Biofunctional Miso-Type Sauce Enhanced with Biocarotenoids: How Does Its Habitual Consumption Affect Lipidemic, Glycemic, and Oxidative Stress Markers? A Pilot Cross-Over Clinical Study
Abstract
1. Introduction
2. Materials and Methods
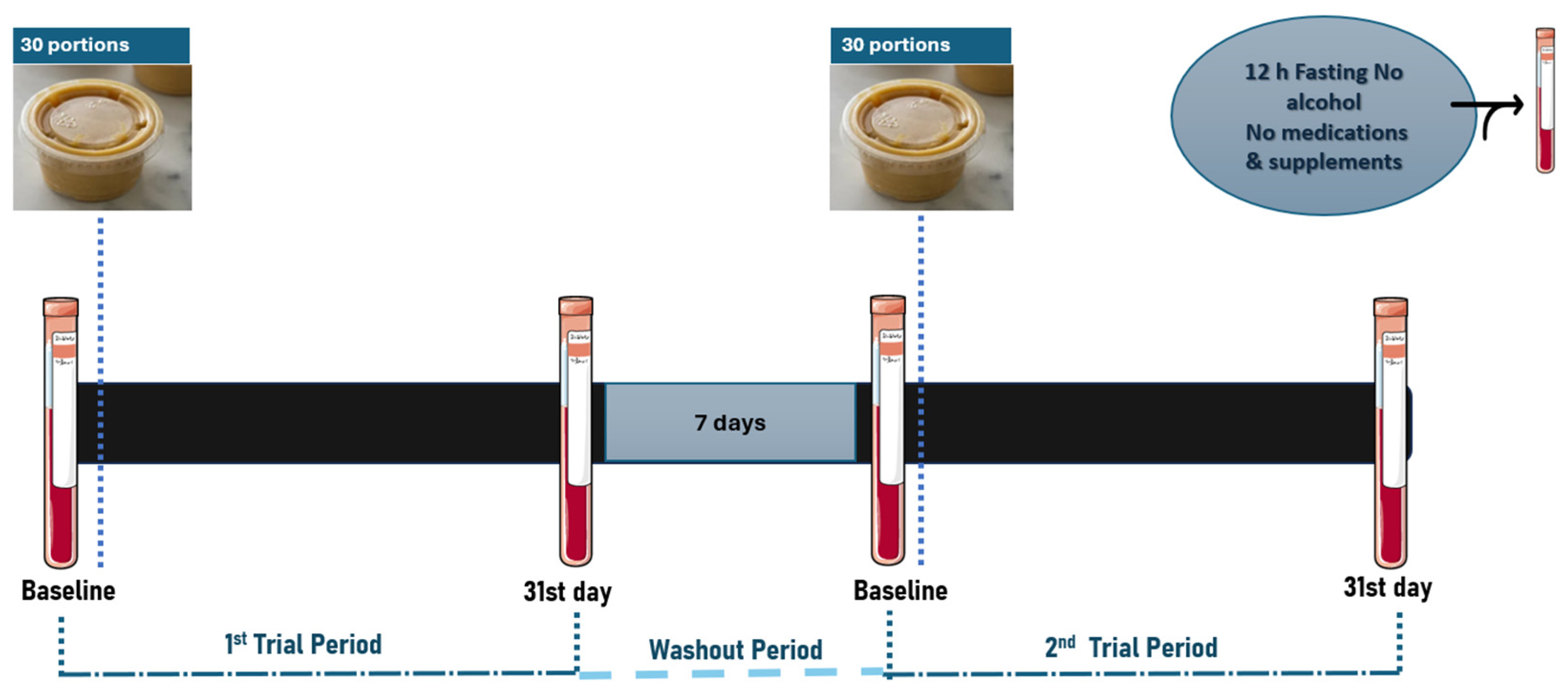
2.1. Study Design
2.2. Participants
2.3. Group Assignment
2.4. Experimental Sauces Composition
- (A)
- Control sauce: Legume-based, made from 50% Greek legume paste (a 1:1 ratio of Afkos and chickpeas), combined with 50% boiled water.
