Abstract
The number of deaths due to malignant neoplasms is increasing year by year. For this reason, new ways of preventing them and supporting treatment are being sought. One of them is adding plant extracts to food to increase its antioxidant, anti-inflammatory and anti-cancerogenic activity. The aim of the study was to examine the effect of different wild-grown fruits (chokeberry, elderberry, hawthorn and sea-buckthorn) added to wheat-flour cookies on the proliferation of: (i) normal BJ lines (fibroblasts); (ii) tumor cells of the MCF-7 (breast cancer) and (iii) WM793 (melanoma) lines. Methanol-acetone extracts were prepared from previously baked wheat-flour cookies fortified with fruits in order to use them in the further part of the research to prepare mixtures with concentrations of 0.5 mg/mL; 1 mg/mL; 1.5 mg/mL; 2.5 mg/mL. The viability and cytotoxicity of normal and neoplastic cells was examined. It was observed that the WM793 melanoma tumor line appeared to be more susceptible to the action of the tested extracts with the addition of selected wild-grown fruits compared to MCF7 breast cancer cells. Moreover, the greatest significant effect on the inhibition of WM793 cells among extracts with a concentration of 2.5 mg/mL was proved in the case of sea-buckthorn (p < 0.05). In terms of the inhibition of the MCF7 line, the effect was proved only in the case of sea buckthorn (p < 0.05), while the viability of these neoplastic cells was at most affected by elderberry and chokeberry extracts (p < 0.05).
Keywords:
wheat-flour cookies; wild grown fruits; cytotoxicity; viability; WM793; MCF7; cancer cells; melanoma; breast cancer 1. Introduction
Cookies made from wheat have gained popularity as a snack food because of their several advantages, such as their ready-to-eat and storable convenience, their wide availability in various forms and their low price. In time, there is a constant increasing tendency in the consumption of this range of food products together with changing consumer preferences and awareness about the strong correlation between improper eating habits and the development of diet-related diseases, including some type of cancers [1,2]. Considerable research over the last 35 years has shown that rates of several forms of cancers are consistently linked to certain aspects of diet [2]. In order to meet the growing demands of the consumers, there has been a tendency to fortify confectionery products with fruit ingredients in order to improve their functional properties, enrich them with fiber, vitamins and minerals, reduce the use of artificial food preservatives and improve health-promoting characteristics [2,3]. Therefore, the food industry introduces modernized products into the market, enriched with plant and plant by-products. So far in the literature the enrichment of cakes and cookies with different fruits and fruit by-products has been proposed, such as: apple pomace, berry, sour cherry pomace, mango peel, banana peel, green banana flour, pumpkin and carrot pomace, pomegranate peel powder, grapefruit and citrus fiber, potato peel, watermelon rind and recently with wild species of chokeberry, elderberry, hawthorn, rowan or sea-buckthorn [3]. Especially, the last ones seem to have a great promising application in the recommended sustainable consumption pattern for the planet’s good and human health. Different wildly grown fruits can be gathered locally, especially from unpolluted rural areas, used as a source of the natural bioactive components, such as anthocyanins, organic acids, pectins (mainly protopectin), vitamins (A, C, P and B vitamins), folic acid, mineral salts or polyunsaturated fatty acids (α-linolenic, linoleic and oleic acid) [3], in order to fulfill the lack of some essential amino acids in other food products such as cereals by delivering a high quality proteins (sea-buckthorn fruits, elderberry) [4] or to help to reduce the overall meat consumption. It is well known and evidenced in the literature that regular consumption of fresh fruits, vegetables and whole grains is beneficial in reducing the likelihood of cancer as a preventive agent. Combining chemotherapy with complementary and alternative medicine has created a new approach to cancer treatment that has led to more holistic approaches [5,6]. Prevention of cancer is also of great importance.
In the available literature, the anticancer and antitumor properties of the selected wildly grown berries have been documented to a greater or less extent. For instance, black chokeberry has been used to treat breast cancer, colon cancer, and leukemia, with some selective effects on cancer stem cells [7]. Concerning anticancer properties of elderberry fruits, in vitro experiments have demonstrated the potential of elderberry extract from flowers or its components on hormone production and receptor expression of trophoblast tumor cells [8], on bladder cancer cells and fibroblasts [9], as well as the influence of cyanidin-3-glucoside in attenuation the angiogenesis of breast cancer [10]. However, more data is still needed, especially on the fruit extracts from elderberry on the propagation and viability of different cancer cells. In the case of sea-buckthorn, many studies have shown that its bioactive components may have anticancer activity against colon cancer—sea-buckthorn polyphenols, mainly of kaempferol and its derivatives [11], glioma tumor—sea buckthorn leaf extract [12], prostate cancer—sea buckthorn leaf aqueous extract [2], and gastric cancer [13]. Despite this, the existing literature data is incomplete and needs to be supplemented in other cancer types and other kind of sea-buckthorn bioactive compounds, e.g., sea-buckthorn carotenoids [14]. There is still little information and research on the anticancer effects of hawthorn. However, the bioactive compounds in hawthorn are thought to have a positive impact on human tumor cells [15,16]. Since the wildly grown fruits usually possess an unpleasant bitter and sour taste in the raw form, they are mainly eaten processed as fruit preserves, teas, bakery and brewery products, beverages, wine, meat and sugar products or dietary supplements [3,13,16,17,18,19,20]. Therefore, their possible anticancer properties should also be evaluated in a processed form consumed with different food products, especially with those which ingestion is possibly equal and popular, such as wheat-flour cookies. Until now, there is no information in the literature data about the anticancer properties of cookies fortified with wild-grown fruits.
Therefore, the purpose of the present study was to investigate the antitumor properties of wheat-flour cookies fortified with the above-mentioned wild-grown fruits, in particular their cytotoxicity properties and the effect on the viability of MCF7 breast cancer and WM893 melanoma cell lines, one of the most common and deadly types of cancer worldwide.
2. Results and Discussion
In this study, five types of wheat cookies were tested: (1) wheat-flour cookies without addition of lyophilised fruits; this was used as a control (2) wheat-flour cookies with 5% of sea-buckthorn (Hippophae rhamnoides L.); (3) cookies with 5% of elderberry (Sambucus nigra L.); (4) cookies with 5% of hawthorn (Crataegus L.); (5) cookies with 5% of chokeberry (Aronia melanocarpa) (Table 1).

Table 1.
The recipe formulation of different kinds of cookies.
