Abstract
Objectives: The aim of this systematic review was to analyze the effect of keratinized mucosa (KM) on different peri-implant health-related parameters and on patient-reported outcome measures (PROMs). Material and methods: Randomized controlled trials, cohort, cross-sectional and case–control human studies with a follow-up period of at least 6 months comparing two groups of patients with presence or absence of KM, or with KM < 2 mm or ≥2 mm were included. Primary outcomes were implant failures, PROMs and BoP (BoP/mBI). Additional outcomes were PPD, plaque accumulation (mPI/PI), gingival inflammation (GI/mGI), marginal bone loss (MBL), soft tissue recession (REC) and biological complications. Results: Fifteen studies were included (one RCT, two cohort prospective and twelve cross-sectional). Meta-analysis was performed for cross-sectional studies. Implant failure and complications were not presented as outcome measures, and five studies analyzed PROMs. Results from the meta-analysis reported no evidence of any statistical significant difference between groups in PPD, BoP and MBL, while a statistical significant difference in GI/BI, PI and REC was present in favor of the group with KW ≥ 2 mm. More biological complications were present in the group with no KM/KM < 2 mm but few cases were present to draw any conclusions. Although a meta-analysis could not be performed, a consistent trend toward the worst pain/discomfort in KM < 2 mm was observed. Conclusions: No clear evidence was found supporting the role of KM in peri-implant health and PROMs, even if more plaque and marginal inflammation were present in the KM < 2 mm group. Clinical relevance: KM could have a role in patients with erratic maintenance and patient comfort.
1. Introduction
The use of dental implants to replace missing teeth has become an increasingly common and predictable clinical practice [1,2,3]. Nowadays, the long-term maintenance of healthy peri-implant tissues and of the esthetic of implant rehabilitation is considered of primary importance [4,5]. Additionally, patient satisfaction and patient ease in maintaining good oral hygiene are key factors for the success of the treatment. In this respect, the role of keratinized mucosa (KM) around implants is still controversial [6,7,8].
The 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions stated that a lack of KM is among the principal factors associated with the recession of peri-implant mucosa [9]. Furthermore, it affirmed that the evidence is equivocal regarding the effect of KM on the long-term wellbeing of the peri-implant tissues. The results of the studies on this topic are divergent and without consensus [10,11,12,13,14,15,16,17]. It seems, however, that KM may have advantages regarding patient comfort and the ease of plaque removal [9]. This is in line with the review paper of Wennström & Derks [6] suggesting that although the lack of an “adequate” width of KM per se is not harmful to peri-implant health, it may hamper the performance of proper oral hygiene.
Other systematic reviews have tried to clarify the importance of KM, addressing its ideal width in relation to health and esthetics with controversial results [6,7,8,18].
The aim of this systematic review was to update the knowledge about the influence of KM on peri-implant health and esthetics, also addressing patient-related variables that were never considered in previous reviews.
2. Material and Methods
2.1. Protocol Registration and Reporting Format
This review was written following the PRISMA Statement for reporting Systematic Reviews and Meta-Analyses [19] and following the Cochrane Collaboration guidelines [20]. The protocol was registered in the PROSPERO database, hosted by the National Institute for Health Research, University of York, Center for Reviews and Dissemination (CRD 42021231674).
2.2. Focused Question
The PICOT format [21] was followed in structuring the research questions.
The focused question was
“In patients having at least one implant-supported restoration under functional loading for at least 6 months does the presence of keratinized mucosa influence soft tissue health, bone levels, esthetics and patient-related variables around implants against the null hypothesis of no influence?”
2.3. Inclusion Criteria
- –
- Randomized controlled trials, cohort, cross-sectional and case–control human studies;
- –
- Studies comparing two groups of patients with a presence or absence of KM, or with KM < 2 mm or ≥2 mm;
- –
- At least 6 months of follow-up;
- –
- Reports of correlation with at least one of the outcome measures considered.
2.4. Reasons for Article Exclusion Included
- (i)
- Retrospective cohort and cross-sectional studies;
- (ii)
- No full text availability;
- (iii)
- Inability to use the KM data or analysis provided.
2.5. Primary Outcome Measures
- –
- Implant failures, defined as the removal of a previously osseointegrated implant due to biological or prosthetic complications;
- –
- Patient-related outcomes: pain, patient satisfaction regarding esthetics, and quality of life;
- –
- Bleeding on probing (BoP or mBI), recorded as bleeding at the bottom of the implant sulcus/pocket with the help of a periodontal probe.
- –
- Biological complications: peri-implantitis, and mucositis.
2.6. Secondary Outcome Measures
- –
- Probing pocket depth (PPD) measured with a periodontal probe, as the distance from the gingival margin to the bottom of the pocket;
- –
- Gingival index (GI), modified gingival index (mGI), bleeding index (BI) or modified sulcular bleeding index (msBI), recorded as marginal bleeding on buccal and lingual/palatal surfaces of the study implants with the help of a periodontal probe;
- –
- Plaque index (PI) or modified plaque index (mPI), recorded as the presence of plaque on buccal and lingual/palatal surfaces of the study implants with the help of a periodontal probe;
- –
- Marginal bone loss (MBL) assessed on periapical radiographs;
- –
- Soft tissue recession (REC) measured clinically with a probe or on hard plaster models with a digital caliper or a probe;
2.7. Search Strategy and Study Selection
A systematic literature search was conducted through the following electronic databases: The Cochrane Central Register of Controlled Trials, EMBASE, and the National Library of Medicine via Pubmed. The search technique was initially created with a list of headings for medical issues and free text terms for the MEDLINE database, and then properly modified for other databases. No limitations were placed on the publishing date, the journal, or the language. A bibliographic database was downloaded with the search results to make it easier to remove duplicates and check cross-references. Appendix A contains information on the search method and how the databases’ search key phrases were created. In May 2021, the final electronic search was carried out.
2.8. Selection of Studies
The titles and abstracts (if available) of the items found through the search were double-checked and independently reviewed by two examiners (L.G., and P.G.). The examiners were specifically trained and calibrated with the first 10 consecutive publications. The entire text of all studies that might have met the eligibility requirements or for which the title and abstract did not provide enough information to make a judgment call were then collected. The next screening process included any article that at least one reviewer thought might be relevant. The full-text publication was then independently and twice reviewed by the same review examiners. Any discrepancy in the studies’ eligibility was settled either through open discussion between the two reviewers until a consensus was established or via arbitration by a third party (M.G.G.). The reasons for the exclusion of any article that did not adhere to the eligibility requirements were highlighted. Any disagreements regarding the studies’ inclusion were settled in the same way as described above.
