Abstract
The continuous development of the industry of composite materials and epoxy resins requires the development of components that modify these systems. It is extremely beneficial to modify functionality by using one or two substances instead of modifying only one system parameter. Typically, this end-use will determine the key parameters of the resin system that should be modified and the modification systems designed as such. In this study, we introduce novel systems utilizing ionic liquids, strategically designed to concurrently alter multiple system parameters, including: (i) flexibility, (ii) crosslinking density, and (iii) fire resistance. The following techniques were used in the research: (i) Differential Scanning Calorimetry (DSC), (ii) Thermogravimetric Analysis (TGA), (iii) Dynamic Mechanical Analysis (DMA) and (iv) fire performance tests (UL-94, Limiting Oxygen Index and Mass loss type cone calorimetry (MLC)) to show as much dependence of material parameters on the type of modifying additive as possible. Both the cured resin and the curing process as well as a single-layer composite reinforced with carbon fiber were tested. The results show that properly designed ionic liquids are able to perform many functions in the composite material and simultaneously affect several parameters, both by lowering and increasing them. In addition, they can exhibit activity in the field of flame-retardant composites.
1. Introduction
Composite materials based on epoxy resins and reinforcing fibers are the backbone of polymer materials used widely around the world. It is a valuable construction material in areas such as electronics (equipment housings, electronic components) [1], paints and coatings [2], construction (construction material used both indoors and outdoors) [3], or transportation (construction materials in aviation, automotive, marine or railroad industries) [4]. It owes its popularity due to its high tensile strength, high adhesion to substrates, good corrosion resistance, chemical resistance, and moisture resistance [5,6].
The biggest disadvantage of this group of materials is their flammability, resulting in the generation of large amounts of heat and the formation of a number of combustion products that are harmful to human health, such as: (i) toxic gases (HBr, HCl), (ii) benzene derivatives and higher aromatic hydrocarbons (PAHs), (iii) non-flammable gases (CO2, NOx, SOx), (iv) flammable VOCs (methane, ethane) or (v) organic irritants, the composition of which is determined by the components of the resin and the overall composite [6,7,8,9,10].
Glass and carbon fibers are the most widely used fiber reinforcement in polymer composites. Although both reinforcements can be considered inert materials that significantly reduce the heat release rate compared to the polymer matrix, they often need additional flame retardancy to comply with the relevant fire safety standards. Flame retardant compounds containing halogens are known, but their use is not friendly to health and the environment as their combustion products are mainly toxic gases (HCl, HBr, HCN). Restrictive requirements for flame retardants and their effectiveness and properties (especially in terms of environmental and health impacts) are forcing the search for halogen-free solutions. Organophosphorus compounds used in composites to increase their thermal stability can be described as reactive (such as 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide, DOPO [9,11]) or non-reactive (such as triphenyl phosphate, TPP [12,13,14,15]). Their flame-retardant effect is directly related to the form in which phosphorus is present in them and can sometimes consist, for example, of generating large amounts of foam during combustion, which prevents further ignition (swelling when exposed to fire). Silicon-based flame retardants (e.g., borosilicates) are preferred to reduce flammability and suppress smoke, which is extremely beneficial in the case of epoxy resins [6]. These flame retardants are widely regarded as environmentally friendly and can be combined with each other: (i) synergy between silicon and phosphorus compounds, (ii) synergy between phosphorus and nitrogen compounds, and (iii) synergy of organophosphorus compounds with inorganic filler (metal oxides and hydroxides) [6,16,17,18]. Admittedly, inorganic fillers such as magnesium hydroxide and magnesium oxide can impart flame-retardant properties of epoxy resin, but to meet the flame-retardancy criterion they need to be used in high concentrations, which deteriorates the mechanical properties of the final material. The search for effective flame retardants, using synergistic interaction between elements that are biodegradable or environmentally friendly and easily scalable from the lab to industry, is currently a challenge in many research groups around the world [6].
Ionic liquids, denoted as ILs, constitute a unique category of chemical compounds defined by their distinctive ionic architecture. These compounds consist of a cation and an anion, each endowed with specific and remarkable attributes that set them apart from other chemicals. Typically, the cation component of ionic liquids originates from organic sources and manifests a wide array of structural variability. Of particular scientific interest is the fact that the positive charge of the cation may be localized on a variety of atoms, frequently encompassing nitrogen, phosphorus, or sulfur, and affording the potential for diverse functional groups within the cation’s molecular framework. Additionally, ILs offer the flexibility to incorporate anions spanning the spectrum from organic to inorganic, and the choice of anionic species exerts a profound influence on the nuanced properties exhibited by the entire IL compound [5].
So, a small group of imidazolium ILs is known, which in their structure have organosilicon groups, mainly based on siloxanes [19,20]. Recent papers propose the use of this type of compound as electrolytes in lithium-ion batteries [19] and discuss their potential areas of application as surfactants with the ability to specific aggregation in aqueous solutions [21]. In addition, the ability to store hydrogen in this type of ionic liquid was also presented [22], but in most published works, attention is focused on the synthesis and properties of these quaternary salts, and the scope of structures is limited to the basic ones, i.e., imidazolium and ammonium ionic liquids.
