Abstract
Sex differences in response to hypovolemia are still an open issue, which is readdressed here by exploiting the potential of near-infrared spectroscopy (NIRS) to monitor the response of lower body negative pressure (LBNP) in upper and lower limbs. In 28 subjects in a supine position, non-invasive arterial blood pressure was continuously monitored along with NIRS parameters from the forearm and thigh during randomized 90 s exposure to LBNP at −10, −20, −30, and −40 mmHg, followed by one 5 min exposure to −30 mmHg. LBNP did not affect arterial blood pressure, slightly increased the heart rate, and affected blood volume in both limbs (p < 0.005). Slopes of tissue oxygenation and deoxy-hemoglobin revealed pressure (p < 0.001) and sex (p < 0.05) dependences for the vasoconstrictive response to LBNP in both arms and legs, with some evidence of larger vasoconstriction in legs. Most variables reached a stable value within 90 s in the arm, while longer time courses were observed in the leg. NIRS is a valuable methodology to detect early LBNP-induced hemodynamic changes, providing that blood volume and blood flow contribution are discriminated. A comparative analysis of time courses proved useful in revealing stronger vasoconstrictive responses in males than in females and in lower limbs than in upper limbs. The same approach could be applied to other experimental contexts.
1. Introduction
Lower body negative pressure (LBNP) has been adopted since the 1960s as a means to induce hypovolemic stimuli in the absence of the hydrostatic gradients that develop with maneuvers, such as standing or head-up tilt. For this reason, it has been conveniently employed for the investigation of autonomic reflexes, namely, the cardio-pulmonary and the arterial baroreceptor reflexes, and their capacity to prevent cardiovascular collapse []. In general terms, it is agreed that women present a lower tolerance to hypovolemic stimuli then men [,,] although the underlying mechanisms are still debated []. For instance, contrasting results concern the extent of peripheral vasoconstriction, which has been reported to be both stronger [] and lower in women than men [,]. In this respect, near-infrared spectroscopy (NIRS) represents an attractive non-invasive methodology for the investigation of peripheral vascular effects, allowing the simultaneous detection of changes in blood volume and tissue oxygenation as well as the inference of changes in blood flow and vascular resistance in different body areas. In fact, tissue oxygenation essentially reflects the balance between blood flow and metabolism, providing that the blood volume remains constant []; thus, changes in blood flow can be inferred from corresponding changes in tissue oxygenation, as reported in different conditions [,,]. However, the interpretation of NIRS signals is complicated by several factors, including (1) dependence on contributions from superficial (cutaneous) and deep (muscle/brain) tissue layers, which may respond differently to autonomic and environmental challenges [,], and (2) dependence on changes in blood volume, perfusion, and metabolism, which may occur at the same time and complicate the interpretation of NIRS signals []. This latter issue is particularly relevant in LBNP studies in which marked changes in blood volume take place (increases and decreases in lower and upper body areas, respectively) along with vascular reflexes affecting local perfusion. Moreover, it must be reiterated that although this blood volume shift is likely to mainly affect the largest and most compliant veins, these may also affect NIRS measurements, as recently demonstrated during venous dilatation produced by venous occlusion tests [] and remote external heating []. On this basis, it is crucial to know the full time course of NIRS signals for their correct interpretation. However, to the best of our knowledge, with the exception of a few original recordings from single subjects, the time course of blood volume and oxygenation changes in response to LBNP has never been reported. In addition, most studies have adopted LBNP protocols in which the pressure progressively decreased, usually in steps of 10 mmHg and 5 min duration. Therefore, the hemodynamic effects observed at a given negative pressure are not representative of that pressure level, being affected by LBNP exposure to all previous pressure steps. To precisely relate the hemodynamic changes to the different pressure levels, distinct LBNP stimuli should be separated by resting intervals (with no LBNP applied). We hypothesized that (1) by examining the time course of NIRS signals in response to distinct LBNP stimuli of different magnitudes, it would be possible to identify the time intervals in which tissue blood volume exhibits the fastest transients and those in which it is relatively stable, thus allowing the discrimination of blood volume displacements from changes in blood flow; (2) upper and lower limbs would exhibit opposite changes in blood volume but a similar reduction in tissue oxygenation as a result of vasoconstriction in a pressure-dependent way; and (3) females would exhibit weaker signs of vasoconstriction than males, which would explain their lower tolerance to the hypovolemic challenge.
To address these hypotheses, the present study investigated the full time course of NIRS signals in response to short-duration isolated LBNP stimuli of different magnitudes (90 s at −10, −20, −30, and −40 mmHg). This allowed us to quantify the duration and extent of blood volume changes as well as the extent of vasoconstriction, as inferred from the slopes of tissue oxygenation and deoxy-hemoglobin signals. The development of slower hemodynamic changes was also investigated during a longer-lasting LBNP exposure (300 s; −30 mmHg).
2. Materials and Methods
2.1. Subjects
Twenty-eight subjects were enrolled, including sixteen males (age: 25 ± 9 years, weight: 75 ± 9 kg, height: 177 ± 6 cm, and BMI: 23.8 ± 3.0 kg/m2) and twelve females (age: 23 ± 3 years, weight: 57 ± 8 kg, height: 164 ± 3 cm, and BMI: 21.2 ± 3.2 kg/m2). Participants were excluded from the study if they presented a prior history of cardiovascular disease or susceptibility to hypotensive crises and fainting episodes. The study was conducted in agreement with the principles of the Declaration of Helsinki (2000) and under the approval of the Ethics Committee of the University of Torino (no. 0059551; 30 January 2023). All subjects gave their written informed consent.
2.2. Experimental Set-Up
Mean arterial blood pressure (MAP) was continuously and non-invasively monitored by finger photo-plethysmography (CNAP System, CNSystems Medizintechnik, Graz, Austria), which also yielded the continuous monitoring of cardiac output (CO) and heart rate (HR). The finger cuff was applied to the third finger of the right hand and a calibration cuff was placed around the ipsilateral arm (Figure 1A).
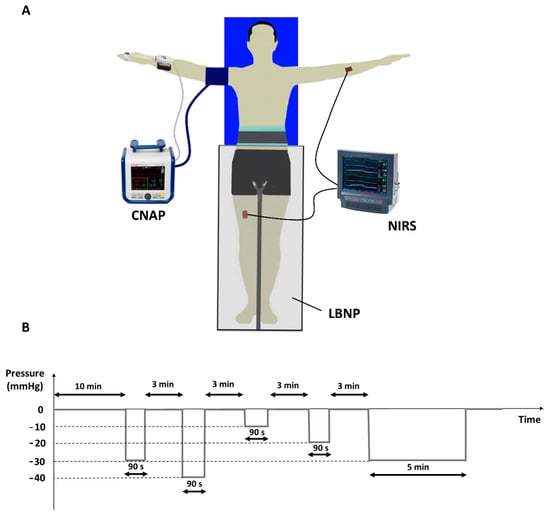
Figure 1.