2.9. Data Extraction and Management
Using a data extraction sheet created especially for this review, two examiners (M.S., and A.P.) independently retrieved all pertinent information from the included publications. The data extraction form including a quality assessment and risk of bias assessment were piloted for 5 papers and modified as required. Calibration of the examiners was conducted on the same papers. Disagreements between the reviewers were always settled via a consensus and open debate. If a dispute persisted, a neutral third party (M.G.G.) resolved it.
2.10. Quality Assessment, Risk of Bias and Data Analysis
Risk of bias for the included studies was evaluated independently by two authors (M.S, and A.P.). The appropriate Joanna Briggs Appraisal Checklist was used for cross- sectional and cohort prospective studies [22]. Possible disagreements were discussed between the same authors. The third author (M.G.G.) was consulted if no agreement was reached. The risk of bias of the study was judged as follows:
- High risk if at least two domains were considered at high risk (no);
- Moderate risk if one domain was considered at high risk or if two or more domains were considered unclear;
- Low risk if no domains were considered at high risk (yes).
For RCTs, it was performed in accordance with the method suggested by the Cochrane collaboration group [20].
2.11. Strategy for Data Synthesis
The analysis was performed by comparing data according to the cutoff used to define the adequate width of keratinized mucosa (KM) (≥2 mm of KM is currently the cut-off value most frequently adopted in the literature). Studies providing data on the presence or absence of KM were analyzed separately for each outcome. If the results were not significantly different from those of studies using 2 mm as a cut-off, all studies were aggregated. Data on GI, mGI/BI, msBI and mPI/PI were aggregated and to be able to compare them the data were normalized.
The following confounding factors were considered: smoking habits, history or the actual presence of periodontal disease, the type of implant surface (rough or machined), the type of implant-supported reconstruction, implant position and the maintenance protocol (Table description).
Whenever possible, the patient was used as the statistical analysis unit, unless all of the comparative studies reported their findings using implants as the statistical analysis unit. Each outcome variable underwent meta-analysis, and the I2 statistic was used to evaluate heterogeneity. To summarize the data for the continuous primary outcomes, weighted mean difference (WMD) or standardized mean difference (MD) with 95% confidence intervals (CI) were chosen. If no substantial heterogeneity was found (i2 = 60%; p > 0.05), mean differences for continuous data were integrated using fixed-effects models; otherwise (i2 > 60%; p < 0.05), a random-effect model was used. The analysis was undertaken using Review Manager software (RevMan 5.4, Version 5.4.1 Copenhagen, Denmark: The Nordic Cochrane Centre, The Cochrane Collaboration, 2020).
2.12. Sensitivity Analysis
In order to investigate if study quality was a factor affecting the results, two sensitivity analyses were performed, one excluding the studies that were judged at high risk of bias and one including only low-risk-of-bias studies. Moreover, a sensitivity analysis was performed excluding a study [23] presenting data of patients with erratic maintenance which were different from those of all the other patients examined in the other included studies.
3. Results
3.1. Search Results and Study Selection
Figure 1 shows the process for performing a literature search. Briefly, 1427 records were left after duplicates were removed for title and abstract screening, 74 studies’ full-text evaluations were completed, and 15 papers were included in the analysis (Table 1) based on preset inclusion criteria. Table 2 lists excluded studies and the explanations for exclusion.
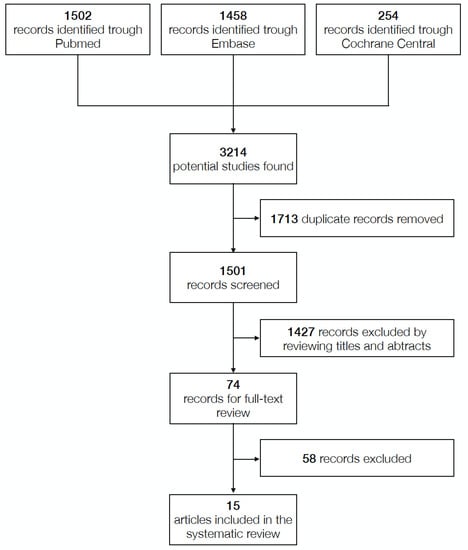
Figure 1.
Literature research process.

Table 1.
Included studies in the analysis.

Table 2.
Excluded studies and reasons for exclusion.
3.2. Description of Studies
One study was a randomized controlled clinical trial [30] (Supplementary Table S1), two studies were cohort prospective studies [30,31,32] (Supplementary Table S1), ten were cross-sectional studies [11,12,23,24,26,27,28,29,34,35] and two studies presented cross-sectional data on patients followed for 10 years [25] and 4 years [33] (Supplementary Table S2).
The total sample population consisted of 1325 patients and 4005 implants. The follow-up of the included cohort prospective studies ranged from 6 months to 4 years.
Two studies [24,25] analyzed the presence or absence of KM, while one study analyzed KM ≥ 1 and no KM [28]. Eleven studies [11,12,23,26,27,29,30,32,33,34,35] were stratified into two groups KM ≥ 2 and KM < 2 mm, while one study included both KM > 2 and KM ≤ 2 [31].
Implant failure and complications were not presented as outcome measures.
Only four studies analyzed patient-reported outcomes [23,25,32,34].
All the included studies reported the values of parameters related to peri-implant soft tissue health. PPD and PI (or mPI) were reported in all studies. BoP was reported in seven studies [24,25,27,30,31,32,34], while mBI was reported in seven studies [11,23,24,26,28,33,35], GI in was reported in seven studies [11,12,29,30,31,33,34], and mGI was reported in one study [26].
Radiographic bone level was reported in all included studies except one [26].
Mucosal recession was reported in six studies [25,26,28,31,33,35].
Smoking habits were reported in all included studies except one [24]. Smokers were excluded in three studies [12,30,31], and heavy smokers were excluded in other three studies [23,32,33].
History of periodontal disease was reported in eight studies [23,25,27,28,30,32,34,35].
In the cross-sectional studies, time point assessment ranged from more than 6 months after loading to 15 years. The cohort prospective studies of Buyukozdemir et al. [31] had a follow-up of six months, while those of Perussolo et al. [32] had a follow-up of 4 years. In the RCT study [30], the reported follow-ups were at 6, 12 and 18 months after the free gingival graft.