- (B)
- Miso-type sauce: The novel sauce from fermented Greek legumes, enhanced with biocarotenoids. It was created by fermenting a blend of cereal byproducts and chickpeas (50% of total weight) with 0.05% (w/w) Aspergillus oryzae spores. This innovative sauce was further enhanced with a fruit byproduct extract, comprising 42.5% of the total formulation. The extract is a combination of carrot (30%), orange (30%), apple (20%), banana (10%), and kiwi peel (5%) extracts, making it particularly rich in biocarotenoids.
2.5. Experimental Design
2.6. Statistical Analysis
3. Results
3.1. Initial Characteristics
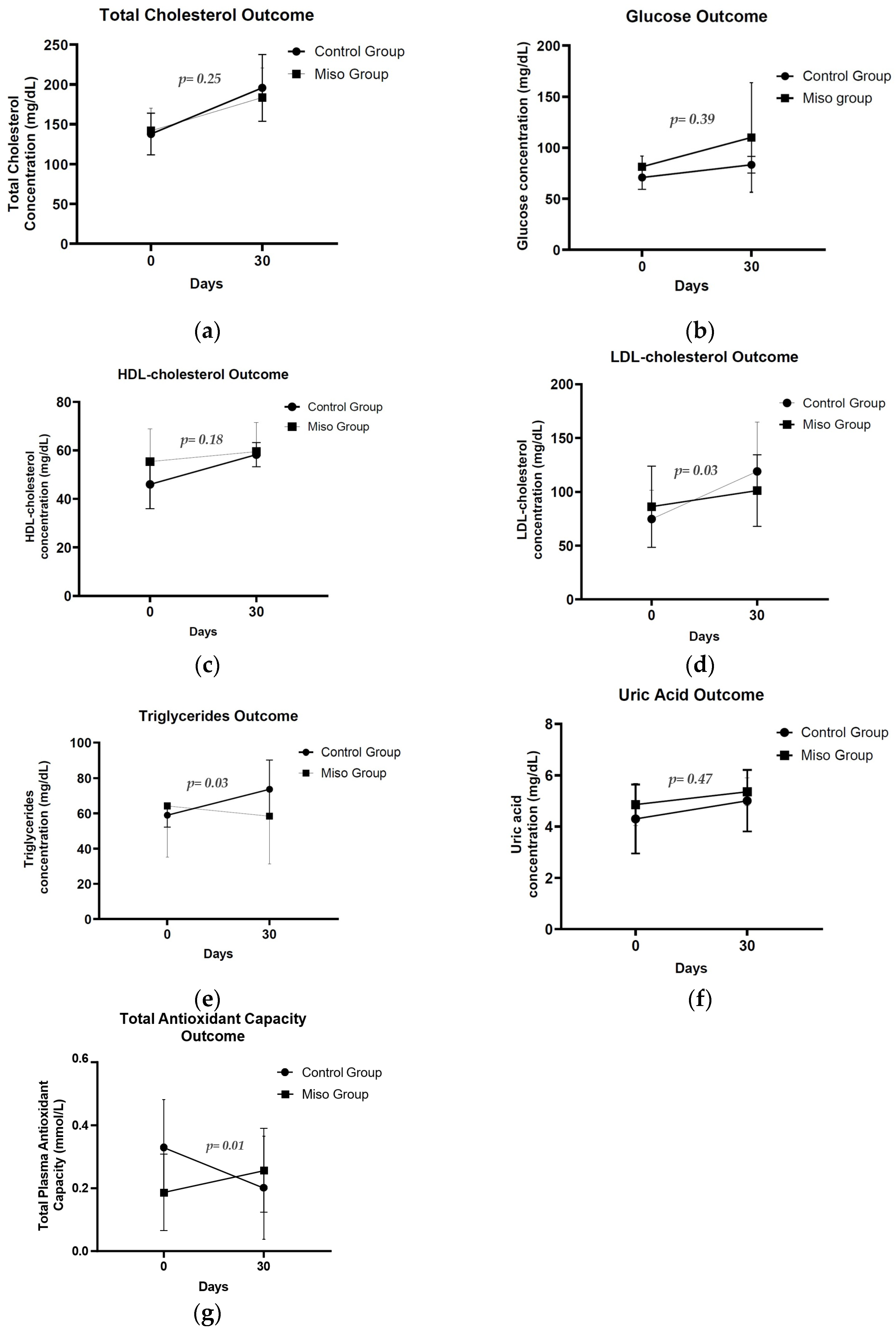
3.2. Biochemical and Antioxidant Responses
3.2.1. Plasma TAC
3.2.2. Serum Lipids
3.2.3. Serum Glucose
3.2.4. Serum Uric Acid
4. Discussion
5. Conclusions
Author Contributions
Funding
 .
.Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khayatan, D.; Nouri, K.; Momtaz, S.; Roufogalis, B.D.; Alidadi, M.; Jamialahmadi, T.; Abdolghaffari, A.H.; Sahebkar, A. Plant-Derived Fermented Products: An Interesting Concept for Human Health. Curr. Dev. Nutr. 2024, 8, 102162. [Google Scholar] [CrossRef] [PubMed]
- Harahap, I.A.; Suliburska, J.; Karaca, A.C.; Capanoglu, E.; Esatbeyoglu, T. Fermented Soy Products: A Review of Bioactives for Health from Fermentation to Functionality. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70080. [Google Scholar] [CrossRef]
- Yang, X.; Nakamoto, M.; Shuto, E.; Hata, A.; Aki, N.; Shikama, Y.; Bando, Y.; Ichihara, T.; Minamigawa, T.; Kuwamura, Y.; et al. Introduction Associations Between Intake of Dietary Fermented Soy Food and Concentrations of Inflammatory Markers: A Cross-Sectional Study in Japanese Workers. J. Med. Investig. 2018, 65, 74–80. [Google Scholar] [CrossRef]
- Papagianni, O.; Delli, E.; Vasila, M.; Loukas, T.; Magkoutis, A.; Dimou, C.; Karantonis, H.C.; Koutelidakis, A.E. The Acute Effect of a Novel Miso-Type Sauce, Enhanced with a Carotenoid-Rich Extract from Fruit By-Products, on Postprandial Biomarkers of Oxidative Stress and Inflammation. Nutrients 2022, 14, 1316. [Google Scholar] [CrossRef] [PubMed]
- Koutelidakis, A.E.; Dimou, C.; Konstantina, N.; Dimou, C.M. Designing, Developing and Optimizing a Two-Stage Solid State Fermentation Process through Valorization of Cereal Milling and Legume By-Products and Waste Streams for the Production of Novel Functional Protein Rich Type-Miso Nutri-Powder, Using Aspergillus Oryzae. Biomed. J. Sci. Tech. Res. 2022, 44, 35763–35771. [Google Scholar] [CrossRef]
- Charalampia, D.; Antonios, K.Ε.; Constantina, N.; Haralabos, K.C. Current Trends and Emerging Technologies in Biopigment Production Processes: Industrial Food and Health Applications. Int. J. Hortic. Agric. Food Sci. 2017, 1, 33–46. [Google Scholar]
- On, S.; Na, W.; Sohn, C. Relationship Between Fermented Food Consumption Patterns, Hs-CRP, and Chronic Diseases Among Middle-Aged Koreans: Data from the 2015–2018 Korea National Health and Nutrition Examination. Nutrients 2025, 17, 1343. [Google Scholar] [CrossRef]
- Takahashi, F.; Hashimoto, Y.; Kaji, A.; Sakai, R.; Miki, A.; Okamura, T.; Kitagawa, N.; Okada, H.; Nakanishi, N.; Majima, S.; et al. Habitual Miso (Fermented Soybean Paste) Consumption Is Associated with Glycemic Variability in Patients with Type 2 Diabetes: A Cross-Sectional Study. Nutrients 2021, 13, 1488. [Google Scholar] [CrossRef]
- Leonard, W.; Zhang, P.; Ying, D.; Adhikari, B.; Fang, Z. Fermentation Transforms the Phenolic Profiles and Bioactivities of Plant-Based Foods. Biotechnol. Adv. 2021, 49, 107763. [Google Scholar] [CrossRef]
