Abstract
The quality and freshness of fruits and vegetables are critical factors in consumer acceptance and are significantly affected during transport and storage. This study aimed to evaluate the quality of greenhouse-grown tomatoes stored for 24 days by combining non-destructive image analysis, spectrophotometric assays (including total phenolic content and antioxidant and antiradical activity assessments), and attenuated total reflectance–Fourier transform infrared (ATR-FTIR) spectroscopy. Additionally, water activity, moisture content, total soluble solids, texture, and color were evaluated. Most physicochemical changes occurred between days 14 and 17, without major impact on overall fruit quality. A progressive transition in peel hue from orange to dark orange, and increased surface irregularity of their textural image were noted. Moreover, the combined use of instrumental and image analyses results via multivariate analysis allowed the clear discrimination of tomatoes according to storage days. In this sense, tomato samples were effectively classified by ATR-FTIR spectral bands, linked to carotenoids, phenolics, and polysaccharides. Machine learning (ML) models, including Random Forest and Gradient Boosting, were trained on image-derived features and accurately predicted shelf life and quality traits, achieving R2 values exceeding 0.9. The findings demonstrate the effectiveness of combining imaging, spectroscopy, and ML for non-invasive tomato quality monitoring and support the development of predictive tools to improve postharvest handling and reduce food waste.
1. Introduction
Tomato (Solanum lycopersicum L.) is the second most important vegetable crop after potato. According to the Food and Agriculture Organization (FAO, 2023) [1], Asia accounts for about 64% of world production. About 190 million tonnes of tomatoes are produced annually, led by China, followed by India and Turkey (https://www.fao.org/home/en/) (accessed on 26 December 2024) [1]. Greece ranks 24th with an annual production of 909,470 tonnes. Although the tomato is classified as a vegetable, it is botanically a fruit, as it consists of seeds and the surrounding parts, the ovary, and the flowering plant. It belongs to the Solanaceae family, is classified as a climacteric fruit, and its origin remains unclear. It is rumored to originate from the Andean region, i.e., several Latin American countries such as Chile, Colombia, and Peru [2]. The cultivated tomato was probably introduced from Mexico to Spain in the 16th century, following the discovery and conquest of America by the Spanish, and spread from there to the rest of Europe [3].
Tomatoes are an important source of minerals, vitamins, essential amino acids, carbohydrates, and fiber. According to the literature, tomato consumption can prevent the development of pathological conditions and lower the likelihood of heart diseases and different types of cancer [4,5,6], due to its high concentration of antioxidant compounds such as vitamin C, vitamin E, phenolic compounds, and carotenoids [5,7]. As for the tomato peel, it is highly nutritious because, in addition to antioxidants such as phenolic acids, ascorbic acid, and lycopene, it contains essential fatty acids and several minerals such as kalium, calcium, and magnesium [8].
Fruit and vegetable producers, food retailers, scientists, and consumers are increasingly interested in the scientific, nutritional, and economic aspects of the post-harvest management of fruit and vegetables to maintain or improve their quality, delay spoilage, and prevent microbial contamination. As tomatoes are a perishable fruit, postharvest losses remain a major issue in global food supply chains, often resulting in substantial economic and nutritional waste due to spoilage during storage and distribution [9,10]. Developing reliable and rapid tools to monitor tomato quality in real time is essential for mitigating these losses.
Numerous scientific studies have already documented the effect of a number of post-harvest manipulations on extending the preservability and improving the quality of tomato fruit, including the storage conditions (temperature, humidity, and duration) [9,11,12,13,14,15,16,17,18,19], the transport distance and duration [9], the ripening or maturation stage of the fruit during the harvest [12,14,19,20,21,22,23], the use of edible films as packaging material [24,25,26,27,28], the presence or absence of suitable packaging [16,29], the post-harvest processing approaches [30,31,32,33,34] and more. However, most of these studies focus on individual techniques or assess only specific quality parameters, limiting their ability to provide a complete picture of tomato quality dynamics over time. Moreover, destructive methods commonly used are often time-consuming and not suitable for real-time or continuous monitoring.
In addition to the above studies, the development of a methodology to evaluate different post-harvest treatments of fruit and vegetables is of particular interest, offering the possibility of predicting their storability in combination with their quality, in order to select the best practices [35,36]. This study addresses these gaps by integrating non-destructive image analysis and ATR-FTIR spectroscopy with traditional spectrophotometric and physicochemical assays, combined with machine learning techniques for predictive modeling. Therefore, the study aims to develop a methodology that correlates quality attributes, such as physicochemical parameters, chemical profile, antioxidant activity, etc., of hydroponically grown tomatoes of the Brioso variety with their external appearance, in order to predict their storage time and preservation. Specifically, the quality of tomatoes throughout storage was evaluated using both non-destructive and destructive techniques, such as image analysis, ATR-FTIR spectroscopy, physicochemical measurements, and spectrophotometric analysis, coupled with statistical methods, in particular discriminant and regression analysis. Finally, despite previous studies highlighting examples of postharvest tomato handling and assessment practices, a more systematic/holistic approach is required that evaluates both surface and internal quality characteristics over time. In the present study, image analysis, spectrophotometric techniques, and ATR-FTIR spectroscopy were combined with machine learning algorithms to comprehensively assess tomato quality during storage in a comprehensive manner and to develop predictive models for estimating This approach builds on existing research by integrating multiple analytical techniques and statistical tools to enhance the monitoring and forecasting of tomato quality under realistic storage conditions.
2. Materials and Methods
2.1. Tomato Sampling
Fruits were harvested on day 0 (1 April 2024) in Xanthi and provided by ‘Thrace Greenhouses’ (https://www.thracegreenhouses.com/ (accessed on 6 October 2024)). The tomatoes harvested were fully ripe and showed no signs of physical or physiological damage. The samples were monoculture mini tomatoes of the Brioso variety, which are grown in bunches under hydroponic conditions, and distributed in packaged form (Figure 1). The packaging is perforated, consists of a simple cardboard and plastic cover, and is designed to extend tomatoes shelf life. In particular, the fruit is protected from any mechanical damage, is not stressed, contamination from other foods is avoided, and exposure to ethylene is limited, delaying ripening [37]. At the same time, the fruit continues to breathe, and moisture transfer could be reduced. The transportation of the tomato samples to the laboratory was completed on day 2, and twenty bunches of eight tomatoes each, with no visual defects, were placed in laboratory incubators (POL-EKO Cooled incubator ST 3, POL-EKO-APARATURA), with controlled temperature and relative humidity, until the end of the experimental procedure. The conservation temperature of the samples was set at 15 ± 0.5 °C to simulate supermarket conditions, while the relative humidity was set at 60 ± 2%, as commonly used in postharvest research and supermarket settings [9,29], and according to the supplier’s suggestion. Six (6) scheduled analyses were carried out every few days, namely on days 3, 7, 10, 14, 17, and 24, and the whole procedure from the time of harvest and thereafter took a total of 24 days. The frequency of experiments is every 3 and 4 days for the first 5 time points. The last time point was chosen 7 days later in order to cover a longer storage period. On each scheduled day of the experiment, random samples were taken from the tomato bunches, and each analysis was performed on ten individual tomato samples. The selection is considered adequate for replicability, as all fruits originated from the same harvest day, shared identical growing and storage conditions, and belonged to the same variety.

Figure 1.
Packaging of tomato samples.
2.2. Image Analysis of the Surfaces of Tomato Peels
For image analysis of tomato samples, an already published methodology of our group was followed [38]. Briefly, three color parameters (L*, a*, and b*) were derived from the colored images, while fifteen textural features were extracted from the grayscale versions. These features were used to analyze the color and textural changes in tomatoes throughout the study. To extract the relevant features, a series of regions of interest (ROIs) (Figure 2) were selected from each image of all samples around the tomato stalk region, and saved as 21 × 21 pixels color images in BMP format, using the approach previously described by Ladika et al. (2024) [36]. The software’s design involved the use of the Python programming language and employing image processing functions from the scikit-image image processing library (https://scikit-image.org/ (accessed on 9 December 2024)). The colored version of the stored ROIs was used to produce the L*a*b* features, while the grayscale version of the ROIs was used to generate the textural features.