3.3. Risk of Bias Assessment
Table 3 presents the findings of the risk of bias assessment (The Joanna Briggs Institute (JBI) Critical Appraisal Checklist for analytical cross-sectional studies (last amended in 2017), Table 4 (The Joanna Briggs Institute (JBI) Critical Appraisal Checklist for cohort studies (last amended in 2017) and Table 5 (The Cochrane Risk of bias for RCTs)). Among the cross-sectional group, a study was deemed to be at high risk [24], and two were deemed to be at low risk [25,27]. In the cohort prospective group, one study was judged to be at high risk [32]; the RCT [30] was judged to be at high risk too. The risk of bias for each of the other studies was considered to be moderate.

Table 3.
The Joanna Briggs Institute (JBI) Critical Appraisal Checklist for analytical cross-sectional studies (last amended in 2017).

Table 4.
The Joanna Briggs Institute (JBI) Critical Appraisal Checklist for cohort study (last amended in 2017).

Table 5.
The Cochrane Risk of bias for RCT.
3.4. Description of Results and Meta-Analysis
Meta-analysis could be conducted only for cross-sectional studies. For the cohort prospective studies, a meta-analysis could be not performed due to the limited number of included studies, the different time points of the follow-up evaluations and the different outcome measures. Confounding factors could not be analyzed since only a few papers reported all this information [25,35], and data could not be used.
3.4.1. Implant Failures
Implant failure was not presented as an outcome measure; only in one study, four implants were lost (two to infection and one to fracture in the no KM group; one to fracture in the KM group) and presented as drop outs [32].
3.4.2. Patient-Related Outcomes
Three cross-sectional studies reported on pain. Roccuzzo et al. [25] reported the presence of soreness/discomfort dichotomously as present in 0 patients in the KM group and 5 patients in the no KM group; Monje and Blasi [23] reported V0AS discomfort (VAS = 0 extreme discomfort; VAS = 100 maximum comfort) in the KM ≥ 2 group as 97.0 ± 8.5 while in the KM < 2 mm group it was 53.8 ± 30. Gharpure et al. [34] reported awareness of food impaction and pain/discomfort. They reported that implants with inadequate KM had greater food impaction (53% versus 40%, p = 0.193) and pain/discomfort during oral hygiene practices (28% versus 10%, p = 0.027) than those with adequate KM.
Perussolo et al. [32] reported at 18 months of discomfort during brushing measured on a VAS (VAS = 0 no discomfort, VAS = 100 extreme discomfort) in the KM < 2 group as 12.28 ± 17.59 while that in the KM ≥ 2 group was 4.25 ± 8.39. Although a meta-analysis could not be performed, a consistent trend toward the worst pain/discomfort in the KM < 2 mm group was observed.
None of the included studies reported on patient satisfaction and quality of life.
3.4.3. BoP (Figure 2 and Figure 3)
Four cross-sectional studies presented data on BoP. Three studies [25,26,27] were included in the meta-analysis (Figure 2). The study of Ladwein et al. [24] was excluded because S.D. was not given.
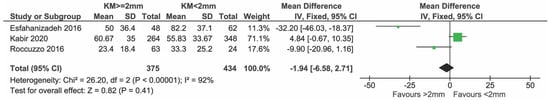
Figure 2.
Cross-sectional studies reporting on BoP included in the meta-analysis [25,26,27].
In total, 375 and 434 implants with KM ≥ 2 mm and KM < 2 mm, respectively, were compared. There was no evidence of a difference in BoP% between groups (mean difference = −1.94%; 95% CI = −6.58; −92.71, p = 0.41). High heterogeneity in effects was detected among the studies (I2 = 92%; p < 0.00001).
Sensitivity analysis including only low-risk studies showed no evidence of any effect and did not change the results substantially (mean difference = 1.91%; CI = −3.02; −6.84, p = 0.45). Heterogeneity decreased but not significantly (I2 = 82% p = 0.02) (Figure 3).
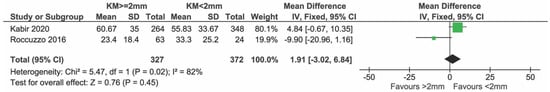
Figure 3.
Sensitivity analysis including only low risk studies reporting on BoP [25,27].
Both cohort prospective clinical studies [31,32] reported data on BoP and found a statistical significant difference in favor of KM ≥ 2 mm (p < 0.05). In the RCT of Oh et al. [30], a statistical significant difference in favor of KM ≥ 2 mm was reported.
3.4.4. Biological Complications
Roccuzzo et al. [25] described, over ten years of follow-up, the percentage of biological complications (51.4% in the no KM group and 12.7% in the KM group) that required additional treatment (antibiotics or surgical therapy). Crespi et al. [33] reported, at 2 years after placement, two implants with a peri-implantitis process with 2 mm of bone loss in the group with KM < 2 mm. Oh et al. [30] reported two subjects with one implant each in the KM < 2 mm group who were excluded from the study due to complications that required additional treatments (curettage with antibiotic prescription). In the KM ≥ 2 mm group, one subject with one implant was excluded due to curettage for biological complication performed before the free gingival graft.
3.4.5. PPD (Figure 4, Figure 5, Figure 6 and Figure 7)
All the twelve cross-sectional studies reported on PPD and were included in the meta-analysis (Figure 4). In total, 2402 and 1281 implants with KW ≥ 2 mm and KW < 2 mm, respectively, were compared (Figure 4). There was a non-significant trend of a lower PPD in favor of the group with KM < 2 mm/absence, with a mean difference (MD) of 0.03 mm (95% CI = −0.02; 0.09 mm, p = 0.23).
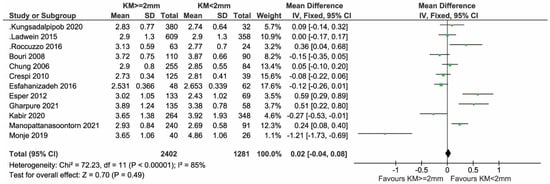
Figure 4.
Cross-sectional studies reporting on PPD included in the meta-analysis [11,12,23,24,25,26,27,28,29,33,34,35].
Sensitivity analysis after removing the study by Ladwein et al. [24], judged to be at high risk, showed no evidence of any effect due to KM (mean difference = 0.02 mm; 95% CI = −0.04, 0.08 mm; p = 0.08). Significant heterogeneity in effects was observed among the studies (I2 = 86%; p < 0.001) (Figure 5).
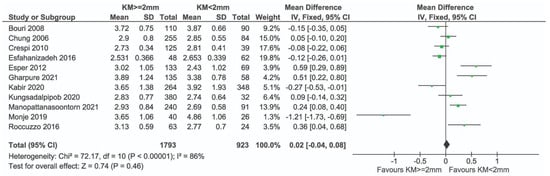
Figure 5.
