Noble Nanofluids and Their Hybrids for Heat Transfer Enrichment: A Review and Future Prospects Coverage
Abstract
:1. Introduction
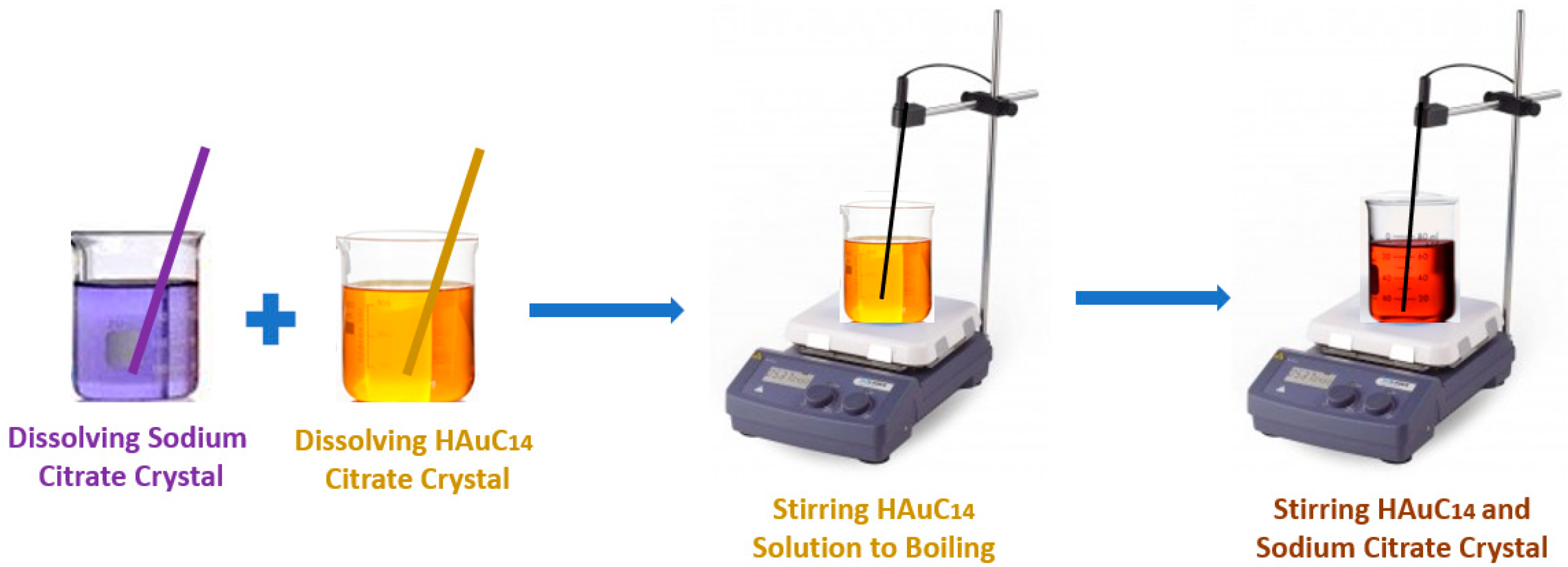
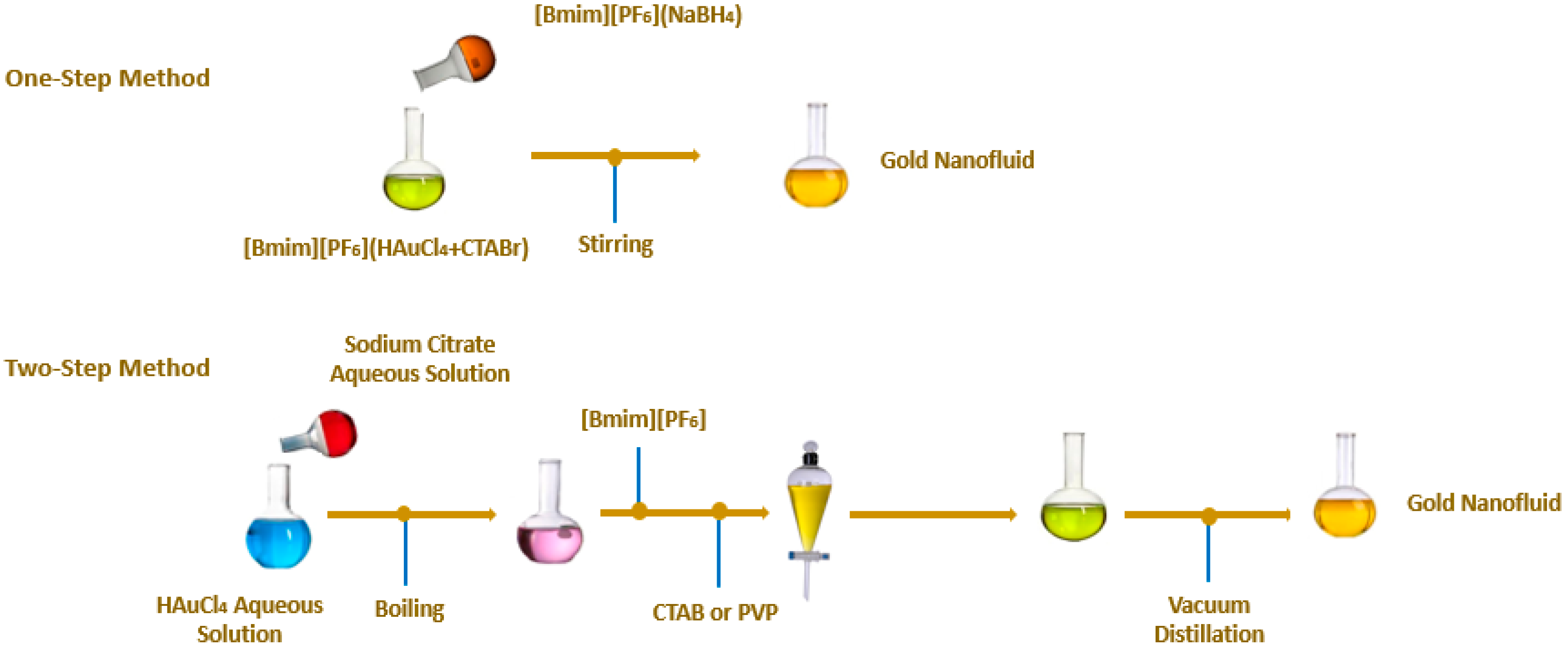
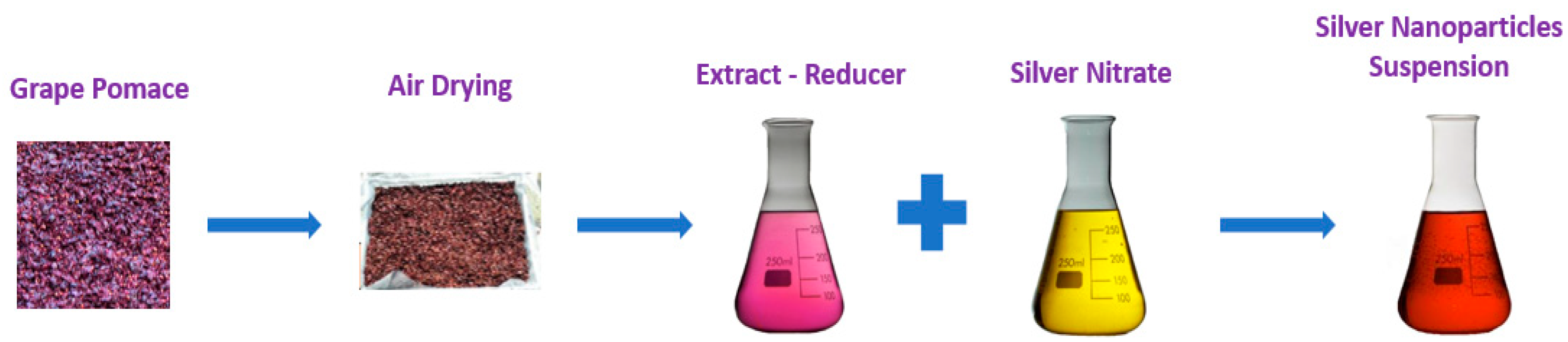
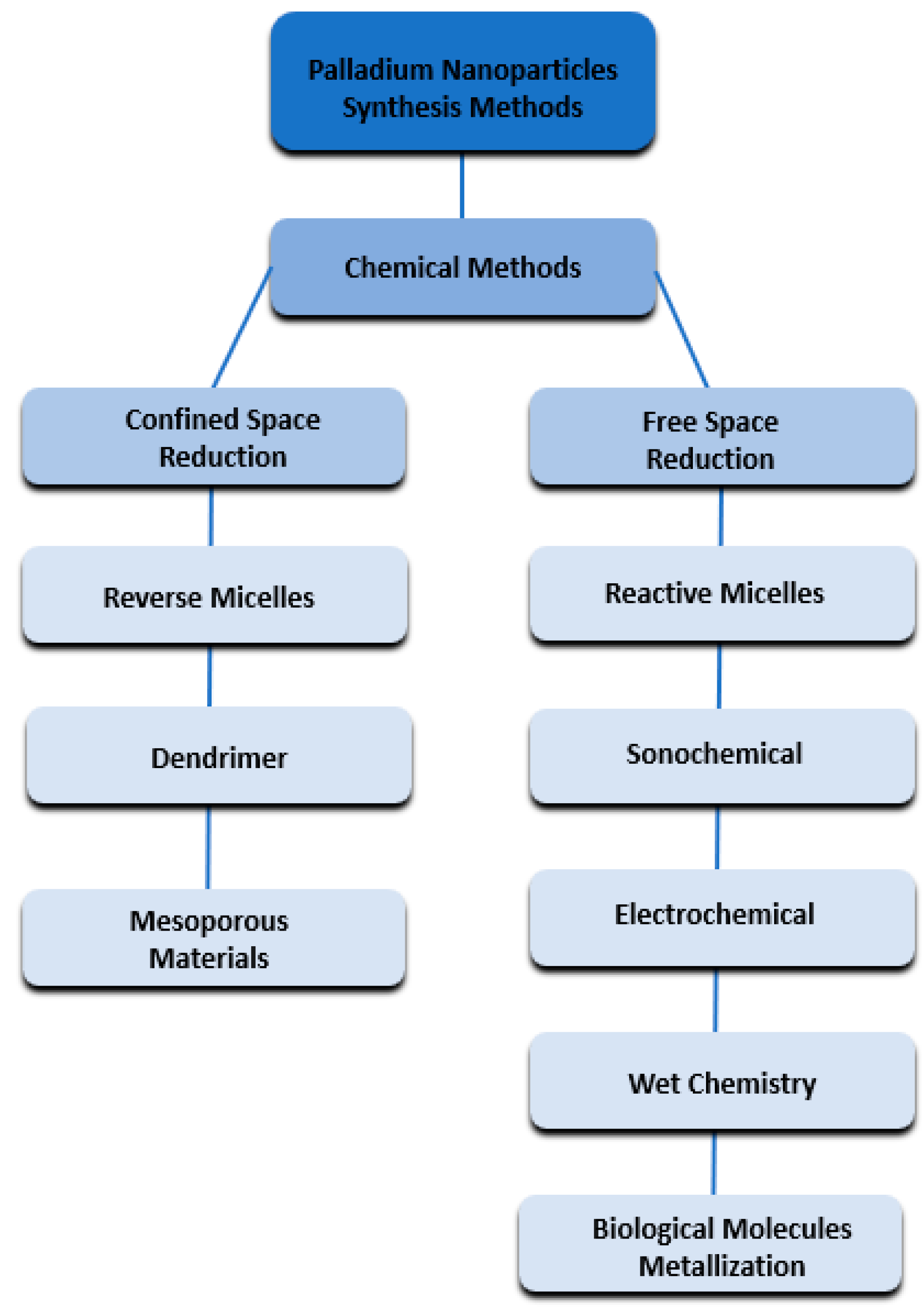