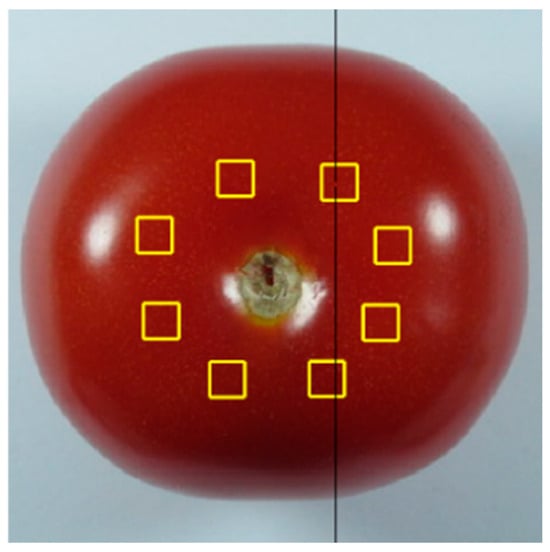
Figure 2.
Extraction of ROIs from the color image of a tomato sample, indicated by the yellow boxes, for textural features calculation.
2.3. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR) of the Outer Surfaces of Tomato Peels
ATR-FTIR spectroscopy was conducted according to the method reported by Christodoulou et al. (2024) [35]. Also, the spectra for each sample and the background were recorded and pre-processed according to the same publication [35].
2.4. Physicochemical Measurements of Tomato Samples
The moisture content was determined by measuring the weight loss of the tomato flesh after drying it in a vacuum oven at 70 °C for 24 h under reduced pressure [39].
Water activity, as well as total soluble solids (TSS), were measured as described by Giannakourou et al. [40].
A tristimulus chromatometer (CR-400, Minolta, Tokyo, Japan) was used to measure the color parameter hue (h) angle in degrees (position of a color on a color wheel), in peel and flesh of the tomato samples. A typical white plate was used for calibration (L*: 97.83, a*: −0.45, b*: +1.88).
A TA-XTplusC texture analyzer (Stable Micro Systems, Godalming, UK) was utilized to measure the firmness of tomatoes during storage. Two 20 mm thick slices were taken from each tomato sample and subjected to texture profile analysis (TPA), causing a double-repeated compression to simulate the chewing process. The texture sensor used was a 6 mm diameter cylindrical compression probe. The penetration depth was selected after preliminary experiments and according to the literature data and was set to 1/3 of the diameter of each sample, while the descent speed, penetration speed, and ascent speed were set to 10, 5, and 10 mm/s, respectively, for all samples [41].
For all experiments, replicates of each measurement were applied using ten individual tomato fruits.
2.5. Extraction of Phenolic Compounds and Spectrophotometric Assays in Phenolic Extracts
For the extraction of phenolic compounds, approximately 3 g of tomato flesh was mixed with 80% v/v aqueous methanol at a 1:10 (w/v) mass-to-volume ratio. The mixture was kept in sealed flasks at 20 °C for 24 h. Following extraction, the solution was filtered using a Buchner funnel and diluted with 80% v/v aqueous methanol. Total phenolic content (TPC), antiradical activity (ABTS), and ferric reducing antioxidant power (FRAP) of the tomato flesh extracts were measured using standard spectrophotometric methods. TPC was assessed using the Folin–Ciocalteu assay, ABTS activity was expressed in Trolox equivalents, and FRAP was calculated based on Fe (II) equivalents. All analyses were performed in triplicate using established protocols [35,36,42].
2.6. Statistical Analysis and Regression Models
The Mann–Whitney–Wilcoxon non-parametric test was used to check the differences in the features of images of tomatoes stored for different lengths of time, applying the Python scipy.stats library (https://docs.scipy.org/doc/scipy/tutorial/stats.html) accessed on 11 December 2024.
In addition, SPSS (IBM SPSS Statistics, version 29.0, Chicago, IL, USA) was used to analyze the results of ATR-FTIR spectroscopy and physicochemical measurements in tomato samples and of spectrophotometric assays in phenolic extracts. The analysis was conducted at a significance level of p < 0.05, via one-way ANOVA and post hoc analysis.
Furthermore, regression analysis was conducted using image-derived features, employing the Random Forest Regressor to predict the storage duration of tomatoes and the Gradient Boosting Regressor to estimate physicochemical properties such as moisture, firmness, and antioxidant/antiradical activity. To develop the regression models, 70% of the dataset was randomly allocated for training, while the remaining 30% was used for validation. Various feature combinations were tested, with a maximum of six features (representing 10% of the dataset size) included in each model. The approach ensured that the model with the highest predictive accuracy, evaluated through the R2 score, was selected.
2.7. Discriminant Analysis
To discriminate tomatoes of different storage days, the features extracted from the tomato images, the spectrophotometric data, the physicochemical attributes, and the ATR-FTIR spectroscopy were subjected to ML methods. Ten (10) different machine learning classifiers from the scikit-learn Python library (https://scikit-learn.org/, accessed on 11 December 2024) were tested on the six (6) categories corresponding to days 3, 7, 10, 14, 17, and 24. The ten machine learning algorithms tested in the study were: Classification and Regression Decision-Tree (CART), K-Nearest Neighbor (KNN), Linear Discriminant Analysis (LDA), Logistic Regression (LogReg), Nearest Centroid, Perceptron, Naïve Bayesian, Random Forest (RF), Support Vector Machines (SVM), and ExtraTreesClassifier. According to the detailed explanation provided by Sinanoglou et al. (2023), the model with the highest accuracy in correctly assigning the tomatoes to their respective storage days was selected [38]. The CART (Classification and Regression Tree) classifier gave the best performance on our data. The CART classifier was designed using all possible feature combinations up to a maximum of three features (1/3 of the minimum class size). Principal component analysis (PCA) was used to reduce each combination into two components, designated PCA1 and PCA2. Two-dimensional PCA scatterplots were used to demonstrate the discriminant analysis results, which were evaluated using the CART machine learning classifier and the ‘K-Fold’ cross-validation function from Python’s Scikit-Learn module. Class-separation surfaces were used in the plots to highlight the differences between different classes. The dataset was divided into K subsets for K-fold cross-validation. The model was then trained and evaluated five times (K = 5), with a different subset used as the test set each time, while the remaining subsets were used for training.
3. Results and Discussion
3.1. Characterization of Tomato During Storage Using Textural-Image Analysis of the Outer Surfaces of Tomato Peels
Image analysis is a non-destructive, accurate, and effective method of assessing the freshness and appearance of fruits and vegetables, improving their quality and optimizing storage conditions [35,36,38]. To assess the effect of storage time and conditions on tomato samples, textural features extracted from colored and grayscale images of the outer surface of the tomato peels were statistically evaluated. The features with significantly changed values from different storage days were selected. The changes in textural features during the 24-day storage period are shown in Figure 3.
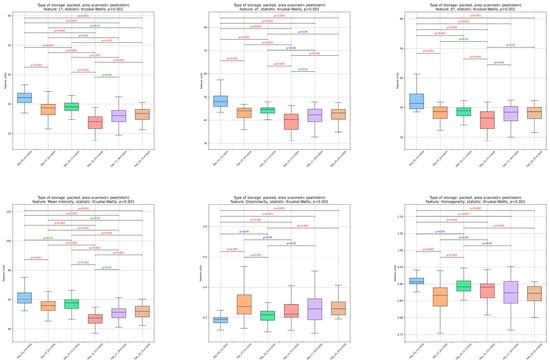
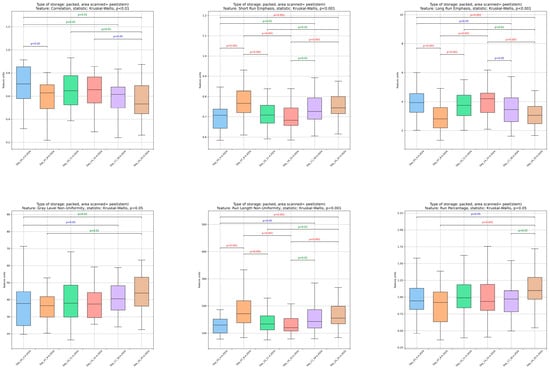
Figure 3.
Changes in image analysis-derived features (L*, a*, b*, mean, dissimilarity, homogeneity, correlation, short run emphasis (SRE), long run emphasis (LRE), grey level non-uniformity (GLN), run length non-uniformity (RLN), and run percentage (RP)) in tomato peels over storage days 3, 7, 10, 14, 17, and 24.
The L* (lightness), a* (red-green), and b* (yellow-blue) parameters, extracted from the tomato peel images, significantly (p < 0.05) decreased until day 14, followed by an increase until day 17, after which they remained stable until the end of storage. The hue angle (h) values (from day 3 to day 24: 43.39 ± 0.80a, 43.71 ± 0.74a, 44.07 ± 0.67a, 41.57 ± 0.50b, 41.87 ± 0.78b, 41.80 ± 0.77b) exhibited a significant (p < 0.05) reduction on day 14, stabilizing thereafter. When comparing the initial and final storage days, a significant (p < 0.05) decrease in lightness was observed, accompanied by a gradual color shift from orange to dark orange. In accordance with these results, Al-Dairi et al. (2021) reported a significant reduction in tomato lightness during a 12-day storage period at 10 and 22 °C [9]. Color changes in tomato peel can be attributed to many factors, such as the ripening stage of the tomato, changes in moisture content, oxygen levels, respiration rate, ethylene production and transpiration rate, storage temperature and duration, relative levels of lycopene, beta-carotene and chlorophyll and their differentiation during storage, accumulation of flavonoids pigments, and the interactions among them [14,24,29,34,43,44,45,46].