Sensitivity analysis excluding high-risk studies reporting on PPD (Ladwein et al. [24]) and [11,12,23,25,26,27,28,29,33,34,35].
Sensitivity analysis after removing the study by Monje et al. [23] (Figure 6) and with only low-risk-of-bias studies did not change the results substantially (Figure 7).
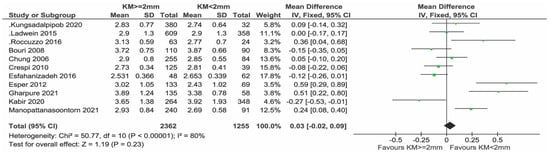
Figure 6.
Sensitivity analysis of PPD excluding the study of Monje & Blasi [11,12,24,25,26,27,28,29,33,34,35].
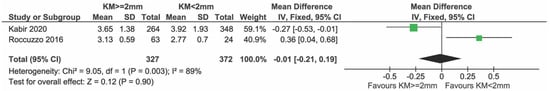
Figure 7.
Sensitivity analysis including only low-risk studies reporting on PPD [25,27].
In the two cohort prospective studies and in the RCT, no significant differences between groups in PPD were reported.
3.4.6. GI, mGI/BI, and msBi (Figure 8 and Figure 9)
Briefly, 9 out of 12 gave data on GI, mGi/BI, and msBI, and were included in the meta-analysis (Figure 8). The data taken on a 0–3 scale were normalized to a 0–4 scale to be able to compare all the data. In total, 1595 and 841 implants with KM ≥ 2 mm and KM < 2 mm, respectively, were compared. There was evidence of a difference in GI, mGI/BI, and msBI in favor of the group with KM ≥ 2 mm (mean difference = −0.26; 95% CI = −0.47, −0.05; p = 0.01). Highly significant heterogeneity in effects was detected among the studies (I2 = 97%, p < 0.0001).
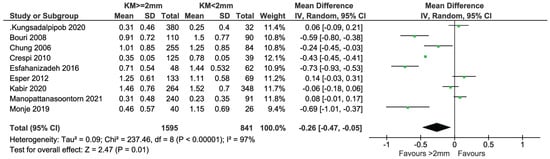
Figure 8.
Cross-sectional studies reporting on GI, mGI/BI, and msBi included in the meta-analysis [11,12,23,26,27,28,29,33,35].
Sensitivity analysis after removing the study by Monje et al. [23] showed weak evidence of a difference in GI, mGI/BI, and msBI between the two groups (In Figure 9 p = 0.05). Heterogeneity in effects among the studies was unchanged (I2 = 97%; p < 0.0001) (Figure 9).
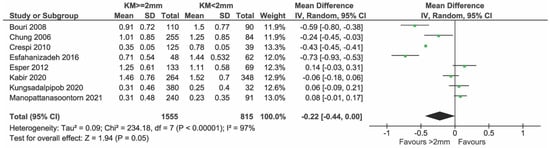
Figure 9.
Sensitivity analysis of GI, mGI/BI, msBi excluding the study of Monje & Blasi [11,12,26,27,28,29,33,35].
Both Oh et al. [30] and Buyukozdemir et al. [31] reported on GI with statistically significant differences in favor of the presence of KM.
3.4.7. PI and mPI (Figure 10 and Figure 11)
All cross-sectional studies reported on PI, but only nine were included in the meta-analysis because Gharpure et al. [34], Ladwein et al. [24] and Roccuzzo et al. [25] provided data in a non-usable/comparable manner (Figure 10). In total, 1595 and 841 implants with KM ≥ 2 mm and KM < 2 mm, respectively, were compared. There was evidence of a difference in PI in favor of the group with KM ≥ 2 mm (mean difference = −0.25; 95% CI = −0.47, −0.04; p = 0.02). Highly significant heterogeneity in effects was detected among the studies (I2 = 98%; p < 0.0001). The sensitivity analysis performed after removing the study of Monje et al. [23] showed no statistical difference between groups (p = 0.08) (Figure 11).
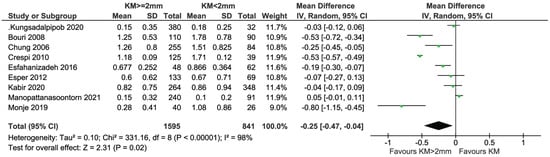
Figure 10.
Cross-sectional studies reporting on PI/mPI included in the meta-analysis [11,12,23,26,27,28,29,33,35].
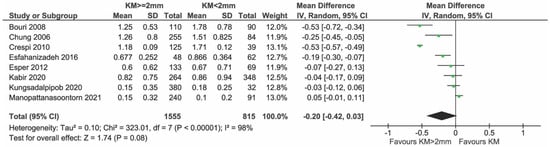
Figure 11.
Sensitivity analysis of PI and mPI excluding the study of Monje & Blasi [11,12,26,27,28,29,33,35].
In the cohort studies, only Buyukozdemir et al. [31] reported a statistically significant difference in PI in favor of the presence of KM.
The RCT of Oh et al. [30] reported no significant differences between groups.
3.4.8. Bone Level (Figure 12, Figure 13 and Figure 14)
Eight studies presented data on the bone level and all were included in the meta-analysis (Figure 12). In total, 1894 and 778 implants with KM ≥ 2 mm and KM < 2 mm, respectively, were compared. There was weak evidence of a reduced bone level change in the group with KM ≥ 2 mm (mean difference = −0.18 mm; 95% CI = −0.36, 0.00 mm; p = 0.05). Significant heterogeneity in effects was detected among the studies (I2 = 80%; p < 0.0001). Sensitivity analysis after removing the study by Ladwein et al. [24] showed no evidence of a difference in bone level change between the two groups (Figure 13, p = 0.08). Heterogeneity was reduced, but still significant (I2 = 82%, p = 0.08) while the sensitivity analysis performed after removing Monje et al. [23] showed a heterogeneity reduction from 80% to 70% (Figure 14).
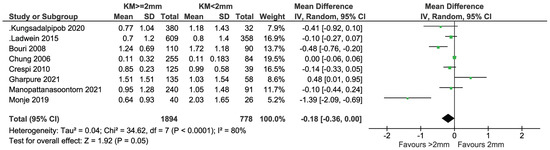
Figure 12.
Cross-sectional studies reporting on MBL included in the meta-analysis [11,23,24,28,29,33,34,35].
