Abstract
In this work, a metal–organic framework (MOF), copper benzene dicarboxylate (Cu-MOF), was tested for the adsorptive recovery of organic dyes (Sunset Yellow FCF, Tartrazine, Orange II, and Methyl Orange) from aqueous solutions. Studies were also carried out to determine the effects of various parameters, and isothermal and kinetic models were proposed. The adsorption capacity of Cu-MOF was much higher than that of activated carbon. The experimental data are best described by the Langmuir isotherm model (R2 > 0.997) and show the ability of Cu-MOF to adsorb 435 mg/g of the dye under optimal conditions. The study of the kinetics of the dye adsorption process followed a pseudo-second-order kinetic model indicating the coexistence of physical and chemisorption, with diffusion within the particles being the rate-limiting step. Thermodynamic studies were also carried out, and they led to the conclusion that the adsorption of the dye was a feasible, spontaneous, and exothermic process (−25.53 kJ mol−1). The high organic dye recovery shows that Cu-MOF can be used as an efficient and reusable adsorbent for the extraction of dyes from aqueous solutions. These studies may lead to economic interest in this adsorbent material for environmental purposes.
1. Introduction
Environmental pollution is increasing all over the world due to population growth and industrial development. Dyes are considered one of the leading pollutants, and they have every chance of affecting the surrounding environment in several ways []. They have a profound and lasting effect on exposed animals, depending on the concentration and duration of exposure, stimulating the evolution of bacteria that interfere with photosynthesis, absorbing and reflecting light from incoming water, and preventing the growth of aquatic plants []. In addition, dyes are used in the food industry to improve the appearance, smell, taste, color, texture, calorie content and shelf life of food. Most dyes are considered mutagenic and carcinogenic due to the presence of aromatic rings in their structure. The presence in food and the appearance of these dyes in surface and groundwater are considered unsafe for human well-being. High doses can cause a variety of health problems, including allergic reactions, skin irritation, gastrointestinal problems, and overactivity in children. Consequently, the presence of dyes in foods is highly regulated, and scientists are developing simple and inexpensive methods for detecting and extracting dyes to minimize their contamination of wastewater.
The problem of detecting and identifying the content of organic dyes in food products is relevant, and, therefore, various methods are used for this purpose, including UV spectrophotometry, high-performance liquid chromatography, spectrofluorometry, electrochemical voltammetry, and mass spectrometry [,,,,,,,,,,,]. However, when determining the concentration of the dye, various limitations and obstacles are observed, such as the low concentration of the dye in food samples, which cannot be detected using instruments, and the positive or negative interference effect of the matrix components. Because of this, the concentrations of dyes in foods cannot be accurately determined. Therefore, separation and pre-concentration methods are needed.
There are several methods most suitable for the extraction, separation, and concentration of dyes. One of them is the solid-phase extraction (SPE) method. This method is common and is used to isolate and concentrate synthetic food colorings. Metal–organic frameworks (MOFs), which are used as sorbents in the SPE method, are new porous compounds, which are also called hybrid porous coordination polymers []. MOFs are types of adsorbents that exhibit unique physical and chemical properties by combining metal-containing units with organic linkers, using strong bonds to create open crystalline frameworks with a constant porosity [,,,,,,,]. A consistent porosity, low density, high surface area, suitable pore size, metal node functionality, and structural flexibility make MOFs unique and well suited for a wide range of applications. In addition, the high surface area and porosity of MOFs can facilitate the diffusion of contaminants through the framework and the presence of an adsorption site. Thus, MOFs are among the most suitable structures in the field of adsorption and separation [,,,].
The terephthalate ion is used as a ligand in MOF chemistry because functional groups in the para-position contribute to the formation of polymer chains that form both monometallic and heterometallic two- or three-dimensional coordination compounds. Based on the possibilities of the structure of the terephthalate ion, it can be assumed that it can function as a bi-, tri-, or tetra-dentate ligand []. For example, copper terephthalate exhibits a high adsorption capacity with respect to various dyes and metal ions [,,,].
An important task today is the study and development of simple and efficient methods for the synthesis of these compounds. The aim of this study was to develop a simple sorption–spectrophotometric method for the determination of food additives using MOF based on copper terephthalate as an adsorbent. To achieve this goal, the adsorption on the sorbent was determined (the dependence of adsorption on time, concentration, and pH); the mechanism of adsorption was studied; and food additives were determined separately and together in real objects (the drink “Orange Fresh”), as well as their analytical characteristics.
2. Materials and Methods
2.1. Initial Materials
A sorbent was prepared with benzene dicarboxylic acid (H2BDC, chemically pure), hydrochloric acid (chemically pure), sodium hydroxide (analytically pure), n-octanol, and copper(II) sulfate purchased from Sigma-Aldrich.
2.2. Dyes
Organic dyes were purchased from Sigma-Aldrich. Figure 1 shows the chemical structures of the dye molecules.
