Abstract
For oenological products, most of the intrinsic and extrinsic drivers of perceived quality are associated with specific aromatic profiles. Aromatic diversity has been recognized as a central element in perceived quality as it is able to transmit the complex interactions between grape variety, geographical characteristics, and viticultural and winemaking practices, including the fermentative process. A comprehensive characterization of flavour compounds by headspace solid-phase microextraction (HS-SPME) and gas chromatography coupled to mass spectrometric analysis is often needed in order to ascertain the quality of wine. HS-SPME requires a proper optimization that can be achieved through an adequate experimental design. Here, a HS-SPME/GC-MS based method was developed to investigate the volatile compounds of wine samples obtained by laboratory-scale fermentations. This was performed by inoculating a commercial Saccharomyces cerevisiae strain, which is used both as single starter and as mixed starter, with an indigenous Hanseniaspora osmophila strain. The experimental conditions of HS-SPME (extraction temperature and time) were optimized by applying a face-centred composite experimental design. Up to 95% of the total variance was explained by the proposed model. The optimized method allowed us to confirm the usefulness of combining the inoculation of grapes with selected yeast strains in co-culture situations in order to improve the wine bouquet.
1. Introduction
The overall aroma of wine is determined by a complex pool of volatile compounds, many of which are present at levels above their olfactory thresholds, and the aroma is one of the most striking features out of those that determine the consumers’ choice. In order to address the consumers’ preferences, wineries continuously adapt their business strategies and production practices [1]. Among the factors of the winemaking processes contributing to the sensorial complexity of the oenological products, i.e., fermentation strategies, grape varieties, geographical origin and wine aging [2], the selection of a suitable starter culture is a useful biotechnological tool with which to increase the number of odorant compounds responsible for the wine organoleptic characteristics. The use of controlled multi-starter fermentations based on non-Saccharomyces and Saccharomyces strains is currently considered as a valid tool for enhancing wine complexity, even though the antagonistic interactions among these kind of yeasts are complex and still not entirely clear.
Other advantages associated with the multi-starter fermentation in winemaking are the possibility of increasing the total acidity, of reducing the ethanol content and of controlling the spoilage of the microflora in the wine [3]. The successful evolution of mixed starter fermentations is correlated with the active participation of non-Saccharomyces strains in the fermentative process, as these yeasts are usually less competitive than those of S. cerevisiae. Among the practices which are useful in increasing the persistence of non-Saccharomyces strains during the fermentation process, the use of immobilized cells rather than free cells gave promising results. Microbial cells can be immobilized through adsorption, covalent binding, gel entrapment into inert natural polymers, such as alginate, agarose, chitosan and pectin; these techniques defend the microbial cells from stress factors emerging during the fermentative process, preserving the metabolic activities correlated to the production of aroma compounds [4]. The effect of different starter formulations on wine bouquet can be studied through an untargeted profiling of the volatile organic compounds (VOCs) which occur in the samples after their preliminary extraction via gas chromatography coupled to mass spectrometry (GC-MS). Mass spectrometry coupled to chromatographic separation techniques have become the techniques of choice for the identification of unknown compounds in several fields, including the field of food chemistry [5,6,7,8]. Although some researchers have realized a VOC extraction by solid-phase extraction (SPE) or liquid–liquid extraction (LLE) using dichloromethane or ether/pentane as solvent [9,10,11,12], the best option for obtaining a complete volatile fraction is the headspace solid-phase microextraction (HS-SPME). HS-SPME is associated with a low risk of overvaluing odorants which have been poorly transferred to the headspace. Moreover, the volatile compounds are not lost during the evaporation, as in LLE, and the extracts are completely free from non-volatile material [13]. The HS-SPME conditions, such as fibre type, extraction temperature and time, widely influence the efficiency of the extraction and, above all, the vapor pressure and equilibrium of the aroma compounds in the headspace of the sample. The selection of the most appropriate SPME fibre depends on the target compounds and the studied matrix. In general, 100 µm of PDMS fibre allows for the adsorption of a higher number of analytes for wine samples and will allow fora higher degree of reproducibility and chemical and mechanical robustness than other compounds, such as the triphasic ones [14,15,16,17]. In detail, Bianco et al. [17] achieved a better precision with the non-polar PDMS phase compared to the DVB/CAR/PDMS phase. On the other hand, Sagratini et al. [14] found that PDMS fibre is particularly selective for esters which positively contribute to the quality of wine products, such as ethyl octanoate, ethyl-9-decenoate and ethyl decanoate. In addition to fibre type selection, extraction time and temperature optimization, sample saturation with a salting out agent has also been proven to enhance the extraction efficiency. This occurs due to the increase in the ionic strength of the solution and the decreased solubility of the apolar analytes in the solution [18].
Most of the practical work involving SPME optimization uses strategies based on systematic studies of one variable at a time (OVAT), during which all the variables affecting the SPME efficiency (i.e., fibre coating, extraction time and temperature, ionic strength and sample volume) are maintained at a constant level during test runs, except for the one being studied. However, the use of the classical univariate approach could result in incorrect optimizations since it does not consider the interactions between different factors, assuming instead that all the variables are independent and the effect, at a given set condition, is also the same if the remaining variables are changed [19]. In order to avoid erroneous run tests, an optimization planned carried out according to an experimental design (DoE) is the best option. Indeed, in DoE all variables are studied at the same time, enabling a reduction in the number of experiments with a complete exploration of the experimental domain [20,21]. Thus, it is a very suitable means with which to investigate most of the variables involved in the HS-SPME process. Anyway, it should be considered that the quality of the results of an experimental design depends on the distribution of experiments in the experimental domain. When DoE does not allow the resolution of the problem, a redefinition of the experimental domain or a change in the postulated model could be needed [22].