Research indicated that maintaining storage temperatures near 15 °C, along with the use of fruit packaging or coatings, reduces ethylene production, physiological respiration, and transpiration in tomatoes. This process also slows down chlorophyll degradation and carotenoid synthesis [29,44,47], which in turn delays the progression of tomato maturity.
By the end of the storage (Figure 3), a significant (p < 0.05) decline was noted in mean (average pixel intensity), homogeneity (the consistency of pixel values), correlation (the relationship between gray levels), and long run emphasis (the dominance of extended sequences of similar intensity pixels). Conversely, dissimilarity (variations in texture), short run emphasis (short sequences of similar intensity pixels), gray level non-uniformity (differences in gray level distribution), run length non-uniformity (RLN), variability in the lengths of intensity runs), and run percentage (RP), the overall distribution of runs) showed a significant (p < 0.05) increase toward the end of storage. Notably, there was a distinct and reversible variation in textural features on day 7, likely due to short-term redistribution of water inside the fruit peel or a delayed reaction of the fruit to storage conditions [48]. The most pronounced changes in most features were detected between days 14 and 17 of storage. The results showed a deterioration in the textural structural integrity and an increase in the inhomogeneity of the tomato peel image, which became more irregular and fragmented after 17 days of storage. Comparable effects of texture deterioration and increased peel dissimilarity during storage have been documented for a variety of vegetables and fruits, such as bananas, strawberries, and cucumbers [35,36,38,49,50,51], and are also associated with a reduction in fruit appearance.
In a further step, discriminant analysis was applied to determine the appropriate groupings of the textural features to cluster the tomato samples based on different days of storage. One of the most indicative scatter diagrams describes, with an accuracy of 90.8%, the discrimination of tomato peel samples among storage days 3, 14, and 24, through texture characteristics, mean, skewness (pixel intensities’ asymmetry within a picture), short run emphasis (SRE), L*, and a* (Figure 4). The first cluster of tomato peels from day 3 (blue circles) occupies the left region of the diagram, the second cluster of tomato peels from day 14 (green squares) extends across the lower-right region, while the third cluster of tomato peels from day 24 (red triangles) covers the upper-central region. Out of 40 samples, 38 samples from the 3rd day, 36 samples from the 14th day, and 35 samples from the 24th day were correctly classified by the machine learning CART (Classification and Regression Tree) classifier.
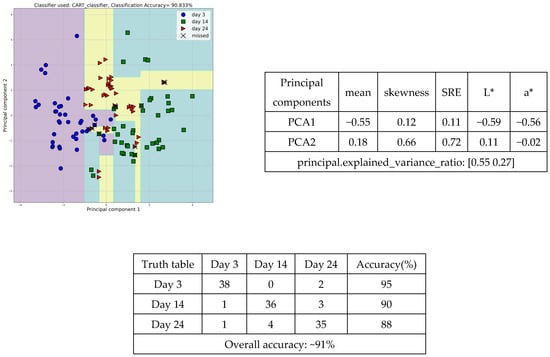
Figure 4.
Scatter diagram, principal component, and truth tables of the tomato peel samples discrimination, employing the textural features, among days 3, 14, and 24.
3.2. Interpretation of ATR-FTIR Spectra from Tomato Peels
The ATR-FTIR spectra absorption bands detected on the outer surfaces of tomato peels during storage (as shown in Table 1 and Figure 5) were in the 4000–499 cm−1 spectral range, and their study led to several important discoveries. The band observed at 3356 cm−1, which is linked to the stretch of O–H found in water, organic acids, carbohydrates, and phenols [38,52], showed a significant (p < 0.05) increase from day 10 to day 14, and then stabilized until the end of the storage period. The band at 1602 cm−1, associated with the bending vibrations of the hydroxyl groups, and correlated to the moisture content [53] of the tomatoes’ peels, showed insignificant variations during the storage period. The bands observed at 2922 and 2854 cm−1, presented the asymmetric and symmetric stretch of C(sp3)-H in methylene present in triglycerides, carboxylic and fatty acids, carbohydrates, free amino acids, waxes, and especially the waxy polymer cutin [36,52,54], demonstrated a consistent (p < 0.05) decrease from day 17 and day 14, respectively, which continued until the end of storage. In full agreement with the above findings, the absorbance at 1460 cm−1, ascribed to the bending vibrations of methylene groups [55,56] in cutin, lipids, carbohydrates, and proteins, showed a meaningful (p < 0.05) decrease from day 14 to 17 of the storage period. In accordance with the above findings, it has been reported that during the gradual ripening of tomatoes in storage, the biopolymers such as cutin and polysaccharides such as pectin—which constitute the cell walls—are depolymerized or converted into more water-soluble structures, which results in the fruit softening [57,58,59].

Table 1.
Mean intensities of the ATR-FTIR spectral bands of tomato peels during storage.
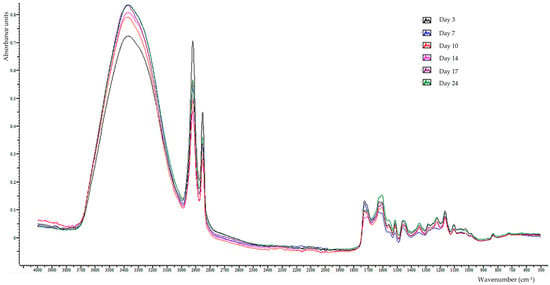
Figure 5.
An overlay of each storage day’s representative ATR–FTIR spectra of tomato peels.
The band at 1726 cm−1, associated with the stretch of C=O in cutin, triglycerides, polysaccharides, and phenolic esters [35,54,55], exhibited a decrease (p < 0.05) on both day 7 and day 17. In contrast, the band at 1708 cm−1, related to the stretching of C=O in carboxylic and fatty acids [55], first appeared on day 10 of storage and showed stable absorption until the end of storage. The absorbance at 1624 cm−1, which is related to the stretch of cis-vinylene in carotenoids or in the (C–C) ring of pectin or the amide I band [54,60], exhibited a progressive and substantial (p < 0.05) decrease until the 10th day and then disappeared. Absorbances at 1558 and 1512 cm−1, which are attributed to the C=C–C stretch in phenolic compounds’ aromatic ring [55], revealed a noteworthy (p < 0.05) increase on the 7th day of storage and then the absorbance of the first band remained constant, while that of the second band decreased significantly on the 17th day of storage and returned to its initial value. Interestingly, the absorption intensities of the band at 833–831 cm−1, assigned to the C–H bend of the para-substituted aromatic rings [55], showed exactly the same changes as the absorption at 1512 cm−1. The band at 550–542 cm−1 (ring torsion of the phenyl group) [56] presented insignificant variations during storage.
The absorbance at 1344 cm−1, related to the C–H bending vibration of polysaccharides and cellulose ring [56] exhibited an increase (p < 0.05) on the 7th day of storage and subsequently remained stable till the end of the storage duration. The band at 1280 cm−1, attributed to the amide III band due to the combined C–N stretch and N–H and O=C–N bend in peptides and proteins [56], or to O–H in-plane bending in primary or secondary hydroxyl compounds (cutin and hydroxy fatty acids) [55], showed a gradual and marked (p < 0.05) increase on the 7th and 10th day.
The band at 1220 cm−1, which is ascribed to the C–O stretch of carbohydrates, phenolics, and steroidal alkaloids and their glycosylated forms [31,38], presented a significant (p < 0.05) increase on the 7th and 10th day of storage. The absorptions at 1165 and 1105 cm−1, due to the C–O and C–C stretches of the glycosidic linkage in polysaccharides and pectin and C–O stretching vibrations of monosaccharides [61,62], showed no significant variations except for the decrease in intensity at 1165 cm−1 from day 17 of storage. The bands at 1056–1050 and 1028–1022 cm−1 are connected to the C–O bending and stretching vibration of the carbohydrate’s C–OH groups, including pectin substances, glucose, and fructose [43,56]. The increase (p < 0.05) in intensity at 1056–1050 cm−1 on day 7 could be linked to pectin hydrolysis into simple sugars. In view of the above results, it is reported [9] that tomato storage at 10 and 24 °C leads to carbohydrate conversion, biosynthesis, and hydrolysis to simple sugars. Furthermore, the decrease (p < 0.05) in intensities at 1165, 1056–1050, and 1028–1022 cm−1 at day 17 or 24 could be attributed to the consumption of total sugars by respiration during storage [29].