Experimental setup including CNAP and NIRS devices along with the LBNP chamber (A). Experimental protocol (B): the sequence of the 90 s lasting stimuli was randomized, while the 5 min lasting stimulus always followed at the end.
The non-invasive monitoring of muscle blood volume and oxygenation was performed using near-infrared spectroscopy (NIRS) (NIRO-200X, Hamamatsu Photonics K.K, Shizuoka, Japan). Two NIRS probes were employed, with an inter-optode distance of 3 cm. The first one was positioned over the flexor muscles of the left forearm and the second one over the right vastus lateralis muscle. The device measures changes in concentration of deoxygenated (HHb) and oxygenated (O2Hb) hemoglobin + myoglobin, expressed in µM·cm, based on the standard Beer–Lambert (BL) methodology. The sum of these two terms yields the total hemoglobin (tHb) concentration, which is an index of blood volume change. Additionally, the NIRS device calculates the tissue hemoglobin index (THI), which reveals relative changes in blood volume, and the tissue oxygenation index (TOI), which represents the percentage of oxygen-saturated (hemoglobin + myoglobin) over total (hemoglobin + myoglobin). These latter parameters are based on the spatially resolved methodology, which focusses the measurement in depth (muscle tissue) so they are thus little affected by hemodynamic changes occurring in the more superficial tissue layers [].
The negative pressure to the lower limbs was delivered through a lower body negative pressure (LBNP) chamber (LBNP 1100, TECHNAVANCE, Austin, TX, USA) []. The device, controlled by a web interface, was used to deliver negative step changes in pressure of different magnitudes and durations. The value of the actual pressure inside the chamber is returned through an analog output.
All signals were digitally sampled (1401micro, CED, Cambridge, UK) at a sampling frequency of 1024 Hz and stored on a PC for offline analysis (Spike2, CED, Cambridge, UK). Data acquisition was continuously performed throughout the whole experimental session.
2.3. Experimental Protocol
Participants were first instrumented with the CNAP and NIRS probes and, after that, they were settled in the supine position within the LBNP chamber, which was sealed at the level of the iliac crest. Each experimental session started with a rest period lasting at least 10 min to allow for fluid redistribution between vascular and interstitial compartments following the change in posture. The LBNP protocol (Figure 1B) consists of a sequence of 4 negative pressure stimuli (−10, −20, −30, and −40 mmHg), each lasting 90 s and separated by a minimum time interval of 3 min (at atmospheric pressure) allowing the reestablishment of the initial hemodynamic conditions. This sequence was followed by a final long-lasting LBNP stimulus (5 min at −30 mmHg) to explore the slower hemodynamic responses to LBNP. The entire experimental protocol lasted about 35 min.
2.4. Data Analysis
All signals were visually inspected to ensure the good quality of the recordings. Tracings were occasionally excluded from the analysis in case of corruption or artifacts.
Average response curves to LBNP were calculated for all variables and all stimuli; then, the quantitative assessment of changes was performed as follows.
For each hypovolemic stimulus, two intervals were identified, corresponding to the 20 s before the beginning of the stimulus and to the last 5 s before its end, i.e., at 90 s (T90s) or at 5 min (T5min), and time averages were calculated. For HR, MAP, and TOI, an average baseline value was obtained from the four baselines pertaining to the four 90 s stimuli. For blood volume indices, THI and tHb, changes with respect to each of the relevant baselines were calculated. Additionally, the slope of the quasi-linear trend of TOI and HHb, exhibited after the initial transient, was calculated over the interval ranging from 30 s after the beginning to the end of the stimulus. To quantify the further changes taking place when extending the duration of the stimulus, average values collected at T90s and at T5min of the 5 min stimulus were compared to baseline. Finally, a repeatability test was conducted by comparing the measurements collected from the first 90 s of the two 30 mmHg stimuli (of 90 s and 5 min duration).
The whole signal processing was performed in MATLAB (The MathWorks Inc. (2022). MATLAB Version: 23.2.0.2668659 (R2023b), Natick, MA, USA: The MathWorks Inc.).
2.5. Statistical Analysis
All the statistical analyses were carried out using IBM® SPSS® Statistics 28.0 (IBM corp., Armonk, NY, USA). Normality of data was checked by Shapiro–Wilk’s test, while homoscedasticity was tested by Levene’s test. Subjects’ age, weight, height, and BMI were statistically compared between groups (male vs. female) using either the Mann–Whitney U test or the independent t-test, depending on the results of the normality and homoscedasticity tests. Furthermore, we compared the basal values in males and females for MAP, HR, and TOI using a two-tailed unpaired sample t-test. A two-way mixed ANOVA was performed to investigate the effects of LBNP (0, −10, −20, −30, and −40 mmHg) and sex on CNAP variables (MAP and HR) and the effects of pressure (−10, −20, −30, and −40 mmHg) and sex on NIRS variables (TOI, THI, HHb, tHb). A two-way mixed ANOVA was performed to calculate the effects of time (baseline, T90s, and T5min) and sex on NIRS and CNAP variables during the 5 min stimulus. Then, if an interaction or main effect was present, an LSD post hoc test was performed to analyze specific differences. In addition, the slopes of TOI and HHb were compared between arms and legs through a three-way mixed ANOVA with factors limb (arm/leg), sex (M/F), and pressure (−10, −20, −30, and −40 mmHg).
Repeatability was assessed by the Pearson’s R correlation coefficient from measurements collected at T90s, from the 5 min and the 90 s exposures at −30 mmHg. Results are presented as mean ± standard deviation; p-values less than 0.05 were considered significant.
3. Results
Some tracings (2 THI, 1 TOI, 2 HHb, 2 tHb) were excluded from the analysis of the responses to the 90 s stimuli because of either the presence of visible artifacts or a failure of the NIRS device. In addition, three subjects did not undergo the 5 min stimulus. Compared to females, males presented with higher weight (75 ± 9 vs. 57± 8 kg, p < 0.01), height (177 ± 6.1 vs. 164 ± 3.4 cm, p < 0.01), and BMI (24 ± 3 vs. 21± 3 kg/m2, p < 0.05) and with similar age (24 ± 9 vs. 23± 3 years, p > 0.05).
In resting conditions, males were also characterized by lower HR (65.3 ± 10.8 vs. 73 ± 12.0 BPM, p = 0.085) and TOI in the thigh (76.4 ± 7.5 vs. 81.1 ± 7.1%, p = 0.015), while no difference was detected in MAP (87.1 ± 7.4 vs. 86.9 ± 10.1 mmHg, p = 0.945) and in TOI in the forearm (72.5 ± 7.3 vs. 76.7 ± 5.0%, p = 0.107).