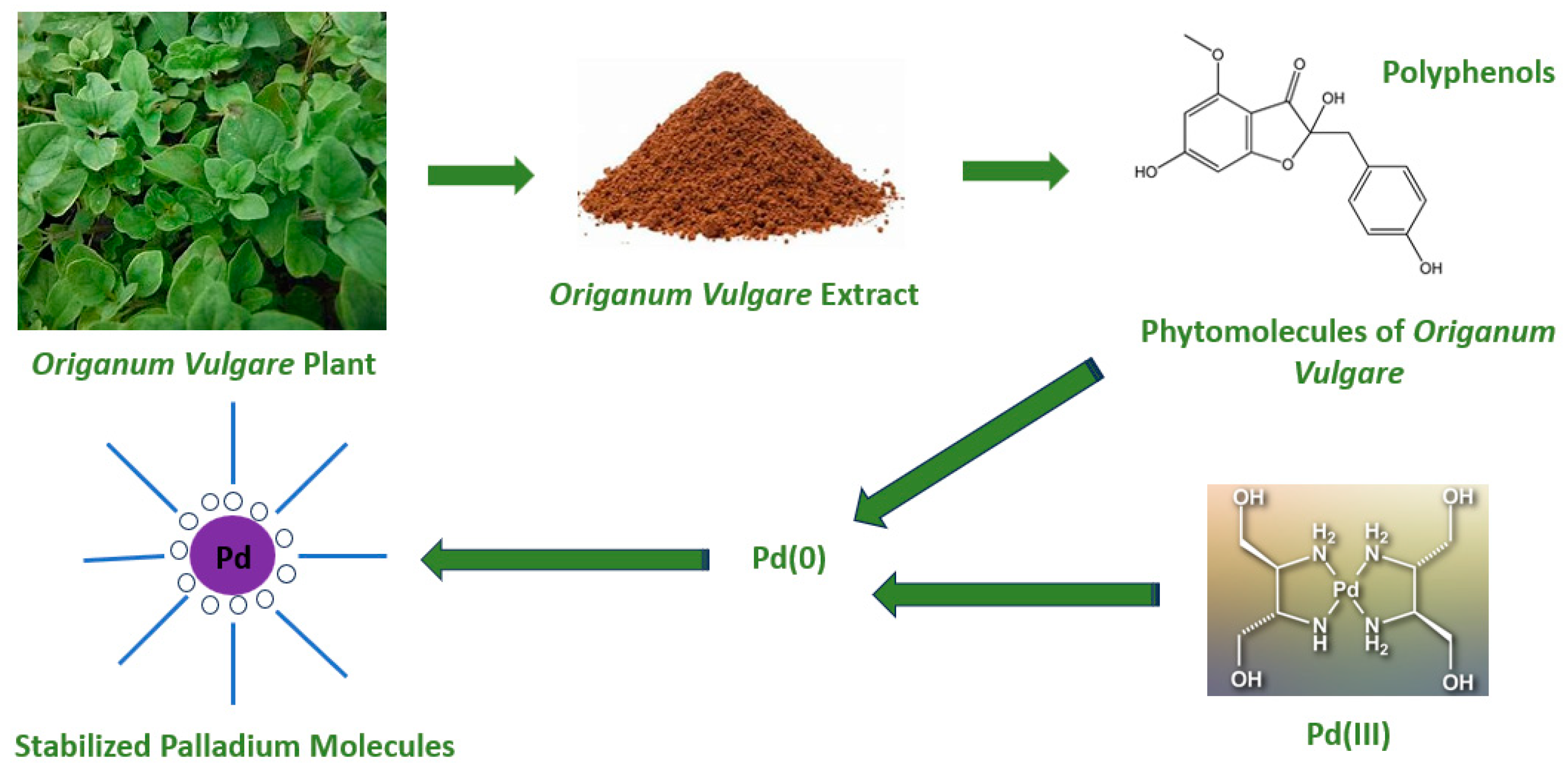
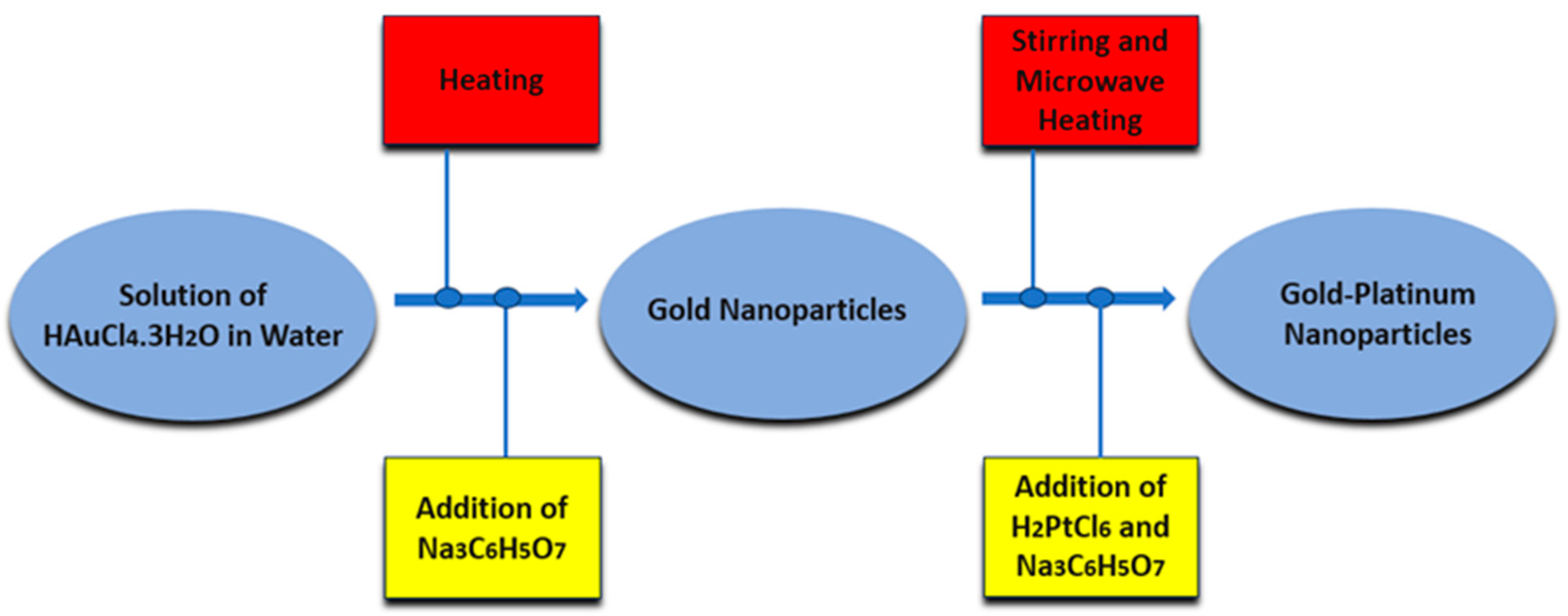
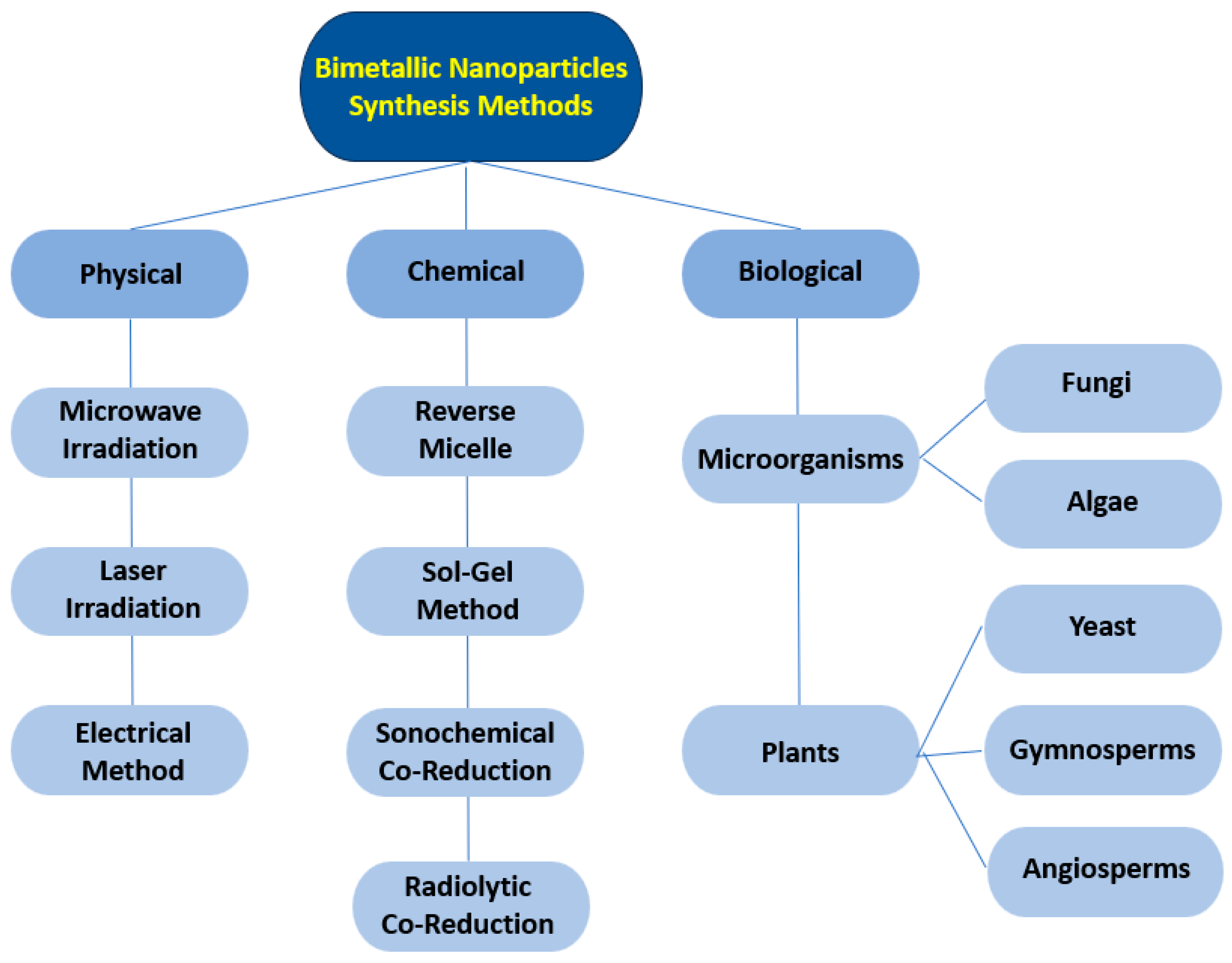
2. Main Preparation Methods
3. Comparison with Other Nanofluids
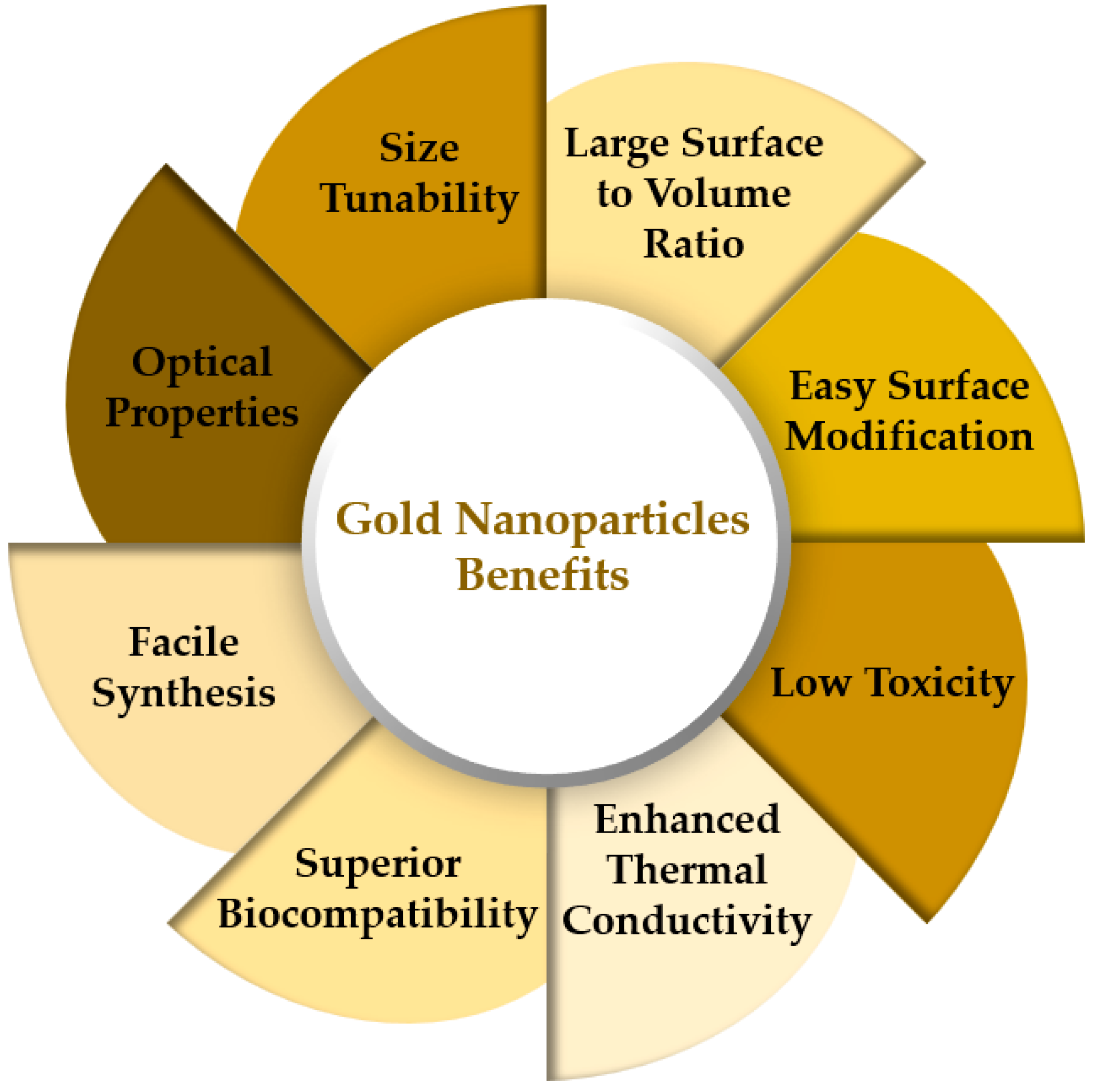
- Better Optical and photothermal conversions, which can be of great usefulness in solar energy systems.