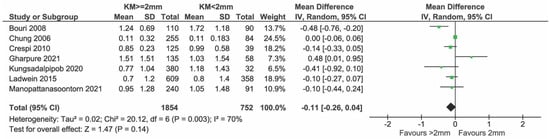
Figure 13.
Sensitivity analysis of MBL excluding the study of Monje & Blasi [11,24,28,29,33,34,35].
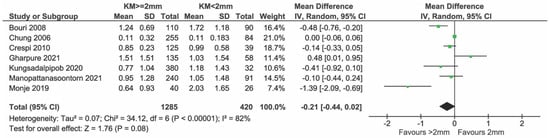
Figure 14.
Sensitivity analysis excluding high-risk studies reporting on MBL (Ladwein et al. [24]) and [11,23,28,29,33,34,35].
The RCT and one cohort study [30,32] reported a significant difference in bone levels between the groups in favor of the presence of KM ≥ 2 mm, while Buyukozdemir et al. [31] reported no differences.
3.4.9. REC (Figure 15)
Five cross-sectional studies reported on REC and were included in the meta-analysis (Figure 15). In total, 856 and 248 implants with KM ≥ 2 mm and KM < 2 mm, respectively, were compared. There was evidence of a difference in REC in favor of the group with KM ≥ 2 mm (mean difference = −0.33 mm; 95% CI = −0.40, −0.25; p < 0.0001). Highly significant heterogeneity in effects was detected among the studies (I2 = 98%; p < 0.0001). No sensitivity analysis was performed.
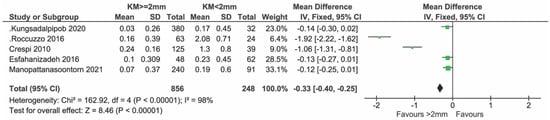
Figure 15.
Cross-sectional studies reporting on REC included in the meta-analysis [25,26,28,33,35].
REC was reported only in one cohort prospective study [31] showing no statistically significant difference between the groups.
4. Discussion
The present systematic review aimed at assessing the role of KM on peri-implant tissue health, esthetics and patient-related variables. The presence of KM ≥ 2 mm was associated with less marginal inflammation (GI, mGI, BI, and msBi), plaque accumulation (PI, mPI), recession, biological complications and less soreness or discomfort during oral hygiene procedures, while no differences were found for implant failures, PPD and bone levels. BoP resulted in being significantly lower in the KM ≥ 2 mm groups in the three prospective studies [30,31,32]. These results should be considered with caution. Only one RCT study with 18 months [30] of follow-up could be included and we decided to analyze studies with data given at the implant level since very few studies used the patient level. Only two studies [25,27] at low risk of bias were included, while studies presented high heterogeneity and few had long follow-ups.
Even if some differences between groups were statistically significant, their clinical importance should be determined, since small differences between groups were found. These differences were even smaller when excluding one study [23] in which patients with erratic compliance were included. In particular, no differences were found for PPD and bone loss. The mean values reported for these variables showed that the population examined presented shallow PPD and little bone loss, so the results should be applied to patients with generally healthy peri-implant tissue. The only exception was presented by Monje et al. [23] where pockets deeper than 4 mm were present in the group with KM < 2 mm and its results suggest that the presence of KM could be important in patients in which compliance is suboptimal.
A reason behind the small differences found between groups could be the choice of the cut-off for sorting a patient into one group or another. Most of the studies used the presence of at least 2 mm of KM as the criteria for describing one group [7,25]. This measure could be difficult to record and similar cases could be assigned to different groups, altering the results. Additionally, using this definition, cases with a presence of KM were pooled with cases with an absence of KM. A minimal presence of KM could have different clinical significance with respect to its complete absence. Only three studies considered the presence or absence of KM as the case definition [24,25,28]. The meta-analysis conducted without these studies did not alter the results. Moreover, the presence of attached mucosa was never analyzed and this could be an important issue when evaluating the results. If KM plays a role in maintaining a peri-implant soft tissue seal that contributes to maintaining the state of health, it ideally means that KM should have characteristics of stability and that it must adhere to the underlying hard tissues. KM consists histologically of a dense, collagen-rich connective tissue covered by a keratinized epithelium. Adherent KM has a lamina propria firmly connected to the underlying periosteum, while free KM lacks this connection. This can be due to tghe implant position or bone resorption leaving supracrestal peri-implant soft tissue of an adequate thickness to avoid recession but without attachment to the underlying periosteum. This lack of attachment could negatively affect the soft tissue seal around implants. Therefore, measuring a vertical dimension of keratinized tissue without defining its characteristics (free or adherent) may not be sufficient. From a clinical point of view, in fact, it may be sufficient to have 1 mm of adherent KM while perhaps the presence of >2 mm of non-adherent KM may not be adequate.
The presence of KM should be evaluated also in relation to other clinical conditions. None of the included studies reported on the vestibule depth. A shallow vestibule, often present in the lower jaw and particularly in patients who underwent tooth extraction for periodontal reasons, should be taken into consideration as it hampers oral hygiene procedures resulting in plaque accumulation and inflammation. Future studies should take into account the presence or absence of attached keratinized mucosa and report on the depth of the vestibule to better define the role of KM in peri-implant health. Interestingly, no studies clearly indicated implant failure and complications as outcome variables. Even if failure is a rare occurrence, it should be analyzed and reported. Two studies [30,32] reported on complications but they were recorded as drop outs, which could alter the real results. In cases without adequate KM, four complications linked to infection (two in Crespi et al. [33], two in Oh et al. [30]), two failures for infection and one failure due to fracture [32] were present, while in the group with KM >2 mm only one implant was lost due to fracture and one complication was due to infection [30]. In one study in the cross-sectional groups, biological complications were reported in 51.4% of cases without KM and in 12.7% of cases with KM [25].
Other systematic reviews [7,8,18,36] on this topic reached conclusions similar to the ones reported in the current manuscript, although differences in the inclusion criteria, methods of analysis, outcomes analyzed and risk of bias assessments were present. In this review, only RCT, prospective cohort and cross-sectional studies were considered, excluding studies with a retrospective design (for example, [15,17]). These strict eligibility criteria were set to improve the quality of the included studies. The original plan, as described in the statistical analysis, was also to only include studies that performed analysis at the patient level, but since only two papers fulfilled this criteria [25,27], we decided to include implant-level analysis studies and meta-analysis was conducted at the implant level. This is a clear limitation of both the included studies and of the current review. All the studies in which the analysis was conducted at the implant level were judged to be at0 least at moderate risk of bias.