On the other hand, ionic liquids containing a phosphorus atom in their structure can be divided into two groups: (i) phosphonium ionic liquids (PhILs) in which the positive charge of the cation is located on the phosphorus atom, and (ii) ionic liquids in which the phosphorus atom is located in the alkyl chain or the anion and is devoid of a direct charge. The precursors of PhILs are mostly various types of phosphines. However, the high sensitivity of phosphines to air requires advanced synthetic equipment that provides an appropriate environment for the process. This feature significantly limits the possibilities of free design of this type of ILs, and only a small group of phosphines is known that show low sensitivity to air. Nevertheless, the applications of PhILs are diverse and in recent years the areas of their use have been presented as catalysts for the cycloaddition of carbon dioxide to epoxides [23], anticorrosive additives for pigments in epoxy coatings [24] or in the extraction of gold from thiosulfate solutions [25]. Moreover, many applications of this group of compounds in catalysis and carbon dioxide absorption are known [26,27]. In contrast, ionic liquids containing charge-free phosphorus in their structure are simpler to synthesize and, in most cases, do not require special conditions. This group of compounds has found use primarily in cellulose dissolution processes [28,29] and electrochemistry [30].
ILs have a number of advantages for use in composite materials and resin systems themselves. One of their most important properties is their ability to initiate the polymerization process of the epoxy resin [5,31,32]. ILs can be designed to predict their performance as cure initiators in a selected range of temperatures and intensities. The curing process can be either cationic or anionic, and the amount of ionic liquid required for the curing process is much smaller than for classical amine curing agents. Critical to the success of the process is the temperature reached by the resin system, as the system itself is inactive at room temperature, which also brings the advantages of a very long gelation time (up to 60 days) and the possibility of long-term storage at room temperature of the already finished resin/hardener mixture in the form of ionic liquid. Ionic liquids affect the properties of the final material through the reduction in the crosslinking density of the resin after curing, resulting in an increase in the stress intensity factor and tensile strength without significant loss of other mechanical properties [33]. In addition, ionic liquids have a scientifically proven effect on fibers of various types. Flax fibers functionalized using an ionic liquid—for example, didecyldimethylammonium nitrate—become resistant to the growth of microorganisms [34]. The antifungal efficacy is derived from a lengthy alkyl chain. However, the most important ability of ionic liquids with respect to different types of fibers is their ability to initiate and stimulate the fibrillation process of the fiber [35,36]. This phenomenon involves the splitting of the main structure of the fiber with the simultaneous formation of a network of microfibers that diverge from the main stem. During fibrillation, the fibers additionally undergo twisting by which a layer of microfibers is formed around the shaft. So far, the effect of ionic liquids in initiating fiber fibrillation has been successfully confirmed for both: natural [37] (silk, cellulose) and synthetic (aramid) fibers [38]. Recent studies show the potential of ionic liquids to impart fire-resistant material characteristics to composites. For this purpose, work is carried out with the use of PhILs, from which it is known to use, for example, ethyltributylphophonium diethyl phosphate [39], 1-vinyl-3-(diethoxyphosphoryl)-propylimidazolium bromide [40] or imidazolium diphenylphosphinate [41], which can act as both a flame-retardant and an epoxy resin hardener. Sometimes, to increase these properties, admixtures of graphene suspended in an ionic liquid are used [16].
Research on flame retardants in recent years has focused on the search for new compounds with effective action and has largely consisted of organic synthesis of substances based on previously known flame retardants (structural improvements of substances, new derivatives) or synthesis of completely new molecules. The mechanism of flame retardancy is, in general, a set of physicochemical reactions and processes that can occur in the gaseous (flame) or condensed (plastic, decomposition products) phase [12,18]. Predicting and designing the mechanisms of flame retardancy is a difficult task [42], and studies show that even compounds containing the same amount of phosphorus, with very similar spatial structures, can flame retard the material in different ways. This is due to dependencies in the molecule such as (i) susceptibility to esterification reactions (hydroxyl groups, acid groups) or (ii) the ability of the flame retardant to migrate inside the resin network [18]. Typically, a flame retardant can simultaneously act in both the gaseous and condensed phases but maintain a preference for one or the other. The mechanism of flame retardancy focuses on the following effects: (i) reducing heat release during combustion, (ii) reducing the amount of smoke and gas released during combustion, and (iii) restricting oxygen, not sustaining combustion [43,44]. However, research on flame retardants has centered around the use of additive-type flame retardants, such as cyclophosphazene derivatives [7], DOPO (9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide), or ammonium polyphosphates and resorcinol bis(diphenylphosphate) [15]. Although the synergistic effect between ionic liquids and inert compounds has been studied [13,14,16], it has not been considered in the context of using as flame retardants only ionic compounds such as ionic liquids, which, containing phosphorus, silicon, and nitrogen atoms, may also exhibit a synergistic effect.
The examples cited illustrate the extensive impact of ionic liquids on resin systems and composites, highlighting the multitude of factors that must be carefully considered to accurately evaluate their cumulative effects on specific materials. At the same time, the cited examples inspire and justify the study of ionic liquids in the context of the search for multifunctional compounds that can perform a function in the area of composite materials on many levels. The aim of the study was to explore the possibility of creating a multifunctional system that modifies both the properties of the epoxy resin and the composite itself on multiple levels. The key task was to identify the areas of influence of the modifications and to assess the intensity of their impact. As a result, a resin system based on an epoxy resin and three ionic liquids was developed: (i) an ionic liquid that is a curing agent, and (ii) a system of two ionic liquids containing silicon and phosphorus in their structure, affecting the parameters of the resin and the carbon composite produced on its basis.
2. Materials and Methods
2.1. Materials
All organic reagents were purchased from Merck, Germany (3-Chloropropyl)trimethoxysilane 97%, 1-methylimidazole 99%, organic solvents with a minimum HPLC grade). Ionic liquids were purchased from IoLiTec GmbH, Heilbron, Germany. Epoxy resin, EnviPoxy 525 (Bisphenol A (BPA) type resin with biocarbon content) was purchased from Spolchemie a.s., Usti nad Labem, Czech Republic. Twill (2 × 2) carbon fabric with a weight of 600 g/m2 was purchased from G. Angeloni S.r.l., Venice, Italy. For all ionic liquids, both purchased and synthesized, Karl-Fisher water content titration was performed as a routine procedure. The water content value for purchased ILs did not exceed 100 ppm and for the synthesized IL the water content value did not exceed 150 ppm. Therefore, they were considered dry, and the water content was negligible during the evaluation of the results.