2.1. WM793 Human Melanoma Line
When analyzing the effect of different extracts obtained from cookies with the addition of selected wild-grown fruits on their anticancer properties against human skin cancer (melanoma) WM793 cells, statistically significant differences were observed between the mean values of cytotoxicity and viability (p < 0.05) (Table 2). Cookies’ extracts containing sea buckthorn berries were the most effective in defending WM793 melanoma cells at three concentrations added (0.5 mg/mL; 1.5 mg/mL, and 2.5 mg/mL) (p < 0.05) (Figure 1). This results in 89.95% cytotoxicity and the lowest viability (45.93%) at the highest concentration applied (2.5 mg/mL) (Table 2, Figure 1). The inhibiting potential of cancer cell growth upon lower concentrations of sea-buckthorn extracts was 0.5 mg/mL—27% cytotoxicity and 76% viability; 1.5 mg/mL—26% cytotoxic force and 70% viability (Table 2). The remaining extracts containing other berries were definitely less efficient (p < 0.05) than the fruits of Hippophae rhamnoides L. depending on the dose used (Figure 1). Elderberry showed the greatest cytotoxicity (3.84%) potential at the highest concentration (2.5 mg/mL) while cookies baked with the addition of hawthorn fruits showed the highest activity at a concentration of 1 mg/mL—5.19% and with the addition of chokeberry fruit at 1.5 mg/mL—1.99% (Table 2).

Table 2.
Cytotoxicity assay after 24 h incubation of extracts with different wild-grown fruits enriched cookies on WM793 human melanoma cell line.
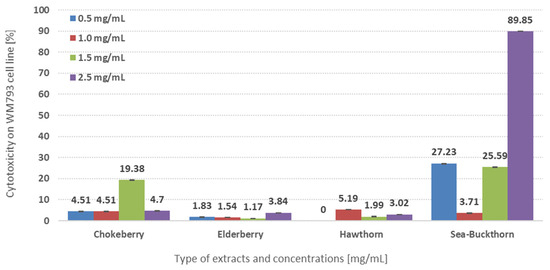
Figure 1.
Cytotoxicity of different plant extracts on WM793 cell line depending on the kind of wild grown-fruits enriched cookies.
2.2. MCF7 Human Breast Cancer Cell Line
Analyzing the effect of different extracts from cookies with the addition of selected wild grown fruits on their antitumor capability against the human breast MCF-7 cancer line, no effect of the tested extracts was observed (p > 0.05). In most cases, null values were obtained (Table 3). As in the previous cancer cell line, sea buckthorn extracts were an exception again. Although relatively low, the cytotoxic effect of it was variable within the concentrations used (Table 3). The highest cytotoxic effect was shown by its extracts at concentrations of 0.5 mg/mL—3.87% and 1.5 mg/mL—5.77% (p < 0.05). Interestingly, when assessing the viability of MCF7 cancer cells, a differentiated effect of individual extracts was documented (Table 3, Figure 2). Samples containing chokeberries and elderberries had the greatest effect on cancer cell viability, regardless of the dose used, resulting in the lowest percentage of cell vitality, average 38% and 39%, respectively (Table 3).

Table 3.
Cytotoxicity assay after 24 h incubation of different wild-grown fruits enriched cookies on MCF7 human breast cell line.
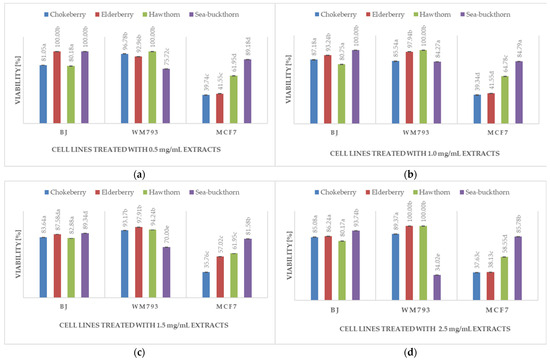
Figure 2.
The comparison of different extracts containing wild grown fruits on human normal fibroblasts BJ, melanoma WM793 and breast tumor MCF7 cell lines depending on the extract concentration: (a) 0.5 mg/mL; (b) 1 mg/mL; (c) 1.5 mg/mL; (d) 2.5 mg/mL. The different letters by the number value of the graph bars are significantly different at p < 0.05.
2.3. BJ Human Normal Fibroblasts Cell Line
Analyzing the effect of different cookies with the addition of selected wild berries on their cytotoxic properties against the human normal fibroblast BJ cell line, it was observed that higher concentrations (1.5–2.5 mg/mL) of extracts with the addition of wild berries were characterized by greater cytotoxic properties (Table 4). However, generally, cell viability of the BJ line was high, reaching more than 85% in most cases, with a few exceptions where it was 65–73% after the use of elder and hawthorn berries extracts at 2.5 mg/mL concentration (Table 4). When the viability of normal BJ fibroblasts was compared with the vitality of the two cancer cells, MCF7 and WM793, treated with the same extracts, the activity of the latter was in most cases statistically significantly lower (p < 0.05), especially in the case of the MCF7 breast tumor cell line for all types of extracts (up to 40–50% lower for chokeberry and elderberry in the concentration of extracts). Sea buckthorn extracts greatly reduced the shelf life of the WM793 melanoma cell line (p < 0.05). This may indicate that wild-grown berries have different anti-tumor health promoting properties, and their efficacy in this regard depends on the type of tumor cell line they act on (Figure 2).

Table 4.
Cytotoxicity assay after 24 h incubation of different wild-grown fruits enriched cookies on BJ human normal fibroblasts.
3. Discussion
To our best knowledge, this is the first study that investigates the anticancer activity of extracts obtained from wheat-flour cookies with an addition of different wild-grown fruits: chokeberry, elderberry, hawthorn and sea-buckthorn at four different concentrations: 0.5 mg/mL; 1.0 mg/mL; 1.5 mg/mL and 2.5 mg/mL on WM793 melanoma and MCF7 cancer cell lines. Melanoma is the most invasive skin cancer with the most significant number of mutations and the ability to escape the immune system and is known for its metastatic capacity and lethality [21]. This type of cancer invades melanocytes, the cells that synthesize the skin pigment melanin [22]. Although melanoma cases represent only 3% of all skin cancers, it is linked to the highest fatal rate (about 65%) [23]. Similarly, breast cancer is the most often occurring cancer. Despite significant advances in early detection and treatment methods, it is still regarded as a leading cause of death in females with 2.3 million new cases (11.7%) [24,25,26,27]. According to the American Cancer Society, the global cancer burden would be 28.4 million cases by 2040, a 47% increase from in the year 2020 [28]. Among the risk factors, 30% of breast cancer cases are attributed to an inappropriate lifestyle, including improper diet leading to excess body weight, lack of physical activity, or alcohol consumption. Despite the unfavorable prognosis in later stage disease, mainly due to metastasis, the recommended treatment in both cases, melanoma and breast cancer, is immunotherapy in combination with other chemotherapeutic drugs [23,29]. Chemotherapy drugs are used for treating people with an advanced cancer or after surgery. However, cancers frequently build up a tolerance to the medicines, leading to treatment failure and disease return. Hence, the need for innovative plant-based pharmaceuticals as complementary and alternative medicine that support the traditional therapies and as a component of daily diets to prevent against development of certain types of cancers. The anticancer effects of wild grown edibles can be divided into two main areas: the impact of extracts from different raw materials (leaves, flowers, stems, berries, seeds) and isolated compounds. No data were available on the effects of extracts from ready-to-eat foods, such as cookies fortified with isolated compounds or wild-growing shrubs’ whole berries on the antitumor properties. Therefore, the results of the study have been referred to the available data on extracts from raw plants or isolated compounds.
