Abstract
This article presents the history of zinc, its production and demand. The quantity of zinc production, both primary zinc from ores and concentrates, and secondary zinc from scrap and zinc-rich waste, was discussed. A comprehensive economic analysis covers zinc prices in the years 1960–2021. The basic methods of obtaining zinc from ores, including pyrometallurgical (Imperial Smelting Process ISP, Kivcet, Ausmelt) and hydrometallurgical (roasting–leaching–electrowinning RLE, atmospheric direct leaching ADL, Engitec Zinc Extraction EZINEX, zinc pressure leach) and their short characteristics, are presented. The global zinc market and the main areas of its application were analyzed. Technologies used for the recovery of zinc from scrap are discussed along with their characteristics. Galvanized steel is the main source of secondary zinc, both in the galvanizing process and in the remelting of galvanized steel. It can be easily recycled with other scrap steel in the electric arc furnace (EAF) for steel production. Currently, with high volatility in the price of zinc, as well as its natural resources in the earth’s crust, recycling is an important activity, despite the fact that zinc concentrates have a relatively constant chemical composition, while the resulting zinc waste contains zinc in varying amounts.
1. Introduction
Zinc is one of the most important non-ferrous metals used in society. Its properties that protect steel against corrosion give immense economic and environmental effects [1]. However, the increasing demand for this metal may limit its availability [2,3], as zinc has been used since ancient times. Romans produced significant amounts of brass products, i.e., an alloy of copper and zinc, around 200 BC. However, the ancient Greeks knew this metal under the name “pseudargyras”, which can be translated as “fake silver”. Archaeological discoveries confirm its presence from around 300 BC in the form of artifacts made of zinc, including a sheet of zinc from Athens [4]. However, a rational and efficient method of producing zinc was not known at that time, which significantly limited its use.
Zinc was probably first obtained in India. Already in ancient Indian writings, the first descriptions of the processes of producing metallic zinc from ores were found [5]. In 1374 it was identified as a new metal. Wastes from the zinc smelter in Zawar, Rajasthan (India) testify to the large scale of zinc production in the period from 1100 to 1500 [6]. Sources report that during this period, this smelter could produce from 60,000 tons of metallic zinc [7] to about a million tons of metallic zinc and zinc oxide [8]. At the end of the thirteenth century, Marco Polo described the production of zinc oxide in Persia, where it was used in the form of pills to treat eye infections [5]. These pills, made of hydrozincite and smithsonite, were found aboard the Roman ship Relitto del Pozzino, which was shipwrecked in 140 BC [9]. At the same time, zinc production also developed in China [10]. In Europe first descriptions of experiments with calamine and the resulting product can be found in the works of Albertus Magnus (ca. 1248), Biringuccio (ca. 1540), Agricola (1546), Paracelsus (1540) and Andreas Libavius (1596) [11,12,13]. In 1742 work on the distillation of zinc from calamine was carried out by the Swedish chemist Anton von Swab [13]. William Champion developed zinc production in vertical retorts and patented it. In 1743, the first zinc smelter with an annual capacity of about 200 tons was built in Bristol [14]. Oddly enough, the official discovery of zinc as a metal dates back three years later and is attributed to the German chemist Andreas Marggraf, who described the zinc production process in great detail [6]. The improved retort process was used by Johann Ruberg, who, in 1798, built a zinc smelter in Wesoła in Upper Silesia [14]. The main advantage of this technique was that the retorts were mounted horizontally in the furnace, which allowed them to be loaded and unloaded without cooling. The Ruberg method became a rational and economical method for large-scale zinc production in Europe. Further zinc smelters were established in Upper Silesia, but zinc production also developed in the Liège region in Belgium, in Aachen, in the Rhineland and in the Ruhr area in Germany [6,12]. Zinc production in the United States began in 1850 with the retort process, but progress was made in the 1920s with the patenting of the continuous retort process. In 1917, a process for the electrowinning of zinc was developed (roasting–leaching–electrowinning). Another modification of the zinc production process was the introduction of pressure leaching in the 1980s [13].
The presented description of zinc production in the old days indicates problems with its acquisition. With the development of civilization, there was a leap in the industry’s interest in zinc, which entailed the need to develop rational and efficient methods of obtaining it, which consequently made this metal one of the most important metals used today in technology. Zinc is a component of many alloys, including brass, aluminum zinc alloy. Rolled zinc sheets with the addition of titanium are used for roofing. It is used in electrical cells and in the form of zinc compounds. Zinc oxide ZnO (zinc white) is produced from primary zinc and is used in the chemical industry, as an additive to paints and varnishes and as a filler and stabilizer for rubber and plastics. Zinc sulfate has strong anti-inflammatory properties. Although zinc has been used in medicine for thousands of years, it was not until 1957 that zinc was officially recognized as one of the essential micronutrients [15]. In industry, zinc is most commonly used to coat steel products to protect against corrosion. Coatings produced by the hot dip galvanizing (HDG) method are the oldest known method of producing anti-corrosion coatings, patented in 1836 [12], and currently the most economical and effective anti-corrosion protection.
The article presents the current state and development of primary and secondary zinc production, taking into account the global demand and consumption of zinc, and reviews the trends of changes in the supply and demand for zinc in the world over the last several decades.
2. Zinc Production, Demand and Applications
2.1. Zinc Production
The good properties of zinc and the growing demand for this metal result in a continuous increase in zinc production throughout the 20th and 21st centuries. Table 1 shows the amount of zinc production in the years 1871–1927. Until 1840, Germany was the world’s leading producer of zinc [13]. In 1907, world production was 737,500 tons, of which the United States produced 31%, Germany 28%, Belgium 21%, Great Britain 8% and all other countries 12% [13]. During World War I, zinc production increased significantly, reaching more than a million tons (1,079,155 tons in 1916 and 1,094,888 tons in 1917, respectively). In the post-war period, zinc production fell by almost half (520,418 tons in 1921), which was caused by the economic crisis. In the 1920s and 1930s, a continuous increase in zinc production was observed, which in 1944 reached the level of about 2 million tons. The increase in zinc production at that time was associated with the coming conflict and the outbreak of World War II. After the war, zinc production steadily increased, exceeding 3 million tons in 1960, 4 million tons in 1965 and 6 million tons in 1975 [16]. This resulted from the post-war reconstruction and the progressing economic development of the countries.

Table 1.
The volume of global zinc production in the years 1871–1927, worked based on [17].
However, the greatest increase in zinc production is observed in the 1990s and in the early 21st century. Table 2 shows the amount of zinc production in the years 1991–2015 (data taken from [18,19,20,21,22,23,24,25,26]). In 2015, global zinc production increased to 13.9 million tons, mainly due to an increase in production in China. Zinc production indicators are the following: mine production (production of zinc contained in ores and concentrates), refined zinc production (production of metallic zinc quantifying the amount of zinc processed in the rectification process) and zinc usage (including both refined zinc and zinc extracted from other sources). World zinc production in 2016–2022, presented by the International Lead and Zinc Study Group (ILZSG) (Table 3), show that over 13 Mt of refined metallic zinc is currently produced annually, of which over 12 Mt comes from mine production [27].

Table 2.
The volume of global zinc production in 1991–2015, worked based on [18,19,20,21,22,23,24,25,26].

Table 3.
World zinc production and usage in years 2016–2022, working out based on [28].
