Abstract
Mirror neurons (MNs), a set of neurons that are activated during the processes of observation and execution of actions, have drawn significant attention in the research of neurodegenerative and psychological disorders. Research in the field of Autism Spectrum Disorder (ASD) and schizophrenia demonstrates evidence in favour of common underlying neural mechanisms underlying the two conditions, especially with respect to mu rhythm suppression, a proxy for MN activation and socio-cognitive impairments. This paper aims to review the most recent studies on the neurological underpinnings of social cognition deficits and cognitive discrepancies shared by ASD and schizophrenia, as detected by measuring the functionality and activation of the mirror neuron system. The findings of the review reveal a lack of consensus with respect to the validity of the “broken mirror” theory. The review also shows that further research is warranted to shed light on the implications of mirror neuron dysfunction in neuropsychiatric conditions and assist the development of technological interventions and treatments.
1. Introduction
Mirror neurons (MNs), a set of neurons that are activated during the processes of observing and executing actions, have drawn significant attention in the research of neurodegenerative and mental disorders. Mirror neurons are located in the inferior parietal cortex, the premotor cortex, and the primary somatosensory cortex [], and they are hypothesised to play a significant role in the comprehension and action of imitation, since the activation patterns of MNs during the process of observing an action are very comparable to those observed when an individual is performing an action []. The mechanism of mirroring elements of motion and behaviour in social contexts and the ability to reproduce these patterns in an imitative manner have been attributed to the firing of this set of neurons [].
Mirror neurons were originally described in 1992 as a set of cells located in the premotor cortex of primates (macaque monkeys) []. Recent research has investigated the involvement of MNs in a number of cognitive and social functions. In humans, as well as in primates, this set of neurons has been found to be linked to social cognition that includes empathy and emotion understanding, intention understanding [], imitation and language processing [,]. Due to their strong relation with the ability to acquire knowledge through imitation processes [], MNs are activated during the action of the sensorimotor system for perceiving facial expressions and gesturing []. Research alleges that this set of neurons can be found in brain regions that include the inferior frontal gyrus [], the superior temporal sulcus [] and the ventral premotor cortex [], which are dynamically involved in the processing of facial expressions and in higher-level cognitive abilities, such as Theory of Mind and empathy []. The aforementioned cerebral distribution advocates the functionality of MNs in processes that go beyond simple motor imitation.
However controversial, it has been theorised that the evolution of MNs also relates to language development by enabling complex communication systems []. Broca’s area, which plays a crucial role in language in both production and perception, has been suggested to be a critical MN region. Nevertheless, research using neuroimaging techniques has shown that MNs are most probably associated with mechanisms implicated in speech articulation and not the semantic representation of human language [].
Brodmann area 2 (BA2) has also been identified as having a relation with the function of the MN system (MNS defined as the group of MNs) []. BA2 has been determined as the locus of mu rhythm generation []. Measuring mu rhythm suppression has been shown to be a valid method for examining the level of activation of the MNS. Mu rhythm suppression indicates reduction in the amplitude of mu waves, which are cerebral oscillations in the frequency band of 8–13 Hz observed in an electroencephalogram (EEG) in the sensorimotor cortex. This type of modulation indicates the difference between active and baseline condition in MN activation. Mu suppression generally emerges during the performance of motion or the observation of such actions []. In addition, mu rhythm suppression has been linked to socio-cognitive mechanisms responsible for the development of intention understanding, inference making, emotion recognition and empathy []. Mu suppression therefore seems to be the neural basis for both social cognition and sensorimotor abilities. Figure 1 below illustrates the parietal mirror neuron (MN) system located in the inferior parietal lobule, as well as the frontal MN system situated in the inferior frontal gyrus.
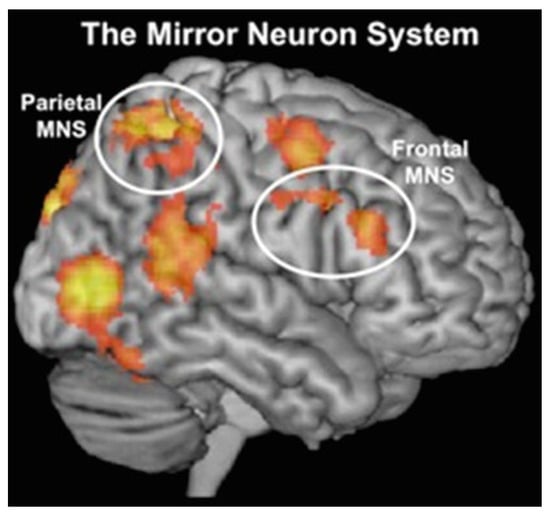
Figure 1.
Schematic illustration of the mirror neuron system and its brain correlates, depicting parietal MN system in the inferior parietal lobule and frontal MN system in the inferior frontal gyrus ([], p.186). Reproduced with permission from Liew & Aziz-Zadeh, Handbook of Neurosociology; published by Springer, Dordrecht, 2013.
Mu rhythm suppression has been a fruitful area of study in the examination of the neurobiological underpinnings of Autism Spectrum Disorder (ASD) and schizophrenia. ASD and schizophrenia are two distinct conditions in terms of clinical profiling and diagnostic traits, which nevertheless demonstrate particular commonalities, such as deficits in social cognition and difficulties with social navigation. These impairments have been associated with the function of the MNS and reduced mu rhythm suppression that individuals with ASD and schizophrenia exhibit while performing in social observation, imitation and empathy tasks []. In addition, both conditions present altered connectivity in specific cerebral regions, such as the superior temporal sulcus and sensorimotor cortex. These regions are related to mu rhythm activity, and contribute to discrepancies identified in both ASD and schizophrenia when processing and responding to social stimuli. The aim of this review is to provide insight into both the distinct and shared impairments in social cognition that characterise the two conditions, as reflected in the aberrant activation of mu rhythm suppression. The examination of mu rhythm suppression in ASD and schizophrenia seems to be crucial for comprehending the neurobiological mechanisms underlying social cognition impairments in these disorders.
Although previous reviews have examined the function of MNs in both ASD and schizophrenia [,], the present review will focus on investigating the phenomenon of mu rhythm suppression in both conditions through studies that have employed the electroencephalography (EEG) technique. This review will visit upon findings of studies within the past 10 years, while selection criteria involve the administration of EEG tests to measure mu rhythm suppression in both conditions with relevance to social dysfunction and impairments in social cognition. To secure the transparent selection of data, this review adheres to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) 2020 checklist (www.prisma-statement.org, accessed on 15 January 2025). Table 1 below demonstrates the selection and rejection criteria for the studies reviewed in the current paper according to PRISMA guidelines. This review hypothesises that both ASD and schizophrenic individuals demonstrate abnormal mu suppression activation and similarities in the hypofunction of MNs.

Table 1.
Inclusion and exclusion criteria.
2. Autism Spectrum Disorder (ASD) vs. Schizophrenia: General Overview
2.1. Autism Spectrum Disorder
Autism Spectrum Disorder (ASD) is characterised by marked phenotypic heterogeneity in terms of manifestation of symptoms, level of severity and traits []. It is defined as a pervasive neurodevelopmental disorder that demonstrates impairments in social interaction, restricted or/and repetitive behavioural patterns, limited interests, and deficits in communication, both verbal and non-verbal []. The autistic spectrum reflects a set of characteristics that correspond to a range between high- and low-functioning individuals []. The classification of DSM-5 (Diagnostic and Statistical Manual of Mental Disorders) for ASD has replaced the term “Pervasive Developmental Disorders” with a single category that comprises previous mentions of diagnostic classes (i.e., Asperger’s Syndrome, Pervasive Developmental Disorder not otherwise specified (PDD-NOS), Rett Syndrome, and Childhood Disintegrative Disorder). It also identifies three severity levels that rely on the extent of support required for each individual in the spectrum [].