In the cytotoxicity and viability assay of the tested extracts with wild grown fruits on WM793 melanoma and cytotoxicity on the MCF7 breast cell line, sea-buckthorn was documented to possess the strongest activity in this regard (p < 0.05) (Table 2 and Table 3, Figure 1 and Figure 2). Sea buckthorn is a deciduous shrub or tree of the genus Hippophae in the family Elaeagnaceae [14]. It is rich in approximately 200 nutritional and bioactive compounds and is therefore often referred to as a “natural vitamin treasure house” or “source of nutrition and health care” [14]. It is significant that the World Health Organization has ranked sea buckthorn among the top ten raw materials with health-promoting properties [4,30]. Sea buckthorn berries are considered to be a wealth source of bioactive compounds, including vitamins, carotenoids (-carotene, zeaxanthin, lutein, lycopene, etc.), polyphenols, flavonoids, organic acids, pectin, carbohydrates, polyunsaturated fatty acids, and essential amino acids [31]. It is also mentioned that the fruit flesh of sea buckthorn produces oil rich in valuable ω-7 palmitoleic acid and carotenoids [30].
Sea buckthorn leaf aqueous extract efficiently affected androgen receptor (AR) and markedly reduced androgen response genes, prostate specific antigen (PSA), elevated lysine-rich leukemia 2 (ELL2), ELL-associated factor 2 (EAF2), and calreticulin (CALR) in vitro. Aqueous extract of sea buckthorn leaves efficiently suppressed the growth and progression of prostate cancer cells [2]. Kim et al. [12] reported that sea buckthorn leaf extract at concentrations of 6.2 and 62 mg/mL significantly decreased the generation of intracellular ROS by 16.3 and 42.3%, respectively; increased the expression of the pro-apoptotic protein B-cell lymphoma-2 (BCL2)-associated X (Bax); and suppressed the fast multiplication of C6 glioma cells (11 and 49.5%). Authors concluded that sea buckthorn could be a potential source of pharmacological intervention for treating glioma. On the other hand, sea buckthorn isolated polyphenols, namely the active component of kaempferol and its derivatives, have exhibited significant anti-colon cancer effects in vitro and in vivo. Mechanistically, sea buckthorn polyphenols enhance the expression of microRNA (miR)-195-5p and miR- 497-5p and suppress the expression of miR-1247-3p to downregulate the expression of cyclins, thereby inhibiting cell cycle arrest in the G1 phase and further proliferation of colon cancer. In addition, sea buckthorn polyphenols (50 mg/kg) significantly decreased tumor volume and controlled its proliferation in xenografted BALB/c nude mice in vivo [11]. In another study, polyphenolic compound isorhamnetin enhanced expression of the mitochondrial pathway pro-apoptotic protein (cytochrome c-caspase 9-caspase 3) in gastric cancer cells in hypoxic conditions, considerably inhibited the autophagy of MKN-45 gastric cancer cells and favored the programmed death of gastric cancer cells by activating the phosphoinositide 3-kinase (PI3K)-protein kinase B (AKT)-mammalian target of rapamycin (mTOR) signaling pathway [13]. In our previous study, we tested the content of flavonoids, phenolic acids and anthocyanins in the extracts obtained from the cookies with wild-grown fruits with the application of Reversed Phase-High Performance Liquid Chromatography (RP-HPLC) and High Performance Liquid Chromatography (HPLC) [3]. Six different polyphenolic compounds have been found in sea-buckthorn cookies and their presence were of rather moderate quantities (mg·100 g−1 dm) (Table S1): chlorogenic acid (51.00 ± 0.87); catechin (11.70 ± 0.52); quercetin (200.73 ± 0.76); quercetin-3-galactoside (190.86 ± 1.79); rutin (304.96 ± 2.06); luteolin 7-O-glucoside (294.36 ± 0.26) [3]. No isorhamnetin or kaempferol was detected. Nevertheless, as shown in this study, sea buckthorn was the plant with the most outstanding potential. This may indicate a need for further analyses with the use of other bioactive compounds (i.e., carotenoids) from sea buckthorn for their antitumor activity in ready-to-eat food products, including cookies.
Apart from sea buckthorn extracts, the samples containing chokeberry and elderberry had a positive influence on the viability of MCF7 cancer cells, regardless of the dose used, resulting in the lowest percentage of neoplastic cell viability, averaging 38% and 39%, respectively (Table 3). On the contrary, the cytotoxicity of the MCF7 breast cancer cell lines was not affected by these two types of extracts.
In the available literature, the effective anticancer activity of both plants has been described. For instance, aronia berries and aronia juice showed promising activity against several different cancer cell lines, including human colon cancer cells HT-29 cells [32] and Caco-2 human colon cancer [33,34], but were not toxic to human NCM460 normal colon cells at the same time [20]. The berry anthocyanidins exhibited selective cell growth inhibitory activity against A549 and H1299 human non-small-cell lung cancer (NSCLC) cells [35]. Aronia melanocarpa polyphenols were assumed to regulate the expression of key regulators of G2/M cell cycle transition and apoptosis. The antitumor activity was associated predominantly with chlorogenic acids, some cyanidin glycosides, and derivates of quercetin [36]. Aronia or chokeberry (Aronia melanocarpa L.) is a shrub, the fresh fruits of which are not usually consumed directly due to their unpleasant bitter taste [20]. Phytochemical studies have shown that Aronia melanocarpa fruits are abundant in phenolic compounds (from 10 to 5500 mg per 100 g of dried fruits): procyanidins, anthocyanins (cyanidin-3-O-galactoside), phenolic acids (chlorogenic acid), quercetin, a fused flavanol-coumarin-phenol unit, also possess hydroxyl radical scavenging and quinone reductase inducing activities [18,37]. In our previous study [3], the contents of flavonoids, phenolic acids and anthocyanins tested by RP-HPLC and HPLC methods in the extracts from the cookies with chokeberry were the most impressive among all kinds and equaled (mg·100 g−1 dm) (Table S1): neochlorogenic acid (919.28 ± 4.85), protocatechuic acid (269.20 ± 0.62), chlorogenic acid (1026.18 ± 4.94), cinnamic acid (3.32 ± 1.85), catechin (21.82 ± 1.23); epicatechin (792.48 ± 0.53); quercetin (202.66 ± 1.71); quercetin-3-galactoside (237.19 ± 3.17), rutin (461.74 ± 10.92) and cyanidin-3-glucoside (78.05 ± 0.36) [3]. The greatest amounts of polyphenols, especially chlorogenic acid, rutin and quercetin-3-galactoside together with one of the strongest antioxidant activities (µmol·g−1 dm) (Table S1): ABTS—15.22 ± 0.05; FRAP—17.47 ± 0.05 might be strongly linked to the low viability (mean 38%) of MCF7 human breast cells which were treated with chokeberry extracts in this study (Table 3). One more possible anticancer mechanism might be the presence of triterpene ester derivatives of ursolic acid in chokeberry fruits, namely, 3-O-trans- and -cis-p-coumaroyl tormentic which were found to inhibit MCF7 and MDA-MB-231 human breast cancer cell proliferation and mammosphere creation through deregulation of the expression of c-Myc protein, a cancer stem cell survival factor [38].
