Featured Application
Factors influencing Vitamin D and its receptor polymorphisms on risk level of dental caries.
Abstract
Dental caries is a multifactorial disease with multiple risk factors. Vitamin D levels (VDLs) and vitamin D receptor polymorphisms (VDRPs) have been investigated for this reason. The aim of this narrative review is to investigate the relation and the factors affecting vitamin D deficiency (VDD), VDRP, Early Childhood Caries (ECC) and Severe Early Childhood Caries (S-ECC) in children (primary and mixed dentition) and dental caries risk in adults (permanent dentition). Additionally, we present a model incorporating factors and interactions that address this relationship. Methods: Three databases (PubMed/MEDLINE, Web of Science, Cochrane Library) were comprehensively searched until 17 January 2023 using the following keywords: “vitamin D”, “vitamin D receptor polymorphism”, “dental caries”, and “dental caries risk”, finding 341 articles. Two reviewers searched, screened, and extracted information from the selected articles. All pooled analyses were based on random-effects models. Eligibility criteria were articles using dmft/DMFT diagnostic criteria with calibrated examiners, probability sampling, and sample sizes. We excluded studies conducted on institutionalized patients. A total of 32 studies were finally used. Results: In most studies, TaqI, FokI, and BsmI polymorphisms affected the prevalence of dental caries. A strong correlation between ECC, S-ECC, and the prevalence of dental caries was reported in association with VDD and maternal intake of VD in primary dentition. Regarding the influence in mixed dentition, the results were found to be inconclusive. A slight positive influence was reported for permanent dentition. Conclusions: Factors affecting caries risk were maternal intake, socioeconomic factors, and level of VD. There is a certain need for more well-conducted studies that will investigate the association between VDR gene polymorphisms and the prevalence of dental caries in mixed and permanent dentition, specifically in adult patients.
Keywords:
vitamin D receptor; polymorphism; dental caries; dentistry; prevalence; prevention; dmft/DMFT index 1. Introduction
Dental caries is a multifactorial disease with multiple risk factors [1]. Vitamin D levels (VDLs) and vitamin D receptor polymorphisms (VDRPs) have been investigated for this reason [2]. Vitamin D is a fat-soluble steroid hormone which regulates calcium and phosphorus levels through the intestine [3]. This vitamin can be found in the human body as vitamin D3 (cholecalciferol) and vitamin D2 [2]. Both are converted to 25-hydroxyvitamin D (25(OH)D), which acts as a biological marker for vitamin D levels in serum. When 25(OH)D reaches the kidneys, it is converted to calcitriol (1,25(OH)2D), the most active form of vitamin D, with a shorter half-life [3].
Vitamin D deficiency (VDD) is defined when levels of 25(OH)D are below 20 ng/mL (50 nmol/L) and there is an insufficiency between 21–29 ng/mL (50–75 nmol/L) [4]. VDD has, therefore, abnormality in calcium, phosphorus, and bone metabolism [4] and is associated with increased risk of neoplastic, metabolic, and immune disorders [5,6]. Tooth mineralization co-occurs to skeletal mineralization, so disturbances in mineral metabolism affect bone and teeth. As VD has a crucial role in tooth and bone mineralization, low levels of VD may lead to a defective and hypomineralized tooth [6]. The main mechanism related to severe VDD causes hypophosphatemia and hypocalcemia with secondary hyperparathyroidism [7]. Hyperparathyroidism, in turn, stimulates renal production of 1,25(OH)2D and absorption of calcium in intestines. It increases bone turnover and may lead to increased serum levels of calcium ions and decreased serum levels of inorganic phosphate [8]. Therefore, proper mineralization of teeth is inhibited due to the loss of VD signaling pathways in tooth cells as concentrations of calcium ions and phosphate ions are low [9,10,11]. The biological activity of VD is modulated and modified by the vitamin D receptor (VDR) protein. VDR is responsible for the expression of many genes involved in cellular proliferation and differentiation, calcium–phosphate homeostasis, and immune response [12]. VDR is regulated by the VDR gene, whose polymorphisms affect the function of the VDR protein. Polymorphisms are genetic variations in non-coding parts of the gene (introns) or in the exonic parts of the DNA that influence at least 1% of the population [13]. Changes in introns do not influence the protein product but can modify the degree of gene expression. On the other hand, changes in exonic parts of the gene affect the protein sequence, except for synonymous polymorphisms, which are alterations in exonic parts that do not affect the protein structure [13]. These variations are responsible for the creation or removal of restriction enzyme sites in DNA, which, in turn, create DNA fragments with various lengths [14]. The most common polymorphisms (VDRP) of the VDR gene, which have been related to oral and systemic conditions, are BsmI (rs1544410), Taql (rs731236), BglI (rs739837), ApaI (rs7975232), FokI (rs10735810), and FokI (rs2228570) [14,15].
VDD has been associated with Early Childhood Caries (ECC), and Severe Early Childhood Caries (S-ECC) [10]. ECC is a global health problem, affecting almost half of preschool children, defined as the presence of one or more decayed, missing, or filled primary teeth in children aged 71 months (5 years) or younger [11]. ECC has a multi-factorial etiology, including susceptible teeth due to colonization with high levels of cariogenic bacteria, such as S. mutans, enamel hypoplasia, and sugar metabolism, and by bacteria that produce acid, which, in turn, demineralizes tooth structure [10]. A subtype of ECC is Severe Early Childhood Caries (S-ECC). S-ECC is defined as any sign of smooth-surface caries in a child under the age of three and, from ages three to five, one or more cavitated, missing, or filled smooth surfaces in primary maxillary anterior teeth or a decayed, missing, or filled score greater than or equal to 4 (for 3 years of age), 5 (for 4 years of age), or 6 (for 5 years of age) [11].