2.2. Ionic Liquids
A total of seven ionic liquids were selected for the study. Five of them are liquids containing phosphorus atoms in their structure, while the sixth is an ionic liquid containing silicon and the seventh is an ionic liquid curing agent. All the structures of the ionic liquids are shown in Figure 1.
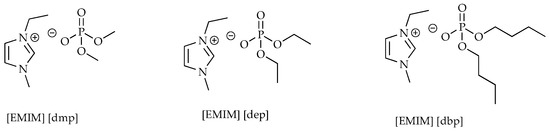

Figure 1.
Structures of ionic liquids.
2.2.1. Synthesis and Analysis of 1-methyl-3-(3-(trimethoxysilyl)propyl)imidazolium Chloride
- Synthesis and analysis
A solution of 1-methylimidazole (10 g, 0.122 mol, 1.0 eq.) in acetonitrile (50 mL) was heated up to 65 °C and then (3-chloropropyl)trimethoxysilane (24.24 g, 0.122 mol, 1.0 eq., 27.6 mL) was added dropwise. The reaction scheme is shown on Figure 2. The reaction mixture was stirred under a reflux condenser for 72 h. Afterwards, the acetonitrile was evaporated, and the resultant liquid was dissolved in water and then washed three times with diethyl ether. The water solution was evaporated, resulting in a viscous, yellow liquid (25 g, 73.11%).

Figure 2.
Reaction scheme of [TMSPrMIM] [Cl] synthesis.
1H NMR (400 MHz, CDCl3): δ = 9.67 (s, 1H), 7.32 (d, J = 1.8 Hz, 1H,), 7.16 (d, J = 1.8 Hz, 1H,), 3.75 (dt, J = 14.7 Hz, J = 7.0 Hz, 2H), 3.49 (s, 3H), 2.90 (s, 9H), 1.37 (dd, J = 9.7 Hz, J = 6.0 Hz, 2H), 0.01 (ddd, J = 16.7 Hz, J = 10.2 Hz, J = 6.6 Hz, 2H)
13C {1H} NMR (100 MHz, CDCl3): δ = 133.56, 122.39, 120.78, 48.95, 48.77, 34.71, 22.47, 4.25.
2.2.2. Preparation of Modification Formulations and Epoxy System
Six of the seven ionic liquids were used to prepare modification systems (Table 1, A–E) for the epoxy resin. For this purpose, each ionic liquid containing phosphorus in its structure was used to create a mixture (at an elevated temperature around 50 °C) with an ionic liquid containing silicon in equimolar ratios.

Table 1.
Parameters of modification systems based on ILs.
2.3. Preparation of Carbon Fiber-Reinforced Epoxy Composites
A resin system consisting of: (i) an epoxy resin (ER), (ii) an ionic liquid acting as a curing agent (CA, 2 g/98 g of ER), and (iii) a modification system based on silicon- and phosphorus-containing ionic liquids (A–E, 10 g/90 g of ER with CA), has been used to prepare prototype structures of carbon composites, using hand lamination. The prepared composites were in the form of monolayers with dimensions of 30 × 30 cm and a thickness of about 1 to 2 mm. The ratio of fiber saturation with resin was 40 to 42% wt. Then, carbon preimpregnates were annealed at 120 °C for a period of 4 h. In addition, a reference (R) composite, devoid of modifying ionic liquids, was also produced.
2.4. Test Methods
2.4.1. Thermal Analysis
Both differential Scanning Calorimetry and thermogravimetric analysis were performed using a method described in previous papers by the authors. Detailed measurement parameters and curves are presented in the Supplementary Materials.
2.4.2. Dynamic Mechanical Analysis (DMA)
Dynamic Mechanical Analysis was performed using a method described in previous papers by the authors. Detailed measurement parameters and curves are presented in Supplementary Materials.
2.4.3. Fire Behavior
UL-94 flammability tests, limiting oxygen index (LOI) tests, and Mass loss type calorimetry (MLC) were performed according to: (i) ASTM D3081 and ASTM D635 (UL-94), (ii) ASTM D2863 (LOI), and (iii) ISO 13927 (MLC). Detailed measurement parameters are presented in Supplementary Materials.
3. Results and Discussion
3.1. Synthesis and Preparation of Modification Formulations Based on Ionic Liquids
In the first stage of the research, modification systems based on six ionic liquids were produced. The goal was to produce such systems that would differ in (i) the chemical structure of the components, (ii) the content of phosphorus and silicon and the ratio of the two to each other, and (iii) the form of phosphorus bonding in the ionic liquid molecule (tertiary phosphorus atom in the cation or phosphorus in the form of organic phosphate). This goal was achieved, and the mixtures produced remained stable throughout the research period.
Nevertheless, it should be noted that all the ionic liquids selected in the study were present at room temperature in the liquid state, which greatly facilitated their preparation. On the basis of the conducted studies, it was observed that the greater the similarity of the cation structure of the two components of the mixture, the more homogeneous it was (mixtures A, B, and C). In contrast, when the ionic liquids differ significantly in their structure (D and E), the formation of emulsions, rather than homogeneous systems, was observed. The emulsion phenomenon is more pronounced, the longer a given mixture is stored and manifests itself in the gradual disintegration of the emulsion into individual phases. Despite the use of elevated temperatures during the preparation of mixtures, this effect could not be eliminated. The observed phenomenon seems to be typical for these types of systems and well understood, if only during studies considering formation of eutectic mixtures of ionic liquids. Two or more ionic liquids will therefore form more stable and homogeneous systems with each other if their structural similarity is greater and they can commonality the individual ions in their composition.