The autistic spectrum is very often linked to motor sensory deficits and problems with coordination, as well as mental disabilities, attentional impairments and anxiety []. However, despite the commonality of traits, individuals with ASD demonstrate individual variation in their general skills and severity of symptomatology. The aetiology of the disorder is multifaceted, encompassing epigenetic factors that stem both from environmental and biological origins or genetic predisposition. Nevertheless, it is still unclear which of those factors mostly contribute to the emergence of ASD, and to what extent. In addition, comorbidity between ASD and other disorders, such as Attention Deficit Hyperactivity Disorder (ADHD), Developmental Language Disorder (DLD), learning disabilities or/and neuropsychological conditions, is very often evinced in ASD, making it difficult to detect symptoms that are specific to the autistic spectrum [].
Research on functional connectivity in ASD demonstrates atypical brain connectivity and deviations from neurotypical populations in terms of brain structure, such as differences in connectivity in the prefrontal cortex, amygdala and cerebellum []. Despite impairments in the neurobiological basis of autism and the neural circuits that are associated with social understanding, some individuals with ASD demonstrate strong abilities in several cognitive areas such as mathematics, art or music []. ASD is more frequently diagnosed in male (vs. female) individuals with a ratio of 4:1, and also more frequently observed in individuals who have siblings with the disorder. Research reveals a large increase in the reported diagnosed cases of ASD within the past 30 years, which is attributed to the existence of standardised diagnostic tools that can more accurately detect autistic traits and separate the diagnostic criteria for ASD from symptoms ascribed to other conditions [].
2.2. Schizophrenia
Schizophrenia is one of the most common mental and neuropsychiatric disorders that affects in approximation a minimum of 0.7% of the global population [,]. Characteristics of the disorder encompass a variety of symptoms, i.e., hallucinations in the sense of seeing non-existing things or people or hearing voices, delusions and unrealistic thoughts, disoriented thinking and language, a sense of disconnection and disassociation from interests, feelings, people, personal care and emotions []. The disorder has an impact on the affected individuals in day-to-day life, negatively influencing their functioning, communication, and social interaction skills, and can result into severe disability, while in many cases, it can also be fatal [].
The onset of the disease emerges early in adulthood, or late adolescence, and it tends to prevail more often in male individual than females. Its aetiology lies in a combination of factors that involve genetic, biological and environmental origins []. Research in the field of genetics and genome-wide association studies (GWAS) has identified genes associated with neural connectivity, synaptic function, calcium signalling and neurotransmitter systems []. Prenatal and perinatal factors as well as abusive experiences constitute environmental factors that have also been found to be involved in the occurrence of schizophrenia [,]. In terms of the pathophysiology of the disorder, neuroimaging studies show that individuals with schizophrenia exhibit cerebral abnormalities [,] that encompass alterations in connectivity between brain regions whose grey matter volume is pathologically reduced, such as the temporal lobes, the prefrontal cortex and the hippocampus. In addition, irregularities in terms of dopamine and glutamatergic neurotransmission [] have been hypothesised to have an impact on the positive and negative symptoms of the disorder.
The symptomatologic classification of schizophrenia reflects the heterogeneity that defines it, and it involves positive, negative and cognitive symptoms. In terms of positive symptoms, they normally appear when the individual experiences a psychotic episode, and involve delusional situations, hallucinations, inappropriate behaviours, and distorted thoughts. Negative symptoms fall into five distinctive categories, namely anhedonia, apathy, avolition, asociality alogia and blunted affect []. Cognitive symptoms can be detected in about 70% of the individuals diagnosed with schizophrenia, and in most cases, they are present before the onset of the disease []. This category of symptoms comprises impairments in verbal and non-verbal memory, attention, reasoning, visual processing especially when experiencing visual illusions, and general executive functioning []. Social cognition is also impaired in individuals with schizophrenia: these individuals have problems with emotion recognition, phacial emotion perception, intention attribution, mentalising, perception of non-verbal social cues, non-literate language and general social contexts [,].
2.3. Commonalities Between ASD and Schizophrenia
When comparing the two conditions, ASD and schizophrenia share significant similarities with respect to deficits in social cognition, despite the fact that they are both separately diagnosed neuropsychiatric disorders. Individuals with either of these conditions demonstrate impairments in mentalising and Theory of Mind, which refers to the ability to comprehend and infer the mental states of others and of oneself, to take other people’s perspectives, and interpret others’ emotions, beliefs and intentions [,]. Theory of Mind deficits result in multiple challenges in everyday social navigation. In ASD, individuals display problems with reasoning and interpreting social cues and behavioural patterns that are impelled by internal mental states and emotions [,]. In individuals with schizophrenia, on the other hand, social and cognitive symptoms are manifested as the negative symptoms of the disorder, and involve a lack of motivation for social integration, problems with emotion perception and expression, and impaired Theory of Mind and sense of empathy [].
Neuroimaging studies have demonstrated impairments in the MNS in both ASD and schizophrenia [,], further supporting the hypothesis that socio-cognitive deficits stem from common neural mechanisms across the two disorders. Shared neural dysfunctions, for instance, irregular function in cerebral areas such as the amygdala, prefrontal cortex and superior temporal sulcus, indicate shared dysfunctional social cognition. The results of recent studies underscore the notable commonality of neuro- and socio-cognitive impairments in ASD and schizophrenia, placing emphasis on the individuals’ emotion recognition and irregular processing speed [].
3. The Mechanisms Behind Broken MNs in ASD and Schizophrenia
3.1. Mu Rhythm Suppression in ASD
Attenuations in the function of the MNS in autism have been included in the theory of “broken mirror neurons”, which has been researched over recent decades to explore possible links between atypical MN functionality and ASD symptomatology. Cognitive deficits in individuals with autism, and impairments in communication and social integration may be traced back to these links []. Dysfunctions in MNs have received attention in the field of autism research; nevertheless, they have also received criticism, mostly due to the mixed findings. It has been claimed that deficits in the sensorimotor system in individuals with ASD is a more plausible reasoning for explaining problems in mentalizing, imitation, and action interpretation. In addition, it has been proposed that the activation of MNS is fundamental for social cognition and reasoning [,] as well as the understanding of mental and emotional states []. These abilities have been well researched in ASD, which is often characterized by considerable discrepancies in internal state reasoning and Theory of Mind [].
Measurements of suppression of the mu wave EEG have been prominent in exploring MN function in individuals. In clinical populations, however, and in ASD in particular, mu rhythm suppression has not been the most effective method. This may be attributed to various reasons ranging from sample sizes to issues with electrode selection for data collection. Neuroimaging studies provide insights into abnormalities in ASD in cerebral areas that are crucially implicated in social cognition and processing of social stimuli, for instance, the prefrontal cortex and the superior temporal sulcus [].
One of the most prominent components that is hypothesised to trigger the activation of MNs is the observation of an action. Action observation involves, among others, the processing of facial expressions further related to ToM skills and emotion recognition []. Deschrijver et al. [] critically examined the motor interference effect in ASD, which corresponds to more errors and slower reaction times when an individual visually perceives a movement that contradicts the movement of intention. A group of 23 adults with high-functioning autism and 23 age-, handedness- and sex-matched healthy controls participated in an EEG paradigm. The study focused on the P3 (a positive deflection in EEG recordings that occurs approximately 300 ms after stimulus onset, and is associated with attention and stimulus evaluation), the N190 (a negative ERP component occurring around 190 ms after stimulus onset, commonly linked to early visual processing and face recognition), and the RP (“Readiness Potential”, a slow negative shift in EEG activity preceding voluntary movement, indicating motor preparation) that were congruent to the conditions of the stimulus. The participants were instructed to visually follow the gestural movements of a videotaped hand. Directly after the end of the observation, the participants were instructed to perform a pre-instructed gesture. The experiment involved a congruent condition, under which the required action matched the shown hand movement; an incongruent condition, where the observed gesture did not match the one for execution; and a baseline condition (hand in resting state). The aim of the study was to examine whether impairments in the processing of self- and other-action performances could provide a justification for imitation attenuations in high-functioning ASD. The results demonstrated that, in contrast with the predictions of the researchers of the study, there was no reported difference between the high-functioning autistic group and the control group in the P3 ERP component. In particular, both groups presented larger numbers of the P3 component when observing the congruent hand movement condition, so distinguishing between congruent and incongruent hand movements with high processing levels was intact. On the other hand, the clinical group demonstrated a larger value of RP for the congruent condition in comparison to the baseline, similarly to the incongruent condition, which implies elevated brain activation for motor preparation in both congruent and incongruent observations, while the baseline condition remained neutral. Comparing the compatible and non-compatible trials, no particular difference was reported. Crucially, the outcomes of the study show that the neural mechanisms underlying the motor interference effect in high-functioning ASD manifest discrepancies in the early stages of motor preparation rather than in higher-level cognitive processes, e.g., interpreting social cues, which contradicts the theory of “broken” MNs in ASD.