In the review by Gobbato et al. and Longoni et al. [7,18], recessions and bone level were not analyzed as they recognized difficulties in standardizing measurement methods. We agree with their considerations, but since we believe that both parameters are important to evaluate, we have decided to analyze these data. Bone level can be considered a surrogate outcome of implant failure, which requires a longer follow-up. Since none of the included papers reported on implant failure, it appeared reasonable to analyze bone stability over time. Interestingly, no differences were found in terms of bone level in patients with or without KM.
In accordance with Longoni et al. [18], to increase the statistical power of the analysis, we pooled outcomes that measured the same clinical parameters as a group (GI, mGI, BI, mBI and PI, mPI). Since we recognized that the indices were different, we normalized them to be able to perform the statistical analysis. We observed that definitions were often used incorrectly and or associated with a wrong reference [26,27,29,33]. We advise a uniform use of indices. A dichotomous index indicating the presence or absence of marginal inflammation, BoP and plaque would be easier to apply and more useful for the comparison of different studies. Data presented as percentages would be easier to interpret clinically.
Moraschini et al. [37] performed an overview to assess the methods, quality, and outcomes of the systematic reviews on the importance of KM. The major limitations that were highlighted consisted of the lack of standardization methods and the absence of evaluation of possible confounding factors. The study in the present manuscript was performed according to PRISMA guidelines and a standardized tool for the risk of bias assessment was applied. Gobbato et al. [7] mentioned the risk of bias in the methods section but it was not reported in the final results and Brito et al. [36] did not perform a risk of bias evaluation. Lin et al. [8] used a non-standardized risk of bias, while Longoni et al. [18] applied the Newcastle–Ottawa modified scale. In the present review, the Joanna Briggs tool was chosen as it includes two questions focused on the presence of confounding factors and strategies for their management. In particular, referring to KM significance, we considered the following as major confounding factors: smoking habits, a history or actual presence of periodontal disease, the type of implant surface (rough or machined), the type of implant-supported reconstruction, the implant position and the maintenance protocol. Only a few papers reported all this information [25,35]; apart from when data were given at the implant level, patient-related variables were impossible to apply to the analysis. Roccuzzo et al. [25] provided data on smoking habits but very few smokers were included to allow a powerful statistical analysis. Manopatonasoontorn et al. [35] performed a univariate and multivariate regression analysis adjusted for confounding factors (oral hygiene, smoking status, history of chronic periodontitis, implant prosthesis type, and diabetes). This type of analysis could be useful for data interpretation. The type of maintenance was rarely reported [25] even if it represents a key aspect of peri-implant health. In our manuscript, a sensitivity analysis was performed, excluding the paper by Monje & Blasi [23] in which only erratic maintenance patients were included. In this group of patients, the presence of KM demonstrated a crucial role. On the other side, Manoppatanasoorn et al. [35] concluded that no association was observed between keratinized mucosa width and plaque accumulation, mucosal inflammation, and interproximal bone level in a population that adhered to implant maintenance therapy and demonstrated optimal oral hygiene. Only Roccuzzo et al. [25] provided detailed information on the maintenance care protocol. We believe that the maintenance protocol after implant positioning is a crucial point for implant survival. When strict maintenance schedules and oral hygiene were consistently adhered to by patients, no association between keratinized mucosa width and peri-implant diagnostic parameters were observed in many studies [12,38,39,40].
Brito et al. [36] included only studies with more than 12 months of follow-up, while Longoni et al. [18] examined studies with at least 4 months of follow-up. We decided to include studies with at least 6 months of follow-up, in accordance with Gobbato et al. [7] and Lin et al. [8], since this allowed the inclusion of a larger number of studies, given that few studies have long follow-ups. Fifteen studies were included in the final analysis while only eight studies had a follow-up lasting longer than 3 years [11,23,24,28,29,32,33,34] and only one study had a 10-year follow- up [25] so data on implant survival were difficult to explore. A long follow-up was deemed necessary to adequately answer the review question and in particular to evaluate one of the most important primary outcomes of this review that is implant failure. According to the data in the literature, indeed, the likelihood of complications increases after 5 years from loading. If we could analyze data from studies with follow-ups of more than 5 years, we could perhaps achieve results that are very different and more significant than those reported in this review.
PROMs have become a crucial endpoint of clinical studies and should be included in every implant-related clinical study to capture patient satisfaction and quality of life [41]. None of the included studies evaluated the quality of life of patients with or without KM and none reported on esthetic perception. The only parameter assessed regarding the patient-related outcome was brushing discomfort and this was reported in four studies [23,25,32,34]. It was not possible to perform a meta-analysis because data were analyzed in different ways. In the literature, there is a trend towards greater discomfort in the absence of mucosa but more studies are needed to evaluate this variable and there is a need for more uniformity in data collection.
4.1. Main Limitations
Several limitations of the present review are worth mentioning.
Even if RCTs give stronger evidence of the effect of the presence or absence of KM, cross-sectional and prospective control studies were included. Additionally, different time point assessments in relation to implant placement were pooled and analyzed together.
Studies with data given at the implant level were included and the analysis was performed at the implant level. Even if potential confounding factors were presented in some studies, we were not able to analyze their influence on the effect of the presence or absence of KM.
In the case of data presented in a non-usable manner, the authors were not contacted and therefore some studies were excluded even though they could have potentially been of interest.
4.2. Recommendation for Future Research
The definition of KM should be more accurate. The description of a definite amount of attached KM versus that of non-attached KM seems more useful clinically. The depth of the vestibulum should be reported. A uniform use of indices for describing outcome measures is advisable, with an emphasis on data that could be easily interpreted clinically, such as the percentage of the presence of plaque, bleeding on probing, and marginal inflammation. Data should be given at the patient level and the mean and SD should be provided. Implant failures and complications should be clearly described. PROMSs should be routinely included in clinical trials.
Confounding factors should be recorded and evaluated when performing statistical analyses. Long follow-ups should be preferred because the effect of the presence or absence of KM needs time for an adequate clinical evaluation.
5. Conclusions
No statistically significant differences between the presence of KM ≥ 2 mm or <2 mm were found for implant failure, PPD or bone loss. BoP resulted in being significantly lower in the KM ≥ 2 mm group only in the three prospective studies.
