Abstract
Modern and contemporary artworks, due to technological developments in the synthesis of new pigments, are characterized by a pictorial palette composed of a wide variety of pigments. This diversity makes it increasingly important to carry out thorough characterization studies in order to gain a complete and comprehensive understanding of the properties of the numerous pigments in use today. In this work, 18 modern acrylic paints were characterized using micro-Raman spectroscopy with two laser excitation lines (532 nm and 633 nm). The analysed pigments can be classified as organic, inorganic, or mixtures, depending on their chemical composition. Specifically, the following pigments were investigated: Cobalt Blue, Permanent Blue Light, Ultramarine, Primary—Cyan Blue, Cerulean Blue, Cobalt Blue (Hue), Indanthrene Blue, Phthalo Blue, Van Dyke Brown, Permanent Green Light, Phthalo Green, Primary Red—Magenta, Cadmium Red Medium, Lemon Yellow, Cadmium Yellow Medium, Zinc White, Titanium White and Iridescent Silver. The pigments were applied to wooden and glass substrates to simulate the creative processes of actual modern and contemporary artworks. The aim was to define their composition, as this knowledge is essential for the protection, conservation and enhancement of cultural heritage.
1. Introduction
The use of acrylics in the artistic field dates back to the early 1950s. They were met with great enthusiasm by artists of the time. Helen Frankenthaler, a prominent American painter active in the 1960s, stated the following:
I changed to acrylics for a number of reasons. Once, I was told that they dry faster, which they do, and that they retain their original color, which they do. I would say durability and light and the fact that one can use water instead of turpentine: all that makes it easier given the abstract image … As painting needed less and less drying time, depth, and so forth, the materials came along that made that more obvious [1,2].
Today, as in the past, acrylic paints remain widely used in artworks due to their ease of application, ability to adhere directly to various supports and rapid drying. Furthermore, once dried, the paint becomes insoluble, allowing for layer superposition without reactivating the underlying paint [3].
Acrylic polymers are characterized by an optimal combination of chemical and optical properties, resulting in durable, high-performance materials that can be produced cost-effectively. These polymers are typically formulated as aqueous dispersion, which exhibit low viscosity, facilitating easy application. In addition, they are non-flammable and non-toxic, making water−based acrylic paints highly suitable as binding media in paintings. When applied to a surface, the water evaporates, causing the polymer particles to coalesce and form a solid, continuous film [4].
Acrylic paints often consist of complex mixtures of organic and inorganic compounds. Consequently, studying their internal interactions is of fundamental importance. The characterization of pigments is essential for planning conservation strategies. A comprehensive understanding of their composition enables the resolution of conservation issues through appropriate interventions.
Indeed, only by fully understanding the properties of acrylic components and additives can the mechanisms of aging and degradation be elucidated [4]. This is achievable through advanced analytical techniques that allow for the precise identification and characterization of the constituent materials.
In this study, acrylic pigments from the Maimeri Brera Acrylics Extrafine line were applied to wood and glass substrates to simulate real artworks and were analysed using µ−Raman spectroscopy. The systematic application of μ-Raman spectroscopy in the characterization of modern acrylic pigments provides a valuable resource for conservation science, offering significant practical implications for the identification and conservation of contemporary artworks, while contributing to the expansion and refinement of existing Raman spectroscopic data on these pigments.
Raman spectroscopy has been extensively used in the field of material science for studying graphene [5], polymers [6], thin films [7], bi-dimensional systems [8], biological [9] and surfaces for Surface Enhanced Raman Spectroscopy (SERS) applications [10].
Among various diagnostic methods, Raman spectroscopy is recognized for its high sensitivity and specificity [11,12,13,14,15,16,17,18]. This vibrational spectroscopy technique is based on Raman scattering—the inelastic scattering of light resulting from the interaction of a monochromatic, coherent beam of light with matter [19].
A conventional Raman setup consists of a spectrometer equipped with a light source, monochromator, sample holder and detector. In contrast, a micro-Raman spectroscopy integrates a confocal microscope with the spectrometer. The ability to operate at the submicrometer scale in a non-invasive and non-destructive manner makes micro-Raman spectroscopy a powerful tool for characterizing materials in cultural heritage studies [19]. The first applications of Raman spectroscopy in cultural heritage conservation date back to the late 1990s [19]. Initially, a major challenge was the lack of reference Raman spectral databases. The first online Raman databases became available only in the early 2000s, and comprehensive pigment and dye-specific spectral libraries have been published more recently [20]. This work thus contributes to expanding and refining the existing Raman spectroscopic data on modern acrylic pigments.
Historically, the range of available pigments was limited. Until the early 19th century, artists’ palettes were restricted to mineral-based pigments, some plant- and insect-derived dyes and a small number of synthetic materials [16]. After 1900, the introduction of synthetic pigments significantly expanded the available colour range. As a result, identifying pigments in modern artwork can be particularly challenging due to their vast diversity [21]. Raman spectroscopy serves as a powerful tool in this endeavour, enabling accurate identification. This technique is highly effective for detecting both organic and inorganic pigments, providing critical information for art restoration. Furthermore, pigment characterization can aid in determining the provenance of art objects [22].
Micro-Raman spectroscopy is an exceptionally versatile technique. Beyond pigment and dye analysis, its applications in cultural heritage include the study of inks, photographic materials, gemstones, glass, ceramics, resins, fibres and ancient organic and fossil compounds [12,14,17,23]. It also assists in investigating degradation and corrosion processes affecting artworks. Additionally, it has forensic applications, enabling the authentication of artworks and archaeological artifacts by detecting forgeries [24].
2. Experimental
2.1. Materials
Figure 1 and Figure 2 show the analysed samples, which consist of eighteen pigments, mixed with acrylic binder, (supplied in tubes form by Maimeri, from the Brera Acrylics Extrafine line) deposited on wooden and glass supports, respectively.

Figure 1.
Acrylic colours from the Acrylics Brera line supplied by Industria Maimeri: Cadmium Red Medium (CRM), Cadmium Yellow Medium (CYM), Cerulean Blue (CeB), Cobalt Blue (CB), Cobalt Blue (Hue) (CB(H)), Indanthrone Blue (IB), Iridescent Silver (IS), Lemon Yellow (LY), Permanent Blue Light (PBL), Permanent Green Light (PGL), Phthalo Blue (PB), Phthalo Green (PG), Primary—Cyan Blue (PBC), Primary Red Magenta (PRM), Titanium White (TW), Ultramarine (UL), Van Dyke Brown (VB), Zinc White (ZW) deposited on wooden panels.

Figure 2.
Acrylic colours from the Acrylics Brera line supplied by Industria Maimeri: Cadmium Red Medium (CRM), Cadmium Yellow Medium (CYM), Cerulean Blue (CeB), Cobalt Blue (CB), Cobalt Blue (Hue) (CB(H)), Indanthrone Blue (IB), Iridescent Silver (IS), Lemon Yellow (LY), Permanent Blue Light (PBL), Permanent Green Light (PGL), Phthalo Blue (PB), Phthalo Green (PG), Primary—Cyan Blue (PBC), Primary Red Magenta (PRM), Titanium White (TW), Ultramarine (UL), Van Dyke Brown (VB), Zinc White (ZW) deposited on glass support.
To simulate real cases, the pigments were applied onto three wooden supports (30 cm × 3 cm), each precoated with four layers of an acrylic plaster preparatory base (produced by Zecchi, product code n. 3030/750). To ensure homogeneity, the preparatory layer was sanded using varying grit sizes of sandpaper. Each pigment covers an area of 3 × 2.5 cm2 on the wood substrate, with varying layer thicknesses [25]. The beechwood support was used. A detailed description of how the samples have been made can be found elsewhere [25]. The same pigments have been deposited on glass support for studying the effect of the substrate on the pigments. Key properties of the studied pigments are summarised in Table 1. A subset of these pigments, previously investigated via optical techniques and discussed elsewhere [25], were also examined in this work using Raman spectroscopy. The molecular structures drawn in this work were made by using ChemDraw Ultra software version 12.0.2.1076, CambridgeSoft.com.

Table 1.
Summary of the studied pigments with their characteristics.
2.2. Experimental Instrument
The µ−Raman spectroscopic analyses were performed using a Horiba−Jobin Yvon−LabRAM µ−Raman spectrometer, controlled by Labspec 5 and equipped with two laser sources: a He-Ne laser (633 nm emission wavelength) of 17 mW of power and a Nd:YAG laser (532 nm wavelength) of 50 mW of power. In order to reduce the power of the laser on the samples, when deemed necessary, in the laser ray path have been introduced filters for the attenuation of the laser power with dif-ferent optical densities (OD) which is obtained by the following relationship:
%Transmission = T = 10−OD∙100%
Thus, when the filter used has an OD = 0.3, then the percentage of the power transmitted is T% = 50%; for OD = 0.6%, then %T = 25%; for OD = 1, then %T = 10%; for OD = 2, then %T = 1%; for OD = 3, then %T = 0.1%; and for OD = 4, then %T = 0.01%. The number of the scans and the acquisition time used to collect the Raman spectra were not the same for all the pigments but were modified on the basis of the analysed pigment in order to maximize the detectability of Raman features shown by the representative Raman spectra. Table 2 summarizes the experimental conditions for the collection of the Raman spectra. Measurements were conducted using athe confocal microscope BX40 (by Olympus, Tokyo, Japan) with a 50× objective. Holographic grating of 1800 groves/mm was used during the Raman measurements. The spectral resolution was 1 cm−1. All Raman spectra presented in this work were baseline-corrected using OriginPro software (OriginPro 2018 (64 bit) SR1 b9.5.1.195).

Table 2.