Another study that measures mu rhythm suppression in ASD through the observation of gestures is that of Fan et al. []. The objective of their study was to test the integrity of the MN hypothesis in ASD by administering an EEG experiment that measured mu rhythm suppression over the sensorimotor cortex while the participants observed and later imitated gestural hand movements. The study involved two groups of participants, 20 male adolescents and young adults, and 20 age-matched neurotypical controls. The two groups were required to complete an eye-tracking and an EEG test that included four conditions: baseline condition (observation of a static object on a screen), hand condition (observation of a video-recorded gesturing action), dot condition (observation of a video with a white dot), and execution condition (manipulation of an object in the same manner as in the hand condition). The study also controlled for fixation duration and normalised fixation number on the stimuli to account for potential differences in visual attention. The results strongly support the inefficacy of the theory of dysfunction in the MNS in ASD. Specifically, mu suppression over the sensorimotor cortex in the observation and imitation conditions did not present differences between the two groups. Essentially, mu rhythm suppression was stronger in both groups during the observation of the hand rather than the moving dot. Contrasting previous research [], no correlation between mu rhythm suppression and imitation was detected. Although the ASD participants scored lower than neurotypicals in the imitation condition, the mu rhythm suppression was not different from controls. The outcomes highlighted the heterogeneity of ASD in terms of traits, and concluded that the MNS activation appears to be intact to a certain extent.
A more recent study by Sotoodeh et al. [] examined the way mu suppression is modulated in ASD by visual attention during the process of perceiving biological motion. The researchers recruited 20 participants diagnosed with high-functioning ASD, and 20 age- and sex-matched healthy controls. An EEG paradigm was conducted to measure the suppression of mu rhythm over the sensorimotor cortex while participants were required to watch point-light displays presenting biological motions, e.g., walking, cartwheeling, free-throwing and underarm throwing, as well as non-biological motion, e.g., moving dots. Eye-tracking technology was used to monitor visual attention and ensure that participants were fixating on the stimuli. The EEG findings showed that visual attention in both groups had a significant impact on mu suppression; nevertheless, mu rhythm suppression in the ASD group was reduced compared to that of the control group during biological motion observation. This finding can be interpreted as a cue for neural deviations in biological motion processing in ASD, and that visual attention is not the only determining factor for impaired mu rhythm suppression. Furthermore, a positive correlation was detected between level of mu suppression and social abilities in the group with ASD, thus revealing an association between social cognition and mu suppression.
The study by Cole et al. [] aimed to investigate MN activation in individuals with ASD in the process of making inferences about other people’s intentions. In total, 43 participants took part in the study, distributed in two groups in compliance with the level of autistic traits. The 20 neurotypical controls that participated in the study reported no history of mental or neurological illness. The study used both transcranial magnetic stimulation (TMS) and EEG experiments to measure MNS activity, giving emphasis on the connection between autistic traits and neural responses during mentalizing tasks. The participants were instructed to watch short clip videos with mentalizing and non-mentalising actions, and place a judgement afterward with respect to the intention of the action performed. The study employed three screening tasks: an EEG task that was conducted during the projection of the videos, an eye-tracking task and a TMS-induced EMG (electromyography) task. In terms of mu rhythm suppression, this was found to be at a lower level in the right hemisphere among participants with higher level of autistic traits when performing in the mentalizing task. Nevertheless, mu rhythm suppression on the right hemisphere did not present significant correlation with how well the participants performed in the task. Notably, there was a positive correlation between lower MN activation in the left hemisphere and poor performance in the mentalising task, with no present effect occurring from the autistic traits. TMS-induced MEP did not present any significant variation between ASD individuals and controls. Similarly, no visual processing differences were reported for the eye-tracking task. The authors concluded that attenuated MNs and impaired ToM in ASD are the reasons behind the irregularities of MN activation in the right hemisphere.
Dumas et al. [] revisited the MN hypothesis aiming by placing emphasis on two main aspects of the existing EEG literature: the functional segregation of mu rhythm into two discrete sub-bands (8–10 Hz and 10–12/13 Hz), and the focus on central electrodes (C3/C4) in previous studies in ASD. Ten high-functioning individuals with ASD and 30 age-matched healthy controls participated in the study. The two groups were tested on a task involving three conditions, namely simple observation of gestures, free imitation of gestures, and imitation of a pre-recorded video. The outcomes of the study revealed a distinct pattern of mu suppression in the ASD group compared to the TD controls. Specifically, when the mu rhythm was analysed as a homogeneous phenomenon covering the 8–13 Hz frequency range, no significant differences were found between the groups. However, when the two sub-bands were distinguished, a different mu response to observation appeared for subjects with ASD in the upper sub-band (10–12/13 Hz) over the sensorimotor cortex, while the lower sub-band (8–10 Hz) responded similarly in both groups. Source reconstructions demonstrated that this effect was related to a joint mu-suppression deficit over the occipito-parietal regions and an increase over the frontal regions in the ASD group. The researchers argues that mu suppression deficits in individuals with ASD do not support a global irregular functionality of the MNS but are rather specific to the neural mechanisms underlying the observation of intentions.
3.2. Mu Rhythm Suppression in Schizophrenia
Mirror neurons have received quite some criticism in research in recent years due to controversial findings of studies and the soundness of the hypotheses concerning the exact function and significance of the MNS [,]. Nevertheless, they have also drawn significant attention, as they have been shown to be actively involved in complex cognitive processes, such as empathy, mentalising, perspective taking, language and imitation []. As mentioned before, the MNS has mostly been researched in ASD, mainly due to the hypothesis of the “broken mirror neurons” which negatively affect language, Theory of Mind and social navigation []. Nevertheless, as shown in the previous section, the results do not demonstrate a clear consensus as to the role of the MNS in social and cognitive discrepancies in autism [].
Research on the connection between the functionality of the MNS and schizophrenic symptoms has gained ground over the last decade. Schizophrenia is often characterised as the disorder of the “social brain” [], which underlines the attenuations in social cognition that schizophrenic patients exhibit. Similarly to ASD, the MNS system has also been investigated in schizophrenia using a variety of neuroimaging methods, each of which measures different aspects of the functions of the MNS (ex. fMRI measuring fluctuations in blood oxygenation, MEG measuring alpha band suppression and gamma band amplification, TMS, EEG measuring mu rhythm suppression, and electromyography to measure rapid face muscle activation during observation of facial expressions).
With respect to mu rhythm suppression as a proxy for MNS activation, research demonstrates abnormalities in the MNS in schizophrenia, which are mainly reflected in varying levels of mu suppression. Variation in mu rhythm suppression has in turn been linked to deficits in Theory of Mind, empathy, emotion recognition and processing of social cues []. Several studies have explored mu rhythm suppression in patients with schizophrenia; however, the outcomes are heterogeneous.
Horan et al. [] conducted an EEG study to explore impairments in the functioning of the MNS in patients with schizophrenia as indexed by mu suppression. The participants of their study included 32 outpatients diagnosed with schizophrenia, and 26 controls with no clinical diagnosis The two groups took part in an EEG paradigm, which involved six conditions of an either observed or executed action. These conditions contained varying levels of social interaction. In addition, validated empathy questionnaires were collected from the participants. The results revealed that both groups exhibited a crucial linear increase in mu rhythm suppression with respect to those conditions that contained a higher demand for socialisation; however, neither interactions nor group differences were found to be significant. In the questionnaires, patients described their empathy and mentalising ability as being low, nevertheless, as evinced from the mu suppression measurements, their MNS functioning appeared to be relatively intact. The researchers concluded that there was an impairment in emotion recognition processing in schizophrenia, with no evidence in favour of inability of experiencing them. However, it should be mentioned that the tasks in Horan et al.’s [] study involved non-complex social and emotional conditions, which may have biased the interpretation of the results.