- Ikeda, K.; Sato, T.; Nakayama, T.; Tanaka, D.; Nagashima, K.; Mano, F.; Joo, E.; Fujimoto, S.; Takahashi, Y.; Kosugi, S.; et al. Dietary Habits Associated with Reduced Insulin Resistance: The Nagahama Study. Diabetes Res. Clin. Pract. 2018, 141, 26–34. [Google Scholar] [CrossRef]
- Saeed, F.; Afzaal, M.; Shah, Y.A.; Khan, M.H.; Hussain, M.; Ikram, A.; Ateeq, H.; Noman, M.; Saewan, S.A.; Khashroum, A.O. Miso: A Traditional Nutritious & Health-Endorsing Fermented Product. Food Sci. Nutr. 2022, 10, 4103–4111. [Google Scholar] [PubMed]
- Chusak, C.; Pasukamonset, P.; Chantarasinlapin, P.; Adisakwattana, S. Postprandial Glycemia, Insulinemia, and Antioxidant Status in Healthy Subjects after Ingestion of Bread Made from Anthocyanin-Rich Riceberry Rice. Nutrients 2020, 12, 782. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.S.; Beaman, J.L.; He, Y.; Guo, Z.; Sun, H. Reduction of Body Fat and Improved Lipid Profile Associated with Daily Consumption of a Puer Tea Extract in a Hyperlipidemic Population: A Randomized Placebo-Controlled Trial. Clin. Interv. Aging 2016, 11, 367–376. [Google Scholar] [CrossRef]
- Nozue, M.; Shimazu, T.; Charvat, H.; Mori, N.; Mutoh, M.; Sawada, N.; Iwasaki, M.; Yamaji, T.; Inoue, M.; Kokubo, Y.; et al. Fermented Soy Products Intake and Risk of Cardiovascular Disease and Total Cancer Incidence: The Japan Public Health Center-Based Prospective Study. Eur. J. Clin. Nutr. 2021, 75, 954–968. [Google Scholar] [CrossRef] [PubMed]
- Tlais, A.Z.A.; Fiorino, G.M.; Polo, A.; Filannino, P.; Cagno, R. Di High-Value Compounds in Fruit, Vegetable and Cereal Byproducts: An Overview of Potential Sustainable Reuse and Exploitation. Molecules 2020, 25, 2987. [Google Scholar] [CrossRef]
- Erskine, E.; Ozkan, G.; Lu, B.; Capanoglu, E. Effects of Fermentation Process on the Antioxidant Capacity of Fruit Byproducts. ACS Omega 2023, 8, 4543–4553. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Hamaguchi, M.; Fukui, M. Fermented Soybean Foods and Diabetes. J. Diabetes Investig. 2023, 14, 1329–1340. [Google Scholar] [CrossRef]
- Ito, K. Review of the Health Benefits of Habitual Consumption of Miso Soup: Focus on the Effects on Sympathetic Nerve Activity, Blood Pressure, and Heart Rate. Environ. Health Prev. Med. 2020, 25, 45. [Google Scholar] [CrossRef]
- Nurmilah, S.; Frediansyah, A.; Cahyana, Y.; Utama, G.L. Biotransformation and Health Potential of Isoflavones by Microorganisms in Indonesian Traditional Fermented Soy Products: A Review. J. Agric. Food Res. 2024, 18, 101365. [Google Scholar] [CrossRef]
- Rizzo, G. Soy-Based Tempeh as a Functional Food: Evidence for Human Health and Future Perspective. Front. Biosci.-Elite 2024, 16, 3. [Google Scholar] [CrossRef]