Here, a face-centred composite experimental design has been developed, taking advantage of information gained from previously acquired experimental data. This design was used to optimize the headspace solid-phase microextraction of the volatile compounds occurring in experimental wine samples. The samples were obtained by laboratory-scale fermentations performed in Aglianico del Vulture grape must, one of the most diffuse grape varieties in the Basilicata region of Southern Italy [23,24]. The grape must was inoculated with a commercial Saccharomyces cerevisiae strain, used both as a single starter and as a mixed starter in combination with an indigenous Hanseniaspora osmophila strain, as both free and immobilized cells.
The optimized SPME-GC/MS method was used to ascertain the aromatic compounds of the experimental wines obtained by different starter cultures.
2. Materials and Methods
2.1. Chemicals and Reagents
Glacial acetic acid (≥99.99%), sodium chloride, absolute ethanol (EtOH, 99.8%), alginic acid sodium salt and the analytical standards of nerolidol (98%) and 1-butanol, 3-methyl-acetate (≥99%) were purchased from Sigma Aldrich (Milan, Italy). The analytical standard of 2-phenylethyl alcohol (98%) was obtained from Carlo Erba (Milan, Italy). A Milli-Q RG system (Millipore, Bedford, MS, USA) was used to produce ultrapure water. A standard mixture of acetic acid (3 mg/mL), 1-butanol, 3-methyl-acetate (3 mg/mL), phenylethyl alcohol (17 mg/mL) and nerolidol (0.070 mg/mL) was freshly prepared in EtOH/H2O (13% v/v) and was used as model to optimize the main parameters affecting the headspace solid-phase microextraction efficiency for subsequent wine flavour analyses.
2.2. Wine Samples
Three wine samples were analyzed in this work which belonged to the same year and batch, namely: W1 and W2, i.e., wine samples obtained by fermentation with S. cerevisiae in co-culture with free and immobilized cells of the H. osmophila strain, respectively; wine sample C, i.e., the control obtained by fermentation with free cells of S. cerevisiae alone. The wild strain of H. osmophila ND1 belonged to the Yeast Collection of the University of Basilicata (UBYC), while the commercial strain of S. cerevisiae EC1118 was purchased from Lallemand Inc. (Toulose, France). The fermentations were performed in 2 L of pasteurized natural grape must (Aglianico del Vulture variety) at 26 °C; they were monitored by determination of weight loss due to CO2 production during sugar fermentation. Grape must was pasteurized at 90 °C for 20 min in order to avoid the growth of undesirable microorganisms [25]. The fermentation process was stopped when weight and °Brix reductions were constant for three consecutive days [26]. Around 20 days were taken by each starter to complete the process. After headspace solid-phase microextraction optimization, all the wine samples were analyzed in triplicate by HS-SPME/GC-MS.
2.3. Optimization of Headspace Solid-Phase Microextraction
For the headspace solid-phase microextraction optimization, 3 mL of standard mixture were placed into 5 mL vials and saturated with 0.6 g of sodium chloride, according to the recommendations of a previously reported method [17], in order to obtain a liquid-phase-to-headspace-volume ratio, 1/β, of 0.6. The vials were sealed with polytetrafluoroethylene/silicone septum caps and equilibrated in a reacti Therm heating stirring module (Thermo Scientific, Pierce Protein Research Products, Rockford, IL, USA) for 10 min at the set temperature (as reported in Table 1) before the headspace sampling was performed. After the sample/headspace equilibration period, the septum of vials was pierced with the needle containing the fibre retracted. Then, the fibre was exposed to the headspace for the fixed time and temperature. In total, 100 µm PDMS fibre was used. The extraction time and temperature were optimized by DoE.

Table 1.
Levels of the variables (extraction time and extraction temperature) explored in the face-centred composite experimental design experiments (DoE).
