Abstract
The lipid, fatty acid, protein, and carbohydrate contents in cyanobacterial strains and biomass can vary by orders of magnitude. Many publications (thousands of peer-reviewed articles) require more work to extract their precise concentration values (i.e., different units, inaccurate data), which makes them not easily exploitable. For this purpose, tables have been compiled from the literature data, including lipids, fatty acids, proteins, and carbohydrates composition and quantities in cyanobacteria. A lot of data (323) were collected after careful a literature search, according to selected criteria in order to distinguish separately cyanobacteria, and according to categories of genus and species and generate average values of the contents of these cell components. These data are exploited in a first systematic analysis of the content in types of strains. Our database can be a powerful tool for biologists, chemists, and environmental agencies to determine the potential concentration of high-value chemical building blocks directly from low-value bloom biomass, cell cultures, or debris in the sediment, offering the potential to minimize environmental waste and add value to the agro-industrial residues. The database can also support strategies for food manufacturers to develop new products with optimized properties for veterinarian applications.
1. Introduction
Cyanobacteria can form blooms (excessive proliferation) according to changes in the natural environmental conditions (i.e., temperature, light, and nutrients) [1,2]. The leading causes of this increase are the environmental impacts caused by anthropic actions that promote the eutrophication of aquatic ecosystems, combined with climate change, increased water temperature, and the increased atmospheric levels of carbon dioxide [1,3]. As a result, the frequency, distribution, intensity, and duration of these blooms have been increasing worldwide. Among the organisms present in the blooms, cyanobacteria stand out, as they can produce toxic substances, which in large quantities can affect aquatic fauna, causing imbalances in the ecosystem [4,5,6].
On the other hand, non-toxic cyanobacterial species have great potential for biotechnological application. They can be used in several industrial sectors, for example, in the food, energy, and pharmaceutical industries, among others, adding value to a raw material that is still little explored [7,8,9]. The study of different strains of cyanobacteria is important due to the different characteristics that these microorganisms present, in addition to their capacity of producing different primary and secondary metabolites. For example, according to Rodolfi et al. [10], some species can fix carbon dioxide (CO2) directly and produce cell biomass suitable for an economically viable dense culture of cyanobacteria [11]. Depending on the conditions, strains of Microcystis aeruginosa (i.e., CCIBt 3106, LTPNA 03, LTPNA 01, and LTPNA 05) can produce or not microcystins [12,13,14], and this suggests that without the extra energy cost of synthesizing cyanotoxin, these non-toxic strains could invest in nutrient reserves [13].
The main primary metabolites produced by cyanobacteria are lipids, carbohydrates, and proteins [15]. Lipids are essential chemical compounds in cyanobacteria, which can be used as a source of food, animal feed, and biodiesel [14,16,17]. According to Sinensky [18], the ability to modify the type and amount of cell lipids is one of the reasons that can explain the fact that cyanobacteria manage to survive under diverse and extreme conditions (e.g., extremophile species in Antarctica and hot springs).
The production of carbohydrates In cyanobacteria for industrial applications is a promising area for biotechnology [19]. For example, sucrose and glycogen from cyanobacteria can be considered good sources for the production of biofuels [20,21]. Since these microorganisms are also interesting producers of proteins, they can be used in food as valuable ingredients [22]. For example, cyanobacteria rich in proteins can be used in the food industry as a protein extract, which may have emulsifying or gelling properties [22]. Several studies have analyzed protein concentration in cyanobacteria [13,23,24,25].
Due to the satisfactory concentrations of nutrients in cyanobacteria, studies on the characterization of fatty acids, lipids, carbohydrates, and proteins are abundant. The large number of studies related to cyanobacteria in the molecular, environmental, and biotechnological areas, among others, makes it difficult to search for specific information on the amount of these macronutrients. Thus, the objective was to collect data on these biocompounds to create a database that compiles the important data for different researches.
2. Materials and Methods
Data selection depends on the purpose of the study and data availability [26]. From this premise, data were selected from published research articles using the Google Scholar, Scielo, PubMed, Science Direct, and Web of Science databases. The main inclusion criteria were the impact factor of journals and the publication of articles in the period from 2000 to 2023. The keywords cyanobacteria, lipids, lipid content, fatty acids, carbohydrates, proteins, and biofuel were used for the search (Figure 1). Review articles with consistent and clear data were considered; however, the concentration values were extracted from the original articles.
Figure 1.
Platforms used in the search for scientific articles for review, date of chosen scientific articles, and primary metabolites revised.
Independent researchers extracted the necessary information from eligible articles, such as the cyanobacteria genus and species, investigated compounds’ content, collection place, authors, year of publication, and digital object identifier (DOI). The details of the articles included were typed into an Excel spreadsheet (Office 2013, Microsoft, Redmond, WA, USA), contemplating the information mentioned in columns. The studies displayed on the different search platforms, according to the search criteria, were extracted in the “ris” format.
We also researched and compiled the data found in the Platform of National Center for Biotechnology Information (NCBI) of the species and/or genera selected for this review. These data are presented in the Supplementary Material (Table S1).
3. Results
Relevant studies on the concentration of fatty acids, lipids, carbohydrates, and proteins in cyanobacteria were selected, totaling 111 data on fatty acids, 119 on lipids, 60 on carbohydrates, and 33 on proteins (Figure 2). A total of 323 data were analyzed and presented in seven tables, gathering data on fatty acids (Table 1, Table 2, Table 3 and Table 4), lipids (Table 5), carbohydrates (Table 6), and proteins (Table 7). We included articles with consistent or clear data and review articles. The fatty acid structures are presented in the Supplementary Material. Saturated fatty acids have simple structures with only single C–C bonds with a terminal carboxylic group (Table S2), while unsaturated fatty acids have more complex structures containing at least one or more C=C double bonds in the carbon backbone (Tables S3 and S4).
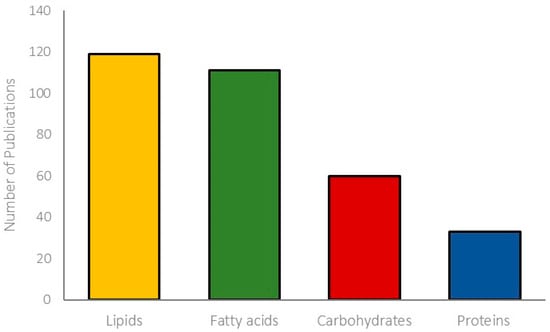
Figure 2.