The decrease in zinc production estimated for 2021 due to the instability and uncertainty of the rate of economic growth rate caused by the development of the pandemic and the increase in COVID-19 infections [18] did not, however, result in a decrease in refined zinc production (Table 3). However, government lockdowns and a drop in zinc prices after the outbreak of the global COVID-19 pandemic caused a decrease in mine production in many countries in 2021, especially in South America [19]. This year, in the period from January to September, a clear decrease in both mine production, refined zinc production and zinc usage was observed compared to the same period in 2021 (Table 3) [28]. It is estimated that world mine production will peak in 2024, exceeding 14 million tons. After this period, mining is likely to decline due to the deteriorating quality of ores and the depletion of zinc reserves [18]. Total global zinc reserves are estimated at around 250 million tons. Due to the relatively high demand for this metal, it has been calculated that zinc reserves will probably only last for the next 17 years [26]. Figure 1 shows countries with large reserves of zinc deposits. Australia has the world’s largest zinc reserves (68 million tons), followed by China, Mexico, Russia and Peru [26].
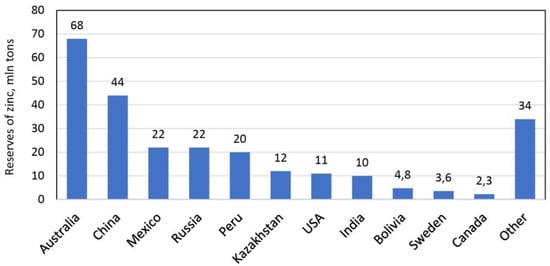
Figure 1.
World reserves of zinc ore deposits in 2020, figure worked out based on data from [19].
Figure 2 shows the production of zinc from ores and concentrates in the years 1995–2020. Since 1995, there has been a continuous increase in mining in China. In 1995, about 12% of mine production was produced by China. Within 25 years, the extraction of zinc ores in China increased to 35% of global extraction, reaching the level of 4.2 Mt in 2020 [26]. The main producers of zinc from ores and concentrates are also Australia (11% of production in 2020), Peru (10%) and Mexico (5%) [19].
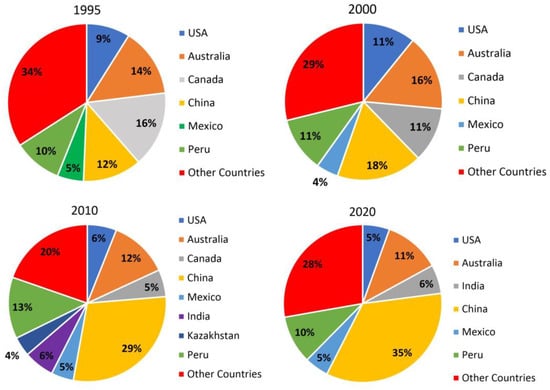
Figure 2.
Zinc mine production in 1995–2020, figures worked out based on data from [19,29,30,31].
Table 4 shows the global producers of refined zinc [19,29,30,31]. In 2020, China’s share of total global zinc production was 46.9%. The next places are taken by South Korea with a share of 7% and India with 5% of global production. Refined zinc production in China increased from 550,000 tons in 1990 to 6425 thousand tons in 2020. Zinc production in China has increased by 1168% over the past 30 years.

Table 4.
Refined zinc production by country, thousands of tones, worked out based on data from [19,29,30,31,32].
Over several decades, a continuous increase and high volatility of the price of zinc has been observed. Even in the 1970s, the price of zinc was at the level of USD 260–270 per ton, reaching a historical record of USD 4442 per ton in November 2006 (Figure 3). In 2021, the price of zinc was USD 2989 per ton (1 September 2021) [33]. This price was practically half the value from the same period in 2020. It was estimated that the price of zinc in 2022 would fall to approx. USD 2400 per ton, with a slight increase in subsequent years [33]. However, the change in the geopolitical situation in February 2022 and the instability of the global economy meant that in April this year, zinc prices exceeded USD 4500 per ton. At the moment (December 2022), the price of zinc is USD 3200 per ton [34].
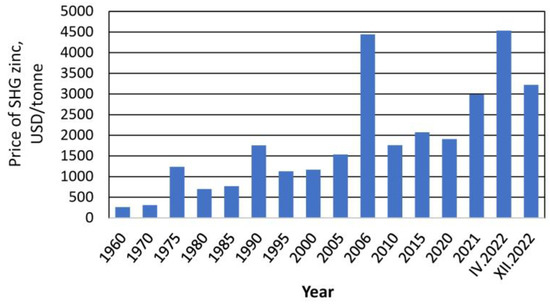
Figure 3.
Zinc price in 1960–2022, figure prepared based on data from [32,33].
2.2. Application and Demand
Zinc usage in 2015–2022 was presented in Table 3. In 2016, the demand for zinc exceeded 13.67 million tons, reaching even 14 million tons in 2021. However, the year 2022 resulted in a decrease in the demand for zinc due to the downturn in the economy related to the lockdown and the effects of COVID-19. Although, according to the ILZSG, global zinc consumption exceeded production in the first 10 months of 2022. Therefore, annual zinc consumption also includes zinc from secondary or recycled sources, such products are collected and processed based on scrap availability, metal composition (e.g., purity, alloy, etc.) and ease of processing [27].
Zinc is now the fourth most used metal in the world after iron, aluminum and copper. It has strong anti-corrosion properties. Demand for zinc is mainly driven by the production of galvanized steel, which is widely used in many industrial sectors. As a result, about 50% of the currently produced zinc is used to produce anti-corrosion coatings by hot dip galvanizing method (Figure 4). About 1/3 of zinc production is used for the production of alloys. This application includes both zinc and aluminum casting alloys (approx. 17%) as well as zinc used as an alloying additive, mainly in brasses and bronzes (approx. 17%). The third significant application of zinc is the production of chemical compounds, including the most important in terms of the production volume of zinc oxide (6%). A similar share (6%) is the production of zinc semi-finished products, of which zinc anodes used in electrochemical corrosion protection and rolled zinc sheets with the addition of Ti are the most important for production [33,35].
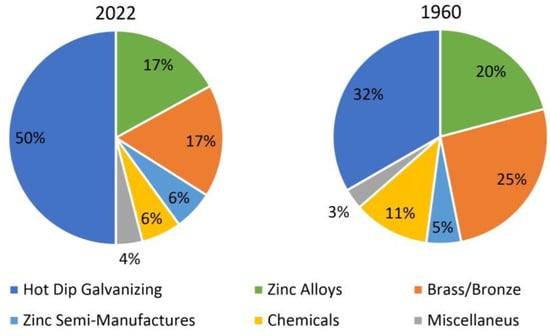
Figure 4.
End use zinc consumption distribution in 1960 and 2022, figure worked out on data taken from [28].
Hot dip galvanizing technology has been dynamically developing over the last few decades. Although this process has been known for over 150 years (in 1836, Sorel patented the hot dip galvanizing process), the consumption of zinc for the hot dip galvanizing process is still increasing. In 1960, only 32% of global zinc production was used for the production of coatings (Figure 4b). Taking into account the global production of zinc in 1960 at the level of 6.8 million tons and over 13 million tons in 2021, this is a significant increase in the consumption of zinc, mainly by the galvanizing industry. Zinc coatings obtained by the hot dip method are currently the most effective and economical anti-corrosion protection. According to statistics, the demand for this type of corrosion protection is constantly growing. In 2019, over 8.1 million tons of structural steel were galvanized in Europe and only compared to the data from 2018, an increase in production by nearly 1% was recorded [36]. In North America, in 2019, 4.9 million tons of steel were galvanized, which gives an approx. 10% increase in the production of galvanized products compared to 2014 [37]. However, by far, the largest HDG market in the world is China. The estimated production of galvanized steel in 2017 was 44 million tons, including 25 million tons of structural steel, 15 million tons of pipes and 4 million tons of small assortment (bolts, nuts, etc.). Zinc consumption for galvanizing in China is currently 58.2% of the country’s total zinc consumption, which is higher than the world average (about 50%) [38]. It should be noted that the demand for galvanized products in China was about 200,000 tons of steel in 1982, about 300,000 tons in 1992 and about 9,000,000 tons of steel in 2002 [39]. These data show that in the last few decades, the amount of galvanized steel in China has been growing exponentially, reflecting the global increase in zinc consumption.