Figure 1.
Structural formulas of dyes.
Stock solutions of dyes (1000 mg/L) were prepared by dissolving the required amount of dye in bidistilled water, and a test solution was prepared daily with the required dilution.
2.3. Characterization
Elemental analyses were carried out on a CHNOS vario EL cubic analyzer (Elementar Analysensysteme GmbH, Langenselbold, Germany), and their results are in good agreement (±0.3%) with the calculated values. Copper was determined on an X-Art M energy-dispersive X-ray fluorescence spectrometer (Comita, Russia) or an MGA-915 atomic absorption spectrometer (Lumex, Russia). IR spectra were recorded on a Nicolet 380 FTIR spectrometer using KBr beads and Softspectra data analysis software. An X-ray diffraction analysis (XRD) was performed on a DRON-UM-2 diffractometer with CuKa radiation (λCu = 1.54184 Å) in the range 2θ = 5–80° of 2θ angles at a scanning rate of 5°/min and a temperature of 25 °C. Scanning electron microscopy (SEM) images were obtained on a Zeiss LEO SUPRA 25 scanning electron microscope.
2.4. Synthesis of MOF
The synthesis of copper terephthalate took place in a medium containing a surfactant (n-octanol) to give it a bulk structure. A total of 2.4 g (0.06 mol) of sodium hydroxide was dissolved in 150 mL of hot water, and 5 g (0.03 mol) of terephthalic acid was added, achieving the complete dissolution of the latter. In another vessel, a solution was prepared from 120 mL of hot water and 15 g of CuSO4·5H2O. Both solutions were filtered using the hot filtration method. Then, 15 mL of n-octanol was added to a solution of sodium terephthalate with constant stirring, and at the same time, the number of revolutions of the magnetic stirrer was increased to form an emulsion. Next, a hot solution of CuSO4·5H2O was added dropwise, while the solution was stirred. As a result of this reaction, a voluminous precipitate of a bright blue color formed. The crystals were filtered on a porous glass plate and dried in air and then at 60 °C for 6 h. This method allowed us to obtain 6.25 g of an almost colorless substance with a slight bluish tint, which corresponds to a yield of 91%, calculated as terephthalic acid. Elemental analysis: Found (%) C—28.9; H—2.5; Cu—40.2. Calculated for Cu(BDC)·2H2O (%): C—29.26; H—2.4; Cu—39.
2.5. Dye Adsorption Study
For the analysis of all the dyes, three series of 5 solutions of 40 mL, each with different concentrations of the additive, were prepared: 20; 10; 5; 2.5; and 1.25 mg/L. Next, the initial solutions were placed in 100 mL beakers, and adsorption was studied at temperatures of 5, 20, and 35 °C. To do this, after reaching the required temperature, a weighed portion of the sorbent (20 mg) was placed in each of the analyzed solutions, and the solutions were placed on a magnetic stirrer, adjusting the rotation speed for good mixing. After 3 h of active stirring, 10 mL of the resulting suspension was taken from each solution and centrifuged for 10 min. The equilibrium concentration of the additive was studied using a spectrophotometer at certain wavelengths. The wavelengths were determined individually for each dye.
The adsorption capacity (qe) of Cu-MOF was calculated using Equation (1):
where C0 is the initial concentration of the dye (mg/L), Ce is the equilibrium concentration of the dye (mg/L), V is the volume of the dye solution, and m is the weight of the sorbent.
Recovery factors (R, %) were calculated using Formula (2):
where C is the concentration of the dye solution after sorption (mg/L).
2.6. Study of Dye Adsorption on a Real Object
To carry out adsorption on real objects, it is necessary to detect the studied dyes in the analyzed objects. For the analysis of the content of synthetic dyes, the method of thin-layer chromatography (TLC) was used. STX-1VE Sorbfil silica gel plates were used as the stationary phase in the experiment, and the mobile phase was a mixture of n-butanol–ethanol–water in a ratio of 60:60:150. The carbonated drink “Orange Fresh” was used as the analyte.
To determine the presence of synthetic dyes in a beverage, they were separated from the matrix solution into an organic layer, which was toluene, using an extraction method. To do this, 40 mL of toluene solution and 5 mL of the test drink were mixed in a separating funnel with a capacity of 100 mL. Standard dye solutions and a solution of the analyzed object were applied with a microtool, 1 μL each, onto an STX-1VE silica gel plate. Chromatographic separation occurs due to the transfer of the components of the mobile phase along the layer of the stationary phase at different speeds in accordance with the distribution coefficients of the separated components and zones of different colors.
To separate the dyes and determine their concentrations in the drink, a column 1.7 cm in diameter and 15 cm in height was used. A sealant layer was placed in the column, and then the column was filled with a sorbent (Cu-MOF), densely filling the sorbent layer until the layer height was 7 cm. Then, 20 mL of the analyzed object (orange juice drink) was poured into the column. After the complete adsorption of the test solution, the eluent (n-butanol–ethanol–water in a ratio of 60:60:150) was passed through the sorbent layer, and fractional samples of 10 mL were taken. Dye concentrations were determined using the above method.