Similarly, the aqueous acetone extract of elderberry fruits in the LoVo human colon adenocarcinoma cell line and MCF7 human breast cancer had a lower value of IC50 compared to the ethyl acetate extract, and its major triterpenoid ursolic acid exhibited the highest cytotoxic activity, with IC50 values of 10.7 and 7.7 µg/mL on MCF-7 and LoVo cells, respectively [39]. The cytotoxic properties of ethanolic extracts from elderflowers, leaves and stems were also documented in human liver cell carcinoma, lung carcinoma and colon cancer cells [9]. The probable explanation was the synergistic actions of (poly)phenolic components, and the molecular docking study indicated that chlorogenic acid and rutin had the ability to interact with important amino acids in the Bcl-2 binding site [39]. Elderberry (Sambucus nigra L.) is the most common species in the family Adoxaceae and the genus Sambucus L. [39]. The fruits have a variety of beneficial components, mainly polyphenolic, including phenolic acids (gallic acid and gentisic acid), flavanones, flavonols and anthocyanins (cyanidin 3-O-sambubioside and cyanidin 3-O-glucoside) together with terpenoid compounds. The major flavonoids were rutin and quercetin [40,41]. RP-HPLC and HPLC determinations of polyphenolic compounds [3] in the extracts from elderberry cookies were as follows (mg·100 g−1 dm) (Table S1): protocatechuic acid (131.23 ± 0.72); catechin (14.05 ± 0.12); quercetin (156.04 ± 0.23) and rutin (1083.20 ± 1.40) [3]. No chlorogenic acid was detected in the cookies prepared from elderberry fruits, but rutin content was statistically significantly highest as evidenced by RP-HPLC determinations (Table S1). Other studies indicated that elderberry agglutinin could selectively identify ovarian carcinoma via specific recognition of α-2,6-linked sialic acids and was capable of activating AKT, launching cytochrome c, provoking mitochondrial dysfunction, increasing the mitochondria oxidative phosphorylation, finally leading to cell cycle arrest at the G2/M phase and the apoptosis of ovarian cancer cells [42]. On the other hand, Ma and Ning [10] proved that elderberry fruit extract and its anthocyanin compound cyanidin 3-O-glucoside enhanced the packing order of polar heads of membrane lipids and changed the structure of membranes as well as decreased the fluidity of human breast adenocarcinoma cells membranes [10]. The statistically significant greatest amounts of cyanidin 3-O-glucoside and rutin detected by RP-HPLC and HPLC determinations (Table S1) might be positively correlated with the reduced viability of MCF7 breast cell lines by elderberry cookies (Table 3). Cytotoxic activity of elderberry flower extracts could only be observable in T24 bladder carcinoma cells but not in MRC-5 human normal fibroblast cells [9]. On the contrary, elderberry and chokeberry extracts exerted greater cytotoxic properties on BJ normal human fibroblasts (mean values for all extracts’ concentrations—46.35%) than on the WM793 melanoma cell line (mean values for all extracts’ concentrations—36.60%) as documented in this study (Table 2 and Table 3). However, this phenomenon has been observed and described in the literature by several researchers for dietary polyphenols [43,44,45,46,47,48,49,50]. Some polyphenols may be carcinogenic or genotoxic at high concentrations [43,45]. Caffeic acid, for instance, induced gastric and renal tumors in rats and mice at a dietary dose of 2% [50]. Quercetin inactivated the O-methylation of catechol estrogens and elevates the urinary concentration of 2- and 4-hydroxyestrodiol by 60–80%, which may lead to increased redox cycling of catechol estrogens and estradiol-induced tumor development, indicating that the genotoxic effects reported in vitro may be due to the high levels used, at which polyphenols can act as prooxidants [48,49,51]. The RP-HPLC determinations in the tested cookies indicated significantly highest concentrations of quercetin in chokeberry and sea-buckthorn extracts (p < 0.05) together with considerable amounts of quercetin-3-O-galactoside in chokeberry, hawthorn and sea-buckthorn extracts. In elderberry extracts the latter has not been detected, while quercetin was found in moderate amounts (Table S1). The formation of glutathionyl-quercetin adducts has been demonstrated in tyrosinase-rich B16F-10 melanoma cells and in the myeloperoxidase-rich human HL-60 cell line, which provide important evidence for the prooxidative metabolism of quercetin in cellular in vitro models. This also implies that tissues abundant in oxidative enzymes may be especially vulnerable to the prooxidative damage caused by quercetin [44]. And finally, green tea catechins (1% or 0.1% of the diet) have been shown to promote tumor formation in the colon of F344 male rats, and though quercetin may reduce cancer cell propagation at high concentrations, it has been found to promote cell growth at low concentrations (1–5 mol/L) [46,47]. The existing contradicted results among the pro-healthy and potential toxic properties of dietary polyphenols and containing them edibles need thorough research in order to estimate the proper pharmaceutical dosages.
Last, the extracts from hawthorn enriched cookies were least effective in inhibiting proliferation of MCF7 human breast and WM793 melanoma cell lines (Table 2, Table 3 and Table 4; Figure 1 and Figure 2). It is surprising because this plant belonging to the Rosaceae family, has a great nutritious value, rich in bioactive components (chlorogenic acid, proanthocyanidin B2, epicatechin, proanthocyanidins, mucoxanthin, quercetin, rutin, ursolic acid, hawthornic acid, oleanolic acid) and is characterized by a high therapeutic and health value [16]. In the literature Zhou et al. [52] found that ursolic acid, a triterpenoid, induced apoptosis of human breast and hepatocellular carcinoma cells via the mitochondrial pathway. Hawthorn polysaccharide inhibited the proliferation of human colon cancer cells by increasing the rate of cancer cell apoptosis [53], while isolated compounds have shown anti-tumor activity both in vivo and in vitro against melanoma [54]. In the present study, these beneficial properties of hawthorn extracts have not been observed. One of the possible explanations is the form of fruits used for extract preparations (raw plant materials vs. processed in the form of baked cookies). The data of polyphenolic compounds given in the literature are greater in raw fruits than in cookies prepared with the added wild fruits [3], what was explained by the baking process at high temperatures (>200 °C) which might have a significant impact on polyphenolic molecules, resulting in some changes in their content and structure [55] hence reducing possible antitumor properties.