The number of publications (2000–2023) referring to the concentrations of lipids, fatty acids, carbohydrates, and proteins in freshwater cyanobacteria.

Table 1.
Main information about classification, collection point, and the literature reference relating to the fatty acid composition of the cyanobacteria selected for this review [14,16,17,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46].

Table 2.
Saturated fatty acid composition of cyanobacteria selected for this review 1,2.

Table 3.
Monounsaturated fatty acid composition of cyanobacteria selected for this review 1,2.

Table 4.
Polyunsaturated fatty acid composition of cyanobacteria selected for this review 1,2.

Table 5.
Lipid content in cyanobacteria selected for this review [17,28,32,35,36,40,41,45,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67] 1.

Table 6.
Carbohydrate content in cyanobacteria selected for this review [15,41,49,64,68,69,70,71,72,73,74,75] 1.

Table 7.
Protein content in cyanobacteria selected for this review [41,46,64,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92] 1.
4. Discussion
Cyanobacteria are rich in primary metabolites and have biotechnological potential for energy production and the pharmaceutical and food industries [14,25,93,94]. There are many works in the literature about cyanometabolites. However, some articles need to present the data clearly and concisely. This work proposes setting up a review of the data published in scientific journals making use of important scientific platforms to facilitate finding this information.
Many studies have analyzed how environmental conditions (e.g., temperature, pH, and nitrogen and phosphate levels) can increase the biochemical composition of microalgae and cyanobacteria, mainly fatty acids and lipids [48]. These compounds are essential for cyanobacteria. In cells, lipids are found mainly in the cell membranes, featuring mainly polyunsaturated fatty acids (FAs) in their structure. The unsaturated FAs play an essential role in membrane physiology, and the proportion of unsaturated and saturated FA determines membrane fluidity [95]. Several authors have been quantifying the concentration of fatty acids and lipids in cyanobacteria worldwide [17,27,28,30,36,47,48,96].
Cyanobacteria exhibit high lipid production, as observed in the data collected in Table 5 (119 strains of cyanobacteria). These microorganisms, which can adapt themselves to culture conditions and exhibit high cell growth, are considered ideal lipid sources for pharmaceutical and biofuel production [97]. For example, they produce a wide variety of lipids with antibiotic and antibiofilm activity [98]. Using these compounds in clinical treatments alone or in association with antibiotics can be considered an alternative to current treatments for human diseases. Examples of commercially important lipids produced by cyanobacteria are polyhydroxyalkanoates (PHAs) and polyhydroxybutyrates (PHBs), which are considered a good alternative to synthetic plastics due to their natural origin, optical purity, thermoplasticity, and biodegradability [99].
Cyanobacteria are among the third-generation raw materials that are viable and increasingly studied for use in biodiesel production [100,101]. Large-scale biodiesel production directly depends on the availability of interesting fatty acids in the raw material. Lipids and fatty acids’ total content may depend on the species and strain studied (Table 1, Table 2, Table 3, Table 4 and Table 5), and their content may be altered or induced by different abiotic factors (e.g., pH, mode of operation, photobioreactor configuration, light, and temperature) [102,103].
Some species of cyanobacteria, such as Oscillatoria sp. FW01, can optimize their yield when cultivated under specific conditions. According to the study by Yadav et al. [17], the cultivation of this strain under controlled light and temperature showed a 12% increase in the production of lipids, as well as a 57% increase in that of fatty acids. Thus, the authors considered Oscillatoria sp. FW01 as a raw material to be potentially used for the sustainable production of biodiesel [17].
According to the data gathered in Table 2, it is possible to observe quite a high content of palmitic acid (C16:0) (approximately 36.5%) in the reviewed cyanobacterial species. One of the characteristics of this acid is its small saturated carbon chain with its low oxidation and melting point [104]. These characteristics make this type of acid especially suitable for biodiesel production. The demand for lipids from microorganisms as possible substitutes for fossil fuels has stimulated research into synthetic biofuels. Oliveira et al. [35] investigated the lipid profile of three strains of Amazonian cyanobacteria (Cyanobium sp., Limnothrix sp., and Nostoc sp.), among which Limnotrix sp. showed the best lipid profile and highest amount of C16:0, which are favorable properties for biodiesel production. In addition, it also showed good values of biodiesel quality parameters, i.e., a high oxidative stability (34.9 h) and a cetane number (58.06) above the minimum established by the American Society for Tests and Materials (ASTM).
In the work by Santana-Sánchez et al. [105], the Synechococcus strains were the only ones that exhibited fatty acid profiles mainly composed of C14:0, C16:0, and C16:1 and without polyunsaturated fatty acids. Boutarfa et al. [27] also analyzed the fatty acid profile of the strains of Mastigocladus laminosus (an extremophile found in hot springs), which revealed C16:0 as the main fatty acid (51–53%) and a medium length chain (from C14 to C20). Nostoc sp. MCC41 presents high concentrations of palmitic acid, can grow under mixotrophic conditions, and fixes atmospheric nitrogen [36]. Thanks to these properties, they may represent excellent raw materials for the production of biodiesel.
Carbohydrates are among the leading products of photosynthesis, and in some species of cyanobacteria, their content can reach up to 50% of the dry weight [106]. These compounds are present in the cell wall (structural support) in addition to being stored as an energy source for the cell [97]. A possible biotechnological application of carbohydrates from cyanobacteria is in the area of biofuels, due to the high content of fermentable sugars and low hemicellulose and lignin contents [15,107]. In particular, the feasibility of producing bioethanol from the cyanobacterial biomass depends on the content and composition of the carbohydrates in the cell, both varying and depending on factors such as cultivation conditions and species type. Therefore, the production of carbohydrates by cyanobacteria has become the focus of much research [15,72,73,108] due to their potential application as a substrate for biofuels [109].
Some cultivation conditions favor the accumulation of carbohydrates in cyanobacterial cells, including the limitation of nitrogen in the medium where it is cultivated [72]. In Table 6, where the data referring to the accumulation of total carbohydrates can be found, we can observe a large variability according to the species, i.e., from 15% in Synechococcus sp. [69] up to 70% in Spirulina maxima [70]. However, the best carbohydrate-accumulating species were also grown in nitrogen-poor media (e.g., wastewater-borne cyanobacteria, Arthrospira platensis NIES-39, and Spirulina platensis) [15,21,70]. In other words, some species are able to accumulate carbohydrates more than others, but this capacity can be influenced by the medium in which they are grown.
