Panels a-z present bar plots generated for samples from Visit 1 (bar “before”), Visit 3 (bar “after”), and Visit 4 (bar “washout”) (with exception for panel h, where data for patient withdrawn from the study are presented).
Author Contributions
Conceptualisation, K.B., K.K., and A.K.; methodology, K.B. and K.K.; bioinformatic analysis, K.K.; validation, K.B., K.K., and M.S.; formal analysis, M.S., K.K., and K.A.C.; investigation, K.B.; resources, K.B.; data curation, K.K. and M.S.; writing—original draft preparation, K.A.C.; writing—review and editing, K.A.C. and K.B.; visualisation, K.A.C. and K.K.; supervision, K.B. and A.K.; project administration, K.B.; funding acquisition, A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding (Probiome sp. z o. o. was the only sponsor).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Independent Ethics Committee of the Regional Medical Chamber in Warsaw (resolution of the IEC No. 58/21 approved 16 December 2021) for studies involving humans.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All materials, datasets, and analysis pipelines associated with this study will be made available to qualified researchers upon reasonable request.
Acknowledgments
The authors would like to thank Magdalena Jander for her invaluable inspiration, enthusiasm, and vision, which were instrumental in initiating and guiding this project. We are also grateful to Mariusz Cięciara for his consistent support, motivation, and dedication to ensuring that the study stayed on course and met its objectives. The authors acknowledge the assistance of ChatGPT (OpenAI, GPT-5, 2025) in the preparation of this manuscript. ChatGPT was used to support linguistic editing and grammar refinement and to assist in the generation of illustrative materials and formatting. The AI model did not participate in the analysis, interpretation of results, or formulation of scientific conclusions. All data analysis, study design, and final interpretations were conducted exclusively by the authors.
Conflicts of Interest
Adam Kiciak is affiliated with Probiome sp. z o. o., which contributed to the development of the tested formulation. The study was designed and conducted independently, and the company did not influence the analysis or interpretation of the results. The remaining authors declare that they have no relevant financial or non-financial interests to disclose.
Abbreviations
The following abbreviations have been used in this manuscript:
| SUDD | Symptomatic Uncomplicated Diverticular Disease |
| PCR | Polymerase Chain Reaction |
| SST | Stool Sample Timepoint |
| SCAD | Segmental Colitis Associated with Diverticulosis |
| ECG | Electrocardiogram |
| ASV | Amplicon Sequence Variant |
| AE | Adverse Event |
| SAE | Serious Adverse Event |
| CE | Clinical Evaluation |
| CRP | C-Reactive Protein |
| VAS | Visual Analogue Scale |
| QoL | Quality of Life |
| IBS | Irritable Bowel Syndrome |
References
- Krogsgaard, L.R.; Lyngesen, M.; Bytzer, P. Systematic review: Quality of trials on the symptomatic effects of the low FODMAP diet for irritable bowel syndrome. Aliment. Pharmacol. Ther. 2017, 45, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- Peery, A.F.; Keku, T.O.; Galanko, J.A.; Sandler, R.S. Colonic diverticulosis is not associated with painful abdominal symptoms in a US population. Gastro Hep Adv. 2022, 1, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Violi, A.; Cambiè, G.; Miraglia, C.; Barchi, A.; Nouvenne, A.; Capasso, M.; Leandro, G.; Meschi, T.; de’ Angelis, G.L.; Di Mario, F. Epidemiology and Risk Factors for Diverticular Disease. Acta Bio Medica Atenei Parm. 2018, 89, 107–112. [Google Scholar] [CrossRef]