As awareness of VDD has increased among patients and the health community, many authors have conducted clinical trials to study a potential association between VDD and dental caries [12,13,14]. In addition, there is a systematic review investigating vitamin D receptor (VDR) gene polymorphisms in relation to increased risk of dental caries only in children [15,16,17]. The aim of this study was to further investigate the relation between VDD, VDRP, ECC, and S-ECC in children (primary and mixed dentition) and dental caries risk in adults (permanent dentition). Additionally, we present a model incorporating factors and interactions that address this relationship.
2. Materials and Methods
For this study, an extended review of the relevant literature was performed. The algorithm of the search was “vitamin D and Polymorpisms and dental caries and abstract and vitamin D receptor”. Records have been selected from three databases (PubMed/Medline, Web of Science, Cochrane Library). Two reviewers searched, screened, and extracted information from the selected articles. All pooled analyses were based on random-effects models. Eligibility criteria were articles using dmft/DMFT diagnostic criteria with calibrated examiners, probability sampling, and sample sizes. We excluded studies conducted on institutionalized patients. A total of 341 articles were reported in the initial search, of which 104 were removed as duplicates. A total of 40 articles were excluded for other reasons, such as inadequate methodology or irrelevance to our search content through abstract evaluation. In the second phase of the study, from the remaining 197 articles, 145 were excluded due to inadequate methodology. In the third phase of the screening, 32 studies were finally included in the review, as seen in detail in the following Prisma flow chart (Figure 1).
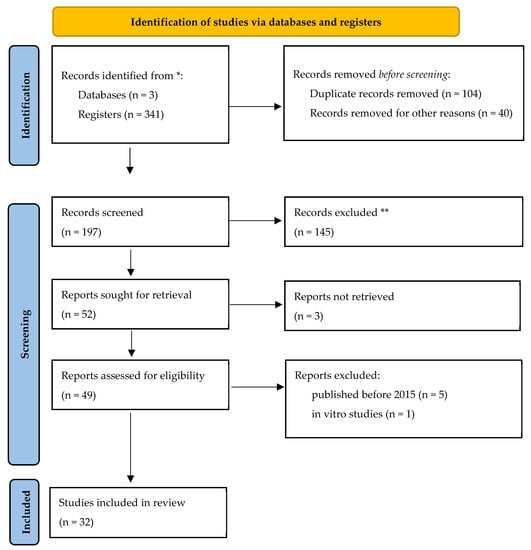
Figure 1.
Prisma flow chart of the present study. * Eligibility criteria were articles using DMFT diagnostic criteria with calibrated examiners, probability sampling, and sample sizes.** We excluded studies conducted on institutionalized patients.
3. Results
Vitamin D Receptor Gene Polymorphisms
To discuss possible relations between VDPP and caries, we needed to report on the possible alleles of the polymorphisms of the VDR gene, presented in Table 1.

Table 1.
Different types of alleles and genotypes of the VDR gene polymorphisms.
From our findings we can support the fact that all relevant studies are differentiated in both methodology and sample size, thus revealing differences in the hypothesis that certain polymorphisms may play a specific role in caries risk assessment (Table 2).
For example, Kong et al. found a correlation between the BsmI polymorphism containing the Bb genotype and increased risk of caries in deciduous teeth of Chinese children [18]. On the other hand, two studies in the Chinese population did not show a statistically significant difference between the caries-free and caries-active groups [19,20]. In numerous studies, no statistically significant difference was found for a higher caries prevalence in the population with ApaI polymorphism [18,19,20,21]. In the case of the TaqI polymorphism, one study suggested the allele ‘t’ as a possible genetic factor for determining dental caries in Chinese individuals because the ‘t’ allele was more frequent in the caries-active group [22]. Similar results were found by Cogulu et al. [19] concerning the TaqI polymorphism, showing a statistically significant difference in TaqI genotypes between the case and control groups. On the other hand, many other case–control studies did not report significant differences in allele and genotype frequency of the TaqI polymorphism between individuals with and without caries [18,19,20,23,24]. Further, the BgII polymorphism has been studied by two research groups in the Brazilian population. The authors concluded that the BgII polymorphism was not associated with an increased risk of dental caries in the respective population [25,26]. From all polymorphisms studied, the FokI polymorphism, distinguished in rs10735810 and rs2228570, is reported to play a significant role in the issue studied here. Although two studies supported that different alleles and genotypes of rs10735810 were not associated with tooth decay in Chinese and Turkish populations [18,19], two other studies found a statistically significant difference when the FokI rs10735810 genotype was present in Chinese and Brazilian populations [20,25]. In terms of the rs2228570 polymorphism, no correlation was found regarding a higher dental caries risk in the Brazilian population [25,26]. A systematic review and meta-analysis compared the influence of the ApaI, FokI (rs10735810), TaqI, BsmI, FokI (rs2228570), and BglI polymorphisms of the VDR gene on dental caries risk [15]. Based on nine studies, the meta-analysis reported an association between the FokI (rs10735810) polymorphism and dental caries risk in children, with the f allele and the ff genotype reported as having a protective role. Additionally, authors found that there was no association between the ApaI (rs7975232), TaqI (rs731236), BsmI (rs1544410), FokI (rs2228570), and BglI (rs739837) polymorphisms of the VDR gene and the prevalence of dental caries in children [15]. The authors claimed that the protective role of the f allele and ff genotype might be due to their interactions with co-transcription factors and location [15]. It is then concluded that there is a lack of consistency among the case–control studies reported here, their results related to the different VDR gene polymorphisms, and their influence on dental caries risk [27,28,29]. This fact is attributed to the statistical heterogeneity among studies, the small number of existing relative studies, and the small sample sizes. Thus, there is a significant need for additional, well-conducted research that will investigate the association between VDR gene polymorphisms and the prevalence of dental caries in mixed and permanent dentition, specifically in adult patients.