2.4. Experimental Design
A face-centred composite experimental design was applied in order to perform an optimization of the HS-SPME. The variables investigated, i.e., extraction temperature and extraction time, were evaluated at three different levels. The independent variables and their related codes and levels are displayed in Table 1. The response evaluated during all experiments was the total sum of peak areas of the standard mixture, and relevant data were obtained in the GC-MS analysis. Three replicates were performed at the central point in order to quantify that the experimental error and response surface methodology were realized to choose the optimum values of temperature and extraction time for the GC-MS analysis of the aroma compounds of the three wine samples. The statistical experimental design and optimization calculations were performed using the R-based software CAT [27]. Regression analysis for the experiment data was performed and the results were fitted into a second-order polynomial model:
2.5. GC-MS Analysis
The extracted analytes were desorbed into the injection port of the GC–MS system for 15 min at 260 °C. GC-MS analyses were performed on an Agilent Hewlett Packard 6890 plus gas chromatograph. This equipment was fitted with a split/splitless injector and an Agilent 5975 mass spectrometer (MS) detector, equipped in turn with a single quadrupole analyzer. The chromatographic separation was carried out on a HP-5MS capillary column 30 m × 0.25 mm i.d. × 0.25 µm film thickness (Agilent Technologies, Santa Clara, CA, USA). A deactivated glass liner 105 × 8.0 × 0.75 mm (Supelco, Milan, Italy) was installed in the injector which was used in splitless mode. Helium was used as the carrier gas at a flow rate of 1 mL min−1. The oven temperature was adapted from Sagratini et al. [14] and programmed as follows: from 35 °C (hold for 0.5 min) to 50 °C (3 °C/min, hold time 2 min) and up to 250 °C (8 °C/min, hold time 2 min). Finally, a temperature of 270 °C was reached at 8 °C/min and held for 2 min. The electron ionization (EI) mode was used for the MS analysis, with an electron energy of 70 eV. The MS acquisition was performed in full-scan mode in the range m/z 30–400. The source temperature and the transfer line temperature were set, respectively, at 230 °C and 300 °C. Enhanced data analysis (Agilent Software, Santa Clara, CA, USA) was performed for the chromatographic data acquisition and processing, while Sigma Plot 11.0 (Systat Software, London, UK) was used for data elaboration and plotting.
3. Results and Discussion
3.1. HS-SPME Optimization by Experimental Design
The flavour profile, alongside the sugar–acid balance, is among the most important parameters contributing to the overall quality of wine products [28,29]. The qualitative determination of wine volatile compounds allows researchers to define an aromatic fingerprint. This is characteristic of a specific oenological product and is the result of several factors, including grape variety, fermentative and post-fermentative processes. The volatile fraction fingerprinting, performed by solid-phase microextraction, combined with direct analysis via gas chromatography coupled to mass spectrometry needs a preliminary optimization of the experimental parameters. A standard solution containing acetic acid, 1-butanol 3-methyl-acetate, 2-phenylethanol and nerolidol in EtOH/H2O was employed for HS-SPME optimization. Since the analytical standards used belonged to the main four classes of volatile compounds occurring in the wine, namely acids, esters, alcohols and terpenes, they can be properly used as a model to mimic the effects of the studied matrix [30]. A 100 µm PDMS fibre was used for the adsorption of a higher number of analytes for wine samples.
The optimization of the extraction time and temperature was conducted by running experiments according to a face-centred composite design to select the best conditions of wine aroma compounds extraction with the lowest number of experiments. The extraction time was varied between 10 and 30 min, while the temperature ranged from 40 °C to 60 °C, as higher temperatures could change the composition of the wine sample by producing artifacts [15]. In total, 9 runs were carried out in triplicate to study the influence of the selected factors and their interactions on the HS-SPME. The results obtained for the responses, i.e., the total sum of peak areas of the standard mixture, are reported in Table 2.

Table 2.
Values of the response obtained at the variation levels chosen for the variables selected for HS-SPME optimization by experimental design, i.e., extraction temperature and extraction time.
The quadratic equation obtained using coded values for the variables was given by:
where R is the dependent response, i.e., the total sum of peaks areas of the standard mixture, T is the extraction temperature, t the extraction time, and T × t the interaction between extraction temperature and time.
R = 5.524 × 109 + 3.024 × 109 T + 1.026 × 109 t + 1.250 × 109 t × T + 2.014 × 109 T2 − 4.269 t2
All the coefficients were found to be statistically significant. A total of 95% of the total variance was explained by this model. To validate the model, three replicates in the central point were performed in order to estimate the experimental error and to detect any lack of fit. The response surface obtained by the use of DoE face-centred composite design is reported in Figure 1. The estimated optimum value range, obtained for the extraction temperature using a response surface methodology, was at around 60 °C (Figure 1). In general, in the SPME experiments, the temperature parameter decreases the partition coefficient between the analyte and the extraction polymer [31]. However, on the other hand, it acts on the extraction by increasing the diffusion of the compounds and therefore increasing the extraction rate. The extraction time was found to have a statistically important positive effect on the total area and number of volatile compounds. The increase in extraction time improves the efficiency of extraction of compounds which have high boiling points and increases the detected peak area. However, the increase in extraction time does not show the same effect on compounds with low boiling point [32]. This could presumably explain the optimum values range found by response surface methodology for the extraction time, which was at intermediate time, i.e., around 20 min. Thus, further experiments were carried out to evaluate the flavour profile of the three wine samples by setting the extraction temperature and the extraction time of the SPME at 60 °C and 20 min, respectively.
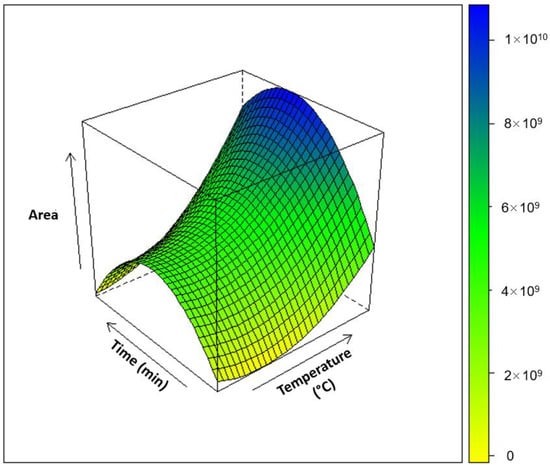
Figure 1.