The galvanizing industry has a decisive influence on shaping global zinc production and market. Commonly used for galvanizing in the 80 s of the last century, metallurgical zinc Good Ordinary Brand (GOB) with a lower purity of 98.5 wt.% contained up to 1.5 wt.% impurities, including up to approx. 1.4 wt.% Pb [40]. The galvanizing bath always contained lead with a content close to the solubility limit in liquid zinc of 1.2 wt.% [41]. Lead was willingly used as an alloying additive to the bath because it lowers the surface tension of liquid zinc and improves the castability of the bath [42]. This results in better zinc flow from the surface of the product, which results in reduced zinc losses and its lower consumption during the galvanizing process. However, the presence of Pb in the bath is very controversial due to its negative impact on the environment and human health [43,44]. In the 80 s of the last century [40], the production of Special High Grade (SHG) zinc with high purity of 99.995 wt.%, in which the level of all impurities does not exceed 0.005 wt.%, significantly increased [45]. The use of GOB zinc for galvanizing systematically decreased, and SHG zinc took its place. Currently, practically only SHG zinc is used for galvanizing in Europe. Although Pb is sometimes added to baths, its content does not exceed 0.4–0.5 wt.% [46]. However, most galvanizing plants reduce or completely eliminate the addition of Pb to the bath, replacing it with the addition of Bi [47] or BiSn [48,49].
The specificity of the hot dip galvanizing process causes the efficiency of zinc used to be lower compared to other areas of zinc production and consumption. Zinc consumption depends not only on the thickness of the coating but also on the losses resulting from the specificity of the technology, i.e., the formation of hard zinc and galvanizing ashes, oxidation of the bath surface and formation of precipitations of solidified zinc. Studies have shown that one ton of zinc used in the hot dip galvanizing process generates 147.6 kg of hard zinc and 139.3 kg of zinc ashes. The average zinc content in hard zinc ranges from 94 to 96 wt.%, while the average zinc content in galvanizing ashes ranges from approximately 70 wt.%. This means that for each tone of SHG zinc delivered to the galvanizing plant, on average, approx. 240 kg of zinc is ineffectively used to create waste in the form of hard zinc and zinc ashes [49]. These are, of course, by-products rich in zinc, which is the raw material for the production of secondary zinc.
The main areas of application of zinc are the following: construction and infrastructure (66%), automotive industry and industrial machinery (28%), consumer goods and electrical appliances (23%) and general engineering (7%) (Figure 5). It should be noted that both steel structures, infrastructure and car bodies are commonly protected against corrosion by hot dip galvanizing coating. This explains such a large share of these areas of application in the global structure of zinc consumption. However, in recent years, new directions for zinc applications have emerged. Agriculture may be a growing end market for zinc applications. Zinc is currently recognized, together with potassium, as one of the most intensive organic fertilizers. It is predicted that China and India will be potential areas for the application of zinc-based fertilizers in the future [50].
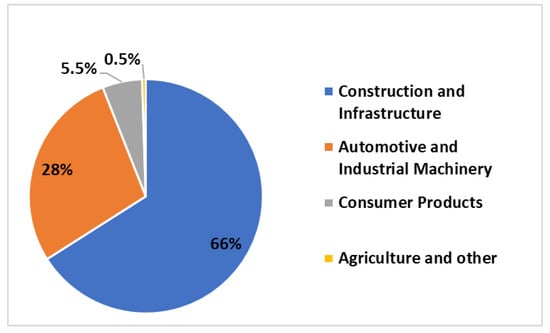
Figure 5.
Zinc end use by applications in 2018, figure prepared based on data taken from [50,51].
3. Zinc Production from Primary Resources
Of the many zinc minerals, only a few are of economic importance. Currently, up to 85% of all zinc production uses sphalerite (ZnS). Its world reserves are enough for several decades. Zinc oxide minerals are zinc carbonates, including smithsonite and hydrozincite and the zinc silicates hemimorphite and willemite. Zinc oxide ores are generally less of a metallurgical and environmental problem than zinc sulphide ores due to the absence or relatively low amounts of elements such as arsenic, cadmium, lead and sulfur. Due to the depletion of sulphide ores and the reduction of sulfur emissions during the technological process, there is an increasing interest in oxide ores, e.g., carbonates and silicates [52,53]. The first stage of obtaining zinc from such ores is the production of a concentrate by flotation or by gravity method. If sulphide or oxide raw materials are processed, an average SO2 content of 5% is obtained in the waste gases, and these gases are used in the sulfuric acid plant. When processing oxide-type raw materials, the SO2 content in the flue gas is too low (0.3–0.5 wt.%) to be used in the sulfuric acid plant, so the gases must be treated in a different way.
The next stage is the processing of the concentrate by pyrometallurgical or hydrometallurgical means. The use of the pyrometallurgical path is not suitable for the processing of low-grade oxide ore due to environmental pollution, high capital expenditures and significant energy consumption [54,55]. Pyrometallurgical methods have various disadvantages, including high energy consumption, significant capital investment and the production of harmful greenhouse gases. The advantage of hydrometallurgical processes is that, on a small scale, the process can be economical due to low capital and operating costs and the recovery of valuable metal-containing products. In addition, the hydrometallurgical processing of zinc minerals has some environmental benefits. Figure 6 presents the available methods of processing zinc ores in the world, while Table 5 presents the characteristics of the main processes used for the processing of zinc ores. Primary zinc is supplied to the market in various qualities, with the highest quality being special high-grade (SHG) zinc or Z1, which contains 99.995 wt.% zinc, followed by Z2 (99.99 wt.%), Z3 (99.95 wt.%), Z4 (99.5 wt.%); while the lowest quality good ordinary brand (GOB) or Z5 is around 98.5 wt.% purity. In addition, this zinc contains such impurities as Pb up to 1.4 wt.%, Cd up to 0.005 wt.%, Fe up to 0.05 wt.%, Sn up to 0.001 wt.%, Cu up to 0.002 wt.% and Al up to 0.001 wt.%. Simultaneously, secondary zinc grades are divided into the following three categories: ZSA (obtained mainly by zinc-bearing process scrap, e.g., Zn ashes)—98.5 wt.% with impurities such as Pb (1.3 wt.%), Cd (0.02 wt.%), Fe (0.05 wt.%) and Al (0.05 wt.%); ZS1 obtained mainly by recycling of scrap containing 98 wt.% Zn and ZS2 containing 97.5% Zn.
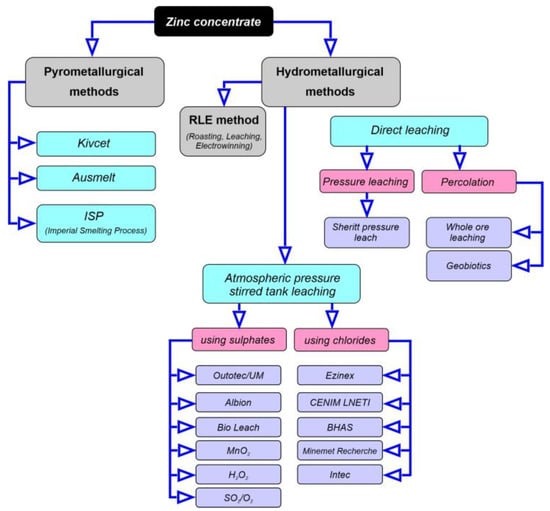
Figure 6.