Table 2.
Studies investigating correlation between VDR polymorphisms and prevalence of dental caries.
Table 2.
Studies investigating correlation between VDR polymorphisms and prevalence of dental caries.
| Authors Publication Year | ApaI rs7975232 | TaqI rs731236 | FokI rs10735810 | FokI rs2228570 | Cdx2 rs11568820 | BglI rs739837 | BsmI rs1544410 | Type of Study/Country | Results |
|---|---|---|---|---|---|---|---|---|---|
| Hu et al., 2015 [22] | + | Case (264)–control (219) study/China Permanent dentition |
| ||||||
| Holla et al., 2017 [23] | + | Case (235)–control (153) study/Czech Permanent dentition | No significant differences in allele and genotype frequency of TaqI between case and control group | ||||||
| Cogulu et al., 2016 [19] | + | + | + | + | Case (57)–control (38) study/Turkey Primary dentition |
| |||
| Kong et al., 2017 [18] | + | + | + | + | Case (249)–control (131) study/China Primary dentition |
| |||
| Yu et al., 2017 [20] | + | + | + | + | Case (200)–control (200) study/China Permanent dentition |
| |||
| Qin et al., 2019 [21] | + | + | + | + | + | Case (304)–control study (245)/China Primary dentition |
| ||
| Barbosa et al., 2020 [25] | + | + | Case–control study/Brazil Permanent dentition | FokI, BgII VDR gene polymorphisms were not associated with dental caries | |||||
| Aribam et al., 2020 [24] | + | Case (60)–control (60) study/India Permanent dentition |
| ||||||
| Fatturi et al., 2020 [26] | + | + | Case–control study/Brazil Permanent dentition |
| |||||
| Sadeghi et al., 2021 [15] | + | + | + | + | + | + | Systematic review, meta-analysis Primary and permanent dentition |
| |
| Nireeksha et al., 2022 [7] | + | Case (239)–control (138) study/India Permanent dentition |
|
The effect of vitamin D serum levels on the prevalence of dental caries has also been studied in many scientific papers among different ages, populations, and dentitions. Table 3 presents studies correlating vitamin D serum levels with the prevalence of dental caries.
From the studies presented in Table 3, we can report on 10 studies addressing the issue on primary dentition, 8 on mixed, 3 on permanent, and one on mixed and permanent dentition. Results from the above-mentioned 10 studies on primary dentition indicate an association between prenatal levels of 25(OH)D and caries risk in primary dentition. It is mentioned that maternal 25(OH)D, during pregnancy, diffuses across the placental barrier, while 25(OH)D cord blood concentrations are 75–90% of maternal concentrations at delivery [30]. Thus, in four out of ten studies, there was an inverse relation between maternal intake of VD and dental caries in children. In favor of this correlation, we refer to the study of Tanaka et al., which examined 1210 Japanese mother–child pairs and agreed on the issue [31]. This inverse relation was also verified by another study, in which the authors found that insufficient levels of 25(OH)D (<50 nmol/L) during the third semester of pregnancy is associated with higher caries experience in primary teeth by the age of six [32]. In a third study, data were extracted from an extensive cohort survey in Austria on VDLs during the 12th week of gestation and after gestation at 4 and 8 years of age, while a dental examination was held at 6 and 10 years of age. Although sugar consumption and incorrect brushing were the main factors associated with dental caries in children participating in the study, low levels of 25(OH)D (<20 ng/mL) during pregnancy magnified the effect of cariogenic parameters. For this reason, the authors claimed that supplementing VD for pregnant women and children could be an option [33]. These findings agree with a 6-year follow-up randomized clinical trial, which concluded that supplementation with a high dose of VD during pregnancy leads to a lower probability of enamel defects in the offspring [34]. On the contrary, Schroth et al. did not find a statistically significant difference when they administered prenatally two oral doses of 50,000 (IU) of VD in the prevalence of Early Childhood Caries [35].
Furthermore, nine studies have investigated the relationship between VD deficiency and ECC or S-ECC. More specifically, there was a negative correlation between VDD and increased risk of ECC [36,37,38,39,40,41,42,43,44,45]; for example, Singleton et al. [41] stated that deficient cord blood 25(OH)D levels (<30 nmol/L) had a significant impact on the dmft in the follow-up period between 12 and 35 months of infants’ age, with dmft scores that were double in relation to nondeficient concentrations. In the same study, no significant association between insufficient levels of 25(OH)D (<50 nmol/L) and ECC was reported. In another study, with a significant sample size of 1510 Chinese children, VD deficiency and insufficiency led to an increased risk of ECC [38]. Chhonkar et al. suggested supplementation of VD to prevent dental caries because VDD is a significant risk factor for dental caries in children [39]. In addition to VDD, children with S-ECC showed significantly lower levels of Ca and serum albumin and higher levels of PTH [40]. Deficient and insufficient levels of VD led to a higher odds ratio for S-ECC in children compared to optimal VD levels, according to two studies that have been conducted in Canada and North America [43,44]. Only one case–control study reported levels of VD in children without caries comparatively higher than in children with severe caries at a mean age of 40.82 months, even if the mean levels of VD were close to optimal [42]. A cross-sectional study with a sample of 1638 children from Poland concluded that the prevalence of ECC and S-ECC was significantly lower in children receiving VD supplementation. The authors also reported that children and mothers of higher education received VD supplementation and had fewer caries, highlighting the socioeconomic background of ECC [45]. In six out of nine studies addressing the issue, it is reported that socioeconomic factors, and especially the mothers’ educational background, have a negative impact on caries risk and a positive effect on levels of VD.