- Superior plasmonic nanofluid filtering with superior surface plasmon resonance.
- Superior biocompatibility. For instance, the gold nanoparticles dispersed in blood can act as drug carriers, destroy cancer cells, and other bio-medical purposes.
- Absence of fouling and Nanoparticles deposition or settlement of the nanoparticles, as in the case of silver Nanoparticles most likely due to their diamagnetic nature.
- Much less concentrated than the other nanofluids. Hence, they require less material. This can be very suitable for large-scale applications using many liters of nanofluid. Nonetheless, an economic analysis upon investment cost/heat transfer capability improvement should be always performed.
- Being the concentrations extremely small, there can be performed experiments with higher concentrations without the risk of fouling, erosion, or clogging of the equipment as it frequently happens with the other nanofluids. Also, the required pumping power and pressure drop associated with these noble metals nanofluids with Higher concentrations is not expected to raise so sharply as it occurs with the other nanofluids. Higher concentrations are expected, according to a major part of the published works, to considerably improve the heat transfer performance of the noble metals nanofluids.
- All the mentioned noble metals nanofluids can be prepared by eco-friendly methods, which does not always happen with the other nanofluids.
4. Heat Transfer Mechanisms and Influencing Factors
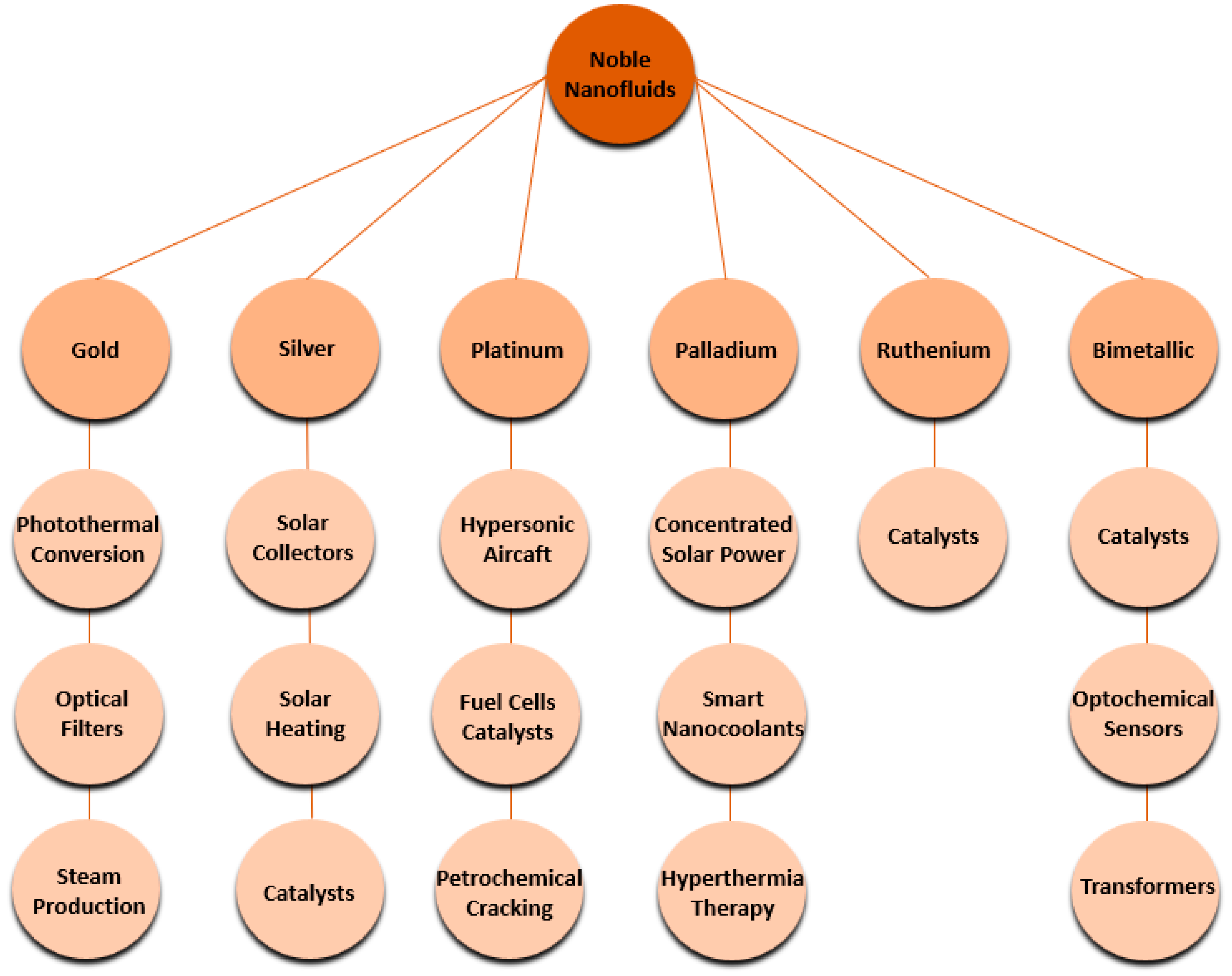
5. Noble Nanofluids
5.1. Gold Nanofluids
5.2. Silver Nanofluids
5.3. Platinum Nanofluids
5.4. Palladium Nanofluids
5.5. Ruthenium Nanofluids
5.6. Iridium, Osmium, and Rhodium Nanofluids
5.7. Bimetallic Nanofluids
5.8. Hybrid Nanofluids
6. Limitations and Challenges
- Further analysis should be performed with the noble metals nanofluids to address the impact of the type and morphology of the nanoparticles on the thermal conductivity and specific heat. Such works will bring some more light on the mechanistic understanding of the thermal conductivity influencing factors.
- There should be conducted more works on the morphology and rheological characteristics of silver nanorods nanofluids to evaluate their ability as heat transfer fluids in large-scale applications.
- Given that it can be one of the main benefits of the noble metals nanofluids in general and of the silver nanofluids in particular, it is highly recommended to carry out long-term pool boiling experiments using silver nanofluids to assess the absence of fouling and nanoparticle deposition onto the boiling surface. The reason behind this fact has been appointed to the diamagnetic character of silver nanoparticles.
- Further research works on the use of gold nanofluids for the heat transfer of thermal management equipment to evaluate their performance in laminar and turbulent regions at high heat fluxes.
- There should be synthesized more gold-ionic liquids nanofluids to infer their stability, thermophysical features and application as heat transfer fluids.
- It is highly recommended to perform preliminary tests with noble metals nanofluids in direct absorption solar collectors to avert the great dispersion of results and advance on their potential application.
- Further research works on the optical and photothermal conversion efficiencies of gold-palladium bimetallic nanofluids with different ratios are most welcome to better understand their suitability for solar thermal conversion processes.
- There should be performed more tests of prolonged storage, continuous and cycling heating, flowing, and solar irradiation tests to evaluate the stability of the gold nanofluids with surface modification.
- Since the plasmonic nanofluid filtering has become increasingly more common in solar energy systems because of their adjustable thermophysical and optical properties, there should be further conducted more works to optimize the features of the filters of the plasmonic nanofluids.
- It is suggested to carry out more on-sun experimental works and economic analysis on the solar thermal conversion of the noble metals nanofluids having higher concentrations than the published ones, to infer whether the cost increase will be compensated by more thermally performant nanofluids.
- The influence of the ultrasonic wave propagation on the thermal conductivity of the noble metals nanofluids or vice versa requires further in-depth research.
- There should be conducted more numerical and experimental works on the heat transfer capability in the solar thermal harvesting and conversion of iridium, osmium, and rhodium nanofluids and hybrids.
- Since the main published findings are not consistent with each other, there should be performed more experimental works of comparison under the same operating conditions between the hybrid noble metals nanofluids and the corresponding mono nanofluids.
- There is a lack of accurate evaluations on how can the microstructure and aggregation morphologies of the noble metals nanofluids affect their thermophysical properties like the thermal conductivity, specific heat, and latent heat of evaporation. Hence, further experimental and numerical studies on the subject are recommended.
7. Conclusions
- The surface plasmon resonance effect provided by the gold nanofluids led to a superior photothermal conversion efficiency even at a low concentration of 10 ppm and this parameter decreased with increasing size of the nanoparticles.
- The trisodium citrate is an excellent reducing agent for the preparation of gold nanofluids to be applied in the solar thermal energy conversion systems. An energy efficiency enhancement of approximately 95% was attained with a 0.04% wt. gold nanofluid.
- The impact of the morphology of the gold nanoparticles on their photothermal conversion rate is still being examined by the research community. As an example, it was already verified that the photothermal conversion efficiency decreased with increasing size of the nanoparticles in a flat shaped direct absorption solar collector, which was caused by the interactions between the optical property, heat loss, and thermal conductivity increase, but the impact of the size of the nanoparticles in a cube-shaped collector was lower than the one in the flat shaped collector model.
