Abstract
This current review aims to provide an overview of the most recent research from the last 10 years on the potential of low-level light therapy (LLLT) in the orthodontic field, particularly focusing on studies about tooth movement, root resorption, pain perception during treatment, and the stability of orthodontic miniscrews. “Low-level laser,” “orthodontic,” and “LLLT” were the search terms utilized on the databases Scopus, Web of Science, and PubMed, and the Boolean operator “AND” was utilized. Of the 974 studies found, 41 publications related to our topic were included in this review. Many authors agree that LLLT could trigger an enhanced biological reaction next to the tooth in the periodontium, promoting osteoblast proliferation and differentiation, while it could also have a positive impact on bone regeneration and on increasing the rate of tooth movement, enhancing the stability of miniscrews and minimizing the occurrence of root resorption. Regarding pain management during treatment studies, the results have been controversial. Conclusions: even though further studies are still needed, the use of LLLT can improve both clinical results and patient comfort during treatment by reducing treatment duration, improving clinical aspects, such as miniscrew stability, and minimizing root resorption. Further investigations are needed to assess whether LLLT offers any real benefits regarding pain relief.
1. Introduction
LLLT in dentistry makes different dental procedures more comfortable for the patient since it is a potential alternative to some standard devices and techniques, such as local anesthesia, scalpels, and drills [1]. LLLT is a “cold light” therapy because it maintains a constant temperature throughout the procedure, which is in contrast to others that are utilized for thermal coagulation or tissue cutting and involve increasing the temperature in tissues [2,3]. Regarding the biomolecular pathway of action, LLLT employs a photochemical mechanism in which energy is transferred to intracellular mitochondrial chromophores, i.e., light-absorbing molecules, such as endogenous porphyrins and respiratory chain components such as cytochrome-C oxidase, which are capable of transferring absorbed laser energy to the mitochondria; at this level, laser energy is converted into metabolic energy via the respiratory chain with the production of adenosine triphosphate (ATP) [4,5]. The mitochondrial respiratory chains act as the main photoreceptors for LLLT at wavelengths in the visible spectrum, whereas the calcium channels at the cell membrane level serve as the key photoreceptors for LLLT at wavelengths in the infrared spectrum [6,7]. The short-term activation of the respiratory chain and oxidation of nicotinamide adenine dinucleotide (NADH) brought on by the respiratory chain’s absorption of light results in modifications to the mitochondrial and cytoplasmic redox states. The electron transport chain becomes active and causes the cytoplasm to become more alkaline, the electrical potential of the mitochondrial membrane to increase, the reserve of ATP to increase, and then the synthesis of nucleic acids to begin [8,9].
LLLT activates cells through two mechanisms:
- A direct mechanism through photobiological action (respiratory chain), where intracellular signaling is activated in redox chains (ATP increase);
- An indirect mechanism of the activation of cells via secondary messengers released by the activated cells directly [10].
Past results have demonstrated great LLLT compliance, no invasiveness, no traumatic side effects, and no collateral impacts [11,12]. These numerous advantages have made it possible to intervene in a safer and easier way, especially in patients with special needs and in small children [13]. Therefore, over recent decades, the results of LLLT have been studied in many branches of dentistry. For example, it can be used to alleviate the painful sensation caused by the needle insertion of needed anesthesia [14] or to disinfect carious lesions or root canals during ongoing devitalization through photoactivation [15], and it may also be useful in the treatment of sore mouth syndrome, although this has yet to be demonstrated with large-scale clinical trials [16]. Thanks to its effect on osteoblast proliferation and bone formation, its ability to interact selectively with the mouth cavity’s tissues, its healing effect, and its biostimulating effect favoring the healing process, the dental laser is particularly suitable for surgery/implantology [9,17]. In recent years, it has also gained popularity in the orthodontic branch, particularly for tooth movement, root resorption, pain perception during treatment, and the stability of orthodontic miniscrews.
Orthodontics has grown considerably in recent decades due to the development of extremely high-performance alloy wires and low-friction brackets. However, for most patients undergoing orthodontic treatment, pain persists as a common problem, particularly in the first 3 to 4 days after fixed orthodontic appliances have been fitted [18,19]. It is clear that this suggested symptom could reduce patients’ compliance [20] or discourage them from treatment [21]. Moreover, the average time for orthodontic treatment varies considerably from case to case, ranging from 12 to 24/36 months. The mechanical pressures used in orthodontic therapy encourage alveolar bone remodeling and consequent tooth movement, the acceleration of which is desirable, obviously within a framework of biocompatibility that does not involve damage to the tooth and supporting tissues, in order to precisely reduce the time of the therapy in question.
In this regard, due to its anti-inflammatory, analgesic, and biostimulatory effects on the tooth and periodontium, the use of LLLT has an impact on the results of orthodontic therapy [22]. Several studies have investigated whether LLLT can promote the epithelialization of the treated tissues, accelerate bone remodeling at the extraction site, improve (accelerate) tooth movement in orthodontics, promote collagen formation, including through the breakdown of pro-inflammatory cascades with analgesic/anti-inflammatory effects [5,6] and, finally, minimize pain [18,19].
In this review, we address current knowledge of the application and use of orthodontic LLLT, with a focus on studies reporting contradictory results, in an attempt to outline future perspectives and pathways.
Limitations of LLLT
Low-level light therapy (LLLT) is a promising technique, but it has some limitations:
- Variable effectiveness: it varies depending on the individual, the correct application, and the choice of light parameters;
- Results are not immediate: it often takes many operating sessions before you see significant results;
- Depth limitations: LLLT is effective primarily in treating superficial conditions, but it has limitations in deep tissue penetration;
- Risk of misuse: excessive or improper use of lasers can cause tissue damage, so they should be administered by experienced operators;
- Cost and affordability: some laser equipment can be expensive and not always affordable for everyone;
- Ongoing research: despite promising results, some applications of LLLT require further research to confirm long-term efficacy and safety.
LLLT is a complementary therapy that may be beneficial for some conditions but should be used with care and under the supervision of experienced healthcare professionals [23,24].