Another critical question is the demand for food, which is a worrying factor in the world because of the growing population. According to the United Nations, the world population could reach 8.5 billion in 2030 and increase even more to 9.7 billion in 2050, creating a significant challenge related to food production. In this way, the food sector looks for foods or inputs that can add nutritional value and benefit human and animal health. These products are called functional foods, which provide metabolic and nutritional effects on health and essential physiological functions [80,83,107,109].
The search for a healthy diet and lifestyle causes consumers to purchase products that complement their physiological and metabolic needs. As a result, there has been an increase in this food sector, which focuses on consuming carbohydrates, lipids, and proteins. However, food alternatives have been sought as a source of protein, replacing animal sources [80,83,107,110]. Algae and microalgae have emerged as promising alternative sources of macronutrients. However, one of the problems encountered is the high cost of producing biomass, limiting the applications of its use. Due to this problem, research is being carried out on cyanobacteria, mainly due to the ease of their proliferation, generating much biomass.
As the results collected in Table 7 show, cyanobacteria are an excellent source of proteins, either as a food supplement or as an input to increase the concentration of this nutrient in food. Among these microorganisms, Spirulina sp. have stood out due to their excellent properties. They can be applied as biostimulants or biofertilizers, animal feed, or to produce human foods enriched in Spirulina sp., which are already commercially available. In addition, they are used in cosmetics, medicines, and functional foods. These applications, mainly as a source of protein, are possible because they are safe, nutritious, and sustainable raw materials.
For this reason, there is much research into the literature on cyanobacteria, as seen in the above tables. Data related to Spirulina sp. can be compared with those of other species and strains of cyanobacteria, demonstrating that it is still an area to be explored [111]. In addition to those presented, cyanobacteria produce other metabolites that can improve and contribute to a healthy diet, adding value to different products or these raw materials [107,110]. However, as seen in this review article, there are still few reports on the concentrations of proteins in different species and strains of cyanobacteria.
Since proteins have different functions in microorganisms, cyanobacteria show a significant variation in their total content (2.5–66.7%), with an average concentration of 36.9% (Table 7). However, there are still few reports on protein concentration in cyanobacterial biomass. This small overview on protein content demonstrates that this is an area of research still to be explored, mainly by the food industry [77,80,83].
5. Conclusions
The biochemical diversity presented by cyanobacteria has favored the study of these microorganisms in several areas of science. This review is essential to facilitate the consultation and location of data from scientific articles on the composition of cyanobacterial species and strains, including the contents of fatty acids (111), lipids (119), carbohydrates (60), and proteins (33). It was also possible to discuss how these characteristics can be commercially relevant since cyanobacteria have been considered good candidates for several applications; for example, as a source of food supplements for humans and animals and in the production of biofuels.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app13053162/s1, Table S1: NCBI (National Center for Biotechnology Information) data for genera and/or species of cyanobacteria selected for this review; Table S2: Main saturated fatty acids detected in cyanobacteria selected for this review; Table S3: Main monounsaturated fatty acids detected in cyanobacteria selected for this review; Table S4: Main polyunsaturated fatty acids detected in cyanobacteria selected for this review.
Author Contributions
Conceptualization, L.S.P. and E.P.; methodology, L.S.P., P.N.N.d.F., A.O.d.S., and E.P.; investigation, L.S.P., P.N.N.d.F., R.B.M. and A.O.d.S.; resources, E.P.; data curation, L.S.P., P.N.N.d.F., R.B.M., M.F.d.S. and A.O.d.S.; writing—original draft preparation, L.S.P., P.N.N.d.F., R.B.M. and A.O.d.S.; writing—review and editing, A.C., M.F.d.S. and E.P.; visualization, L.S.P.; supervision, E.P.; project administration, E.P.; funding acquisition, E.P. and A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the São Paulo State Research Foundation (FAPESP), grant numbers 2013/07914-8, 2021/00149-0, and 2021/14239-1, the University of São Paulo Foundation (FUSP), grant number 1979, the Coordination for the Improvement of Higher-Level Personnel (CAPES), grant number 88887483720/2020-00, and the University of São Paulo—USPSusten Program of the Superintendence of Environmental Management (Supplementary Notice DOE 13 July 2022).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef]
- Sotton, B.; Paris, A.; le Manach, S.; Blond, A.; Duval, C.; Qiao, Q.; Catherine, A.; Combes, A.; Pichon, V.; Bernard, C.; et al. Specificity of the metabolic signatures of fish from cyanobacteria rich lakes. Chemosphere 2019, 226, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, R.P.; Egli, T.; Hofstetter, T.B.; von Gunten, U.; Wehrli, B. Global water pollution and human health. Annu. Rev. Environ. Resour. 2010, 35, 109–136. [Google Scholar] [CrossRef]