3.1. Exposure to 90 s LBNP
Exposure to −10 to −40 mmHg LBNP for 90 s was well tolerated by all subjects. No significant changes from baseline and no sex differences were detected in ABP, although females exhibited a slight decreasing trend at increasing LBNP magnitude. Minor but significant increases were detected in HR at −40 mmHg (females: 75.3 ± 14.7 bpm; males: 69.2 ± 14.9 BPM), with no sex differences. The full time course of the responses of NIRS variables to the 90 s exposure to different levels of LBNP is described by the average tracings of Figure 2, for both the forearm and the thigh (males and females being pooled together). It can be observed that TOI and HHb exhibited a qualitatively similar (but specular) pattern in arms and legs, consisting, after an initial transient, in an almost linear decrease in TOI and increase in HHb, with slopes increasing with the magnitude of the LBNP. Conversely, blood volume indices THI and tHb both exhibited opposite patterns: a decrease in the arm, starting immediately with application of the stimulus, completing in 10–20 s and then remaining stable until the end of the stimulus, and an increase in the leg, initially rapid and later slow, not achieving a stable level within the 90 s duration of the stimulus. Also in this case, the effects were related to the magnitude of the stimulus. Comparison of responses for male and females at the different LBNP magnitudes, along with statistical significance, is reported in Figure 3. It can be observed that a main effect of pressure is exhibited by all NIRS variables, while a significant dependence on sex results only for TOI and HHb slopes and not for blood volume changes. Note that the TOI and HHb slopes were calculated over the time interval following the rapid blood volume shift, where the variables exhibit a mostly linear trend.
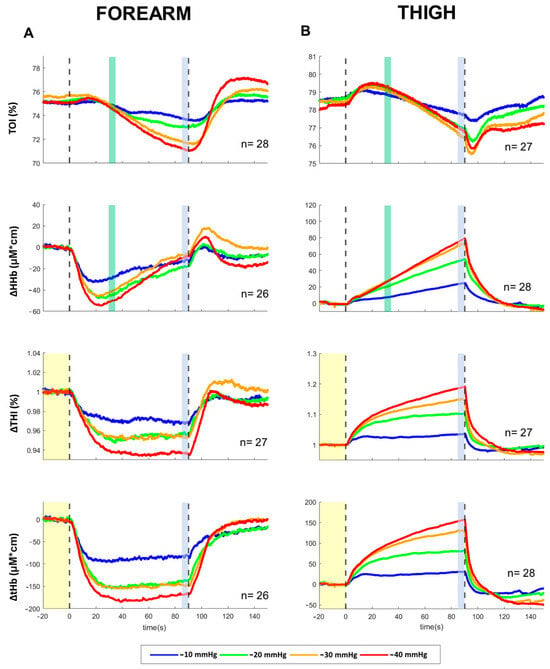
Figure 2.
Averaged response of NIRS parameters recorded from the forearm (A) and the thigh (B) during 90 s exposure to LBNP of −10, −20, −30, −40 mmHg. Vertical dashed lines indicate the start and the end of the LBNP stimulus; vertical colored bands indicate where the measurements were taken to quantify average effects and slopes (Figure 3), the yellow band indicating the baseline. TOI: tissue oxygenation index; HHb: deoxy-hemoglobin concentration; THI: tissue hemoglobin index; tHb: total hemoglobin concentration. The THI tracings were normalized to baseline; HHb and tHb baselines were set to 0. The number of subjects (n) includes males and females pooled together.
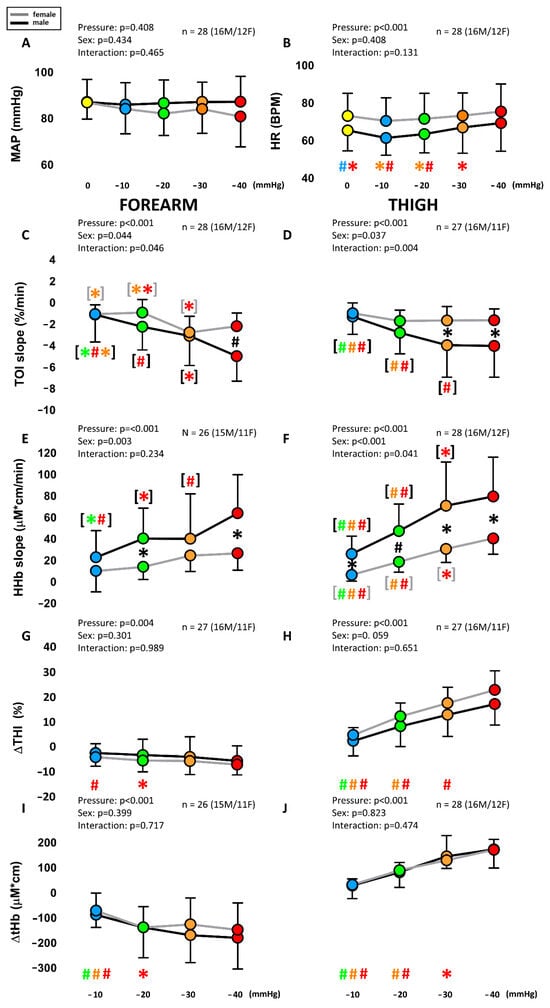
Figure 3.
Average of LBNP on systemic variables (A,B) and local NIRS variables from the forearm (C,E,G,I) and the thigh (D,F,H,J) in males and females. MAP: mean arterial pressure; HR: heart rate; TOI: tissue oxygenation index; HHb: deoxy-hemoglobin concentration; THI: tissue hemoglobin index; tHb: total hemoglobin concentration. Different colors are associated with the different pressure levels, while the black and gray lines indicate males and females, respectively. Main effects and interaction are reported in text form and post hoc tests are reported with symbols (#: p < 0.001; * p > 0.05), the color identifying the pressure level considered in the comparison, e.g., a red asterisk over the green dot indicates a significant difference between the red (40 mmHg) and green (20 mmHg) conditions. If # and * are within black (gray) square brackets they refer specifically to the male (female) group. Otherwise, they refer to the whole group (males + females). The total number of subjects (n) is also distinctly specified for males (M) and females (F).
3.2. Exposure to −30 mmHg LBNP for 5 min
The 5 min LBNP exposure at −30 mmHg was well tolerated by all subjects. No significant effect of sex or time and no sex–time interaction were detected in MAP, while a main effect of time was detected in HR, slightly increased after 5 min compared to baseline (78.5 ± 11.8 bpm for females and 78.8 ± 16.5 for males, p < 0.01). The time course of NIRS variables is shown in Figure 4. It can be observed that the TOI decreased, eventually reaching a plateau, earlier in the arm than in the leg. Apart from the large initial transient in the arm, HHb follows a quite similar pattern: a short-lasting increase followed by a plateau in the arm (after the initial drop) and a longer-lasting, although progressively slower, increase in the leg. Differently from the TOI, the HHb in the leg does not reach a plateau but maintains a slow and continuous rise for the whole 5 min exposure to LBNP. This late slow rise is also exhibited by THI and tHb in the leg, and in the female arm, while males maintained a stable reduction in blood volume in the arm throughout the 5 min duration of the test. The comparison of the early (90 s) and late (5 min) effects of exposure to −30 mmHg, in males and females, is shown in Figure 5. A main effect of time was observed for all NIRS variables, except for HHb in the arm. A main effect of sex was found only for TOI, and a time–sex interaction was observed in THI, in the thigh.