Response surface plot for the total sum of peaks areas of the standard mixture used as model for the HS-SPME optimization according to the DoE face-centred composite design (95% of the explained variance).
3.2. Wine Flavour Profiling
After the optimization of the HS-SPME/GC-MS method, the best conditions of the analysis were applied to the untargeted flavour profiling of the wines which had been obtained by fermentation with mixed cultures of yeasts and which were composed of the S. cerevisiae strain in association with the ND1 strain. This was tested both in free and immobilized cells, in comparison to the control, which was the experimental wine that had been obtained using single fermentation with the EC1118 strain. The SPME-GC/MS analysis allowed the identification of the 21 volatile organic compounds in the wine sample obtained by the mixed starters of S. cerevisiae and the free cells of H. osmophila (sample W1), which was a considerably greater number compared to the 8 compounds identified in the W2 wine sample that had been fermented with immobilized cells of the ND1 strain in co-culture with EC1118, and of the 6 compounds identified in the control (C), i.e., the wine sample obtained through the fermentation with S. cerevisiae alone. Such a difference in the number of VOCs detected in W1 and W2 wines could be due to a remarkable reduction in the enzymatic activity of yeasts, potentially caused by the diffusion limitations or the breakage of the microcapsules [33]. Figure 2 shows the total ion current chromatograms, obtained at optimized conditions, of W1 and W2 wine samples and of the control C.
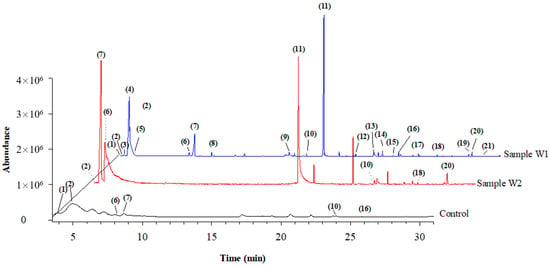
Figure 2.
Total ion current chromatograms obtained by the HS-SPME/GC-MS analysis of the three wine samples, i.e., Aglianico wine obtained by fermentation with the mixed starters S. cerevisiae and free cells of H. osmophila (W1); Aglianico wine obtained by fermentation with the mixed starters S. cerevisiae and immobilized cells of H. osmophila (W2); Aglianico wine obtained by fermentation with S. cerevisiae alone (control C). SPME parameters: 100 µm PDMS fibre, extraction time 20 min, extraction temperature 60 °C, salinity 0.6 g of NaCl in 3 mL of sample volume.
All the volatile organic compounds detected in the three samples were identified by comparing their mass spectra with those available in the literature [34,35,36] and in the NIST electronic Mass Spectral Database. They belonged to five classes, namely higher alcohols, esters, volatile acids, terpenes, aldehydes and volatile phenols. The retention times, the quality match values with the NIST data and the main chemical-physical characteristics of the identified compounds are reported in Table 3, along with the occurrence or not of each VOC in the two wine samples and in the control. The wine obtained by co-inoculation of the free cells of H. osmophila and S. cerevisiae had a high number of higher alcohols, representing about the 44.9% of the total volatile organic compounds (Figure 3) and includ ethyl acetate (compound 2), 2-butanol-3-methyl (compound 3), 1-butanol, 3-methyl- (compound 4), 1-heptanol (compound 5), 2,3-butanediol (compound 8), phenylethyl alcohol (compound 9) and 1-dodecanol (compound 11). Higher alcohols are also referred to as fusel alcohols and are alcohols which have more than two carbons in their chain; thus, they have higher molecular weights and higher boiling points than ethanol. The discussion regarding the role that these compounds play in improving the quality of oenological products remains controversial. Higher alcohols impart a range of organoleptic attributes, ranging from solvent-like to floral. which could positively influence wine flavour [37]. However, several authors attributed to higher alcohols the pungent and unpleasant character of some wines, especially when present at high levels [38,39]. This is the case of phenylethyl alcohol and 1-butanol, 3-methyl-, known also as isoamyl alcohol, both among the major fusel alcohols occurring in the W1 Aglianico wine analyzed in this study.

Table 3.
Retention times, quality match values with the NIST library data, molecular formula, molecular weight, boiling points, odour threshold values (OTVs) of the organic volatile compounds identified in the Aglianico wine sample obtained by fermentation with the mixed starters S. cerevisiae and H. osmophila and in the control, i.e., Aglianico wine sample obtained by fermentation with S. cerevisiae alone.
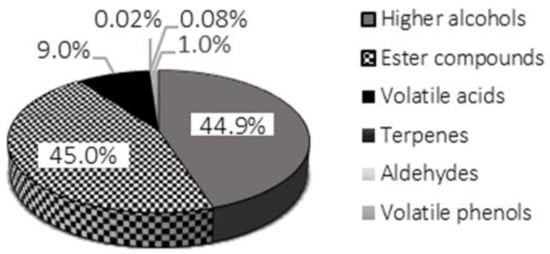
Figure 3.
Pie chart reporting the percentage composition of higher alcohols, esters, volatile acids, terpenes, aldehydes and volatile phenols detected in the headspace of W1 wine sample, based on average chromatographic peaks areas.