Furthermore, many studies have investigated the relation between 25(OH)D levels and dental caries risk in mixed dentition, of which eight are included in this study. In all studies included here, there seems to be a weak indication that improving children’s VD status might be an additional preventive measure against caries during the period of mixed dentition. In more detail, a cross-sectional study with a sample of 1017 Canadian children from 6 to 11 years of age stated that optimal levels of 25(OH)D (>75 nmol/L) had a significantly lower odds ratio for caries [46]. Further, Navarro et al. reported a weak association between the risk of dental caries and 25(OH)D serum, and the authors did not recommend supplementation of VD as a preventive measure for managing dental caries [47]. A weak inverse correlation between VD and dental caries risk in mixed dentition was found also in another study with a sample of 206 Swedish children [48]. Supplementation of VD and fluoride during the entire first year of a child’s life led to a lower probability of caries-related restorations at the age of 10 in primary teeth in relation to children who received the same supplementation for less than 6 months [49]. A cohort study with a sample of 335 Portuguese children and a mean age of 7 years reported that 25(OH)D levels less than 30 ng/mL were associated with advanced dental caries in permanent teeth, while no statistically significant difference was found in terms of 25(OH)D levels and dental caries risk in mixed dentition [50]. The same results, regarding mixed dentition, were reported in a study with a sample of 121 Polish children with growth hormone deficiency. There was no statistically significant impact of VD on the mean DMFT index, but the authors recommended VD as a potentially effective agent in reducing dental caries, especially for growth hormone deficiency patients [51].
Apart from primary and mixed dentitions, a possible correlation between levels of 25(OH)D and dental caries risk has been investigated for permanent dentition. In our study, we report on three studies regarding permanent dentition and one with both mixed and permanent ones. From our findings in all four relevant studies, it seems that permanent dentition presents a higher correlation with VD deficiency than the mixed one. To support this, we can report that in a cross-sectional study with a sample of 2579 American adolescents, VD deficiency in permanent dentition has a limited but statistically non-significant association with caries in permanent dentition of adolescents [52]. A cross-sectional study on 1688 children from Korea found that children with serum 25(OH)D levels lower than 50 nmol/L were 1.29 times more likely to develop dental caries and that 25(OH)D concentration and DMFT were negatively correlated. However, the authors reported that there is difficulty in confirming the association between dental caries experience and 25(OH)D levels [53]. Another study that has investigated permanent dentition and 25(OH)D levels was conducted in the USA with 4244 participants with a mean age of 51.22 years. This study stated that the severely deficient group was strongly correlated with dental caries and that 25(OH)D is significantly associated with the prevalence of dental caries among US adults [54].
Concerning the VD supplementation for caries prevention in permanent dentition, we found data in all three relevant studies. Firstly, a systematic review of control clinical trials studied the influence of VD supplementation in two different forms (vitamin D2, D3) on dental caries prevention [55]. The results showed that VD supplementation reduced the risk of dental caries by about 47%, but with low certainty [55]. In two different mendelian randomization studies, the results failed to find a strong and statistically significant causal relationship between vitamin D concentrations and risk of dental caries [56,57].
Driven by the analysis performed in this study, we present a relevant model of the VD, VDRP, and dental caries equation. The model was designed with the Vensim program (Vensim PLE 8.1.0), showing factors influencing the equation. Vensim, developed by Ventana Systems (a simulation system employing causal tracing; US Patent Application EP19910909851, 26 February 1991), is precision simulation software. It primarily shows continuous simulation (system dynamics), with agent-based modeling capabilities. This modeling language supports arrays (subscripts) and permits mapping among factors. The built-in allocation functions satisfy, in our case, constraints that may not be met by conventional approaches (Figure 2).


Table 3.
Studies investigating the relation between 25(OH)D levels and dental caries.
Table 3.
Studies investigating the relation between 25(OH)D levels and dental caries.