Experimental conditions for the collection of the representative Raman spectra.
All pigments were analysed with both the lasers at 532 nm and 633 nm. In the cases where the collected spectra, at each laser, show the same Raman features, only the representative Raman spectrum collected with the better signal to noise ratio will be displayed.
3. Results and Discussion
The results are organized according to the material sequence listed in Table 1. The observed Raman lines for all materials are reported in Table 3, categorized by substrate (wood and glass) and laser wavelength (green, 532 nm; red, 633 nm). In several cases, no meaningful spectra were obtained with one of the two lasers.

Table 3.
Observed Raman bands in the representative Raman spectra collected on wood and glass substrates.
Figure 3 displays a photograph of the Phthalo Blue (PB) pigment (Figure 3a), optical microscope images of the pigment on wood (Figure 3b) and glass (Figure 3c) and Raman spectra acquired at 532 nm (blue line) and 633 nm (red open circle-line) for PB on wood (Figure 3d) and glass (Figure 3e). Figure 4 shows the molecular structure of phthalocyanine.
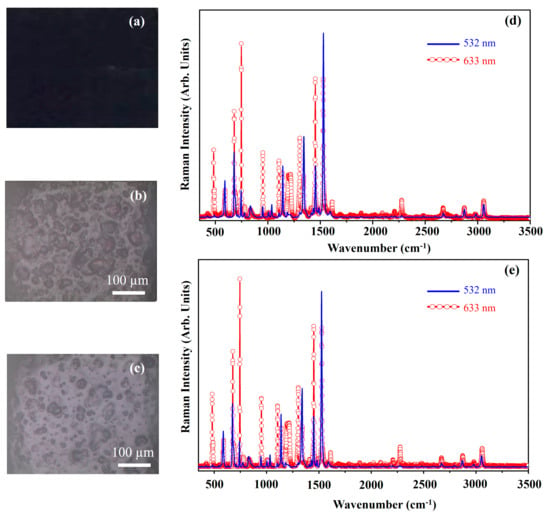
Figure 3.
Photograph of Phthalo Blue (PB) pigment (a); optical microscope image of PB pigment on wood (b) and on glass (c) substrates; representative Raman spectra collected with 532 nm laser (blue line) and 633 nm laser (red open circle-line) of PB pigment on wood (d) and on glass substrates (e).
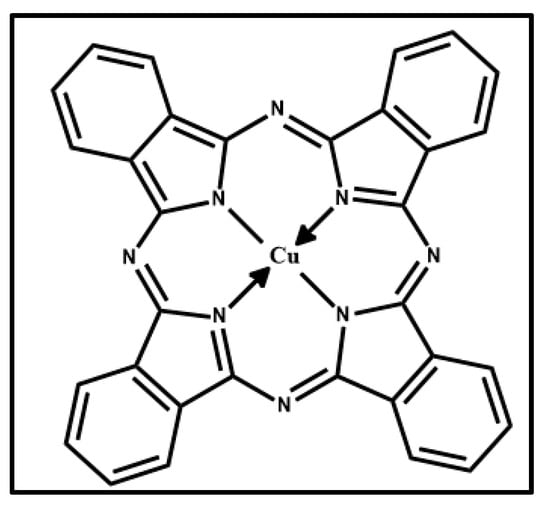
Figure 4.
Molecular structure of copper phthalocyanine.
The PB pigment exhibits a dark blue hue (Figure 3a), and its morphology appears unaffected by the substrate (Figure 3b,c). The Raman spectra collected on the two different substrates with either 633 nm or 532 nm excitation wavelength show no significant differences. Nevertheless, spectra acquired with different lasers show different spectral patterns. Such differences are ascribed to a Raman Resonance effect that often occurs during the Raman investigations on phthalocyanine systems. This effect explains why certain Raman bands appear prominently with higher-energy laser excitation (532 nm) but are weak or absent at lower energy (633 nm), and vice versa. The observed spectral variations depend on the absorption profile of the material. A detailed explanation of the Raman resonance effect can be found elsewhere [26,27,28]. The stronger bands fall at about 484 cm−1, 680 cm−1, 748 cm−1, 954 cm−1, 1107 cm−1, 1305 cm−1, 1453 cm−1 and 1525 cm−1. Although phthalocyanine exists in two main allotropic forms, α (alpha) and β (beta), the PB pigment (Table 1), is made by phthalocyanine α. Thus, the Raman band at 484 cm−1 is assigned to the A2g symmetry described as the deformation of isoindole; the Raman band at 680 cm−1 is attributed to the A1g symmetry and it is relative to the breathing mode of nitrogen atoms of the ring.
Figure 5 shows a photograph of the pigment Primary—Cyan Blue pigment (PBC) (Figure 5a), optical microscope images of the pigment on wood (Figure 5b) and glass (Figure 5c) and Raman spectra collected with the 532 nm (blue line) and the 633 nm (red open circle-line) on the pigment deposited on wood (Figure 5d) and on glass (Figure 5e). The molecular structure of PBC is phthalocyanine β different from the pigment PB that is a phthalocyanine α.
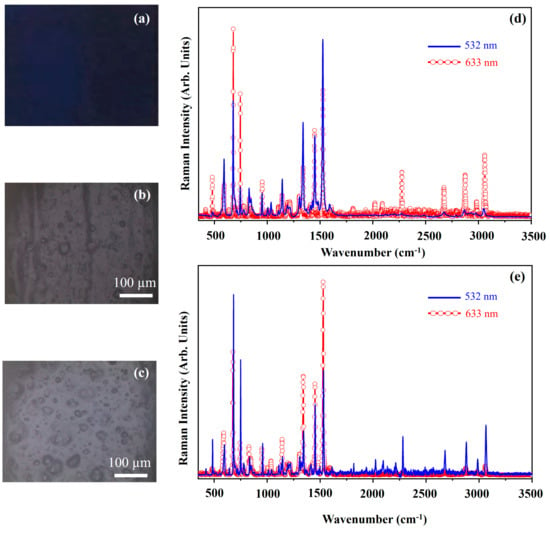
Figure 5.
Photograph of Primary−Cyan Blue (PBC) pigment (a); optical microscope images of PBC pigment on wood (b) and on glass (c) substrates; representative Raman spectra collected with 532 nm laser (blue-line) and 633 nm laser (red-open circle line) of PBC pigment on wood (d) and on glass substrates (e).
The PBC pigment exhibits a dark blue colour (Figure 5a) and the optical microscope images (Figure 5b,c) show that the morphology of the pigment deposited on wood and glass substrates are the same. The substrates do not affect the Raman spectra either. In fact, the spectra shown in Figure 5d,e are similar if collected with the same laser source. The main differences that can be seen between the two kinds of spectra are assigned to the different Resonant Raman effect induced by using the red laser sources with respect to that (Resonant effect) induced by using the green laser source. The main Raman features fall at 591 cm−1, 677 cm−1, 744 cm−1, 1142 cm−1, 1339 cm−1, 1451 cm−1 and 1527 cm−1. The band assignments are similar to that made above for the PB pigment.
The Raman band at 677 cm−1 is attributed to the A1g symmetry and it is relative to the breathing mode of nitrogen atoms of the ring; the Raman band at 744 cm−1, is assigned to the B1g symmetry concerning the antisymmetric deformation of the macrocycle. The Raman bands at 954 cm−1 and 1107 cm−1 assigned to the B2g symmetry (related to a deformation mode that includes the nitrogen atoms of the ring) and to A1g symmetry (concerning the deformation modes of C−H bonds), respectively, result to be medium bands and not strong as they were in the case of the PB pigment. The Raman band at 1142 cm−1 is assigned to the breathing of pyrrole group [29]. The Raman band at 1339 cm−1 is due to the C–C stretching of the same group [29]. The band at 1451 cm−1 is ascribed to the B2g symmetry described as deformation of the isoindole ring system and, at the end, the band at 1527 cm−1 is ascribed to the A1g symmetry described as the symmetric motion of all four isoindole groups [27,30,31].
Figure 6 shows the photograph of the pigment Indanthrone Blue (IB) (Figure 6a), the optical microscope images of the pigment on wood (Figure 6b) and on glass (Figure 6c) and the Raman spectra collected on the pigment deposited on wood (Figure 6d) and on glass (Figure 6e). The molecular structure of 6,15-Dihydro-5,9,14,18-anthrazinetetrone, the component of IB pigment, is shown in Figure 7.
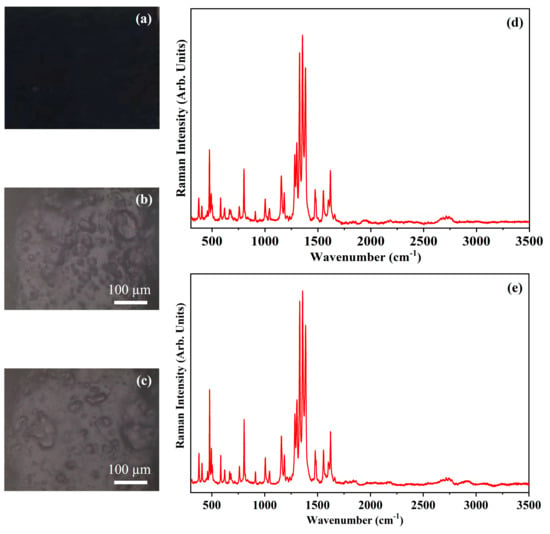
Figure 6.
Photograph of Indanthrone Blue (IB) pigment (a); optical microscope images of IB pigment on wood (b) and on glass (c) substrates; representative Raman spectra collected with red laser of IB pigment on wood (d) and on glass substrates (e).