The absorptions at 970 and 721 cm−1, linked to trans- and cis-C–H out-of-plane bending vibrations of the carotenoids, respectively [55], showed a gradual and significant (p < 0.05) increase until day 17, and then remained constant or decreased until the end of storage, respectively. The carotenoid content of tomatoes, especially lycopene and beta-carotene, increased with increasing storage time and was significantly influenced by storage conditions (temperature and light presence) and the ripening stage of tomato fruits [9,24]. In addition, Choi et al. (2010) reported that lycopene content in tomato peel significantly decreased in overripe tomatoes [23].
Finally, the bands at 630–623 and 518–514 cm−1 related to the O–H out-of-plane and to the glycosidic linkage (C–O–C) in-plane bending of pectin, respectively [38], displayed insignificant changes during storage.
Additionally, the tomatoes were grouped according to the different storage days using the intensities of the spectral bands at 721, 1165, and 1624 cm−1, achieving a discrimination accuracy of 100.0%. The scatter diagram (Figure 6) shows, the correct classification of all samples on days 3 (blue circles), 7 (green squares), 10 (red triangles), 14 (pine green pentagons), 17 (purple triangles), and 24 (yellow pentagons). All samples were correctly classified by the machine learning CART classifier.
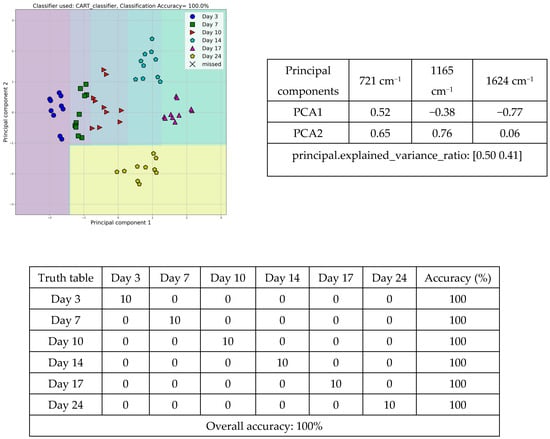
Figure 6.
Scatter diagram, principal component, and truth tables of the discrimination, among tomato peel samples at days 3, 7, 10, 14, 17, and 24, employing the intensities of the ATR-FTIR spectral bands.
3.3. Physicochemical Parameters of Tomato Flesh During Storage
The physicochemical parameters of the tomatoes, such as water activity, moisture content, total soluble solids (Brix), firmness (N), and hue angle (h) were evaluated and are presented in Table 2. These parameters were measured 6 times during 24 days of storage at 15 ± 0.5 °C and relative humidity of 60 ± 2%.

Table 2.
Water activity, moisture content, brix, firmness, and hue angle (h) of tomato flesh over storage of 3, 7, 10, 14, 17, and 24 days.
Water activity demonstrated a significant (p < 0.05) decrease at day 10, stabilization until day 17, and then increased until the end of the storage period, maintaining a significantly higher value at the end of storage. Moisture content (%) showed a significant increase and decrease on days 17 and 24, respectively, remaining statistically higher at the end of the storage period. According to the literature data, the high relative humidity at 60 ± 2% in the tomato samples’ preservation oven could be responsible for the lack of moisture loss and the slight but noticeable increase in both moisture and water activity during storage [9,46]. Moreover, the packaging material could prevent moisture loss by limiting water dehydration [9,29], increasing the storability of the fresh vegetables and fruits. In addition, Breda et al. (2017) reported that water loss from fruits and vegetables leads to undesirable metabolic changes in cells and activation of enzymatic processes, accelerating ageing and reducing nutritional value [48].
The level of total soluble solids (TSS) is indicative of the taste quality of the fruit and is representative of the quantity of soluble minerals and sugars existing in the fresh fruit [63]. The TSS of tomatoes are strongly influenced by storage temperature and moisture content [9,64]. Total soluble solids of tomatoes increased (p < 0.05) between days 3 and 7 of storage and decreased (p < 0.05) between days 7 and 10. Then, TSS increased (p < 0.05) again from day 10 to 14, and significantly declined from day 14 to day 17. After that, there was no longer any discernible statistically significant difference. Consistent with our results, Tigist et al. (2013) described that the maximum TSS content of fresh tomato varieties was recorded on day 16 of storage under ambient temperature, after which a decreasing trend in solids was observed for all varieties [64]. The increase in TSS during storage is mainly due to polysaccharides (hemicellulose and pectin) conversion to oligosaccharides and simple sugars [9,65], whereas the reduction may be linked to the use of total sugars through respiration during the storage period [29]. Furthermore, the TSS results fully confirm the results of ATR-FTIR spectroscopy.
Tomato firmness is a crucial quality trait that affects consumer acceptance and is influenced by ripening at the harvest stage, storage temperature and duration, transpiration-induced moisture loss, and enzymatic changes [63]. At the fourteenth day, the firmness, which represents the greatest force needed to chew the tomato flesh, showed a substantial (p < 0.05) decrease before stabilizing until the end of storage. The degradation of polysaccharides in the fruit flesh and the rise in moisture content may be the causes of tomato fruit softening during storage [66]. Additionally, ethylene is a critical factor in the ripening and ageing of tomato, regulating enzyme activity and causing several physiological and biochemical changes, including aroma generation and softening [64,67]. Pila et al. (2010) claim that the increased sensitivity of the fruit during storage is caused by the metabolism of cell wall carbohydrates, which is responsible for the breakdown and weakening of the cell walls and the firmness decrease [68].
The color parameter h angle of the tomato flesh samples showed an increase (p < 0.05) from day 17 to 24. All the previous days, the changes that occurred were not statistically significant. Based on the results of our research team’s previous study on cucumbers [35], it appears that variations in moisture content caused corresponding variations in hue angle values. Moreover, similarly to our findings, Tadesse et al. (2015) pinpointed that, in comparison to fruits kept at chilling temperature (4 °C), those held at higher temperatures maintained their distinctive hue [45].
Many factors, including maturity stage, variety, seasonal conditions, agricultural practices, light, temperature, geographical origin, and post-harvest treatment, have a notable impact on total phenolic content [68,69,70]. The TPC, antioxidant, and antiradical activity of the extracts from the tomato flesh samples, as well as their changes during storage, are listed in Table 3. The total phenolic content results of this study are in line with those of other researchers [68,71,72]. The predominant phenolic compounds in tomatoes are flavonoids and hydroxycinnamic acids, especially caffeic and chlorogenic acids [5,31,73,74]. On days 10 and 14, the TPC of tomato pulp increased gradually and significantly (p < 0.05), and from day 17 it decreased (p < 0.05) until the end of storage. On days 14 and 17, antiradical activity increased (p < 0.05) and then decreased on day 24. Until the tenth day, the antioxidant activity fluctuated negligibly. It then decreased (p < 0.05) on day 14, and a relatively constant level was maintained until the end of the storage period. A strong positive correlation (R2 = 0.8188, p < 0.001) was found among TPC and antiradical activity, while TPC and antioxidant activity and antiradical and antioxidant activity also showed high correlations (R2 = 0.7355, p < 0.001 and R2 = 0.7406, p < 0.001, respectively). The increase in total phenolic constituents during storage, namely on the 14th day, is probably due to the hydrolysis of their glycosidic derivatives and the production of more active aglycon substances [35]. Moreover, the autoxidation reaction of phenolic compounds by oxygen may possibly be the cause of the reduction in phenolic compounds after a period of storage [44]. It has also been reported that the increase in TPC during storage might be attributed to the activation of their biosynthesis by enzymes, as well as the inhibition of their oxidation [75,76]. This is probably associated with variations in storage conditions, such as different temperature and moisture levels, post-harvest irradiation treatment, and the use of controlled or modified atmosphere packaging.

Table 3.
Total phenolic content (TPC), antiradical activity (ABTS), and antioxidant activity (FRAP) of tomato flesh during storage.
To a further step, the tomato samples were grouped according to the different storage days using moisture content, brix, and TPC (mg/kg), achieving a discrimination accuracy of 93.0%. The above combination of three features was used to design a high-performance machine learning model, utilizing the CART classifier, that correctly classified 9, 9, 8, 10, 10, and 10 tomato samples on days 3, 7, 10, 14, 17, and 24, respectively, out of 10 samples. The scatter diagram (Figure 7) presents the correct classification of all samples at days 3rd (blue circles), 7th (green squares), 10th (red triangles), 14th (pine green pentagons), 17th (purple triangles), and 24th (yellow pentagons).