In addition to the higher alcohols, a significant number of ester compounds were identified in the W1 wine sample, mainly ethyl esters. These specific compounds are biosynthesized through a condensation between ethanol and acyl-CoA, a process which is mediated by acyltransferases [40]. Ethyl esters and acetates represented the largest group (45%, Figure 3) of the total volatiles occurring in the wine obtained by mixed yeast inoculation. Many of these ester compounds are considered as varietal markers which allow the red wines to display varietal differentiation [41]. Moreover, they widely contribute to the fruity attributes of the oenological products. As evidence, the sensory evaluations reported in the literature revealed a stronger fruity character in wines fermented with mixed cultures than in control wines [42]. For example, 1-butanol, 3-methyl-, acetate (compound 6), which was also identified in the wine sample that had been obtained by the co-inoculation of immobilized cells of H. osmophila and S. cerevisiae (W2) and in the control (C), was found to confer the typical banana flavour. This is characteristic of the S. cerevisiae metabolism; however, when present in excess, it could mask key varietal characters of wines [43]. Among the ester aromatic compounds detected in W1 sample, the most important was acetic acid, 2-phenylethyl ester (compound 11). As described in previous studies, its concentration was higher in wines obtained with mixed cultures than in wines produced by S. cerevisiae pure cultures and it was modulated by changing the initial yeast ratio [42]. Acetic acid, 2-phenylethyl ester imparts “raspberry”- and ‘honey’-like aromas to wine [44]. Ethyl esters of fatty acids also contribute with pleasant fruity and floral odours to wine aroma. Seven ethyl esters of straight-chain fatty acids with an even number of carbon atoms (C8-C18) were identified in sample W1, i.e., octanoic acid, ethyl ester (compound 10), decanoic acid, ethyl ester (compound 12), dodecanoic acid, ethyl ester (compound 16), tetradecanoic acid, ethyl ester (compound 18), ethyl 9-hexadecenoate (compound 19), hexadecanoic acid, ethyl ester (compound 20) and linoleic acid ethyl ester (compound 21). The concentration of fatty acids ethyl esters is dependent on wine aging: it generally decreases as the storage time gets longer, especially at low pH and high temperature values [45,46]. Although their production has been shown to be lower when apiculate yeasts are inoculated, like those belonging to the genus Hanseniaspora [47], a greater presence of these compounds was found in this study in W1 wine compared to the control, in which only octanoic acid, ethyl ester and dodecanoic acid, ethyl ester occurred. As regards to W2 wine, octanoic acid, ethyl ester; acetic acid, 2-phenylethyl ester; tetradecanoic acid, ethyl ester; and hexadecanoic acid, ethyl ester were identified as representative compounds belonging to the ester class (Table 3). All the volatile fatty acids detected in the three samples occurred in their esterified form. The exception was acetic acid, which was also detected in its free form (compound 7). It should be noted that a decrease in the retention time of acetic acid was observed for the W2 wine, which may have been due to the hydrolysis of ethyl acetate. As representative compounds belonging to the class of aldehydes and terpenes, acetaldehyde (compound 1) and nerolidol (compound 15) were, respectively, identified in the W1 wine. Moreover, the first one was also detected in the W2 wine sample which had been fermented with immobilized cells of H. osmophila in co-cultures with S. cerevisiae and in the control. Acetaldehyde is derived from alcohol fermentation by yeasts. In this study, no intense chromatographic peaks were observed which related to other aldehydes in the total ion current chromatograms of the three analyzed samples. This was most likely because they were involved in several reactions with wine phenolics, whose products impacted wine colour, flavour and astringency [48]. Nerolidol (compound 15), a compound with a characteristic floral odour similar to that of rose [49], was not detected in the W2 sample, nor was it found in the control. This occurred because, in general, the presence of flavour compounds belonging to the terpenes class is due to some specific enzymatic activities of the yeasts, usually those activities linked to non-Saccharomyces species which can contribute to increasing the sensory profile of the wine [50]. Instead, compound 14, i.e., phenol, 2,5 bis (1,1-dimethylethyl) was the only volatile phenol detected in the co-fermented wine W1. Such a compound has been previously identified by Lu et al. [51] in the Changyu wine. It is produced by wild yeast and, at concentrations higher than 600 μg/L, it confers a fishy smell to wine [52]. As regards saturated cyclic alkanes, a cyclododecane (compound 17), previously found in Nero di Troia wine by Baiano et al. [53], has been detected in W1 wine sample, probably as a contaminant.
4. Conclusions
If properly optimized through experimental design, headspace solid-phase microextraction, coupled to GC-MS analysis, ensures a comprehensive characterization of the aroma compounds responsible for wine complexity. Here, a face-centred composite experimental design matrix and response surface methodology were applied to designing the experiments and evaluating the interactive effects of the two studied parameters, i.e., extraction time and extraction temperature. The optimum conditions which were suggested by the second-order polynomial regression model for the obtention of higher amounts of VOCs were 20 min and 60 °C. The optimized HS-SPME/GC-MS-based method allowed us to ascertain the flavour complexity of wine which had been obtained by controlled multi-starter fermentations between non-Saccharomyces and Saccharomyces strains. H. osmophila, used as free cells in co-inoculated fermentation with a commercial strain of S. cerevisiae, proved able to positively modulate the flavour profile of Aglianico wine as the number of volatile organic compounds detected in the headspace of the wine sample was greatly higher (21 compounds) compared to the wine control, which had been obtained by fermentation with S. cerevisiae alone (6 compounds).