| Authors Publication Year | Type of Study | Population | Age | Dentition | Results | Conclusions |
|---|---|---|---|---|---|---|
| Schroth et al., 2013 [40] | Case–control study |
|
| Primary |
|
|
| Tanaka et al., 2015 [31] | Prospective study | 1210 Japanese mother–child pairs | 36–46 months postnatal for the evaluation | Primary |
| Association between higher maternal vitamin D intake during pregnancy and a reduced risk of dental caries |
| Schroth et al., 2016 [46] | Cross-sectional study | 1017 Canadian children | 6 to 11 years | Mixed |
| Association between caries and lower serum vitamin D in a sample of Canadian children |
| Kühnisch et al., 2017 [49] | Clinical trial |
|
| Mixed |
|
|
| Chhonkar et al., 2018 [39] | Case–control study |
|
| Primary |
|
|
| Deane et al., 2018 [44] | Case–control study |
|
| Primary |
|
|
| Gyll et al., 2018 [48] | Intervention study |
|
| Mixed |
|
|
| Wójcik et al., 2019 [51] |
|
| Mixed |
|
| |
| Akinkugbe et al., 2018 [52] | Cross-sectional |
| Two age groups:
| Permanent | Participants with insufficiency and deficiency of vitamin D had non-statistically significant adjusted estimates of 1.02 (0.72, 1.44) and 1.23 (0.7, 2.16), respectively, for caries experience | Deficiency of vitamin D appears to have limited but statistically non-significant association with adolescent caries |
| Kim et al., 2018 [53] | Cross-sectional study |
| 10–12 years of age | Permanent |
|
|
| Singleton et al., 2019 [41] | Cohort study |
| Two periods for follow-up of the infants:
| Primary |
|
|
| Schroth et al., 2020 [35] | Prospective cohort |
| Primary |
|
| |
| Zhou et al., 2020 [54] | Cross-sectional study |
|
| Permanent |
|
|
| Jha et al., 2021 [42] | Case–control study | 266 children from India | Mean age 40.82 +/− 14.09 months | Primary | Children with severe caries had significantly lower vitamin D3 in very young childhood (68.87 +/− 28.04 vs. 82.89 +/− 31.12 nmol/L, p < 0.001) | Levels of vitamin D3 in children without caries were comparatively higher than in children with severe caries |
| Navarro et al., 2021 [47] | Prospective cohort study | 5257 multi-ethnic children | Mean age 6.1 (4.8–9.1) | Mixed |
|
|
| Silva et al., 2021 [50] | Cohort study | 335 Portuguese children | Mean age 7 years | Mixed and permanent |
|
|
| Williams et al., 2021 [43] | Case–control study | 144 caries-free children 200 children with S-ECC from North America | 42.1 +/− 14.6 months | Primary |
|
|
| Olczak-Kowalczyk et al., 2021 [45] | Cross-sectional study | 1638 children from Poland | 3 years of age | Primary |
|
|
| Suárez-Calleja et al., 2021 [33] | Cohort study | 188 children | Dental examination 6–10 years of age | Mixed |
|
|
| Chen et al., 2021 [38] | Cross-sectional | 1510 Chinese children | 44 +/− 8.2 months | Primary |
|
|
| Beckett et al., 2022 [32] | Observational study | 81 children from New Zealand | Mean age of 6.6 years +/− 0.6 | Mixed |
|
|
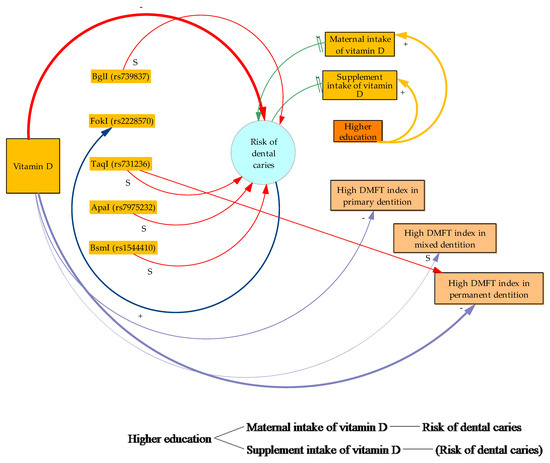
Figure 2.
A Vensim diagram provides a graphical modeling interface with basic VDRPs and risk of dental caries. The diagram presents flows and causal loops among the basic factors affecting VD serum levels and risk of caries reported in our study. It also reveals a text-based system of equations in a declarative programming language. (+) positive or in tandem influence, (−) negative or inverse influence, (S) neutral relationship, (=) delayed response among factors, (…) border lines with dots support lack of sufficient data. Border lines support a quantitatively stronger relationship among factors. Red causal loops show the VDRP relationship with risk of dental caries; purple causal loops show VD serum levels and type of dentition; green ones show the way specific factors relate to risk of dental caries; orange ones show the interconnection between higher education and factors affecting dental caries; and blue is a reverse loop showing the interconnection of FokI (rs2228579) and risk of dental caries.
4. Discussion
Dental caries incidence can derive from a host of factors that may be related to the structure of dental enamel, immunologic response to cariogenic bacteria, or the composition of saliva. Dental caries causes a modern problem even in developed countries, leading to a decrease in the quality of life of the affected individuals and high economic costs for both individuals and society, with disparities related to well-known issues of socioeconomics, immigration, lack of preventive efforts, and dietary changes [58]. The burden of dental caries in children is incredibly high, causing pain that can affect school attendance, eating and speaking, and impair growth and development [21]. More data on the correlation of dental caries with VDD and polymorphisms were derived from studies conducted in children. However, it is not only in children that this problem could cause dysfunction in social life and diet. Related problems can occur in young or middle-aged adults due to the cumulative and chronic nature of the disease [59]. Our study reveals the positive correlation of the DMFT index in permanent dentition with VDLs both in maternal and early birth stages. Our model also shows the importance of the FokI (rs2228570) polymorphism, in conjunction with the risk of dental caries, thus suggesting that relevant blood and genetic examinations can arise awareness of ECC, S-ECC, and high dental caries risk in permanent dentition, as already supported elsewhere [15].
As a higher risk of carious lesions has been associated with lower socioeconomic levels [60,61], we could expect further reductions in preventive care and frequency of dental visits [62]. According to the new Global Oral Health Status Report from the WHO [63], it is estimated that almost half of the world’s population (45%, or 3.5 billion people) suffer from oral diseases such as caries, with 3 out of every 4 affected people living in low- and middle-income countries. Global cases of dental caries have increased by 1 billion over the last 30 years. This clearly indicates that many people do not have access to prevention measures and treatment of oral diseases such as dental caries. Socioeconomic factors should then be considered when planning preventive programs for caries. Higher education is a positive factor for controlling caries risk and an important part of the relevant model.