- The ketone and alcohol solvents form spherical gold nanoparticles, whereas the acetonitrile and tetrahydrofuran form rods or triangular gold nanoparticles. The rods and triangles are more prone to clustering and sedimentation producing unstable nanofluids. Hence, it is recommended to avoid the use of acetonitrile and tetrahydrofuran or to add protective agents.
- The bio-friendly silver-coconut oil nanofluid is a promising solution for thermal management systems working with increased heat fluxes. Also, the fouling formation inherent to the use of this nanofluid can be taken as negligible in comparison with other nanofluids like the alumina and copper oxide aqueous nanofluids.
- The microwave-assisted one-step method has already proved to be able to produce stable silver nanofluids having nanoparticles between 30 nm and 60 nm of dimensions. The microwave methodology is facile, rapid, and scalable.
- It was demonstrated that silver nanofluids at only 1% vol. could increase the base fluid effective thermal conductivity approximately by 1.6 times at 50 °C.
- It was already confirmed that small silver nanoparticles at very low concentrations exhibit higher thermal conductivity and diffusivity values than water.
- The platinum-hydrocarbon nanofluids with hyperbranched polymer as initiator and stabilizer are very promising fuels having enhanced cracking behavior and endothermic ability for the heating/cooling in hypersonic aircraft.
- The nanofluids containing palladium nanoplatelets can ameliorate the thermal performance of surface solar collectors by up to nearly 32%, whilst decreasing the required pumping power by 15%.
- The palladium nanoparticles stabilized by long-chain alkyl-modified hyperbranched polyglycol present enhanced thermal conductivity and over time stability.
- The incorporation of gold, silver, and palladium in polyethylene glycol showed improved light-to-heat conversion efficiencies. The large size spectrum of the silver nanoparticles enhanced their capability to absorb and convert a broader spectrum of sunlight wavelengths. The nanofluids were stable in time due to the interactions between the molecules of the nanoparticles and the polyethylene glycol molecules.
- The gold-platinum nanofluids demonstrated to possess slightly lower thermal conductivity in reference to the gold nanofluids. This can be interpreted based on the hybrid metallic nanostructure, where the platinum having much lower thermal conductivity than gold will decrease the overall thermal conductivity of the bimetallic nanofluids.
- The gold-palladium bimetallic nanofluids exhibit better photothermal conversion than that of monometallic gold or palladium nanofluids.
- Further research works should be performed on the noble metal nanofluids with different formulations and extremely low concentrations to address the impact on the heat transfer capability and photothermal conversion efficiency of the systems incorporating such type of nanofluids, together with an accurate investment cost-benefits analysis.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations/Nomeclature
| ACN | Acetonitrile |
| B | Constant Rate of Heat Dissipation |
| [Bmim]PF6 | 1-butyl-3-methylimidazolium hexafluorophosphate |
| CHF | Critical Heat Flux |
| CTAB | Cetyltrimethylammonium bromide |
| EGMUDE | Triethyleneglycolmono-11-mercaptoundecylethe |
| ETOTAL | Total stored Energy |
| E% | Photothermal Conversion Energy |
| HTC | Heat Transfer Coefficient |
| LSPR | Localized Surface Plasmon Resonance |
| LAL | Laser Ablation in Liquids |
| MWCNT | Muti-walled carbon nanotubes |
| PBS | Polybutylene succinate |
| PEG | Polyethylene glycol |
| PVP | Polyvinylpyrrolidone |
| SER | Stored Energy Ratio |
| SH | Specific Heat |
| TC | Thermal Conductivity |
| TD | Thermal Diffusivity |
| THF | Tetrahydrofuran |
| TOAB | Tetraoctylammonium bromide |
| Base Fluid | Liquid that integrates a nanofluid in which the incorporated nanoparticles are dispersed. Some examples of base fluids are water, ethylene glycol, and oil. |
| Bimetallic Nanofluids | Nanofluids where the nanoparticles dispersed in the base fluid are the composite mixture of two or more metallic elements. |
| Critical Heat Flux | Point at which a fluid reaches its maximum heat transfer rate. In other words, is the heat flux at which the boiling process ceases to be effective in transferring heat from a solid heating surface to the heat transfer fluid. |
| Heat Transfer Coefficient | Is the quantitative measure of the convective heat transfer between the thermal fluid medium and the heating surface (wall), which is flowed over by the fluid. |
| Hybrid | is the composite form having or produced by the combination of two or more distinct elements and characterized by a heterogeneous origin, composition, and appearance. |
| Hybrid Nanofluids | Nanofluids where the nanoparticles dispersed in the base fluid are the composite mixture of metallic, non-metallic, ceramic, and polymeric elements. |
| Nanofluids | The nanofluids are engineered colloidal suspensions of solid nanostructures (e.g., nanoparticles) in a base fluid. |
| Nanoparticles | Ultrafine particles of matter that has from 1 to 100 nanometers of characteristics dimension (e.g., diameter). |
| Noble Metals | Metallic chemical elements having superior resistance to oxidation or corrosion even at high temperatures. Examples of the noble metals are gold, silver, platinum, palladium, ruthenium, iridium, among others. |
| Noble Metals Nanofluids | Nanofluids where the nanoparticles added to the base fluid are made of noble metals. |
| Sonication | Or ultra-sonication is the process that apply ultrasonic frequencies superior to 20 KHz to agitate and disperse the nanoparticles in the base fluid. The sonication can be conducted employing either an ultrasonic bath or an ultrasonic probe designated by sonicator. |
| Surfactants | Chemical compounds that reduce the surface tension or interfacial tension between the liquid and solid that composes the nanofluids. The surfactants can be divided into non-ionic, anionic, cationic, and amphoteric. |
References
- Seifikar, F.; Azizian, S.; Eslamipanah, M.; Jaleh, B. One step synthesis of stable nanofluids of Cu, Ag, Au, Ni, Pd, and Pt in PEG using laser ablation in liquids method and study of their capability in solar-thermal conversion. Sol. Energy 2022, 246, 74–88. [Google Scholar] [CrossRef]
- Urnukhsaikhan, E.; Bold, B.-E.; Gunbileg, A.; Sukhbaatar, N.; Mishig-Ochir, T. Antibacterial activity and characteristics of silver nanoparticles biosynthesized from Carduus crispus. Sci. Rep. 2021, 11, 21047. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.-Y.; Huang, J.; Chen, C.-Y.; Wang, Z.-X.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Jacob, M.V.; Antunes, E. A critical review on silver nanoparticles: From synthesis and applications to its mitigation through low-cost adsorption by biochar. J. Environ. Manag. 2021, 281, 111918. [Google Scholar] [CrossRef] [PubMed]
- Nadaroğlu, H.; Alayli, G.A.; İnce, S. Synthesis of Nanoparticles by Green Synthesis Method. Int. J. Innov. Res. Rev. 2017, 1, 6–9. Available online: https://www.injirr.com/article/view/4 (accessed on 12 July 2023).
- Salehi, J.M.; Heyhat, M.M.; Rajabpour, A. Enhancement of thermal conductivity of silver nanofluid synthesized by a one-step method with the effect of polyvinylpyrrolidone on thermal behavior. Appl. Phys. Lett. 2013, 102, 231907. [Google Scholar] [CrossRef]
- Liu, D.; Chen, X.; Xu, G.; Guan, J.; Cao, Q.; Dong Bo Qi, Y.; Li, C.; Mu, X. Iridium nanoparticles supported on hierarchical porous N-doped carbon: An efficient water-tolerant catalyst for bio-alcohol condensation in water. Sci. Rep. 2016, 6, 21365. [Google Scholar] [CrossRef]
- Neumman, O.; Urban, A.S.; Day, J.; Lal, S.; Nordlander, P.; Halas, N.J. Solar Vapor Generation Enabled by Nanoparticles. ACS Nano 2013, 7, 42–49. [Google Scholar] [CrossRef]
- Otanicar, T.; Dale, J.; Orosz, M.; Brekke, N.; DeJarnette, D.; Tunkara, E.; Roberts, K.; Harikumar, P. Experimental evaluation of a prototype hybrid CPV/T system utilizing a nanoparticle fluid absorber at elevated temperatures. Appl. Energy 2018, 228, 1531–1539. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, T.; Jin, J. Nanoparticle pollution and associated increasing potential risks on environment and human health: A case study of China. Environ. Sci. Pollut. Res. Int. 2015, 22, 19297–19306. [Google Scholar] [CrossRef]
- Elsaid, K.; Olabi, A.G.; Wilberforce, T.; Abdelkareem, M.A.; Sayed, E.T. Environmental impacts of nanofluids: A review. Sci. Total Environ. 2021, 763, 144202. [Google Scholar] [CrossRef] [PubMed]
- Nakjavani, M.; Nikkah, V.; Sarafraz, M.M.; Shoja, S.; Sarafraz, M. Green synthesis of silver nanoparticles using green tea leaves: Experimental study on the morphological, rheological and antibacterial behavior. Heat Mass Transf. 2017, 53, 3201–3209. [Google Scholar] [CrossRef]
- Hjerrild, N. Nanofluid Optical Filters for Photovoltaic/Thermal Collectors. Ph.D. Thesis, UNSW Faculty Sydney, Sydney, Australia, 2018. [Google Scholar] [CrossRef]
- Du, J.Y.; Su, Q.M.; Li, L.; Wang, R.J.; Zhu, Z.F. Evaluation of the influence of aggregation morphology on thermal conductivity of nanofluid by a new MPCD-MD hybrid method. Int. Commun. Heat Mass Transf. 2021, 127, 105501. [Google Scholar] [CrossRef]
- Song, D.; Jing, D.; Ma, W.; Zhang, X. Effect of particle aggregation on thermal conductivity of nanofluids: Enhancement of phonon MFP. J. Appl. Phys. 2019, 125, 015103. [Google Scholar] [CrossRef]
- Wang, R.J.; Qian, S.; Zhang, Z.Q. Investigation of the aggregation morphology of nanoparticle on the thermal conductivity of nanofluid by molecular dynamics simulations. Int. J. Heat Mass Transf. 2018, 127, 1138–1146. [Google Scholar] [CrossRef]