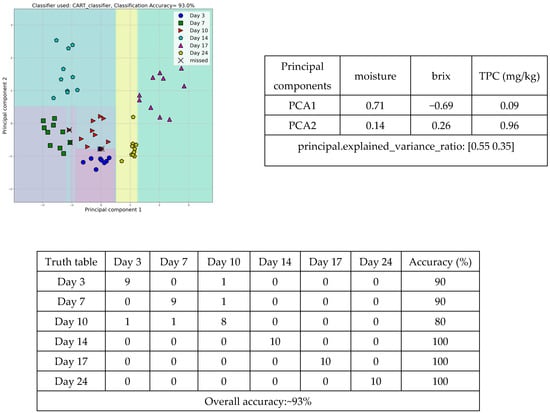
Figure 7.
Scatter diagram, principal component, and truth tables of the discrimination among tomato samples at days 3, 7, 10, 14, 17, and 24, employing the physicochemical parameters of tomato flesh.
3.4. Prediction of Storage Period and Tomato Quality During Storage by Regression Analysis
The necessity of studying tomato quality during storage is twofold: to preserve their freshness and to minimize post-harvest spoilage. To this end, predictive models were developed to assess storage time and flesh quality parameters using textural information obtained from image analysis of tomato peels. A gradient boosting regression model was selected for this specific challenge. The process involved the construction of multiple decision trees in succession, with the errors in each tree being corrected to enhance the accuracy of the final model. The model was trained and evaluated K times by using different parts of the dataset for training and testing in several iterations, ensuring that the model generalizes effectively to unknown data. This method helps to provide a more accurate estimate of the model’s performance. The degree to which the model fits the data is measured by the coefficient of determination (R-squared, R2). The models focusing on the predictability of the tomato’s storability were built using regression analysis and the features extracted from the tomato images.
The features that exhibited the highest R2 (R2 > 0.952), were identified as being long-run emphasis (LRE), correlation, angular second moment (ASM), RLN, skewness, and RP (Figure 8). The findings demonstrated that the storage duration of tomato samples can be accurately predicted using a gradient-boosting regression model that was trained using the extracted features of tomato peel images. This finding aligns with the results of similar studies conducted by our research team and other researchers [35,36,77,78,79], who have investigated various vegetable products. The findings underscore the importance of leveraging machine learning methodologies, underpinned by image texture and instrumental analysis outcomes, to ensure the conservation of vegetables and fruits.
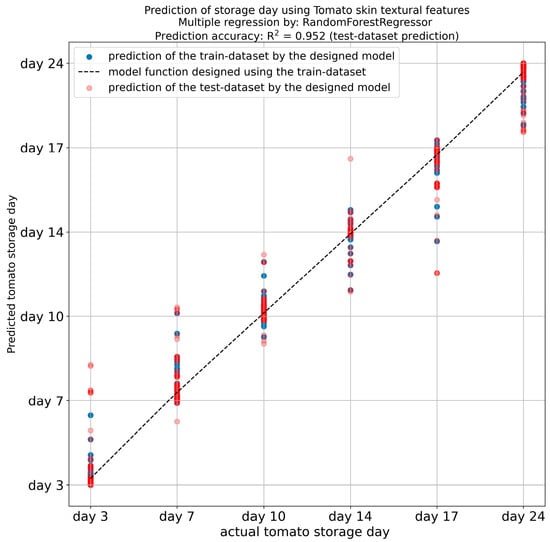
Figure 8.
Prediction of tomato storage days from textural features of tomato peel.
To further estimate the tomatoes’ quality during 24-day storage period, textural features derived from grayscale and colored images of the tomato peel were correlated with physicochemical attributes (brix, firmness, and moisture) as well as the TPC, antioxidant, and antiradical activity of tomato (see Figure 9), using ML algorithms.
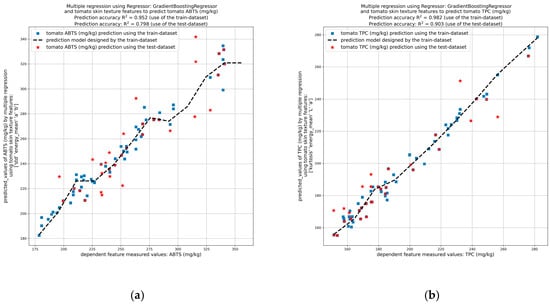
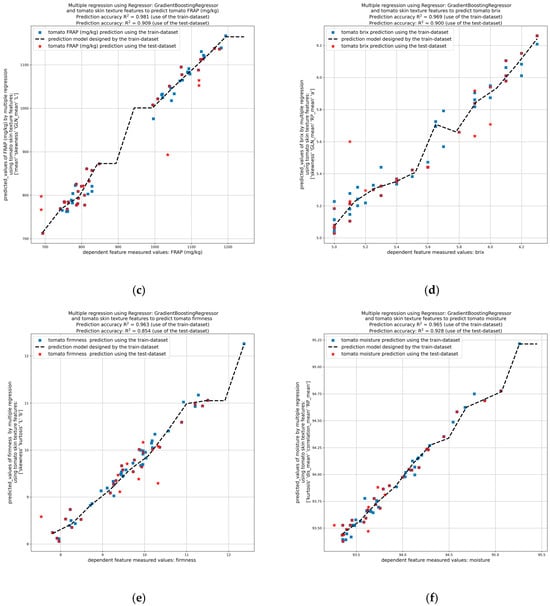
Figure 9.
Prediction of tomato quality parameters by image analysis of tomato peel using the testing dataset. Prediction of tomato (a) antiradical activity (ABTS), (b) TPC, (c) antioxidant activity (FRAP), (d) Brix, (e) firmness, and (f) moisture.
The results demonstrated that the standard deviation, energy, mean, a*, and b* values of the tomato peel have been found to be reliable indicators of the antiradical activity of the tomato flesh towards ABTS free radical (with a mean R2 > 0.952 and a test R2 > 0.798) (Figure 9a). Regarding TPC (Figure 9b) and antioxidant activity via FRAP assay (Figure 9c) of the tomato flesh, the indicators found were kurtosis, energy, mean, L*, and a*, plus mean, skewness, grey level non-uniformity (GLN), and L*, respectively, with prediction accuracy R2 higher than 0.9. Moreover, the skewness, GLN, mean, RP and a* values of the tomato peel have been shown to be effective indicators of the brix values of the tomato flesh (mean R2 > 0.969 and test R2 > 0.900) (Figure 9d), whereas the skewness, kurtosis, L* and b*, have been demonstrated to be significant indicators of firmness values (mean R2 > 0.963 and test R2 > 0.854) (Figure 9e). Finally, the kurtosis, standard deviation, mean, correlation, and RP values of the tomato peel have been shown to be effective indicators of the moisture content of the tomato flesh (mean R2 > 0.965 and test R2 > 0.928) (Figure 9f).
The results collected illustrate the effectiveness of ML applied to image texture data in revealing associations with fruit and vegetable quality attributes. These quality parameters include physicochemical attributes, antioxidant and antiradical activities, and phytochemical content, among others. The findings suggest that these algorithms can serve as a tool for the prediction and assessment of vegetable and fruit quality and shelf life. However, a limitation of the present study is its focus on a single tomato cultivar from one geographic region. Future studies should explore different varieties and production systems to ensure broader applicability of the proposed approach.
4. Conclusions
In order to prevent post-harvest deterioration and ensure consistent quality for consumers, one of the most important issues to be addressed is the quality of fruit and vegetables in relation to storage time. On this basis, the effects of storage at 15 °C on the peel image texture, physicochemical quality, and antioxidant/antiradical properties of hydroponic tomatoes of the Brioso variety were evaluated. This objective was achieved by applying several analytical methods, both destructive and non-destructive, to tomato peel and flesh over a 24-day storage duration. In addition to image analysis and mid-infrared spectroscopy, total soluble solids expressed as Brix, water activity, moisture content, texture, color, total phenolics, and antiradical/antioxidant activity (ABTS and FRAP) were measured after 3, 7, 10, 14, 17, and 24 days of storage. The most important findings are highlighted below. For the tomato peels, storage caused a decrease in lightness and a gradual change in color from orange to dark orange, as well as an increase in the inhomogeneity of the tomato peel image, especially after 17 days of storage. The tomato samples were successfully classified using the FTIR spectral bands at 721, 1165, and 1624 cm−1, associated with carotenoids and polysaccharides, during different storage days. With regard to the physicochemical properties of the tomato flesh, the most significant changes were observed after 17 days of storage and were mainly associated with moisture content increase and Brix and firmness decrease. Furthermore, there were strong correlations between the TPC and the antioxidant and antiradical activity data, which showed higher levels between the tenth and seventeenth days of storage. As a further step, the study aimed to develop predictive models to evaluate how well textural variables from tomato peel image analysis could predict fruit quality parameters and storage duration. The findings indicated that the features of colored and grayscale images of tomato peel are good indicators of physicochemical attributes and antiradical/antioxidant activity of tomato flesh (R2 > 0.9). The storage day of tomato samples was accurately predicted using a random forest regressor model trained on extracted features from tomato peel images. Finally, in terms of limitations of our study, the potential impact of tomato variety on the measured quality parameters, as different cultivars may exhibit variations in physicochemical properties and storage behavior. Future studies could explore multiple varieties from different regions to further validate the applicability of our findings across a broader range of tomato types. Importantly, this integrated method could support real-time quality monitoring during distribution, helping to flag early signs of deterioration and reduce food waste in tomato supply chains.