Author Contributions
C.T.: Investigation; Data curation; M.A.A.: Data curation; Writing—Original Draft; B.G.: Formal analysis; G.B.: Supervision; R.P. (Raffaella Pascale): Review and Editing; F.L.: Supervision; R.C.: Supervision; A.C.: Investigation; Review and Editing; R.P. (Rocchina Pietrafesa): Review and Editing; G.S.: Investigation; A.D.C.: Review and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request due to privacy restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stanco, M.; Lerro, M.; Marotta, G. Consumers’ preferences for wine attributes: A best-worst scaling analysis. Sustainability 2020, 12, 2819. [Google Scholar] [CrossRef]
- Fischer, U. Wine Aroma. In Flavours and Fragrances; Springer: Berlin/Heidelberg, Germany, 2007; pp. 241–267. [Google Scholar]
- Ciani, M.; Comitini, F. Yeast interactions in multi-starter wine fermentation. Curr. Opin. Food Sci. 2015, 1, 1–6. [Google Scholar] [CrossRef]
- Kosseva, M.R. Immobilization of Microbial Cells in Food Fermentation Processes. Food Bioprocess Technol. 2011, 4, 1089–1118. [Google Scholar] [CrossRef]
- Acquavia, M.A.; Pascale, R.; Pappalardo, I.; Santarsiero, A.; Martelli, G.; Bianco, G. Characterization of quercetin derivatives in crossing combination of habanero white and capsicum annuum peppers and of anti-inflammatory and cytotoxic activity. Separations 2021, 8, 90. [Google Scholar] [CrossRef]
- Pascale, R.; Acquavia, M.A.; Onzo, A.; Cataldi, T.R.I.; Calvano, C.D.; Bianco, G. Analysis of surfactants by mass spectrometry: Coming to grips with their diversity. Mass Spectrom. Rev. 2021, 1–32. [Google Scholar] [CrossRef]
- Acquavia, M.A.; Pascale, R.; Foti, L.; Carlucci, G.; Scrano, L.; Martelli, G.; Brienza, M.; Coviello, D.; Bianco, G.; Lelario, F. Analytical methods for extraction and identification of primary and secondary metabolites of apple (Malus domestica) fruits: A review. Separations 2021, 8, 91. [Google Scholar] [CrossRef]
- Onzo, A.; Acquavia, M.A.; Cataldi, T.R.I.; Ligonzo, M.; Coviello, D.; Pascale, R.; Martelli, G.; Bondoni, M.; Scrano, L.; Bianco, G. Coceth sulfate characterization by electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2020, 34, e8884. [Google Scholar] [CrossRef]
- Abbott, N.; Etiévant, P.; Langlois, D.; Lesschaeve, I.; Issanchou, S. Evaluation of the Representativeness of the Odor of Beer Extracts Prior to Analysis by GC Eluate Sniffing. J. Agric. Food Chem. 1993, 41, 777–780. [Google Scholar] [CrossRef]
- Ortega-Heras, M.; González-SanJosé, M.L.; Beltrán, S. Aroma composition of wine studied by different extraction methods. Anal. Chim. Acta 2002, 458, 85–93. [Google Scholar] [CrossRef]
- Castro, R.; Natera, R.; Durán, E.; García-Barroso, C. Application of solid phase extraction techniques to analyse volatile compounds in wines and other enological products. Eur. Food Res. Technol. 2008, 228, 1–18. [Google Scholar] [CrossRef]
- López, R.; Aznar, M.; Cacho, J.; Ferreira, V. Determination of minor and trace volatile compounds in wine by solid-phase extraction and gas chromatography with mass spectrometric detection. J. Chromatogr. A 2002, 966, 167–177. [Google Scholar] [CrossRef]
- De-La-fuente-blanco, A.; Ferreira, V. Gas chromatography olfactometry (Gc-o) for the (semi)quantitative screening of wine aroma. Foods 2020, 9, 1892. [Google Scholar] [CrossRef]
- Sagratini, G.; Maggi, F.; Caprioli, G.; Cristalli, G.; Ricciutelli, M.; Torregiani, E.; Vittori, S. Comparative study of aroma profile and phenolic content of Montepulciano monovarietal red wines from the Marches and Abruzzo regions of Italy using HS-SPME-GC-MS and HPLC-MS. Food Chem. 2012, 132, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Perestrelo, R.; Silva, C.L.; Silva, P.; Câmara, J.S. Establishment of the volatile signature of wine-based aromatic vinegars subjected to maceration. Molecules 2018, 23, 499. [Google Scholar] [CrossRef]
- Tao, Y.S.; Li, H.; Wang, H.; Zhang, L. Volatile compounds of young Cabernet Sauvignon red wine from Changli County (China). J. Food Compos. Anal. 2008, 21, 689–694. [Google Scholar] [CrossRef]
- Bianco, G.; Novario, G.; Zianni, R.; Cataldi, T.R.I. Comparison of two SPME fibers for the extraction of some off-flavor cork-taint compounds in bottled wines investigated by GC-HRMS. Anal. Bioanal. Chem. 2009, 393, 2019–2027. [Google Scholar] [CrossRef]