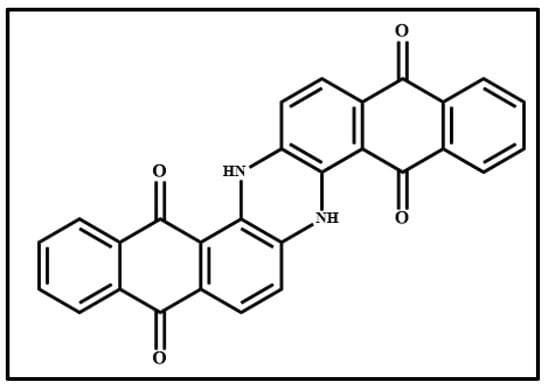
Figure 7.
Molecular structure of 6,15-Dihydro-5,9,14,18-anthrazinetetrone, named simply as anthrazinetetrone (Indanthrone Blue, IB, pigment).
The photograph (Figure 6a) shows the very dark blue colour of the pigment IB and the other optical micrographs (Figure 6b,c) show that the morphology of the pigment is not affected by the substrate.
Also, in this case, there are no differences between the spectra collected on the pigment deposited on the wood substrates with respect to those deposited on glass substrates. The main Raman bands of IB pigment fall at 476 cm−1, 802 cm−1, 1154 cm−1, 1281 cm−1, 1301 cm−1, 1327 cm−1, 1355 cm−1, 1383 cm−1 and 1619 cm−1. The Raman band at 476 cm−1 is due to the bending of the C–C–C bond and the bending of the C–N–C bond [32]. The Raman band at 802 cm−1 concerns the breathing mode of the C–C–C bond and the bending of the C–C–C bonds [32]. The Raman band at 1154 cm−1 is ascribed to the bending of the C–C(–H)–C bonds. The Raman band at 1281 cm−1 is attributed to several modes of vibration: the stretching mode of C=C bonds, the breathing of the C–C–C bond and the bending of the C–N(H)–C, C–C(H)–C and C–C(=O)–C bonds. The Raman band at 1301 cm−1 is attributed to the C–C stretching and to the bending of C–C–C, C–C(H)–C and C–C(=O)–C bonds. The Raman band at 1327 cm−1 is ascribed to the stretching of the C=C and C–N groups. The Raman band at 1355 cm−1 is assigned to the stretching of the C–C bond and to the bending of C–C(H)–C and C–C(=O)–C bonds [32]. The Raman band at 1383 cm−1 is ascribed to the C–C stretching and to the bending of C–N(H)–C, C–C(H)–C and C–C(=O)–C bonds. The Raman band at 1619 cm−1 is due to the C=O and C=C stretching and to the C–N(H)–C bending [32].
Figure 8 shows the photograph of the pigment Phthalo Green (PG) (Figure 8a), the optical microscope images of the pigment on wood (Figure 8b) and on glass (Figure 8c) and the Raman spectra collected with the 532 nm (blue line) and the 633 nm (red open circle-line) on the pigment deposited on wood (Figure 8d) and on glass (Figure 8e). The molecular structure of chlorinated phthalocyanine is shown in Figure 9.
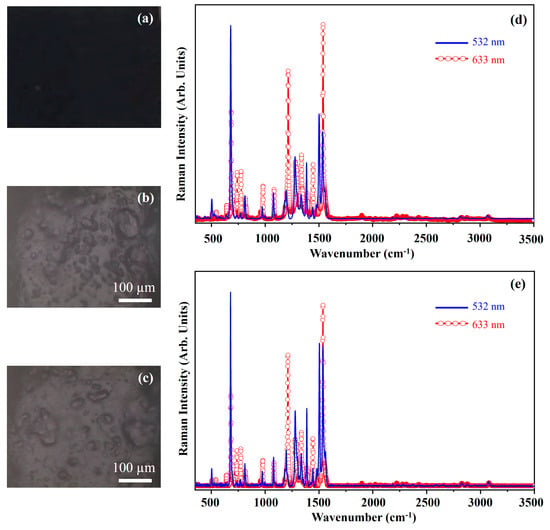
Figure 8.
Photograph of Phthalo Green (PG) pigment (a); optical microscope images of PG pigment on wood (b) and on glass (c) substrates; representative Raman spectra collected with 532 nm laser (blue line) and 633 nm laser (red open circle-line) of PG pigment on wood (d) and on glass substrates (e).
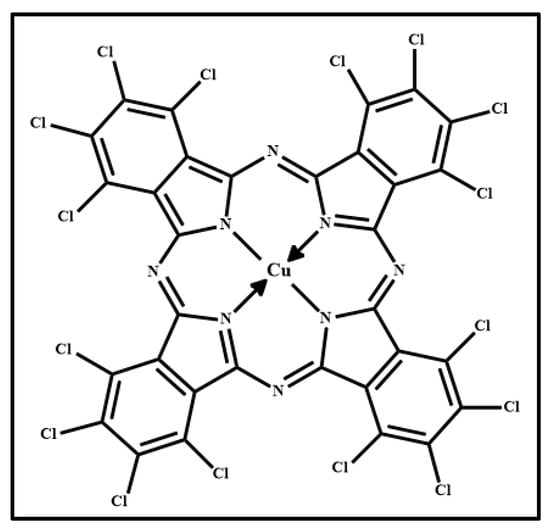
Figure 9.
Molecular structure of chlorinated phthalocyanine.
The photograph (Figure 8a) shows the dark green colour of the pigment PG and the other optical micrographs (Figure 8b,c) show that the morphology of the pigment is not affected by the substrate.
The Raman spectra collected with the same laser sources on pigment deposited on wood substrate (Figure 8d) and on glass substrate (Figure 8e) are quite similar each other. Chlorinated phthalocyanine spectra (Figure 8) show Raman features shifted compared with those of the spectra collected on the alpha and beta forms (Figure 3 and Figure 5, respectively), due to the presence of chloro substituents on the benzene rings of the isoindole groups [31,33]. As it can be seen by the comparison between the spectra of Figure 8 with those shown in Figure 3 and Figure 5, the chloride atoms also affect, in addition to the Raman shift, the intensity ratios of some bands [33], as could be expected.
In Figure 8, the main Raman bands fall at 684 cm−1, 740 cm−1, 776 cm−1, 980 cm−1, 1084 cm−1, 1214 cm−1, 1282 cm−1, 1339 cm−1, 1446 cm−1 and 1538 cm−1. The Raman band at 684 cm−1 is ascribed to the breathing mode of nitrogen atoms of the ring [33]. The Raman band at 740 cm−1 is assigned in this case to vibrational modes of C–N–C bonds, and to the benzene deformation. The Raman band at 776 cm−1 is ascribed to C–N–C bonds vibrational modes, to the benzene deformation and to C–Cl modes [33]. The Raman band that falls at 980 cm−1 is due to the contribution of several vibrational modes, that is the C–N–C between two isoindole units, the isoindole deformation and the C–Cl bonds [33]. The Raman band at 1084 cm−1 is ascribed to C–N, the benzene deformation and the C–Cl bonds [33]. Also, to the C–N bond, the C–Cl bonds, and in this case, the isoindole deformation, is ascribed to the Raman band that falls at 1214 cm−1. The Raman bands at 1282 cm−1 and at 1339 cm−1 are assigned to the C–C bonds and isoindole deformation [33]. The Raman band at 1446 cm−1 is assigned to several vibrational modes relative to the C–N, C–N–C and C–C bonds. At last, the Raman band at 1538 cm−1, the most intense of the spectrum, is assigned to C–C and C–N bonds [33].
Figure 10 shows the photograph of the pigment Primary Red Magenta (PRM) (Figure 10a), the optical microscope images of the pigment on wood (Figure 10b) and on glass (Figure 10c) and the Raman spectra collected on the pigment deposited on wood (Figure 10d) and on glass (Figure 10e). PRM pigment is constituted by an organic molecule whose IUPAC name is 5,12-Dihydroquinolino[2,3-b]acridine-7,14-dione and that is usually named as quinacridone. The quinacridone molecular structure is shown in Figure 11.
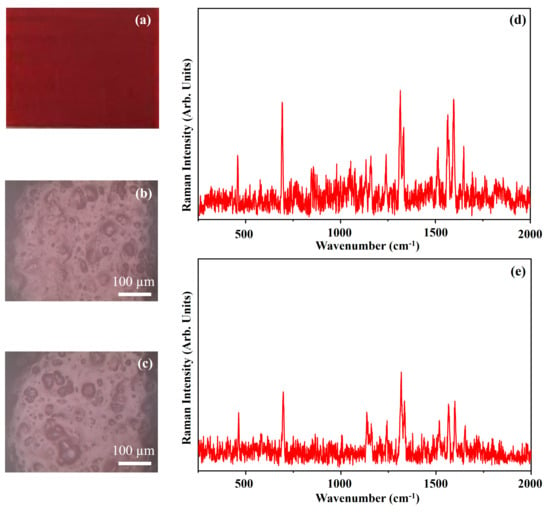
Figure 10.
Photograph of Primary Red—Magenta (PRM) pigment (a); optical microscope images of PRM pigment on wood (b) and on glass (c) substrates; representative Raman spectra collected with red laser of PRM pigment on wood (d) and on glass substrates (e).
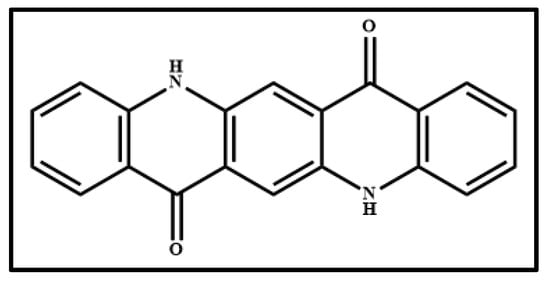
Figure 11.