4. Materials and Methods
4.1. Wheat Cookie Preparation
Fresh samples of the ripe, edible parts of wild fruits (sea buckthorn, elderberry, chokeberry and hawthorn) were collected from unpolluted rural areas of the Malopolska region (Poland), frozen and lyophilised (ALPHA 1–4, Martin Christ, Osterode am Harz, Germany). The lyophilisate was then ground in a laboratory grinder to obtain a sample no larger than 0.5 mm (Knifetec Sample Mill 1095, FOSS Tescator, Hoeganaes, Sweden) and used in this form as a bakery ingredient.
The dough components were as shown in Table 1: wheat flour type 450 (500 g) (“Złote Pola”, Polish Plants Grains S.A., Krakow, Poland), margarine (250 g) (“Kasia” Unile-ver, Warsaw, Poland), sucrose (200 g) (“Królewski” Südzuker Polska S.A., Wroclaw, Poland), eggs (150 g) (“Ale jaja” Poultry firm Woźniak Sp. z o.o., Rawicz, Poland), baking powder (6 g) (Dr. Oetker Poland Sp. z o.o., Gdansk, Poland). Subsequently, 5 g of the freeze-dried fruits were added to the biscuit dough instead of 5 g of wheat flour for each type of cookie. The control sample used 5 g of wheat flour instead of freeze-dried fruit. After combining, the ingredients were formulated into a dough and left in the refrigerator (4 °C, 30 min). The dough was then shaped to a thickness of 7 mm and sliced into round cookies of the same size (3.5 cm). These cookies were then baked (200 °C, 8 min) and chilled. The cookies were kept in tightly closed bags in a dark, dry place. Before each analysis, the cookies were crushed in a laboratory grinder (Knifetec Sample Mill 1095, FOSS Tescator, Sweden).
Preparation of the Methanol-Acetone Extracts
Fresh samples (5 g) were inoculated with a solution of 80% acidified methanol (methanol HCl 2% (95/5 v/v) (Merck, St. Louis, MO, USA) for 2 h at 25 °C and then sedimented by centrifugation (1500× g, 15 min). The supernatant was stored, and the pellet was re-extracted with 40 mL solution of 70% acetone (POCH, Gliwice, Poland) for the next 2 h and then subjected to centrifugation (1500× g, 15 min). The supernatants from both sets of centrifugations were mixed and used for further assay. The obtained methanol-acetone extracts were evaporated in a vacuum evaporator to eliminate solvents and then dried using a lyophiliser (ALPHA 1–4, Martin Christ, Germany), and the final powder was used to prepare the following solutions at concentrations of 0.5 mg/mL; 1.5 mg/mL and 2.5 mg/mL.
4.2. Cell Cultures
4.2.1. Cell Lines
The following cell lines were used for in vitro testing of anticancer properties:
- line BJ (control)—a line of human normal fibroblasts, obtained from the American Type Culture Collection (ATCC, Manassasm, VA, USA);
Medium: Eagle’s Minimum Essential Medium (M3024, Merck, Darmstadt, Germany) supplemented with 2 mM L-glutamine (25030149, ThermoFisher, Waltham, MA, USA), 1 mM sodium pyruvate (11360070 ThermoFisher, Waltham, MA, USA), antibiotics (A5955, Merck, Darmstadt, Germany) and 10% fetal bovine serum (FBS) (F7524 Merck, Darmstadt, Germany);
- A line WM793—a human skin cancer (melanoma) line, obtained from The European Searchable Tumour Line Database (ESTDAB, Tubingen, Germany)
Medium: RPMI-1640 (R4130, Merck, Darmstadt, Germany) supplemented with antibiotics (A5955, Merck, Darmstadt, Germany) and 10% fetal bovine serum (FBS) (F7524 Merck, Darmstadt, Germany);
- A line MCF7—a human breast cancer line, obtained from the American Type Culture Collection (ATCC, Manassasm, VA, USA);
Medium: Eagle’s Minimum Essential Medium (M3024, Merck, Darmstadt, Germany) supplemented with 2 mM L-glutamine (25030149, ThermoFisher, Waltham, MA, USA), 1 mM sodium pyruvate (11360070 ThermoFisher, Waltham, MA, USA), 0.01 mg/mL human recombinant insulin (407709, Merck, Darmstadt, Germany), antibiotics (A5955, Merck, Darmstadt, Germany) and 10% fetal bovine serum.
4.2.2. Cells Preparation
Cells were cultured in an incubator with controlled conditions (temp. 37 °C, 95% humidity, 5% CO2) in the cell culture medium by the manufacturer’s recommendations. Three times during the week, the medium was changed into fresh one in an amount of 5 mL. A passage of cells was performed once a week or at confluence greater than 70%. The medium was poured off, the cells were washed with 1 mL of PBS solution (D8537, Merck, Darmstadt, Germany) and 0.5 mL of trypsin (D8537, Merck, Darmstadt, Germany) was added. They were incubated for about 5 min until the cells detached from the medium, then trypsin was inactivated by adding 1 mL of medium. 2 drops of the suspension were left or transferred to another bottle and 5 mL of fresh medium was added. Cultures were continued at 37 °C and 5% CO2 until the desired number of cells was reached.
4.2.3. Cells Seeding
After the cells were counted using an automatic counter from Bio-Rad (TC20 automatic cell counter (Bio-Rad, Hercules, CA, USA), they were seeded into 96-well plates (351172, Corning, Corning, NY, USA). A solution of 14.4 mL of cell suspension after passage with fresh medium was prepared and each cell line was seeded into an individual plate at 150 μL per well, then incubated for 24 h at 37 °C and in an atmosphere of 5% CO2.
4.2.4. Adding Experiments Conditions
After 24 h of incubation, the experimental conditions were added to the plates, which were mixtures of individual cookie extracts with medium at final concentrations of 0.5 mg/mL; 1 mg/mL; 1.5 mg/mL and 2.5 mg/mL, and medium without added extract at the same concentrations was used as a control. Each extract concentration was added to the cells in triplicate, and the remaining 3 wells were left without added conditions. The cells prepared in this way were further incubated for 24 h.