Less statistical marginal inflammation, plaque accumulation, and recession were associated with the presence of KM ≥ 2 mm, but the differences were clinically small. More biological complications were described in the no KM/ KM < 2 mm group but the reduced number of cases does not allow us to draw any conclusions. Although a meta-analysis could not be performed, a consistent trend toward the worst pain/discomfort in KM < 2 mm was observed. These results should be considered with caution, since most of the studies were at a moderate and high risk of bias, follow-ups were short and data were given at the implant level. Furthermore, most of the included patients had low plaque levels and PPD values so these results may not be valid for patients with erratic compliance that presented as having higher benefits from KM presence.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app13158631/s1, Table S1: Cohort prospective studies and randomized controlled clinical trials included in the analysis.; Table S2: Cross-sectional studies included in the analysis.
Author Contributions
Conceptualization, M.S. and M.G.G.; methodology, A.P.; software, M.D.F.; validation, L.G., P.G. and G.L.; formal analysis, M.D.F.; investigation, M.S.; resources, A.P.; data curation, M.D.F.; writing—original draft preparation, M.S. and M.G.G; writing—review and editing, M.S., A.P., L.G, P.G. and M.G.G.; visualization, G.Z.; supervision, A.S., G.L. and G.Z.; project administration, M.S.; funding acquisition, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was self-funded by the authors and their institutions, and no external funding was received.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest related to the contents of this study.
Appendix A
Search strategy
PubMed research: (“dental implants”[mh] OR “dental implantation”[mh] OR ((“implant”[tiab] OR “implants”[tiab]) AND (dental[tiab] OR oral[tiab] OR tooth [tiab]))) AND (“mouth mucosa”[mh] OR ((“peri-implant”[tiab] OR “masticatory”[tiab] OR “attached”[tiab] OR “keratinized”[tiab] OR “keratinised”[tiab] OR “KT”[tiab]) AND (“mucosa”[tiab] OR “gingiva” [tiab]))).
EMBASE research: (dental:ab,ti AND implant:ab,ti OR ‘tooth implant’/exp/mj) AND (keratinized:ab,ti AND mucosa:ab,ti OR (keratinized:ab,ti AND tissue:ab,ti) OR (keratinized:ab,ti AND tissues:ab,ti) OR ‘attached gingiva’:ab,ti OR ‘attached mucosa’:ab,ti OR ‘soft tissue’/exp/mj) AND [embase]/lim.
Cochrane Central research: (“dental implants” AND “mucosa”).
References
- Quirynen, M.; Herrera, D.; Teughels, W.; Sanz, M. Implant therapy, 40 years of experience. Periodontology 2000 2014, 66, 7–12. [Google Scholar] [CrossRef]
- Buser, D.; Sennerby, L.; De Bruyn, H. Modern implant dentistry based on osseointegration, 50 years of progress, current trends and open questions. Periodontology 2000 2017, 73, 7–21. [Google Scholar] [CrossRef]
- Block, M.S. Dental Implants, The Last 100 Years. J. Oral. Maxillofac. Surg. 2018, 76, 11–26. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Miron, R.J. Health, Maintenance, and Recovery of Soft Tissues around Implants. Clin. Implant Dent. Relat. Res. 2016, 18, 618–634. [Google Scholar] [CrossRef]
- Rodriguez, A.M.; Rosenstiel, S.F. Esthetic considerations related to bone and soft tissue maintenance and development around dental implants, Report of the Committee on Research in Fixed Prosthodontics of the American Academy of Fixed Prosthodontics. J. Prosthet. Dent. 2012, 108, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Wennström, J.L.; Derks, J. Is there a need for keratinized mucosa around implants to maintain health and tissue stability? Clin. Oral. Implants Res. 2012, 23 (Suppl. 6), 136–146. [Google Scholar] [CrossRef]
- Gobbato, L.; Avila-Ortiz, G.; Sohrabi, K.; Wang, C.W.; Karimbux, N. The effect of keratinized mucosa width on peri-implant health, A systematic review. Int. J. Oral Maxillofac. Implants 2013, 28, 1536–1545. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.-H.; Chan, H.-L.; Wang, H.-L. The significance of keratinized mucosa on implant health: A systematic review. J. Periodontol. 2013, 84, 1755–1767. [Google Scholar] [CrossRef]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S313–S318. [Google Scholar] [CrossRef] [PubMed]
- Boynueğri, D.; Nemli, S.K.; Kasko, Y.A. Significance of keratinized mucosa around dental implants: A prospective comparative study. Clin. Oral Implants Res. 2013, 24, 928–933. [Google Scholar] [CrossRef]
- Chung, D.M.; Oh, T.-J.; Shotwell, J.L.; Misch, C.E.; Wang, H.L. Significance of keratinized mucosa in maintenance of dental implants with different surfaces. J. Periodontol. 2006, 77, 1410–1420. [Google Scholar] [CrossRef] [PubMed]
- Esper, L.A.; Ferreira, S.B., Jr.; de Oliveira Fortes Kaizer, R.; de Almeida, A.L.P.F. The role of keratinized mucosa in peri-implant health. Cleft Palate Craniofac. J. 2012, 49, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-S.; Kim, Y.-K.; Yun, P.-Y.; Yi, Y.J.; Lee, H.J.; Kim, S.G.; Son, J.S. Evaluation of peri-implant tissue response according to the presence of keratinized mucosa. Oral Pathol. Oral Radiol. Endodontol. 2009, 107, e24–e28. [Google Scholar] [CrossRef] [PubMed]
- Wennström, J.L.; Bengazi, F.; Lekholm, U. The influence of the masticatory mucosa on the peri-implant soft tissue condition. Clin. Oral Implant. Res. 1994, 5, 1–8. [Google Scholar] [CrossRef]
- Zigdon, H.; Machtei, E.E. The dimensions of keratinized mucosa around implants affect clinical and immunological parameters. Clin. Oral Implants Res. 2008, 19, 387–392. [Google Scholar] [CrossRef]
- Schrott, A.R.; Jimenez, M.; Hwang, J.-W.; Fiorellini, J.; Weber, H.P. Five-year evaluation of the influence of keratinized mucosa on peri-implant soft-tissue health and stability around implants supporting full-arch mandibular fixed prostheses. Clin. Oral Implants Res. 2009, 20, 1170–1177. [Google Scholar] [CrossRef]