Author Contributions
Conceptualization, V.J.S., P.C., P.Z. and D.C.; methodology P.C., G.L., K.T., D.C., V.J.S., P.T. and E.K.; software D.C., P.C.; validation T.T. and E.K.; formal analysis, T.T., P.C., P.T. and E.K.; investigation V.J.S., P.Z., D.C., P.C., E.K., T.T., P.T., G.L. and K.T.; data curation, P.C. and D.C.; writing—original draft preparation V.J.S., P.C. and D.C.; writing—review and editing E.K., D.C., V.J.S., T.T. and P.Z.; visualization, P.C. and D.C.; supervision V.J.S. and P.Z.; project administration, V.J.S.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We are grateful to “Thrace Greenhouses S.A” for kindly providing the tomato samples.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Food and Agriculture Organization. FAOSTAT. Available online: https://www.fao.org/home/en/ (accessed on 26 December 2024).
- Bai, Y.; Lindhout, P. Domestication and Breeding of Tomatoes: What Have We Gained and What Can We Gain in the Future? Ann. Bot. 2007, 100, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Van Andel, T.; Vos, R.A.; Michels, E.; Stefanaki, A. Sixteenth-Century Tomatoes in Europe: Who Saw Them, What They Looked like, and Where They Came From. PeerJ 2022, 10, e12790. [Google Scholar] [CrossRef] [PubMed]
- Tsaniklidis, G.; Charova, S.N.; Fanourakis, D.; Tsafouros, A.; Nikoloudakis, N.; Goumenaki, E.; Tsantili, E.; Roussos, P.A.; Spiliopoulos, I.K.; Paschalidis, K.A.; et al. The Role of Temperature in Mediating Postharvest Polyamine Homeostasis in Tomato Fruit. Postharvest Biol. Technol. 2021, 179, 111586. [Google Scholar] [CrossRef]
- Ali, M.Y.; Sina, A.A.I.; Khandker, S.S.; Neesa, L.; Tanvir, E.M.; Kabir, A.; Khalil, M.I.; Gan, S.H. Nutritional Composition and Bioactive Compounds in Tomatoes and Their Impact on Human Health and Disease: A Review. Foods 2020, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Añibarro-Ortega, M.; Pinela, J.; Ćirić, A.; Martins, V.; Rocha, F.; Soković, M.D.; Barata, A.M.; Carvalho, A.M.; Barros, L.; Ferreira, I.C.F.R. Valorisation of Table Tomato Crop By-Products: Phenolic Profiles and In Vitro Antioxidant and Antimicrobial Activities. Food Bioprod. Process. 2020, 124, 307–319. [Google Scholar] [CrossRef]
- Elbadrawy, E.; Sello, A. Evaluation of Nutritional Value and Antioxidant Activity of Tomato Peel Extracts. Arab. J. Chem. 2016, 9, S1010–S1018. [Google Scholar] [CrossRef]
- Al-Dairi, M.; Pathare, P.B.; Al-Yahyai, R. Chemical and Nutritional Quality Changes of Tomato during Postharvest Transportation and Storage. J. Saudi Soc. Agric. Sci. 2021, 20, 401–408. [Google Scholar] [CrossRef]
- Sibomana, M.S.; Workneh, T.S.; Audain, K. A Review of Postharvest Handling and Losses in the Fresh Tomato Supply Chain: A Focus on Sub-Saharan Africa. Food Secur. 2016, 8, 389–404. [Google Scholar] [CrossRef]
- Tao, J.; Zuo, J.; Watkins, C.B.; Bai, C.; He, X.; Liu, S.; Han, L.; Zhao, X.; Liu, Y.; Li, J.; et al. Low Storage Temperature Affects Quality and Volatile Compounds in Fresh Tomatoes. Food Chem. 2024, 460, 140400. [Google Scholar] [CrossRef] [PubMed]
- Famuyini, M.J.; Olalusi, A.P.; Sedara, A.M. Effect of Maturity Stage on Quality and Shelf Life of Tomato (Lycopersicon esculentum Mill.) Using the Refrigerator Storage System. Eurasian J. Agric. Res. 2020, 4, 23–44. [Google Scholar]
- Sinha, S.R.; Singha, A.; Faruquee, M.; Jiku, A.S.; Rahaman, A.; Alam, A.; Kader, M.A. Post-Harvest Assessment of Fruit Quality and Shelf Life of Two Elite Tomato Varieties Cultivated in Bangladesh. Bull. Natl. Res. Cent. 2019, 43, 185. [Google Scholar] [CrossRef]
- Park, M.-H.; Sangwanangkul, P.; Baek, D.-R. Changes in Carotenoid and Chlorophyll Content of Black Tomatoes (Lycopersicone sculentum L.) during Storage at Various Temperatures. Saudi J. Biol. Sci. 2018, 25, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Tilahun, S.; Park, D.S.; Taye, A.M.; Jeong, C.S. Effects of Storage Duration on Physicochemical and Antioxidant Properties of Tomato (Lycopersicon esculentum Mill.). HST 2017, 35, 89–97. [Google Scholar] [CrossRef]
- Sualeh, A.; Daba, A.; Kiflu, S.; Mohammed, A. Effect of Storage Conditions and Packing Materials on Shelf Life of Tomato. Food Sci. Qual. Manag. 2016, 56, 60–67. [Google Scholar]
- Farneti, B.; Alarcón, A.A.; Papasotiriou, F.G.; Samudrala, D.; Cristescu, S.M.; Costa, G.; Harren, F.J.M.; Woltering, E.J. Chilling-Induced Changes in Aroma Volatile Profiles in Tomato. Food Bioprocess Technol. 2015, 8, 1442–1454. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.D.C.; Rodrigues, D.; Pantazis, V.; Cavaco, A.M.; Siomos, A.S.; Miguel, G. Nutritional Quality Changes of Fresh-Cut Tomato during Shelf Life. Food Sci. Biotechnol. 2013, 22, 1–8. [Google Scholar] [CrossRef]
- Takahashi, N.; Maki, H.; Nishina, H.; Takayama, K. Evaluation of Tomato Fruit Color Change with Different Maturity Stages and Storage Temperatures Using Image Analysis. IFAC Proc. Vol. 2013, 46, 147–149. [Google Scholar] [CrossRef]
- Nguyen, T.M.V.; Hertog, M.L.A.T.M.; Van de Poel, B.; Tran, D.T.; Nicolaï, B. Targeted System Approach to Ethylene Biosynthesis and Signaling of a Heat Tolerant Tomato Cultivar; the Impact of Growing Season on Fruit Ripening. Front. Plant Sci. 2023, 14, 1195020. [Google Scholar] [CrossRef] [PubMed]
- Quintero Ramírez, M.; Alvarez Valdez, E.; Ceballos Aguirre, N.; Duno, D.; Taborda Ocampo, G. Volatilomic Profile of the Tree Tomato (Solanum betaceum Cav.) Pulp during Ripening and Senescence Using HS–SPME with GC–MS. LWT 2023, 186, 115213. [Google Scholar] [CrossRef]
- Kushwaha, R.; Singh, M.; Singh, V.; Puranik, V.; Kaur, D. Variation of Bioactive Compounds and Antioxidant Activity during Ripening of Tomato Cultivars. J. Food Agric. Res. 2021, 1, 15–29. [Google Scholar]
- Choi, S.-H.; Lee, S.-H.; Kim, H.-J.; Lee, I.-S.; Kozukue, N.; Levin, C.E.; Friedman, M. Changes in Free Amino Acid, Phenolic, Chlorophyll, Carotenoid, and Glycoalkaloid Contents in Tomatoes during 11 Stages of Growth and Inhibition of Cervical and Lung Human Cancer Cells by Green Tomato Extracts. J. Agric. Food Chem. 2010, 58, 7547–7556. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, K.; Lekjing, S.; Noonim, P.; Charoenphun, N. Extension of Quality and Shelf Life of Tomatoes Using Chitosan Coating Incorporated with Cinnamon Oil. Foods 2024, 13, 1000. [Google Scholar] [CrossRef] [PubMed]