4.2.5. Cytotoxicity Assay
The effect of cytotoxicity of extracts on cells performed using the Cytotoxicity Detection Kit (LDH) (Cat no: 4744934001, Roche, Basel, Switzerland) according to manufacturer’s instruction. Cytotoxicity was determined after 24 h. Assessment of LDH activity in cell supernatant was the measure of toxicity of investigated substance in relation to cultured cells. It was a colorimetric method, and the assays were determined using an ELISA Multiskan GO Microplate Spectrophotometer) (Thermo Scientific, Waltham, MA, USA).
4.2.6. Cell Viability Assay
Evaluation of the cell vitality was performed with the colorimetric method using crystalline violet. This test involves quantitatively measuring crystal violet associated with fixed cellular structures. After incubation of the cells with the extracts, the medium from the cells was removed and the cells were carefully washed two times with cold PBS solution (D8537, Merck, Darmstadt, Germany). The cells were then fixed for 10 min with cold 100% methanol (D8537, Merck, Darmstadt, Germany). After fixation, the methanol was removed, and crystal violet (0.5% crystal violet in 25% methanol) was added to the wells for 10 min. After incubation, the dye was removed, and any residual dye was removed by rinsing the plates three times with distilled water. Then 200 µL of methanol was added to the wells and incubated for 20 min at room temperature. After incubation, spectrophotometric measurements were made at a wavelength of 590 nm using an ELISA Multiskan GO Microplate Spectrophotometer (Thermo Scientific, Waltham, MA, USA).
4.3. Data Analyses
The results were presented as ranges of at least three parallel repetitions with standard deviation around the mean. One-way analysis of variance was applied in order to assess the influence of different wild grown fruit additions on the tested parameters. The Duncan test was used in order to evaluate the significance of differences at a level of p < 0.05. All the calculations were carried out using Statistica v. 10 software (Statsoft, Inc., Tulsa, OK, USA).
5. Conclusions
In the cytotoxicity and viability assay of the tested extracts with wild grown fruits on WM793 melanoma and cytotoxicity on the MCF7 breast cell line, sea-buckthorn was documented to possess the strongest activity in this regard (p < 0.05), while hawthorn enriched cookies were least effective in inhibiting proliferation of neoplastic cell lines (p < 0.05). Cookies enriched with wild grown fruits have the potential to become a novel snack food with anticancer properties, especially fortified with sea-buckthorn fruits. At the same time, the samples containing chokeberry and elderberry positively influenced the viability of MCF7 cancer cells, regardless of the dose used, resulting in the lowest percentage of cancer cell viability, averaging 38% and 39%, respectively. On the contrary, these two types of extracts did not affect the cytotoxicity of MCF7 breast cancer cell lines. The decrease in viability without a cytotoxic effect is desirable because it may suggest such processes as cell cycle/proliferation arrest, apoptosis or autophagy—i.e., removal cancer cells without affecting the surrounding cells. The phenomenon needs further studies, however, especially because the extracts from these berries exerted greater cytotoxic properties on BJ normal human fibroblasts (mean values for all extracts’ concentrations—46.35%) than on WM793 melanoma cell line (mean values for all extracts’ concentrations—36.60%).
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/app132212256/s1: Table S1: Total polyphenols, polyphenolic compounds’ profile, antioxidant activity and acrylamide content of the tested cookies [3].
Author Contributions
Conceptualization, B.B.; methodology, D.D.; software, B.B. and J.K.-D.; validation, I.D; formal analysis, D.D.; investigation, D.D. and B.B.; data curation, B.B. and J.K.-D.; resources, D.D. and I.D.; writing—original draft preparation, B.B.; visualization, J.K.-D.; supervision, I.D. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partly supported by the research subsidy 070015-D020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article and Supplementary Materials.
Acknowledgments
The Authors would like to thank to METROFOOD-CZ research infrastructure project (MEYS Grant No: LM2023064) including access to its facilities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Krajewska, A.; Dziki, D. Enrichment of Cookies with Fruits and Their By-Products: Chemical Composition, Antioxidant Properties, and Sensory Changes. Molecules 2023, 28, 4005. [Google Scholar] [CrossRef] [PubMed]
- Masoodi, K.; Wani, W.; Dar, Z.; Mansoor, S.; Anam-ul-Haq, S.; Farooq, I.; Khursheed, H.; Shafiq, A.W.; Firdos, A.; Nehvi, N.A. Sea buckthorn (Hippophae rhamnoides L.) inhibits cellular proliferation, wound healing and decreases expression of prostate specific antigen in prostate cancer cells in vitro. J. Funct. Foods 2020, 73, 104102. [Google Scholar] [CrossRef]
- Borczak, B.; Sikora, M.; Kapusta-Duch, J.; Fołta, M.; Szewczyk, A.; Zięć, G.; Doskoĉil, I.; Leszczyńska, T. Antioxidative Properties and Acrylamide Content of Functional Wheat-Flour Cookies Enriched with Wild-Grown Fruits. Molecules 2022, 27, 5531. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Feng, X.; Dorjsurend, B.; Chimedtseren, C.; Damd, T.A.; Zhang, C. Traditional food, modern food and nutritional value of Sea buckthorn (Hippophae rhamnoides L.): A review. J. Future Foods 2023, 3, 191–205. [Google Scholar] [CrossRef]
- Bottomley, A.; Coens, C.; Mierzynska, J.; Blank, C.U.; Mandalà, M.; Long, G.V.; Mukhametshina, G. Adjuvant pembrolizumab versus placebo in resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): Health-related quality-of-life results from a double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 655–664. [Google Scholar] [CrossRef]