Molecular structure of 5,12-Dihydroquinolino[2,3-b]acridine-7,14-dione named as quinacridone.
The photograph (Figure 10a) shows the Ruby Red colour of the pigment PRM and the other optical microscope images (Figure 10b,c) show that the morphology of the pigment it is not affected by the substrate. The Raman spectra of PRM show few Raman bands in the range between 150 cm−1 and 1650 cm−1 as it is possible to see in Figure 10, because of the presence of luminescence during the acquisition of the spectra. The stronger Raman bands observed in the two Raman spectra of Figure 10 fall at 693 cm−1, 1316 cm−1, 1565 cm−1, 1597 cm−1 and 1650 cm−1. The Raman band at 693 cm−1 is ascribed to the in-plane deformation of the C–C–C bond. The Raman band at 1316 cm−1 is assigned to the stretching of C–C bond, the Raman band at 1565 cm−1 is ascribed to the stretching of the C=C bond. The Raman band at 1597 cm−1 is attributed to the stretching of the C=C and C=O bonds and the Raman band at 1650 cm−1 is ascribed to the symmetric stretching of the C=O [34].
Figure 12 shows the photograph of the pigment Lemon Yellow (LY) (Figure 12a), the optical micrographs of the pigment on wood (Figure 12b) and on glass (Figure 12c), and the Raman spectra collected on the pigment deposited on wood (Figure 12d) and on glass (Figure 12e).
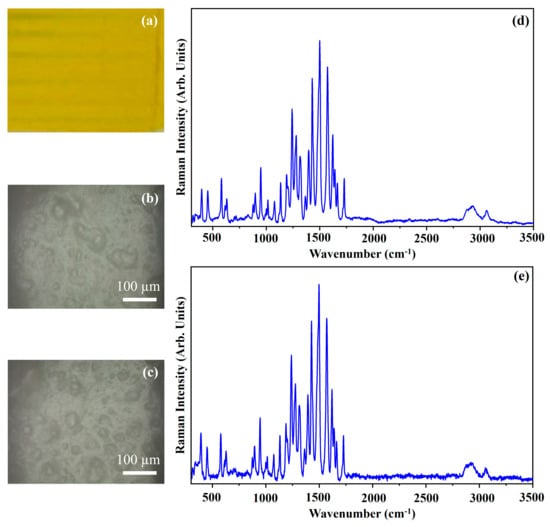
Figure 12.
Photograph of Lemon Yellow (LY) pigment (a); optical microscope images of LY pigment on wood (b) and on glass (c) substrates; representative Raman spectra collected with green laser of LY pigment on wood (d) and on glass substrates (e).
The photograph (Figure 12a) shows the yellow colour of the pigment LY and the other optical microscope images (Figure 12b,c) show that the morphology of the pigment is not affected by the substrate.
As it can be seen in Figure 12, the Raman spectra of LY on wood and glass substrates are very similar to each other. The more intense Raman bands observed in the spectra are at 1241 cm−1, 1279 cm−1, 1318 cm−1, 1396 cm−1, 1430 cm−1, 1500 cm−1, 1573 cm−1 and 1622 cm−1. As reported in Table 1, the composition of Lemon Yellow pigment is benzimidazolone. On the other hand, however, the molecular structure of the pigment shown in Figure 13 is slightly different from benzimidazolone (Figure 13). In fact, the LY pigment is part of a family called Hansa yellow pigments, whose members are also called azo pigments for the presence of N=N bond in their structure (Figure 13). Benzimidazolone pigment is the pure benzimidazolone molecule that is substituted in position 5 of the ring with an acetoacetylamine bound to dimethyl terephthalate through the azo bond (Figure 13). Therefore, the Raman assignment of the bands follows the knowledge of the structure of the pigment described above.
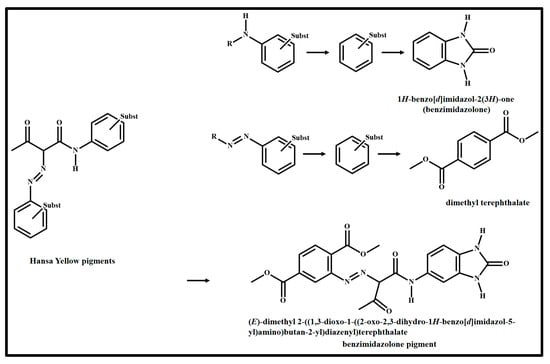
Figure 13.
The mono azo Hansa yellow family pigments (on the left) and the molecular structure of benzimidazolone pigment Lemon Yellow (LY, C.I. 11,784) on the right.
The Raman band at 1241 cm−1 is ascribed to the amide group of acetoacetylamine and in particular, to the amide III band [13,22]. The Raman bands at 1279 cm−1 and 1318 cm−1 are in the range assigned to aromatic ring vibrations and to C−H deformations of aliphatic side chains of Hansa yellow pigments (Figure 13) [22]. The Raman bands at 1396 cm−1 and at 1430 cm−1 are assigned to the stretching mode of the N=N bond with a contribution to the last band of the stretching vibration of the COO group and of the deformation vibration of the methyl group of the dimethyl terephthalate of the pigment [13,22,35]. The Raman band at 1500 cm−1 is related to the azo-benzyl group while the other bands at 1573 cm−1 and 1622 cm−1 are ascribable to the aromatic stretching of benzene derivatives rings [13,22]. (In the region of the last band, bending in the plane of the N−H bond of the benzimidazolone molecule [36] and the ring stretching of the p-disubstituted benzene [35] are reported).
Figure 14 shows the photograph of the pigment Cobalt Blue (CB) (Figure 14a), the optical microscope images of the pigment on wood (Figure 14b) and on glass (Figure 14c) and the Raman spectra collected on the pigment deposited on wood (Figure 14d) and on glass (Figure 14e). The photograph (Figure 14a) shows the Bright Blue colour of the pigment CB and the other optical images (Figure 14b,c) show that the morphology of the pigment it is not affected by the substrate. Cobalt Blue is an inorganic pigment made by cobalt aluminium oxides (CoAl2O4) crystallized in its spinel structure (spinel concerns the structures with the following formula, AB2X4 crystallized in the cubic crystal system) [37].
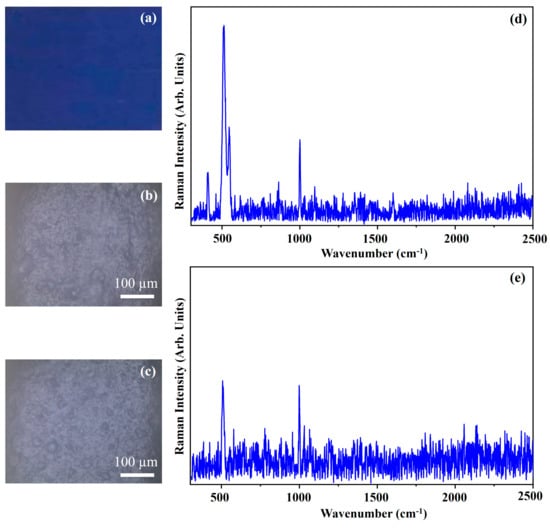
Figure 14.
Photograph of Cobalt Blue (CB) pigment (a); optical microscope images of CB pigment on wood (b) and on glass (c) substrates; representative Raman spectra collected with green laser of CB pigment on wood (d) and on glass substrates (e).
The Raman spectra of the CB pigment present the typical spectra of a spinel structure with a strong band that falls approximatively at 512 cm−1 and another band that falls at about 407 cm−1 [20,37,38] (Figure 14). In the spectrum collected on the CB pigment deposited on glass (Figure 14e), the band at 407 cm−1 is hidden in the noise signal.
Figure 15 shows the photograph of the pigment Ultramarine (UL) (Figure 15a), the optical microscope images of the pigment on wood (Figure 15b) and on glass (Figure 15c), and the Raman spectra collected on the pigment deposited on wood (Figure 15d) and on glass (Figure 15e).
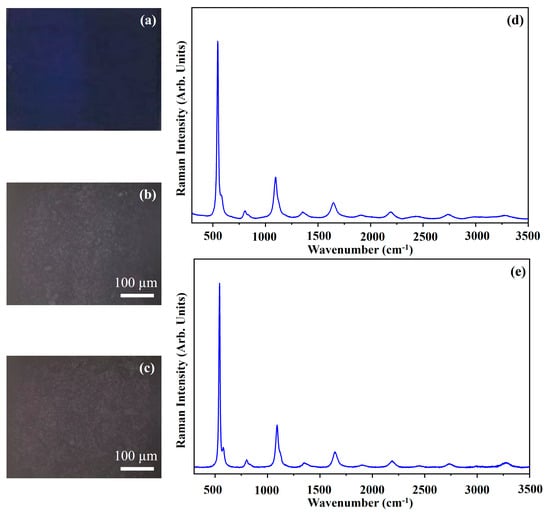
Figure 15.
Photograph of Ultramarine (UL) pigment (a); optical microscope images of UL pigment on wood (b) and on glass (c) substrates; representative Raman spectra collected with green laser of UL pigment on wood (d) and on glass substrates (e).
The photograph (Figure 15a) shows the intense blue colour of the pigment UL and the other optical microscope images (Figure 15b,c) show that the morphology of the pigment is not affected by the substrate.
The Ultramarine pigment (UL) is a sodium polysulfide–aluminosilicate mineral (Na8–10Al6Si6O24S2–4). The Raman spectra show the typical spectra of the UL pigment with its bands at 548 cm−1, 579 cm−1, 806 cm−1, 1096 cm−1, 1353 cm−1, 1648 cm−1, 2195 cm−1 and 2732 cm−1 [20,39]. In particular, the Raman band at 548 cm−1 is due to the stretching mode
of lazurite blue chromophores radical ions while the Raman band at 1096 cm−1 is due to the first harmonic mode of the band at 548 cm−1 [20,39].