Available methods of processing zinc ores, figure prepared based on data taken from [56,57].

Table 5.
Characteristics of basic methods of obtaining zinc from ores and concentrates, worked out based on data from [58,59,60,61,62,63,64,65,66,67].
4. Zinc Production from Secondary Resources
Products containing zinc, e.g., galvanized steel, become a source of raw materials for recycling (this is the so-called old scrap). These products are collected and processed based on scrap availability, alloy composition (e.g., purity, etc.) and ease of processing [68]. Currently, more and more zinc is produced, and this entails the generation of many zinc-containing wastes at various stages of its production (new scrap). Table 6 presents the formation of new and old zinc scrap at different stages of zinc production, while Figure 7 shows the material cycle in zinc recycling.

Table 6.
Sources and types of zinc scrap, worked out based on [68].
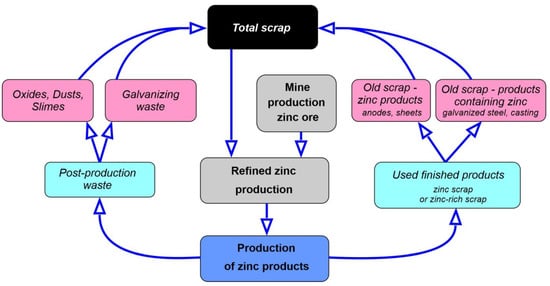
Figure 7.
Material cycle in zinc recycling, figure prepared based on data taken from [69].
The primary energy Intensity of zinc production ranges from 49 to 55 GJ/tone. Energy savings due to the recycling of steel (mainly in the case of galvanized steel) and zinc can range from 60 to 75% [57]. Worldwide, approximately 20% of zinc becomes new scrap in batch and continuous galvanizing processes.
Zinc concentrates have a relatively constant chemical composition, while the resulting zinc wastes contain zinc in varying amounts. Table 7 shows the characteristics of various zinc precipitations generated in the industry and during the production process. Galvanized steel is the main source of secondary zinc, both in the galvanizing process and in the remelting of galvanized steel. The specificity of the hot dip galvanizing process causes the efficiency of zinc use is lower compared to other areas of zinc production and consumption. Zinc consumption depends not only on the thickness of the coating but also on the losses resulting from the specificity of the technology, i.e., the formation of hard zinc and galvanizing ashes, oxidation of the bath surface and formation of precipitations of solidified zinc. Studies have shown that one ton of zinc used in the hot dip galvanizing process generates 147.6 kg of hard zinc and 139.3 kg of zinc ashes. The average zinc content in hard zinc ranges from 94 to 96 wt.%, while the average zinc content in galvanizing ashes ranges from approximately 70 wt.%. This means that for each tone of SHG zinc delivered to the galvanizing plant, on average, approx. 240 kg of zinc is ineffectively used to create waste in the form of hard zinc and zinc ashes [49]. These are, of course, by-products rich in zinc, which is the raw material for the production of secondary zinc.

Table 7.
Characteristics of various zinc wastes generated in the industry and during the production process, worked out based on [57,71,72].
Zinc oxidation on the surface of the galvanizing bath creates zinc oxide (galvanizing ash) contaminated with flux decomposition products. The need to clean the surface of the bath before each immersion and the emergence of the charge generates a significant amount of galvanizing ashes. Ensuring a high-quality coating requires continuous cleaning of the bath surface and periodic removal of galvanizing ashes from it. During the collection of ashes, it is also inevitable that significant amounts of “pure” zinc will be removed from the surface. Galvanizing ashes are, therefore, a zinc-rich waste. During galvanizing, the iron is dissolved in the bath. The source of bath contamination with iron is galvanized products, but also the dissolving galvanizing kettle and flux contaminated with iron chloride. After the zinc bath reaches the state of saturation with iron (0.03 wt.% at 450 °C), excess iron reacts with liquid zinc and is released in the form of fine particles of intermetallic phases δ1 (FeZn7) or ζ (FeZn13) forming the so-called hard zinc. When the galvanizing bath contains an Al additive above 0.14 wt.% (continuous galvanizing), the reaction of Al with dissolved iron leads to the formation of particles of the Fe2Al5Znx phase. Hard zinc, which can be identified as surface dross (mainly Fe2Al5Znx) and bottom dross (mainly δ1 (FeZn7) or ζ (FeZn13) phase), hinders the galvanizing process and requires periodic removal—the surface dross is skimmed off by labor or machine at intervals, while the bottom dross can be removed when the operation is shut down. One novel efficient method called the supergravity method for recovering zinc from galvanizing dross has been proposed by the University of Science and Technology Beijing in China [70].
The hot dip galvanizing process also provides zinc-rich waste as a residue from the purification of finished products. Zinc thickening and drips formed on the surface are removed by mechanical grinding, and the removed zinc is re-melted. Zinc-rich wastes are also zinc sludges formed when wet fluxing is used. However, wet galvanizing is now much less frequently used.
Another important source of zinc is electric arc furnace (EAF) dust. It is estimated that the EAF furnace generates 15–25 kg of dust per ton of raw steel, the zinc content varies from 10 to 40%. The minimum zinc content necessary for the process of zinc recovery from steelmaking dust to be profitable depends on various factors, such as the proximity of the steelworks, energy costs, slag cost; however, it should be at the level of 15% [73]. Zinc is also contained in steelmaking dust from the converting process (BOF). However, the content of zinc in it varies between only 0.5 and 6%. This value is too small for the profitable recovery of zinc from these dusts. Zinc is not recovered from the BOF process dusts; the suggested solution, in this case, is to collect galvanized sheet scrap and send it to the EAF process or to dezincify steel scrap [74,75,76].
The hydrometallurgical production of zinc produces deposits and leaching and purification residues. In the production of rayon fiber yarn, zinc is used as a modifier for cellulose regeneration. After the rayon is made, it is washed and the solution contains 30–50 ppm of zinc, which precipitates in the form of zinc hydroxide [71].
Pyrometallurgical methods used to treat secondary zinc waste include the following: electrothermic retort processes (ERP), Imperial Smelting Process (ISP) and Waelz furnace. Hydrometallurgical processes include the following: molecular recognition technology (MRT), EZINEX process, Zincex process, CASHMAN process, sulfuric acid leaching process, chloride leaching process, chloride–sulfate leaching process, alkaline leaching process, crystallization and solvent extraction [77]. Table 8 presents the basic characteristics of the mentioned processes. One of the most commonly used processes for recycling today is the Waelz process. The recovery of zinc from this process is 91 to 93% [78], and the remaining 7–9% remains in the slag. This slag is directed to landfills. The product obtained in the rolling furnace is zinc oxide, which is the raw material for the process of obtaining metallic zinc in the ISP and solvent extraction-electrowinning processes, while up to 20% of the charge in the roasting–leaching–electrowinning (RLE) process. The use of zinc oxide as a feedstock in zinc smelters has been increasing in recent years; in 2016, it amounted to 878 thousand tons, while in 2019 already 1448 thousand tons [78]. This indicates an average annual increase in the use of ZnO at the level of 20% [36,38].

Table 8.
Characteristics of metallurgical methods for processing secondary zinc waste [77,79,80,81,82].