Other studies, on the other hand, have reported reduced mu rhythm suppression in patients with schizophrenia. Mitra et al. [] investigated the efficacy of the MNS operation in individuals with schizophrenia. The researchers’ objective was to detect whether the occurrence of psychotic episodes of the illness was attributed to attenuations of the MNS system by measuring mu rhythm suppression. Mu suppression was measured through an EEG task, in which the participants were presented with a clip containing short sequences involving white background, biological motion with social inferences, and white visual noise. The study recruited 30 participants: 15 drug-naïve or drug-free patients with schizophrenia at the early stages of the illness, and 15 controls with no record of neuropsychiatric disorder, matched on age, sex and educational level. According to the results, the level of mu rhythm suppression over the sensorimotor cortex (SMC) among patients with schizophrenia and healthy controls presented significant deviations, which as interpreted as an indicator for the MNS dysfunction. In addition, the findings highlighted a significant negative correlation between mu suppression over the right sensorimotor cortex (rSMC) and the thought disturbance cluster on PANSS (Positive and Negative Syndrome Scale) []. Thought disorder refers to cognitive discrepancies that attenuate communication, language and thought []. Mitra et al.’s [] results link irregularities in the function of the MNS to cognitive deficits in patients with schizophrenia.
Differences in research outcomes across studies may be attributed to differences in study design, population samples, and/or the tasks administered to measure the suppression of the mu wave. Minichino et al. [] conducted an analysis of relevant studies in order to investigate the activation of MNs in schizophrenia and ASD by measuring deviations in mu rhythm suppression during the observation of biological motion. For their analysis, they selected three groups of participants: individuals with high-functioning ASD, individuals with schizophrenia in the early stages of psychosis (EP) that had active negative symptoms, and a group of controls with no clinical diagnoses. The authors examined neural commonalities between the disorders that might stem from the negative symptoms of schizophrenia. The studies selected had all used EEG during the observation of biological motion. The analysis involved four conditions: baseline/ball condition [], moving hand condition [], social interactive condition [] and a biological motion/point light display animation []. The results indicated that reactions to the observation of biological movement resulted in crucially reduced mu rhythm suppression in both clinical groups (i.e., individuals with ASD and individuals with early psychosis experiencing negative symptoms) as opposed to the healthy control group. For the EP group, the findings showed that individuals that exhibited more severe negative symptoms demonstrated less mu suppression. As mentioned before, negative symptoms in schizophrenia correspond to impairments in social cognition and executive functioning. Minichino et al.’s [] study has highlighted the similarities between ASD and schizophrenia with respect to dysfunctional neural mechanisms associated with impaired socio-cognitive performance.
The relationship between mu rhythm suppression and degree of severity of symptoms in schizophrenia has also been a fruitful area of research. For instance, Singh et al. [] tested 20 first-episode psychosis patients with schizophrenia for Event Related Desynchronisation (ERD) of mu waves, comparing their performance to 12 healthy controls. Their findings demonstrated that reduced mu suppression was associated with more severe negative symptoms and poorer social functioning in first-episode psychosis patients, further implying that deficits with respect to mu suppression may be related to the social and cognitive impairments observed in the early stages of schizophrenia.
Similarly, the objective of the study by McCormick et al. [] was to explore the connection among MN function, psychosis and empathic processes in patients with schizophrenia, by measuring mu rhythm suppression and its correlation with empathy and psychotic episodes. A group of 32 participants were recruited for this study: 8 patients with active schizophrenic psychosis, 8 patients in the residual phase of the disease, and 16 controls with no reported neurological or psychiatric disorders. An EEG paradigm was administered that measured mu rhythm suppression over the sensorimotor cortex while the participants were observing their own hand movements and hand movements of other individuals. Furthermore, levels of empathy were evaluated by the implementation of the Interpersonal Reactivity Index (IRI), which involves a classification of empathy and perspective taking (Perspective Taking subscale, Empathic Concern subscale, Personal Distress subscale and Fantasy subscale). The study revealed that the levels of mu rhythm suppression in the schizophrenic patients that experience active psychotic episodes were greater over the left sensorimotor cortex, when compared to the other groups. Healthy controls and patients with schizophrenia in the residual phase exhibited no considerable differences in the levels of mu rhythm suppression. McCormick et al. [] attributed the higher levels observed in the first group to the occurrence of psychotic episodes. In particular, the findings have shown that the more severe the psychotic symptoms, the greater the left-sided mu rhythm suppression. In addition, according to the findings from the IRI, the degree of personal distress was significantly stronger in patients with schizophrenia than controls. The outcomes of the study suggest that impaired social cognition and empathy can be attributed to abnormal function of the MNS in schizophrenic patients, and can be further negatively impacted by increased levels of distress.
A study by Wynn et al. [] has investigated the optimal dose of oxytocin (OT) for boosting the processing of social cues and contexts in patients with schizophrenia. The main aim of the study was to measure mu rhythm suppression as a proxy for the function of the MNS, and pupil dilation, which served as an index for emotion recognition and processing. The researchers recruited 47 individuals diagnosed with schizophrenia that were randomly instructed to receive one of eight doses of OT (8, 12, 24, 36, 48, 60, 72, or 84 IU), or the placebo substance. The experiment involved two tasks associated with social cues: an EEG task that measure mu rhythm suppression in reaction to biological motion, and a pupillometry task that demonstrated the level of the dilation of the pupil when observing faces that present emotion. The outcomes of the study were that OT doses of 36 and 48 IU substantially boosted mu suppression, as opposed to the placebo. Crucially, the study demonstrated no effect of the OT on the face affect pupillometry test, which can be attributed to the well-established impairment that individuals with schizophrenia present in face emotion processing and recognition []. Table 2 provides an overview of the studies used in this review, the methodological approach and their main findings in both conditions.

Table 2.
Overview of the selected research studies for this review.
3.3. Methodological Limitations and Strengths of the Included Studies
The selected studies that have investigated the MNS function in ASD and schizophrenia present a range of methodological strengths and limitations, particularly concerning the EEG protocols, sample sizes, and statistical rigor. Deschrijver et al. [] employed high-density EEG with appropriate artifact correction to localize motor interference effects in high-functioning ASD, but their modest sample size (n ≈ 20 per group) limits generalisability of findings and statistical power. Fan et al.’s [] study lacked detailed reporting on EEG preprocessing parameters, raising concerns about the reproducibility of the findings and signal quality, however, the particular study has been notable for combining behavioural and EEG measures to argue against the MNS dysfunction in ASD. Sotoodeh et al.’s [] methodological design represents a methodological advance by integrating eye-tracking with EEG to control for visual attention, which has been a critical confound in MNS research, however, the relatively large dataset would benefit from more transparent reporting of effect sizes and corrections for multiple comparisons. Finally, Cole et al. [] demonstrated methodological rigor through the integration of EEG and TMS, offering multimodal evidence for intact MNS activity in ASD. Nevertheless, the small sample sizes and the complexity of dual-modality analyses introduce further potential noise and interpretation challenges.