- Bohn, T. Carotenoids and Markers of Oxidative Stress in Human Observational Studies and Intervention Trials: Implications for Chronic Diseases. Antioxidants 2019, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Enciso-Martínez, Y.; Zuñiga-Martínez, B.S.; Ayala-Zavala, J.F.; Domínguez-Avila, J.A.; González-Aguilar, G.A.; Viuda-Martos, M. Agro-Industrial By-Products of Plant Origin: Therapeutic Uses as Well as Antimicrobial and Antioxidant Activity. Biomolecules 2024, 14, 762. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Bottalico, L.; Charitos, I.A.; Castellaneta, F.; Gaxhja, E.; Topi, S.; Palmirotta, R.; Jirillo, E. Exploitation of Natural By-Products for the Promotion of Healthy Outcomes in Humans: Special Focus on Antioxidant and Anti-Inflammatory Mechanisms and Modulation of the Gut Microbiota. Antioxidants 2024, 13, 796. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Jung, E.S.; Choi, E.K.; Jeong, D.Y.; Jo, S.W.; Jin, J.H.; Lee, J.M.; Park, B.H.; Chae, S.W. Supplementation with Aspergillus Oryzae-Fermented Kochujang Lowers Serum Cholesterol in Subjects with Hyperlipidemia. Clin. Nutr. 2015, 34, 383–387. [Google Scholar] [CrossRef]
- Patil, N.D.; Bains, A.; Sridhar, K.; Rashid, S.; Kaur, S.; Ali, N.; Chawla, P.; Sharma, M. Effect of Sustainable Pretreatments on the Nutritional and Functionality of Chickpea Protein: Implication for Innovative Food Product Development. J. Food Biochem. 2024, 2024, 5173736. [Google Scholar] [CrossRef]
- Hong, L.; Fan, L.; Wu, J.; Yang, J.; Hou, D.; Yao, Y.; Zhou, S. Pulse Proteins and Their Hydrolysates: A Comprehensive Review of Their Beneficial Effects on Metabolic Syndrome and the Gut Microbiome. Nutrients 2024, 16, 1845. [Google Scholar] [CrossRef]
- do Prado, F.G.; Pagnoncelli, M.G.B.; de Melo Pereira, G.V.; Karp, S.G.; Soccol, C.R. Fermented Soy Products and Their Potential Health Benefits: A Review. Microorganisms 2022, 10, 1606. [Google Scholar] [CrossRef]
- Khosravi, A.; Razavi, S.H. Therapeutic Effects of Polyphenols in Fermented Soybean and Black Soybean Products. J. Funct. Foods 2021, 81, 104467. [Google Scholar] [CrossRef]
- Li, J.; Bollati, C.; d’Adduzio, L.; Fanzaga, M.; Cruz-Chamorro, I.; Arnoldi, A.; Sirtori, C.R.; Lammi, C. Food-Derived Peptides with Hypocholesterolemic Activity: Production, Transepithelial Transport and Cellular Mechanisms. Trends Food Sci. Technol. 2024, 143, 104279. [Google Scholar] [CrossRef]
- Gugliucci, A. The Chylomicron Saga: Time to Focus on Postprandial Metabolism. Front. Endocrinol. 2023, 14, 1322869. [Google Scholar] [CrossRef]
- Shi, W.; Hou, T.; Liu, W.; Guo, D.; He, H. The Hypolipidemic Effects of Peptides Prepared from Cicer Arietinum in Ovariectomized Rats and HepG2 Cells. J. Sci. Food Agric. 2019, 99, 576–586. [Google Scholar] [CrossRef]
- Angeles, J.G.C.; Villanueva, J.C.; Uy, L.Y.C.; Mercado, S.M.Q.; Tsuchiya, M.C.L.; Lado, J.P.; Angelia, M.R.N.; Bercansil-Clemencia, M.C.M.; Estacio, M.A.C.; Torio, M.A.O. Legumes as Functional Food for Cardiovascular Disease. Appl. Sci. 2021, 11, 5475. [Google Scholar] [CrossRef]