The process of formation of the final epoxy system is shown graphically in Figure 3. The total content of ionic liquids in the system varies at about 12% by weight, with about 2% by weight being [EMIM] [dca], which acts as a cure initiator (CA) in the epoxy system. The mode of action and properties of this liquid as a curing agent have been studied by numerous groups of scientists [32,45,46,47,48], and it is because this substance is so well known that it was chosen for this study. The polymerization of the epoxy resin initiated by [EMIM] [dca] starts around 80–100 °C, depending on the type of resin. The process is characterized by the presence of two thermal peaks on the DSC thermogram, which can indicate simultaneous cationic polymerization (initiated by IL cation) and anionic polymerization (initiated by [dca] anion). The polymerization peak occurs in the temperature range of 140–180 °C. After the epoxy resin system is properly prepared with CA, a previously prepared modifying system based on phosphorus- and silicon-donor ILs is added with an amount of 10% by weight. Therefore, five homogeneous epoxy systems were obtained that remained stable for the duration of the conducted tests and did not cure at room temperature for at least 30 days (the test period). The systems containing phosphonium ionic liquids were characterized by the presence of a light-yellow full color, which limited the transparency parameters of the system.

Figure 3.
Schematic presentation of epoxy systems prepared with ionic liquids.
Table 2 summarizes the most important parameters of the obtained systems. The systems were characterized by a relatively equal mass content of phosphorus (0.5–0.6 g in terms of pure phosphorus for every 100 g of resin system). However, noticeable differences are observed in the case of silicon content, which varies between 0.2 and 0.5 g for every 100 g of the resin system. Such an assumption made it possible to thoroughly investigate the effect of the ratio of the various elements on the achieved modification effect of both the cured resin and the carbon fiber composite.

Table 2.
Composition of the epoxy systems with ionic liquids.
3.2. Curing Properties of Modified Epoxy Resin Formulations
Thermal Analysis
DSC analysis was performed for all resin systems, the results of which are summarized in Table 3. All modified systems relative to the reference, containing only [EMIM] [dca] as a curing agent, showed a decrease in onset temperature values, even by 24–25% (C and E). At the same time, there was also a decrease in the temperature of the maximum thermal peak in all modified systems relative to the reference. A proposed explanation for this phenomenon is the aggregate increase in the concentration of 1-ethyl-3-methylimidazolium cation in the systems, thereby shifting the polymerization equilibrium toward cationic polymerization. This fact also seems to be confirmed by the decrease in intensity or even the almost complete disappearance of the second thermal peak observed in the case of thermograms for the modified systems, indicating the almost complete elimination of anionic polymerization (C and D, thermograms in Supplementary Materials). In addition, compounds with long alkyl chains introduced into the systems can also affect the reduction of the temperatures of thermal transformations occurring during the polymerization of the epoxy resin. In the case of the total enthalpy of the process, a decrease in its value was observed in all cases, with a marked change of 14% for system E.

Table 3.
Characteristic of the curing process, determined with DSC.
Similarly, according to the results obtained, a decrease in the glass transition temperature values of the modified systems was observed. The average decrease in the Tg value was 41% with respect to the reference value, reaching the largest decrease for system B. At the same time, systems D and E, containing phosphonium ionic liquids, recorded the lowest decrease at 33 and 34%, respectively, regarding the reference. The results obtained allow us to conclude that, despite the generally observed plasticizing effect achieved by modifying the resin system with ILs, this phenomenon can be somewhat controlled and due to the long-chain alkyl compounds, be significantly reduced.
The effect of modifying the resin system with ionic liquids on thermal stability was investigated using thermogravimetric analysis (TGA), the results of which are presented in Table 4. The following parameters were used in the analysis of the results: (i) the temperature at which the mass of the sample is reduced by 5% (T5%), (ii) the temperature when 50% of the sample decays (T50%), (iii) the maximum mass loss rate (dTGmax), and (iv) the maximum mass loss rate temperature (TdTGmax). For all the tested systems, the thermal stability decreases, however, in the case of system E, these changes are practically imperceptible.

Table 4.
Thermogravimetric analysis results of the reference and samples modified with ionic liquids.
For system E, containing a PhIL and [TMSPrMIM] [Cl] and at the same time a two-fold excess of total phosphorus in relation to silicon, a decrease in the T50% value by only 2% was observed, while a decrease in TdTGmax by 1%. In addition, T5% decreased by 18% compared to the reference, and the maximum mass loss rate was reduced to 2%/°C). In all systems, the decrease in the value of T5% was at the level of 18–20%. In turn, the results obtained for system B, which is characterized by the highest total content of phosphorus and silicon (with a predominance of phosphorus), significantly differ from the entire matrix. The T5% and T50% values, reduced by 29% and 11% respectively, indicate that this system can exhibit flame retardant activity in the gas phase, unlike the other systems where this activity is expected rather in the solid phase. For all systems, the maximum mass loss rate was reduced by an average of 41%.