2. Materials and Methods
2.1. Protocol and Registration
This systematic review was conducted by the standards of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) 2020 statement [25]. The review protocol was registered at PROSPERO under the unique number 461827.
2.2. Search Processing
LLLT and Orthodontic were the search terms utilized on the databases (Scopus, Web of Science, and PubMed) to select the papers under evaluation, with the Boolean operator “AND.” The search was restricted to just items released in English during the previous ten years (March 2013–March 2023) (Table 1).

Table 1.
Database search indicators.
2.3. Eligibility Criteria
The reviewers, who worked in pairs, chose works that satisfied the following criteria for inclusion: (1) human subjects-only research, (2) clinical studies or case reports, and (3) research conducted on people receiving LLLT during orthodontic treatment.
Exclusion criteria were (1) in vitro studies, (2) animal studies, and (3) systematic reviews, narrative reviews, and meta-analyses. Duplicate studies were removed manually.
The review was conducted using the PICO criteria:
- − Population: adults and children, both male and female, who received LLLT treatment;
- − Intervention: LLLT during orthodontics;
- − Comparison: orthodontics without LLLT;
- − Outcome: effectiveness of the LLLT in orthodontic treatment, in particular regarding tooth movement, root resorption, and pain perception during treatment and the stability of orthodontic miniscrews.
2.4. Data Processing
The screening procedure, which was carried out by reading the article titles and abstracts chosen in the earlier identification step, allowed for the exclusion of any publications that varied from the themes looked at.
The complete text of publications that had been determined to match the predetermined inclusion criteria was then read.
Reviewer disagreements on the choice of the article were discussed and settled.
Quality Assessment
The quality of the included papers was assessed by two reviewers, RF and EI, using the reputable Cochrane risk-of-bias assessment for randomized trials (RoB 2). The following six areas of possible bias are evaluated by this tool: random sequence generation, allocation concealment, participant and staff blinding, outcome assessment blinding, inadequate outcome data, and selective reporting. A third reviewer (FI) was consulted in the event of a disagreement until an agreement was reached.
3. Results
Keyword searches of the Web of Science (264), Scopus (355), and PubMed (362) databases yielded a total of 981 articles. The subsequent elimination of duplicates (391) resulted in the inclusion of 590 articles. Of these 590 studies, 555 were excluded because they deviated from the previously defined inclusion criteria. The screening phase ended with the selection of 35 publications for this work (Figure 1). The results of each study are reported in Table 2.
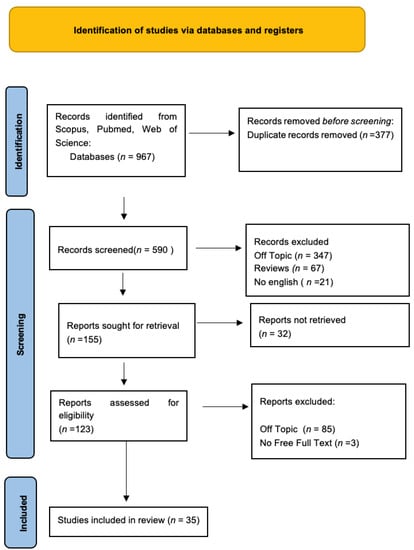
Figure 1.
PRISMA flowchart diagram of the inclusion process. The literature search’s preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram.

Table 2.
Characteristics of the studies included in the analysis.
Quality Assessment and Risk of Bias
The risk of bias in the included studies is reported in Figure 2. Regarding the randomization process, 50% of studies present a high risk of bias and allocation concealment. All other studies ensure a low risk of bias. In total, 75% of studies exclude a performance; half of the studies confirm an increased risk of detection bias (self-reported outcome), and 75% of the included studies present a low detection bias (objective measures) (Figure 2). A total of 75% of studies ensure a low risk regarding attrition and reporting bias.
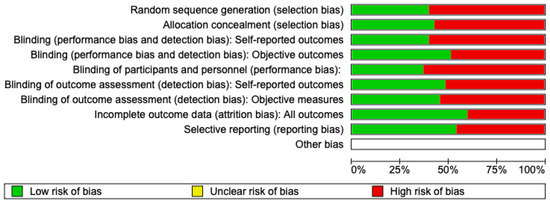
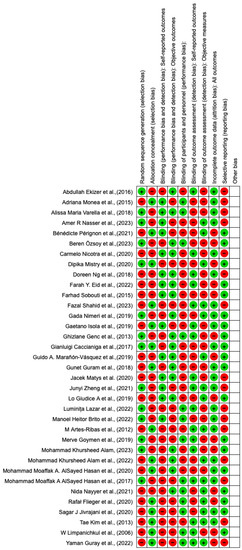
Figure 2.
Risk of bias: red indicates high risk, and green indicates low risk.
4. Discussion
4.1. LLLT and Pain
The pain caused by the use of orthodontic force originates from the biological response generated by Prostaglandin E2 and Interleukin-1b acting in the gingival crevicular fluid 1 h after the application of force [44,46]. Through LLLT, an attempt is made to modulate pain without the use of drugs that can cause tissue reactions, side effects, and inhibition of root movements [44]. LLLT is performed with lasers at about 800 nm and with minimum doses of 6 joules and no more than 10 joules [47]. A high dose should be avoided because it reduces the anti-inflammatory and analgesic effects and increases the heat of the locoregional mucosal tissues [31]. The visual analog scale (VAS) is used to assess pain, which is a subjective method but the most reliable way of assessing it [46].
Analyzing the VAS values, pain increases in the first few hours and peaks within 24 h and then decreases towards day seven [45]. Hypotheses made about the action potential of LLLT are that it modulates inflammation by reducing cytokines and mRNA and COX-2 levels, alters the conduction of peripheral nerve action potentials, reduces endogenous endorphins, and has anti-nociceptive properties [45,46]. Studies can be performed between experimental and placebo groups [45]. It should be borne in mind that the perception of pain is variable and subjective. This bias occurs when the difference is compared between groups [34,52]. Environmental, anatomical, sociocultural, and genetic factors can influence studies. For this, it would be suitable to apply a split-mouth assessment method [56]. In one study, pain intensity was assessed by comparing a laser-treated hemi-arch and the contralateral hemi-arch without laser treatment. The resulting VAS reported lower pain values for the LLLT-treated hemi-arch [45].