All galvanized scrap, regardless of grade, is now recycled in the electric arc furnace. Currently, many methods are also used to recover zinc from the waste generated in the hot dip galvanizing process. An economic method of removing the zinc coating before re-melting the galvanized scrap is also being sought. This is not a new concept. Tin-plated steels are regularly subjected to a metal removal process prior to the recycling of tin-plated products. In fact, some original dezincification solutions were based on industrial processes for removing tin coatings [83]. Tin-coated steel is usually recycled in an alkaline detinning process, leaving the steel substrate to be re-melted.
The conducted studies on the recycling of galvanized steel scrap mainly concern alkaline dezincification, i.e., zinc dissolution by hot caustic soda (NaOH) to the form of sodium zincates (Na2ZnO2 and NaHZnO2)—Figure 8a. Zinc is recovered electrolytically in the form of powder, while uncoated steel is readily used for re-melting [84]. Spontaneous alkaline dissolution of zinc (without heating or using an external electric current source) is thermodynamically possible but kinetically limited [85,86]. Dissolution will only occur when the zinc is in electrical contact with a metal with a lower hydrogen overpotential, such as platinum, iron, or nickel, or if a strong oxidizing agent is added. Recently, acid dezincification has been quite popular—Figure 8b. Typically very low concentrations of acid make the process safe, and the dissolution reaction occurs spontaneously, without the need for heating or the use of an external source of electricity. The advantage of using dilute acids is the complete dissolution of the zinc coating with little or no attack on the steel substrate, which is eventually recycled while the zinc is recovered from the solution either in metallic form or as a metal salt depending on the type of acid used.
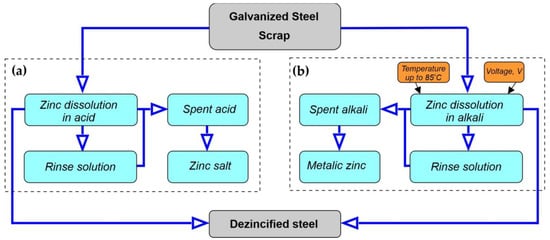
Figure 8.
Scheme: (a) acidic and (b) alkaline dezincification of galvanized products.
The following reactions occur in the acid dezincification process:
- Zinc dissolution:
- Hydrogen release:
- Formation of zinc salts:
In the alkaline dezincification process, the following reactions take place:
- Oxygen reduction:
- Formation of zinc hydroxide:
- Formation of zinc salts:
In the case of the acid dezincification process, it was found that more concentrated acids (more than 10%) tend to accelerate the dezincification rate but also have an intense effect on the dissolution of the steel substrate. Dilute acids (2% or less) reduce the rate of dissolution but tend to dezincify partially or even ineffectively [84].
To sum up, in hydrometallurgical processes, valuable metals with a high mass fraction can be obtained. The energy consumption of the processes is low due to their low temperature. Unfortunately, these methods require high corrosion resistance of the devices. Slag obtained after leaching is also problematic due to its complex composition, and it cannot meet the storage requirements of environmental protection law. Pyrometallurgical methods are also used for dezincification (e.g., in Japan, there are two plants based on this solution), but they are much more expensive. They focus on steel scrap, and the recovery of zinc products is practically low [87]; what is more, these processes are highly energy-intensive and require large equipment.
Figure 9 shows the flow of recycled zinc, taking into account the hot dip galvanizing process and finished products withdrawn from circulation [88]. Currently, about 70% of the zinc comes from primary refining of zinc ores (including 10–15% from recycling sources), and about 30% comes directly from recycled zinc (accounting for 80% of the zinc available for recycling). The long life of galvanized steel products in the construction industry makes predicting their appearance in waste streams difficult.
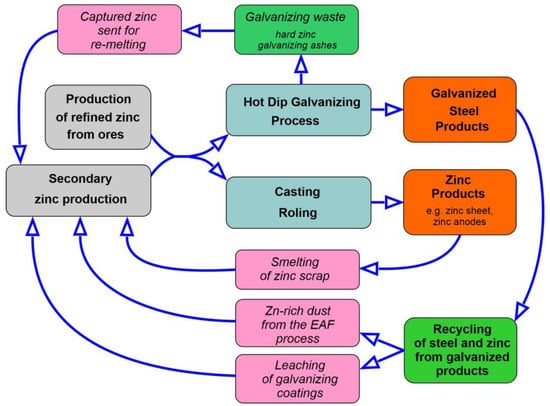
Figure 9.
Recycling zinc flow, including the hot dip galvanizing process and finished products withdrawn from circulation.
In the case of zinc, the following two recycling rates are used: end of life (EOL) and recycled content (RC). The EOL indicator offers a very comprehensive approach by taking into account recycling efficiency, product lifetime and historical production patterns; quantifies the value of the zinc actually recovered at the end of the product’s life (‘old scrap’) and recycled into new metallic zinc. It is estimated that around 45% of the available zinc at the end of the life of various zinc-containing products is recovered and actually recycled (Table 9). In developed regions such as Europe and North America, the EOL rate may be 50% or greater. For developing regions, the rate is 30–40% due to the lack of developed recycling networks and regulatory initiatives aimed at reducing industrial waste [71]. Recycled content RC is a measure of the recycled content in a product. The recycled content for refined zinc is currently around 8% (scrap combined with concentrate as raw material in the smelter).

Table 9.
Recycling process indicators, worked out based on [71].
Table 10 shows the production of zinc, taking into account the method of obtaining it. It can be seen that, over the years, more attention has been paid to the amount of recycled zinc. However, the lifetime of zinc-containing products is variable. In particular, this applies to galvanized products, the specificity of which uses zinc to extend their durability. The durability of zinc coating protection depends on the needs of the market and the application. Then the service life may be from 10 to 15 years in the case of, for example, the service life of cars or household appliances. These products are scrapped due to the wear of other elements and subassemblies, although the zinc in these products still fulfills its protective role. The situation is different in the case of anti-corrosion protection of steel structures and products operated in an atmospheric environment with different corrosive aggressiveness. The aggressiveness of the atmospheric environment depends on many factors, e.g., temperature, humidity, and content of aggressive components, i.e., chlorides, sulfur compounds, weather factors, etc. The aggressiveness of the natural atmospheric environment may change over time. Then the durability of galvanized elements ranges from several to even 100 years and is difficult to predict.

Table 10.
Total zinc production broken down into primary and secondary zinc production and non-differentiated production in thousands of tons, based on data from [89,90,91].
5. Summary
Zinc can be produced from primary raw materials by pyrometallurgical or hydrometallurgical methods. Pyrometallurgical methods are still used in different parts of the world but gradually lose their importance and are no longer used, e.g., in the EU for the treatment of zinc concentrates, except in Poland. The main factors determining this are the need for an additional distillation step in order to obtain high-quality zinc and the relatively low efficiency of zinc extraction. In hydrometallurgical methods, there are problems with the emission of residual sulfur dioxide, NOX and acidic effluents and the release of metals such as arsenic, mercury and cadmium, and additionally organic pollutants. Therefore, emissions to air and water, waste management and energy considerations are among the key factors influencing the choice of techniques used in the processing of zinc-bearing ores.