Thus, new ways of identifying high-risk individuals are future tools in controlling caries. As observed in our study, genetic variation in host factors such as VDPs may contribute to increased risks of the disease. Thus, it seems imperative that VDR gene polymorphisms can be proposed as a marker for identifying patients with high caries risk [21]. As mentioned by Di Spigna et al. [64], the genetic screening of VDRPs could be a valuable tool for the early identification of other health problems too, such as osteoporosis in female patients with rheumatoid arthritis (RA). Commercial kits, based on the Restriction Fragment Length Polymorphism (RFLP) method for VDR polymorphisms detection, could then be used in patients at high risk for dental caries too. It is proposed, then, that “the clinician and the lab manager may join to evaluate costs and availability, of the appropriate methods to setting molecular diagnostics of VDR Genotyping” [64]. Soon, genetic tests, now performed either by academic ultra-specialized labs or custom service laboratories using certified commercial kits [65], could be performed widely, in relation to demand in dental settings, under a protected insurance policy that could provide low-income citizens with cost-effective and accurate diagnostic and preventive tools for dental caries. In these cases, future studies should focus on the importance of the application of fluorides [66] or other remineralizing biomimetic materials [67] for prevention purposes.
This review highlights the need for more detailed and extensive studies to establish the cost and effectiveness of genotyping, introducing it more in everyday dental clinical practice in terms of caries prevalence. The limitations of the present study include the risk of misinterpreting data from clinical studies that have different methodology due to inadequate explanations and the possible incomplete retrieval of identified relevant research. In the future, with new genetic markers being identified and validated, dentists will have new ways and means to tailor specific dental therapy to individual caries risk genetic profiles [65]. Overall, this would reduce the final functional treatment costs within national health systems while further enhancing the economic status of lower- and middle-class individuals. Consequently, pharmaceutical and biotechnology companies must join their future investments to develop accurate and low-cost genetic tests for routine dental diagnostics.
5. Conclusions
This study strengthens the relationship of dental caries with VD levels, particularly in primary dentition and less in mixed and permanent dentition. VD levels in children are directly related to the maternal intake and socioeconomic factors and education of the mother.
There is an inconsistency among the case–control studies and their results regarding the different VDR gene polymorphisms and their influence on ECC and S-ECC for primary and mixed dentition and dental caries risk in permanent dentition. This fact is attributed to the statistical heterogeneity between studies, the small number of existing relative studies, and the small sample sizes. There is a need for more well-conducted studies that will investigate other factors possibly influencing the current suggested model as well as the association between VDR gene polymorphisms and the risk of dental caries. VDR gene polymorphisms could be a marker for identifying not only children but also adult patients with high caries risk.
Author Contributions
Conceptualization, M.A., E.P. and C.R.; methodology, M.P. and M.A.; validation, M.P. and M.A.; formal analysis, M.P. and M.A.; investigation, M.P.; writing—original draft preparation, M.P. and M.A.; writing—review and editing, M.P., M.A., E.P., C.R. and T.V.; visualization, M.P., M.A. and E.P.; supervision, M.A., C.R. and T.V.; project administration, M.A. and T.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
Vitamin D (VD), vitamin D deficiency (VDD), vitamin D receptor (VDR), vitamin D receptor polymorphism (VDRP), Restriction Fragment Length Polymorphism (RFLP), Early Childhood Caries (ECC), Severe Early Childhood Caries (S-ECC), Decayed, Missing, and Filled Teeth (DMFT) index.
References
- Norman, A.W. From Vitamin D to Hormone D: Fundamentals of the Vitamin D Endocrine System Essential for Good Health. Am. J. Clin. Nutr. 2008, 88, 491S–499S. [Google Scholar] [CrossRef]
- Borel, P.; Caillaud, D.; Cano, N.J. Vitamin D Bioavailability: State of the Art. Crit. Rev. Food Sci. Nutr. 2015, 55, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Ajibade, D.V.; Dhawan, P.; Fechner, A.J.; Mady, L.J. Vitamin D: Metabolism. Endocrinol. Metab. Clin. N. Am. 2010, 39, 243–253. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Pfotenhauer, K.M.; Shubrook, J.H. Vitamin D Deficiency, Its Role in Health and Disease, and Current Supplementation Recommendations. J. Osteopath. Med. 2017, 117, 301–305. [Google Scholar] [CrossRef]