- Comparato, G.; Fanigliulo, L.; Aragona, G.; Cavestro, G.M.; Cavallaro, L.G.; Leandro, G.; Pilotto, A.; Nervi, G.; Soliani, P.; Sianesi, M.; et al. Quality of Life in Uncomplicated Symptomatic Diverticular Disease: Is It Another Good Reason for Treatment? Dig. Dis. 2007, 25, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, B.M.; Reid, M.W.; Bolus, R.; Whitman, C.B.; Talley, J.; Dea, S.; Shahedi, K.; Karsan, H.; Teal, C.; Melmed, G.Y.; et al. Development and validation of a disease-targeted quality of life instrument for chronic diverticular disease: The DV-QOL. Qual. Life Res. 2015, 24, 163–179. [Google Scholar] [CrossRef]
- Tursi, A.; Scarpignato, C.; Strate, L.L.; Lanas, A.; Kruis, W.; Lahat, A.; Danese, S. Colonic diverticular disease. Nat. Rev. Dis. Primers 2020, 6, 20. [Google Scholar] [CrossRef]
- Barbara, G.; Scaioli, E.; Barbaro, M.R.; Biagi, E.; Laghi, L.; Cremon, C.; Marasco, G.; Colecchia, A.; Picone, G.; Salfi, N.; et al. Gut Microbiota, Metabolome and Immune Signatures in Patients with Uncomplicated Diverticular Disease. Gut 2017, 66, 1252–1261. [Google Scholar] [CrossRef]
- Maccaferri, S.; Candela, M.; Turroni, S.; Centanni, M.; Severgnini, M.; Consolandi, C.; Cavina, P.; Brigidi, P. IBS-Associated Phylogenetic Unbalances of the Intestinal Microbiota Are Not Reverted by Probiotic Supplementation. Gut Microbes 2012, 3, 406–413. [Google Scholar] [CrossRef]
- Tursi, A.; Mastromarino, P.; Capobianco, D.; Elisei, W.; Miccheli, A.; Capuani, G.; Tomassini, A.; Campagna, G.; Picchio, M.; Giorgetti, G.; et al. Assessment of Fecal Microbiota and Fecal Metabolome in Symptomatic Uncomplicated Diverticular Disease of the Colon. J. Clin. Gastroenterol. 2016, 50, S9–S12. [Google Scholar] [CrossRef]
- Linninge, C.; Roth, B.; Erlanson-Albertsson, C.; Molin, G.; Toth, E.; Ohlsson, B. Abundance of Enterobacteriaceae in the Colon Mucosa in Diverticular Disease. World J. Gastrointest. Pathophysiol. 2018, 9, 18–27. [Google Scholar] [CrossRef]
- Gueimonde, M. Qualitative and Quantitative Analyses of the Bifidobacterial Microbiota in the Colonic Mucosa of Patients with Colorectal Cancer, Diverticulitis and Inflammatory Bowel Disease. World J. Gastroenterol. 2007, 13, 3985. [Google Scholar] [CrossRef]
- Daniels, L.; Budding, A.E.; de Korte, N.; Eck, A.; Bogaards, J.A.; Stockmann, H.B.; Consten, E.C.; Savelkoul, P.H.; Boermeester, M.A. Fecal Microbiome Analysis as a Diagnostic Test for Diverticulitis. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1927–1936. [Google Scholar] [CrossRef]
- Schieffer, K.M.; Sabey, K.; Wright, J.R.; Toole, D.R.; Drucker, R.; Tokarev, V.; Harris, L.R.; Deiling, S.; Eshelman, M.A.; Hegarty, J.P.; et al. The Microbial Ecosystem Distinguishes Chronically Diseased Tissue from Adjacent Tissue in the Sigmoid Colon of Chronic, Recurrent Diverticulitis Patients. Sci. Rep. 2017, 7, 8467. [Google Scholar] [CrossRef]
- Kvasnovsky, C.L.; Leong, L.E.X.; Choo, J.M.; Abell, G.C.J.; Papagrigoriadis, S.; Bruce, K.D.; Rogers, G.B. Clinical and Symptom Scores Are Significantly Correlated with Fecal Microbiota Features in Patients with Symptomatic Uncomplicated Diverticular Disease: A Pilot Study. Eur. J. Gastroenterol. Hepatol. 2018, 30, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Hullar, M.A.J.; Sandstrom, R.S.; Stamatoyannopoulos, J.A.; Lampe, J.W.; Strate, L.L. The Fecal Microbiome in Diverticulitis and Asymptomatic Diverticulosis: A Case-Control Study in the US. medRxiv 2019. [Google Scholar] [CrossRef]