- Wang, R.J.; Feng, C.; Zhang, Z.; Shao, C.; Du, J.Y. What quantity of charge on the nanoparticle can result in a hybrid morphology of the nanofluid and a higher thermal conductivity? Powder Technol. 2023, 422, 118443. [Google Scholar] [CrossRef]
- Jin, X.; Guan, H.Q.; Wang, R.J.; Huang, L.Z.; Shao, C. The most crucial factor on the thermal conductivity of metal-water nanofluids: Match degree of the phonon density of state. Powder Technol. 2022, 412, 117969. [Google Scholar] [CrossRef]
- Cui, X.; Wang, J.; Xia, G.D. Enhanced thermal conductivity of nanofluids by introducing Janus particles. Nanoscale 2021, 14, 99–107. [Google Scholar] [CrossRef]
- Guan, H.Q.; Su, Q.M.; Wang, R.J.; Huang, L.Z.; Shao, C.; Zhu, Z.F. Why can hybrid nanofluid improve thermal conductivity more? A molecular dynamics simulation. J. Mol. Liq. 2023, 372, 121178. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, H.-J.; Du, X.; Wen, D. Photothermal conversion characteristics of gold nanoparticle dispersions. Sol. Energy 2014, 100, 141–147. [Google Scholar] [CrossRef]
- Sabir, R.; Ramzan, N.; Umer, A.; Muryan, H. An experimental study of forced convective heat transfer characteristic of gold water nanofluid in laminar flow. Sci. Int. (Lahore) 2015, 27, 235–241. [Google Scholar]
- Chen, M.; He, Y.; Zhu, J.; Kim, D.R. Enhancement of photo-thermal conversion using gold nanofluids with different particle sizes. Energy Convers. Manag. 2016, 112, 21–30. [Google Scholar] [CrossRef]
- Burgos, J.; Mondragón, R.; Elcioglu, E.B.; Fabregat-Santiago, F.; Hernández, L. Experimental Characterization and Statistical Analysis of Water-Based Gold Nanofluids for Solar Applications: Optical Properties and Photothermal Conversion Efficiency. Sol. RRL 2022, 6, 2200104. [Google Scholar] [CrossRef]
- López-Munoz, G.A.; Balderas-López, J.A.; Ortega-Lopez, J.; Pescador-Rojas, J.A.; Salazar, J.S. Thermal diffusivity measurement for urchin-like gold nanofluids with different solvents, sizes and concentrations/shapes. Nanoscale Res. Lett. 2012, 7, 667. Available online: https://www.nanoscalereslett.com/content/7/1/667 (accessed on 12 July 2023.). [CrossRef] [PubMed]
- Carrilo-Berdugo, I.; Sampaio-Guzmán, J.; Dominguez-Nunez, A.; Aguilar, T.; Martinez-Merino, P.; Navas, J. Are Nanofluids Suitable for Volumetric Absorption in PTC-CSP Plants? An Exemplified, Realistic Assessment with Characterized Metal–Oil Nanofluids. Energy Fuels 2022, 36, 8413–8421. [Google Scholar] [CrossRef]
- Han, X.; Yao, Y.; Zhao, X.; Huang, J.; Khosa, A.A. Investigations of stable surface-modified gold nanofluids optical filters based on optical optimization for photovoltaic/thermal systems. Sustain. Energy Technol. Assess. 2023, 57, 103203. [Google Scholar] [CrossRef]
- Amjad, M.; Raza, G.; Xin, Y.; Pervaiz, S.; Xu, J.; Du, X.; Wen, D. Volumetric solar heating and steam generation via gold nanofluids. Appl. Energy 2017, 206, 393–400. [Google Scholar] [CrossRef]
- Du, M.; Tang, G.H. Plasmonic nanofluids based on gold nanorods/nanoellipsoids/nanosheets for solar energy harvesting. Sol. Energy 2016, 137, 393–400. [Google Scholar] [CrossRef]
- Shalkevich, N.; Escher, W.; Burgi, T.; Bruno, M.; Si-Ahmed, L.; Poulikakos, D. On the thermal conductivity of gold nanoparticle colloids. Langmuir 2009, 26, 663–670. [Google Scholar] [CrossRef]
- Kim, H.J.; Bang, I.C.; Onoe, J. Characteristic stability of bare Au-water nanofluids fabricated by pulsed laser ablation in liquids. Opt. Lasers Eng. 2009, 47, 532–538. [Google Scholar] [CrossRef]
- Abdelhalim, M.A.K.; Mady, M.M.; Ghannam, M.M. Rheological and dielectric properties of different gold nanoparticle sizes. Lipid Health Dis. 2011, 10, 208. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Wen, D. Ultrasonic-aided fabrication of gold nanofluids. Nanoscale Res. Lett. 2011, 6, 198. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, X.; Lou, W.; Hao, J. Gold-ionic liquid nanofluids with preferably tribological properties and thermal conductivity. Nanoscale Res. Lett. 2011, 6, 259. [Google Scholar] [CrossRef]
- Lopez-Munoz, G.; Pescador-Rojas, J.; Ortega, J.; Santoyo, J.; Balderas-Lopez, J. Thermal diffusivity measurement of spherical gold nanofluids of different sizes/concentrations. Nanoscale Res. Lett 2012, 7, 423. [Google Scholar] [CrossRef]
- Torres-Mendieta, R.; Mondragón, R.; Juliá, E.; Mendoza-Yero, O.; Cordoncillo, E.; Lancis, J.; Mínguez-Vega, G. Fabrication of gold nanoparticles in Therminol VP-1 by laser ablation and fragmentation with fs pulses. Laser Phys. Lett. 2014, 11, 126001. [Google Scholar] [CrossRef]
- John, J.; Thomas, L.; Kurian, A.; George, S.D. Modulating fluorescence quantum yield of highly concentrated fluorescein using differently shaped green synthesized gold nanoparticles. J. Lumin. 2016, 172, 39–46. [Google Scholar] [CrossRef]
- Zeiny, A.; Jin, H.; Lin, G.; Song, P.; Wen, D. Solar evaporation via nanofluids: A comparative study. Renew. Energy 2018, 122, 443–454. [Google Scholar] [CrossRef]
- Yuan, M.; Li, Y.-B.; Guo, J.; Zhong, R.-B.; Wang, L.-P.; Zhang, F. Solvent effects on gold nanoparticle formation from pho-to-chemical reduction of Au(III) by UV irradiation. Nucl. Sci. Tech. 2018, 29, 158. [Google Scholar] [CrossRef]
- Nimmagadda, R.; Reuven, R.; Asirvatham, L.G.; Wongwises, S. Thermal Management of Electronic Devices Using Gold and Carbon Nanofluids in a Lid-Driven Square Cavity Under the Effect of Variety of Magnetic Fields. IEEE Trans. Compon. Packag. Manuf. Technol. 2020, 10, 1868–1878. [Google Scholar] [CrossRef]
- Zaaroura, I.; Harmand, S.; Carlier, J.; Toubal, M.; Fasquelle, A.; Nongaillard, B. Experimental studies on evaporation kinetics of gold nanofluid droplets: Influence of nanoparticle sizes and coating on thermal performance. Appl. Therm. Eng. 2021, 183, 116180. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, M.; Yan, H.; Zhou, P.; Chen, X.-Y. Enhanced solar thermal conversion performance of plasmonic gold dimer nanofluids. Appl. Therm. Eng. 2020, 178, 115561. [Google Scholar] [CrossRef]
- Fogaça, M.B.; Dias, D.T.; Gómez, S.L.; Behainne, J.J.R.; Turchiello, R.F. Effectiveness of a Shell and Helically Coiled Tube Heat Exchanger Operated With Gold Nanofluids at Low Concentration: A Multi-Level Factorial Analysis. J. Thermal Sci. Eng. Appl. 2021, 13, 021029. [Google Scholar] [CrossRef]
- Sharma, P.; Baek, I.H.; Cho, T.; Park, S.; Lee, K.B. Enhancement of thermal conductivity of ethylene glycol based silver nanofluids. Powder Technol. 2011, 208, 7–19. [Google Scholar] [CrossRef]
- Warrier, P.; Teja, A. Effect of particle size on the thermal conductivity of nanofluids containing metallic nanoparticles. Nanoscale Res. Lett. 2011, 6, 247. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef]
- Pourhoseini, S.H.; Naghizadeh, N.; Hoseinzadeh, H. Effect of silver-water nanofluid on heat transfer performance of a plate heat exchanger: An experimental and theoretical study. Powder Technol. 2018, 332, 279–286. [Google Scholar] [CrossRef]
- Oliveira, G.A.; Filho, E.P.B.; Wen, D. Synthesis and characterization of silver/water nanofluids. High Temp. High Press. 2014, 43, 69–83. [Google Scholar]
- Gupta, M.; Singh, V.; Kumar, S.; Dibaghi, N. Experimental analysis of heat transfer behavior of silver, MWCNT and hybrid (silver + MWCNT) nanofluids in a laminar tubular flow. J. Therm. Anal. Calorim. 2020, 142, 1545–1559. [Google Scholar] [CrossRef]
- Khamliche, T.; Khamlich, S.; Doyle, T.B.; Makinde, D.; Maaza, M. Thermal conductivity enhancement of nano-silver particles dispersed ethylene glycol based nanofluids. Mater. Res. Express 2018, 5, 035020. [Google Scholar] [CrossRef]
- Singh, A.K.; Raykar, V.S. Microwave synthesis of silver nanofluids with polyvinylpyrrolidone (PVP) and their transport properties. Colloid Polym. Sci. 2008, 286, 1667–1673. [Google Scholar] [CrossRef]
- Tamjid, E.; Gunther, B. Rheology and colloidal structure of silver nanoparticles dispersed in diethylene glycol. Powder Technol. 2010, 197, 49–53. [Google Scholar] [CrossRef]
- Zhu, D.; Huang, G.; Zhang, L.; He, Y.; Xie, H.; Yu, W. Silver Nanowires Contained Nanofluids with Enhanced Optical Absorption and Thermal Transportation Properties. Energy Environ. Mater. 2019, 2, 22–29. [Google Scholar] [CrossRef]
- Wang, H.; Qiao, X.; Chen, J.; Ding, S. Preparation of silver nanoparticles by chemical reduction method. Colloids Surf. A Physicochem. Eng. Asp. 2005, 256, 111–115. [Google Scholar] [CrossRef]
- Shameli, K.; Ahmad, M.B.; Jazayeri, S.D.; Sedaghat, S.; Shabanzadeh, P.; Jahangirian, H.; Mahdavi, M.; Abdollahi, Y. Synthesis and Characterization of Polyethylene Glycol Mediated Silver Nanoparticles by the Green Method. Int. J. Mol. Sci. 2012, 13, 6639–6650. [Google Scholar] [CrossRef] [PubMed]
- Nyamgoudar, S.M.; Silaparasetti, V.P.; Shilpa, M.P.; Pavithra, K.S.; Mundinamani, S.; Eshwarappa, K.M.; Surabhi, S.; Raman, K.; Ravikanara Ganesha, A.; Gurumurthy, S.C. Analysis of shape dependency of thermal conductivity of silver-based nanofluids. J. Therm. Anal. Calorim. 2022, 147, 14031–14038. [Google Scholar] [CrossRef]
- Hasan, I.A.; Sultan, K.F. Performance Evaluation of the V–basin Tube Solar Collector by using different Nanoparticles and Base Fluids. Eng. Technol. J. 2018, 36, 461–470. [Google Scholar] [CrossRef]
- Seyhan, M.; Altan, C.L.; Gurten, B.; Bucak, S. The effect of functionalized silver nanoparticles over the thermal conductivity of base fluids. AIP Adv. 2017, 7, 045101. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, W.; Zhu, D.; Xie, H.; Huang, G. Enhanced Thermal Conductivity for Nanofluids Containing Silver Nanowires with Different Shapes. J. Nanomater. 2017, 2017, 5802016. [Google Scholar] [CrossRef]
- Iyahraja, S.; Rajadurai, J.S. Stability of aqueous nanofluids containing PVP-coated silver nanoparticles. Arab. J. Sci. Eng. 2016, 41, 653–660. [Google Scholar] [CrossRef]