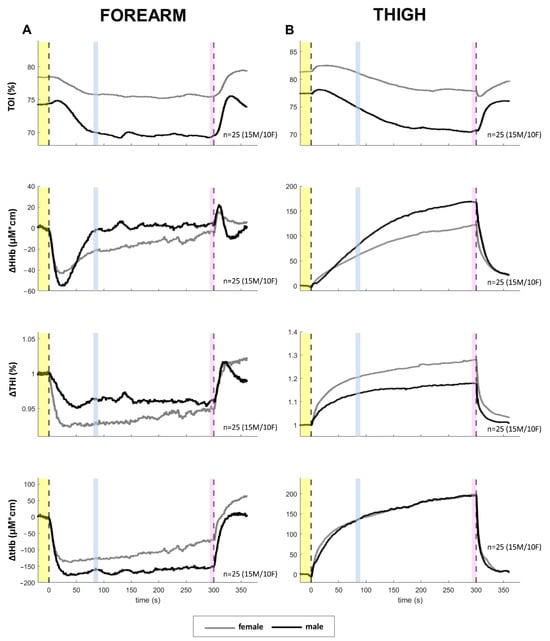
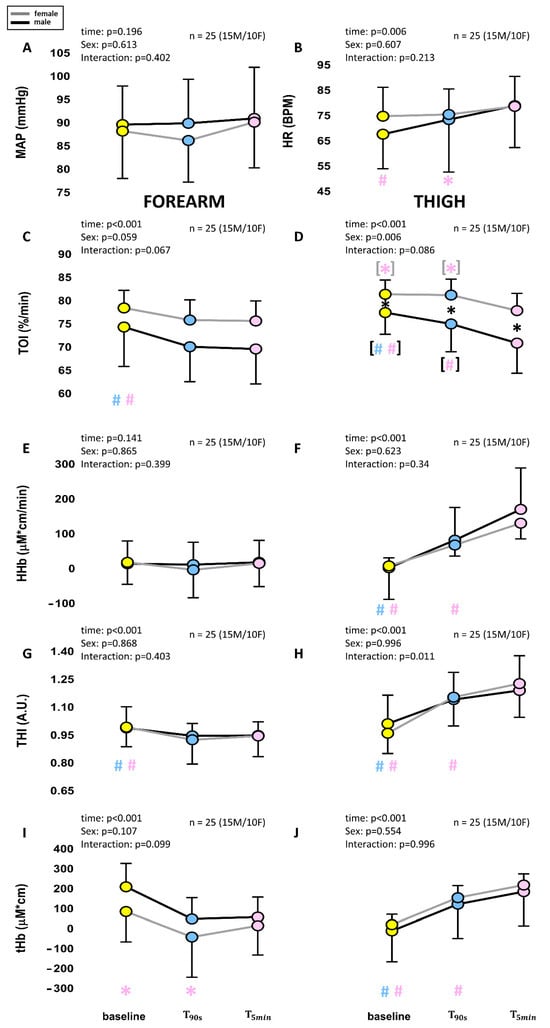
Figure 5.
Comparison of average changes at 90 s (T90s) and 5 min (T5min) from the beginning of a −30 mmHg LBNP stimulus. The diagrams show changes in systemic (A,B) and NIRS variables col-lected from the forearm (C,E,G,I) and the thigh (D,F,H,J) as shown in Figure 4. Different colors are associated with the different times, while the black and gray lines indicate males and females, re-spectively. Main effects and interaction are reported in text form and post hoc tests are reported with symbols (#: p < 0.001; * p > 0.05), the color identifying the pressure level considered in the comparison, e.g., a red asterisk over the green dot indicates a significant difference between the red (40 mmHg) and green (20 mmHg) conditions. If # and * are within black (gray) square brackets they refer specifically to the male (female) group. Otherwise, they refer to the whole group (males + females). The total number of subjects (n) is also distinctly specified for males (M) and females (F).
3.3. Comparison Between Forearm and Thigh
Blood volume changes have opposite signs in arms and legs, exhibiting consistent decreases and increases, respectively. Conversely, the slopes of TOI and HHb exhibit a similar trend with no significant difference between arms and leg and no limb–pressure interaction as revealed by the three-way ANOVA (which confirmed a main effect of sex and pressure).
3.4. Repeatability of Hemodynamic Changes at −30mmHg, 90 s
Repeatability of NIRS measurements was assessed by the correlation of measurements taken after 90 s of exposure to −30 mmHg, collected from the responses to 90 s and 5 min stimuli. The results indicate a generally good correlation, as indicated by Pearson’s correlation coefficient (Table 1).

Table 1.
Repeatability test of measurements related to 90 s, −30 mmHg stimuli.
4. Discussion
4.1. Summary of Results
For the first time, a description of the full time course of the response of NIRS variables to LBNP is provided. The effects are described for different pressure levels, tested individually through isolated stimuli (−10 to −40 mmHg), for both upper and lower limbs. The main findings may be synthesized as follows: (1) blood volume indices reveal a fast volume depletion at the forearm and a volume increase in the leg developing in about 20 s; (2) in the leg, this first transient is followed by a further increase in volume which progresses over more than 5 min; (3) after the initial volume transient, the typical signs of vasoconstriction, a decrease in TOI and an increase in HHb, can be detected in both arms and legs, with the slope related to the magnitude of LBNP; (4) females exhibit lower slopes than males in both TOI and HHb, suggesting a reduced vasoconstriction.
4.2. Isolated vs. Progressive LBNP Applications
Most LBNP studies applied different pressure levels by progressively increasing the negative pressure [,,,], in some cases until reaching a pre-syncope state [,,]. However, this approach does not allow a comparison of the effects obtained at the different pressure levels since a cumulative effect takes place with the progression of the stimulus, i.e., the effects observed at a certain pressure level are affected by the exposure to preceding pressure levels. To account for this effect and to improve comparison between studies that may have followed different pressure steps or different step durations, the cumulative stress index (CSI), defined as the pressure time integral [], is often adopted. In the present study, isolated and randomized stimuli at the different pressure levels were delivered, thus minimizing the CSI. This approach allowed us to detect the true dependence of the different variables on the LBNP.