- Tursi, A.; Brandimarte, G.; Di Mario, F.; Lanas, A.; Scarpignato, C.; Bafutto, M.; Walker, M. International consensus on diverticulosis and diverticular disease. Statements from the 3rd international symposium on diverticular disease. J. Gastrointest. Liver Dis. 2019, 28, 57–66. [Google Scholar] [CrossRef]
- Hall, J.; Hardiman, K.; Lee, S.; Lightner, A.; Stocchi, L.; Paquette, I.M.; Feingold, D.L. The American Society of Colon and Rectal Surgeons clinical practice guidelines for the treatment of left-sided colonic diverticulitis. Dis. Colon Rectum 2020, 63, 728–747. [Google Scholar] [CrossRef]
- Cuomo, R.; Barbara, G.; Pace, F.; Annese, V.; Bassotti, G.; Binda, G.A.; Annibale, B. Italian consensus conference for colonic diverticulosis and diverticular disease. United Eur. Gastroenterol. J. 2014, 2, 413–442. [Google Scholar] [CrossRef] [PubMed]
- Lahner, E.; Bellisario, C.; Hassan, C.; Zullo, A.; Esposito, G.; Annibale, B. Probiotics in the Treatment of Diverticular Disease. A Systematic Review. J. Gastrointest. Liver Dis. 2016, 25, 79–86. [Google Scholar] [CrossRef]
- Binda, G.A.; Cuomo, R.; Laghi, A.; Nascimbeni, R.; Serventi, A.; Bellini, D.; Gervaz, P.; Annibale, B. Practice parameters for the treatment of colonic diverticular disease: Italian Society of Colon and Rectal Surgery (SICCR) guidelines. Tech. Coloproctology 2015, 19, 615–626. [Google Scholar] [CrossRef]
- Calini, G.; Abd El Aziz, M.A.; Paolini, L.; Abdalla, S.; Rottoli, M.; Mari, G.; Larson, D.W. Symptomatic uncomplicated diverticular disease (SUDD): Practical guidance and challenges for clinical management. Clin. Exp. Gastroenterol. 2023, 16, 29–43. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The Role of Short-Chain Fatty Acids in Health and Disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Hodgkinson, K.; El Abbar, F.; Dobranowski, P.; Manoogian, J.; Butcher, J.; Figeys, D.; Mack, D.; Stintzi, A. Butyrate’s role in human health and the current progress towards its clinical application to treat gastrointestinal disease. Clin. Nutr. 2023, 42, 61–75. [Google Scholar] [CrossRef]
- Deleu, S.; Machiels, K.; Raes, J.; Verbeke, K.; Vermeire, S. Short chain fatty acids and its producing organisms: An overlooked therapy for IBD? eBioMedicine 2021, 66, 103293. [Google Scholar] [CrossRef]
- Facchin, S.; Vitulo, N.; Calgaro, M.; Buda, A.; Romualdi, C.; Pohl, D.; Perini, B.; Lorenzon, G.; Marinelli, C.; D’Incà, R.; et al. Microbiota changes induced by microencapsulated sodium butyrate in patients with inflammatory bowel disease. Neurogastroenterol. Motil. 2020, 32, e13914. [Google Scholar] [CrossRef]
- Banasiewicz, T. Badanie porównujące profil uwalniania się w jelicie aktualnie dostępnych na rynku polskim produktów zawierających maślan sodu. Med. Faktów 2017, 4, 37. [Google Scholar]
- Kaehler, B.D.; Bokulich, N.A.; Caporaso, J.G.; Huttley, G.A. Species-level microbial sequence classification is improved by source-environment information. bioRxiv 2018. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Robeson, M.S.; O’Rourke, D.R.; Kaehler, B.D.; Ziemski, M.; Dillon, M.R.; Foster, J.T.; Bokulich, N.A. RESCRIPt: Reproducible sequence taxonomy reference database management. PLOS Comput. Biol. 2021, 17, e1009581. [Google Scholar] [CrossRef]
- Prjibelski, A.; Antipov, D.; Meleshko, D.; Lapidus, A.; Korobeynikov, A. Using SPAdes de novo assembler. Curr. Prot. Bioinf. 2020, 70, e102. [Google Scholar] [CrossRef] [PubMed]