- Alcover, S.; Ramos-Regalado, L.; Girón, G.; Muñoz-García, N.; Vilahur, G. HDL-Cholesterol and Triglycerides Dynamics: Essential Players in Metabolic Syndrome. Antioxidants 2025, 14, 434. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wang, W.; Li, S.; Li, J.; Zhao, R.; Liu, D.; Wu, J. Glucoregulatory Properties of Fermented Soybean Products. Fermentation 2023, 9, 254. [Google Scholar] [CrossRef]
- Jiang, C.; Ci, Z.; Kojima, M. Antioxidant Activity, α-Glucosidase and Lipase Inhibitory Activity in Rice Miso with Kidney Bean. J. Food Nutr. Res. 2018, 6, 504–508. [Google Scholar] [CrossRef]
- Laya, A.; Wangso, H.; Fernandes, I.; Djakba, R.; Oliveira, J.; Carvalho, E. Bioactive Ingredients in Traditional Fermented Food Condiments: Emerging Products for Prevention and Treatment of Obesity and Type 2 Diabetes. J. Food Qual. 2023, 2023, 5236509. [Google Scholar] [CrossRef]
- Alshaalan, R.A.; Charalambides, M.N.; Edwards, C.H.; Ellis, P.R.; Alrabeah, S.H.; Frost, G.S. Impact of Chickpea Hummus on Postprandial Blood Glucose, Insulin and Gut Hormones in Healthy Humans Combined with Mechanistic Studies of Food Structure, Rheology and Digestion Kinetics. Food Res. Int. 2024, 188, 114517. [Google Scholar] [CrossRef]
- Menzel, A.; Samouda, H.; Dohet, F.; Loap, S.; Ellulu, M.S.; Bohn, T. Common and Novel Markers for Measuring Inflammation and Oxidative Stress Ex Vivo in Research and Clinical Practice—Which to Use Regarding Disease Outcomes? Antioxidants 2021, 10, 414. [Google Scholar] [CrossRef]
- Jiang, C.; Ci, Z.; Kojima, M. α-Glucosidase Inhibitory Activity in Rice Miso Supplementary with Black Soybean. Am. J. Food Sci. Technol. 2019, 7, 27–30. [Google Scholar] [CrossRef]
| Nutritional Composition per 20 g | Control Sauce | Miso-Type Sauce |
|---|---|---|
| Energy (kcal) | 67.4 | 67.4 |
| Carbohydrates (g) | 7.45 | 7.45 |
| Fat, total (g) | 0.42 | 0.42 |
| Protein (g) | 3.90 | 3.99 |
| Saturated fat (g) | 0.05 | 0.05 |
| Unsaturated fat (g) | 0.37 | 0.37 |
| Cholesterol (mg) | 0.00 | 0.00 |
| Dietary fiber, total (g) | 0.92 | 0.92 |
| Sugar, total (g) | 0.60 | 0.60 |
| Phenolics, total (mg) | 1.40 | 57.60 |
| Biocarotenoids, total (mg) | 0.04 | 48.16 |
| Antioxidant capacity, total (mg) | 0.11 | 165.18 |
| Variable | Mean ± SD | p Value 1 |
|---|---|---|
| Age (years) | 22.80 ± 4.02 | 0.68 |
| Anthropometric Characteristics | ||
| Body Weight (kg) | 71.73 ± 19.09 | 0.08 |
| Height (cm) | 168.40 ± 7.76 | 0.071 |
| Body Mass Index (index) | 24.97 ± 4.27 | 0.14 |
| Fat Mass (kg) | 22.38 ± 8.42 | 0.42 |
| Muscle Mass (kg) | 52.25 ± 12.45 | 0.01 2 |
| Body Water (kg) | 53.85 ± 8.79 | 0.27 |
| Bone Mass (kg) | 2.78 ± 0.60 | 0.01 2 |
| Waist/Hip Circumference Ratio (index) | 0.68 ± 0.22 | 0.08 |