Figure 16 shows the photograph of the pigment Cerulean Blue (CeB) (Figure 16a), the optical microscope images of the pigment on wood (Figure 16b) and on glass (Figure 16c), and the Raman spectra collected on the pigment deposited on wood (Figure 16d) and on glass (Figure 16e).
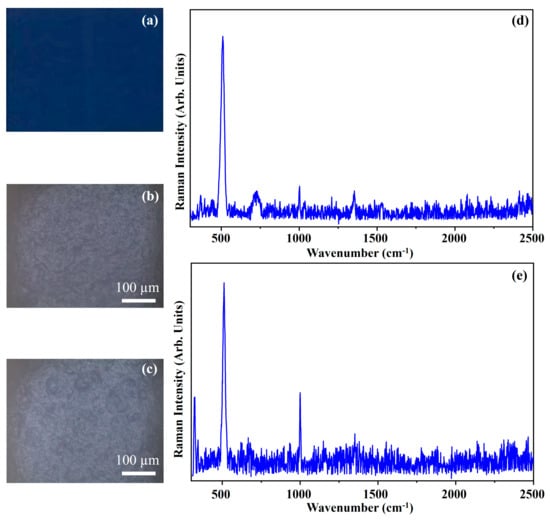
Figure 16.
Photograph of Cerulean Blue (CeB) pigment (a); optical microscope images of CeB pigment on wood (b) and on glass (c) substrates; representative Raman spectra collected with green laser of CeB pigment on wood (d) and on glass substrates (e).
The photograph (Figure 16a) shows the dark blue colour of the pigment CeB and the other optical microscope images (Figure 16b,c) show that the morphology of the pigment is not affected by the substrate.
Cerulean Blue is an inorganic pigment made up of Cobalt Aluminium Chromium Oxides [Co(Al,Cr)2O4] and the Raman spectra collected on such a pigment show its typical band at about 510 cm−1 [39].
Figure 17 shows the photograph of the pigment Cadmium Red Medium (CRM) (Figure 17a), the optical micrographs of the pigment on wood (Figure 17b) and on glass (Figure 17c), and the Raman spectra collected on the pigment deposited on wood (Figure 17d) and on glass (Figure 17e).
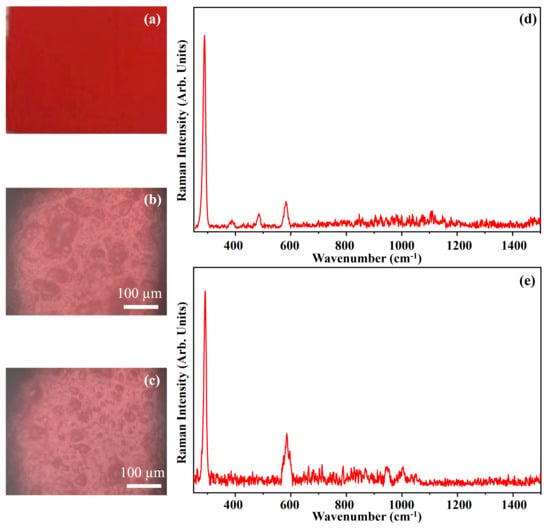
Figure 17.
Photograph of Cadmium Red Medium (CRM) pigment (a); optical microscope images of CRM pigment on wood (b) and on glass (c) substrates; representative Raman spectra collected with green laser of CRM pigment on wood (d) and on glass substrates (e).
The photograph (Figure 17a) shows the intense red colour of the pigment CRM and the other optical microscope images (Figure 17b,c) show that the morphology of the pigment is not affected by the substrate.
The pigment CRM is made by Cadmium Sulfoselenide mineral, CdS∙CdSe, and in Figure 17, the main Raman features, which fall at 288 cm−1, 484 cm−1 and 582 cm−1 [20], are shown.
Figure 18 shows the photograph of the pigment Zinc White (ZW) (Figure 18a), the optical microscope images of the pigment on wood (Figure 18b) and on glass (Figure 18c), and the Raman spectra collected on the pigment deposited on wood (Figure 18d) and on glass (Figure 18e).
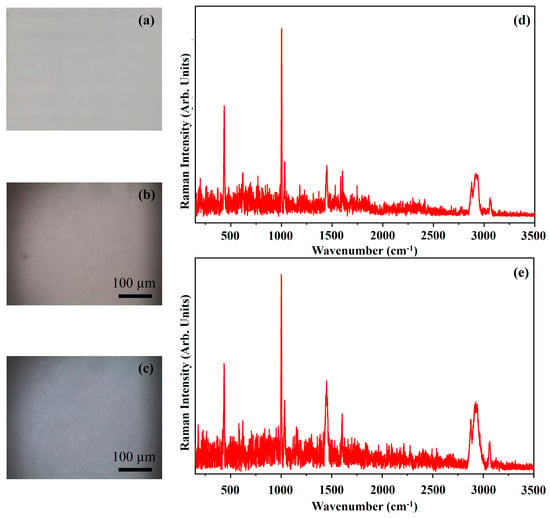
Figure 18.
Photograph of Zinc White (ZW) pigment (a); optical microscope images of ZW pigment on wood (b) and on glass (c) substrates; representative Raman spectra collected with red laser of ZW pigment on wood (d) and on glass substrates (e).
The photograph (Figure 18a) shows the light grey colour of the pigment ZW and the other optical microscope images (Figure 18b,c) show that the morphologies of the pigment deposited on wood seem to be light brown while those obtained when the pigment is deposited on glass substrate seem to be dark grey. It is due to the different contrast that the substrates induce on the pigment.
Zinc oxide (ZnO) has characteristic bands in the low wavenumber region. In the reported spectrum of Figure 18d, it is possible to observe a characteristic band of zinc oxide at approximately 436 cm−1 [40]. The same band is present on the spectrum of Figure 18e at 435 cm−1. The other bands present on the spectra, including those assigned at the C−H stretching at about 3000 cm−1, are due to some organic binder present on the pigment.
Figure 19 shows the photograph of the pigment Titanium White (TW) (Figure 19a), the optical microscope images of the pigment on wood (Figure 19.b) and on glass (Figure 19c) and the Raman spectra collected on the pigment deposited on wood (Figure 19d) and on glass (Figure 19e).
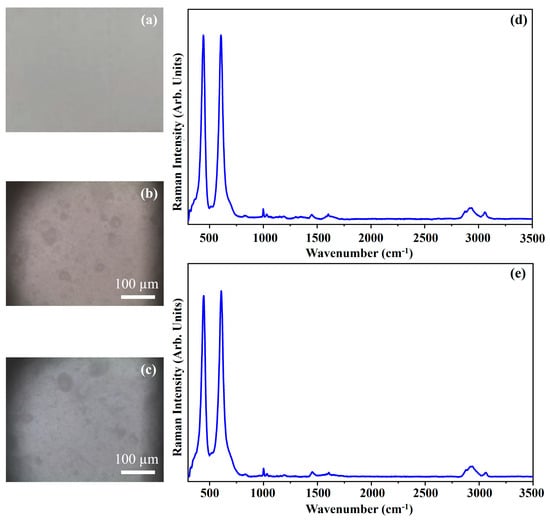
Figure 19.
Photograph of Titanium White (TW) pigment (a); optical microscope images of TW pigment on wood (b) and on glass (c) substrates; representative Raman spectra collected with red laser of TW pigment on wood (d) and on glass substrates (e).
The photograph (Figure 19a) shows the light grey colour of the pigment TW and the other optical microscope images (Figure 19b,c) show that the morphologies have different colours depending on if the pigment is deposited on the wood or on glass, where it results in being light brown and dark grey, respectively.
The inorganic pigment Titanium White, TW presents Raman modes at 443 cm−1 and 606 cm−1 as shown in the Raman spectra of Figure 19. Those bands are typical of Titanium dioxide (TiO2) in rutile form [41].
Figure 20 shows the photograph of the pigment Iridescent Silver (IS) (Figure 20a), the optical microscope images of the pigment on wood (Figure 20b) and on glass (Figure 20c) and the Raman spectra collected on the pigment deposited on wood (Figure 20d) and on glass (Figure 20e).
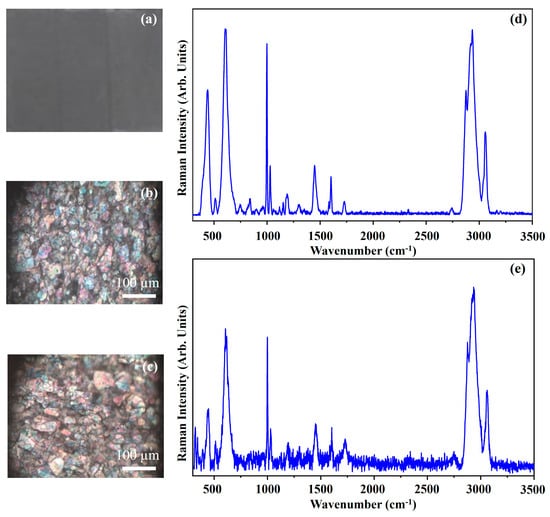
Figure 20.
Photograph of Iridescent Silver (IS) pigment (a); optical microscope images of IS pigment on wood (b) and on glass (c) substrates; representative Raman spectra collected with green laser of IS pigment on wood (d) and on glass substrates (e).
The photograph (Figure 20a) shows the dark grey colour of the pigment IS and the other optical micrographs (Figure 20b,c) show that the morphology of the pigment it is not affected by the substrate.