- Parametthanuwat, T.; Bhuwakietkumjohn, N.; Rittidech, S.; Ding, Y. Experimental investigation on thermal properties of silver nanofluids. Int. J. Heat Fluid Flow 2015, 56, 80–90. [Google Scholar] [CrossRef]
- Asmat-Campos, D.; Avalos, V.; Delgado, A.; Gutierrez, H.; Jacinto, P.; Reyes, Z. Synthesis and characterization of nanofluids from the biosynthesis of nanoparticles and their evaluation in solar thermal systems. E3S Web Conf. 2020, 167, 05003. [Google Scholar] [CrossRef]
- Kathiravan, R.; Kumar, R.; Gupta, A.; Chandra, R. Preparation and Pool Boiling Charcatreistics of Silver Nanofluids Over a Flat Plate Heater. Heat Transf. Eng. 2012, 33, 69–78. [Google Scholar] [CrossRef]
- Moreira, L.M.; Carvalho, E.A.; Bell, M.J.; Anjos, V.; Sant’Ana, A.C.; Alves, A.P.; Fragneaud, B.; Sena, L.Á.; Archanjo, B.S.; Achete, C.A. Thermo-optical properties of silver and gold nanofluids. J. Therm. Anal. Calorim. 2013, 114, 557–564. [Google Scholar] [CrossRef]
- Maddah, H.; Rezazadeh, M.; Maghsoudi, M.; Nasirikokhdan, S. The effect of silver and aluminum oxide nanoparticles on thermophysical properties of nanofluids. J. Nanostruct. Chem. 2013, 3, 28. [Google Scholar] [CrossRef]
- Sarafraz, M.M.; Arya, A.; Nikkhah, V.; Hormozi, F. Thermal Performance and Viscosity of Biologically Produced Silver/Coconut Oil Nanofluids. Chem. Biochem. Eng. Q. 2016, 30, 489–500. [Google Scholar] [CrossRef]
- Zeroual, S.; Estellé, P.; Cabaleiro, D.; Vigolo, B.; Emo, M.; Halim, W.; Ouaskit, S. Ethylene glycol based silver nanoparticles synthesized by polyol process: Characterization and thermophysical profile. J. Mol. Liq. 2020, 310, 113229. [Google Scholar] [CrossRef]
- Walshe, J.; Carron, P.M.; McLoughlin, C.; McCormack, S.; Doran, J.; Amarandei, G. Nanofluid Development Using Silver Nanoparticles and Organic-Luminescent Molecules for Solar-Thermal and Hybrid Photovoltaic-Thermal Applications. Nanomaterials 2020, 10, 1201. [Google Scholar] [CrossRef]
- Contreras, E.M.C.; Oliveira, G.A.; Filho, E.P.B. Experimental analysis of the thermohydraulic performance of graphene and silver nanofluids in automotive cooling systems. Int. J. Heat Mass Transf. 2019, 132, 375–387. [Google Scholar] [CrossRef]
- Selvam, C.; Irshad, E.C.M.; Lal, D.M.; Harish, S. Convective heat transfer characteristics of water–ethylene glycol mixture with silver nanoparticles. Exp. Therm. Fluid Sci. 2016, 77, 188–196. [Google Scholar] [CrossRef]
- Wu, X.; Chen, X.; Jin, S.; He, G.; Guo, Y.; Fang, W. Highly stable macroinitiator/platinum/hydrocarbon nanofluids for efficient thermal management in hypersonic aircraft from synergistic catalysis. Energy Convers. Manag. 2019, 198, 111797. [Google Scholar] [CrossRef]
- Wu, X.; Ye, D.; Jin, S.; Guo, Y.; Fang, W. Cracking of platinum/hydrocarbon nanofluids with hyperbranched polymer as stabilizer and initiator. Fuel 2019, 255, 115782. [Google Scholar] [CrossRef]
- Carrillo-Berdugo, I.; Midgley, S.D.; Grau-Crespo, R.; Zorrilla, D.; Navas, J. Understanding the Specific Heat Enhancement in Metal-Containing Nanofluids for Thermal Energy Storage: Experimental and Ab Initio Evidence for a Strong Interfacial Layering Effect. ACS Appl. Energy Mater. 2020, 3, 9246–9256. [Google Scholar] [CrossRef]
- Carrilo-Berdugo, I.; Estellé, P.; Sani, E.; Mercatelli, L.; Grau-Crespo, R.; Zorrila, D.; Navas, J. Optical and Transport Properties of Metal−Oil Nanofluids for Thermal Solar Industry: Experimental Characterization, Performance Assessment, and Molecular Dynamics Insights. ACS Sustain. Chem. Eng. 2021, 9, 4194–4205. [Google Scholar] [CrossRef]
- Qin, X.; Yang, S.; Chen, Y.; Qin, X.; Zhao, J.; Fang, W.; Luo, D. Thermal Conductivity and Stability of Hydrocarbon-Based Nanofluids with Palladium Nanoparticles Dispersed by Modified Hyperbranched Polyglycerol. ACS Omega 2020, 5, 31156–31163. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.C.R.; Da Silva, N.P.; Castilho, A.V.; Favre-Nicolin, V.A.; Cesar CLOrlande, H.R.B.; Dos Santos, D.S. Synthesis, characterization and photothermal analysis of nanostructured hydrides of Pd and PdCeO2. Sci. Rep. 2020, 10, 17561. [Google Scholar] [CrossRef]
- Yue, L.; Wub, J.; Gonga, Y.; Fang, W. Thermodynamic properties and pyrolysis performances of hydrocarbon-fuel-based nanofluids containing palladium nanoparticles. J. Anal. Appl. Pyrolysis 2016, 120, 347–355. [Google Scholar] [CrossRef]
- Patil, V.S.; Patil, S.H.; Patil, K.R.; Rode, C.V.; Coronas, A.; Nieto de Castro, C.A. Study of anion effect and heat transfer properties of Ru Ionanofluid. In Proceedings of the Solar Absorption Refrigeration Systems Operating with Ionic Liquids, an Indo-Spanish Workshop, IIT MADRAS, Chennai, India, 21–22 February 2014. [Google Scholar]
- Bonet, F.; Delmas, V.; Grugeon, S.; Urbina, R.H.; Silvert, P.Y.; Tekaia-Elhissen, K. Synthesis of monodisperse Au, Pt, Pd, Ru and Ir nanoparticles in ethylene glycol. Nanostruct. Mater. 1999, 11, 1277–1284. [Google Scholar] [CrossRef]
- Zhang, T.; Li, S.-C.; Zhu, W.; Ke, J.; Yu, J.-W.; Zhang, Z.-P.; Dai, L.-X.; Gu, J.; Zhang, Y.-W. Iridium ultrasmall nanoparticles, worm-like chain nanowires, and porous nanodendrites: One-pot solvothermal synthesis and catalytic CO oxidation activity. Surf. Sci. 2016, 648, 319–327. [Google Scholar] [CrossRef]
- Santacruz, L.; Donnici, S.; Granados, A.; Shafir, A.; Vallribera, A. Fluoro-tagged osmium and iridium nanoparticles in oxidation reactions. Tetrahedron 2018, 74, 6890–6895. [Google Scholar] [CrossRef]
- Tanaka, H.; Orita, K.; Maede, A.; Ishikawa, H.; Miura, M.; Arai, S.; Higushi, T.; Otha, S.; Muto, S. Dynamics of Rh nanoparticle surface structure during NO reduction revealed by operando transmission electron microscopy. Appl. Catal. A Gen. 2021, 626, 118334. [Google Scholar] [CrossRef]
- Swiatkowska-Warkocka, Z. Bimetal CuFe Nanoparticles—Synthesis, Properties, and Applications. Appl. Sci. 2021, 11, 1978. [Google Scholar] [CrossRef]
- Zhou, G.; Lu, M.; Yang, Z. Aqueous Synthesis of Copper Nanocubes and Bimetallic Copper/Palladium Core−Shell Nanostructures. Langmuir 2006, 22, 5900–5903. [Google Scholar] [CrossRef] [PubMed]
- Behera, A.; Mittu, B.; Padhi, S.; Patra, N.; Singh, J. Chapter 25—Bimetallic nanoparticles: Green synthesis, applications, and future perspectives. In Multifunctional Hybrid Nanomaterials for Sustainable Agri-Food and Ecosystems, Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 639–682. [Google Scholar] [CrossRef]
- Loza, K.; Heggen, M.; Epple, M. Synthesis, Structure, Properties, and Applications of Bimetallic Nanoparticles of Noble Metals. Adv. Funct. Mater. 2020, 30, 1909260. [Google Scholar] [CrossRef]
- Singh, S.K.; Iizuka, Y.; Xu, Q. Nickel-palladium nanoparticle catalyzed hydrogen generation from hydrous hydrazine for chemical hydrogen storage. Int. J. Hydrogen Energy 2011, 36, 11794–11801. [Google Scholar] [CrossRef]
- Feng, R.; Li, M.; Liu, J. Synthesis of core–shell Au@Pt nanoparticles supported on Vulcan XC-72 carbon and their electrocatalytic activities for methanol oxidation. Colloids Surf. A 2012, 406, 6–12. [Google Scholar] [CrossRef]
- Kumari, M.; Jacob, J.; Philip, D. Green synthesis and applications of Au-Ag bimetallic nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 137, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Hammami, I.; Alabdallah, N.M.; Jomaa, A.A.; Kamoun, M. Gold nanoparticles: Synthesis properties and applications. J. King Saud Univ. Sci. 2021, 33, 101560. [Google Scholar] [CrossRef]
- Gad, G.M.A.; Hegazy, M.A. Optoelectronic properties of gold nanoparticles synthesized by using wet chemical method. Mater. Res. Express 2019, 6, 085024. [Google Scholar] [CrossRef]
- Jan, H.; Gul, R.; Andleeb, A.; Ullah, S.; Shah, M.; Khanum, M.; Ullah, I.; Hano, C.; Abbasi, B.H. A detailed review on biosynthesis of platinum nanoparticles (PtNPs), their potential antimicrobial and biomedical applications. J. Saudi Chem. Soc. 2021, 25, 101297. [Google Scholar] [CrossRef]
- Ledendecker, M.; Paciok, P.; Osowiecki, W.T.; Pander, M.; Heggen, M.; Göhl, D.; Kamat, G.A.; Erbe, A.; Mayrhofer, K.J.; Alivisatos, A.P. Engineering gold-platinum core-shell nanoparticles by self-limitation in solution. Commun. Chem. 2022, 5, 71. [Google Scholar] [CrossRef]
- Lucas, G.L.L.; Romanus, H.; Ispas, A.; Bund, A. Hollow platinum-gold and palladium-gold nanoparticles: Synthesis and characterization of composition-structure relationship. J. Nanopart. Res. 2022, 24, 245. [Google Scholar] [CrossRef]
- Wang, S.-S.; Wu, C.-W.; Chen, P.-Y.; Lee, C.-L. Preferential deposition of gold and platinum atom on palladium nanocubes as catalysts for oxidizing glucose in the phosphate-buffered solution. J. Electroanal. Chem. 2023, 930, 117142. [Google Scholar] [CrossRef]
- Lou, Z.; Fujitsuka, M.; Majima, T. Pt–Au triangular nanoprisms with strong dipole plasmon resonance for hydrogen generation studied by single-particle spectroscopy. ACS Nano 2016, 10, 6299–6305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Toshima, N. Synthesis of Au/Pt bimetallic nanoparticles with a Pt-rich shell and their high catalytic activities for aerobic glucose oxidation. J. Colloid Interface Sci. 2013, 394, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Mallah, A.R.; Zubir, M.N.M.; Alawi, O.A.; Newaz, K.S.; Badry, A.B.M. Plasmonic nanofluids for high photothermal conversion efficiency in direct absorption solar collectors: Fundamentals and applications. Sol. Energy Mater. Sol. Cell. 2019, 201, 110084. [Google Scholar] [CrossRef]
- Verma, A.K.; Yadav, N.; Sing, S.P.; Dey, K.K.; Singh, D.; Yadav, R.R. Study of Ultrasonic Attenuation and Thermal Conduction in Bimetallic Gold/Platinum Nanofluids. Johnson Matthey Technol. Rev. 2021, 65, 556–567. [Google Scholar] [CrossRef]