4.3. Analysis of Time Courses Reveals Blood Volume and Oxygenation Changes
The full time course of the hemodynamic responses to LBNP has rarely been reported; knowledge of time courses is, however, necessary to disentangle the effects of coexisting changes in blood volume and blood flow. First of all, it is possible to observe that spatially resolved spectroscopy (SRS) and BL parameters provide qualitatively similar patterns of response (THI parallels tHb, and TOI is specular to HHb) suggesting that skin and muscle tissues do not respond in a conflicting way. In fact, it has been shown in several contexts that SRS parameters focus the measurement at depth while BL ones are affected by both cutaneous and deep (brain or muscular) circulation and that a different response from superficial and deep tissues may result in different or even opposite changes in BL and SRS variables [,,]. It is reasonable to expect that muscles and skin exhibit similar passive and myogenic responses to blood pressure while the reflex sympathetic response to mild hypovolemic stimuli mostly concerns the muscular circulation [].
The rapid blood volume change is detected by tHb and THI (Figure 2 and Figure 4): as expected a decrease occurs in the arm, as a consequence of lowered central venous pressure [,,], and an increase occurs in the leg, as a consequence of vessel dilatation stimulated by the increased transmural pressure [,]. However, while in the arm it completes within 15–20 s, in the leg it continues at a slower rate for more than 5 min, documenting the occurrence of a slow stress relaxation of vessel walls. The relevance of stress relaxation was pointed out with model fitting the hemodynamic response to passive head-up tilt []. In the cited study a time constant of 30 s was assumed for this phenomenon, based on previous studies on animal models. The present results suggest a slower time course (with a time constant of about 90 s), as can be roughly estimated from the THI and tHb recordings of Figure 4 (right). However, it must be reminded that the present measurements are representative of a small sample volume, not the entire lower limb, and that the largest and fastest volume increase is exhibited by the large veins rather than smaller vessels within the muscle tissue [].
After the initial transient, the effect of vasoconstriction and blood flow reduction may be analyzed. To this aim, the attention has been focused on TOI and HHb whose slopes are indicative of a perfusion/metabolism mismatch [], while O2Hb was not considered due to its larger dependence on confounding factors, such as the cutaneous circulation []. Since blood pressure is substantially unchanged, as well as the metabolism of resting limbs, the negative TOI and positive HHb slopes reveal a reduction in blood flow, as likely induced by the sympathetically mediated increase in vascular resistances. Notably, this effect is LBNP-dependent and occurs in both arms and legs. It can be observed that this slow TOI decrease (and HHb increase) is much slower in the leg than in the arm. This is likely due to the longer time required for the deoxygenated blood to invade the enlarged venous compartment of the legs as compared to the emptied compartment of the arms, as well as to a hyperemic response affecting the lower limbs (see below). It has been proposed that the myogenic constriction in response to the increase in transmural pressure is primarily responsible for the increase in vascular resistance of lower limbs []. However, this mechanism does not explain the similar response to LBNP of upper limbs, not concerned by changes in transmural pressure. The vasoconstriction in upper and, at least in part, in lower limbs must be attributed to increases in sympathetic activity as documented also by direct recording of muscle sympathetic nerve activity in the same conditions [,]. Based on the lack of a visible drops in arterial blood pressure and increases in heart rate, at these low levels of LBNP stimuli (as observed also in the present study), it is generally considered that the sympathetic nervous system is primarily activated by the cardiopulmonary reflex [,], with only minor contributions from the arterial baroreflex []. Notably, hemodynamic changes in the upper limbs almost fully develop in about 90 s while accommodation of blood volume in the lower limbs takes more than 5 min.
One aspect that remains to be discussed is the transient TOI increase that takes place at the beginning of the LBNP stimulus. In the arm, it is small and short lasting (<20 s) and is likely due to the emptying of the venous compartment, i.e., it does not mean a real increase in oxygenation in the tissues but simply reflects the reduced amount of deoxygenated hemoglobin in the sample volume. The same explanation does not apply to the leg, where venous blood volume increases. The larger and longer-lasting TOI increase in the leg reflects, in fact, a real increase in oxygenation, caused by a transient hyperemia. This phenomenon was documented by Lott et al. [] who observed a short-lasting (10–15 s) femoral blood flow increase when exposing one leg, inserted into a limb tank, to negative pressure stimuli. The magnitude of the peak hyperemia increased with the magnitude of the negative pressure, almost doubling resting blood flow, at −50 mmHg. Not surprisingly, the effect on tissue oxygenation largely outlasts the duration of the hyperemia, as has been observed with compressive stimuli and short contractions, eliciting hyperemic responses lasting 10–20 s and a TOI increase of up to 100 s in duration [,]. In the present data, this TOI increase is particularly prominent and longer lasting in females (see, Figure 4), possibly due to a weaker constrictory response (see male–female comparison below).
Failing to discriminate blood volume from oxygenation changes may limit the understanding of the underlying physiological processes, leading to possible misinterpretations. For instance, HHb in the forearm was reported to be unaffected by LBNP up to −50 mmHg []. However, the present results clearly indicate a prominent vasoconstriction-induced HHb increase, compensating the initial drop caused by venous emptying. Curiously, since both phenomena are dependent on the magnitude of the LBNP, such compensation is reproduced at the different pressures so that HHb returns close to control levels within 90 s from the beginning of the LBNP, in all conditions (Figure 2).
A specular pattern can be exhibited by O2Hb in lower limbs whereby an initial increase due to blood volume increase and hyperemia is followed by a decrease due to reduced blood flow and increased Hb desaturation. The two changes may compensate each other, leading to an overall non-significant change in O2Hb (with LBNP up to −30 mmHg) and to the conclusion that vasoconstriction in lower legs does not take place or is delayed compared to other compartments []. On the contrary, the present study documents consistent constriction at the thigh already at −10 mmHg, as revealed by TOI and HHb slopes.
4.4. Sex Differences
It is generally accepted that women exhibit a lower tolerance to orthostatic stress and hypovolemic stimuli [,,]. Specifically, when exposed to LBNP women were shown to exhibit pre-syncopal symptoms earlier than men [,,,]. For example, in a progressively increasing LBNP protocol women reported pre-syncopal symptoms at 462 mmHg·min compared to 1070 mmHg·min in men. In the present study, employing mild LBNP stimuli (<150 mmHg·min), we did not evidence significant changes in ABP but only minor changes in HR, with no differences between males and females, in agreement with previous studies []. However, clear evidence of sympathetic vasoconstriction was consistently detected in terms of reduced tissue oxygenation already at −10 mmHg. This effect, quantified in terms of TOI and HHb slope, was significantly larger in males than females in both arms and legs. There are, however, contrasting results in the literature about possible gender differences in the extent of peripheral vasoconstriction. Convertino [] reported a higher decrease in forearm vascular conductance (FVC) in women than men exposed to progressive LBNP; the difference remained even when changes in vascular conductance were related to changes in central venous pressure (CVP). With a similar protocol, Franke et al. reported, instead, absence of differences in ΔFVC/ΔCVP up to −20 mmHg []; however, in their figure, women appear to reach the minimum FVC already at −20 mmHg, while males progressively decreased FVC up to −90 mmHg, thus demonstrating a stronger vasoconstrictive capacity. Interestingly, Hudson et al. [] reported a higher vasoconstrictor response in a group including trained and untrained women compared to men, who, however, also exceptionally exhibited higher tolerance than men. On the other hand, several studies reported larger constrictor responses in men in both the upper [] and lower limbs [,,]. In addition, women appeared to have a reduced myogenic constrictor response in limbs exposed to negative pressure [] and lower muscle sympathetic nerve activity in response to LBNP []. In the cited studies, blood flow measurements, generally based on venous occlusion plethysmography, may be difficult to interpret, given the need to account for differences in body weight, tissue composition, etc. In this respect, the presently observed decrease in tissue oxygenation, reflecting the increased Hb desaturation, may better represent the actual hypoperfusion undergone by the tissues. Our data support the concept that males have a more prominent capacity to reduce peripheral blood flow, which may contribute to their generally observed greater tolerance to LBNP.