Currently, around 30–35% of the zinc consumed annually comes from recycling. Production of zinc from secondary sources brings a number of measurable benefits, such as saving primary resources and fossil resources used to provide energy in primary mining processes, reducing zinc storage and losses (maintenance of landfills and increasing the amount of waste cost a lot), reclamation of waste, mitigation of environmental effects and health, and improving the economic efficiency of existing infrastructure. Dust, metal particles, chemicals and harmful gases are released into the environment during the zinc mining and refining process. Although companies try to keep emissions to a minimum, the process still harms the environment. The recycling process is also safer and cheaper. Recycling rates are increasing with advances in zinc production and zinc recycling technologies. However, it is estimated that this level will not increase rapidly due to the large dispersion of zinc products and the problematic collection of zinc scrap.
For the recycling process, one of the problems is the service life of the products, which is relatively long for steel, e.g., bridges, and steel structures that are in constant use. At present, the service life of zinc-coated steel products is extended. The long service life of products, as well as changes in the corrosiveness of atmospheric environments at that time, make the life of products less predictable. Both in the case of zinc and steel itself, the amount of scrap for secondary production may change, and shortages are observed periodically. In this case, primary production from ores and concentrates is based. Both steel and zinc are 100% recyclable with no loss of chemical or physical properties. For steel, it is estimated that virtually 100% of all structural steel is recovered for reuse; the same index for zinc is over 80%. Galvanized steel can be easily recycled with other scrap steel in the electric arc furnace (EAF) steelmaking process. Zinc evaporates in the initial phase of the process and is collected in EAF dust, which is then recycled at specialist facilities and often returned to the production of refined zinc. More problematic are the dusts from the converting process (BOF), where the zinc content is up to 6%, which is below the profitability of their recovery. Currently, the International Zinc Association (IZA) has developed models to quantify zinc recycling rates to demonstrate the recyclability of products and the effectiveness of recycling programs. However, one proposed solution for galvanized products is to collect them, and use acid or alkali dezincification as a pre-treatment, and then direct the cleaned steel scrap to an electric furnace or converter.
The level of recycling of zinc from ashes and hard zinc after galvanizing is currently high, but there is still a problem related to the collection and sorting of used zinc or galvanized items. In the future, this will be a necessity in some industry segments, but at the same time offers opportunities to improve zinc recycling rates. The most obvious opportunity to improve recycling rates is in developing regions, where advanced recycling networks and infrastructure investments continue to expand. The International Zinc Association constantly monitors the efficiency of zinc recycling and on the basis of material flow nalysis (MFA), characterizes the life cycle of products containing zinc in various applications. However, one of the problems is still the lack of regulations to encourage the collection and processing of scrap metal. Currently, with the high volatility of the price of this metal, as well as the dwindling natural resources of zinc in the earth’s crust, this is a necessary course of action.
To sum up, the issue of zinc recovery from waste materials gives significant environmental and economic effects compared to primary production from ores. However, it faces also many problems related to the preparation of waste materials, the content of zinc in them, and the content of other metals in them, which results in solving the problems of complex structure and correlation of metal-containing phases or re-oxidation of non-ferrous metals after reduction. However, due to the increasing demand for zinc and the decreasing resources of this metal, the production of zinc from waste materials becomes a necessity, and the above-mentioned problems are important issues to be solved.
Author Contributions
Conceptualization, M.S. and H.K.; formal analysis, H.K. and M.S.; investigation, H.K. and M.S.; resources, H.K.; writing—original draft preparation, M.S.; writing—review and editing, H.K.; visualization, H.K.; supervision, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sverdrupa, H.U.; Olafsdottira, A.H.; Ragnarsdottir, K.V. On the long-term sustainability of copper, zinc and lead supply, using a system dynamics model. Resour. Conserv. Recycl. 2019, 4, 100007. [Google Scholar] [CrossRef]
- Ragnarsdottir, K.V.; Sverdrup, H.; Koca, D. Substitution of metals in times of potential supply limitations: What are the mitigation options and limitations? In In Proceedings of the 2015 World Resources Forum, Davos, Switzerland, 11–15 September 2015. [Google Scholar]
- Graedel, T.E.; Erdmann, L. Will metal scarcity impede industrial use? Met. Res. Soc. Bull. 2012, 37, 325–333. [Google Scholar] [CrossRef]
- Available online: https://www.livescience.com/29378-zinc.html (accessed on 24 August 2021).
- Available online: https://www.spotlightmetal.com/history-deposits-and-production-of-zinc-a-917750/ (accessed on 24 August 2021).
- Available online: https://www.rsc.org/periodic-table/element/30/zinc (accessed on 25 August 2021).
- Gandhi, S.M. Ancient mining and metallurgy in Rajasthan. In Crustal Evolution and Metallogeny in the Northwestern Indian Shield: A Festschrift for Asoke Mookherjee; Deb, M., Ed.; Alpha Science Int. Ltd.: Oxford, UK, 2000; pp. 29–51. ISBN 1-84265-001-7. [Google Scholar]
- Emsley, J. Nature’s Building Blocks: An A-Z Guide to the Elements; Oxford University Press: Oxford, UK, 2001; pp. 499–505. ISBN 978-0-19-850340-8. [Google Scholar]
- Giachi, G.; Pallecchi, P.; Romualdi, A.; Ribechini, E.; Lucejko, J.J.; Colombini, M.P.; Mariotti Lippi, M. Ingredients of a 2000-y-old medicine revealed by chemical, mineralogical, and botanical investigations. Proc. Natl. Acad. Sci. USA 2013, 110, 1193–1196. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.britannica.com/science/zinc/History (accessed on 25 August 2021).
- Marggraf, J. Experiments on a way of extracting zinc from its true mineral, i.e., the stone calamine. Hist. L’académie R. Sci. Belles-Lett. Berl. 1746, 2, 49–57. (In French) [Google Scholar]
- Weeks, M.E. III. Some Eighteenth-Century Metals. The Discovery of the Elements. J. Chem. Educ. 1932, 9, 22. [Google Scholar] [CrossRef]
- Habashi, F. Zinc—The Metal from the East. Bull. Can. Inst. Min. Met. 2001, 94, 71–76. [Google Scholar]
- Wilczok, E. 150 lat Hutnictwa Metali Nieżelaznych w Szopienicach; Zakłady Graficzne: Katowice, Poland, 1984. (In Polish) [Google Scholar]
- Ostrowska-Popielska, P.; Sorek, A. Przegląd i wstępny dobór technologii odzysku cynku ze szlamów i pyłów stalowniczych. Pr. Inst. Metal. Żelaza 2012, 64, 39–46. (In Polish) [Google Scholar]
- Grandell, L.; Höök, M.; Höök, M. Assessing Rare Metal Availability Challenges for Solar Energy Technologies. Sustainability 2015, 7, 11818–11837. [Google Scholar] [CrossRef]
- Pehrson, E.W. Summarized Data of Zinc Production; United States Government Printing Office: Washington, DC, USA, 1929; pp. 1–54. [Google Scholar]
- Available online: https://www.spglobal.com/platts/en/market-insights/latest-news/metals/031021-global-zinc-demand-to-outpace-production-growth-in-2021-narrowing-surplus-ugmk (accessed on 25 August 2021).
- Available online: https://pubs.usgs.gov/periodicals/mcs2021/mcs2021-zinc.pdf (accessed on 25 August 2021).
- Plachy, J. Zinc. U.S. Geological Survey Minerals Yearbook; U.S. Geological Survey: Washington, DC, USA, 2000; pp. 86.1–86.8. [Google Scholar]
- Available online: https://www.usgs.gov/centers/nmic/zinc-statistics-and-information (accessed on 25 August 2021).