- Chen, M.; He, Y.; Zhu, J. Preparation of Au–Ag bimetallic nanoparticles for enhanced solar photothermal conversion. Int. J. Heat Mass Transf. 2017, 114, 1098–1104. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, L.; Bing, N.; Xie, H.; Yu, W. Enhancement of photothermal conversion performance using nanofluids based on bimetallic Ag-Au alloys in nitrogen-doped graphitic polyhedrons. Energy 2019, 183, 747–755. [Google Scholar] [CrossRef]
- Sánchez-Ramírez, J.F.; Jiménez Pérez, J.L.; Cruz Orea, A.; Gutiérrez Fuentes, R.; Bautista-Hernández, A.; Pal, U. Thermal diffusivity of nanofluids containing Au/Pd bimetallic nanoparticles of different compositions. J. Nanosci. Nano-Technol. 2006, 63, 685–690. [Google Scholar] [CrossRef]
- Gutierrez Fuentes, R.; Pescador Rojas, J.A.; Jiménez-Pérez, J.L.; Sanchez Ramirez, J.F.; Cruz-Orea, A.; Mendoza-Alvarez, J.G. Study of thermal diffusivity of nanofluids with bimetallic nanoparticles with Au(core)/Ag(shell) structure. Appl. Surf. Sci. 2008, 255, 781–783. [Google Scholar] [CrossRef]
- Dsouza, A.; Shilpa, M.P.; Gurumurthy, S.C.; Nagaraja, B.S.; Mundinamani, S.; Raman, K.; Gedda, M.; Murari, M.S. CuAg and AuAg bimetallic nanoparticles for catalytic and heat transfer applications. Clean Technol. Environ. Policy 2021, 23, 2145–2155. [Google Scholar] [CrossRef]
- Makishima, A. Possibility of hybrids materials. Ceram. Jpn. 2004, 39, 90–91. [Google Scholar]
- Gamachu, D.; Ibrahim, W. Mixed convection flow of viscoelastic Ag-Al2O3/water hybrid nanofluid past a rotating disk. Phys. Scr. 2021, 96, 125205. [Google Scholar] [CrossRef]
- Hayat, T.; Nadeem, S. Heat transfer enhancement with Ag–CuO/water hybrid nanofluid. Results Phys. 2017, 7, 2317–2324. [Google Scholar] [CrossRef]
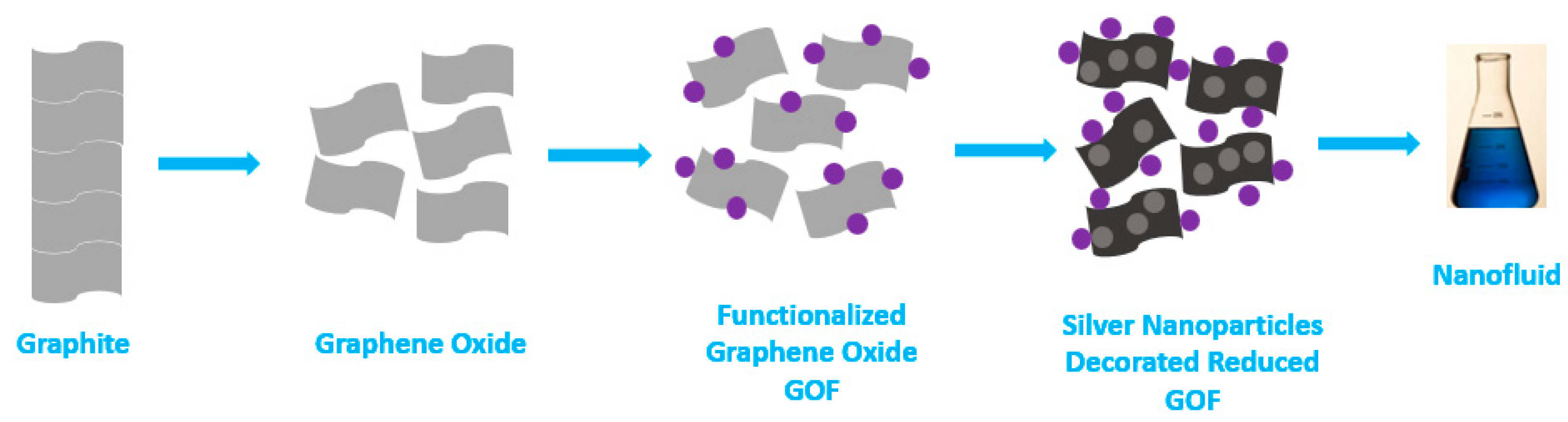
- Singh, J.P.; Nandi, T.; Ghosh, S.K. Structure-property relationship of silver decorated functionalized reduced graphene oxide based nanofluids: Optical and thermophysical aspects and applications. Appl. Surf. Sci. 2021, 542, 148410. [Google Scholar] [CrossRef]
- Naderi, A.; Gazori, H.; Bozegi, M. Experimental Analysis PVP Coated Silver Nanofluid Properties for Application in Photovoltaic/Thermal (PVT) Collectors. J. Model Based Res. 2020, 1, 28–40. [Google Scholar] [CrossRef]
- Mbambo, M.C.; Madito, M.J.; Khamliche, T.; Mtshali, C.B.; Khumalo, Z.M.; Madiba, I.G.; Mothudi, B.M.; Maaza, M. Thermal conductivity enhancement in gold decorated graphene nanosheets in ethylene glycol based nanofluid. Sci. Rep. 2020, 10, 14730. [Google Scholar] [CrossRef]
- Yarmand, H.; Gharehkhani, S.; Shirazi, S.F.S.; Goodarzi, M.; Amiri, A.; Sarsam, W.S.; Alehashem, M.S.; Dahari, M.; Kazi, S.N. Study of synthesis, stability and thermo-physical properties of graphene nanoplatelet/platinum hybrid nanofluid. Int. Commun. Heat Mass Transf. 2016, 77, 15–21. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, G.; Shen, L.; Yu, W.; Zhu, D.; Zhang, Y.; Zhang, L.; Xie, H. Enhanced solar energy absorption on nitrogen-doped carbon nanotubes decorated with gold-palladium bimetallic nanoparticles. Therm. Sci. 2018, 22, S701–S708. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Wan, M.; Singh, S.; Singh, D.K.; Yadav, R.R.; Singh, D.; Mishra, G. Experimental Investigation of Thermal Conduction in Copper-Palladium Nanofluids. J. Nanofluids 2016, 5, 496–501. [Google Scholar] [CrossRef]
- Aberoumand, S.; Jafarimoghaddam, A. Tungsten (III) oxide (WO3)—Silver/transformer oil hybrid nanofluid: Preparation, stability, thermal conductivity and dielectric strength. Alex. Eng. J. 2018, 57, 169–174. [Google Scholar] [CrossRef]
- Ma, M.; Zhai, Y.; Yao, P.; Li, Y.; Wang, H. Effect of surfactant on the rheological behavior and thermophysical properties of hybrid nanofluids. Powder Technol. 2021, 379, 373–383. [Google Scholar] [CrossRef]
- Li, H.; He, Y.; Wang, C.; Wang, X.; Hu, Y. Tunable thermal and electricity generation enabled by spectrally selective absorption nanoparticles for photovoltaic/thermal applications. Appl. Energy 2019, 236, 117–126. [Google Scholar] [CrossRef]
- Hjerrild, N.E.; Mesgari, S.; Crisostomo, F.; Scott, J.A.; Amal, R.; Taylor, R.A. Hybrid PV/T enhancement using selectively ab-sorbing Ag–SiO2/carbon nanofluids. Sol. Energy Mater. Sol. Cells 2016, 147, 282–287. [Google Scholar] [CrossRef]
- Munkhbayar, B.; Tanshen, M.R.; Jeoun, J.; Chung, H.; Jeong, H. Surfactant-free dispersion of silver nanoparticles into MWCNT-aqueous nanofluids prepared by one-step technique and their thermal characteristics. Ceram Int. 2013, 39, 6415–6425. [Google Scholar] [CrossRef]
- Baby, T.T.; Ramaprabhu, S. Synthesis and nanofluid application of silver nanoparticles decorated graphene. J. Mater. Chem. 2011, 21, 9702–9709. [Google Scholar] [CrossRef]
- Chen, L.; Yu, W.; Xie, H. Enhanced thermal conductivity of nanofluids containing Ag/MWNT composites. Powder Technol. 2012, 231, 18–20. [Google Scholar] [CrossRef]
- Botha, S.S.; Ndungu, P.; Bladergroen, B.J. Physicochemical properties of oil-based nanofluids containing hybrid structures of silver nanoparticles supported on silica. Ind. Eng. Chem. Res. 2011, 50, 3071–3077. [Google Scholar] [CrossRef]
- Han, W.S.; Rhi, S.H. Thermal characteristics of grooved heat pipe with hybrid nanofluids. Therm. Sci. 2011, 15, 195–206. [Google Scholar] [CrossRef]
- Dinarvand, S.; Rostami, M.N. An innovative mass-based model of aqueous zinc oxide–gold hybrid nanofluid for von Kármán´s swirling flow. J. Therm. Anal. Calorim. 2019, 138, 845–855. [Google Scholar] [CrossRef]
- Jin, X.; Lin, G.; Zeiny, A.; Jin, H.; Bai, L.; Wen, D. Solar photothermal conversion characteristics of hybrid nanofluids: An ex-perimental and numerical study. Renew. Energy 2019, 141, 937–949. [Google Scholar] [CrossRef]
- Baniamerian, Z.; Mashayekhi, M.; Mehdipour, R. Evaporative Behavior of Gold-Based Hybrid Nanofluids. J. Thermo-Phys. Heat Transf. 2017, 32, 284–291. [Google Scholar] [CrossRef]




| Authors | Morphology of the Nanoparticles | Concentration of the Nanoparticles | Base Fluid | Main Findings | Observations | Reference |
|---|---|---|---|---|---|---|
| Shalkevich et al. | Spherical with 2 to 45 nm | 0.00025% vol.–1% vol. | Water | TC increase of 1.4% at 0.11% vol. and 40 nm | Addition of EGMUDE surfactant | [30] |
| Kim et al. | Near-Spherical with 7.1 to 12.1 nm | 0.018% vol. | Water | TC increase of 9.3% | Stable after one month without any surfactant | [31] |
| Abdelhalim et al. | Spherical with 10/20 nm Hexagonal with 50 nm | 0.01% vol. | Water | The larger nanoparticles with 50 nm exhibited higher viscosity than the smaller ones with 10 nm and 20 nm | The electrical conductivity increased with increasing size of the nanoparticles | [32] |
| Chen and Wen | Spherical and Plate-Shaped with 10 to 300 nm | 764 μM/L | Water | TC increase of around 65% | The ultrasonication is a very powerful tool in engineering particle size and shape The TC was dependent on the particle shape and size | [33] |
| Wang et al. | Spherical with 5.2 ± 1.2 nm | 0.000255% vol., 0.00051% vol., and 0.00102% vol. | [Bmim]PF6 | TC increase of 13.1% vol. | Addition of CTABr surfactant | [34] |
| Lopez-Munoz et al. | Near- Spherical with 16 nm to 125 nm | 0.2 mg/mL to 1 mg/ml | Water | TD increase of 4.5% at 1 mg/mL with 55 nm | The TD ratio increased with increasing concentration | [35] |
| Torres-Mendieta et al. | Spherical with 20 nm to nearly 750 nm | --- | Therminol VP-1 and TOAB | TC increase of around 4.1% | Addition of PBS surfactant | [36] |
| John et al. | Spherical, Star-shaped and Bean-shaped | 0.26 nM to 2.31 nM | Water | TD depended on the shape and concentration of the nanoparticles | The TD values indicate an enhancement in the TC and a decrease in the SH | [37] |
| Zeiny et al. | Spherical with 2.5 nm to 9 nm | 30 mg/L to 150 mg/L | Water | Photothermal conversion increase of around 72% | The specific absorption rate of the gold nanofluids was superior to that of copper nanofluids | [38] |
| Yuan et al. | Spheres, Triangles, and Rods | Ketone, alcohol, ACN, THF | Ketone and alcohol formed gold nano-scaled spheres ACN and THF formed rods and triangles | The gold triangles and rods had more tendency to aggregate | [39] | |
| Nimmagadda et al. | Spherical with 50 nm and 70 nm | 2% vol. | Water | The nanofluid at 2 vol.% with 50 nm caused an increase in the convective heat transfer efficiency around 13% | The convective heat transfer efficiency increased with increasing Reynolds number and it was optimal in the magnetic field located along the X-axis | [40] |
| Zaaroura et al. | Spherical with 2.2 nm, 5 nm, and 10 nm | 1% vol. | Water | The uncapped nanofluids with PBS solution showed that the nanoparticle size of 5 nm caused the greatest increase in the evaporation rate of 35% than the ones with 10 nm The citrate-capped nanofluids showed opposite results, the larger nanoparticles of 10 nm had the greatest increase in the evaporation rate of 15% but still less than the solution with PBS only | Addition of citrate-capping PBS and no citrate-PBS | [41] |
| Chen et al. | Sphere and rod with 20 nm | 0 ppm to 200 ppm | Water | The solar absorption efficiency of the dimer nanofluid was enhanced by 21.2% when compared with the blended nanofluid with the same number of sphere and rod | Utilization of a thin layer of a plasmonic dimer nanofluid composed of a sphere and rod in water | [42] |
| Fogaça et al. | Spherical with 14 ± 2 nm | Two concentrations around 10−5% vol. | Water | The less concentrated nanofluids were more efficient, suggesting the presence of a range of gold concentration for improving the heat transfer | Use of a shell and helically coiled tube heat exchanger with flowrates of 20 l/h, 30 l/h, and 40 l/h | [43] |
| Han et al. | Spherical with 20 nm, 40 nm, 60 nm, and 80 nm | 1 ppm to 80 ppm | Water and Ethylene Glycol | The optimal Merit Function of 1.95 and exergy efficiency of around 14% was achieved filtered by gold + PVP/ethylene glycol nanofluid at 29.2 ppm and 2 mL/min. | Addition of PVP or PEG | [27] |