With respect to the studies that have investigated the MNS in schizophrenia, Horan et al. [] applied a well-established mu rhythm suppression paradigm, and included a healthy control group enhancing interpretability. Crucially, their moderate sample size and lack of detailed artifact rejection procedures may limit replicability. Mitra et al. [] contributed valuable cross-cultural data using EEG to examine mu rhythm suppression activity in schizophrenia, but their relatively small and clinically heterogeneous sample, along with limited statistical correction for multiple comparisons, weakens the robustness of their findings. Minichino et al. [] demonstrated methodological strength by comparing both early psychosis (EP) and ASD participants, yet the EEG analysis focused narrowly on sensor-level mu rhythm suppression without source localization, potentially reducing neuroanatomical specificity. McCormick et al. [] incorporated both fMRI and EEG measures, offering a multimodal perspective on empathy and the MNS function in schizophrenia; nevertheless, their modest sample and the limited temporal resolution of fMRI reduced the study’s capacity to link neural activity directly to mirroring processes. Finally, Wynn et al. [] employed a pharmacological intervention design using oxytocin, and incorporated neurophysiological measures to examine social processing, showcasing innovative methodology. Nonetheless, EEG outcomes were secondary to the pharmacological aims, and the study lacked mu rhythm-specific analyses, making their study less directly informative about the MNS function. Collectively, while these studies provide valuable insights into mu rhythm suppression activity and its link to socio-cognitive deficits in ASD and schizophrenia, future work would benefit from larger cohorts, standardized EEG processing pipelines, and robust statistical corrections to improve replicability and validity.
4. Conclusions
Research in the field of MNs has advanced in the past years. However, the findings with respect to ASD and schizophrenia do not reach a consensus with respect to the functionality of this set of neurons and/or the validity of the theories around it. Mirror neurons are a class of neurons that fire both during the performance of an action or action observation, and have considerable impact on the individuals’ social cognition skills, including imitation, empathy, intention, emotion recognition and Theory of Mind. In both ASD and schizophrenia, research has shown that the MNS may be dysfunctional, indicating abnormal activation and impaired perception of motion activity, which has in turn detrimental effects on the social cognitive skills of the individuals. On the other hand, the two disorders differ markedly in terms of the neural abnormalities they demonstrate, despite the shared social cognitive deficits that are often linked to MN dysfunction. In individuals with ASD, MN dysfunction is frequently associated with reduced mu rhythm suppression during both action observation and execution tasks, suggesting impaired resonance mechanisms that may hinder the development of imitation and Theory of Mind []. By contrast, schizophrenia patients may exhibit more variable MN responses; while some studies show preserved mu suppression, others report reduced MN activity specifically in relation to negative symptoms and disrupted intention attribution [,]. This dissociation may reflect differences in the timing and nature of the neurodevelopmental disruptions that occur in the two conditions: in ASD, MNS anomalies often emerge early and are relatively stable, whereas in schizophrenia, they may develop later and interact with broader network dysfunctions, such as impaired salience attribution and executive functioning. Moreover, compensatory mechanisms such as attentional engagement, may differentially influence MN responses in each disorder [].
The MN hypothesis, which posits that dysfunctions in the MNS underlie the social and communicative deficits observed in ASD and schizophrenia, has garnered significant attention but remains highly controversial. Initial support for the hypothesis stemmed from findings of reduced mu suppression in individuals with ASD, which was interpreted as a manifestation of impaired MNS activity []. However, subsequent research has challenged this interpretation, suggesting that such findings may be confounded by atypical attentional engagement rather than specific MNS dysfunctions []. Moreover, alternative models posit that deficits in ASD and schizophrenia may instead reflect broader disruptions in large-scale neural networks, such as the default mode or salience networks, which are crucial for social cognition [,]. In schizophrenia, for instance, impaired social cognition has been linked to dysfunctional connectivity in frontotemporal and limbic circuits, rather than isolated MN abnormalities []. Research also suggests that observed MNS abnormalities may instead be secondary to deficits in visual attention. With respect to ASD, studies have indicated that reduced mu suppression during biological motion perception correlates with decreased visual attention, as measured by eye-tracking metrics. This suggests that attentional factors, rather than intrinsic MNS dysfunction, may underlie observed neural differences in ASD []. Similarly, in schizophrenia, impairments in social attention and deficits in processing salient stimuli have been found to influence neural responses during action observation tasks [], raising questions about the specificity of the MNS dysfunction. This counter-evidence underscores the necessity of cautiously interpreting MNS -related findings, and further advocates for a more integrative, network-based approach to understanding social deficits in neurodevelopmental and psychiatric disorders.
Table 3 illustrates the supporting and opposing evidence of the “broken” MN hypothesis as explored by the studies reviewed in this paper:

Table 3.
Comparative evidence supporting and opposing the broken mirror theory in ASD and schizophrenia.
The studies selected for this paper have investigated mu rhythm suppression in schizophrenia and ASD as a proxy for discrepancies in the function and activation of the MNS, implementing EEG paradigms. In the case of ASD, numerous research studies have presented evidence for reduced mu rhythm suppression during tasks that require observation of biological actions. The theory of broken MNs has been approached through a critical lens in this review, including studies that report no links between mu rhythm suppression and task performance, whereas others present findings that support this hypothesis and attribute the core deficits in social navigation and communication in ASD to a dysfunctional MNS.
A thematic comparison of MNS abnormalities in ASD and schizophrenia on the basis of the studies that have been reviewed, reveals both overlapping features and condition-specific differences. In ASD, Oberman and Ramachandran [] proposed widespread MNS dysfunction as a core mechanism behind social and communicative impairments. However, more recent investigations offer a more nuanced picture. Fan et al. [] and Cole et al. [] found intact MNS activity under certain task conditions, suggesting that task effects, such as task demands and cognitive engagement, may influence MNS responses. Furthermore, Sotoodeh et al. [] demonstrated that visual attention plays a key modulatory role in mu rhythm suppression in autistic individuals, indicating that attentional deficits may underlie MNS anomalies. Deschrijver et al. [] and Cole et al. [] also emphasized that while automatic motor resonance may be atypical in ASD, higher-order inferential processes remain relatively preserved.
In schizophrenia, the picture is similarly complex. Horan et al. [] observed preserved mu suppression in individuals with schizophrenia, challenging the assumption of a pervasive MNS dysfunction. Yet, Mitra et al. [] and McCormick et al. [] reported deficits in mu rhythm modulation and empathy-related activation, suggesting that MNS anomalies may emerge more prominently in relation to negative symptoms or reduced social functioning in schizophrenia. Minichino et al. [] further demonstrated that biological motion-induced mu rhythm suppression was diminished in early psychosis patients with concurrent ASD traits, thus highlighting potential transdiagnostic features of MNS dysfunction. Wynn et al. [], while not directly measuring mu rhythm suppression, contributed to the debate by showing that oxytocin modulates social brain activity, which may interact with MNS-related pathways. Overall, both disorders show altered MNS-related activity, but ASD appears to be more influenced by attentional and contextual modulation, while MNS changes seem to be more variable in schizophrenia and may depend on symptom profile and disease phase. The overall findings support a model in which MNS abnormalities in ASD and schizophrenia are neither uniform nor wholly distinct, but shaped by broader cognitive and neurophysiological contexts.
The controversies evident in the literature might stem from a variety of factors, such as the marked heterogeneity in terms of symptomatology and traits in ASD, as well as the small sample sizes and the different methodological designs across studies, or/and the targeting of limited cerebral areas. Similarly, the studies that have examined mu suppression in schizophrenia also present controversies in their outcomes. Reduced mu suppression in schizophrenia has been linked to negative symptoms, severity of symptoms and also thought disorder. In addition, divergent findings in the case of schizophrenia range from outcomes that depict intact mu suppression and MN activation, to severe MNS dysfuntions. Divergence in the results may be attributed to differences in study design, task selection, and size and representability of the samples. In total, the present review has demonstrated impairments in the MNS in both ASD and schizophrenia. Correlations between severity of symptoms or frequency of traits and level of mu rhythm suppression have been detected. In the case of schizophrenic patients, reduced levels of mu rhythm suppression were found to correlate with the severity of negative symptoms, whereas in ASD, reduced levels of mu rhythm suppression correlated with intense deficits in social cognition.
Crucially, the overall findings of the selected studies demonstrate robust deficits in socio-cognitive understanding and Theory of Mind, i.e., the ability to mentalise, infer and understand emotions while observing actions in both clinical conditions. Further research in the field is of essential importance in order to reach consensus for the neural operations that determine the function of the MNS in both schizophrenia and ASD.