- Kvasnovsky, C.L.; Bjarnason, I.; Donaldson, A.N.; Sherwood, R.A.; Papagrigoriadis, S. A randomized double-blind placebo-controlled trial of a multi-strain probiotic in treatment of symptomatic uncomplicated diverticular disease. Inflammopharmacology 2017, 25, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Tursi, A.; Brandimarte, G.; Giorgetti, G.M.; Elisei, W. Mesalazine and/or Lactobacillus casei in preventing recurrence of symptomatic uncomplicated diverticular disease of the colon: A prospective, randomized, open-label study. J. Clin. Gastroenterol. 2006, 40, 312–316. [Google Scholar] [CrossRef]
- Lahner, E. High-Fibre Diet and Lactobacillus Paracasei B21060 in Symptomatic Uncomplicated Diverticular Disease. World J. Gastroenterol. 2012, 18, 5918. [Google Scholar] [CrossRef]
- Annibale, B.; Maconi, G.; Lahner, E.; De Giorgi, F.; Cuomo, R. Efficacy of Lactobacillus Paracasei Sub. Paracasei F19 on Abdominal Symptoms in Patients with Symptomatic Uncomplicated Diverticular Disease: A Pilot Study. Minerva Gastroenterol. Dietol. 2011, 57, 13–22. [Google Scholar] [PubMed]
- Ojetti, V.; Saviano, A.; Brigida, M.; Petruzziello, C.; Caronna, M.; Gayani, G.; Franceschi, F. Randomized control trial on the efficacy of Limosilactobacillus reuteri ATCC PTA 4659 in reducing inflammatory markers in acute uncomplicated diverticulitis. Eur. J. Gastroenterol. Hepatol. 2022, 34, 496–502. [Google Scholar] [CrossRef]
- Krokowicz, L.; Stojcev, Z.; Kaczmarek, B.F.; Kociemba, W.; Kaczmarek, E.; Walkowiak, J.; Krokowicz, P.; Drews, M.; Banasiewicz, T. Microencapsulated Sodium Butyrate Administered to Patients with Diverticulosis Decreases Incidence of Diverticulitis—A Prospective Randomized Study. Int. J. Colorectal Dis. 2014, 29, 387–393. [Google Scholar] [CrossRef]
- Tursi, A.; Procaccianti, G.; De Bastiani, R.; Turroni, S.; D’Amico, F.; Allegretta, L.; Antonino, N.; Baldi, E.; Casamassima, C.; Casella, G.; et al. Micro-encapsulated and colonic-release sodium butyrate modulates gut microbiota and improves abdominal pain in patients with symptomatic uncomplicated diverticular disease. Front. Med. 2025, 12, 1487892. [Google Scholar] [CrossRef]
- Gąsiorowska, A.; Romanowski, M.; Walecka-Kapica, E.; Kaczka, A.; Chojnacki, C.; Padysz, M.; Siedlecka, M.; Banasik, J.; Sobolewska-Włodarczyk, A.; Wiśniewska-Jarosińska, M.; et al. Efficacy and Safety of a Mixture of Microencapsulated Sodium Butyrate, Probiotics, and Short Chain Fructooligosaccharides in Patients with Irritable Bowel Syndrome—A Randomized, Double-Blind, Placebo-Controlled Study. J. Clin. Med. 2025, 14, 6. [Google Scholar] [CrossRef]
- Bolster, L.T.; Papagrigoriadis, S. Diverticular disease has an impact on quality of life—results of a preliminary study. Colorectal Dis. 2003, 5, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Canani, R.B.; Costanzo, M.D.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011, 17, 1519–1528. [Google Scholar] [CrossRef]
- Scarpignato, C.; Barbara, G.; Lanas, A.; Strate, L.L. Management of colonic diverticular disease in the third millennium: Highlights from a symposium held during the United European Gastroenterology Week. Ther. Adv. Gastroenterol. 2018, 11, 1756284818771305. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, A.C.; Salminen, S.; Isolauri, E. Probiotics: An overview of beneficial effects. Antonie Van Leeuwenhoek 2002, 82, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Geirnaert, A.; Calatayud, M.; Grootaert, C.; Laukens, D.; Devriese, S.; Smagghe, G.; Vos, M.D.; Boon, N.; Wiele, T.V. Butyrate-producing bacteria supplemented in vitro to Crohn’s disease patient microbiota increased butyrate production and enhanced intestinal epithelial barrier integrity. Sci. Rep. 2017, 7, 11450. [Google Scholar] [CrossRef]