3. Results and Discussion
3.1. Synthesis and Characterization of the Sorbent
The preparation of Cu-MOF was carried out by mixing a metal salt and an acid in water in the presence of a surfactant to impart a bulk structure to MOF (Figure 2). Cu-MOF was analyzed using XRD, SEM, and IR spectroscopy.

Figure 2.
Powder of the obtained Cu-MOF.
X-ray diffraction was performed to identify the obtained compounds. The observed sharp and distinct diffraction peaks indicate that the prepared samples of copper terephthalates have good crystallinity and phase purity. All positions of the diffraction peaks are in good agreement with the results of the simulation performed in the “MatH-3”program used for processing XRD data. Figure 3 shows the diffraction pattern of the synthesized Cu-MOF; the lower part of the figure shows lines corresponding to the calculated peaks, which satisfactorily coincide with the values on the experimental curve and are in good agreement with previously published data [,,,].
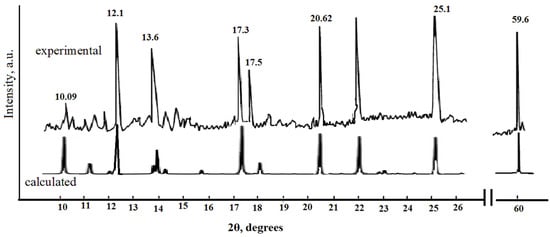
Figure 3.
XRD patterns of a copper terephthalate sample.
An SEM image is shown in Figure 4, which clearly shows prismatic crystals with a length of 30 to 100 µm and a thickness of 10 µm.
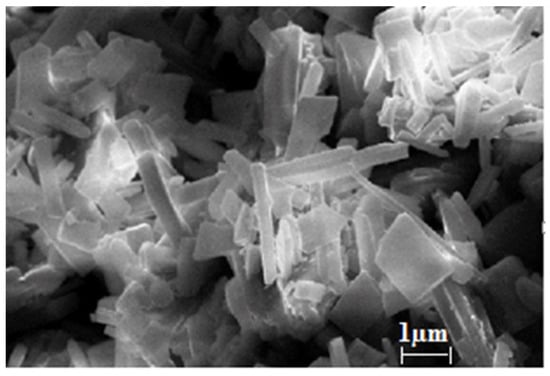
Figure 4.
SEM image of copper terephthalate crystals.
The IR spectrum of Cu-MOF (Figure 5) contains absorption bands corresponding to the crystallization water, carboxylate ions, C=C bonds of the aromatic ring, and Me-O bonds. In the region of 1720 cm−1, there is no absorption band characteristic of the carboxyl group. The difference between the asymmetric and symmetric vibrations of the carboxyl groups indicates that carboxyl groups can function as both monodentate and bidentate ligands.
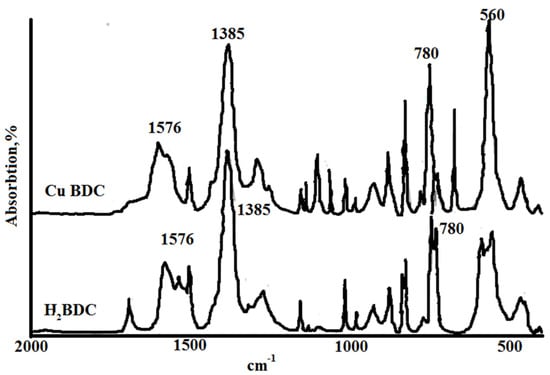
Figure 5.
IR spectra of copper terephthalate and terephthalic acid.
3.2. Solid-Phase Extraction of Dyes
In the course of this work, studies were carried out that made it possible to evaluate the ability of copper terephthalate to adsorb dyes in aqueous solutions. A study was made of the effect of the initial concentration, pH, contact time on the adsorption of dyes, and the kinetics of the process.
With an increase in the initial concentration, the degree of adsorption increased significantly, and with an increase in temperature, it decreased (Figure 6).
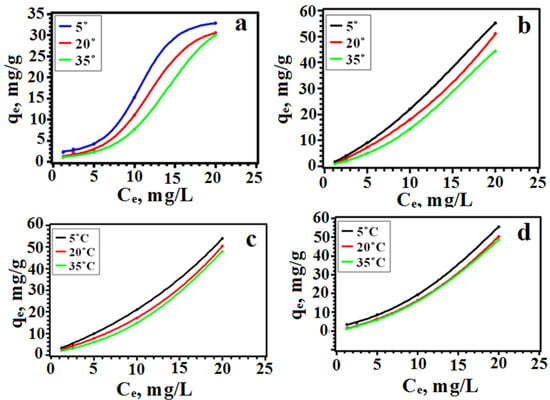
Figure 6.