- Fiorini, D.; Pacetti, D.; Gabbianelli, R.; Gabrielli, S.; Ballini, R. A salting out system for improving the efficiency of the headspace solid-phase microextraction of short and medium chain free fatty acids. J. Chromatogr. A 2015, 1409, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Parameswaran, R.; Box, G.E.P.; Hunter, W.G.; Hunter, J.S. Statistics for Experimenters: An Introduction to Design, Data Analysis, and Model Building. J. Mark. Res. 1979, 16, 291. [Google Scholar] [CrossRef]
- Bouaid, A.; Ramos, L.; Gonzalez, M.J.; Fernández, P.; Cámara, C. Solid-phase microextraction method for the determination of atrazine and four organophosphorus pesticides in soil samples by gas chromatography. J. Chromatogr. A 2001, 939, 13–21. [Google Scholar] [CrossRef]
- Varrone, C.; Giussani, B.; Izzo, G.; Massini, G.; Marone, A.; Signorini, A.; Wang, A. Statistical optimization of biohydrogen and ethanol production from crude glycerol by microbial mixed culture. Int. J. Hydrogen Energy 2012, 37, 16479–16488. [Google Scholar] [CrossRef]
- Leardi, R. Experimental design in chemistry: A tutorial. Anal. Chim. Acta 2009, 652, 161–172. [Google Scholar] [CrossRef]
- Boscaino, F.; Ionata, E.; La Cara, F.; Guerriero, S.; Marcolongo, L.; Sorrentino, A. Impact of Saccharomyces cerevisiae and Metschnikowia fructicola autochthonous mixed starter on Aglianico wine volatile compounds. J. Food Sci. Technol. 2019, 56, 4982–4991. [Google Scholar] [CrossRef]
- Alba, V.; Anaclerio, A.; Gasparro, M.; Caputo, A.R.; Montemurro, C.; Blanco, A.; Antonacci, D. Ampelographic and molecular characterisation of Aglianico accessions (Vitis vinifera L.) collected in Southern Italy. S. Afr. J. Enol. Vitic. 2011, 32, 164–173. [Google Scholar] [CrossRef]
- Mok, C.; Song, K.T.; Park, Y.S.; Lim, S.; Ruan, R.; Chen, P. High hydrostatic pressure pasteurization of red wine. J. Food Sci. 2006, 71, M265–M269. [Google Scholar] [CrossRef]
- Alberico, G.; Capece, A.; Mauriello, G.; Pietrafesa, R.; Siesto, G.; Garde-cerd, T.; Maresca, D.; Romano, R.; Romano, P. Influence of Microencapsulation on Fermentative Behavior of Hanseniaspora osmophila in Wine Mixed Starter Fermentation. Fermentation 2021, 7, 112. [Google Scholar] [CrossRef]
- Leardi, R.; Melzi, C.; Polotti, G. CAT (Chemometric Agile Tool). Available online: http://gruppochemiometria.it/index.php/software (accessed on 30 June 2021).
- Chidi, B.S.; Bauer, F.F.; Rossouw, D. Organic acid metabolism and the impact of fermentation practices on wine acidity–A review. S. Afr. J. Enol. Vitic. 2018, 39, 315–329. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Esteban-Fernández, A.; Navascués, E.; Marquina, D.; Santos, A.; Moreno-Arribas, M.V. Microbial contribution to Wine aroma and its intended use for Wine quality improvement. Molecules 2017, 22, 189. [Google Scholar] [CrossRef] [PubMed]
- Romano, P.; Caruso, M.; Capece, A.; Lipani, G.; Paraggio, M.; Fiore, C. Metabolic diversity of Saccharomyces cerevisiae strains from spontaneously fermented grape musts. World J. Microbiol. Biotechnol. 2003, 19, 311–315. [Google Scholar] [CrossRef]
- Rocha, S.; Ramalheira, V.; Barros, A.; Delgadillo, I.; Coimbra, M.A. Headspace solid phase microextraction (SPME) analysis of flavor compounds in wines. Effect of the matrix volatile composition in the relative response factors in a wine model. J. Agric. Food Chem. 2001, 49, 5142–5151. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.W.; Wan Aida, W.M.; Maskat, M.Y.; Osman, H. Optimization of headspace solid phase microextraction (HS-SPME) for gas chromatography mass spectrometry (GC-MS) analysis of aroma compound in palm sugar (Arenga pinnata). J. Food Compos. Anal. 2006, 19, 822–830. [Google Scholar] [CrossRef]
- Benucci, I.; Cecchi, T.; Lombardelli, C.; Maresca, D.; Mauriello, G.; Esti, M. Novel microencapsulated yeast for the primary fermentation of green beer: Kinetic behavior, volatiles and sensory profile. Food Chem. 2021, 340, 127900. [Google Scholar] [CrossRef]
- De Hoffmann, E.; Stroobant, V. Mass Spectrometry Principles and Applications; John & Wiley and Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Fu, M.; Duan, P.; Gao, J.; Kenttämaa, H.I. Ion-molecule reactions for the differentiation of primary, secondary and tertiary hydroxyl functionalities in protonated analytes in a tandem mass spectrometer. Analyst 2012, 137, 5720–5722. [Google Scholar] [CrossRef] [PubMed]