The Iridescent Silver pigment, IS, (also called iridescent pearl white), is an inorganic pigment made of mica + synthetic titanium oxide. The main Raman features of such pigment (Figure 20) are 440 cm−1, 606 cm−1, 999 cm−1, 1030 cm−1, 1189 cm−1, 1449 cm−1, 1603 cm−1, 2876 cm−1, 2919 cm−1, 2936 cm−1 and 3056 cm−1 [42]. The presence of the Raman bands at 440 cm−1 and 606 cm−1 suggests that the IS pigment contains titanium white too [42].
Figure 21 shows the photograph of the pigment Cadmium Yellow Medium (CYM) (Figure 21a), the optical images of the pigment on wood (Figure 21b) and on glass (Figure 21c), and the Raman spectra collected on the pigment deposited on wood (Figure 21d) and on glass (Figure 21e).
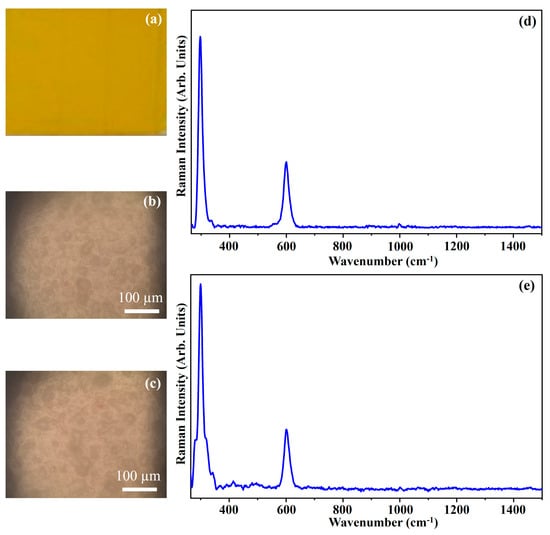
Figure 21.
Photograph of Cadmium Yellow Medium (CYM) pigment (a); optical microscope images of CYM pigment on wood (b) and on glass (c) substrates; representative Raman spectra collected with green laser of CYM pigment on wood (d) and on glass substrates (e).
The photograph (Figure 21a) shows the dark yellow colour of the pigment CYM and the other optical microscope images (Figure 21b,c) show that the morphology of the pigment is not affected by the substrate.
The Raman spectra show the characteristic features of a pigment made up by Cadmium Sulphide (CdS), with the presence of two bands at 297 cm−1 and 600 cm−1 assigned to the longitudinal optical phonon (LO) and to the 2LO overtone of the CdS crystal lattice [16]. These bands are typically assigned to a crystalline structure similar to the Wurtzite [43].
The shoulder at 337 cm−1 seems to indicate that in the pigment, traces of zinc sulphide are present [44].
Figure 22 shows the photograph of the pigment Cobalt Blue (Hue) (CB(H)) (Figure 22a), the optical microscope images of the pigment on wood (Figure 22b) and on glass (Figure 22c), and the Raman spectra collected with the 532 nm (blue line) and the 633 nm (red open circle-line) on the pigment deposited on wood (Figure 22d) and on glass (Figure 22e).
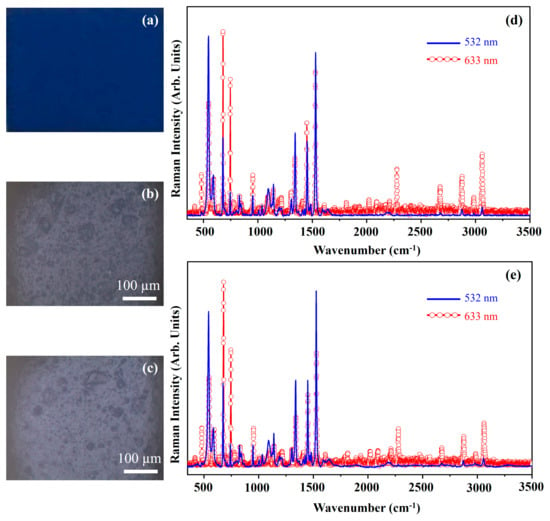
Figure 22.
Photograph of Cobalt Blue (Hue) (CB(H)) pigment (a); optical microscope images of CB(H) pigment on wood (b) and on glass (c) substrates; representative Raman spectra collected with 532 nm laser (blue line) and 633 nm laser (red open circle-line) of CB(H) pigment on wood (d) and on glass substrates (e).
The photograph (Figure 22a) shows the intense blue colour of the pigment CB(H) and the other optical microscope images (Figure 22b,c) show that the morphology of the pigment is not affected by the substrate.
The CB(H) pigment is a mixture of sodium polysulfide-aluminosilicate, β-phthalocyanines and titanium dioxide. The main Raman features fall at 483 cm−1, 546 cm−1, 591 cm−1, 640 cm−1, 678 cm−1, 746 cm−1, 804 cm−1, 830 cm−1, 845 cm−1, 953 cm−1, 1008 cm−1, 1038 cm−1, 1096 cm−1, 1144 cm−1, 1196 cm−1, 1215 cm−1, 1308 cm−1, 1343 cm−1, 1373 cm−1, 1413 cm−1, 1431 cm−1, 1454 cm−1, 1472 cm−1, 1485 cm−1, 1531 cm−1, 1593 cm−1, 1610 cm−1, 1641 cm−1, 2188 cm−1, 2211 cm−1, 2279 cm−1, 2677 cm−1, 2797 cm−1, 2873 cm−1, 2984 cm−1 and 3061 cm−1. Among all such Raman features, there are vibrational modes attributed to beta phthalocyanine (observed in the Raman spectrum of PBC pigment, see Figure 5) and to sodium polysulfide-aluminosilicate (observed in the Raman spectra of UL pigment, see Figure 15) [15,20,39]. As stated above, the different Raman spectra collected by using two laser sources are due to a Resonance effect that improves the detectability of some bands.
Figure 23 shows the photograph of the pigment Permanent Blue Light (PBL) (Figure 23a), the optical microscope images of the pigment on wood (Figure 23b) and on glass (Figure 23c), and the Raman spectra collected with the 532 nm (blue line) and the 633 nm (red open circle-line) on the pigment deposited on wood (Figure 23d) and on glass (Figure 23e).
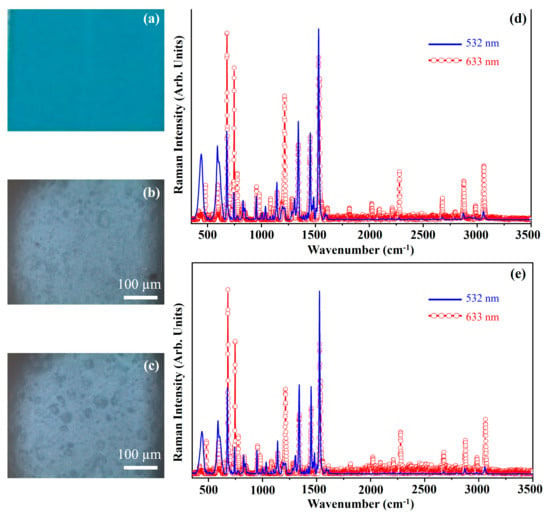
Figure 23.
Photograph of Permanent Blue Light (PBL) pigment (a); optical microscope images of PBL pigment on wood (b) and on glass (c) substrates; representative Raman spectra collected with 532 nm laser (blue line) and 633 nm laser (red open circle-line) of PBL pigment on wood (d) and on glass substrates (e).
The photograph (Figure 23a) shows the blue colour of the pigment PBL and the other optical micrographs (Figure 23b,c) show that the morphology of the pigment is not affected by the substrate.
The pigment Permanent Blue Light, PBL, is a mixture of chlorinated phthalocyanines, β phthalocyanines and titanium dioxide.
Therefore, the Raman spectra show characteristic Raman features attributed to the phthalocyanines at approximately 483 cm−1, 594 cm−1, 681 cm−1, 747 cm−1, 777 cm−1, 955 cm−1, 1145 cm−1, 1342 cm−1, 1452 cm−1 and 1531 cm−1 [20,39]. Of course, as state above, to the Resonance Raman effect is ascribed the reason why the spectra collected with different laser sources show different Raman spectral patterns. The bands assigned to titanium dioxide are detectable at 440 cm−1 and 588 cm−1.
Figure 24 shows the photograph of the pigment Van Dyke Brown (VB) (Figure 24a), the optical microscope images of the pigment on wood (Figure 24b) and on glass (Figure 24c) and the Raman spectra collected on the pigment deposited on wood (Figure 24d) and on glass (Figure 24e).
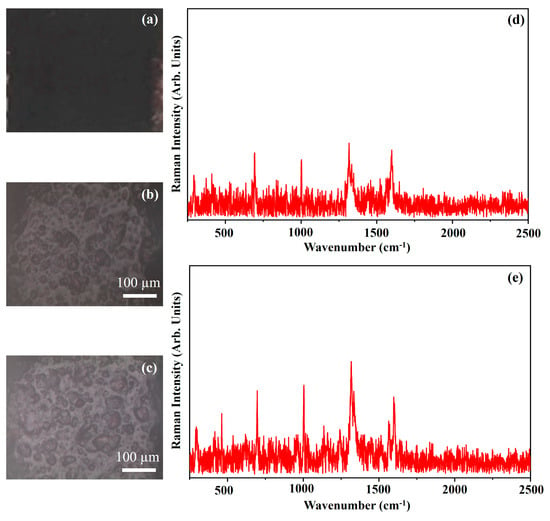
Figure 24.