Dependence of solid-phase extraction on the initial concentration of the dye (a—SY, b—TAR, c—Orange II, d—Methyl Orange).
Figure 7 shows photographs of the dye solutions before and after adsorption.
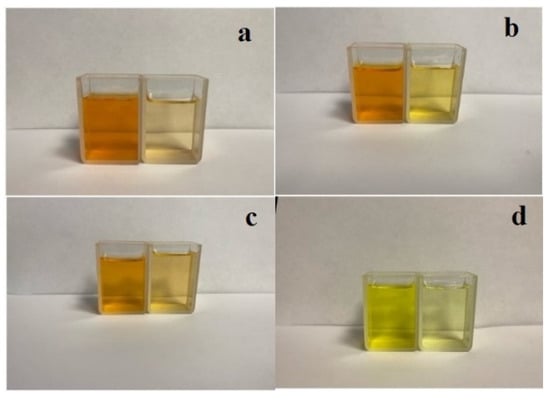
Figure 7.
Comparison of dye solutions and dye adsorbed on the sorbent (a—SY, b—TAR, c—Orange II, d—Methyl Orange).
The initial pH of the dye solution is a key factor controlling the adsorption process. The pH was studied in the range from 3.0 to 11.0, as shown in Figure 8. The degree of the extraction of dyes increases with increasing pH from 3.0 to 7.0. However, when the pH was between 7.0 and 11.0, the recovery decreased. It can be concluded that the surface of copper terephthalate can acquire a positive charge, which can interact with anionic dyes via various binding mechanisms and pathways, such as electrostatic attraction or hydrogen bonding. Dyes are best extracted in an environment close to neutral. However, in an acidic environment, extraction processes are better than in an alkaline one.
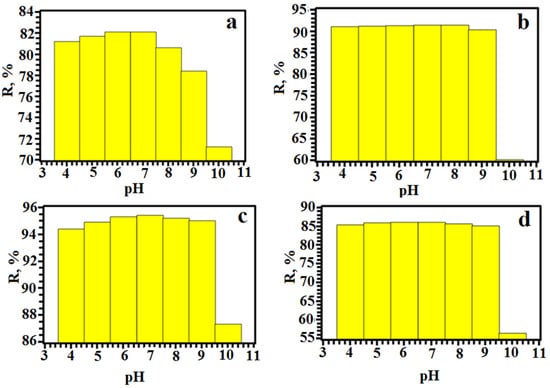
Figure 8.
Dependence of the degree of extraction on the pH of the medium (a—SY, b—TAR, c—Orange II, d—Methyl Orange).
The results obtained in the study of the adsorption of various azo dyes on copper terephthalate were analyzed using various adsorption models, particularly the Langmuir and Freundlich models.
The scheme of the Langmuir isotherm is based on the hypothesis of monolayer adsorption, where all sorption centers are dynamically and identically equivalent []. The linearized Langmuir isotherm can be expressed using Equation (3):
where KL is the Langmuir adsorption constant (mg/L), and qmax is the maximum adsorption capacity of the monolayer (mg/g).
The Freundlich isotherm shows adsorption on a heterogeneous surface with the same energy and in the presence of interactions between adsorbed molecules. This means that it is not limited to building a monolayer. The linearized Freundlich isotherm can be expressed using Equation (4):
The parameters of the Langmuir and Freundlich adsorption isotherms are summarized in Table 1.

Table 1.
Calculated parameters of the Langmuir and Freundlich isotherms for the adsorption of dyes by copper terephthalate.
High KF and 1/n values were obtained. This indicates that the adsorption of dyes by copper terephthalate promotes positive cooperative binding and its inherent heterogeneity []. The correlation coefficient (R2) shows that the adsorption of the Sunset and Tartrazine dyes is better described by the Freundlich isotherm than by the Langmuir model, which indicates that adsorption occurs on a heterogeneous surface with the same energy, interactions between adsorbed molecules are possible, and adsorption is not limited to building a monolayer. However, it can be concluded that the adsorption of the Orange II dye is better described by the Langmuir model, which indicates that all sorption sites are energetically equivalent and identical and that adsorption is based on monolayer adsorption. It was also concluded that both models are applicable for Methyl Orange.
To determine the spontaneity of the adsorption process and verify the experimental data, three main thermodynamic parameters were used: the change in enthalpy (ΔH0), entropy (ΔS0), and Gibbs free energy (ΔG0).
Adsorption as a spontaneous process of the accumulation of dye molecules on the surface of a sorbent is accompanied by a decrease in the entropy of the system. Since the criterion for the spontaneity of the process is
then adsorption is possible only at ∆H < 0 (exothermic process). Equilibrium is determined by the condition ∆H = T·∆S. With an increase in temperature, the equilibrium shifts towards the endothermic process, i.e., desorption.