- Aljohani, A.S.M.; Ahmed, A.A.; Althwab, S.A.; Alkhamiss, A.S.; Rasheed, Z.; Fernández, N.; Al Abdulmonem, W. Gene expression of glutathione S-transferase alpha, glutathione S-transferase rho, glutathione peroxidase, uncoupling protein 2, cytochrome P450 1A, heat shock protein 70 in liver of Oreochromis niloticus upon exposure of microcystin-LR, microcystin-RR and toxic cyanobacteria crude. Gene Rep. 2022, 26, 101498. [Google Scholar] [CrossRef]
- Arismendi-González, L.; Sepúlveda-Sánchez, M.; Arboleda-Baena, C.M.; Palacio-Betancur, H.; Murillo Ramos, E.; Muskus-López, C.E.; Pohlon, E.; Flórez Molina, M.T.; Betancur Uran, J.; Palacio Baena, J. Evidence for toxic cyanobacteria in sediments and the water-sediment interface of a tropical drinking water reservoir. Limnologica 2021, 91, 125924. [Google Scholar] [CrossRef]
- Vilar, M.C.P.; da Silva Ferrão-Filho, A.; Azevedo, S.M.F.O. Single and mixed diets of the toxic cyanobacteria Microcystis aeruginosa and Raphidiopsis raciborskii differently affect Daphnia feeding behavior. Food Webs 2022, 32, e00245. [Google Scholar] [CrossRef]
- Pulz, O.; Gross, W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648. [Google Scholar] [CrossRef]
- Tamagnini, P.; Leitão, E.; Oliveira, P.; Ferreira, D.; Pinto, F.; Harris, D.J.; Heidorn, T.; Lindblad, P. Cyanobacterial hydrogenases: Diversity, regulation and applications. FEMS Microbiol. Rev. 2007, 31, 692–720. [Google Scholar] [CrossRef]
- Parmar, A.; Singh, N.K.; Pandey, A.; Gnansounou, E.; Madamwar, D. Cyanobacteria and microalgae: A positive prospect for biofuels. Bioresour. Technol. 2011, 102, 10163–10172. [Google Scholar] [CrossRef]
- Rodolfi, L.; Chini Zittelli, G.; Bassi, N.; Padovani, G.; Biondi, N.; Bonini, G.; Tredici, M.R. Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 2009, 102, 100–112. [Google Scholar] [CrossRef]
- Zahra, Z.; Choo, D.H.; Lee, H.; Parveen, A. Cyanobacteria: Review of current potentials and applications. Environments 2020, 7, 13. [Google Scholar] [CrossRef]
- Bortoli, S.; Oliveira-Silva, D.; Krüger, T.; Dörr, F.A.; Colepicolo, P.; Volmer, D.A.; Pinto, E. Growth and microcystin production of a Brazilian Microcystis aeruginosa strain (LTPNA 02) under different nutrient conditions. Rev. Bras. Farmacogn. 2014, 24, 389–398. [Google Scholar] [CrossRef]
- Jacinavicius, F.R.; Pacheco, A.B.F.; Chow, F.; Verissimo da Costa, G.C.; Kalume, D.E.; Rigonato, J.; Schmidt, E.C.; Sant’Anna, C.L. Different ecophysiological and structural strategies of toxic and non-toxic Microcystis aeruginosa (cyanobacteria) strains assessed under culture conditions. Algal Res. 2019, 41, 101548. [Google Scholar] [CrossRef]
- Passos, L.S.; Almeida, É.C.; de Pereira, C.M.P.; Casazza, A.A.; Converti, A.; Pinto, E. Chemical characterization of Microcystis aeruginosa for feed and energy uses. Energies 2021, 14, 3013. [Google Scholar] [CrossRef]
- Arias, D.M.; Ortíz-Sánchez, E.; Okoye, P.U.; Rodríguez-Rangel, H.; Balbuena Ortega, A.; Longoria, A.; Domínguez-Espíndola, R.; Sebastian, P.J. A Review on cyanobacteria cultivation for carbohydrate-based biofuels: Cultivation aspects, polysaccharides accumulation strategies, and biofuels production scenarios. Sci. Total Environ. 2021, 794, 148636. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa, F.; de Troch, M.; Malanga, G.; Hernando, M. Differential sensitivity of fatty acids and lipid damage in Microcystis aeruginosa (cyanobacteria) exposed to increased temperature. Comp. Biochem. Physiol. 2020, 235, 108773. [Google Scholar] [CrossRef]
- Yadav, G.; Sekar, M.; Kim, S.-H.; Geo, V.E.; Bhatia, S.K.; Sabir, J.S.M.; Chi, N.T.L.; Brindhadevi, K.; Pugazhendhi, A. Lipid content, biomass density, fatty acid as selection markers for evaluating the suitability of four fast growing cyanobacterial strains for biodiesel production. Bioresour. Technol. 2021, 325, 124654. [Google Scholar] [CrossRef] [PubMed]
- Sinensky, M. Homeoviscous adaptation—A homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc. Natl. Acad. Sci. USA 1974, 71, 522–525. [Google Scholar] [CrossRef]
- Keshari, N.; Gugger, M.; Zhu, T.; Lu, X. Compatible solutes profiling and carbohydrate feedstock from diversified cyanobacteria. Algal Res. 2019, 43, 101637. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H. Recent trends in global production and utilization of bio-ethanol fuel. Appl. Energy 2009, 86, 2273–2282. [Google Scholar] [CrossRef]
- Aikawa, S.; Joseph, A.; Yamada, R.; Izumi, Y.; Yamagishi, T.; Matsuda, F.; Kawai, H.; Chang, J.-S.; Hasunuma, T.; Kondo, A. Direct conversion of Spirulina to ethanol without pretreatment or enzymatic hydrolysis processes. Energy Environ. Sci. 2013, 6, 1844–1849. [Google Scholar] [CrossRef]
- Grossmann, L.; Hinrichs, J.; Weiss, J. Cultivation and downstream processing of microalgae and cyanobacteria to generate protein-based technofunctional food ingredients. Crit. Rev. Food Sci. Nutr. 2020, 60, 2961–2989. [Google Scholar] [CrossRef]
- Chagas, B.M.E.; Mullen, C.A.; Dorado, C.; Elkasabi, Y.; Boateng, A.A.; Melo, M.A.F.; Ataíde, C.H. Stable bio-oil production from proteinaceous cyanobacteria: Tail gas reactive pyrolysis of Spirulina. Ind. Eng. Chem. Res. 2016, 55, 6734–6741. [Google Scholar] [CrossRef]
- Kebelmann, K.; Hornung, A.; Karsten, U.; Griffiths, G. Intermediate pyrolysis and product identification by TGA and Py-GC/MS of green microalgae and their extracted protein and lipid components. Biomass Bioenergy 2013, 49, 38–48. [Google Scholar] [CrossRef]