- Flores-López, M.L.; Vieira, J.M.; Rocha, C.M.R.; Lagarón, J.M.; Cerqueira, M.A.; Jasso De Rodríguez, D.; Vicente, A.A. Postharvest Quality Improvement of Tomato (Solanum lycopersicum L.) Fruit Using a Nanomultilayer Coating Containing Aloe Vera. Foods 2023, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, Y.; Ye, M.; Zhu, L.; Zhang, L.; Zhu, S. Improvement in Storage Quality of Postharvest Tomato Fruits by Nitroxyl Liposomes Treatment. Food Chem. 2021, 359, 129933. [Google Scholar] [CrossRef] [PubMed]
- Patanè, C.; Malvuccio, A.; Saita, A.; Rizzarelli, P.; Siracusa, L.; Rizzo, V.; Muratore, G. Nutritional Changes during Storage in Fresh-Cut Long Storage Tomato as Affected by Biocompostable Polylactide and Cellulose Based Packaging. LWT 2019, 101, 618–624. [Google Scholar] [CrossRef]
- Mirdehghan, S.H.; Valero, D. Bioactive Compounds in Tomato Fruit and Its Antioxidant Activity as Affected by Incorporation of Aloe, Eugenol, and Thymol in Fruit Package during Storage. Int. J. Food Prop. 2016, 20, 1798–1806. [Google Scholar] [CrossRef]
- Shehata, S.A.; Abdelrahman, S.Z.; Megahed, M.M.A.; Abdeldaym, E.A.; El-Mogy, M.M.; Abdelgawad, K.F. Extending Shelf Life and Maintaining Quality of Tomato Fruit by Calcium Chloride, Hydrogen Peroxide, Chitosan, and Ozonated Water. Horticulturae 2021, 7, 309. [Google Scholar] [CrossRef]
- Wang, S.; Qiang, Q.; Xiang, L.; Fernie, A.R.; Yang, J. Targeted Approaches to Improve Tomato Fruit Taste. Hortic. Res. 2023, 10, uhac229. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, M.; Duan, X.; Abu-Izneid, T.; Rauf, A.; Khan, Z.; Mitra, S.; Emran, T.B.; Aljohani, A.S.M.; Alhumaydhi, F.A.; et al. Phytochemical and Nutritional Profiling of Tomatoes; Impact of Processing on Bioavailability—A Comprehensive Review. Food Rev. Int. 2023, 39, 5986–6010. [Google Scholar] [CrossRef]
- Lima, G.P.P.; Gómez, H.A.G.; Seabra Junior, S.; Maraschin, M.; Tecchio, M.A.; Borges, C.V. Functional and Nutraceutical Compounds of Tomatoes as Affected by Agronomic Practices, Postharvest Management, and Processing Methods: A Mini Review. Front. Nutr. 2022, 9, 868492. [Google Scholar] [CrossRef] [PubMed]
- Pathare, P.B.; Al-Dairi, M.; Al-Mahdouri, A. Effect of Storage Conditions on Postharvest Quality of Tomatoes: A Case Study at Market-Level. J. Agric. Mar. Sci. 2021, 26, 13–20. [Google Scholar]
- Luna-Guevara, M.L.; Jimenez-Gonzalez, O.; Luna-Guevara, J.J.; Hernandez-Carranza, P.; Ochoa-Velasco, C.E. Quality Parameters and Bioactive Compounds of Red Tomatoes (Solanum lycopersicum L.) Cv Roma VF at Different Postharvest Conditions. J. Food Res. 2014, 3, 8. [Google Scholar] [CrossRef][Green Version]
- Christodoulou, P.; Ladika, G.; Tsiantas, K.; Kritsi, E.; Tsiaka, T.; Cavouras, D.; Zoumpoulakis, P.; Sinanoglou, V.J. Quality Assessment of Greenhouse-Cultivated Cucumbers (Cucumis sativus) during Storage Using Instrumental and Image Analyses. Appl. Sci. 2024, 14, 8676. [Google Scholar] [CrossRef]
- Ladika, G.; Strati, I.F.; Tsiaka, T.; Cavouras, D.; Sinanoglou, V.J. On the Assessment of Strawberries’ Shelf-Life and Quality, Based on Image Analysis, Physicochemical Methods, and Chemometrics. Foods 2024, 13, 234. [Google Scholar] [CrossRef] [PubMed]
- Pathare, P.B.; Opara, U.L. Structural Design of Corrugated Boxes for Horticultural Produce: A Review. Biosyst. Eng. 2014, 125, 128–140. [Google Scholar] [CrossRef]
- Sinanoglou, V.J.; Tsiaka, T.; Aouant, K.; Mouka, E.; Ladika, G.; Kritsi, E.; Konteles, S.J.; Ioannou, A.-G.; Zoumpoulakis, P.; Strati, I.F.; et al. Quality Assessment of Banana Ripening Stages by Combining Analytical Methods and Image Analysis. Appl. Sci. 2023, 13, 3533. [Google Scholar] [CrossRef]
- Helrich, K. Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; Association of Official Analytical Chemists: Arlington, TX, USA, 1990; ISBN 978-0-935584-42-4. [Google Scholar]
- Giannakourou, M.C.; Stavropoulou, N.; Tsironi, T.; Lougovois, V.; Kyrana, V.; Konteles, S.J.; Sinanoglou, V.J. Application of Hurdle Technology for the Shelf Life Extension of European Eel (Anguilla anguilla) Fillets. Aquac. Fish. 2023, 8, 393–402. [Google Scholar] [CrossRef]
- Chen, L.; Opara, U.L. Texture Measurement Approaches in Fresh and Processed Foods—A Review. Food Res. Int. 2013, 51, 823–835. [Google Scholar] [CrossRef]
- Kritsi, E.; Tsiaka, T.; Sotiroudis, G.; Mouka, E.; Aouant, K.; Ladika, G.; Zoumpoulakis, P.; Cavouras, D.; Sinanoglou, V.J. Potential Health Benefits of Banana Phenolic Content during Ripening by Implementing Analytical and In Silico Techniques. Life 2023, 13, 332. [Google Scholar] [CrossRef] [PubMed]
- Anđelini, M.; Major, N.; Išić, N.; Kovačević, T.K.; Ban, D.; Palčić, I.; Radunić, M.; Goreta Ban, S. Sugar and Organic Acid Content Is Dependent on Tomato (Solanum lycoperiscum L.) Peel Color. Horticulturae 2023, 9, 313. [Google Scholar] [CrossRef]
- Jati, I.R.A.P.; Setijawaty, E.; Utomo, A.R.; Darmoatmodjo, L.M.Y.D. The Application of Aloe Vera Gel as Coating Agent to Maintain the Quality of Tomatoes during Storage. Coatings 2022, 12, 1480. [Google Scholar] [CrossRef]
- Tadesse, T.N.; Ibrahim, A.M.; Gebreselas, W. Degradation and Formation of Fruit Color in Tomato (Solanum lycopersicum L.) in Response to Storage Temperature. Am. J. Food Technol. 2015, 10, 147–157. [Google Scholar] [CrossRef]
- Ayomide, O.B.; Ajayi, O.O.; Ajayi, A.A. Advances in the Development of a Tomato Postharvest Storage System: Towards Eradicating Postharvest Losses. J. Phys. Conf. Ser. 2019, 1378, 022064. [Google Scholar] [CrossRef]
- Tolasa, M.; Gedamu, F.; Woldetsadik, K. Impacts of Harvesting Stages and Pre-Storage Treatments on Shelf Life and Quality of Tomato (Solanum lycopersicum L.). Cogent Food Agric. 2021, 7, 1863620. [Google Scholar] [CrossRef]
- Breda, C.A.; Morgado, D.L.; De Assis, O.B.G.; Duarte, M.C.T. Effect of Chitosan Coating Enriched with Pequi (Caryocar Brasiliense Camb.) Peel Extract on Quality and Safety of Tomatoes (Lycopersicon esculentum Mill.) during Storage. J. Food Process. Preserv. 2017, 41, e13268. [Google Scholar] [CrossRef]
- Palumbo, M.; Cefola, M.; Pace, B.; Attolico, G.; Colelli, G. Computer Vision System Based on Conventional Imaging for Non-Destructively Evaluating Quality Attributes in Fresh and Packaged Fruit and Vegetables. Postharvest Biol. Technol. 2023, 200, 112332. [Google Scholar] [CrossRef]
- Apte, S.K.; Patavardhan, P.P. Feature Fusion Based Orange and Banana Fruit Quality Analysis with Textural Image Processing. J. Phys. Conf. Ser. 2021, 1911, 012023. [Google Scholar] [CrossRef]
- Subhashree, S.N.; Sunoj, S.; Xue, J.; Bora, G.C. Quantification of Browning in Apples Using Colour and Textural Features by Image Analysis. Food Qual. Saf. 2017, 1, 221–226. [Google Scholar] [CrossRef]