- Plachy, J. Zinc. U.S. Geological Survey—Minerals Information; Mineral Commodity Summaries; U.S. Geological Survey: Washington, DC, USA, 1996. [Google Scholar]
- Bi, X. Zinc, U.S. Geological Survey Minerals Yearbook—2005; U.S. Geological Survey: Washington, DC, USA, 2007; pp. 84.1–84.15. [Google Scholar]
- Thomas, C.L. Zinc in August 2020; U.S. Department of the Interior, U.S. Geological Survey: Washington, DC, USA, 2020; pp. 85.1–85.15. [Google Scholar]
- Tolcin, A.C. Zinc Statistics and Information; U.S. Department of the Interior, U.S. Geological Survey: Washington, DC, USA, 2012; pp. 84.1–85.11. [Google Scholar]
- Garside, M.; Global Zinc Reserves by Country 2020. 18 February 2021. Available online: https://www.statista.com/statistics/273639/global-zinc-reserves-by-country/ (accessed on 29 August 2021).
- Available online: http://www.egga.com/wp-content/uploads/2014/06/5.-Recycling_material_supply.pdf (accessed on 27 August 2021).
- Lead and Zinc Study Group (ILZSG). Available online: https://www.ilzsg.org/static/statistics.aspx (accessed on 27 November 2022).
- Available online: https://s3-us-west-2.amazonaws.com/prd-wret/assets/palladium/production/mineral-pubs/zinc/mcs-2011-zinc.pdf (accessed on 30 August 2021).
- Available online: https://s3-us-west-2.amazonaws.com/prd-wret/assets/palladium/production/mineral-pubs/zinc/720301.pdf (accessed on 30 August 2021).
- Available online: https://s3-us-west-2.amazonaws.com/prd-wret/assets/palladium/production/mineral-pubs/zinc/zinc_mcs96.pdf (accessed on 30 August 2021).
- Available online: https://knoema.com/ftmgyvg/zinc-prices-forecast-long-term-2018-to-2030-data-and-charts (accessed on 30 August 2021).
- Available online: https://tradingeconomics.com/commodity/zinc (accessed on 1 September 2021).
- London Metal Exchange (LME). Available online: https://www.lme.com/Metals/Non-ferrous/LME-Zinc#Price+graphs (accessed on 27 November 2022).
- Available online: https://www.essentialchemicalindustry.org/metals/zinc.html (accessed on 1 September 2021).
- European General Galvanizers Association (EGGA). Statistical Report 2019; EGGA: Sutton Coldfield, UK, 2020. [Google Scholar]
- American Galvanizers Association (AGA). Available online: https://galvanizeit.org/about-aga/industry/industry-stats (accessed on 28 November 2020).
- Van Leeuwen, M. Recent developments in the global zinc and HDG markets. In In Proceedings of the 25th Hot Dip Galvanizing Conference, České Budějovice, Czech Republic, 2–4 October 2019; pp. 23–29. [Google Scholar]
- Smale, D. Zinc in galvanizing: A world view, with special reference to China. In In Proceedings of the 21st International Galvanizing Conference, INTERGALVA 2006, Naples, Italy, 12–14 June 2006; pp. 1–6. [Google Scholar]
- Porter, F.C. Zinc Handbook: Properties Processing and Use in Design; Marcel Dekker: New York, NY, USA, 1991. [Google Scholar]
- Moser, Z.; Zabdyr, L.; Gasior, W.; Salawa, J.; Zakulski, W. The Pb-Zn (Lead-Zinc) System. J. Phase Equilibria 1994, 15, 643–649. [Google Scholar] [CrossRef]
- Krepski, R.P. The influence of lead in after-fabrication hot dip galvanizing. In In Proceedings of the 14th International Galvanizing Conference, London, UK, 6–12 June 1986; Zinc Development Association: London, UK, 1986; p. 6/6–6/12. [Google Scholar]
- Beguin, P.; Bosschaerts, M.; Dhaussy, D.; Pankert, R.; Gilles, M. Galveco a solution for galvanizing reactive steel. In In Proceedings of the 19th International Galvanizing Conference, INTERGALVA 2000, Berlin, Germany, 1–8 March 2000; EGGA: Berlin, Germany, 2000. [Google Scholar]
- Reumont, G.; Perrot, P. Fundamental Study of Lead Additions in Industrial Zinc. In In Proceedings of the 18th International Galvanizing Conference, Birmingham, UK, 8–11 June 1997; EGGA: Birmingham, UK, 1997. [Google Scholar]
- EN 1179:2003; Zinc and Zinc Alloys; Primary Zinc, CEN: Brussels, Belgium, 2003.
- Kania, H.; Mendala, J.; Kozuba, J.; Saternus, M. Development of bath chemical composition for batch hot-dip galvanizing-A Review. Materials 2020, 13, 4168. [Google Scholar] [CrossRef]
- Kania, H.; Saternus, M.; Kudláček, J.; Svoboda, J. Microstructure characterization and corrosion resistance of zinc coating obtained in a Zn-AlNiBi galvanizing bath. Coatings 2020, 10, 8–758. [Google Scholar] [CrossRef]
- Kania, H.; Saternus, M.; Kudláček, J. Impact of Bi and Sn on microstructure and corrosion resistance of zinc coatings obtained in Zn-AlNi bath. Materials 2020, 13, 3788. [Google Scholar] [CrossRef]
- Langill, T. North American Galvanizers process Survery. In In Proceedings of the 19th International Galvanizing Conference, INTERGALVA 2000, Berlin, Germany, 1–8 March 2000; EGGA: Berlin, Germany, 2000; pp. 1–7. [Google Scholar]
- Available online: https://investingnews.com/daily/resource-investing/base-metals-investing/zinc-investing/zinc-applications-zinc-demand/ (accessed on 1 September 2021).
- Zinc Market Overview, Nexa Investor Meeting, October 2018. Available online: https://www.annualreports.com/HostedData/AnnualReportArchive/n/TSX_NEXA_2018.pdf (accessed on 1 December 2022).
- Kaksonen, A.H.; Boxall, N.J.; Gumulya, Y.; Khaleque, H.N.; Morris, C.; Bohu, T.; Lakaniemi, A.M. Microwave roasting and leaching of an oxide-sulphide zinc ore. Hydrometallurgy 2016, 166, 243–251. [Google Scholar] [CrossRef]
- Feng, L.; Yang, X.; Shen, Q.; Xu, M.; Jin, B. Pelletizing and alkaline leaching of powdery low grade zinc oxide ores. Hydrometallurgy 2007, 89, 305–310. [Google Scholar] [CrossRef]
- Choi, W.; Torma, A.; Ohline, R.; Ghali, E. Electrochemical aspects of zinc sulphide leaching by Thiobacillus Ferrooxidans. Hydrometallurgy 1993, 33, 137–152. [Google Scholar] [CrossRef]
- Soltani, F.; Darabi, H.; Aram, R.; Ghadiri, M. Leaching and solvent extraction purification of zinc from Mehdiabad complex oxide ore. Sci. Rep. 2021, 11, 1566. [Google Scholar] [CrossRef]
- Fuls, H.; Petersen, F.J. Evaluation of Processing Options for The Treatment Of Zinc Sulphide Concentrates At Skorpion Zinc—Synopsis. J. South. Afr. Inst. Min. Metall. 2013, 113, 423–434. [Google Scholar]
- Grogan, J. Dezincing of Galvanized Steel. Ph.D. Thesis, Colorado School of Mines, Golden, CO, USA, 2015. [Google Scholar]
- Available online: https://www.sciencedirect.com/topics/engineering/hydrometallurgy (accessed on 29 August 2021).