- Pagels, F.; Guedes, A.C.; Amaro, H.M.; Kijjoa, A.; Vasconcelos, V. Phycobiliproteins from cyanobacteria: Chemistry and biotechnological applications. Biotechnol. Adv. 2019, 37, 422–443. [Google Scholar] [CrossRef]
- Gao, P.; Li, Z.; Gibson, M.; Gao, H. Ecological risk assessment of nonylphenol in coastal waters of China based on species sensitivity distribution model. Chemosphere 2014, 104, 113–119. [Google Scholar] [CrossRef]
- Boutarfa, S.; Senoussi, M.M.; González-Silvera, D.; López-Jiménez, J.Á.; Aboal, M. Fatty acids profile of Mastigocladus laminosus Cohn ex Kichner isolated from Algerian hot springs as a biofuel feedstock. Biocatal. Agric. Biotechnol. 2022, 42, 102373. [Google Scholar] [CrossRef]
- Bolatkhan, K.; Sadvakasova, A.K.; Zayadan, B.K.; Kakimova, A.B.; Sarsekeyeva, F.K.; Kossalbayev, B.D.; Bozieva, A.M.; Alwasel, S.; Allakhverdiev, S.I. Prospects for the creation of a waste-free technology for wastewater treatment and utilization of carbon dioxide based on cyanobacteria for biodiesel production. J. Biotechnol. 2020, 324, 162–170. [Google Scholar] [CrossRef]
- Li, R.; Watanabe, M.M. Fatty acid profiles and their chemotaxonomy in planktonic species of Anabaena (Cyanobacteria) with straight trichomes. Phytochemistry 2001, 57, 727–731. [Google Scholar] [CrossRef]
- Devi, N.D.; Sun, X.; Hu, B.; Goud, V.V. Bioremediation of domestic wastewater with microalgae-cyanobacteria co-culture by nutritional balance approach and its feasibility for biodiesel and animal feed production. Chem. Eng. J. 2023, 454, 140197. [Google Scholar] [CrossRef]
- Syrpas, M.; Bukauskaitė, J.; Paškauskas, R.; Bašinskienė, L.; Venskutonis, P.R. Recovery of lipophilic products from wild cyanobacteria (Aphanizomenon flos-aquae) isolated from the Curonian Lagoon by means of supercritical carbon dioxide extraction. Algal Res. 2018, 35, 10–21. [Google Scholar] [CrossRef]
- Anahas, A.M.P.; Muralitharan, G. Isolation and screening of heterocystous cyanobacterial strains for biodiesel production by evaluating the fuel properties from fatty acid methyl ester (FAME) profiles. Biores. Technol. 2015, 184, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Anahas, A.M.P.; Muralitharan, G. Characterization of heterocystous cyanobacterial strains for biodiesel production based on fatty acid content analysis and hydrocarbon production. Energy Convers. Manag. 2018, 157, 423–437. [Google Scholar] [CrossRef]
- Gayathria, M.; Shunmugama, S.; Vanmathi, A.; Rahman, P.K.S.M.; Muralitharan, G. Growth kinetic and fuel quality parameters as selective criterion for screening biodiesel producing cyanobacterial strains. Biores. Technol. 2018, 247, 453–462. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, D.T.; Vasconcelos, C.T.; Feitosa, A.M.; Aboim, J.B.; de Oliveira, A.D.; Xavier, L.P.; Santos, A.S.; Gonçalves, E.C.; da Rocha Filho, G.N.; do Nascimento, L.A. Lipid profile analysis of three new amazonian cyanobacteria as potential sources of biodiesel. Fuel 2018, 234, 785–788. [Google Scholar] [CrossRef]
- Nagappan, S.; Bhosale, R.; Duc Nguyen, D.; Pugazhendhi, A.; Tsai, P.-C.; Chang, S.W.; Ponnusamy, V.K.; Kumar, G. Nitrogen-fixing cyanobacteria as a potential resource for efficient biodiesel production. Fuel 2020, 279, 118440. [Google Scholar] [CrossRef]
- Termina, M.; Rezankova, H.; Rezanka, T.; Dembitsky, V.M. Diversity of the fatty acids of the Nostoc species and their statistical analysis. Microbiol. Res. 2007, 162, 308–321. [Google Scholar] [CrossRef]
- Iliev, I.; Petkov, G.; Lukavsky, J.; Furnadzhieva, S.; Andreeva, R. Do cyanobacterial lipids contain fatty acids longer than 18 carbon atoms? Z. Für Nat. C 2011, 66, 267–276. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Shkrob, I.; Go, J.V. Dicarboxylic and Fatty acid compositions of cyanobacteria of the genus Aphanizomenon. Biochemistry 2001, 66, 72–76. [Google Scholar] [CrossRef]
- Sahu, A.; Pancha, I.; Jain, D.; Paliwal, C.; Ghosh, T.; Patidar, S.; Bhattacharya, S.; Mishra, S. Fatty acids as biomarkers of microalgae. Phytochemistry 2013, 89, 53–58. [Google Scholar] [CrossRef]
- Kim, S.M.; Kim, J.Y.; Park, J.; Bae, E.H.; Kang, J.-S.; Kim, K.Y.; Choi, Y.-E. Volatile fatty acid-treated mixotrophic cultivation of lipid/carbohydrate-rich cyanobacterial species, Pseudanabaena mucicola GO0704, for the enhancement of biofuel production. Bioresour. Technol. 2023, 367, 128066. [Google Scholar] [CrossRef] [PubMed]
- Hernando, M.; De Troch, M.; De la Rosa, F.; Giannuzzi, L. Fatty acid response of the invasive bivalve Limnoperna fortunei fed with Microcystis aeruginosa exposed to high temperature. Comp. Biochem. Physiol. 2021, 240, 108925. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Zhao, H.; Lu, X.; Wang, C.; Yang, M.; Bai, F. Quantitative analysis of fatty-acid-based biofuels produced by wild-type and genetically engineered cyanobacteria by gas chromatography–mass spectrometry. J. Chromatogr. A 2011, 1218, 8289–8293. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, F.S.; Karlberg, M.; Graeve, M.; Wulff, A. Cyanobacteria in Scandinavian coastal waters—A potential source for biofuels and fatty acids? Algal Res. 2014, 5, 42–51. [Google Scholar] [CrossRef]
- Lu, Y.; Zhuo, C.; Li, Y.; Li, H.; Yang, M.; Xu, D.; He, H. Evaluation of filamentous heterocystous cyanobacteria for integrated pig-farm biogas slurry treatment and bioenergy production. Biores. Technol. 2020, 297, 122418. [Google Scholar] [CrossRef]
- De Morais, E.G.; Druzian, J.I.; Nunes, I.L.; De Morais, M.G.; Costa, J.A.V. Glycerol increases growth, protein production and alters the fatty acids profile of Spirulina (Arthrospira) sp LEB 18. Proc. Biochem. 2019, 76, 40–45. [Google Scholar] [CrossRef]