Photograph of Van Dyke Brown (VB) pigment (a); optical microscope images of VB pigment on wood (b) and on glass (c) substrates; representative Raman spectra collected with red laser of VB pigment on wood (d) and on glass substrates (e).
The photograph (Figure 24a) shows the Dark Brown colour of the pigment VB and the other optical microscope images (Figure 24b,c) show that the morphology of the pigment is not affected by the substrate. The pigment Van Dyke Brown, VB, is a mixture of brown iron oxide, carbon black and quinacridone [45].
The Raman spectra are typical of a carbon-based material in which the two characteristic bands of the carbon can be observed: the D and G bands at 1317 cm−1 and 1599 cm−1, respectively. The first band is due to the defects present within the carbon crystalline lattice, while the second one is due to the longitudinal vibrations within it [45]. The Raman band at 693 cm−1 is ascribed to quinacridone [21].
Figure 25 shows the photograph of the pigment Permanent Green Light (PGL) (Figure 25a), the optical microscope images of the pigment on wood (Figure 25b) and on glass (Figure 25c), and the Raman spectra collected with the 532 nm (blue line) and the 633 nm (red open circle-line) on the pigment deposited on wood (Figure 25d) and on glass (Figure 25e).
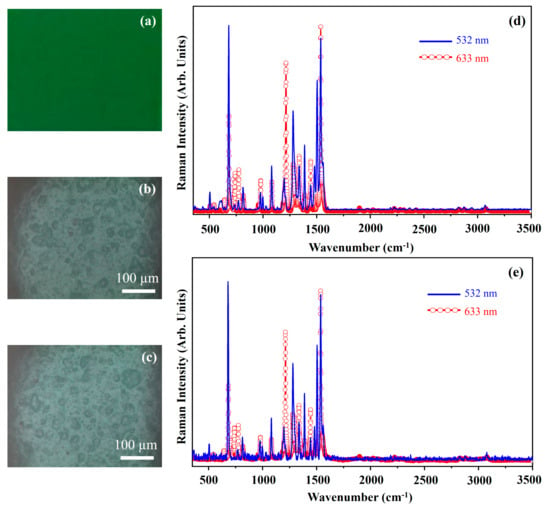
Figure 25.
Photograph of Permanent Green Light (PGL) pigment (a); optical microscope images of PGL pigment on wood (b) and on glass (c) substrates; representative Raman spectra collected with 532 nm laser (blue line) and 633 nm laser (red open circle-line) of PGL pigment on wood (d) and on glass substrates (e).
The photograph (Figure 25a) shows the dark green colour of the pigment PGL and the other optical microscope images (Figure 25b,c) show that the morphology of the pigment is not affected by the substrate.
The pigment Permanent Green Light (PGL) is a mixture of Arylide yellow, Titanium dioxide and chlorinated Phthalocyanines [29]. Arylide yellow is part of the family of the Hansa yellow pigments (see Figure 13). In Figure 26, the arylide molecular structure is shown.
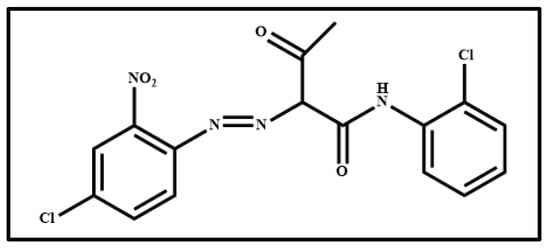
Figure 26.
Molecular structure of (E)-2-((4-chloro-2-nitrophenyl)diazenyl)-N-(2-chlorophenyl)-3-oxobutanamide, named arylide yellow.
The main Raman features fall at about 683 cm−1, 739 cm−1, 775 cm−1, 979 cm−1, 1083 cm−1, 1214 cm−1, 1282 cm−1, 1292 cm−1, 1338 cm−1, 1445 cm−1 and 1538 cm−1 (Figure 25). Such bands are assigned to the chlorinated Phthalocyanine (see Figure 8) [29]. Figure 25 shows the spectra collected with both laser sources because due to the Raman resonance effect, these spectra were different in the presence of pigments that contain phthalocyanine, as mentioned above.
Table 3 lists the conditions of acquisition of the Raman spectra acquired on the pigments and the observed Raman bands.
4. Conclusions
Micro-Raman spectroscopy was employed to characterize the following modern acrylic pigments: Cobalt Blue, Permanent Blue Light, Ultramarine, Primary—Cyan Blue, Cerulean Blue, Cobalt Blue (Hue), Indanthrone Blue, Phthalo Blue, Van Dyke Brown, Permanent Green Light, Phthalo Green, Primary Red—Magenta, Cadmium Red Medium, Lemon Yellow, Cadmium Yellow Medium, Zinc White, Titanium White and Iridescent Silver. These pigments were applied to both wooden and glass substrates, to simulate paint layers in real art-objects in cultural heritage contexts.
The Raman analysis successfully identified the main bands for each pigment studied. In addition, the substrate (wood or glass) was found to have negligible influence on the Raman spectra for nearly all the pigments.
For phthalocyanine-based pigments, however, spectral variations were observed when using different laser sources. This phenomenon was attributed to the resonance Raman effect, which was introduced to explain the experimental data. In conclusion, this study confirms that Raman spectroscopy is a highly sensitive and specific diagnostic tool for art conservation. The results also provide a comprehensive spectral library of acrylic pigments, serving as a valuable reference for future research.
Author Contributions
Conceptualization, I.B.C., M.C., J.S. and R.F.; methodology, A.N., M.C. and R.C.B.; software, I.B.C. and A.N.; validation, M.C., R.C.B., J.S. and R.F.; formal analysis, I.B.C. and M.C.; investigation, I.B.C. and A.N.; resources, M.C. and R.C.B.; data curation, A.N. and M.C.; writing—original draft preparation, A.N. and M.C.; writing—review and editing, I.B.C., A.N., M.C., R.C.B., J.S. and R.F.; visualization, M.C., J.S. and R.F.; supervision, M.C; project administration, M.C. and R.C.B.; funding acquisition, M.C. and R.C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the CHANGES, SPOKE 5 “Science and Technologies for Sustainable Diagnostics of Cultural Heritage”, PE 0000020, CUP B53C22003890006, NRP M4C2 Investment 1.3, funded by the European Union—NextGenerationEU.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request to the corresponding author.
Acknowledgments
The authors thank the project “MOST—Microtomografia, μ−Raman e analisi multimodale del corpus di spade provenienti dalle tombe di Torre Galli e conservate presso Il MArRC”, CUP B53C22003890006.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jablonski, E.; Learner, T.; Hayes, J.; Golden, M. Conservation Concerns for Acrylic Emulsion Paints. Stud. Conserv. 2003, 48, 3–12. [Google Scholar] [CrossRef]
- De Antonio, E.; Tuchman, M. Painters Painting: A Candid History of the Modern Art Scene, 1940–1970; 1. Aufl.; Abbeville Pr: New York, NY, USA, 1984; ISBN 978-0-89659-418-0. [Google Scholar]
- Pugliese, M. Tecnica Mista: Materiali e Procedimenti Nell’arte del XX Secolo; Pearson Italia S.p.a.: Milan, Italy, 2006; ISBN 978-88-424-9239-9. [Google Scholar]
- Chiantore, O.; Scalarone, D.; Learner, T. Characterization of Artists’ Acrylic Emulsion Paints. Int. J. Polym. Anal. Charact. 2003, 8, 67–82. [Google Scholar] [CrossRef]
- Politano, G.G.; Cazzanelli, E.; Versace, C.; Castriota, M.; Desiderio, G.; Davoli, M.; Vena, C.; Bartolino, R. Micro-Raman Investigation of Ag/Graphene Oxide/Au Sandwich Structure. Mater. Res. Express 2019, 6, 075605. [Google Scholar] [CrossRef]
- Rizzuto, C.; Teeters, D.C.; Barberi, R.C.; Castriota, M. Plasticizers and Salt Concentrations Effects on Polymer Gel Electrolytes Based on Poly (Methyl Methacrylate) for Electrochemical Applications. Gels 2022, 8, 363. [Google Scholar] [CrossRef]
- Álvarez-García, J.; Izquierdo-Roca, V.; Pistor, P.; Schmid, T.; Pérez-Rodríguez, A. Raman Spectroscopy on Thin Films for Solar Cells. In Advanced Characterization Techniques for Thin Film Solar Cells; Abou-Ras, D., Kirchartz, T., Rau, U., Eds.; Wiley: Hoboken, NJ, USA, 2016; pp. 469–499. ISBN 978-3-527-33992-1. [Google Scholar]
- Politano, G.G.; Castriota, M.; De Santo, M.P.; Pipita, M.M.; Desiderio, G.; Vena, C.; Versace, C. Variable Angle Spectroscopic Ellipsometry Characterization of Spin-Coated MoS2 Films. Vacuum 2021, 189, 110232. [Google Scholar] [CrossRef]
- Pezzotti, G. Raman Spectroscopy in Cell Biology and Microbiology. J. Raman Spectrosc. 2021, 52, 2348–2443. [Google Scholar] [CrossRef]
- Khonina, S.N.; Kazanskiy, N.L. Trends and Advances in Wearable Plasmonic Sensors Utilizing Surface-Enhanced Raman Spectroscopy (SERS): A Comprehensive Review. Sensors 2025, 25, 1367. [Google Scholar] [CrossRef]
- Lauridsen, C.B.; Sanyova, J.; Simonsen, K.P. Raman Analysis of Complex Pigment Mixtures in 20th Century Metal Knight Shields of the Order of the Elephant. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 150, 54–62. [Google Scholar] [CrossRef]