Therefore, further investigations are needed to assess whether LLLT offers any real benefits in pain relief [59].
4.2. LLLT and Root Resorption
Root resorption (RR) can occur thanks to many factors, such as root morphology, tooth anatomy, genetic factors, therapeutic procedures, and malocclusion [35]. Several studies have shown that through LLLT, RR is reduced due to its biostimulatory and bioinhibitory benefits [12]. LLLT has an effect on the mitochondria of leucocytes and fibroblasts, increasing the production of ATP, growth factors, and reactive oxygen species (ROS) and promoting cell migration. An important role is played by RANKL and osteoprotegrins (OPG) that interact in hard tissue remodeling [48]. Morphological changes are assessed with CBCT [36]. Studies performed with high-reliability micro-CT have not shown a great difference between the groups with and without LLLT [53]. Mini-implants were used in research on intrusive movement, and LLLT verified that the groups treated with LLLT reduced both volumetric and linear root resorption by 10 to 12% [60].
4.3. LLLT and Miniscrew Stability
In a randomized clinical trial by Rafał Flieger et al. in 2020 and in a follow-up study by Jacek Matys et al. in 2020, low-level light therapy was used to assess the impact of photobiomodulation (PBM) on the stability and potential loss of mini-implants (MIs), while also assessing patients’ post-treatment pain experiences. At 60 days after the end of treatment, there was significantly greater stability in the laser group than in the control groups but no significant difference in pain sensation over time [54].
The MIs were placed 2 mm below the mucogingival junction on both sides of the maxilla at the region of adherent gingiva between the second premolar and the first molar. While the implants on the left side were in the control group and therefore not exposed to laser irradiation, the MIs on the right side of the maxilla were exposed to a 635 nm diode laser with a dosage of 10 J and exposure periods of 100 s per spot [54]. Light therapy was performed at different periods during orthodontic treatment: immediately and in different periods. MI stability was also measured at the same time periods. The secondary stability was found to be much higher in the right MIs compared with the left control group. There were no appreciable differences, however, in the level of pain measured on both sides of the jaw. Therefore, it could be concluded that 635 nm diode laser irradiation increases the secondary stability of orthodontic MIs by maintaining their total stability for as long as two months of treatment [42,54].
Also, Abdullah Ekizer et al., in an RCT performed in 2016, studied the effects of light-emitting diode (LPT)-mediated photobiomodulation therapy on the stability of MI, as well as the rate of tooth movement during orthodontic treatment in cases of upper first premolar extraction, in which it was necessary, therefore, to retract the upper canines (Figure 3) [32,40,58].
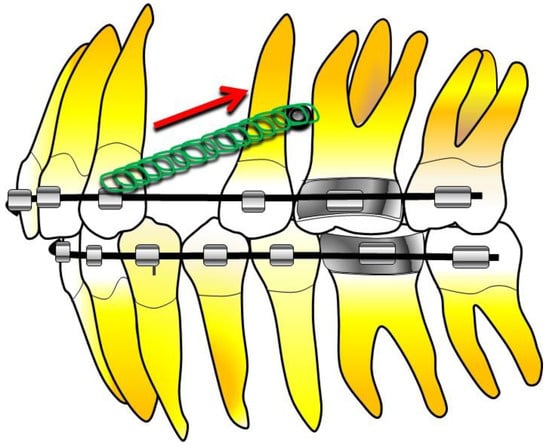
Figure 3.
Stability case of MI in orthodontic treatment with extraction of the upper first premolar [40].
The primary outcome of this study was to evaluate the effect of LPT on the rate of orthodontic tooth movement. The secondary outcome was the measurement of MI stability and interleukin-1β levels in gingival and peri-implant crevicular fluid after LPT [30,40].
With a large increase in tooth movement following LPT, MI stability was dramatically increased in the LPT group in the second and third months. In conclusion, LPT might hasten tooth movement during orthodontic treatment and improve MI stability [40].
In the 2019 clinical study by Guido A. Marañón-Vásquez et al., the effect of photobiomodulation (PBM) on the stability and displacement of orthodontic MIs subjected to loading was evaluated. Cone beam computed tomography images were viewed to determine the extent of MIs’ head displacement. MIs in PBM groups showed less loss of stability [58].
MIs can lose stability and be moved during loading in the various phases of orthodontic treatment, debunking the beliefs that were held until a few years ago, thinking that MIs remained stable throughout the loading period. It has been established that physiological repair processes and a consequent decrease in bone density around MIs could promote their instability over time.
The bone around the MIs may increase its density significantly only 3 months after implantation. For this reason, some authors suggest that several weeks of repair time is needed before applying loading to MIs. Although immediate loading protocols are widely recommended in clinical practice, the literature on this topic still remains unclear. It has been shown that the stability of MIs is subject to change during the repair process and that, after an initial decrease, stability values remain stable only after the fourth week. It has been suggested that photobiomodulation (PBM) can increase the stability of MIs. Non-ionizing light sources in the near-infrared and visible range are used in this therapy to encourage nonthermal biological activities on tissues [40,58].
The PBM light emissions have the potential to permeate into tissues and promote wound healing. PBM enhances the process of bone healing by promoting an increase in vascularization, control of inflammatory processes, proliferation of fibroblasts, keratinocytes, chondrocytes, and osteoblasts, as well as the production of cytokines that trigger matrix formation. As a result, this treatment has been employed to repair the midpalatal suture following fast maxillary growth and to enhance osseointegration following the installation of dental implants. The MIs’ heads were exposed to laser emissions. The MI was placed, and then the diode laser was applied. The MIs’ heads were exposed to laser emissions. The diode laser was used immediately after the MIs were inserted and then every 48–72 h for two weeks. The distance between the light source and the tissues was standardized with a small spacer applied to the laser tip (Figure 4) [40].