- Chen, G.; Ran, X.; Li, B.; Li, Y.; He, D.; Huang, B.; Fu, S.; Liu, J.; Wang, W. Sodium butyrate inhibits inflammation and maintains epithelium barrier integrity in a TNBS-induced inflammatory bowel disease mice model. eBioMedicine 2018, 30, 317–325. [Google Scholar] [CrossRef]
- Lopetuso, L.R.; Petito, V.; Graziani, C.; Schiavoni, E.; Paroni Sterbini, F.; Poscia, A.; Gaetani, E.; Franceschi, F.; Cammarota, G.; Sanguinetti, M.; et al. Gut Microbiota in Health, Diverticular Disease, Irritable Bowel Syndrome, and Inflammatory Bowel Diseases: Time for Microbial Marker of Gastrointestinal Disorders. Dig. Dis. 2018, 36, 56–65. [Google Scholar] [CrossRef]
- van Rossen, T.M.; Ooijevaar, R.E.; Kuyvenhoven, J.P.; Eck, A.; Bril, H.; Buijsman, R.; Boermeester, M.A.; Stockmann, H.B.A.C.; de Korte, N.; Budding, A.E. Microbiota Composition and Mucosal Immunity in Patients with Asymptomatic Diverticulosis and Controls. PLoS ONE 2021, 16, e0256657. [Google Scholar] [CrossRef]
- Tursi, A.; Papa, V.; Lopetuso, L.R.; Settanni, C.R.; Gasbarrini, A.; Papa, A. Microbiota composition in diverticular disease: Implications for therapy. Int. J. Mol. Sci. 2022, 23, 14799. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Louis, P.; Duncan, S.H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 577–589. [Google Scholar] [CrossRef]
- Zmora, N.; Zilberman-Schapira, G.; Suez, J.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Elinav, E. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell 2018, 174, 1388–1405. [Google Scholar] [CrossRef]
- Maldonado-Gómez, M.X.; Martínez, I.; Bottacini, F.; O’Callaghan, A.; Ventura, M.; van Sinderen, D.; Walter, J. Stable engraftment of Bifidobacterium longum AH1206 in the human gut depends on individualized features of the resident microbiome. Cell Host Microbe 2016, 20, 515–526. [Google Scholar] [CrossRef]
- Tursi, A.; Papa, A. The role of gut microbiota in the pathogenesis of diverticular disease: Where are we now? Genome Med. 2024, 16, 153. [Google Scholar] [CrossRef]
- Lamiki, P.; Tsuchiya, J.; Pathak, S.; Okura, R.; Solimene, U.; Jain, S.; Kawakita, S.; Marotta, F. Probiotics in Diverticular Disease of the Colon: An Open Label Study. J. Gastrointest. Liver Dis. 2010, 19, 31–36. [Google Scholar]
- Tursi, A.; Turroni, S.; De Bastiani, R.; Procaccianti, G.; D’Amico, F.; Allegretta, L.; Picchio, M. Gut microbiota in symptomatic uncomplicated diverticular disease stratifies by severity of abdominal pain. Europ. J. Gastroenterol. Hepatol. 2025, 37, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Mining Lactobacillus and Bifidobacterium for organisms with long-term gut colonization potential. Clin. Nutr. 2020, 39, 1315–1323. [Google Scholar] [CrossRef]
- Zucko, J.; Starcevic, A.; Diminic, J.; Oros, D.; Mortazavian, A.M.; Putnik, P. Probiotic–friend or foe? Curr. Opin. Food Sci. 2020, 32, 45–49. [Google Scholar] [CrossRef]
- Kumar, K.; Sharma, R. Role of Probiotics in Treatment of Gut-Related Diseases. Prebiotics Probiotics Dis. Regul. Manag. 2022, 1–26. [Google Scholar] [CrossRef]
- Marlicz, W.; Skonieczna-Żydecka, K.; Krynicka, P.; Łoniewski, I.; Rydzewska, G. Probiotics in irritable bowel syndrome—Is the quest for the right strain over? Rapid review of existing guidelines and recommendations. Gastroenterol. Rev. Przegląd Gastroenterol. 2021, 16, 369–382. [Google Scholar] [CrossRef]
- Głąbień, M.; Miłkowski, P.; Kuśnierz, A.; Kusiak, K.; Aleksandrowicz, D.; Wieczorek, O.; KoSndratowicz, A. Sodium Butyrate as Gut Microbiota Modulators: Mechanisms of Action and Potential Clinical Applications-Literature Review and New Perspectives. Qual. Sport 2024, 27, 55233. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).