∆H − T·∆S = ∆G < 0,
The Gibbs equation was used to study the effect of temperature on equilibrium adsorption (5):
where K is the thermodynamic equilibrium constant, which can be determined using Equation (6):
Considering the change in concentrations in the real-time interval and depending on the dose of the sorbent, Equation (6) can be written as
The Gibbs equation can also be expressed as follows:
Combining the above equations, we obtain
The obtained thermodynamic parameters are presented in Table 2.

Table 2.
Calculated thermodynamic parameters.
The data presented in Table 2 indicate that the adsorption process occurs spontaneously. The process is exothermic because the enthalpy change is negative. A positive entropy change value indicates that the number of degrees of freedom at the solid–liquid interface increases during dye adsorption and reflects the sorbate affinity for sorbent molecules. The values of the Gibbs free energy change also increase. This suggests that an increase in temperature indicates a higher adsorption efficiency at moderately low temperatures.
To achieve equilibrium, it is necessary to provide an appropriate contact time between the dye and the sorbent, as this is one of the main factors in the SPE process based on equilibrium. The results are shown in Figure 9. It can be seen that the degree of adsorption is higher at a lower temperature. However, all three temperatures can be considered satisfactory since the degree of adsorption for each of them exceeds 90%. The dye recovery rates during adsorption turned out to be quantitative: SY ≥ 80%; TAR ≥ 90%; Orange II ≥ 93%; Methyl Orange ≥ 85%.
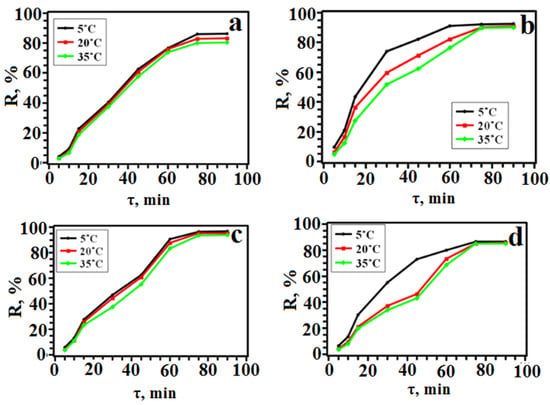
Figure 9.
Dependence of the degree of extraction on the contact time (a—SY, b—TAR, c—Orange II, d—Methyl Orange).
The mechanism of adsorption, particularly the chemical reaction and mass transfer, was analyzed using the pseudo-first-order rate equation and the pseudo-second-order rate equation. The linear form of the pseudo-first-order kinetic model can be expressed using the following equation:
where qe (mg/g) is the amount of dye adsorbed at equilibrium; qt (mg/g) is the amount of dye adsorbed at any given time (t); and k1 (1/min) is the pseudo-first-order absorption rate constant, which can be calculated from the plot of the logarithm (qe − qt) versus time.
The pseudo-second-order kinetic model can be expressed in linear form using Equation (11):
where k2 (g/(mg/min)) is the pseudo-second-order sorption rate constant, which can be calculated from a linear plot of t/qt versus time.
The kinetic parameters under different conditions are given in Table 3. The applied pseudo-first-order equation satisfactorily describes the patterns of dye adsorption at the initial stages of the adsorption process when the film diffusion phenomenon has a significant effect on the process. However, the high values of the correlation coefficients make it possible to judge in favor of the applicability of both the pseudo-first- and pseudo-second-order models for describing the chemical stage of the sorption process, as well as for the possibility of considering intermolecular interactions in the analyzed systems.

Table 3.
Estimated kinetic parameters.
The adsorption of organic dyes by MOFs can occur for various reasons:
- The adsorption of the dye may be due to the electrostatic interaction between MOF and the adsorbate. The neutrality of the studied sorbent means the absence of any active functional groups but does not exclude the occurrence of an electrostatic charge on the surfaces of the sorbent and sorbate. For example, well-vacuum-dried copper terephthalate, placed in a glass storage vessel, easily covers the walls of this vessel, spreading in a relatively thin layer, which we repeatedly observed.
- A sufficiently high oxygen content in the MOF composition allows for the formation of hydrogen bonds with dye molecules.
- The formation of π-π stacking between the benzene rings of the sorbent and sorbate is possible.Cation and anion π interactions are possible.
- Similar examples of interactions between sorbents of similar classes and organic dyes are quite often discussed in the works [,].
The sorbent can be used repeatedly. For this, a study was made of the number of working cycles (Figure 10). The results show that the extraction of the dyes from the aqueous solutions was quantitative over four cycles. The average values of extraction with the standard deviations of the dyes for four cycles were as follows: SY—79.3 ± 3.1%; TAR—89.1 ± 2.3%; Orange II—92.1 ± 3.2%; Methyl Orange—85.4 ± 1.0%. These results suggest that the sorbent was mechanically stable and had excellent recyclability.