Future Research Implications and Clinical Applications
This line of research is fruitful for the development of specific biomarkers in both clinical conditions that can assist diagnostic processes as well as provide information about the overlapping neurodevelopmental origins of those conditions. Further research on mu rhythm suppression on schizophrenia and ASD will offer a more wholesome understanding of the factors that shape the aetiology of the disorders and will aid in the development and design of appropriate interventions. Priority should be given on disentangling core deficits from secondary effects related to attention, motivation, and symptom heterogeneity. Advances in neuroimaging and electrophysiological techniques, particularly when combined with eye-tracking and individualized symptom profiling, may help clarify whether the MNS anomalies reflect a distinct neurobiological marker or are contingent upon broader cognitive disruptions. Longitudinal and transdiagnostic studies are also essential to determine the developmental trajectories and shared versus divergent MNS mechanisms across the two conditions. Clinically, improved understanding of the MNS function may inform targeted interventions aimed at enhancing social cognition, such as neurofeedback protocols based on mu rhythm modulation or/and adjunctive treatments using neuromodulators, like oxytocin. However, translating these findings into practice will require rigorously controlled trials with standardised methodologies to ensure replicability and therapeutic efficacy across diverse clinical populations.
Mirror neurons and mu rhythm suppression contribute to our understanding of the neural basis of communication and language, stipulating valuable insights for natural language processing. It is crucial to find the link and bridge the gap between artificial intelligence (AI) systems and neuroscientific evidence, in order to develop high-order models that mimic human communication, language and interaction in neurotypical and neurodivergent populations, such as ASD and schizophrenia. A deep understanding of the brain functions that define complex cognitive systems in individuals with neurodegenerative and neuropsychiatric disorders will enhance the advancement of innovative technological applications of AI systems on language impairments, and also improve language and communicational interventions and treatments [,].
Author Contributions
Conceptualization, M.A., V.S. and E.P.; investigation, M.A., V.S. and E.P.; writing—original draft preparation, M.A., V.S. and E.P.; writing—review and editing, M.A., V.S. and E.P.; supervision, M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Molenberghs, P.; Cunnington, R.; Mattingley, J.B. Is the mirror neuron system involved in imitation? A short review and meta-analysis. Neurosci. Biobehav. Rev. 2009, 33, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Rizzolatti, G.; Craighero, L. The mirror-neuron system. Annu. Rev. Neurosci. 2004, 27, 169–192. [Google Scholar] [CrossRef]
- Bonini, L.; Rotunno, C.; Arcuri, E.; Gallese, V. Mirror neurons 30 years later: Implications and applications. Trends Cogn. Sci. 2022, 26, 767–781. [Google Scholar] [CrossRef]
- Bonini, L.; Rozzi, S.; Serventi, F.U.; Simone, L.; Ferrari, P.F.; Fogassi, L. Ventral premotor and inferior parietal cortices make distinct contribution to action organization and intention understanding. Cereb. Cortex 2010, 20, 1372–1385. [Google Scholar] [CrossRef]
- Gallese, V.; Keysers, C.; Rizzolatti, G. A unifying view of the basis of social cognition. Trends Cogn. Sci. 2004, 8, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Gallese, V.; Goldman, A. Mirror neurons and the simulation theory of mind-reading. Trends Cogn. Sci. 1998, 2, 493–501. [Google Scholar] [CrossRef]
- de Jong, T.; Van Gog, T.; Jenks, K.; Manlove, S.; Van Hell, J.; Jolles, J.; Van Merrienboer, J.; Van Leeuwen, T.; Boschloo, A. Explorations in Learning and the Brain: On the Potential of Cognitive Neuroscience for Educational Science; Springer Science & Business Media: New York, NY, USA, 2009. [Google Scholar]
- Leslie, K.R.; Johnson-Frey, S.H.; Grafton, S.T. Functional imaging of face and hand imitation: Towards a motor theory of empathy. Neuroimage 2004, 21, 601–607. [Google Scholar] [CrossRef]
- Kilner, J.M.; Neal, A.; Weiskopf, N.; Friston, K.J.; Frith, C.D. Evidence of mirror neurons in human inferior frontal gyrus. J. Neurosci. 2009, 29, 10153–10159. [Google Scholar] [CrossRef] [PubMed]
- de la Rosa, S.; Schillinger, F.L.; Bülthoff, H.H.; Schultz, J.; Uludag, K. fMRI adaptation between action observation and action execution reveals cortical areas with mirror neuron properties in human BA 44/45. Front. Hum. Neurosci. 2016, 10, 78. [Google Scholar] [CrossRef]
- Gazzola, V.; Keysers, C. The observation and execution of actions share motor and somatosensory voxels in all tested subjects: Single-subject analyses of unsmoothed fMRI data. Cereb. Cortex 2009, 19, 1239–1255. [Google Scholar] [CrossRef]
- Singer, T. The neuronal basis and ontogeny of empathy and mind reading: Review of literature and implications for future research. Neurosci. Biobehav. Rev. 2006, 30, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Théoret, H.; Pascual-Leone, A. Language acquisition: Do as you hear. Curr. Biol. 2002, 12, R736–R737. [Google Scholar] [CrossRef]
- Cerri, G.; Cabinio, M.; Blasi, V.; Borroni, P.; Iadanza, A.; Fava, E.; Fornia, L.; Verpozzi, V.; Riva, M.; Casarotti, A.; et al. The mirror neuron system and the strange case of Broca’s area. Hum. Brain Mapp. 2015, 36, 1010–1027. [Google Scholar] [CrossRef] [PubMed]
- Damiani, D.; Nascimento, A.M.; Pereira, L.K. Cortical brain functions–the brodmann legacy in the 21st century. Arq. Bras. Neurocir. Braz. Neurosurg. 2020, 39, 261–270. [Google Scholar] [CrossRef]
- Valizadeh, A.; Mbwogge, M.; Rasouli Yazdi, A.; Hedayati Amlashi, N.; Haadi, A.; Shayestefar, M.; Moassefi, M. The mirror mechanism in schizophrenia: A systematic review and qualitative meta-analysis. Front. Psychiatry 2022, 13, 884828. [Google Scholar] [CrossRef] [PubMed]
- Oberman, L.M.; McCleery, J.P.; Ramachandran, V.S.; Pineda, J.A. EEG evidence for mirror neuron activity during the observation of human and robot actions: Toward an analysis of the human qualities of interactive robots. Neurocomputing 2007, 70, 2194–2203. [Google Scholar] [CrossRef]
- Genzer, S.; Ong, D.C.; Zaki, J.; Perry, A. Mu rhythm suppression over sensorimotor regions is associated with greater empathic accuracy. Soc. Cogn. Affect. Neurosci. 2022, 17, 788–801. [Google Scholar] [CrossRef]
- Liew, S.L.; Aziz-Zadeh, L. The human mirror neuron system, social control, and language. In Handbook of Neurosociology; Springer: Dordrecht, The Netherlands, 2013; pp. 183–205. [Google Scholar]
- Chisholm, K.; Lin, A.; Armando, M. Schizophrenia spectrum disorders and autism spectrum disorder. In Psychiatric Symptoms and Comorbidities in Autism Spectrum Disorder; Springer: Berlin/Heidelberg, Germany, 2016; pp. 51–66. [Google Scholar]
- Gallese, V.; Rochat, M.J.; Berchio, C. The mirror mechanism and its potential role in autism spectrum disorder. Dev. Med. Child Neurol. 2013, 55, 15–22. [Google Scholar] [CrossRef]