- Castro, D.T.H.; Leite, D.F.; da Silva Baldivia, D.; dos Santos, H.F.; Balogun, S.O.; da Silva, D.B.; Carollo, C.A.; de Picoli Souza, K.; dos Santos, E.L. Structural Characterization and Anticancer Activity of a New Anthraquinone from Senna velutina (Fabaceae). Pharmaceuticals 2023, 16, 951. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, Y.; Liua, X.; Chen, X.; Ding, C.; Dong, L.; Zhang, J.; Sun, S.; Ding, S.; Khatoom, S.; et al. Chokeberry (Aronia melanocarpa) as a new functional food relationship with health: An overview. J. Future Foods 2021, 1–2, 168–178. [Google Scholar] [CrossRef]
- Schröder, L.; Richter, D.U.; Piechulla, B.; Chrobak, M.; Kuhn, C.; Schulze, S.; Abarzua, S.; Jeschke, U.; Weissenbacher, T. Effects of Phytoestrogen Extracts Isolated from Elder Flower on Hormone Production and Receptor Expression of Trophoblast Tumor Cells JEG-3 and BeWo, as well as MCF7 Breast Cancer Cells. Nutrients 2016, 8, 616. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.I.; Amparo, T.R.; Almeida, T.C.; Costa, F.S.F.; Brandăo, G.C.; Santos, O.D.H.D.; da Silva, G.N.; Bianco de Souza, G.H. Cytotoxic activity of butanolic extract from Sambucus nigra L. flowers in natura and vehiculated in micelles in bladder cancer cells and fibroblasts. Nat. Prod. Res. 2020, 36, 1100–1104. [Google Scholar] [CrossRef]
- Ma, X.; Ning, S. Cyanidin-3-glucoside attenuates the angiogenesis of breast cancer via inhibiting STAT3/VEGF pathway. Phytother. Res. 2019, 33, 81–89. [Google Scholar] [CrossRef]
- Wu, H.; Li, C.; Cui, M.; Guo, H.; Chen, S.; Du, J.; Li, H.; Zhuoyu, L. Polyphenols from Hippophae rhamnoides suppressed colon cancer growth by regulating miRNA-mediated cel cycle arrest and apoptosis in vitro and in vivo. J. Funct. Foods 2021, 87, 104780. [Google Scholar] [CrossRef]
- Kim, S.; Hwang, E.; Yi, S.; Song, K.; Lee, H.; Heo, T.; Park, S.K.; Jung, Y.J.; Jun, H.S. Sea buckthorn leaf extract inhibits glioma cell growth by reducing reactive oxygen species and promoting apoptosis. Appl. Biochem. Biotechnol. 2017, 182, 1663–1674. [Google Scholar] [CrossRef]
- Li, L.; Gao, X.L.; Liu, J.G.; Chitrakar, B.; Wang, B.; Wang, Y.C. Hawthorn pectin: Extraction, function and utilization. Curr. Res. Nutr. Food Sci. 2021, 4, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, F.; Wei, P.; Chai, X.; Hou, G.; Meng, Q. Phytochemistry, health benefits, and food applications of sea buckthorn (Hippophae rhamnoides L.): A comprehensive review. Front. Nutr. 2022, 9, 1036295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chi, L.; Wu, Y.; Zhang, L.; Xu, C. Quality Comparison of Hawthorn Wines Fermented by Saccharomyces cerevisiae with and without Pulp Contact and Pectase Treatment. J. Chem. 2017, 6431818. [Google Scholar] [CrossRef]
- Zhang, J.; Chai, X.; Zhao, F.; Hou, G.; Meng, Q. Food Applications and Potential Health Benefits of Hawthorn. Foods 2022, 11, 2861. [Google Scholar] [CrossRef] [PubMed]
- Sidor, A.; Drożdżyńska, A.; Gramza-Michalowska, A. Black chokeberry (Aronia melanocarpa) and its products as potential health-promoting factors–an overview. Trends Food Sci. Technol. 2019, 89, 45–60. [Google Scholar] [CrossRef]
- Yang, S.-Q.; Wang, D.; Gao, Y.-X. Advances in studies on the function and application of Aronia melanocarpa. Food Res. Dev. 2021, 42, 206–213. [Google Scholar]
- Li, T.; Fu, S.; Huang, X.; Zhang, X.; Cui, Y.; Zhang, Z.; Ma, Y.; Zhang, X.; Yu, Q.; Yang, S.; et al. Biological properties and potential application of hawthorn and its major functional components: A review. J. Funct. Foods 2022, 90, 104988. [Google Scholar] [CrossRef]
- Ren, Y.; Frank, T.; Meyer, G.; Lei, J.; Grebenc, J.R.; Slaughter, R.; Gao, Y.G.; Kinghorn, A.D. Potential Benefits of Black Chokeberry (Aronia melanocarpa) Fruits and Their Constituents in Improving Human Health. Molecules 2022, 27, 7823. [Google Scholar] [CrossRef]
- Villani, A.; Potestio, L.; Fabbrocini, G.; Troncone, G.; Malapelle, U.; Scalvenzi, M. The Treatment of Advanced Melanoma: Therapeutic Update. Int. J. Mol. Sci. 2022, 23, 6388. [Google Scholar] [CrossRef]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current state of melanoma diagnosis and treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef]
- Lee, C.-S.; Thomas, C.M.; Ng, K.E. An Overview of the Changing Landscape of Treatment for Advanced Melanoma. Pharmacotherapy 2017, 37, 319–333. [Google Scholar] [CrossRef]
- Comşa, S.; Cîmpean, A.M.; Raica, M. The story of MCF-7 breast cancer cell line: 40 years of experience in research. Anticancer. Res. 2015, 35, 3147–3154. [Google Scholar] [PubMed]
- Sung, S.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R. Breast Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 524–541. [Google Scholar] [CrossRef] [PubMed]
- Foudah, A.I.; Alam, A.; Salkini, M.A.; Ross, S.A.; Kumar, P.; Aldawsari, M.F.; Alqarni, M.H.; Sweilam, S.H. Synergistic Combination of Letrozole and Berberine in Ascorbic Acid-Stabilized AuNPs: A Promising Solution for Breast Cancer. Pharmaceuticals 2023, 16, 1099. [Google Scholar] [CrossRef]
- Kashyap, D.; Pal, D.; Sharma, R.; Garg, V.K.; Goel, N.; Koundal, D.; Zaguia, A.; Koundal, S.; Belay, A. Global Increase in Breast Cancer Incidence: Risk Factors and Preventive Measures. BioMed Res. Int. 2022, 2022, 9605439. [Google Scholar] [CrossRef]
- Sung, W.-W.; Chang, C.-H. Nevi, dysplastic nevi, and melanoma: Molecular and immune mechanisms involving the progression. Tzu Chi Med. J. 2022, 34, 1–7. [Google Scholar]
- Mihalcea, L.; Aprodu, I.; Dumitras, L.; Cucolea, E.I.; Danila, G.-M.; Enachi, E.; Barbu, V.; Constantin, O.E.; Grigore-Gurgu, L.; Stanciuc, N. Whey Proteins Isolate-Based Biopolymeric Combinations to Microencapsulate Supercritical Fluid Extracted Oleoresins from Sea Buckthorn Pomace. Pharmaceuticals 2021, 14, 1217. [Google Scholar] [CrossRef]
- Yang, W.; Laaksonen, O.; Kallio, H.; Yang, B. Effects of latitude and weather conditions on proanthocyanidins in berries of Finnish wild and cultivated sea buckthorn (Hippophaë rhamnoides L. ssp. rhamnoides). Food Chem. 2017, 216, 87–96. [Google Scholar] [CrossRef]
- Gill, N.K.; Rios, D.; Osorio-Camacena, E.; Mojica, B.E.; Kaur, B.; Soderstrom, M.A.; Gonzalez, M.; Plaat, B.; Poblete, C.; Kaur, N.; et al. Anticancer effects of extracts from three different chokeberry species. Nutr. Cancer 2021, 73, 1168–1174. [Google Scholar] [CrossRef]