- D’Ortenzio, L.; Kahlon, B.; Peacock, T.; Salahuddin, H.; Brickley, M. The Rachitic Tooth: Refining the Use of Interglobular Dentine in Diagnosing Vitamin D Deficiency. Int. J. Paleopathol. 2018, 22, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Nireeksha, N.; Hegde, M.N.; Shetty, S.S.; Kumari, S.N. FOK l Vitamin D Receptor Gene Polymorphism and Risk of Dental Caries: A Case-Control Study. Int. J. Dent. 2022, 2022, 6601566. [Google Scholar] [CrossRef] [PubMed]
- Dudding, T.; Thomas, S.J.; Duncan, K.; Lawlor, D.A.; Timpson, N.J. Re-Examining the Association between Vitamin D and Childhood Caries. PLoS ONE 2015, 10, e0143769. [Google Scholar] [CrossRef] [PubMed]
- Bergwitz, C.; Jüppner, H. Regulation of Phosphate Homeostasis by PTH, Vitamin D, and FGF23. Annu. Rev. Med. 2010, 61, 91–104. [Google Scholar] [CrossRef]
- AAPD. Policy on Early Childhood Caries (ECC): Classifications, consequences, and preventive strategies. Pediatr Dent. 2017, 39 (Suppl. S7), 59–61. [Google Scholar]
- Uribe, S.; Innes, N.; Maldupa, I. The global prevalence of early childhood caries: A systematic review with meta-analysis using the WHO diagnostic criteria. Int. J. Pediatr. Dent. 2021, 31, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, J.; DeLuca, H.F. Where Is the Vitamin D Receptor? Arch. Biochem. Biophys. 2012, 523, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Valdivielso, J.M.; Fernandez, E. Vitamin D Receptor Polymorphisms and Diseases. Clin. Chim. Acta 2006, 371, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Uitterlinden, A.G.; Fang, Y.; van Meurs, J.B.J.; Pols, H.A.P.; van Leeuwen, J.P.T.M. Genetics and Biology of Vitamin D Receptor Polymorphisms. Gene 2004, 338, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Golshah, A.; Godiny, M.; Sharifi, R.; Khavid, A.; Nikkerdar, N.; Tadakamadla, S.K. The Most Common Vitamin D Receptor Polymorphisms (ApaI, FokI, TaqI, BsmI, and BglI) in Children with Dental Caries: A Systematic Review and Meta-Analysis. Children 2021, 8, 302. [Google Scholar] [CrossRef]
- Cooper, G.S.; Umbach, D.M. Are Vitamin D Receptor Polymorphisms Associated with Bone Mineral Density? A Meta-Analysis. J. Bone Miner. Res. 2010, 11, 1841–1849. [Google Scholar] [CrossRef]
- Gong, G.; Stern, H.S.; Cheng, S.-C.; Fong, N.; Mordeson, J.; Deng, H.-W.; Recker, R.R. The Association of Bone Mineral Density with Vitamin D Receptor Gene Polymorphisms. Osteoporos. Int. 1999, 9, 55–64. [Google Scholar] [CrossRef]
- Kong, Y.; Zheng, J.; Zhang, W.; Jiang, Q.; Yang, X.; Yu, M.; Zeng, S. The Relationship between Vitamin D Receptor Gene Polymorphism and Deciduous Tooth Decay in Chinese Children. BMC Oral Health 2017, 17, 111. [Google Scholar] [CrossRef]
- Cogulu, D.; Onay, H.; Ozdemir, Y.; Aslan, G.I.; Ozkinay, F.; Eronat, C. The Role of Vitamin D Receptor Polymorphisms on Dental Caries. J. Clin. Pediatr. Dent. 2016, 40, 211–214. [Google Scholar] [CrossRef]
- Yu, M.; Jiang, Q.-Z.; Sun, Z.-Y.; Kong, Y.-Y.; Chen, Z. Association between Single Nucleotide Polymorphisms in Vitamin D Receptor Gene Polymorphisms and Permanent Tooth Caries Susceptibility to Permanent Tooth Caries in Chinese Adolescent. BioMed Res. Int. 2017, 2017, 4096316. [Google Scholar] [CrossRef]
- Qin, X.; Shao, L.; Zhang, L.; Ma, L.; Xiong, S. Investigation of Interaction between Vitamin D Receptor Gene Polymorphisms and Environmental Factors in Early Childhood Caries in Chinese Children. BioMed Res. Int. 2019, 2019, 4315839. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.P.; Li, Z.Q.; Zhou, J.Y.; Yu, Z.H.; Zhang, J.M.; Guo, M.L. Analysis of the Association between Polymorphisms in the Vitamin D Receptor (VDR) Gene and Dental Caries in a Chinese Population. Genet. Mol. Res. 2015, 14, 11631–11638. [Google Scholar] [CrossRef] [PubMed]
- Holla, L.I.; Borilova Linhartova, P.; Kastovsky, J.; Bartosova, M.; Musilova, K.; Kukla, L.; Kukletova, M. Vitamin D Receptor TaqI Gene Polymorphism and Dental Caries in Czech Children. Caries Res. 2017, 51, 7–11. [Google Scholar] [CrossRef]
- Aribam, V.; Aswath, N.; Ramanathan, A. Single-Nucleotide Polymorphism in Vitamin D Receptor Gene and Its Association with Dental Caries in Children. J. Indian Soc. Pedod. Prev. Dent. 2020, 38, 8. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.C.F.; Lima, D.C.; Reis, C.L.B.; Reis, A.L.M.; Rigo, D.; Segato, R.A.B.; Storrer, C.L.M.; Küchler, E.C.; Oliveira, D.S.B. Vitamin D Receptor FokI and BglI Genetic Polymorphisms, Dental Caries, and Gingivitis. Int. J. Paediatr. Dent. 2020, 30, 642–649. [Google Scholar] [CrossRef]