- Fuad Hossain, M.; Ratnayake, R.R.; Mahbub, S.; Kumara, K.L.W.; Magana-Arachchi, D.N. Identification and culturing of cyanobacteria isolated from freshwater bodies of Sri Lanka for biodiesel production. Saudi J. Biol. Sci. 2020, 27, 1514–1520. [Google Scholar] [CrossRef]
- Yalcin, D. Growth, lipid content, and fatty acid profile of freshwater cyanobacteria Dolichospermum affine (Lemmermann) Wacklin, Hoffmann, and Komárek by using modified nutrient media. Aquac. Int. 2020, 28, 1371–1388. [Google Scholar] [CrossRef]
- Patel, V.K.; Sundaram, S.; Patel, A.K.; Kalra, A. Characterization of seven species of cyanobacteria for high-quality biomass production. Arab. J. Sci. Eng. 2018, 43, 109–121. [Google Scholar] [CrossRef]
- Singh, P.; Kumar, D. Biomass and lipid production potential of cyanobacteria and microalgae isolated from the diverse habitats of Garhwal Himalaya, Uttarakhand, India. Biomass Bioenergy 2022, 162, 106469. [Google Scholar] [CrossRef]
- Moreira, A.S.P.; Gonçalves, G.; Conde, T.A.; Couto, D.; Melo, T.; Maia, I.B.; Pereira, H.; Silva, J.; Domingues, M.R.; Nunes, C. Chrysotila pseudoroscoffensis as a source of high-value polar lipids with antioxidant activity: A lipidomic approach. Algal Res. 2022, 66, 102756. [Google Scholar] [CrossRef]
- Cardoso, L.G.; Duarte, J.H.; Andrade, B.B.; Lemos, P.V.F.; Costa, J.A.V.; Druzian, J.I.; Chinalia, F.A. Spirulina sp. LEB 18 cultivation in outdoor pilot scale using aquaculture wastewater: High biomass, carotenoid, lipid and carbohydrate production. Aquaculture 2020, 525, 735272. [Google Scholar] [CrossRef]
- Mashayekhi, M.; Sarrafzadeh, M.H.; Tavakoli, O.; Soltani, N.; Faramarzi, M.A. Potential for biodiesel production and carbon capturing from Synechococcus elongatus: An isolation and evaluation study. Biocatal. Agric. Biotechnol. 2017, 9, 230–235. [Google Scholar] [CrossRef]
- Rajeshwari, K.R.; Rajashekhar, M. Biochemical composition of seven species of cyanobacteria isolated from different aquatic habitats of western ghats, Southern India. Braz. Arch. Biol. Technol. 2011, 54, 849–857. [Google Scholar] [CrossRef]
- Da Rós, P.C.M.; Silva, C.S.P.; Silva-Stenico, M.E.; Fiore, M.F.; De Castro, H.F. Assessment of chemical and physico-chemical properties of cyanobacterial lipids for biodiesel production. Mar. Drugs 2013, 11, 2365–2381. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kumar, D. Biomass and lipid productivities of Cyanobacteria—Leptolyngbya foveolarum HNBGU001. Bioenergy Res. 2021, 14, 278–291. [Google Scholar] [CrossRef]
- Aboim, J.B.; Oliveira, D.T.; de Mescouto, V.A.; dos Reis, A.S.; da Rocha Filho, G.N.; Santos, A.V.; Xavier, L.P.; Santos, A.S.; Gonçalves, E.C.; Nascimento, A.S. Optimization of light intensity and NaNO3 concentration in amazon cyanobacteria cultivation to produce biodiesel. Molecules 2019, 24, 2326. [Google Scholar] [CrossRef] [PubMed]
- Hena, S.; Znad, H.; Heong, K.T.; Judd, S. Dairy farm wastewater treatment and lipid accumulation by Arthrospira platensis. Water Res. 2018, 128, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Sharathchandra, K.; Rajashekhar, M. Total lipid and fatty acid composition in some freshwater cyanobacteria. J. Algal Biomass Utln. 2011, 2, 83–97. [Google Scholar]
- Karatay, S.E.; Dönmez, G. Microbial oil production from thermophile cyanobacteria for biodiesel production. Appl. Energy 2011, 88, 3632–3635. [Google Scholar] [CrossRef]
- Senatore, V.; Rueda, E.; Bellver, M.; Díez-Montero, R.; Ferrer, I.; Zarra, T.; Naddeo, V.; García, J. Production of phycobiliproteins, bioplastics and lipids by the cyanobacteria Synechocystis sp. treating secondary effluent in a biorefinery approach. Sci. Total Environ. 2023, 857, 159343. [Google Scholar] [CrossRef] [PubMed]
- Modiri, S.; Sharafi, H.; Alidoust, L.; Hajfarajollah, H.; Haghighi, O.; Azarivand, A.; Zamanzadeh, Z.; Zahiri, H.S.; Vali, H.; Noghabi, K.A. Lipid production and mixotrophic growth features of cyanobacterial strains isolated from various aquatic sites. Microbiology 2015, 161, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Shahid, A.; Malik, S.; Liu, C.-G.; Musharraf, S.G.; Siddiqui, A.J.; Khan, F.; Tarbiah, N.I.; Gull, M.; Rashid, U.; Mehmood, M.A. Characterization of a newly isolated cyanobacterium Plectonema terebrans for biotransformation of the wastewater-derived nutrients to biofuel and high-value bioproducts. J. Water Process. Eng. 2021, 39, 101702. [Google Scholar] [CrossRef]
- Paliwal, C.; Pancha, I.; Ghosh, T.; Maurya, R.; Chokshi, K.; Bharadwaj, S.V.V.; Ram, S.; Mishra, S. Selective carotenoid accumulation by varying nutrient media and salinity in Synechocystis sp. CCNM 2501. Bioresour. Technol. 2015, 197, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Kim, H.S.; Kim, H.; Park, J.; Kim, S.; Choi, Y.-E. Direct removal of harmful cyanobacterial species by adsorption process and their potential use as a lipid source. J. Chem. Eng. 2022, 427, 131727. [Google Scholar] [CrossRef]
- Meixner, K.; Kovalcik, A.; Sykacek, E.; Gruber-Brunhumer, M.; Zeilinger, W.; Markl, K.; Haas, C.; Fritz, I.; Mundigler, N.; Stelzer, F.; et al. Cyanobacteria biorefinery—Production of poly(3-hydroxybutyrate) with Synechocystis salina and utilisation of residual biomass. J. Biotechnol. 2018, 265, 46–53. [Google Scholar] [CrossRef]
- Sarmah, P.; Rout, J.A. Biochemical profile of five species of cyanobacteria isolated from polythene surface in domestic sewage water of Silchar town, Assam (India). Curr. Trends Biotechnol. Pharm. 2018, 12, 2230–7303. [Google Scholar]