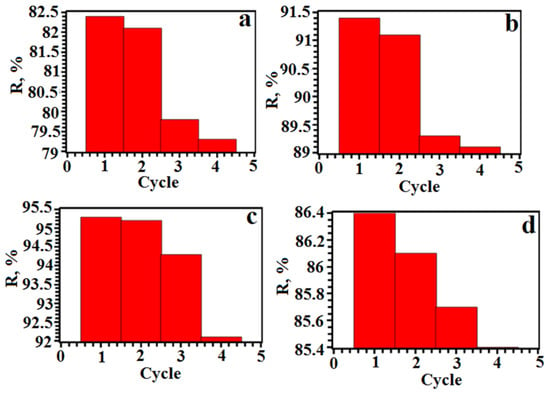
Figure 10.
Number of working cycles (a—SY, b—TAR, c—Orange II, d—Methyl Orange).
3.3. Analysis of a Real Object
A chromatographic analysis confirmed the presence of dyes in the carbonated drinks analyzed in this study. The distribution coefficients Rf calculated as the ratio of the distance l traveled by the dye in a thin sorbent layer to the distance L = 9.3 cm traveled by the solvent from the start to the start line are shown in Table 4.

Table 4.
Rf values for synthetic dyes.
The analyzed object was passed through the column, and the following results were obtained: when the real object was passed through the sorbent, a transparent solution was obtained at the output—the sorbent absorbed both dyes (Figure 11). No dye was detected in this sample, which means that objects such as sugar were present in the clear solution. When the eluent was passed through the sorbent, the dyes were successively washed out. First, the yellow-green dye, TAR, was washed off, followed by the orange dye, SY. When eluting the Orange Fresh drink applied to a chromatographic column, it was possible to clearly separate the dyes. During further elution, the individual dyes were washed out with the eluent in the following sequence: 1—TAR and 2—SY. As a result of the elution, 5 mL of TAR dye and 7 mL of SY dye were collected. The concentration of each dye in a separate sample was also determined, and it was determined that the content of dyes in the original sample was as follows: TAR 10.75 mg/L and SY 12.34 mg/L.

Figure 11.
Separation of dyes in a real object (a—passage of a real object through the column; b—extraction of TAR after passing the eluent; c—isolation of SY after re-eluting; d—collected samples of isolated dyes).
4. Conclusions
The resulting MOF based on Cu-containing terephthalic acid was characterized using XRD, SEM, and IR spectroscopy. In Cu-MOF, the existence of prismatic crystals 30 to 100 µm long and 10 µm thick was found. Copper terephthalate was used for the adsorptive removal of azo dyes from aqueous solutions (SY, TAR, Orange II, and Methyl Orange), as well as for the separation of food additives in real systems. The kinetic data are consistent with both first- and second-order pseudokinetic models. The Langmuir and Freundlich isotherm models were used to describe the adsorption isotherms. According to the correlation coefficients (R2), the Freundlich model fits the data better than the Langmuir model for SY and TAR dyes. The adsorption of the Orange II dye is better described by the Langmuir model. For Methyl Orange, both models are acceptable. Copper terephthalate showed a satisfactory ability to adsorb azo dyes. The values of thermodynamic parameters (ΔG0, ΔH0, and ΔS0) show that the dye adsorption process is spontaneous and exothermic; in the process of sorption, the number of degrees of freedom increases, which characterizes the good affinity of the sorbate with the sorbent. When studying real objects, it was determined that the sorbent under study makes it possible to separate food additives that can be used to detect these dyes and determine their concentrations.