3.3. Carbon Fiber Reinforced Composites’ Properties
DMA
Dynamic Mechanical Analysis was used to investigate the properties of carbon fiber-reinforced epoxy composites produced using ionic liquid systems. Table 5 summarizes the results of the determined glass transition temperatures for the composites, using data on storage modulus (E’), tan delta values (tan δ), and loss modulus (E’’). The value of the Tg, which actually represents a certain range of temperatures, was presented as a specific numerical value in order to evaluate the performance of the obtained materials. The results obtained in the DMA analysis were compared with the Tg result determined during the DSC analysis of the resin systems themselves without carbon fiber reinforcement. The results show how much influence the reinforcement has on the properties of the final material. Moreover, for all the systems modified with ionic liquids, a significant increase in the glass transition temperature value was recorded compared to the reference. Systems A, B, and C show an increase in Tg values of nearly 50%, while for system E the increase was 75%. To assess the effect of the modification on the polymer network inside the material, the crosslinking density was determined, the value of which for each system translates into an increase in the glass transition temperature, which is the expected result. Crosslinking density was determined by a mathematical equation presented in several other works [49,50]. A denser network inside the material reduces the potential of the polymer chain to stress and rotate by making the final material stiffer and stronger. An explanation for this phenomenon may be that ionic liquids supplied to the system build into the polymer structure, and the presence of long chains stabilizes bond rotation. Interestingly, in the case of resin systems without carbon fiber reinforcement, the trend in the change in glass transition temperature values is opposite to that observed for fabricated composite structures. This primarily demonstrates the influence of factors such as reinforcement, fibers, sample size, or, most importantly, the technique of determining the parameter, on the value of glass transition temperatures.

Table 5.
Glass transition temperatures and crosslinking density of the reference and samples modified with ionic liquids. Glass transition for pure epoxy resin with ionic liquids determined with DSC. Glass transition temperatures and crosslinking density for carbon-reinforced composite samples determined with DMA.
Complementing the Tg analysis is the effect of temperature on the E’ value and the intensity of the shift of this value toward lower values depending on the modification system. The results presented in Table 6 indicate that the sample modified with the D system retains the most similar storage modulus parameters at 28 °C compared to the reference. In the case of the other systems, a shift towards more elastic rather than rigid materials is observed. On the other hand, the situation changes dramatically if we consider the rate at which the value of the parameter E’ changes with increasing temperature. In this comparison, the most stable in relation to the reference is the E system, while the fastest change is observed in the D sample, which, as noted earlier, at a temperature of 28 °C retained the value of E’ closest to the reference, while at temperature 75 °C this value decreased by almost half in relation to the reference. We suppose that the modifying system D influences the elasticity of the composite material the most among all those tested, however, systems A, C, and E also show noticeable activity in this direction.

Table 6.
Storage modulus E′ for carbon-reinforced composite samples of the reference and samples modified with ionic liquids.
3.4. Fire Performance
3.4.1. UL-94 and LOI
The final stage of the research was to evaluate the ability of the produced ionic liquid-based modification systems to flame retard the epoxy resin. For this purpose, the UL-94 test was first performed and the LOI was determined, the results of which are summarized in Table 7. Tests were conducted for resin samples without reinforcement. The obtained results allow us to assume that at a concentration of 10% by weight in the epoxy material, the tested modifying systems do not show significant flame-retardant properties towards the resin, which, considering the actual amount of phosphorus and silicon introduced into the system, was an expected result. Nevertheless, some dependencies are observed between the modifying system and the flame-retardant properties. Although, according to the classification, all materials received the HB rating, the LOI value increased to 30 V/V% for the E system. The result is also interesting because it is the E system that contains the smallest total amount of phosphorus and silicon. It is assumed that in the case of the tested systems, the oxygen index was influenced not so much by the content or presence of silicon and phosphorus, but by the extensive structure of the phosphonium ionic liquid molecule with numerous long alkyl chains.

Table 7.
Limiting oxygen index and UL-94 results of the reference and samples modified with ionic liquids.
3.4.2. MLC
For samples of composites reinforced with carbon fiber, a test was carried out using mass loss type cone calorimetry, the results of which are presented in Table 8. In this case, System E showed a 34% increase in pHHR relative to the reference value (143 vs. 107 kW/m2) with a simultaneous shift in time of pHHR from 32 to 36 s, while System A showed a 24% increase (133 vs. 107 kW/m2). A slight decrease of this value was observed for systems B and D and amounted to 7 and 3%, respectively. THR values have been reduced the most in the case of system B (4.8 MJ/m2), however, this difference (16%), compared to the reference (5.7 MJ/m2), proves that the flame-retardant properties are insufficient. No significant effect of modifying systems on the amount of residues was observed. We suppose that, despite the changes in individual values during the analysis, the amount of the modifying system used in the composite and the amounts of silicon and phosphorus related to it were not enough to significantly delay the development of the fire.

Table 8.
Mass loss calorimetry results of the reference and samples modified with ionic liquids.
4. Conclusions
This study presented multifunctional modifying systems based on ionic liquids for epoxy resin. The proposed modifying systems introduced into the epoxy composite in an amount of 10% by weight: (i) increase the degree of crosslinking of the polymer matrix, (ii) increase the glass transition temperature, and (iii) improve the fire resistance of the material. Moreover, the produced systems showed a significant effect on the value of storage modulus, allowing for control of this parameter depending on the modifying system used. The studied systems showed an effect on increasing the flexibility of the fabricated structures. The paper pointed out the determining effect of the structure of the ionic liquids included in the modifying system, and in particular, the influence of long alkyl chains on individual material parameters such as crosslinking density. In addition, all modifying systems contained silicon and phosphorus atoms in various ratios, but due to the limiting content of the modifying system in the resin, it was not possible to thoroughly investigate the effect of the presence of these elements on individual parameters, in particular, on fire resistance. This aspect will be more thoroughly studied in future work. To summarize, the most important result of the present work is the confirmation, that ionic liquids can simultaneously modify many parameters both in the epoxy resin itself and in the composite, and each time the use of these substances and their effect on the final material should be studied in a multifaceted manner. Properly selected ionic liquids can be great alternatives as functional additives in composite materials with more than one functionality, but sometimes the wrong liquid can result in improving some values at the expense of worsening others.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app131910661/s1, 1. NMR spectrum, 2. DSC curves, 3. TGA curves, 4. DMA curves and 5. MLC curve.