- Adibrad, M.; Shahabuei, M.; Sahabi, M. Significance of the width of keratinized mucosa on the health status of the supporting tissue around implants supporting overdentures. J. Oral Implantol. 2009, 35, 232–237. [Google Scholar] [CrossRef]
- Longoni, S.; Tinto, M.; Pacifico, C.; Sartori, M.; Andreano, A. Effect of Peri-implant Keratinized Tissue Width on Tissue Health and Stability: Systematic Review and Meta-analysis. Int. J. Oral Maxillofac. Implants 2019, 34, 1307–1317. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, A.L.; PRISMA-PGroup. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, F.K.; Weeks, L.; Sterne, A.C.J.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Miller, S.A.; Forrest, J.L. Enhancing your practice through evidence-based decision making: PICO, learning how to ask good questions. J. Evid. Based Dent. Pract. 2001, 1, 136–141. [Google Scholar] [CrossRef]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Lisy, K.; Qureshi, R.; Mattis, P.; et al. Chapter 7: Systematic Reviews of Etiology and Risk; JBI Reviewer’s Manual: Adelaide, SA, Australia, 2019. [Google Scholar]
- Monje, A.; Blasi, G. Significance of keratinized mucosa/gingiva on peri-implant and adjacent periodontal conditions in erratic maintenance compliers. J. Periodontol. 2019, 90, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Ladwein, C.; Schmelzeisen, R.; Nelson, K.; Fluegge, T.V.; Fretwurst, T. Is the presence of keratinized mucosa associated with periimplant tissue health? A clinical cross-sectional analysis. Int. J. Implant Dent. 2015, 1, 11. [Google Scholar] [CrossRef]
- Roccuzzo, M.; Grasso, G.; Dalmasso, P. Keratinized mucosa around implants in partially edentulous posterior mandible: 10-year results of a prospective comparative study. Clin. Oral Implants Res. 2016, 27, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Esfahanizadeh, N.; Daneshparvar, N.; Motallebi, S.; Akhondi, N.; Askarpour, F.; Davaie, S. Do we need keratinized mucosa for a healthy peri-implant soft tissue? Gen. Dent. 2016, 64, 51–55. [Google Scholar]
- Kabir, L.; Stiesch, M.; Grischke, J. The effect of keratinized mucosa on the severity of peri-implant mucositis differs between periodontally healthy subjects and the general population: A cross-sectional study. Clin. Oral Investig. 2020, 25, 1183–1193. [Google Scholar] [CrossRef]
- Kungsadalpipob, K.; Supanimitkul, K.; Manopattanasoontorn, S.; Sophon, N.; Tangsathian, T.; Arunyanak, S.P. The lack of keratinized mucosa is associated with poor peri-implant tissue health: A cross-sectional study. Int. J. Implant Dent. 2020, 6, 28. [Google Scholar] [CrossRef]
- Bouri, A., Jr.; Bissada, N.; Al-Zahrani, M.S.; Faddoul, F.; Nouneh, I. Width of keratinized gingiva and the health status of the supporting tissues around dental implants. Int. J. Oral Maxillofac. Implants 2008, 23, 323–326. [Google Scholar]
- Oh, S.-L.; Masri, R.M.; Williams, D.A.; Ji, C.; Romberg, E. Free gingival grafts for implants exhibiting lack of keratinized mucosa: A prospective controlled randomized clinical study. J. Clin. Periodontol. 2017, 44, 195–203. [Google Scholar] [CrossRef]
- Buyukozdemir Askin, S.; Berker, E.; Akincibay, H.; Uysal, S.; Erman, B.; Tezcan, İ.; Karabulut, E. Necessity of keratinized tissues for dental implants: A clinical, immunological, and radiographic study. Clin. Implant Dent. Relat. Res. 2015, 17, 1–12. [Google Scholar] [CrossRef]
- Perussolo, J.; Souza, A.B.; Matarazzo, F.; Oliveira, R.P.; Araújo, M.G. Influence of the keratinized mucosa on the stability of peri-implant tissues and brushing discomfort: A 4-year follow-up study. Clin. Oral Implants Res. 2018, 29, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Crespi, R.; Capparè, P.; Gherlone, E. A 4-year evaluation of the peri-implant parameters of immediately loaded implants placed in fresh extraction sockets. J. Periodontol. 2010, 81, 1629–1634. [Google Scholar] [CrossRef]
- Gharpure, A.S.; Latimer, J.M.; Aljofi, F.E.; Kahng, J.H.; Daubert, D.M. Role of thin gingival phenotype and inadequate keratinized mucosa width (<2 mm) as risk indicators for peri-implantitis and peri-implant mucositis. J. Periodontol. 2021, 92, 1687–1696. [Google Scholar] [PubMed]
- Manopattanasoontorn, S.; Supanimitkul, K.; Tangsathian, T.; Sophon, N.; Arunyanak, S.P.; Kungsadalpipob, K. Association between keratinized mucosa width and peri-implant diagnostic parameters in Asian maintenance compliers: A Cross-sectional study. J. Int. Acad. Periodontol. 2021, 23, 167–178. [Google Scholar]
- Brito, C.; Tenenbaum, H.C.; Wong, B.K.C.; Schmitt, C.; Nogueira-Filho, G. Is keratinized mucosa indispensable to maintain peri-implant health? A systematic review of the literature. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 643–650. [Google Scholar] [CrossRef]
- Moraschini, V.; Luz, D.; Velloso, G.; Barboza, E.d.S.P. Quality assessment of systematic reviews of the significance of keratinized mucosa on implant health. Int. J. Oral Maxillofac. Surg. 2017, 46, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Wennström, J.L. Lack of association between width of attached gingiva and development of soft tissue recession. A 5-year longitudinal study. J. Clin. Periodontol. 1987, 14, 181–184. [Google Scholar] [CrossRef]
- Frisch, E.; Ziebolz, D.; Vach, K.; Ratka-Krüger, P. The Effect of Keratinized Mucosa Width on Peri-Implant Outcome under Supportive Postimplant Therapy. Clin. Implant. Dent. Relat. Res. 2015, 17, e236–e244. [Google Scholar] [CrossRef]
- Lim, H.-C.; Wiedemeier, D.B.; Hämmerle, C.H.F.; Thoma, D.S. The amount of keratinized mucosa may not influence peri-implant health in compliant patients: A retrospective 5-year analysis. J. Clin. Periodontol. 2019, 46, 354–362. [Google Scholar] [CrossRef]
- Feine, J.; Abou-Ayash, S.; Al Mardini, M.; de Santana, R.B.; Bjelke-Holtermann, T.; Bornstein, M.M.; Braegger, U.; Cao, O.; Cordaro, L.; Eycken, D.; et al. Group 3 ITI Consensus Report: Patient-reported outcome measures associated with implant dentistry. Clin. Oral Implants Res. 2018, 29 (Suppl. 16), 270–275. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).