- Jeste, S.S.; Geschwind, D.H. Disentangling the heterogeneity of autism spectrum disorder through genetic findings. Nat. Rev. Neurol. 2014, 10, 74–81. [Google Scholar] [CrossRef]
- Sicile-Kira, C. Autism Spectrum Disorder: The Complete Guide to Understanding Autism; Tarcher Perigee: New York, NY, USA, 2014. [Google Scholar]
- Attwood, T. Asperger’s Syndrome. Tizard Learn. Disabil. Rev. 2006, 11, 3–11. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Goldstein, G.; Johnson, C.R.; Minshew, N.J. Attentional processes in autism. J. Autism Dev. Disord. 2001, 31, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Parellada, M.; Penzol, M.J.; Pina, L.; Moreno, C.; González-Vioque, E.; Zalsman, G.; Arango, C. The neurobiology of autism spectrum disorders. Eur. Psychiatry 2014, 29, 11–19. [Google Scholar] [CrossRef]
- Nicpon, M.F.; Doobay, A.F.; Assouline, S.G. Parent, teacher, and self-perceptions of psychosocial functioning in intellectually gifted children and adolescents with autism spectrum disorder. J. Autism Dev. Disord. 2010, 40, 1028–1038. [Google Scholar] [CrossRef]
- Fombonne, E. The rising prevalence of autism. J. Child Psychol. Psychiatry 2018, 59, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Javitt, D.C. Balancing therapeutic safety and efficacy to improve clinical and economic outcomes in schizophrenia: A clinical overview. Am. J. Manag. Care 2014, 20 (Suppl. S8), S160–S165. [Google Scholar] [PubMed]
- Charlson, F.J.; Ferrari, A.J.; Santomauro, D.F.; Diminic, S.; Stockings, E.; Scott, J.G.; McGrath, J.J.; Whiteford, H.A. Global epidemiology and burden of schizophrenia: Findings from the global burden of disease study 2016. Schizophr. Bull. 2018, 44, 1195–1203. [Google Scholar] [CrossRef]
- McGrath, J.; Saha, S.; Chant, D.; Welham, J. Schizophrenia: A concise overview of incidence, prevalence, and mortality. Epidemiol. Rev. 2008, 30, 67–76. [Google Scholar] [CrossRef]
- Adamu, M.J.; Qiang, L.; Nyatega, C.O.; Younis, A.; Kawuwa, H.B.; Jabire, A.H.; Saminu, S. Unraveling the pathophysiology of schizophrenia: Insights from structural magnetic resonance imaging studies. Front. Psychiatry 2023, 14, 1188603. [Google Scholar] [CrossRef]
- Li, G.; Dai, J.; Liu, H.; Lin, Y.; Liu, Q.; Zheng, K.; Li, S.; Chen, S.; Ye, Y. Association study between genetic variants and the risk of schizophrenia in the Chinese population based on GWAS-implicated 6p21. 3–23.1 human genome region: A case-control study. BMC Psychiatry 2021, 21, 483. [Google Scholar] [CrossRef]
- Jenkins, T.A. Perinatal complications and schizophrenia: Involvement of the immune system. Front. Neurosci. 2013, 7, 110. [Google Scholar] [CrossRef]
- Vila-Badia, R.; Del Cacho, N.; Butjosa, A.; Arumí, C.S.; Santjusto, M.E.; Abella, M.; Cuevas-Esteban, J.; Pardo, M.; Muñoz-Samons, D.; Usall, J. Prevalence and types of childhood trauma in first episode psychosis patients. Relation with clinical onset variables. J. Psychiatr. Res. 2022, 146, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Nyatega, C.O.; Qiang, L.; Adamu, M.J.; Younis, A.; Kawuwa, H.B. Altered dynamic functional connectivity of cuneus in schizophrenia patients: A resting-state fMRI study. Appl. Sci. 2021, 11, 11392. [Google Scholar] [CrossRef]
- Ippolito, G.; Bertaccini, R.; Tarasi, L.; Di Gregorio, F.; Trajkovic, J.; Battaglia, S.; Romei, V. The role of alpha oscillations among the Main neuropsychiatric disorders in the adult and developing human brain: Evidence from the last 10 years of research. Biomedicine 2022, 10, 3189. [Google Scholar] [CrossRef]
- Reddy-Thootkur, M.; Kraguljac, N.V.; Lahti, A.C. The role of glutamate and GABA in cognitive dysfunction in schizophrenia and mood disorders–A systematic review of magnetic resonance spectroscopy studies. Schizophr. Res. 2022, 249, 74–84. [Google Scholar] [CrossRef]
- Correll, C.U.; Schooler, N.R. Negative symptoms in schizophrenia: A review and clinical guide for recognition, assessment, and treatment. Neuropsychiatr. Dis. Treat. 2020, 16, 519–534. [Google Scholar] [CrossRef]
- Bozikas, V.P.; Andreou, C. Longitudinal studies of cognition in first episode psychosis: A systematic review of the literature. Aust. N. Z. J. Psychiatry 2011, 45, 93–108. [Google Scholar] [CrossRef]
- Valentina, C.; De Carolis, A.; Trovini, G.; Dehning, J.; Di Pietro, S.; Curto, M.; Donato, N.; De Pisa, E.; Girardi, P.; Comparelli, A. Neurocognition in schizophrenia: From prodrome to multi-episode illness. Psychiatry Res. 2014, 220, 129–134. [Google Scholar]
- Green, M.F.; Horan, W.P.; Lee, J. Nonsocial and social cognition in schizophrenia: Current evidence and future directions. World Psychiatry 2019, 18, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Savla, G.N.; Vella, L.; Armstrong, C.C.; Penn, D.L.; Twamley, E.W. Deficits in domains of social cognition in schizophrenia: A meta-analysis of the empirical evidence. Schizophr. Bull. 2013, 39, 979–992. [Google Scholar] [CrossRef]
- Matthews, N.L.; Goldberg, W.A. Theory of mind in children with and without autism spectrum disorder: Associations with the sibling constellation. Autism 2018, 22, 311–321. [Google Scholar] [CrossRef]
- Samson, F.; Mottron, L.; Jemel, B.; Belin, P.; Ciocca, V. Can spectro-temporal complexity explain the autistic pattern of performance on auditory tasks? J. Autism Dev. Disord. 2006, 36, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, F.; Hosseini, S.; Tahamtan, M.; Bayat, M. The Impaired Theory of Mind in Autism Spectrum Disorders and the Possible Remediative Role of Transcranial Direct Current Stimulation. J. Adv. Med. Sci. Appl. Technol. 2017, 3, 175–178. [Google Scholar] [CrossRef]
- Begeer, S.; Malle, B.F.; Nieuwland, M.S.; Keysar, B. Using theory of mind to represent and take part in social interactions: Comparing individuals with high-functioning autism and typically developing controls. Eur. J. Dev. Psychol. 2010, 7, 104–122. [Google Scholar] [CrossRef]
- Owen, M.J.; O’Donovan, M.C. The genetics of cognition in schizophrenia. In Genomic Psychiatry; Academic Press: Cambridge, MA, USA, 2024. [Google Scholar]
- Sugranyes, G.; Kyriakopoulos, M.; Corrigall, R.; Taylor, E.; Frangou, S. Autism spectrum disorders and schizophrenia: Meta-analysis of the neural correlates of social cognition. PLoS ONE 2011, 6, e25322. [Google Scholar] [CrossRef] [PubMed]
- King, B.H.; Lord, C. Is schizophrenia on the autism spectrum? Brain Res. 2011, 1380, 34–41. [Google Scholar] [CrossRef]
- Eack, S.M.; Bahorik, A.L.; McKnight, S.A.; Hogarty, S.S.; Greenwald, D.P.; Newhill, C.E.; Phillips, M.L.; Keshavan, M.S.; Minshew, N.J. Commonalities in social and non-social cognitive impairments in adults with autism spectrum disorder and schizophrenia. Schizophr. Res. 2013, 148, 24–28. [Google Scholar] [CrossRef]
- Ramachandran, V.S.; Oberman, L.M. Broken mirrors: A theory of autism. Sci. Am. 2006, 295, 62–69. [Google Scholar] [CrossRef]
- Pacherie, E.; Dokic, J. From mirror neurons to joint actions. Cogn. Syst. Res. 2006, 7, 101–112. [Google Scholar] [CrossRef]
- Praszkier, R. Empathy, mirror neurons and SYNC. Mind Soc. 2016, 15, 1–25. [Google Scholar] [CrossRef]
- Debes, R. Which empathy? Limitations in the mirrored “understanding” of emotion. Synthese 2010, 175, 219–239. [Google Scholar] [CrossRef]