- Chunhua, Y.; Bei, S. Emerging Methodologies and Applications in Modelling, Identification and Control 2021. In Modeling, Optimization, and Control of Zinc Hydrometallurgical Purification Process; Chapter 1–|; Academic Press: Cambridge, MA, USA; pp. 3–14.
- Ozberk, E.; Jankola, W.A.; Vecchiarelli, M.; Krysa, B.D. Commercial operations of the Sherritt zinc pressure leach process. Hydrometallurgy 1995, 39, 49–52. [Google Scholar] [CrossRef]
- Maccagni, M.G. INDUTEC®/EZINEX® Integrate Process on Secondary Zinc-Bearing Materials. J. Sustain. Metall. 2016, 2, 133–140. [Google Scholar] [CrossRef]
- Robinson, D.J.; MacDonald, S.A.; Olper, M. Design details of engitec “EZINEX” electrowinning plant. In CIM Conference on Electrometallurgy; Gonzalez, J.A., Dutrizac, J.E., Kelsall, G.H., Eds.; CIM Conference: Toronto, ON, Canada, 2001; pp. 45–56. [Google Scholar]
- Available online: http://www.engitec.com/en/zinc_and_its_alloys/ (accessed on 1 September 2021).
- Masters, L.B. The Sherritt zinc pressure leach process: Integration applications and opportunities; Researchgate: Vancouver, WA, Canada, 2010; pp. 487–504. [Google Scholar]
- Wood, J.; Coveney, J.; Helin, G.; Xincheng, S. The Outotec ® Direct Zinc Smelting Process. In In Proceedings of the Conference: European Metallurgical Conference 2015, Dusseldorf, Germany, 14–17 June 2015. [Google Scholar]
- Available online: https://www.totalmateria.com/page.aspx?ID=CheckArticle&site=ktn&NM=366 (accessed on 5 September 2021).
- Bernasowski, M.; Klimczyk, A.; Stachura, R. Overview of Zinc Production in Imperial Smelting Process. Available online: https://www.researchgate.net/publication/322386767_Overview_of_Zinc_Production_in_Imperial_Smelting_Process (accessed on 5 September 2021).
- Zinc Recykling–Cloosing Loop, 2011–2015 International Zinc Association. Available online: www.zinc.org (accessed on 1 September 2021).
- Al Sharif, E.S. Recycled zinc: A possible substitute for pure refined zinc? Galvanizing & Coil Coating Conference. Abu Dhabi, United Arab Emirates, 10–11 September 2014; 10–11 September 2014. [Google Scholar]
- Wang, Z.; Gao, J.; Meng, L.; Shi, A.; Guo, Z. Recovery of Zinc from Zn–Al–Fe Melt by Super-gravity Separation. ISIJ Int. 2018, 58, 1175–1177. [Google Scholar] [CrossRef]
- Jha, M.; Kumar, V.; Singh, R.J. R. Review of hydrometallurgical recovery of zinc from industrial wastes. Resour. Conserv. Recycl. 2001, 33, 1–22. [Google Scholar] [CrossRef]
- Sahu, K.K.; Agrawal, A.; Pandey, B.D. Recent trends and current practices for secondary processing of zinc and lead. Part II: Zinc recovery from secondary sources. Waste Manag. Res. 2004, 22, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Southwick, L. Zinc Recovery from Electric Arc Furnace (EAF) Dust; Worldwide Survey, ILZSG: Lisbon, Portugal, 2015. [Google Scholar]
- Ma, N. Toward 100% recycling of steelmaking offgas solid waste by reallocating zinc-bearing materials. In Energy Technology 2020: Recycling, Carbon Dioxide Management and Other Technologies; Chen, X., Zhong, Y., Zhang, L., Howarter, J.A., Baba, A.A., Wang, C., Sun, Z., Zhang, M., Olivetti, E., Luo, A., Eds.; TMS Series; Springer: Berlin/Heidelberg, Germany; pp. 159–168.
- Grogan, J.; Martins, G.M.; Anderson, C.G. Dezincing of galvanized steel by sulpuric acid leaching. In Extraction 2018; Davies, B., Moats, M., Wang, S., Gregurek, D., Kapusta, J., Battle, T., Schlesinger, M., Flores, G.R.A., Jak, E., Goodall, G., Eds.; TMS Series; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1733–1742. [Google Scholar]
- Alam, S.; Lakshmanan, V.I.; Sridhar, R. Recykling of zinc from galvanized steel scrap. In PbZn 2020: 9th international Symposium on Lead and Zinc Processing; Siegmund, A., Alam, S., Grogan, J., Kerney, U., Shibata, E., Eds.; TMS, 2020; Springer: Berlin/Heidelberg, Germany, 2020; pp. 821–824. [Google Scholar]
- Olabiyi, O. Simulation and Modelling of Zinc Recovery Process from Steel Scrap. Master’s Thesis, Department of Chemical and Biological Engineering, University of Saskatchewan, Saskatoon, SK, Canada, October 2020. [Google Scholar]
- Van Leeuwen, M. Increasing the circularity of zinc–pathways to closing the loop for zinc. In In Proceedings of the 26th Hot Dip Galvanizing Conference, Valec, Czech Republic, 21–23 September 2021; pp. 21–31. [Google Scholar]
- Zunkel, D. What to do with your EAF dust. Steel Times Int. 1996, 20, 46. [Google Scholar]
- Available online: https://www.onemine.org/document/abstract.cfm?docid=33171&title=Josephtown-Electrothermic-Zinc-Smelter-Of-St-Joe-Minerals-Corporation-Monaca-Pennsylvania (accessed on 9 September 2021).
- Díaz, G.; Martín, D. Modified Zincex process: The clean, safe and profitable solution to the zinc secondaries treatment. Resour. Conserv. Recycl. 1994, 10, 43–57. [Google Scholar] [CrossRef]
- Available online: https://ddtp.tecnicasreunidas.es/wp-content/uploads/2016/11/P-ZINCEX-technology-information-II.pdf (accessed on 5 September 2021).
- Pflaum, D.A. The scrap industry’s perpective on recyling coated scrap. In Steelmaking Conference Proceedings; SCP: Toronto, ON, Canada, 1992; Volume 75, pp. 713–715. [Google Scholar]
- Ijomah, M.N.C.; Ijomah, A.I. Chemical recycling of galvanized steel scrap. Indian J. Chem. Technol. 2003, 10, 159–165. [Google Scholar]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; NACE International Press: Houston, TX, USA, 1974. [Google Scholar]
- Fontana, M.G.; Grene, N.D. Corrosion Engineering; McGraw-Hill Press: New York, NY, USA, 1967. [Google Scholar]
- Gock, E.; Vogt, V.; Schonfelder, I.; Carlowitz, O.; Zeller, T.; Sauter, A.; Pilkahn, H. Process for the Acid Pre-Dezincification of Steel Scrap. In In Proceedings of the XXVI International Mineral Processing Congress, New Delhi, India, 24–28 September 2012. No. 729. [Google Scholar]
- Available online: https://www.galvanizing.org.uk/sustainable-construction/zinc-is-sustainable/zinc-recycling/ (accessed on 15 September 2021).
- Available online: https://www.usgs.gov/centers/nmic/zinc-statistics-and-information,myb1-2017-zinc%20(2).pdf (accessed on 18 September 2021).
- Available online: https://s3-us-west-2.amazonaws.com/prd-wret/assets/palladium/production/mineral-pubs/zinc/myb1-2011-zinc.pdf (accessed on 18 September 2021).
- Available online: https://s3-us-west-2.amazonaws.com/prd-wret/assets/palladium/production/mineral-pubs/zinc/zincmyb01.pdf (accessed on 18 September 2021).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).