Author Contributions
Conceptualization, D.Z., A.T. and A.S.; Formal analysis, D.Z.; Funding acquisition, M.S.; Investigation, D.Z., A.S., Á.P., Z.K., B.P. and B.S.; Methodology, D.Z., A.T. and M.S.; Software, D.Z., B.S., B.P. and A.S.; Supervision, A.T. and M.S.; Validation, D.Z., A.T. and M.S.; Visualization, D.Z.; Writing—original draft, D.Z.; Writing—review & editing, D.Z., A.T., Á.P., A.S. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Science Centre, grant number 2017/26/E/ST8/01059 and grant number 2019/35/B/ST8/03736. This research was funded by the National Research, Development and Innovation Office (NKFIH K142517).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Qu, L.; Sui, Y.; Zhang, C.; Dai, X.; Li, P.; Sun, G.; Xu, B.; Fang, D. Improved Flame Retardancy of Epoxy Resin Composites Modified with a Low Additive Content of Silica-Microencapsulated Phosphazene Flame Retardant. React. Funct. Polym. 2020, 148, 104485. [Google Scholar] [CrossRef]
- Kumar, S.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent Development of Biobased Epoxy Resins: A Review. Polym. Plast. Technol. Eng. 2018, 57, 133–155. [Google Scholar] [CrossRef]
- Spee, T.; Van Duivenbooden, C.; Terwoert, J. Epoxy Resins in the Construction Industry. Ann. N. Y. Acad. Sci. 2006, 1076, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Khan, R.; Khan, N.; Aamir, M.; Pimenov, D.Y.; Giasin, K. Effect of Seawater Ageing on Fracture Toughness of Stitched Glass Fiber/Epoxy Laminates for Marine Applications. J. Mar. Sci. Eng. 2021, 9, 196. [Google Scholar] [CrossRef]
- Zielinski, D.; Szpecht, A.; Hinc, P.; Maciejewski, H.; Smiglak, M. Mono N-Alkylated DABCO-Based Ionic Liquids and Their Application as Latent Curing Agents for Epoxy Resins. ACS Appl. Polym. Mater. 2021, 3, 5481–5493. [Google Scholar] [CrossRef]
- Zhi, M.; Yang, X.; Fan, R.; Yue, S.; Zheng, L.; Liu, Q.; He, Y. A Comprehensive Review of Reactive Flame-Retardant Epoxy Resin: Fundamentals, Recent Developments, and Perspectives. Polym. Degrad. Stab. 2022, 201, 109976. [Google Scholar] [CrossRef]
- Seraji, S.M.; Gan, H.; Swan, S.R.; Varley, R.J. Phosphazene as an Effective Flame Retardant for Rapid Curing Epoxy Resins. React. Funct. Polym. 2021, 164, 104910. [Google Scholar] [CrossRef]
- Fang, M.; Qian, J.; Wang, X.; Chen, Z.; Guo, R.; Shi, Y. Synthesis of a Novel Flame Retardant Containing Phosphorus, Nitrogen, and Silicon and Its Application in Epoxy Resin. ACS Omega 2021, 6, 7094–7105. [Google Scholar] [CrossRef]
- Varganici, C.-D.; Rosu, L.; Bifulco, A.; Rosu, D.; Mustata, F.; Gaan, S. Recent Advances in Flame Retardant Epoxy Systems from Reactive DOPO–Based Phosphorus Additives. Polym. Degrad. Stab. 2022, 202, 110020. [Google Scholar] [CrossRef]
- Liu, X.-F.; Xiao, Y.-F.; Luo, X.; Liu, B.-W.; Guo, D.-M.; Chen, L.; Wang, Y.-Z. Flame-Retardant Multifunctional Epoxy Resin with High Performances. Chem. Eng. J. 2022, 427, 132031. [Google Scholar] [CrossRef]
- Hu, J.; Shan, J.; Zhao, J.; Tong, Z. Isothermal Curing Kinetics of a Flame Retardant Epoxy Resin Containing DOPO Investigated by DSC and Rheology. Thermochim. Acta 2016, 632, 56–63. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Qi, X.; Qian, L. The Pyrolysis Behaviors of Phosphorus-Containing Organosilicon Compound Modified APP with Different Polyether Segments and Their Flame Retardant Mechanism in Polyurethane Foam. Compos. Part B Eng. 2019, 173, 106784. [Google Scholar] [CrossRef]
- Zhu, T.; Zhou, X.; Guo, G.; Chai, Z.; Gao, M. Synergistic Flame Retardant Effect between Ionic Liquid Functionalized Imogolite Nanotubes and Ammonium Polyphosphate in Epoxy Resin. Polymers 2023, 15, 1455. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.-W.; Zhao, X.; Fu, T.; Li, D.-F.; Guo, Y.; Wang, X.-L.; Wang, Y.-Z. Synergy Effect between Quaternary Phosphonium Ionic Liquid and Ammonium Polyphosphate toward Flame Retardant PLA with Improved Toughness. Compos. Part B Eng. 2020, 197, 108192. [Google Scholar] [CrossRef]