Author Contributions
Conceptualization, V.A.Z. and I.E.U.; investigation, M.A.C. and N.S.K.; methodology, M.A.C.; validation, M.A.C.; visualization, N.S.K.; writing—original draft, V.A.Z. and I.E.U.; writing—review and editing, V.A.Z. and I.E.U. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shabir, M.; Yasin, M.; Hussain, M.; Shafiq, I.; Akhter, P.; Nizami, A.-S.; Jeon, B.-H.; Park, Y.-K. A review on recent advances in the treatment of dye-polluted wastewater. J. Ind. Eng. Chem. 2022, 112, 1–19. [Google Scholar] [CrossRef]
- Slokar, Y.M.; Le Marechal, A.M. Methods of decoloration of textile wastewaters. Dyes Pigm. 1998, 37, 335–356. [Google Scholar] [CrossRef]
- Naseem, T.; Waseem, M. A comprehensive review on the role of some important nanocomposites for antimicrobial and wastewater applications. Int. J. Environ. Sci. Technol. 2022, 19, 2221–2246. [Google Scholar] [CrossRef]
- Włodarczyk, E.; Zarzycki, P.K. Chromatographic behavior of selected dyes on silica and cellulose micro-TLC plates: Potential application as target substances for extraction, chromatographic, and/or microfluidic systems. J. Liq. Chromatogr. Relat. Technol. 2017, 40, 259. [Google Scholar] [CrossRef]
- Kucharska, M.; Grabka, J. A review of chromatographic methods for determination of synthetic food dyes. Talanta 2010, 80, 1045–1051. [Google Scholar] [CrossRef]
- Uddin, M.J.; Ampiaw, R.E.; Lee, W. Adsorptive removal of dyes from wastewater using a metal-organic framework: A review. Chemosphere 2021, 284, 131314. [Google Scholar] [CrossRef]
- Bişgin, A.T.; Uçan, M.; Narin, İ.; Soylak, M. A comparative study for separation, preconcentration and determination of tartrazine (E 102) in soft drink samples by two kinds of amberlite resins. Food Anal. Methods 2015, 8, 2141–2149. [Google Scholar] [CrossRef]
- Mokhtari, P.; Ghaedi, M.; Dashtian, K.; Rahimi, M.R.; Purkait, M.K. Removal of methyl orange by copper sulfide nanoparticles loaded activated carbon: Kinetic and isotherm investigation. J. Mol. Liq. 2016, 219, 299–305. [Google Scholar] [CrossRef]
- Bişgin, A.T. Single and Simultaneous Solid-Phase Extraction and UV–Vis Determination for Monitoring E129, E133 and E110 in Foodstuffs. Iran. J. Sci. Technol. Trans. A Sci. 2021, 45, 163. [Google Scholar] [CrossRef]
- Bişgin, A.T.; Sürme, Y.; Uçan, M.; Narin, İ. Solid-phase extraction and spectrophotometric determination of Allura Red (E129) in foodstuff, soft drink, syrup and energy drink samples: A comparison study. Int. J. Food Sci. Technol. 2016, 51, 2367. [Google Scholar] [CrossRef]
- Iwuozor, K.O.; Ighalo, J.O.; Emenike, E.C.; Ogunfowora, L.A.; Igwegbe, C.A. Adsorption of methyl orange: A review on adsorbent performance. Curr. Res. Green Sustain. Chem. 2021, 4, 100179. [Google Scholar] [CrossRef]
- Marçalm, L.; de Fariam, E.H.; Saltarellim, M.; Calefim, P.S.; Nassarm, E.J.; Ciuffim, K.J. Amine-functionalized titanosilicates prepared by the sol-gel process as adsorbents of the azo-dye Orange II. Ind. Eng. Chem. Res. 2011, 50, 239–246. [Google Scholar] [CrossRef]
- Kousha, M.; Daneshvar, E.; Salar-Sohrabi, M.; Jokar, M.; Bhatnagar, A. Adsorption of acid orange II dye by raw and chemically modified brown macroalga Stoechospermum marginatum. Chem. Eng. J. 2012, 192, 67–76. [Google Scholar] [CrossRef]
- Saleh, M.M.S. Oxidation and complexation-based spectrophotometric methods for sensitive determination of tartrazine E102 in some commercial food samples. Comput. Chem. 2016, 4, 51. [Google Scholar] [CrossRef]
- Dzhardimalieva, G.I.; Uflyand, I.E. Design and synthesis of coordination polymers with chelated units and their application in nanomaterials science. RSC Adv. 2017, 7, 422–488. [Google Scholar] [CrossRef]
- Ba Mohammed, B.; Lgaz, H.; Alrashdi, A.A.; Yamni, K.; Tijani, N.; Dehmani, Y.; El Hamdani, H.; Chung, I.-M. Insights into methyl orange adsorption behavior on a cadmium zeolitic-imidazolate framework Cd-ZIF-8: A joint experimental and theoretical study. Arab. J. Chem. 2021, 14, 102897. [Google Scholar] [CrossRef]
- Wang, P.L.; Xie, L.H.; Joseph, E.A.; Li, J.R.; Su, X.O.; Zhou, H.C. Metal–Organic Frameworks for Food Safety. Chem. Rev. 2019, 119, 10638–10690. [Google Scholar] [CrossRef]
- Uflyand, I.E.; Zhinzhilo, V.A.; Nikolaevskaya, V.O.; Kharisov, B.I.; Oliva González, C.M.; Kharissova, O.V. Recent strategies to improve MOF performance in solid phase extraction of organic dyes. Microchem. J. 2021, 168, 106387. [Google Scholar] [CrossRef]