- Altschuler, M.; Sideridis, G.; Kala, S.; Warshawsky, M.; Gilbert, R.; Carroll, D.; Burger-Caplan, R.; Faja, S. Measuring Individual Differences in Cognitive, Affective, and Spontaneous Theory of Mind Among School-Aged Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2018, 48, 3945–3957. [Google Scholar] [CrossRef] [PubMed]
- Iacoboni, M.; Woods, R.P.; Brass, M.; Bekkering, H.; Mazziotta, J.C.; Rizzolatti, G. Cortical mechanisms of human imitation. Science 1999, 286, 2526–2528. [Google Scholar] [CrossRef] [PubMed]
- Deschrijver, E.; Wiersema, J.R.; Brass, M. Disentangling neural sources of the motor interference effect in high functioning autism: An EEG-study. J. Autism Dev. Disord. 2017, 47, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.T.; Decety, J.; Yang, C.Y.; Liu, J.L.; Cheng, Y. Unbroken mirror neurons in autism spectrum disorders. J. Child Psychol. Psychiatry 2010, 51, 981–988. [Google Scholar] [CrossRef]
- Oberman, L.M.; Ramachandran, V.S. The simulating social mind: The role of the mirror neuron system and simulation in the social and communicative deficits of autism spectrum disorders. Psychol. Bull. 2007, 133, 310. [Google Scholar] [CrossRef]
- Sotoodeh, M.S.; Chien, S.H.L.; Hadjikhani, N. Visual attention modulates mu suppression during biological motion perception in autistic individuals. Eur. J. Neurosci. 2024, 60, 6668–6685. [Google Scholar] [CrossRef]
- Cole, E.J.; Barraclough, N.E.; Enticott, P.G. Investigating mirror system (MS) activity in adults with ASD when inferring others’ intentions using both TMS and EEG. J. Autism Dev. Disord. 2018, 48, 2350–2367. [Google Scholar] [CrossRef]
- Dumas, G.; Soussignan, R.; Hugueville, L.; Martinerie, J.; Nadel, J. Revisiting mu suppression in autism spectrum disorder. Brain Res. 2014, 1585, 108–119. [Google Scholar] [CrossRef]
- Spaulding, S. Mirror neurons and social cognition. Mind Lang. 2013, 28, 233–257. [Google Scholar] [CrossRef]
- Hickok, G. Eight problems for the mirror neuron theory of action understanding in monkeys and humans. J. Cogn. Neurosci. 2009, 21, 1229–1243. [Google Scholar] [CrossRef]
- Rizzolatti, G.; Sinigaglia, C. The functional role of the parieto-frontal mirror circuit: Interpretations and misinterpretations. Nat. Rev. Neurosci. 2010, 11, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Andreou, M.; Skrimpa, V. Theory of mind deficits and neurophysiological operations in autism spectrum disorders: A review. Brain Sci. 2020, 10, 393. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.F.D.C. Reflecting on the mirror neuron system in autism: A systematic review of current theories. Dev. Cogn. Neurosci. 2013, 3, 91–105. [Google Scholar] [CrossRef]
- Jonathan, B. The social brain hypothesis of schizophrenia. World Psychiatry 2006, 5, 77. [Google Scholar]
- Green, M.F.; Horan, W.P. Social cognition in schizophrenia. Curr. Dir. Psychol. Sci. 2010, 19, 243–248. [Google Scholar] [CrossRef]
- Horan, W.P.; Pineda, J.A.; Wynn, J.K.; Iacoboni, M.; Green, M.F. Some markers of mirroring appear intact in schizophrenia: Evidence from mu suppression. Cogn. Affect. Behav. Neurosci. 2014, 14, 1049–1060. [Google Scholar] [CrossRef]
- Mitra, S.; Nizamie, S.H.; Goyal, N.; Tikka, S.K. Mu-wave activity in schizophrenia: Evidence of a dysfunctional mirror neuron system from an indian study. Indian J. Psychol Med. 2014, 36, 276–281. [Google Scholar] [CrossRef]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.; Lewine, R.R. Rethinking thought disorder. Schizophr. Bull. 2017, 43, 514–522. [Google Scholar] [CrossRef]
- Minichino, A.; Singh, F.; Pineda, J.; Friederich, E.; Cadenhead, K.S. Biological Motion induced mu suppression is reduced in Early Psychosis (EP) patients with active negative symptoms and Autism Spectrum Disorders (ASD). Psychiatry Res. 2016, 238, 374–377. [Google Scholar] [CrossRef][Green Version]
- Oberman, L.M.; Hubbard, E.M.; McCleery, J.P.; Altschuler, E.L.; Ramachandran, V.S.; Pineda, J.A. EEG evidence for mirror neuron dysfunction in autism spectrum disorders. Cogn. Brain Res. 2005, 24, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Oberman, L.M.; Pineda, J.A.; Ramachandran, V.S. The human mirror neuron system: A link between action observation and social skills. Soc. Cogn. Affect. Neurosci. 2007, 2, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Ulloa, E.R.; Pineda, J.A. Recognition of point-light biological motion: Mu rhythms and mirror neuron activity. Behav. Brain Res. 2007, 183, 188–194. [Google Scholar] [CrossRef]
- Singh, F.; Pineda, J.; Cadenhead, K.S. Association of impaired EEG mu wave suppression, negative symptoms and social functioning in biological motion processing in first episode of psychosis. Schizophr. Res. 2011, 130, 182–186. [Google Scholar] [CrossRef]
- McCormick, L.M.; Brumm, M.C.; Beadle, J.N.; Paradiso, S.; Yamada, T.; Andreasen, N. Mirror neuron function, psychosis, and empathy in schizophrenia. Psychiatry Res. Neuroimaging 2012, 201, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Wynn, J.K.; Green, M.F.; Hellemann, G.; Reavis, E.A.; Marder, S.R. A dose-finding study of oxytocin using neurophysiological measures of social processing. Neuropsychopharmacology 2019, 44, 289–294. [Google Scholar] [CrossRef]
- Marwick, K.; Hall, J. Social cognition in schizophrenia: A review of face processing. Br. Med. Bull. 2008, 88, 43–58. [Google Scholar] [CrossRef]
- Hyatt, C.J.; Wexler, B.E.; Pittman, B.; Nicholson, A.; Pearlson, G.D.; Corbera, S.; Bell, M.D.; Pelphrey, K.; Calhoun, V.D.; Assaf, M. Atypical dynamic functional network connectivity state engagement during social–emotional processing in schizophrenia and autism. Cereb. Cortex 2022, 32, 3406–3422. [Google Scholar] [CrossRef]
- Uddin, L.Q.; Supekar, K.; Lynch, C.J.; Khouzam, A.; Phillips, J.; Feinstein, C.; Ryali, S.; Menon, V. Salience network–based classification and prediction of symptom severity in children with autism. JAMA Psychiatry 2013, 70, 869–879. [Google Scholar] [CrossRef]
- Yang, G.J.; Murray, J.D.; Wang, X.J.; Glahn, D.C.; Pearlson, G.D.; Repovs, G.; Krystal, J.H.; Anticevic, A. Functional hierarchy underlies preferential connectivity disturbances in schizophrenia. Proc. Natl. Acad. Sci. USA 2016, 113, E219–E228. [Google Scholar] [CrossRef]
- Mehta, U.M.; Thirthalli, J.; Aneelraj, D.; Jadhav, P.; Gangadhar, B.N.; Keshavan, M.S. Mirror neuron dysfunction in schizophrenia and its functional implications: A systematic review. Schizophr. Res. 2014, 160, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Gaspar, P.A.; Bermudez, D.H.; Aburto-Ponce, M.B.; Beggel, O.; Javitt, D.C. Disrupted third visual pathway function in schizophrenia: Evidence from real and implied motion processing. NeuroImage Clin. 2024, 41, 103570. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, A.J.; Darabseh, R.S. Mirror Neuron Cells, Autism, Language Deficit, and the Potential Role of Artificial Intelligence. J. Ecohumanism 2024, 3, 12552–12559. [Google Scholar] [CrossRef]
- Dickerson, K.; Gerhardstein, P.; Moser, A. The role of the human mirror neuron system in supporting communication in a digital world. Front. Psychol. 2017, 8, 698. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).