- De Farias Silva, C.E.; Sforza, E.; Bertucco, A. Chapter 3—Enhancing carbohydrate productivity in photosynthetic microorganism production: A comparison between cyanobacteria and microalgae and the effect of cultivation systems. In Advances in Feedstock Conversion Technologies for Alternative Fuels and Bioproducts. New Technologies, Challenges and Opportunities; Woodhead Publishing Series in Energy; Hosseini, M., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 37–67. [Google Scholar] [CrossRef]
- González-Fernández, C.; Ballesteros, M. Linking microalgae and cyanobacteria culture conditions and key-enzymes for carbohydrate accumulation. Biotechnol. Adv. 2012, 30, 1655–1661. [Google Scholar] [CrossRef]
- Arias, D.M.; García, J.; Uggetti, E. Production of polymers by cyanobacteria grown in wastewater: Current status, challenges and future perspectives. New Biotechnol. 2020, 55, 46–57. [Google Scholar] [CrossRef]
- Papadopoulos, K.P.; Economou, C.N.; Tekerlekopoulou, A.G. Two-step treatment of brewery wastewater using electrocoagulation and cyanobacteria-based cultivation. J. Environ. Manag. 2020, 265, 110543. [Google Scholar] [CrossRef]
- Shahid, A.; Usman, M.; Atta, Z.; Musharraf, S.G.; Malik, S.; Elkamel, A.; Shahid, M.; Abdulhamid Alkhattabi, N.; Gull, M.; Mehmood, M.A. Impact of wastewater cultivation on pollutant removal, biomass production, metabolite biosynthesis, and carbon dioxide fixation of newly isolated cyanobacteria in a multiproduct biorefinery paradigm. Bioresour. Technol. 2021, 333, 125194. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Mallick, N. Bioprospecting marine microalgae and cyanobacteria as alternative feedstocks for bioethanol production. Sustain. Chem. Pharm. 2022, 29, 100798. [Google Scholar] [CrossRef]
- Aikawa, S.; Ho, S.-H.; Nakanishi, A.; Chang, J.-S.; Hasunuma, T.; Kondo, A. Improving polyglucan production in cyanobacteria and microalgae via cultivation design and metabolic engineering. Biotechnol. J. 2015, 10, 886–898. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, D.; Upadhyay, S.N.; Mishra, P.K. Growth of cyanobacteria: Optimization for increased carbohydrate content. Appl. Biochem. Biotechnol. 2018, 184, 1247–1262. [Google Scholar] [CrossRef]
- Perendeci, N.A.; Yılmaz, V.; Taştan, B.E.; Gökgöl, S.; Fardinpoor, M.; Namlı, A.; Steyer, J.P. Correlations between biochemical composition and biogas production during anaerobic digestion of microalgae and cyanobacteria isolated from different sources of Turkey. Bioresour. Technol. 2019, 281, 209–216. [Google Scholar] [CrossRef]
- Hotos, G.; Avramidou, D.; Mastropetros, S.G.; Tsigkou, K.; Kouvara, K.; Makridis, P.; Kornaros, M. Isolation, identification, and chemical composition analysis of nine microalgal and cyanobacterial species isolated in lagoons of Western Greece. Algal Res. 2023, 69, 102935. [Google Scholar] [CrossRef]
- Alvarez, X.; Alves, A.; Ribeiro, M.P.; Lazzari, M.; Coutinho, P.; Otero, A. Biochemical characterization of Nostoc sp. exopolysaccharides and evaluation of potential use in wound healing. Carbohydr. Polym. 2021, 254, 117303. [Google Scholar] [CrossRef]
- Markou, G.; Chatzipavlidis, I.; Georgakakis, D. Cultivation of Arthrospira (Spirulina) platensis in olive-oil mill wastewater treated with sodium hypochlorite. Bioresour. Technol. 2012, 112, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Niccolai, A.; Chini Zittelli, G.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae of interest as food source: Biochemical composition and digestibility. Algal Res. 2019, 42, 101617. [Google Scholar] [CrossRef]
- Teuling, E.; Wierenga, P.A.; Schrama, J.W.; Gruppen, H. Comparison of protein extracts from various unicellular green sources. J. Agric. Food Chem. 2017, 65, 7989–8002. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.; Affan, M.A.; Lee, D.-W.; Kang, D.-H. Characterization of the coccoid cyanobacterium Myxosarcina sp. KIOST-1 isolated from mangrove forest in Chuuk State, Federated States of Micronesia. Ocean Sci. J. 2017, 52, 359–366. [Google Scholar] [CrossRef]
- López-Rodríguez, A.; Mayorga, J.; Flaig, D.; Fuentes, G.; Cotabarren, J.; Obregón, W.D.; Gómez, P.I. Comparison of two strains of the edible cyanobacteria Arthrospira: Biochemical characterization and antioxidant properties. Food Biosci. 2021, 42, 101144. [Google Scholar] [CrossRef]
- Nagle, V.L.; Mhalsekar, N.M.; Jagtap, T.G. Isolation, optimization and characterization of selected Cyanophycean members. Indian J. Mar. Sci. 2010, 39, 212–218. [Google Scholar]
- De Morais, M.G.; da Cruz Reichert, C.; Dalcanton, F.; Durante, A.J.; Marins, L.F.; Costa, J.A.V. Isolation and characterization of a new Arthrospira strain. Z. Für Nat. C 2014, 63, 144–150. [Google Scholar] [CrossRef]
- Gentscheva, G.; Milkova-Tomova, I.; Pehlivanov, I.; Gugleva, V.; Nikolova, K.; Petkova, N.; Andonova, V.; Buhalova, D.; Pisanova, E. Chemical characterization of selected algae and cyanobacteria from Bulgaria as sources of compounds with antioxidant activity. Appl. Sci. 2022, 12, 9935. [Google Scholar] [CrossRef]
- Villaró, S.; Morillas-España, A.; Acién, G.; Lafarga, T. Optimisation of operational conditions during the production of Arthrospira platensis using pilot-scale raceway reactors, protein extraction, and assessment of their techno-functional properties. Foods 2022, 11, 2341. [Google Scholar] [CrossRef]
- Tonietto, A.E.; Lombardi, A.T.; Vieira, A.A.H.; Parrish, C.C.; Choueri, R.B. Cylindrospermopsis raciborskii (cyanobacteria) exudates: Chemical characterization and complexation capacity for Cu, Zn, Cd and Pb. Water Res. 2014, 49, 381–390. [Google Scholar] [CrossRef]