- Pomázi, Á.; Krecz, M.; Toldy, A. The Effect of the Combined Application of Solid- and Gas-Phase Flame Retardants in Epoxy Gelcoats on the Thermal Stability, Fire Performance and Adhesion of Coated Carbon Fibre–Reinforced Epoxy Composites. J. Therm. Anal. Calorim. 2022, 148, 257–270. [Google Scholar] [CrossRef]
- Li, X.; Liang, D.; Li, K.; Ma, X.; Cui, J.; Hu, Z. Synergistic Effect of a Hypophosphorous Acid-Based Ionic Liquid and Expandable Graphite on the Flame-Retardant Properties of Wood–Plastic Composites. J. Therm. Anal. Calorim. 2021, 145, 2343–2352. [Google Scholar] [CrossRef]
- Chen, C.; Song, W.; Jiang, M.; Zhang, R.; Li, S.; Cai, Y.; Yao, J. SiO2/MOFs-Based Synergistic Flame Retardants Provide Enhanced Fire Safety for Epoxy Resins. Mater. Today Commun. 2023, 35, 105805. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, W.; Zhang, M.; Cui, Y.; Zhang, K.; Qu, H. TGA-FTIR and Char Residue Analysis: Studying the Synergistic Flame Retardancy Mechanism of Ionic Liquid-Modified Graphene and Hexaphenoxy Cyclotriphosphazene. J. Therm. Anal. Calorim. 2022, 147, 13253–13259. [Google Scholar] [CrossRef]
- Chavan, S.N.; Tiwari, A.; Nagaiah, T.C.; Mandal, D. Ether and Siloxane Functionalized Ionic Liquids and Their Mixtures as Electrolyte for Lithium-Ion Batteries. Phys. Chem. Chem. Phys. 2016, 18, 16116–16126. [Google Scholar] [CrossRef]
- Kubo, T.; Koge, S.; Ohshita, J.; Kaneko, Y. Preparation of Imidazolium Salt Type Ionic Liquids Containing Cyclic Siloxane Frameworks. Chem. Lett. 2015, 44, 1362–1364. [Google Scholar] [CrossRef]
- Wang, G.; Li, P.; Du, Z.; Wang, W.; Li, G. Surface Activity and Aggregation Behavior of Siloxane-Based Ionic Liquids in Aqueous Solution. Langmuir 2015, 31, 8235–8242. [Google Scholar] [CrossRef] [PubMed]
- Deyko, G.S.; Glukhov, L.M.; Kustov, L.M. Hydrogen Storage in Organosilicon Ionic Liquids. Int. J. Hydrogen Energy 2020, 45, 33807–33817. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Y.; Wang, S.; Dai, Z.; Xiong, Y. Synergistic Catalysis of Metalloporphyrins and Phosphonium Ionic Liquids for the Efficient Transformation of CO2 under Ambient Conditions. J. CO2 Util. 2021, 48, 101519. [Google Scholar] [CrossRef]
- Henriques, R.R.; Soares, B.G. Sepiolite Modified with Phosphonium Ionic Liquids as Anticorrosive Pigment for Epoxy Coatings. Appl. Clay Sci. 2021, 200, 105890. [Google Scholar] [CrossRef]
- Mahandra, H.; Faraji, F.; Ghahreman, A. Novel Extraction Process for Gold Recovery from Thiosulfate Solution Using Phosphonium Ionic Liquids. ACS Sustain. Chem. Eng. 2021, 9, 8179–8185. [Google Scholar] [CrossRef]
- Arkhipova, D.M.; Ermolaev, V.V.; Miluykov, V.A.; Valeeva, F.G.; Gaynanova, G.A.; Zakharova, L.Y.; Minyaev, M.E.; Ananikov, V.P. Tri-Tert-Butyl(n-Alkyl)Phosphonium Ionic Liquids: Structure, Properties and Application as Hybrid Catalyst Nanomaterials. Sustainability 2021, 13, 9862. [Google Scholar] [CrossRef]
- Rahman, M.H.; Khajeh, A.; Panwar, P.; Patel, M.; Martini, A.; Menezes, P.L. Recent Progress on Phosphonium-Based Room Temperature Ionic Liquids: Synthesis, Properties, Tribological Performances and Applications. Tribol. Int. 2022, 167, 107331. [Google Scholar] [CrossRef]
- Zhao, D.; Li, H.; Zhang, J.; Fu, L.; Liu, M.; Fu, J.; Ren, P. Dissolution of Cellulose in Phosphate-Based Ionic Liquids. Carbohydr. Polym. 2012, 87, 1490–1494. [Google Scholar] [CrossRef]
- Isik, M.; Sardon, H.; Mecerreyes, D. Ionic Liquids and Cellulose: Dissolution, Chemical Modification and Preparation of New Cellulosic Materials. Int. J. Mol. Sci. 2014, 15, 11922–11940. [Google Scholar] [CrossRef]
- Tiago, G.A.O.; Matias, I.A.S.; Ribeiro, A.P.C.; Martins, L.M.D.R.S. Application of Ionic Liquids in Electrochemistry—Recent Advances. Molecules 2020, 25, 5812. [Google Scholar] [CrossRef]
- Zielinski, D.; Szpecht, A.; Hinc, P.; Smiglak, M. Synthesis and Behavior of Hexamethylenetetramine-Based Ionic Liquids as an Active Ingredient in Latent Curing Formulations with Ethylene Glycol for DGEBA. Molecules 2023, 28, 892. [Google Scholar] [CrossRef] [PubMed]
- Rahmathullah, M.A.M.; Jeyarajasingam, A.; Merritt, B.; VanLandingham, M.; McKnight, S.H.; Palmese, G.R. Room Temperature Ionic Liquids as Thermally Latent Initiators for Polymerization of Epoxy Resins. Macromolecules 2009, 42, 3219–3221. [Google Scholar] [CrossRef]