As for blood pooling in the legs, a number of studies have reported increased pooling in males compared to females during LBNP [,,]. In the present study, NIRS blood volume indices did not evidence any dependence on sex. These data are not in contrast considering that THI and tHb are related to a small cutaneous-muscular sample volume which is not representative of the entire limb and that the largest blood volume changes occur at the level of the large veins [].
4.5. Limb Differences
Although the present data evidenced similar slopes in TOI and HHb in the forearm and the thigh during LBNP, conclusions about a similar vasoconstrictive action in the upper and lower limbs would not be correct, as the larger blood volume of the lower limbs is expected to slow down the deoxygenation of the whole sample volume compared to upper limbs. In fact, vasoconstriction and the ensuing reduction in blood flow increase Hb desaturation in the capillaries, which in turn reduces the Hb saturation in the venous compartment (and in the whole sample volume); this latter process is slower if the size of the venous compartment is larger. As discussed above, a slower TOI and HHb time course is, in fact, observed in the leg. As a corollary of this consideration, having a similar slope in arms and legs implies that a stronger constriction takes place in the legs. The issue of a possibly stronger sympathetic constriction in lower limbs has long been investigated and is supported by a number of studies [,,] but not all []. In particular, dilatation in the (contralateral) arm and constriction in the leg were observed during a unilateral handgrip exercise [,], while a greater gain of the blood flow response to changes in CVP was reported for the leg compared to the arm by Vissing [] during mild LBNP. In the latter study, the authors were cautious in the interpretation of the result, observing that it could have been caused by the larger muscle/skin ratio in the calf compared to the forearm. This concern is now resolved, considering that the specific focus of the TOI measurement is on the deep muscle tissue, with virtually no effects from the skin. The present data thus reinforce the notion of stronger sympathetic constrictor effectiveness in the lower compared to the upper limbs. Recent investigations on head muscles showed a similar relation between head and upper limb muscles: the former exhibiting a less constrictory action or even dilatation during sympathetic activation tests [,]. Interestingly, dilator responses to endothelium-dependent and -independent vasodilators were reported to be blunted in the legs compared to the arms [] and the myogenic response elicited by application of negative pressure to single limbs produced a stronger vasoconstriction in the legs than in the arms []. All these data support the concept that the constrictor capacity of blood vessels is differentiated in the different body areas according to a vertical gradient, adequate to match the hydrostatic gradient that develops with an erect posture: the constrictor capacity is higher where blood pressure is higher [,].
4.6. Limitations of the Study
A first limitation is related to neglecting the menstrual phase of the female participants. It has been shown that the phase of the menstrual cycle can influence the activation of the sympathetic nervous system under conditions of hypovolemia induced via LBNP [,]. However, other studies have failed to evidence significant effects of menstrual phase on orthostatic tolerance [] as well as on cardiovascular variables such as HR, CO, TPR, and stroke volume, both at rest and in response to hypovolemic stress, induced via LBNP or head-up tilt [,,].
An additional limitation concerns the fact that the influence of adipose tissue thickness (ATT) was not accounted for. It is known that ATT may affect NIRS measurements and complicate the comparison between subject groups []. In particular, van Beekvelt et al. reported that tissue oxygenation changes during ischemia, as assessed by the slope of the BL index Hbdiff, defined as the difference between O2Hb and HHb, negatively correlated with ATT. On this basis, a hypothetically larger ATT in females than in males could have contributed to the lower TOI and HHb slopes observed in the present study. However, several considerations point to excluding a major influence of ATT on the present results: (1) spatially resolved NIRS variables like TOI are intrinsically less affected by superficial tissue layers [,], including the adipose tissue, although some residual influence remains []; (2) the difference in ATT between males and females in the present study was presumably small, given the similarity in BMI between the two groups (24 vs. 21 kg/m2); (3) ATT is considered to predominantly affect the blood volume rather than oxygenation indices [,]; however, no main effect of sex on HHb and THI was observed here. These considerations support the concept that the observed differences in TOI and HHb slopes truly indicate a sex difference in blood flow control.
5. Conclusions
In this study, the time course of NIRS variables collected from arms and legs has been analyzed during short-lasting isolated exposure to mild LBNP. This approach allowed us to disentangle and quantify blood volume from blood flow changes and to detect stronger vasoconstriction in the leg compared to the arms and in males compared to females. The present results may serve as a reference for future studies with LBNP as well as for other experimental challenges.
Author Contributions
M.R. and R.A. contributed equally to this paper. Conceived and designed research: M.R. and S.R.; performed experiments: M.R. and R.A.; analyzed data: M.R. and R.A.; interpreted results of experiments: M.R. and S.R.; prepared figures: M.R. and R.A.; drafted manuscript: M.R. and R.A.; edited and revised manuscript: S.R.; approved final version of manuscript: M.R., R.A. and S.R. All authors have read and agreed to the published version of the manuscript.
Funding
The research activity was supported by Ministero dell’Università e della Ricerca under ‘Dipartimenti di Eccellenza ex L.232/2016’ to Dipartimento di Neuroscienze, Università di Torino (ECCELLENZA2327_D21) and by the European Union, NextGenerationEU, PNRR program (ROAS_PNRR_MIMIT_POC_23_01-C18H23000530002; recipient: S.R.).
Institutional Review Board Statement
All authors have read the journal’s authorship statement. The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Local Ethical Committee of University of Turin 125507, 16 February 2024.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflict of interest. All authors have read the journal’s policy on the disclosure of potential conflicts of interest. Moreover, all authors declare that there are no conflicts of interest to disclose.