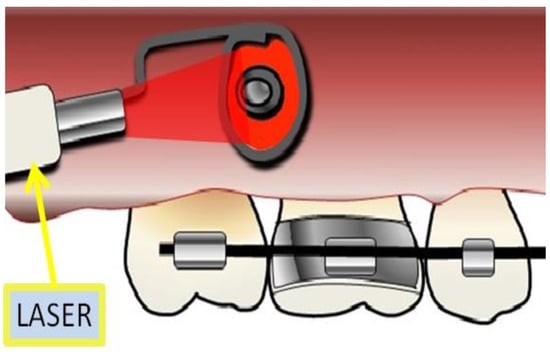
Figure 4.
Laser irradiation by device adapted to laser equipment.
It has been demonstrated that LLLT inhibits mitosis during in vivo cellular division, leading to an accumulation of collagen and nucleic acids. Further research on the LLLT has been conducted in order to improve the repair and safeguarding of fractured tissues as well as the nerve revitalization processes.
The amount of energy used and the length of the laser’s beam are what determine the PBM’s effects at the cellular level. Some studies have examined the effects of PBM on osteoblast differentiation [42]. After a month or two, the stability of the MI has improved thanks to the use of a laser with an 808 nm wavelength on peri-implant materials. The use of a diode laser has not had any appreciable effects on the pain point. The PBM appears to have a beneficial impact on osseous implant healing.
Therefore, clinically, LLLT could be helpful to provide an adequate level of stability of the MI that serves as a scheletric restraining system in complex orthodontic treatment.
4.4. LLLT and Tooth Movement
The primary goal of orthodontic patients is to improve their dental and facial aesthetics as quickly as possible. Typically, patients anticipate a treatment period lasting no more than one year and a half. It is frequently believed that the prolonged duration of orthodontic treatments poses a risk of treatment interruption or results in the patient’s unwillingness to cooperate. Long-term orthodontic treatment entails an increase in the risk of developing cancer, gingivitis, and caries [29]. Additionally, a lengthy course of treatment may have a detrimental impact on the effectiveness of the national health system as well as private research studies. Therefore, a shorter treatment duration by acceleration of dental movement has long been a source of concern for both orthodontists and patients [38,61].
The movement of the teeth during orthodontic treatment, or Orthodontic Tooth Movement (OTM), is a biological response to interference with the dentofacial structure’s physiological balance as determined by an external force. When mechanical forces are applied during OTM, there is periodontal remodeling that is followed by an osseous remodeling that is classified as a biological mechanism that causes an immediate inflammatory response and an improvement in the patient’s perception of pain. This might result in a significantly reduced rate of OTM and related phenomena, such as gingivitis, tooth abscesses, and radicular absorption [37,41].
It is crucial to ease tooth movement, which accelerates osseo-remodeling, in order to shorten the duration of orthodontic treatment [38,61]. To reduce the length of orthodontic therapy, minimally invasive alternative therapies and methods have been developed. Mechanical vibration, corticotomy, piezocision, as well as other pharmaceutical adjuvants have all been proposed as potential methods to speed up OTM. However, despite the fact that the majority of them were effective, their use might not be clinically justified due to their intrusive nature or potential side effects [37].
Recent studies have shown that LLLT is effective in triggering remodeling processes in oral tissues that are hydrophilic and durative by virtue of its photobiostimulatory effects. The use of LLLT during OTM has been demonstrated to be helpful and effective in speeding up dental movement. In addition, it has been shown that LLLT could prevent the release of pain-related analgesic mediators and increase cellular activity during wound healing, including an increase in collagen and elastin production. The laser’s biostimulating effect is most noticeable during the cellular proliferation phase [37,41,49].
Most studies that have evaluated the impact of LLLT on the speed of dental movement have come to the conclusion that LLLT causes an increase in dental movement [49].
The biostimulating effects of LLLT on oral tissues also result from the tissue’s cellular absorption of the laser’s light, which triggers the activation of intracellular signal cascades that increase cellular metabolism and alter the anti-inflammatory properties of soft and hard oral tissues. It has been demonstrated that this procedure promotes, over time, a better dental movement and the formation of osteoclasts on the compression side during the experimental dental movement, determining and improving the OTM time [37,41].
Tooth crowding is believed to be the most common type of malocclusion [43,51]. The leveling and lining up of such cases may take up to 8 months. In general, one of the main reasons why patients hesitate to continue their treatment is the duration of orthodontic treatment [43].
A study by Beren Zsoy and colleagues was launched in 2023 to examine the impact of LLLT on tooth mobility during orthodontic treatment maxillary molar distalization in a 12-week period. A total of 16 different points on the first and second molars have had laser therapy applied for 10 s at a time to an arbitrary molar area (Figure 5). When compared to the contralateral control group, the amount of movement of the subjects’ teeth on the applied side by the laser was much greater. In comparison to moles on the other side, those treated with the laser grew 1.22 times more in 12 weeks [30].
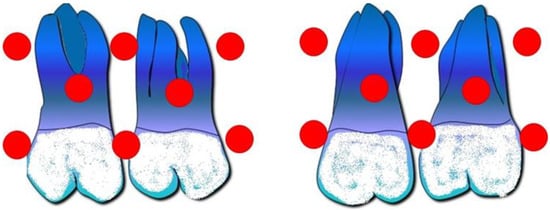
Figure 5.
Laser application points on the first and second molars from the vestibular and palatal views.
Sixteen application points were used on the vestibule and palate [30].
The application of LLLT in orthodontics:
- Could accelerate orthodontic tooth movement [43];
- Could significantly increase treatment efficiency during tooth alignment [39];
- Could provide some efficacy in pain reduction during treatment [28,43,55]
Some studies show that the PBM does not affect the amount of tooth radicular inflammatory response during orthodontic treatment, regardless of whether different laser application protocols increase or decrease its presence [33,62].
LLLT significantly decreases treatment times by accelerating orthodontic alignment and gap closure by an average of 30% [63,64,65].
Despite the fact that the LLLT was statistically significant in terms of accelerating oral movement during orthodontic treatment, its clinical impact was not significant [30,57].