- Malik, M.; Zhao, C.; Schoene, N.; Guisti, M.M.; Moyer, M.P.; Magnuson, B.A. Anthocyanin-rich extract from Aronia meloncarpa E. induces a cell cycle block in colon cancer but not normal colonic cells. Nutr. Cancer 2003, 46, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Bermùdez-Soto, M.J.; Larrosa, M.; Garcia-Cantalejo, J.M.; Espín, J.C.; Tomás-Barberan, F.A.; García-Conesa, M.T. Upregulation tumor suppressor carcinoembryonic antigen-related cell adhesion molecule 1 in human colon cancer Caco-2 cells following repetitive exposure to dietary levels of a polyphenol-rich chokeberry juice. J. Nutr. Biochem. 2007, 18, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Kausar, H.; Jeyabalan, J.; Aqil, F.; Chabba, D.; Sidana, J.; Singh, I.P.; Gupta, R.C. Berry anthocyanidins synergistically suppress growth and invasive potential of human non-small-cell lung cancer cells. Cancer Lett. 2012, 325, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Jurendić, T.; Šćetar, M. Aronia melanocarpa Products and By-Products for Health and Nutrition: A Review. Antioxidants 2021, 10, 1052. [Google Scholar] [CrossRef] [PubMed]
- Naman, C.B.; Li, J.; Moser, A.; Hendrycks, J.M.; Benatrehina, P.A.; Chai, H.; Yuan, C.; Keller, W.J.; Kinghorn, A.D. Computerassisted structure elucidation of black chokeberry (Aronia melanocarpa) fruit juice isolates with a new fused pentacyclic flavonoid skeleton. Org. Lett. 2015, 17, 2988–2991. [Google Scholar] [CrossRef]
- Choi, H.S.; Kim, S.-L.; Kim, J.-H.; Deng, H.-Y.; Yun, B.-S.; Lee, D.-S. Triterpene acid (3-O-p-coumaroyltormentic acid) isolated from Aronia extracts inhibits breast cancer stem cell formation through downregulation of c-Myc protein. Int. J. Mol. Sci. 2018, 19, 2528. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; He, X.-Q.; Wu, D.T.; Li, H.B.; Feng, Y.-B.; Zou, L.; Gan, R.Y. Elderberry (Sambucus nigra L.): Bioactive Compounds, Health Functions, and Applications. J. AgricFood Chem. 2022, 70, 4202–4220. [Google Scholar] [CrossRef]
- Dominguez, R.; Zhang, L.; Rocchetti, G.; Lucini, L.; Pateiro, M.; Munekata, P.E.S.; Lorenzo, J.M. Elderberry (Sambucus nigra L.) as potential source of antioxidants. Characterization, optimization of extraction parameters and bioactive properties. Food Chem. 2020, 330, 127266. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Todorovic, B.; Veberic, R.; Stampar, F.; Ivancic, A. Investigation of anthocyanin profile of four elderberry species and interspecific hybrids. J. Agric Food Chem 2014, 62, 5573–5580. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Ray, U.; Chatterjee, B.P.; Roy, S.S. Targeted apoptosis in ovarian cancer cells through mitochondrial dysfunction in response to Sambucus nigra agglutinin. Cell Death Dis. 2017, 8, 2762. [Google Scholar] [CrossRef]
- Sinha, M.; Sachan, D.K.; Bhattacharya, R.; Singh, P.; Parthasarathi, R. ToxDP2 Database: Toxicity prediction of dietary polyphenols. Food Chem. 2022, 370, 131350. [Google Scholar] [CrossRef] [PubMed]
- Awad, H.M.; Boersma, M.G.; Boeren, S.; van der Woude, H.; van Zandenb, J.; van Bladeren, P.J.; Vervoort, J.; Rietjens, I.M.C.M. Identification of O-quinone/quinone methide metabolites of quercetin in a cellular in vitro system. FEBS Lett. 2002, 520, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Mennen, L.I.; Walker, R.; Bennetau-Pelissero, C.; Scalbert, A. Risks and safety of polyphenol consumption. Am. J. Clin. Nutr. 2005, 81, 326S–329S. [Google Scholar] [CrossRef]
- Van der Woude, H.; Gliszczynska-Swiglo, A.; Struijs, K.; Smeets, A.; Alink, G.M.; Rietjens, I.M. Biphasic modulation of cell proliferation by quercetin at concentrations physiologically relevant in humans. Cancer Lett. 2003, 200, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Hirose, M.; Hoshiya, T.; Mizoguchi, Y.; Nakamura, A.; Akagi, K.; Shirai, T. Green tea catechins enhance tumor development in the colon without effects in the lung or thyroid after pretreatment with 1,2-dimethylhydrazine or 2,2′-dihydroxy-di-n-propylnitrosamine in maleF344 rats. Cancer Lett. 2001, 168, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.T.; Liehr, J.G. Inhibition of catechol O-methyltransferase-catalyzed O-methylation of 2- and 4-hydroxyestradiol by quercetin: Possible role in estradiol-induced tumorigenesis. J. Biol. Chem. 1996, 271, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.T.; Liehr, J.G. Quercetin increases the severity of estradiol-induced tumorigenesis in hamster kidney. Toxicol. Appl. Pharmacol. 1994, 125, 149–158. [Google Scholar] [CrossRef]
- Hagiwara, A.; Hirose, M.; Takahashi, S.; Ogawa, K.; Shirai, T.; Ito, N. Forestomach and kidney carcinogenicity of caffeic acid in F344 rats andC57BL/6N C3H/HeN F1 mice. Cancer Res. 1991, 51, 5655–5660. [Google Scholar]
- Sakihama, Y.; Cohen, M.F.; Grace, S.C.; Yamasaki, H. Plant phenolic antioxidant and prooxidant activities: Phenolics-induced oxidative damage mediated by metals in plants. Toxicology 2002, 177, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Zhang, L.; Zhou, L.M.; Chang, Q.; Chow, M. Intestinal absorption of hawthorn flavonoids-in vitro, in situ and in vivo correlations. Life Sci. 2006, 79, 2455–2462. [Google Scholar] [CrossRef]
- Ma, L.; Xu, G.; Tang, X.; Zhang, C.; Zhao, W.; Wang, J.; Chen, H. Anti-Cancer Potential of Polysaccharide Extracted from Hawthorn (Crataegus) on Human Colon Cancer Cell Line HCT116 via Cell Cycle Arrest and Apoptosis. J. Funct. Foods 2020, 64, 103677. [Google Scholar] [CrossRef]
- Ho, G.T.T.; Bräunlich, M.; Austarheim, I.; Wangensteen, H.; Malterud, K.E.; Slimestad, R.; Barsett, H. Immunomodulating activityof Aronia melanocarpa polyphenols. Int. J. Mol. Sci. 2014, 15, 11626–11636. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Wang, M.; Zheng, J.; Ou, S. Review. Positive and negative effects of polyphenol incorporation in baked foods. Food Chem. 2019, 2984, 90–99. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).