Abbreviations
The following abbreviations are used in this manuscript:
| LBNP | Lower Body Negative Pressure |
| NIRS | Near-Infrared Spectroscopy |
| MAP | Mean Arterial Blood Pressure |
| CNAP | Continuous Non-invasive Arterial Pressure |
| CO | Cardiac Output |
| HR | Heart Rate |
| HHb | Changes in Deoxygenated Hemoglobin + Myoglobin |
| O2Hb | Changes in Oxygenated Hemoglobin + Myoglobin |
| BL | Beer–Lambert |
| tHb | Total Hemoglobin |
| THI | Tissue Hemoglobin Index |
| TOI | Tissue Oxygenation Index |
| ANOVA | Analysis of Variance |
| CSI | Cumulative Stress Index |
| SRS | Spatially Resolved Spectroscopy |
| FVC | Forearm Vascular Conductance |
| CVP | Central Venous Pressure |
| Hbdiff | Difference between O2Hb and HHb |
| ATT | Adipose Tissue Thickness |
References
- Goswami, N.; Blaber, A.P.; Hinghofer-Szalkay, H.; Convertino, V.A. Lower body negative pressure: Physiological effects, applications, and implementation. Physiol. Rev. 2019, 99, 807–851. [Google Scholar] [CrossRef]
- Convertino, V.A. Gender differences in autonomic functions associated with blood pressure regulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1998, 275, R1909–R1920. [Google Scholar] [CrossRef]
- Fu, Q.; Arbab-Zadeh, A.; Perhonen, M.A.; Zhang, R.; Zuckerman, J.H.; Levine, B.D. Hemodynamics of orthostatic intolerance: Implications for gender differences. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H449–H457. [Google Scholar] [CrossRef]
- Montgomery, L.D.; Kirk, P.J.; Payne, P.A.; Gerber, R.L.; Newton, S.D.; Williams, B.A. Cardiovascular responses of men and women to lower body negative pressure. Aviat. Space Env. Med. 1977, 48, 138–145. [Google Scholar]
- Franke, W.D.; Johnson, C.P.; Steinkamp, J.A.; Wang, R.; Halliwill, J.R. Cardiovascular and autonomic responses to lower body negative pressure. Clin. Auton. Res. 2003, 13, 36–44. [Google Scholar] [CrossRef]
- Hachiya, T.; Hashimoto, I.; Saito, M.; Blaber, A.P. Peripheral vascular responses of men and women to LBNP. Aviat. Space Environ. Med. 2012, 83, 118–124. [Google Scholar] [CrossRef]
- Frey, M.A.; Hoffler, G.W. Association of sex and age with responses to lower-body negative pressure. J. Appl. Physiol. 1988, 65, 1752–1756. [Google Scholar] [CrossRef]
- Ferrari, M.; Muthalib, M.; Quaresima, V. The use of near-infrared spectroscopy in understanding skeletal muscle physiology: Recent developments. Phil. Trans. R. Soc. A 2011, 369, 4577–4590. [Google Scholar] [CrossRef]
- Messere, A.; Ceravolo, G.; Franco, W.; Maffiodo, D.; Ferraresi, C.; Roatta, S. Increased tissue oxygenation explains the attenuation of hyperemia upon repetitive pneumatic compression of the lower leg. J. Appl. Physiol. 2017, 123, 1451–1460. [Google Scholar] [CrossRef]
- Rashid, A.; Roatta, S. Differential control of blood flow in masseter and biceps brachii muscles during stress. Arch. Oral Biol. 2022, 141, 105490. [Google Scholar] [CrossRef]
- Ye, S.; Stetter, S.; McCully, K.K. Muscle Metabolism During Multiple Muscle Stimulation Using an Affordable Equipment. JFMK 2024, 9, 248. [Google Scholar] [CrossRef]
- Canova, D.; Roatta, S.; Bosone, D.; Micieli, G. Inconsistent detection of changes in cerebral blood volume by near infrared spectroscopy in standard clinical tests. J. Appl. Physiol. 2011, 110, 1646–1655. [Google Scholar] [CrossRef]
- Messere, A.; Roatta, S. Influence of cutaneous and muscular circulation on spatially resolved versus standard Beer-Lambert near-infrared spectroscopy. Physiol. Rep. 2013, 1, e00179. [Google Scholar] [CrossRef]
- Grassi, B.; Quaresima, V. Near-infrared spectroscopy and skeletal muscle oxidative function in vivo in health and disease: A review from an exercise physiology perspective. J. Biomed. Opt. 2016, 21, 091313. [Google Scholar] [CrossRef]
- Seddone, S.; Ermini, L.; Policastro, P.; Mesin, L.; Roatta, S. Evidence that large vessels do affect near infrared spectroscopy. Sci. Rep. 2022, 12, 2155. [Google Scholar] [CrossRef]
- Messere, A.; Roatta, S. Local and remote thermoregulatory changes affect NIRS measurement in forearm muscles. Eur. J. Appl. Physiol. 2015, 115, 2281–2291. [Google Scholar] [CrossRef]
- Gonzalez, J.E.; Cooke, W.H. Acute fasting reduces tolerance to progressive central hypovolemia in humans. J. Appl. Physiol. 2024, 136, 362–371. [Google Scholar] [CrossRef]
- Hachiya, T.; Blaber, A.P.; Saito, M. Changes in superficial blood distribution in thigh muscle during LBNP assessed by NIRS. Aviat. Space Environ. Med. 2004, 75, 118–122. [Google Scholar]
- Murphy, E.K.; Bertsch, S.R.; Klein, S.B.; Rashedi, N.; Sun, Y.; Joyner, M.J.; Curry, T.B.; Johnson, C.P.; Regimbal, R.J.; Wiggins, C.C.; et al. Non-invasive biomarkers for detecting progression toward hypovolemic cardiovascular instability in a lower body negative pressure model. Sci. Rep. 2024, 14, 8719. [Google Scholar] [CrossRef]
- Soller, B.R.; Ryan, K.L.; Rickards, C.A.; Cooke, W.H.; Yang, Y.; Soyemi, O.O.; Crookes, B.A.; Heard, S.O.; Convertino, V.A. Oxygen saturation determined from deep muscle, not thenar tissue, is an early indicator of central hypovolemia in humans. Crit. Care Med. 2008, 36, 176–182. [Google Scholar] [CrossRef]
- Yang, H.; Cooke, W.H.; Reed, K.S.; Carter, J.R. Sex differences in hemodynamic and sympathetic neural firing patterns during orthostatic challenge in humans. J. Appl. Physiol. 2012, 112, 1744–1751. [Google Scholar] [CrossRef]
- Hachiya, T.; Walsh, M.L.; Saito, M.; Blaber, A.P. Delayed vasoconstrictor response to venous pooling in the calf is associated with high orthostatic tolerance to LBNP. J. Appl. Physiol. 2010, 109, 996–1001. [Google Scholar] [CrossRef]
- Niemeijer, V.M.; Jansen, J.P.; van Dijk, T.; Spee, R.F.; Meijer, E.J.; Kemps, H.M.; Wijn, P.F. The influence of adipose tissue on spatially resolved near-infrared spectroscopy derived skeletal muscle oxygenation: The extent of the problem. Physiol. Meas. 2017, 38, 539. [Google Scholar] [CrossRef]