Therefore, more research is required to determine the impact of LLLT on tooth movement while paying close attention to the laser’s parameters [49].
5. Conclusions
In the context of modern, minimally invasive dentistry, LLLT fits in perfectly and, thanks to its intrinsic characteristics, helps the clinician in his or her daily routine. The goal of dentistry in general, and therefore also of orthodontics, is indeed to achieve the best possible result with the best means available, but at the same time, it is also important to do so while reducing chair time and minimizing patient discomfort.
Unfortunately, some orthodontic procedures—separator placement, banding of fixed orthodontic appliances, removable or orthodontic dental braces—can also be associated in a good number of patients with discomfort and/or pain that can not only reduce patient compliance and thus confidence in the practitioner, but also in some cases, discourage them from starting or continuing with a given treatment. In this regard, LLLT has been found to be useful in modulating pain perception, although there is an underlying subjectivity inherent in the individual, as well as possible bias due to the placebo effect. It also seems to be able to minimize root resorption in a good percentage of studies, although again, factors such as the degree of initial crowding and/or long follow-up must be taken into account. PMB can help in stabilizing MIs in terms of osseointegration, just as it can be a very valuable resource in accelerating OTM.
LLLT, despite its innumerable advantages, still has some limitations, but the current literature is in agreement as to the usefulness and safety of the indications for the use of LLLT, provided that the clinician is properly trained and practiced in the use of this type of light, but it is equally certain that further randomized controlled clinical trials with a larger sample size are needed to increase the strength of the evidence on the beneficial effects of the use of low-intensity light therapy in orthodontics.
Author Contributions
Conceptualization, A.M.I., A.P., G.L., G.D.V. and G.D.; methodology L.F., G.L., A.D.I. and A.P.; software, A.M.I., G.L., F.I., I.T. and L.F.; validation, G.L., F.I., G.D.V. and A.M.I.; formal analysis, A.M.I., I.T., A.D.I., F.I. and A.P.; investigation, G.L., A.M.I. and A.P.; resources, A.D.I., A.M.I., G.L, I.T., L.F. and A.P.; data curation, G.D.V., A.D.I., G.D., G.L. and A.P.; writing—original draft preparation, A.M.I., A.D.I., I.T. and A.P.; writing—review and editing, G.L., G.D., F.I. and A.P.; visualization, G.D.V. and F.I.; supervision, A.M.I., I.T., L.F. and F.I.; project administration, G.L., G.D., L.F. and A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to acknowledge Luis Eduardo Almeida, native English speaker and associate professor at the Department of Surgical Sciences, School of Dentistry, Marquette University, Milwaukee, Wisconsin, USA, for reviewing and correcting the scientific English of the article.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| LLLT | Low-level light therapy |
| ATP | Adenosine triphosphate |
| RR | Root resorption |
| MI | Mini-implants |
| LTP | Photobiomodulation mediated by light-emitting diodes |
| PBM | Photobiomodulation |
| OTM | Orthodontic tooth movement |
| IL-1 | Interleukin-1 |
| VAS | Visual analogue scale |
| CBCT | Cone beam computed tomography |
| LE/P | Laser emission/photobiomodulation |
| RCT | Randomized clinical trial |
References
- Khumaidi, M.A.; Paturusi, I.; Nusdwinuringtyas, N.; Islam, A.A.; Gunawan, W.B.; Nurkolis, F.; Taslim, N.A. Is Low-Level Laser Therapy Effective for Patients with Knee Joint Osteoarthritis? Implications and Strategies to Promote Laser Therapy Usage. Front. Bioeng. Biotechnol. 2022, 10, 1089035. [Google Scholar] [CrossRef]
- Chung, H.; Dai, T.; Sharma, S.K.; Huang, Y.-Y.; Carroll, J.D.; Hamblin, M.R. The Nuts and Bolts of Low-Level Laser (Light) Therapy. Ann. Biomed. Eng. 2012, 40, 516–533. [Google Scholar] [CrossRef]
- Adina, S.; Dipalma, G.; Bordea, I.R.; Lucaciu, O.; Feurdean, C.; Inchingolo, A.D.; Septimiu, R.; Malcangi, G.; Cantore, S.; Martin, D.; et al. Orthopedic Joint Stability Influences Growth and Maxillary Development: Clinical Aspects. J. Biol. Regul. Homeost. Agents 2020, 34, 747–756. [Google Scholar] [PubMed]
- Karu, T. Is It Time to Consider Photobiomodulation as a Drug Equivalent? Photomed. Laser Surg. 2013, 31, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Crimi, S.; Badnjević, A.; Cervino, G.; Bianchi, A.; Cicciù, M. Correlation between Temporomandibular Disorders (TMD) and Posture Evaluated Trough the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD): A Systematic Review with Meta-Analysis. J. Clin. Med. 2023, 12, 2652. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.C. The Photobiological Basis of Low Level Laser Radiation Therapy. Laser Ther. 1991, 3, 19–24. [Google Scholar] [CrossRef]