- Fonseca, E.; Demétrio da Silva, V.; Klitzke, J.S.; Schrekker, H.S.; Amico, S.C. Imidazolium Ionic Liquids as Fracture Toughening Agents in DGEBA-TETA Epoxy Resin. Polym. Test. 2020, 87, 106556. [Google Scholar] [CrossRef]
- Foksowicz-Flaczyk, J.; Walentowska, J. Antifungal Activity of Ionic Liquid Applied to Linen Fabric. Int. Biodeterior. Biodegrad. 2013, 84, 412–415. [Google Scholar] [CrossRef]
- Berglund, L.; Anugwom, I.; Hedenström, M.; Aitomäki, Y.; Mikkola, J.-P.; Oksman, K. Switchable Ionic Liquids Enable Efficient Nanofibrillation of Wood Pulp. Cellulose 2017, 24, 3265–3279. [Google Scholar] [CrossRef]
- Eksiler, K.; Andou, Y.; Yilmaz, F.; Shirai, Y.; Ariffin, H.; Hassan, M.A. Dynamically Controlled Fibrillation under Combination of Ionic Liquid with Mechanical Grinding. J. Appl. Polym. Sci. 2017, 134, 44469. [Google Scholar] [CrossRef]
- Boukhriss, A.; Gmouh, S.; Hannach, H.; Roblin, J.-P.; Cherkaoui, O.; Boyer, D. Treatment of Cotton Fabrics by Ionic Liquid with PF6− Anion for Enhancing Their Flame Retardancy and Water Repellency. Cellulose 2016, 23, 3355–3364. [Google Scholar] [CrossRef]
- Kerche, E.F.; da Silva, V.D.; Fonseca, E.; Salles, N.A.; Schrekker, H.S.; Amico, S.C. Epoxy-Based Composites Reinforced with Imidazolium Ionic Liquid-Treated Aramid Pulp. Polymer 2021, 226, 123787. [Google Scholar] [CrossRef]
- Sonnier, R.; Dumazert, L.; Livi, S.; Nguyen, T.K.L.; Duchet-Rumeau, J.; Vahabi, H.; Laheurte, P. Flame Retardancy of Phosphorus-Containing Ionic Liquid Based Epoxy Networks. Polym. Degrad. Stab. 2016, 134, 186–193. [Google Scholar] [CrossRef]
- Xiao, F.; Wu, K.; Luo, F.; Guo, Y.; Zhang, S.; Du, X.; Zhu, Q.; Lu, M. An Efficient Phosphonate-Based Ionic Liquid on Flame Retardancy and Mechanical Property of Epoxy Resin. J. Mater. Sci. 2017, 52, 13992–14003. [Google Scholar] [CrossRef]
- Xu, Y.-J.; Shi, X.-H.; Lu, J.-H.; Qi, M.; Guo, D.-M.; Chen, L.; Wang, Y.-Z. Novel Phosphorus-Containing Imidazolium as Hardener for Epoxy Resin Aiming at Controllable Latent Curing Behavior and Flame Retardancy. Compos. Part B Eng. 2020, 184, 107673. [Google Scholar] [CrossRef]
- Pomázi, Á.; Toldy, A. Predicting the Flammability of Epoxy Resins from Their Structure and Small-Scale Test Results Using an Artificial Neural Network Model. J. Therm. Anal. Calorim. 2023, 148, 243–256. [Google Scholar] [CrossRef]
- Shi, X.-H.; Li, X.-L.; Li, Y.-M.; Li, Z.; Wang, D.-Y. Flame-Retardant Strategy and Mechanism of Fiber Reinforced Polymeric Composite: A Review. Compos. Part B Eng. 2022, 233, 109663. [Google Scholar] [CrossRef]
- Schartel, B. Phosphorus-Based Flame Retardancy Mechanisms—Old Hat or a Starting Point for Future Development? Materials 2010, 3, 4710–4745. [Google Scholar] [CrossRef]
- Capricho, J.C.; Fox, B.; Hameed, N. Multifunctionality in Epoxy Resins. Polym. Rev. 2020, 60, 1–41. [Google Scholar] [CrossRef]
- Demleitner, M.; Sanchez-Vazquez, S.A.; Raps, D.; Bakis, G.; Pflock, T.; Chaloupka, A.; Schmölzer, S.; Altstädt, V. Dielectric Analysis Monitoring of Thermoset Curing with Ionic Liquids: From Modeling to the Prediction in the Resin Transfer Molding Process. Polym. Compos. 2019, 40, 4500–4509. [Google Scholar] [CrossRef]
- Orduna, L.; Razquin, I.; Aranburu, N.; Guerrica-Echevarría, G. Are Ionic Liquids Effective Curing Agents for Preparing Epoxy Adhesives? Int. J. Adhes. Adhes. 2023, 125, 103438. [Google Scholar] [CrossRef]
- Liu, D.Y.; Krogstad, D.V. Self-Assembly and Phase Transformation of Block Copolymer Nanostructures in Ionic Liquid-Cured Epoxy. Macromolecules 2021, 54, 988–994. [Google Scholar] [CrossRef]
- Barszczewska-Rybarek, I.M.; Korytkowska-Wałach, A.; Kurcok, M.; Chladek, G.; Kasperski, J. DMA Analysis of the Structure of Crosslinked Poly(Methyl Methacrylate)s. Acta Bioeng. Biomech. 2017, 19, 47–53. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, M.; Lee, W.; Kim, H.-G.; Lim, C.-S.; Seo, B. Synthesis and Characterization of a Polyurethane Phase Separated to Nano Size in an Epoxy Polymer. Coatings 2019, 9, 319. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).