- Cazzanelli, E.; Platania, E.; De Santo, G.; Fasanella, A.; Castriota, M. Micro-Spectroscopic Raman Investigation on the Canvas Oil Painting “Rebecca at the Well” of Neapolitan Anonymous. J. Raman Spectrosc. 2012, 43, 1694–1698. [Google Scholar] [CrossRef]
- Ropret, P.; Centeno, S.A.; Bukovec, P. Raman Identification of Yellow Synthetic Organic Pigments in Modern and Contemporary Paintings: Reference Spectra and Case Studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 69, 486–497. [Google Scholar] [CrossRef]
- Pingitore, G.; Cerchiara, T.; Chidichimo, G.; Castriota, M.; Gattuso, C.; Marino, D. Structural Characterization of Corrosion Product Layers on Archaeological Iron Artifacts from Vigna Nuova, Crotone (Italy). J. Cult. Herit. 2015, 16, 372–376. [Google Scholar] [CrossRef]
- Scherrer, N.C.; Stefan, Z.; Francoise, D.; Annette, F.; Renate, K. Synthetic Organic Pigments of the 20th and 21st Century Relevant to Artist’s Paints: Raman Spectra Reference Collection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 73, 505–524. [Google Scholar] [CrossRef]
- Correia, A.M.; Clark, R.J.H.; Ribeiro, M.I.M.; Duarte, M.L.T.S. Pigment Study by Raman Microscopy of 23 Paintings by the Portuguese Artist Henrique Pousão (1859–1884). J. Raman Spectrosc. 2007, 38, 1390–1405. [Google Scholar] [CrossRef]
- Yogurtcu, B.; Cebi, N.; Koçer, A.T.; Erarslan, A. A Review of Non-Destructive Raman Spectroscopy and Chemometric Techniques in the Analysis of Cultural Heritage. Molecules 2024, 29, 5324. [Google Scholar] [CrossRef]
- Saggio, F.; Rizzuto, C.; Teeters, D.; Didik, J.; Barberi, R.C.; Castriota, M. Raman Investigations on the Frame of the Painting “White Man’s Buffalo” by the Artist Charles, M. Russell. Appl. Sci. 2023, 13, 3654. [Google Scholar] [CrossRef]
- Caggiani, M.C.; Colomban, P. Raman Microspectroscopy for Cultural Heritage Studies. Phys. Sci. Rev. 2018, 3, 20180007. [Google Scholar] [CrossRef]
- Caggiani, M.C.; Cosentino, A.; Mangone, A. Pigments Checker Version 3.0, a Handy Set for Conservation Scientists: A Free Online Raman Spectra Database. Microchem. J. 2016, 129, 123–132. [Google Scholar] [CrossRef]
- Schulte, F.; Brzezinka, K.; Lutzenberger, K.; Stege, H.; Panne, U. Raman Spectroscopy of Synthetic Organic Pigments Used in 20th Century Works of Art. J. Raman Spectrosc. 2008, 39, 1455–1463. [Google Scholar] [CrossRef]
- Vandenabeele, P.; Moens, L.; Edwards, H.G.M.; Dams, R. Raman Spectroscopic Database of Azo Pigments and Application to Modern Art Studies. J. Raman Spectrosc. 2000, 31, 509–517. [Google Scholar] [CrossRef]
- Barba Castagnaro, I.; Nucera, A.; Barberi, R.C.; Castriota, M. Study and Micro-Raman Characterization of Pigments Present on Majolicas of Historical and Artistic Interest from Gerace, Italy. Herit. Sci. 2023, 11, 24. [Google Scholar] [CrossRef]
- Ropret, P.; Madariaga, J.M. Applications of Raman Spectroscopy in Art and Archaeology. J. Raman Spectrosc. 2014, 45, 985–992. [Google Scholar] [CrossRef]
- Striova, J.; Dal Fovo, A.; Fontani, V.; Barucci, M.; Pampaloni, E.; Raffaelli, M.; Fontana, R. Modern Acrylic Paints Probed by Optical Coherence Tomography and Infrared Reflectography. Microchem. J. 2018, 138, 65–71. [Google Scholar] [CrossRef]
- Tackley, D.R.; Dent, G.; Smith, W.E. Phthalocyanines: Structure and Vibrations. Phys. Chem. Chem. Phys. 2001, 3, 1419–1426. [Google Scholar] [CrossRef]
- Bovill, A.J.; McConnell, A.A.; Nimmo, J.A.; Smith, W.E. Resonance Raman Spectra of .Alpha.-Copper Phthalocyanine. J. Phys. Chem. 1986, 90, 569–575. [Google Scholar] [CrossRef]
- Anghelone, M.; Jembrih-Simbürger, D.; Schreiner, M. Identification of Copper Phthalocyanine Blue Polymorphs in Unaged and Aged Paint Systems by Means of Micro-Raman Spectroscopy and Random Forest. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 149, 419–425. [Google Scholar] [CrossRef]
- Anghelone, M.; Jembrih-Simbürger, D.; Pintus, V.; Schreiner, M. Photostability and Influence of Phthalocyanine Pigments on the Photodegradation of Acrylic Paints under Accelerated Solar Radiation. Polym. Degrad. Stab. 2017, 146, 13–23. [Google Scholar] [CrossRef]
- Jennings, C.; Aroca, R.; Hor, A.; Loutfy, R.O. Raman Spectra of Solid Films 3—Mg, Cu and Zn Phthalocyanine Complexes. J. Raman Spectrosc. 1984, 15, 34–37. [Google Scholar] [CrossRef]
- Basova, T.V.; Kiselev, V.G.; Schuster, B.; Peisert, H.; Chassé, T. Experimental and Theoretical Investigation of Vibrational Spectra of Copper Phthalocyanine: Polarized Single-crystal Raman Spectra, Isotope Effect and DFT Calculations. J. Raman Spectrosc. 2009, 40, 2080–2087. [Google Scholar] [CrossRef]
- Chang, J.; Cañamares, M.V.; Aydin, M.; Vetter, W.; Schreiner, M.; Xu, W.; Lombardi, J.R. Surface-Enhanced Raman Spectroscopy of Indanthrone and Flavanthrone. J. Raman Spectrosc. 2009, 40, 1557–1563. [Google Scholar] [CrossRef]
- Sukhikh, A.; Bonegardt, D.; Klyamer, D.; Krasnov, P.; Basova, T. Chlorosubstituted Copper Phthalocyanines: Spectral Study and Structure of Thin Films. Molecules 2020, 25, 1620. [Google Scholar] [CrossRef]
- Del Puerto, E.; Domingo, C.; Garcia Ramos, J.V.; Sanchez-Cortes, S. Adsorption Study and Detection of the High Performance Organic Pigments Quinacridone and 2,9-Dimethylquinacridone on Ag Nanoparticles By Surface-Enhanced Optical Spectroscopy. Langmuir 2014, 30, 753–761. [Google Scholar] [CrossRef]
- Rodrígueza, R.; Jiménez, S.; Vargas, S.; Pacheco, S.; Castaño, V.M. Micro-Raman Characterization of Anisotropic Dimethyl Terephthalate Crystallites. Int. J. Polym. Mater. 2001, 49, 1–13. [Google Scholar] [CrossRef]
- Benali, B.; El Assyry, A.; Boucetta, A.; Lazar, Z.; Lakhrissi, B. Thermal, Structural, and Conformational Study of the Benzimidazolone Molecule. Res. Chem. Intermed. 2015, 41, 821–830. [Google Scholar] [CrossRef]
- Bouchard, M.; Gambardella, A. Raman Microscopy Study of Synthetic Cobalt Blue Spinels Used in the Field of Art. J. Raman Spectrosc. 2010, 41, 1477–1485. [Google Scholar] [CrossRef]
- Bell, I.M.; Clark, R.J.H.; Gibbs, P.J. Raman Spectroscopic Library of Natural and Synthetic Pigments (Pre- ≈ 1850 AD). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1997, 53, 2159–2179. [Google Scholar] [CrossRef]
- Burgio, L.; Clark, R.J.H. Library of FT-Raman Spectra of Pigments, Minerals, Pigment Media and Varnishes, and Supplement to Existing Library of Raman Spectra of Pigments with Visible Excitation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2001, 57, 1491–1521. [Google Scholar] [CrossRef]
- Artesani, A.; Ghirardello, M.; Mosca, S.; Nevin, A.; Valentini, G.; Comelli, D. Combined Photoluminescence and Raman Microscopy for the Identification of Modern Pigments: Explanatory Examples on Cross-Sections from Russian Avant-Garde Paintings. Herit. Sci. 2019, 7, 17. [Google Scholar] [CrossRef]
- Yakes, B.J.; Michael, T.J.; Perez-Gonzalez, M.; Harp, B.P. Investigation of Tattoo Pigments by Raman Spectroscopy. J. Raman Spectrosc. 2017, 48, 736–743. [Google Scholar] [CrossRef]
- Schenk, F.; Parker, A. Iridescent Color: From Nature to the Painter’s Palette. Leonardo 2011, 44, 108–115. [Google Scholar] [CrossRef]
- Database of Raman Spectroscopy, X-Ray Diffraction and Chemistry of Minerals. Available online: https://rruff.info/ (accessed on 5 February 2024).
- Aguayo, T.; Clavijo, E.; Villagrán, A.; Espinosa, F.; Sagüés, F.E.; Campos-Vallette, M. Raman vibrational study of pigments with patrimonial interest for the chilean cultural heritage. J. Chil. Chem. Soc. 2010, 55, 347–351. [Google Scholar] [CrossRef]
- Coccato, A.; Jehlicka, J.; Moens, L.; Vandenabeele, P. Raman Spectroscopy for the Investigation of Carbon-based Black Pigments. J. Raman Spectrosc. 2015, 46, 1003–1015. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).