- Yu, W.; Naim, J.O.; McGowan, M.; Ippolito, K.; Lanzafame, R.J. Photomodulation of Oxidative Metabolism and Electron Chain Enzymes in Rat Liver Mitochondria. Photochem. Photobiol. 1997, 66, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, P.M.; Pinheiro AL, B.; Ângelo Castilho Salgado, M.; Pedreira Ramalho, L.M. A Preliminary Report on the Effect of Laser Therapy on the Healing of Cutaneous Surgical Wounds as a Consequence of an Inversely Proportional Relationship between Wavelength and Intensity: Histological Study in Rats. Photomed. Laser Ther. 2004, 22, 513–518. [Google Scholar] [CrossRef]
- Malcangi, G.; Patano, A.; Trilli, I.; Piras, F.; Ciocia, A.M.; Inchingolo, A.D.; Mancini, A.; Hazballa, D.; Di Venere, D.; Inchingolo, F.; et al. Therapeutic and Adverse Effects of Lasers in Dentistry: A Systematic Review. Photonics 2023, 10, 650. [Google Scholar] [CrossRef]
- Sun, G.; Tunér, J. Low-Level Laser Therapy in Dentistry. Dent. Clin. N. Am. 2004, 48, 1061–1076. [Google Scholar] [CrossRef]
- Cruz, D.R.; Kohara, E.K.; Ribeiro, M.S.; Wetter, N.U. Effects of Low-Intensity Laser Therapy on the Orthodontic Movement Velocity of Human Teeth: A Preliminary Study. Lasers Surg. Med. 2004, 35, 117–120. [Google Scholar] [CrossRef]
- Ng, D.; Chan, A.K.; Papadopoulou, A.K.; Dalci, O.; Petocz, P.; Darendeliler, M.A. The Effect of Low-Level Laser Therapy on Orthodontically Induced Root Resorption: A Pilot Double Blind Randomized Controlled Trial. Eur. J. Orthod. 2018, 40, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Fiorillo, L.; Cervino, G.; Cicciù, M. Prevalence of Temporomandibular Disorders (TMD) in Pregnancy: A Systematic Review with Meta-analysis. J. Oral Rehabil. 2023, 50, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Tatullo, M.; Dipalma, G.; Inchingolo, F. Oral Infection by Staphylococcus Aureus in Patients Affected by White Sponge Nevus: A Description of Two Cases Occurred in the Same Family. Int. J. Med. Sci. 2012, 9, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Ronsivalle, V.; Shapira, I.; Cicciù, M. Prevalence of Temporomandibular Disorders in Subjects Affected by Parkinson Disease: A Systematic Review and Metanalysis. J. Oral Rehabil. 2023, 50, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Reyad, A.A.; Mishriky, R.; Girgis, E. Pharmacological and Non-Pharmacological Management of Burning Mouth Syndrome: A Systematic Review. Dent. Med. Probl. 2020, 57, 295–304. [Google Scholar] [CrossRef]
- Doshi-Mehta, G.; Bhad-Patil, W.A. Efficacy of Low-Intensity Laser Therapy in Reducing Treatment Time and Orthodontic Pain: A Clinical Investigation. Am. J. Orthod. Dentofac. Orthop. 2012, 141, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Vermesan, D.; Inchingolo, F.; Patrascu, J.M.; Trocan, I.; Prejbeanu, R.; Florescu, S.; Damian, G.; Benagiano, V.; Abbinante, A.; Caprio, M.; et al. Anterior Cruciate Ligament Reconstruction and Determination of Tunnel Size and Graft Obliquity. Eur Rev Med Pharmacol Sci 2015, 19, 357–364. [Google Scholar]
- Ngan, P.; Kess, B.; Wilson, S. Perception of Discomfort by Patients Undergoing Orthodontic Treatment. Am. J. Orthod. Dentofac. Orthop. 1989, 96, 47–53. [Google Scholar] [CrossRef]
- Sergl, H.G.; Klages, U.; Zentner, A. Functional and Social Discomfort during Orthodontic Treatment–Effects on Compliance and Prediction of Patients’ Adaptation by Personality Variables. Eur. J. Orthod. 2000, 22, 307–315. [Google Scholar] [CrossRef]
- Rapone, B.; Ferrara, E.; Santacroce, L.; Topi, S.; Gnoni, A.; Dipalma, G.; Mancini, A.; Di Domenico, M.; Tartaglia, G.M.; Scarano, A.; et al. The Gaseous Ozone Therapy as a Promising Antiseptic Adjuvant of Periodontal Treatment: A Randomized Controlled Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 985. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.D.; Ceci, S.; Patano, A.; Inchingolo, A.M.; Montenegro, V.; Di Pede, C.; Malcangi, G.; Marinelli, G.; Coloccia, G.; Garibaldi, M.; et al. Elastodontic Therapy of Hyperdivergent Class II Patients Using AMCOP® Devices: A Retrospective Study. Appl. Sci. 2022, 12, 3259. [Google Scholar] [CrossRef]
- Dima, R.; Tieppo Francio, V.; Towery, C.; Davani, S. Review of Literature on Low-Level Laser Therapy Benefits for Nonpharmacological Pain Control in Chronic Pain and Osteoarthritis. Altern. Ther. Health Med. 2018, 24, 8–10. [Google Scholar]
- Grassi, F.R.; Ciccolella, F.; D’Apolito, G.; Papa, F.; Iuso, A.; Salzo, A.E.; Trentadue, R.; Nardi, G.M.; Scivetti, M.; De Matteo, M.; et al. Effect of Low-Level Laser Irradiation on Osteoblast Proliferation and Bone Formation. J Biol Regul Homeost Agents 2011, 25, 603–614. [Google Scholar] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef]
- Ekizer, A.; Türker, G.; Uysal, T.; Güray, E.; Taşdemir, Z. Light Emitting Diode Mediated Photobiomodulation Therapy Improves Orthodontic Tooth Movement and Miniscrew Stability: A Randomized Controlled Clinical Trial. Lasers Surg. Med. 2016, 48, 936–943. [Google Scholar] [CrossRef]
- Monea, A.; Monea, M.; Pop, D.; Bereşescu, G. The Effect of Low Level Laser Therapy on Orthodontic Tooth Movement. Optoelectron. Adv. Mater. 2015, 9, 286–289. [Google Scholar]