- Vissing, S.F.; Scherrer, U.; Victor, R.G. Relation between sympathetic outflow and vascular resistance in the calf during perturbations in central venous pressure. Evidence for cardiopulmonary afferent regulation of calf vascular resistance in humans. Circ. Res. 1989, 65, 1710–1717. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.G.; Mahon, S.; Tromberg, B.J.; Ryan, K.L.; Convertino, V.A.; Rickards, C.A.; Osann, K.; Brenner, M. Tissue hemoglobin monitoring of progressive central hypovolemia in humans using broadband diffuse optical spectroscopy. J. Biomed. Opt. 2008, 13, 064027. [Google Scholar] [CrossRef][Green Version]
- Wolthuis, R.A.; Bergman, S.A.; Nicogossian, A.E. Physiological effects of locally applied reduced pressure in man. Physiol. Rev. 1974, 54, 566–595. [Google Scholar] [CrossRef] [PubMed]
- van Heusden, K.; Gisolf, J.; Stok, W.J.; Dijkstra, S.; Karemaker, J.M. Mathematical modeling of gravitational effects on the circulation: Importance of the time course of venous pooling and blood volume changes in the lungs. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, 2152–2165. [Google Scholar] [CrossRef]
- Buckey, J.C.; Peshock, R.M.; Blomqvist, C.G. Deep venous contribution to hydrostatic blood volume change in the human leg. Am. J. Cardiol. 1988, 62, 449–453. [Google Scholar] [CrossRef]
- Lott, M.E.J.; Hogeman, C.; Herr, M.; Bhagat, M.; Sinoway, L.I. Sex differences in limb vasoconstriction responses to increases in transmural pressures. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H186–H194. [Google Scholar] [CrossRef]
- Rea, R.F.; Hamdan, M.; Clary, M.P.; Randels, M.J.; Dayton, P.J.; Strauss, R.G. Comparison of muscle sympathetic responses to hemorrhage and lower body negative pressure in humans. J. Appl. Physiol. 1991, 70, 1401–1405. [Google Scholar] [CrossRef]
- Furlan, R.; Jacob, G.; Palazzolo, L.; Rimoldi, A.; Diedrich, A.; Harris, P.A.; Porta, A.; Malliani, A.; Mosqueda-Garcia, R.; Robertson, D. Sequential modulation of cardiac autonomic control induced by cardiopulmonary and arterial baroreflex mechanisms. Circulation 2001, 104, 2932–2937. [Google Scholar] [CrossRef][Green Version]
- Messere, A.; Tschakovsky, M.; Seddone, S.; Lulli, G.; Franco, W.; Maffiodo, D.; Ferraresi, C.; Roatta, S. Hyper-Oxygenation Attenuates the Rapid Vasodilatory Response to Muscle Contraction and Compression. Front. Physiol. 2018, 9, 1078. [Google Scholar] [CrossRef]
- Hachiya, T.; Blaber, A.P.; Saito, M. Near-infrared spectroscopy provides an index of blood flow and vasoconstriction in calf skeletal muscle during lower body negative pressure. Acta Physiol. 2008, 193, 117–127. [Google Scholar] [CrossRef]
- Blaber, A.P.; Hinghofer-Szalkay, H.; Goswami, N. Blood volume redistribution during hypovolemia. Aviat. Space Environ. Med. 2013, 84, 59–64. [Google Scholar] [CrossRef]
- White, D.D.; Gotshall, R.W.; Tucker, A. Women have lower tolerance to lower body negative pressure than men. J. Appl. Physiol. 1996, 80, 1138–1143. [Google Scholar] [CrossRef]
- Carter, R.; Hinojosa-Laborde, C.; Convertino, V.A. Sex comparisons in muscle sympathetic nerve activity and arterial pressure oscillations during progressive central hypovolemia. Physiol. Rep. 2015, 3, e12420. [Google Scholar] [CrossRef]
- Rahman, M.A.; Goodhead, K.; Medcalf, J.F.; O’Connor, M.; Bennett, T. Haemodynamic responses to nonhypotensive central hypovolaemia induced by lower body negative pressure in men and women. Eur. J. Appl. Physiol. 1991, 63, 151–155. [Google Scholar] [CrossRef]
- Hudson, D.L.; Smith, M.L.; Raven, P.B. Physical fitness and hemodynamic response of women to lower body negative pressure. Med. Sci. Sports Exerc. 1987, 19, 375–381. [Google Scholar] [CrossRef]
- Eklund, B.; Kaijser, L.; Knutsson, E. Blood flow in resting (contralateral) arm and leg during isometric contraction. J. Physiol. 1974, 240, 111–124. [Google Scholar] [CrossRef]
- Rusch, N.; Shepherd, J.; Webb, R.C.; Vanhoutte, P. Different behavior of the resistance vessels of the human calf and forearm during contralateral isometric exercise, mental stress, and abnormal respiratory movements. Circ. Res. 1981, 48, I118–I130. [Google Scholar]
- Newcomer, S.C.; Leuenberger, U.A.; Hogeman, C.S.; Handly, B.D.; Proctor, D.N. Different vasodilator responses of human arms and legs. J. Physiol. 2004, 556, 1001–1011. [Google Scholar] [CrossRef]
- Proctor, D.N.; Newcomer, S.C. Is There a Difference in Vascular Reactivity of the Arms and Legs? Med. Sci. Sports Exerc. 2006, 38, 1819–1828. [Google Scholar] [CrossRef]
- Usselman, C.W.; Nielson, C.A.; Luchyshyn, T.A.; Gimon, T.I.; Coverdale, N.S.; Van Uum, S.H.M.; Shoemaker, J.K. Hormone phase influences sympathetic responses to high levels of lower body negative pressure in young healthy women. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R957–R963. [Google Scholar] [CrossRef]
- Shankhwar, V.; Urvec, J.; Steuber, B.; Schmid Zalaudek, K.; Saloň, A.; Hawliczek, A.; Bergauer, A.; Aljasmi, K.; Abdi, A.; Naser, A.; et al. Effects of menstrual cycle on hemodynamic and autonomic responses to central hypovolemia. Front. Cardiovasc. Med. 2024, 11, 1290703. [Google Scholar] [CrossRef]
- Meendering, J.R.; Torgrimson, B.N.; Houghton, B.L.; Halliwill, J.R.; Minson, C.T. Menstrual cycle and sex affect hemodynamic responses to combined orthostatic and heat stress. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H631–H642. [Google Scholar] [CrossRef]
- Frey, M.; Mathes, K.L.; Hoffler, G.W. Cardiovascular responses of women to lower body negative pressure. Aviat. Space Environ. Med. 1986, 57, 531–538. [Google Scholar]
- Barstow, T.J. Understanding near infrared spectroscopy and its application to skeletal muscle research. J. Appl. Physiol. 2019, 126, 1360–1376. [Google Scholar] [CrossRef]
- Craig, J.C.; Broxterman, R.M.; Wilcox, S.L.; Chen, C.; Barstow, T.J. Effect of adipose tissue thickness, muscle site, and sex on near-infrared spectroscopy derived total-[hemoglobin+ myoglobin]. J. Appl. Physiol. 2017, 123, 1571–1578. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).