| Biochemical Characteristics | ||
| Total Cholesterol (mg/dL) | 143.70 ± 21.12 | 0.62 |
| Glucose (mg/dL) | 75.70 ± 12.84 | 0.93 |
| HDL Cholesterol (mg/dL) | 45.37 ± 9.36 | 0.08 |
| LDL Cholesterol (mg/dL) | 81.64 ± 33.32 | 0.96 |
| Triglycerides (mg/dL) | 61.20 ± 21.38 | 0.58 |
| Uric Acid (mg/dL) | 4.30 ± 1.34 | 0.69 |
| TAC (mmol/L) | 0.27 ± 0.09 | 0.53 |
| Baseline | 30-Day Follow-Up | p-Value 1 Group Effect | p-Value 1 Time Effect | p-Value 1 Group × Time Interaction | ||
|---|---|---|---|---|---|---|
| Total cholesterol (mg/dL) | Control | 137.70 ± 25.90 | 195.60 ± 42.10 | 0.80 | <0.001 2 | 0.25 |
| Miso | 141.80 ± 28.20 | 183.60 ± 37.08 | ||||
| Glucose (mg/dL) | Control | 70.80 ± 11.69 | 83.75 ± 9.08 | 0.08 | 0.04 2 | 0.39 |
| Miso | 81.30 ± 10.40 | 110.00 ± 53.69 | ||||
| HDL cholesterol (mg/dL) | Control | 46.00 ± 3.53 | 58.25 ± 1.75 | 0.27 | 0.002 2 | 0.18 |
| Miso | 55.37 ± 4.77 | 59.50 ± 4.22 | ||||
| LDL cholesterol (mg/dL) | Control | 74.75 ± 26.69 | 119.05 ± 45.68 | 0.75 | <0.001 2 | 0.03 2 |
| Miso | 86.27 ± 37.68 | 101.17 ± 33.30 | ||||
| Triglycerides (mg/dL) | Control | 58.97 ± 6.76 | 73.70 ± 16.60 | 0.49 | 0.41 | 0.03 2 |
| Miso | 64.20 ± 29.05 | 58.40 ± 27.10 | ||||
| Uric Acid (mg/dL) | Control | 4.30 ± 1.34 | 5.01 ± 1.20 | 0.33 | 0.001 2 | 0.47 |
| Miso | 4.86 ± 0.82 | 5.36 ± 0.54 | ||||
| TAC (mmol/L) | Control | 0.33 ± 0.15 | 0.20 ± 0.15 | 0.50 | 0.001 2 | 0.01 2 |
| Miso | 0.18 ± 0.09 | 0.26 ± 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papagianni, O.I.; Dimou, C.; Koutelidakis, A.E. Biofunctional Miso-Type Sauce Enhanced with Biocarotenoids: How Does Its Habitual Consumption Affect Lipidemic, Glycemic, and Oxidative Stress Markers? A Pilot Cross-Over Clinical Study. Appl. Sci. 2025, 15, 5962. https://doi.org/10.3390/app15115962
Papagianni OI, Dimou C, Koutelidakis AE. Biofunctional Miso-Type Sauce Enhanced with Biocarotenoids: How Does Its Habitual Consumption Affect Lipidemic, Glycemic, and Oxidative Stress Markers? A Pilot Cross-Over Clinical Study. Applied Sciences. 2025; 15(11):5962. https://doi.org/10.3390/app15115962
Chicago/Turabian StylePapagianni, Olga I., Charalampia Dimou, and Antonios E. Koutelidakis. 2025. "Biofunctional Miso-Type Sauce Enhanced with Biocarotenoids: How Does Its Habitual Consumption Affect Lipidemic, Glycemic, and Oxidative Stress Markers? A Pilot Cross-Over Clinical Study" Applied Sciences 15, no. 11: 5962. https://doi.org/10.3390/app15115962
APA StylePapagianni, O. I., Dimou, C., & Koutelidakis, A. E. (2025). Biofunctional Miso-Type Sauce Enhanced with Biocarotenoids: How Does Its Habitual Consumption Affect Lipidemic, Glycemic, and Oxidative Stress Markers? A Pilot Cross-Over Clinical Study. Applied Sciences, 15(11), 5962. https://doi.org/10.3390/app15115962