- Varella, A.M.; Revankar, A.V.; Patil, A.K. Low-Level Laser Therapy Increases Interleukin-1β in Gingival Crevicular Fluid and Enhances the Rate of Orthodontic Tooth Movement. Am. J. Orthod. Dentofac. Orthop. 2018, 154, 535–544.e5. [Google Scholar] [CrossRef] [PubMed]
- Pérignon, B.; Bandiaky, O.N.; Fromont-Colson, C.; Renaudin, S.; Peré, M.; Badran, Z.; Cuny-Houchmand, M.; Soueidan, A. Effect of 970 nm Low-Level Laser Therapy on Orthodontic Tooth Movement during Class II Intermaxillary Elastics Treatment: A RCT. Sci. Rep. 2021, 11, 23226. [Google Scholar] [CrossRef]
- Özsoy, B.; Güldüren, K.; Kamiloğlu, B. Effect of Low-Level Laser Therapy on Orthodontic Tooth Movement during Miniscrew-Supported Maxillary Molar Distalization in Humans: A Single-Blind, Randomized Controlled Clinical Trial. Lasers Med. Sci. 2023, 38, 76. [Google Scholar] [CrossRef] [PubMed]
- Nicotra, C.; Polizzi, A.; Zappalà, G.; Leonida, A.; Indelicato, F.; Caccianiga, G. A Comparative Assessment of Pain Caused by the Placement of Banded Orthodontic Appliances with and without Low-Level Laser Therapy: A Randomized Controlled Prospective Study. Dent. J. 2020, 8, 24. [Google Scholar] [CrossRef]
- Mistry, D.; Dalci, O.; Papageorgiou, S.N.; Darendeliler, M.A.; Papadopoulou, A.K. The Effects of a Clinically Feasible Application of Low-Level Laser Therapy on the Rate of Orthodontic Tooth Movement: A Triple-Blind, Split-Mouth, Randomized Controlled Trial. Am. J. Orthod. Dentofac. Orthop. 2020, 157, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Eid, F.; El-Kenany, W.; Mowafy, M.; Elkalza, A. The Influence of Two Photobiomodulation Protocols on Orthodontically Induced Inflammatory Root Resorption (a Randomized Controlled Clinical Trial). BMC Oral Health 2022, 22, 221. [Google Scholar] [CrossRef] [PubMed]
- Farias, R.D.; Closs, L.Q.; Miguens, S.A.Q. Evaluation of the Use of Low-Level Laser Therapy in Pain Control in Orthodontic Patients: A Randomized Split-Mouth Clinical Trial. Angle Orthod. 2016, 86, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Shahid, F.; Nowrin, S.A.; Alam, M.K.; Khamis, M.F.; Husein, A.; Rahman, N.A. Effects of Low-Level Laser Therapy and Bracket Systems on Root Resorption during Orthodontic Treatment: A Randomized Clinical Trial. Healthcare 2023, 11, 864. [Google Scholar] [CrossRef]
- Nimeri, G.; Kau, C.H.; Corona, R.; Shelly, J. The Effect of Photobiomodulation on Root Resorption during Orthodontic Treatment. Clin. Cosmet. Investig. Dent. 2014, 6, 1–8. [Google Scholar] [CrossRef]
- Isola, G.; Matarese, M.; Briguglio, F.; Grassia, V.; Picciolo, G.; Fiorillo, L.; Matarese, G. Effectiveness of Low-Level Laser Therapy during Tooth Movement: A Randomized Clinical Trial. Materials 2019, 12, 2187. [Google Scholar] [CrossRef]
- Genc, G.; Kocadereli, I.; Tasar, F.; Kilinc, K.; El, S.; Sarkarati, B. Effect of Low-Level Laser Therapy (LLLT) on Orthodontic Tooth Movement. Lasers Med. Sci. 2013, 28, 41–47. [Google Scholar] [CrossRef]
- Caccianiga, G.; Paiusco, A.; Perillo, L.; Nucera, R.; Pinsino, A.; Maddalone, M.; Cordasco, G.; Lo Giudice, A. Does Low-Level Laser Therapy Enhance the Efficiency of Orthodontic Dental Alignment? Results from a Randomized Pilot Study. Photomed. Laser Surg. 2017, 35, 421–426. [Google Scholar] [CrossRef]
- Marañón-Vásquez, G.A.; Lagravère, M.O.; Borsatto, M.C.; de Souza, S.S.; Watanabe, P.C.A.; Matsumoto, M.A.N.; Saraiva, M.d.C.P.; Romano, F.L. Effect of Photobiomodulation on the Stability and Displacement of Orthodontic Mini-Implants Submitted to Immediate and Delayed Loading: A Clinical Study. Lasers Med. Sci. 2019, 34, 1705–1715. [Google Scholar] [CrossRef]
- Guram, G.; Reddy, R.K.; Dharamsi, A.M.; Syed Ismail, P.M.; Mishra, S.; Prakashkumar, M.D. Evaluation of Low-Level Laser Therapy on Orthodontic Tooth Movement: A Randomized Control Study. Contemp. Clin. Dent. 2018, 9, 105–109. [Google Scholar] [CrossRef]
- Matys, J.; Flieger, R.; Gedrange, T.; Janowicz, K.; Kempisty, B.; Grzech-Leśniak, K.; Dominiak, M. Effect of 808 nm Semiconductor Laser on the Stability of Orthodontic Micro-Implants: A Split-Mouth Study. Materials 2020, 13, 2265. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, K. Clinical Research: Low-Level Laser Therapy in Accelerating Orthodontic Tooth Movement. BMC Oral Health 2021, 21, 324. [Google Scholar] [CrossRef]
- Lo Giudice, A.; Nucera, R.; Perillo, L.; Paiusco, A.; Caccianiga, G. Is Low-Level Laser Therapy an Effective Method to Alleviate Pain Induced by Active Orthodontic Alignment Archwire? A Randomized Clinical Trial. J. Evid. Based Dent. Pract. 2019, 19, 71–78. [Google Scholar] [CrossRef]
- Lazăr, A.-P.; Dakó, T.; Bud, A.; Vlasa, A.; Ormenișan, A.; Mârțu, M.-A.; Păcurar, M.; Lazăr, L. The Effects of Periodontal Laser Therapy on Pain in Adult Patients with Orthodontic Treatment: A Randomized Clinical Trial. Appl. Sci. 2022, 12, 3601. [Google Scholar] [CrossRef]
- Artés-Ribas, M.; Arnabat-Dominguez, J.; Puigdollers, A. Analgesic Effect of a Low-Level Laser Therapy (830 nm) in Early Orthodontic Treatment. Lasers Med. Sci. 2013, 28, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Brito, M.H.; Nogueira, C.Q.; Cotrin, P.; Fialho, T.; Oliveira, R.C.; Oliveira, R.G.; Salmeron, S.; Valarelli, F.P.; Freitas, K.M.S.; Cançado, R.H. Efficacy of Low-Level Laser Therapy in Reducing Pain in the Initial Stages of Orthodontic Treatment. Int. J. Dent. 2022, 2022, 3934900. [Google Scholar] [CrossRef]