- Wang, M.; Morón-Ortizc, A.; Zhou, J.; Benítez-González, A.; Mapelli-Brahm, P.; Meléndez-Martínez, A.J.; Barba, F.J. Effects of pressurized liquid extraction with dimethyl sulfoxide on the recovery of carotenoids and other dietary valuable compounds from the microalgae Spirulina, Chlorella and Phaeodactylum tricornutum. Food Chem. 2023, 405, 134885. [Google Scholar] [CrossRef]
- Bortolini, D.G.; Maciel, G.M.; Fernandes, I.A.A.; Pedro, A.C.; Rubio, F.T.V.; Branco, I.G.; Haminiuk, C.W.I. Functional properties of bioactive compounds from Spirulina spp.: Current status and future trends. Food Chem. 2022, 5, 100134. [Google Scholar] [CrossRef]
- Dalla Costa, V.; Filippini, R.; Zusso, M.; Caniato, R.; Piovan, A. Monitoring of Spirulina flakes and powders from Italian companies. Molecules 2022, 27, 3155. [Google Scholar] [CrossRef]
- Hu, H.; Li, Y.; Yin, C.; Ouyang, Y. Isolation and characterization of a mesophilic Arthrospira maxima strain capable of producing docosahexaenoic acid. J. Microbiol. Biotechnol. 2011, 21, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Issa, A.; Ali, E.; Abdel-Basset, R.; Awad, M.F.; Ebied, A.M.; Hassan, S.A. The impact of nitrogen concentrations on production and quality of food and feed supplements from three cyanobacteria and potential application in biotechnology. Biocatal. Agric. Biotechnol. 2020, 24, 101533. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of emerging concern in aquatic systems: Chemistry, occurrence, effects, and removal methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef]
- Altabe, S.G.; Mansilla, M.C.; de Mendoza, D. Remodeling of membrane phospholipids by bacterial desaturases. In Stearoyl-CoA Desaturase Genes in Lipid Metabolism; Springer: New York, NY, USA, 2013; pp. 209–231. [Google Scholar]
- Abd El Fatah, H.M.; El-Baghdady, K.Z.; Zakaria, A.E.; Sadek, H.N. Improved lipid productivity of Chlamydomonas globosa and Oscillatoria pseudogeminata as a biodiesel feedstock in artificial media and wastewater. Biocatal. Agric. Biotechnol. 2020, 25, 101588. [Google Scholar] [CrossRef]
- Singh, H.; Varanasi, J.L.; Banerjee, S.; Das, D. Production of carbohydrate enrich microalgal biomass as a bioenergy feedstock. Energy 2019, 188, 116039. [Google Scholar] [CrossRef]
- Cepas, V.; Gutiérrez-Del-Río, I.; López, Y.; Redondo-Blanco, S.; Gabasa, Y.; Iglesias, M.J.; Soengas, R.; Fernández-Lorenzo, A.; López-Ibáñez, S.; Villar, C.J.; et al. Microalgae and cyanobacteria strains as producers of lipids with antibacterial and antibiofilm activity. Mar. Drugs 2021, 19, 675. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Moreira, J.B.; Lucas, B.F.; Braga, V.S.; Cassuriaga, A.P.; Morais, M.G. Recent advances and future perspectives of PHB production by cyanobacteria. Ind. Biotechnol. 2018, 14, 249–256. [Google Scholar] [CrossRef]
- Mathimani, T.; Mallick, N. A Comprehensive review on harvesting of microalgae for biodiesel—Key challenges and future directions. Renew. Sustain. Energy Rev. 2018, 91, 1103–1120. [Google Scholar] [CrossRef]
- Mathimani, T.; Mallick, N. A Review on the hydrothermal processing of microalgal biomass to bio-oil—Knowledge gaps and recent advances. J. Clean Prod. 2019, 217, 69–84. [Google Scholar] [CrossRef]
- da Silva, M.F.; Casazza, A.A.; Ferrari, P.F.; Perego, P.; Bezerra, R.P.; Converti, A.; Porto, A.L.F. A new bioenergetic and thermodynamic approach to batch photoautotrophic growth of Arthrospira (Spirulina) platensis in different photobioreactors and under different light conditions. Bioresour. Technol. 2016, 207, 220–228. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, P. Effect of CO2 concentration on algal growth: A review. Renew. Sustain. Energy Rev. 2014, 38, 172–179. [Google Scholar] [CrossRef]
- Canakci, M. The potential of restaurant waste lipids as biodiesel feedstocks. Bioresour. Technol. 2007, 98, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Santana-Sánchez, A.; Lynch, F.; Sirin, S.; Allahverdiyeva, Y. Nordic cyanobacterial and algal lipids: Triacylglycerol accumulation, chemotaxonomy and bioindustrial potential. Physiol. Plant. 2021, 173, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.-J.; Chang, J.-S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Li, X.; Yuan, Y.; Yang, C.; Tang, T.; Zhao, Q.; Sun, Y. Adaptive evolution and carbon dioxide fixation of Chlorella sp. in simulated flue gas. Sci. Total Environ. 2019, 650, 2931–2938. [Google Scholar] [CrossRef]
- Silva, C.E.; Abud, A.K.; Silva, I.C.; Andrade, N.P.; Cerqueira, R.B.; Andrade, F.P.; Carvalho, F.D.; Almeida, R.M.; Souza, J.E. Acceptability of tropical fruit pulps enriched with vegetal/microbial protein sources: Viscosity, importance of nutritional information and changes on sensory analysis for different age groups. J. Food Sci. Technol. 2019, 56, 3810–3822. [Google Scholar] [CrossRef]
- Arias, D.M.; Uggetti, E.; García-Galán, M.J.; García, J. Production of polyhydroxybutyrates and carbohydrates in a mixed cyanobacterial culture: Effect of nutrients limitation and photoperiods. New Biotechnol. 2018, 42, 1–11. [Google Scholar] [CrossRef]
- Cerri, R.; Niccolai, A.; Cardinaletti, G.; Tulli, F.; Mina, F.; Daniso, E.; Bongiorno, T.; Chini Zittelli, G.; Biondi, N.; Tredici, M.R.; et al. Chemical composition and apparent digestibility of a panel of dried microalgae and cyanobacteria biomasses in rainbow trout (Oncorhynchus mykiss). Aquaculture 2021, 544, 737075. [Google Scholar] [CrossRef]
- Lafarga, T.; Sánchez-Zurano, A.; Villaró, S.; Morillas-España, A.; Acién, G. Industrial production of Spirulina as a protein source for bioactive peptide generation. Trends Food. Sci. Technol. 2021, 116, 176–185. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).