- Gautam, R.K.; Banerjee, S.; Sanroman, M.A.; Chattopadhyaya, M.C. Synthesis of copper coordinated dithiooxamide metal organic framework and its performance assessment in the adsorptive removal of tartrazine from water. J. Environ. Chem. Eng. 2017, 5, 328–340. [Google Scholar] [CrossRef]
- Oymak, T.; Tokalıoğlu, Ş.; Cam, Ş.; Demir, S. Determination of color additive tartrazine (E 102) in food samples after dispersive solid phase extraction with a zirconium-based metal-organic framework (UiO-66(Zr)-(COOH)2). Food Addit. Contam. Part A 2020, 37, 731–741. [Google Scholar] [CrossRef]
- García, E.R.; Medina, R.L.; Lozano, M.M.; Pérez, I.H.; Valero, M.J.; Maubert Franco, A.M. Adsorption of Azo-Dye Orange II from Aqueous Solutions Using a Metal-Organic Framework Material: Iron-Benzenetricarboxylate. Materials 2014, 7, 8037–8057. [Google Scholar] [CrossRef]
- Beydaghdari, M.; Saboor, F.H.; Babapoor, A.; Karve, V.V.; Asgari, M. Recent Advances in MOF-Based Adsorbents for Dye Removal from the Aquatic Environment. Energies 2022, 15, 2023. [Google Scholar] [CrossRef]
- Ali, A.; Muslim, M.; Neogi, I.; Afzal, M.; Alarifi, A.; Ahmad, M. Construction of a 3D Metal–Organic Framework and Its Composite for Water Remediation via Selective Adsorption and Photocatalytic Degradation of Hazardous Dye. ACS Omega 2022, 7, 24438–24451. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.-P.; Kang, Y.-S.; Guo, F.; Sun, W.-Y. Controlled synthesis of NbO-type metal-organic framework nano/microcrystals with superior capacity and selectivity for dye adsorption from aqueous solution. Microporous Mesoporous Mater. 2019, 273, 60–66. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, Y.; Wang, P.; Yao, Z.-Y.; Zhang, X.-D.; Sun, W.-Y. Coordination polymers with 1,3-bis(1-imidazolyl)-5-(imidazol-1-ylmethyl)benzene and biphenyl-4,4′-dicarboxylate ligands: Selective adsorption of gas and dye molecules. Microporous Mesoporous Mater. 2017, 241, 192–201. [Google Scholar] [CrossRef]
- Liu, Z.-Q.; Zhao, Y.; Wang, P.; Kang, Y.-S.; Azam, M.; Al-Resayes, S.I.; Liu, X.-H.; Lu, Q.-Y.; Sun, W.-Y. Fluorescent sensing and selective adsorption properties of metal–organic frameworks with mixed tricarboxylate and 1H-imidazol-4-yl-containing ligands. Dalton Trans. 2017, 46, 9022–9029. [Google Scholar] [CrossRef]
- Li, Y.-L.; Zhao, Y.; Wang, P.; Kang, Y.-S.; Liu, Q.; Zhang, X.-D.; Sun, W.-Y. Multifunctional Metal–Organic Frameworks with Fluorescent Sensing and Selective Adsorption Properties. Inorg. Chem. 2016, 55, 11821–11830. [Google Scholar] [CrossRef]
- Dzhardimalieva, G.I.; Uflyand, I.E. Chemistry of Polymeric Metal Chelates; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Carson, C.G.; Hardcastle, K.; Schwartz, J.; Liu, X.; Hoffmann, C.; Gerhardt, R.A.; Tannenbaum, R. Synthesis and Structure Characterization of Copper Terephthalate Metal–Organic Frameworks. Eur. J. Inorg. Chem. 2009, 2009, 2338–2343. [Google Scholar] [CrossRef]
- Panasyuk, G.P.; Azarova, L.A.; Budova, G.P.; Savost’yanov, A.P. Copper terephthalate and its thermal decomposition products. Inorg. Mater. 2007, 43, 951–955. [Google Scholar] [CrossRef]
- Phan, N.T.S.; Nguyen, T.T.; Nguyen, K.D.; Vo, A.X.T. An open metal site metal–organic framework Cu(BDC) as a promising heterogeneous catalyst for the modified Friedländer reaction. Appl. Catal. A Gen. 2013, 464–465, 128. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Tan, Q.; Hong, S.; Sun, Z. Facile synthesis of two-dimensional copper terephthalate for efficient electrocatalytic CO2 reduction to ethylene. J. Exp. Nanosci. 2021, 16, 246–254. [Google Scholar] [CrossRef]
- Mittal, A.; Malviya, A.; Kaur, D.; Mittal, J.; Kurup, L. Studies on the adsorption kinetics and isotherms for the removal and recovery of Methyl Orange from wastewaters using waste materials. J. Hazard. Mater. 2007, 148, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Sriram, G.; Bendre, A.; Mariappan, E.; Altalhi, T.; Kigga, M.; Ching, Y.C.; Jung, H.-Y.; Bhaduri, B.; Kurkuri, M. Recent trends in the application of metal-organic frameworks (MOFs) for the removal of toxic dyes and their removal mechanism-A review. Sustain. Mater. Technol. 2022, 31, e00378. [Google Scholar] [CrossRef]
- Shahnawaz Khan, M.; Khalid, M.; Shahid, M. What triggers dye adsorption by metal organic frameworks? The current perspectives. Mater. Adv. 2020, 1, 1575–1601. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).