- Alves, S.P.; Tyburczy, C.; Lawrence, P.; Bessa, R.J.B.; Thomas Brenna, J. Acetonitrile covalent adduct chemical ionization tandem mass spectrometry of non-methylene-interrupted pentaene fatty acid methyl esters. Rapid Commun. Mass Spectrom. 2011, 25, 1933–1941. [Google Scholar] [CrossRef] [PubMed]
- De-La-Fuente-Blanco, A.; Sáenz-Navajas, M.P.; Ferreira, V. On the effects of higher alcohols on red wine aroma. Food Chem. 2016, 210, 107–114. [Google Scholar] [CrossRef]
- San-juan, F.; Ferreira, V.; Cacho, J.; Escudero, A.; Prolabo, B.D.H. Quality and Aromatic Sensory Descriptors (Mainly Fresh and Dry Fruit Character) of Spanish Red Wines can be Predicted from their Aroma-Active Chemical Composition. J. Agric. Food Chem. 2011, 59, 7916–7924. [Google Scholar] [CrossRef] [PubMed]
- Saenz-Navajas, M.-P.; Avizcuri, J.-M.; Ballester, J.; Fernandez-Zurbano, P.; Ferreira, V.; Dominique Peyron, D.V. Sensory-active compounds influencing wine experts’ and consumers’ perception of red wine intrinsic quality. Food Sci. Technol. 2015, 60, 400–411. [Google Scholar] [CrossRef]
- Costello, P.J.; Siebert, T.E.; Solomon, M.R.; Bartowsky, E.J. Synthesis of fruity ethyl esters by acyl coenzyme A: Alcohol acyltransferase and reverse esterase activities in Oenococcus oeni and Lactobacillus plantarum. J. Appl. Microbiol. 2013, 114, 797–806. [Google Scholar] [CrossRef]
- Antalick, G.; Šuklje, K.; Blackman, J.W.; Meeks, C.; Deloire, A.; Schmidtke, L.M. Influence of Grape Composition on Red Wine Ester Profile: Comparison between Cabernet Sauvignon and Shiraz Cultivars from Australian Warm Climate. J. Agric. Food Chem. 2015, 63, 4664–4672. [Google Scholar] [CrossRef]
- Viana, F.; Gil, J.V.; Vallés, S.; Manzanares, P. Increasing the levels of 2-phenylethyl acetate in wine through the use of a mixed culture of Hanseniaspora osmophila and Saccharomyces cerevisiae. Int. J. Food Microbiol. 2009, 135, 68–74. [Google Scholar] [CrossRef]
- Plata, C.; Millán, C.; Mauricio, J.C.; Ortega, J.M. Formation of ethyl acetate and isoamyl acetate by various species of wine yeasts. Food Microbiol. 2003, 20, 217–224. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I.S. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Díaz-Maroto, M.C.; Schneider, R.; Baumes, R. Formation pathways of ethyl esters of branched short-chain fatty acids during wine aging. J. Agric. Food Chem. 2005, 53, 3503–3509. [Google Scholar] [CrossRef] [PubMed]
- Câmara, J.S.; Alves, M.A.; Marques, J.C. Changes in volatile composition of Madeira wines during their oxidative ageing. Anal. Chim. Acta 2006, 563, 188–197. [Google Scholar] [CrossRef]
- Herraiz, T.; Reglero, G.; Herraiz, M.; Martin-Alvarez, P.J.; Cabezudo, M.D. The influence of the yeast and type of culture on the volatile composition of wines fermented without sulfur dioxide. Am. J. Enol. Vitic. 1990, 41, 313–318. [Google Scholar] [CrossRef]
- Han, G.; Webb, M.R.; Waterhouse, A.L. Acetaldehyde reactions during wine bottle storage. Food Chem. 2019, 290, 208–215. [Google Scholar] [CrossRef]
- Drtilová, T.; Ďurčanská, K.; Machyňáková, A.; Špánik, I.; Klempová, T.; Furdíková, K. Impact of different pure cultures of Saccharomyces cerevisiae on the volatile profile of Cabernet Sauvignon rosé wines. Czech J. Food Sci. 2020, 38, 94–102. [Google Scholar] [CrossRef]
- Romano, P.; Braschi, G.; Siesto, G.; Patrignani, F. Role of Yeasts on the Sensory Component of Wines. Foods 2022, 11, 1921. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Mi, J.; Chen, X.; Luo, Q.; Li, X.; He, J.; Zhao, R.; Jin, B.; Yan, Y.; Cao, Y. Analysis on volatile components of co-fermented fruit wines by Lycium ruthenicum murray and wine grapes. Food Sci. Technol. 2021, 42, 1–8. [Google Scholar] [CrossRef]
- Zhang, X.K.; Lan, Y.B.; Zhu, B.Q.; Xiang, X.F.; Duan, C.Q.; Shi, Y. Changes in monosaccharides, organic acids and amino acids during Cabernet Sauvignon wine ageing based on a simultaneous analysis using gas chromatography–mass spectrometry. J. Sci. Food Agric. 2018, 98, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Baiano, A.; Mentana, A.; Quinto, M.; Centonze, D.; Previtali, M.A.; Varva, G.; Del Nobile, M.A.; De Palma, L. Volatile composition and sensory profile of wines obtained from partially defoliated vines: The case of Nero di Troia wine. Eur. Food Res. Technol. 2017, 243, 247–261. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).