- Kozłowicz, K.; Różyło, R.; Gładyszewska, B.; Matwijczuk, A.; Gładyszewski, G.; Chocyk, D.; Samborska, K.; Piekut, J.; Smolewska, M. Identification of Sugars and Phenolic Compounds in Honey Powders with the Use of GC–MS, FTIR Spectroscopy, and X-Ray Diffraction. Sci. Rep. 2020, 10, 16269. [Google Scholar] [CrossRef] [PubMed]
- Bello-Pérez, L.A.; Ottenhof, M.-A.; Agama-Acevedo, E.; Farhat, I.A. Effect of Storage Time on the Retrogradation of Banana Starch Extrudate. J. Agric. Food Chem. 2005, 53, 1081–1086. [Google Scholar] [CrossRef]
- Skolik, P.; McAinsh, M.R.; Martin, F.L. ATR-FTIR Spectroscopy Non-Destructively Detects Damage-Induced Sour Rot Infection in Whole Tomato Fruit. Planta 2019, 249, 925–939. [Google Scholar] [CrossRef] [PubMed]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to Read and Interpret FTIR Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Fourier Transform Infrared (FTIR) Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Li, X.; Huang, H.; Zhang, L.; Zhao, L. Effect of Postharvest Storage Temperature and Duration on Tomato Fruit Quality. Foods 2025, 14, 1002. [Google Scholar] [CrossRef] [PubMed]
- Bu, J.; Yu, Y.; Aisikaer, G.; Ying, T. Postharvest UV-C Irradiation Inhibits the Production of Ethylene and the Activity of Cell Wall-Degrading Enzymes during Softening of Tomato (Lycopersicon esculentum L.) Fruit. Postharvest Biol. Technol. 2013, 86, 337–345. [Google Scholar] [CrossRef]
- Ren, Y.; Sun, P.; Wang, X.; Zhu, Z. Degradation of Cell Wall Polysaccharides and Change of Related Enzyme Activities with Fruit Softening in Annona squamosa during Storage. Postharvest Biol. Technol. 2020, 166, 111203. [Google Scholar] [CrossRef]
- Mohie, M.K.; Mohamed, F.G.; Shaheen, M.S. Fourier Transformer Infrared Spectroscopy for Quality Assurance of Tomato Products. J. Am. Sci. 2011, 7, 559–572. [Google Scholar]
- Hong, T.; Yin, J.-Y.; Nie, S.-P.; Xie, M.-Y. Applications of Infrared Spectroscopy in Polysaccharide Structural Analysis: Progress, Challenge and Perspective. Food Chem. X 2021, 12, 100168. [Google Scholar] [CrossRef] [PubMed]
- Talari, A.C.S.; Martinez, M.A.G.; Movasaghi, Z.; Rehman, S.; Rehman, I.U. Advances in Fourier Transform Infrared (FTIR) Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2017, 52, 456–506. [Google Scholar] [CrossRef]
- Abiso, E.; Satheesh, N.; Hailu, A. Effect of Storage Methods and Ripening Stages on Postharvest Quality of Tomato (Lycopersicom esculentum Mill) Cv. Chali. Annals. Food Sci. Technol. 2015, 16, 127–137. [Google Scholar]
- Tigist, M.; Workneh, T.S.; Woldetsadik, K. Effects of Variety on the Quality of Tomato Stored under Ambient Conditions. J. Food Sci. Technol. 2013, 50, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, F.; Kallarackal, J. Tree Tomato (Solanum betaceum Cav.) Reproductive Physiology: A Review. Sci. Hortic. 2019, 248, 206–215. [Google Scholar] [CrossRef]
- Eboibi, O.; Akpokodje, O.I.; Nyorere, O.; Oghenerukevwe, P.; Uguru, H. Evaluation of Textural Qualities and Chemical Properties of Some Tomato Cultivars. Direct Res. J. Agric. Food Sci. 2019, 7, 147–157. [Google Scholar] [CrossRef]
- Borghesi, E.; Ferrante, A.; Gordillo, B.; Rodríguez-Pulido, F.J.; Cocetta, G.; Trivellini, A.; Mensuali-Sodi, A.; Malorgio, F.; Heredia, F.J. Comparative Physiology during Ripening in Tomato Rich-Anthocyanins Fruits. Plant Growth Regul. 2016, 80, 207–214. [Google Scholar] [CrossRef]
- Pila, N.; Gol, N.B.; Rao, T.V.R. Effect of Post Harvest Treatments on Physicochemical Characteristics and Shelf Life of Tomato (Lycopersicon esculentum Mill.) Fruits during Storage. Am. Eurasian J. Agric. Envion.Sci. 2010, 9, 470–479. [Google Scholar]
- Mouhamed, B.A.; Kasnazany, S.A.S. Impact of Harvesting Stages and Postharvest Treatments on the Quality and Storability of Tomato Fruits (Solanum lycopersicum L.) Cv. Sangaw. Coatings 2024, 14, 1143. [Google Scholar] [CrossRef]
- Cruz-Carrión, Á.; Ruiz De Azua, M.J.; Bravo, F.I.; Aragonès, G.; Muguerza, B.; Suárez, M.; Arola-Arnal, A. Tomatoes Consumed In-Season Prevent Oxidative Stress in Fischer 344 Rats: Impact of Geographical Origin. Food Funct. 2021, 12, 8340–8350. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, R.; Raiola, A.; Tenore, G.C.; Frusciante, L.; Barone, A.; Monti, D.M.; Rigano, M.M. Antioxidant Bioactive Compounds in Tomato Fruits at Different Ripening Stages and Their Effects on Normal and Cancer Cells. J. Funct. Foods 2015, 18, 83–94. [Google Scholar] [CrossRef]
- Ilahy, R.; Hdider, C.; Lenucci, M.S.; Tlili, I.; Dalessandro, G. Antioxidant Activity and Bioactive Compound Changes during Fruit Ripening of High-Lycopene Tomato Cultivars. J. Food Compos. Anal. 2011, 24, 588–595. [Google Scholar] [CrossRef]
- Chaudhary, P.; Sharma, A.; Singh, B.; Nagpal, A.K. Bioactivities of Phytochemicals Present in Tomato. J. Food Sci. Technol. 2018, 55, 2833–2849. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Da Silva, L.; Maria Silva, B. (Eds.) Natural Bioactive Compounds from Fruits and Vegetables as Health Promoters Part II.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2016; ISBN 978-1-68108-243-1. [Google Scholar]
- Hernández-Fuentes, A.; López-Vargas, E.; Pinedo-Espinoza, J.; Campos-Montiel, R.; Valdés-Reyna, J.; Juárez-Maldonado, A. Postharvest Behavior of Bioactive Compounds in Tomato Fruits Treated with Cu Nanoparticles and NaCl Stress. Appl. Sci. 2017, 7, 980. [Google Scholar] [CrossRef]
- Rivero, R.M.; Ruiz, J.M.; García, P.C.; López-Lefebre, L.R.; Sánchez, E.; Romero, L. Resistance to Cold and Heat Stress: Accumulation of Phenolic Compounds in Tomato and Watermelon Plants. Plant Sci. 2001, 160, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Ropelewska, E.; Noutfia, Y. Application of Image Analysis and Machine Learning for the Assessment of Grape (Vitis L.) Berry Behavior under Different Storage Conditions. Eur. Food Res. Technol. 2024, 250, 935–944. [Google Scholar] [CrossRef]
- Li, D.; Bai, L.; Wang, R.; Ying, S. Research Progress of Machine Learning in Extending and Regulating the Shelf Life of Fruits and Vegetables. Foods 2024, 13, 3025. [Google Scholar] [CrossRef] [PubMed]
- Contreras-López, E.; Jaimez-Ordaz, J.; Ugarte-Bautista, I.; Ramírez-Godínez, J.; González-Olivares, L.G.; García-Curiel, L.; Pérez-Flores, J.G. Use of Image Analysis to Determine the Shelf-Life of an Apple Compote with Wine. Food Sci. Technol. 2022, 42, e04122. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).