- Goymen, M.; Gulec, A. Effect of Photobiomodulation Therapies on the Root Resorption Associated with Orthodontic Forces: A Pilot Study Using Micro Computed Tomography. Clin. Oral Investig. 2020, 24, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.K. Effects of Low-Level Laser Therapy on Orthodontic Tooth Movement: Evaluation of Bony Changes via 3DCBCT. Children 2023, 10, 384. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.K.; Ganji, K.K.; Alfawzan, A.A.; Manay, S.M.; Srivastava, K.C.; Chaudhari, P.K.; Hosni, H.A.; Alswairki, H.J.; Alansari, R.A. Ectopic Eye Tooth Management: Photobiomodulation/Low-Level Laser Emission Role in Root Resorption after Fixed Orthodontic Treatment. Healthcare 2022, 10, 610. [Google Scholar] [CrossRef]
- AlSayed Hasan, M.M.A.; Sultan, K.; Hamadah, O. Low-Level Laser Therapy Effectiveness in Accelerating Orthodontic Tooth Movement: A Randomized Controlled Clinical Trial. Angle Orthod. 2017, 87, 499–504. [Google Scholar] [CrossRef]
- AlSayed Hasan, M.M.A.; Sultan, K.; Ajaj, M.; Voborná, I.; Hamadah, O. Low-Level Laser Therapy Effectiveness in Reducing Initial Orthodontic Archwire Placement Pain in Premolars Extraction Cases: A Single-Blind, Placebo-Controlled, Randomized Clinical Trial. BMC Oral Health 2020, 20, 209. [Google Scholar] [CrossRef]
- Nayyer, N.; Tripathi, T.; Rai, P.; Kanase, A. Effect of Photobiomodulation on External Root Resorption during Orthodontic Tooth Movement-a Randomized Controlled Trial. Int. Orthod. 2021, 19, 197–206. [Google Scholar] [CrossRef]
- Flieger, R.; Gedrange, T.; Grzech-Leśniak, K.; Dominiak, M.; Matys, J. Low-Level Laser Therapy with a 635 nm Diode Laser Affects Orthodontic Mini-Implants Stability: A Randomized Clinical Split-Mouth Trial. J. Clin. Med. 2019, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Jivrajani, S.J.; Bhad Patil, W.A. Effect of Low Intensity Laser Therapy (LILT) on MMP-9 Expression in Gingival Crevicular Fluid and Rate of Orthodontic Tooth Movement in Patients Undergoing Canine Retraction: A Randomized Controlled Trial. Int. Orthod. 2020, 18, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.T.; Bayome, M.; Park, J.-B.; Park, J.H.; Baek, S.-H.; Kook, Y.-A. Effect of Frequent Laser Irradiation on Orthodontic Pain. A Single-Blind Randomized Clinical Trial. Angle Orthod. 2013, 83, 611–616. [Google Scholar] [CrossRef]
- Limpanichkul, W.; Godfrey, K.; Srisuk, N.; Rattanayatikul, C. Effects of Low-Level Laser Therapy on the Rate of Orthodontic Tooth Movement. Orthod. Craniofac. Res. 2006, 9, 38–43. [Google Scholar] [CrossRef]
- Güray, Y.; Yüksel, A.S. Effect of Light-Emitting Photobiomodulation Therapy on the Rate of Orthodontic Tooth Movement: A Randomized Controlled Clinical Trial. J. Orofac. Orthop. 2022. [Google Scholar] [CrossRef] [PubMed]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Fiorillo, L.; Cervino, G.; Cicciù, M. Economic Inequalities and Temporomandibular Disorders: A Systematic Review with Meta-analysis. J. Oral Rehabil. 2023, 50, 715–723. [Google Scholar] [CrossRef]
- Nasser, A.R.; Sultan, K.; Hajeer, M.Y.; Hamadah, O. Investigating the Effectiveness of Low-Level Laser in Reducing Root Resorption of the Upper Incisors during Intrusion Movement Using Mini-Implants in Adult Patients with Deep Overbite: A Randomized Controlled Clinical Trial. Cureus 2023, 15, e35381. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Del Corso, M.; Inchingolo, F.; Sammartino, G.; Charrier, J.-B. Platelet-Rich Plasma (PRP) and Platelet-Rich Fibrin (PRF) in Human Cell Cultures: Growth Factor Release and Contradictory Results. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2010, 110, 418–421. [Google Scholar] [CrossRef] [PubMed]
- Qamar, Z.; Alghamdi, A.M.S.; Haydarah, N.K.B.; Balateef, A.A.; Alamoudi, A.A.; Abumismar, M.A.; Shivakumar, S.; Cicciù, M.; Minervini, G. Impact of Temporomandibular Disorders on Oral Health-related Quality of Life: A Systematic Review and Meta-analysis. J. Oral Rehabil. 2023, 50, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.; Ashkar, S.; Hamade, E.; Gutknecht, N.; Lampert, F.; Mir, M. The Effect of Low-Level Laser Therapy during Orthodontic Movement: A Preliminary Study. Lasers Med. Sci. 2008, 23, 27–33. [Google Scholar] [CrossRef]
- Sousa, M.V.d.S.; Scanavini, M.A.; Sannomiya, E.K.; Velasco, L.G.; Angelieri, F. Influence of Low-Level Laser on the Speed of Orthodontic Movement. Photomed. Laser Surg. 2011, 29, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Kreisner, P.-E.; Blaya, D.-S.; Gaião, L.; Maciel-Santos, M.-E.-S.; Etges, A.; Santana-Filho, M.; de Oliveira, M.-G. Histological Evaluation of the Effect of Low-Level Laser on Distraction Osteogenesis in Rabbit Mandibles. Med. Oral Patol. Oral Cir. Bucal 2010, 15, e616–e618. [Google Scholar] [CrossRef] [PubMed][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).