| Authors | Morphology of the Nanoparticles | Concentration of the Nanoparticles | Base Fluid | Main Findings | Observations | Reference |
|---|---|---|---|---|---|---|
| Singh and Raikar | Spherical with 30 nm to 60 nm | 1% vol. | Ethanol | 1.55-fold increase in the TC | Addition of PVP | [51] |
| Kathiravan et al. | Spherical with 15 nm | 0.25% wt., 0.5% wt., and 0.75% wt. | Water | TC was 2.5 times greater at a heat flux of 250 kW/m2 at 0.75% wt. | Addition of sodium lauryl sulfate | [63] |
| Moreira et al. | Spherical with 15.17 nm | 0.000426 | Water | 15.9% increase in the TD | The smaller nanoparticles gave a better enhancement in the heat transfer | [64] |
| Madah et al. | Spherical with 40 nm | 0.25% vol. to 5% vol. | Water | The TC increased linearly with increasing concentration of nanoparticles | The electrical conductivity increased considerably with increasing concentration of nanoparticles | [65] |
| Salehi et al. | Spherical with 3 nm to 11 nm | 0 ppm to 1000 ppm | Water | Maximum increase in the TC of nearly 18% | Addition of PVP | [6] |
| Sarafraz et al. | Spherical with 50 nm | 0.5% wt., 1% wt., and 1.5% wt. | Coconut Oil | Increase in the TC of 60% at 1.5% wt. | The TC slightly increased and the viscosity decreased with increasing temperature The TC and viscosity increased with increasing concentration of nanoparticles | [66] |
| Zhu et al. | Nanowires with 100 nm of diameter and 20 µm and 100 µm of length | 5 mg·mL−1 to 20 mg·mL−1 | Ethylene Glycol | At 20 mg·mL−1 the photothermal conversion efficiency of the 20 µm and 100 µm nanowires were of 43.3% and 46.1%, respectively | The 100 µm long silver nanowires nanofluid had a higher photothermal conversion efficiency than the 20 µm ones | [53] |
| Zeroual et al. | Spherical with 2–3 nm | --- | Ethylene Glycol | Increase in the TC of 2.5–3% | Addition of various contents of latex copolymer | [67] |
| Walshe et al. | Spherical with 5 nm to 110 nm | --- | Water | Enhancements of 5–32% in the photo-thermal conversion efficiency | Addition of PVA | [68] |
| Oliveira et al. | Spherical with 10 nm and 80 nm | 0.1% vol.–0.3% vol. | Water | The maximum increase in the TC was 18% at 0.3% vol. | Little increase in the viscosity at 0.3% vol. | [48] |
| Nakhjavani et al. | Spherical with 25 nm, 45–50 nm, and 75 nm | 0.1% wt.–0.4% wt. | Water | Increase in the TC of approximately 45% | Optimal concentration of 0.1% wt. | [12] |
| Contreras et al. | Spherical | 0.01% vol., 0.05% vol. and 0.1% vol. | Water/Ethylene Glycol 50:50 | Increase of up to 4.4% in the heat transfer rate | The pumping power at high temperatures and mass flow rates increased by up to 4.1% | [69] |
| Selvam et al. | Spherical with diameters up to 100 nm | 0.05% vol., 0.1% vol. and 0.15% vol. | Water/Ethylene Glycol 70:30 | The maximum enhancement of TC was around 12% at 0.15% vol. and 50 °C | The SH decreased with increasing concentration and increased with increasing temperature Maximum decrease in the SH of around 7% at 0.15% vol. and 40 °C | [70] |
| Seifikar et al. | Spherical with 4.5 nm | 0.002 g/100 mL, 0.005 g/100 mL, and 0.008 g/100 mL | Polyethylene Glycol | The surface temperature reached around 52 °C with a ΔT of 24 °C under 1 Sun solar simulator light intensity for 1 h | Highly Stable Nanofluids | [1] |
| Authors | Nanoparticles | Concentration of the Nanoparticles | Base Fluid | Main Findings | Observations | Reference |
|---|---|---|---|---|---|---|
| Chen et al. | Gold-Silver | 0.00025% vol. | Water | Highest solar photothermal conversion efficiency of around 41.4% | The efficiency of the system using bimetallic nanoparticles was higher than that using only gold nanoparticles of around 37.8% with a 40% decrease in the gold consumption | [100] |
| Zhu et al. | Gold-Silver/nitrogen-doped graphitic polyhedrons | 5% wt. | Ethylene Glycol | The photothermal conversion efficiency increased by up to approximatelly 74.4% | Considerable broadband absorption in the visible and near-infrared spectrum at lower concentration | [101] |
| Sanchéz-Ramirez et al. | Gold-Palladium with Au/Pd = 12/1, 5/1, 1/1, 1/5 molar ratios | --- | Water | The maximum diffusivity was achieved for the nanoparticles with highest gold/palladium molar ratio | The TD was strongly dependent on the composition of the nanoparticles | [102] |
| Gutierres Fontes et al. | Gold-Silver with core-shell structure at Au/Ag = 3/1, 1/1, 1/3, and 1/6 molar ratios | --- | Water | Linear increment of the TD when the silver shell thickness is increased | The TD was sensible to the type of the metallic nanoparticles structure The TD was modified by altering the composition of the nanoparticles | [103] |
| Dsouza et al. | Gold-Silver spherical with 30 nm | 0% vol. to 1.0% vol. | Water | Increase in the TC of 7% at 0.6% vol. and the viscosity has nearly attained 6% | The TC and viscosity increases with increasing concentration of nanoparticles | [104] |
| Silver-Copper with 65 nm | 0% vol. to 1.0% vol. | Water | Increase in the TC of 8% at 1% vol. and increase in the viscosity of about 72% at the same concentration | The TC and viscosity increased with increasing concentration of nanoparticles |
| Authors | Nanoparticles | Concentration of the Nanoparticles | Base Fluid | Main Findings | Observations | Reference |
|---|---|---|---|---|---|---|
| Ma et al. | Silver-Graphene | 0.001% wt., 0.002% wt., and 0.003 wt. | Water | Increase in the CHF of around 52.3% at 0.001% wt. Increase in the HTC of around 69.6% at 0.001% wt. | The bubble formation period of hybrid nanofluids was short and the bubble separation diameter was small | [115] |
| Li et al. | Silver-Titanium Oxide | 100 ppm and 200 ppm | Water | 200 ppm nanofluid produced ~1.4 times of the overall efficiency of the PV/T system compared to water. The merit function reached 2.16 at 100 ppm | The nanoparticles exhibited high absorptivity in the visible wavelengths and good transmittance in the spectral response range of the photovoltaic cell Tunable nanoparticle concentrations were adopted to obtain high-efficiency solar energy utilization | [116] |
| Hjerrild et al. | Core–shell silver-silica nanodiscs | 0.026% wt. | Water | Increase in the efficiency by 30% of the PV/T collector by 30% at 0.026% wt. | The silver nanodiscs strongly absorb visible light with minimal scattering, whereas the silica shell maintains the shape and absorption spectrum of the silver cores | [117] |
| Munkhbayar et al. | Silver-Multi- walled carbon nanotubes with 100 nm (silver) | 0.05% wt. MWCNTs–3% wt. silver | Water | Increase in the TC of 14% | Maximum absorbance of 2.506 abs at a wavelength of 264 nm | [118] |
| Baby and Ramaprabhu | Silver-Hydrogen Induced Exfoliated Graphene | 0.05% vol. | Water | Increase in the TC of around 25% | The nanofluids were stable for three months | [119] |
| Chen et al. | Silver-Multi- walled carbon nanotubes | 1% vol. | Water | Increase in the TC of around 24% | Higher TC than that of the MWCNTs nanofluids of around 12% | [120] |
| Botha et al. | Silver-Silica | 0.6% wt. silver–1.4% wt. silica | Transformer Oil | The theoretical increase in the TC was lower than the experimental determined one, suggesting that there is a synergistic effect between the silver nanoparticles and silica Lower dielectric strength | Newtonian behavior at lower silica concentrations and Bingham flow at high concentrations Lower viscosity compared to the one of the silica nanofluids | [121] |
| Han and Rhi | Silver-Alumina | 0.005% vol., 0.05% vol., and 0.1% vol. | Water | Made the heat pipe system deteriorate in terms of thermal resistance | Greater thermal resistance with increasing concentration of nanoparticles | [122] |
| Dinarvand et al. | Gold-Zinc Oxide nanospheres and nanoplatelets | 3.33% vol. | Water | Increase of 40% in the heat transfer rate | The heat transfer rate increases 2.2% more with the nanoplatelets than with the nanospheres | [123] |
| Jin et al. | Gold-Titanium Oxide spherical and oval with 12–15 nm | Gold 5 ppm and 10 ppm | Water | Increase of around 60.8% in the absorption efficiency | The light–to–heat conversion characteristics of hybrid nanofluids are not necessarily stronger than that of single nanofluids The ratio of different concentrations and the content of different components affected the results | [124] |
| Banianerian et al. | Gold-Titanium Oxide | 0% vol. to 3% vol. | Water | Maximum increase in the latent heat of evaporation of around 27% at 0.1% vol. and 150 °C | Use of hybrid nanofluids to enhance the fluid LHE is rational just in high working pressures. (higher than 400 kPa) Increasing the concentration of nanoparticles at low pressures reduced the latent heat of evaporation, whereas at higher pressures the inverse trend was verified | [125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, J.; Moita, A.; Moreira, A. Noble Nanofluids and Their Hybrids for Heat Transfer Enrichment: A Review and Future Prospects Coverage. Appl. Sci. 2023, 13, 9568. https://doi.org/10.3390/app13179568
Pereira J, Moita A, Moreira A. Noble Nanofluids and Their Hybrids for Heat Transfer Enrichment: A Review and Future Prospects Coverage. Applied Sciences. 2023; 13(17):9568. https://doi.org/10.3390/app13179568
Chicago/Turabian StylePereira, José, Ana Moita, and António Moreira. 2023. "Noble Nanofluids and Their Hybrids for Heat Transfer Enrichment: A Review and Future Prospects Coverage" Applied Sciences 13, no. 17: 9568. https://doi.org/10.3390/app13179568
APA StylePereira, J., Moita, A., & Moreira, A. (2023). Noble Nanofluids and Their Hybrids for Heat Transfer Enrichment: A Review and Future Prospects Coverage. Applied Sciences, 13(17), 9568. https://doi.org/10.3390/app13179568