- Fatturi, A.L.; Menoncin, B.L.; Reyes, M.T.; Meger, M.; Scariot, R.; Brancher, J.A.; Küchler, E.C.; Feltrin-Souza, J. The Relationship between Molar Incisor Hypomineralization, Dental Caries, Socioeconomic Factors, and Polymorphisms in the Vitamin D Receptor Gene: A Population-Based Study. Clin. Oral Investig. 2020, 24, 3971–3980. [Google Scholar] [CrossRef]
- Schwendicke, F.; Frencken, J.E.; Bjørndal, L.; Maltz, M.; Manton, D.J.; Ricketts, D.; Van Landuyt, K.; Banerjee, A.; Campus, G.; Doméjean, S.; et al. Managing Carious Lesions: Consensus Recommendations on Carious Tissue Removal. Adv. Dent. Res. 2016, 28, 58–67. [Google Scholar] [CrossRef]
- Kidd, E.A.M. Clinical Threshold for Carious Tissue Removal. Dent. Clin. N. Am. 2010, 54, 541–549. [Google Scholar] [CrossRef]
- Chapple, I.L.C.; Bouchard, P.; Cagetti, M.G.; Campus, G.; Carra, M.-C.; Cocco, F.; Nibali, L.; Hujoel, P.; Laine, M.L.; Lingstrom, P.; et al. Interaction of Lifestyle, Behaviour or Systemic Diseases with Dental Caries and Periodontal Diseases: Consensus Report of Group 2 of the Joint EFP/ORCA Workshop on the Boundaries between Caries and Periodontal Diseases. J. Clin. Periodontol. 2017, 44 (Suppl. S18), S39–S51. [Google Scholar] [CrossRef]
- Keim, S.A.; Bodnar, L.M.; Klebanoff, M.A. Maternal and Cord Blood 25(OH)-Vitamin D Concentrations in Relation to Child Development and Behaviour. Paediatr. Perinat. Epidemiol. 2014, 28, 434–444. [Google Scholar] [CrossRef]
- Tanaka, K.; Hitsumoto, S.; Miyake, Y.; Okubo, H.; Sasaki, S.; Miyatake, N.; Arakawa, M. Higher Vitamin D Intake during Pregnancy Is Associated with Reduced Risk of Dental Caries in Young Japanese Children. Ann. Epidemiol. 2015, 25, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Beckett, D.M.; Broadbent, J.M.; Loch, C.; Mahoney, E.K.; Drummond, B.K.; Wheeler, B.J. Dental Consequences of Vitamin D Deficiency during Pregnancy and Early Infancy—An Observational Study. Int. J. Environ. Res. Public. Health 2022, 19, 1932. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Calleja, C.; Aza-Morera, J.; Iglesias-Cabo, T.; Tardón, A. Vitamin D, Pregnancy and Caries in Children in the INMA-Asturias Birth Cohort. BMC Pediatr. 2021, 21, 380. [Google Scholar] [CrossRef] [PubMed]
- Nørrisgaard, P.E.; Haubek, D.; Kühnisch, J.; Chawes, B.L.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H. Association of High-Dose Vitamin D Supplementation During Pregnancy with the Risk of Enamel Defects in Offspring: A 6-Year Follow-up of a Randomized Clinical Trial. JAMA Pediatr. 2019, 173, 924–930. [Google Scholar] [CrossRef]
- Schroth, R.J.; Christensen, J.; Morris, M.; Gregory, P.; Mittermuller, B.-A.; Rockman-Greenberg, C. The Influence of Prenatal Vitamin D Supplementation on Dental Caries in Infants. J. Can. Dent. Assoc. 2020, 86, k13. [Google Scholar]
- Tinanoff, N. Introduction to the Conference: Innovations in the Prevention and Management of Early Childhood Caries. Pediatr. Dent. 2015, 37, 198–199. [Google Scholar]
- American Academy of Pediatric Dentistry; American Academy of Pediatrics; American Academy of Pediatric Dentistry Council on Clinical Affairs. Policy on Early Childhood Caries (ECC): Classifications, Consequences, and Preventive Strategies. Pediatr. Dent. 2005, 27 (Suppl. S7), 31–33. [Google Scholar]
- Chen, Z.; Lv, X.; Hu, W.; Qian, X.; Wu, T.; Zhu, Y. Vitamin D Status and Its Influence on the Health of Preschool Children in Hangzhou. Front. Public Health 2021, 9, 675403. [Google Scholar] [CrossRef]
- Chhonkar, A.; Arya, V. Comparison of Vitamin D Level of Children with Severe Early Childhood Caries and Children with No Caries. Int. J. Clin. Pediatr. Dent. 2018, 11, 199–204. [Google Scholar] [CrossRef]
- Schroth, R.J.; Levi, J.A.; Sellers, E.A.; Friel, J.; Kliewer, E.; Moffatt, M.E. Vitamin D Status of Children with Severe Early Childhood Caries: A Case–Control Study. BMC Pediatr. 2013, 13, 174. [Google Scholar] [CrossRef]
- Singleton, R.; Day, G.; Thomas, T.; Schroth, R.; Klejka, J.; Lenaker, D.; Berner, J. Association of Maternal Vitamin D Deficiency with Early Childhood Caries. J. Dent. Res. 2019, 98, 549–555. [Google Scholar] [CrossRef]
- Jha, A.; Jha, S.; Shree, R.; Menka, K.; Shrikaar, M. Association between Serum Ferritin, Hemoglobin, Vitamin D3, Serum Albumin, Calcium, Thyrotropin-Releasing Hormone with Early Childhood Caries: A Case–Control Study. Int. J. Clin. Pediatr. Dent. 2021, 14, 648–651. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.L.; Boyle, J.; Mittermuller, B.-A.; Carrico, C.; Schroth, R.J. Association between Vitamin D and Dental Caries in a Sample of Canadian and American Preschool-Aged Children. Nutrients 2021, 13, 4465. [Google Scholar] [CrossRef]
- Deane, S.; Schroth, R.J.; Sharma, A.; Rodd, C. Combined Deficiencies of 25-Hydroxyvitamin D and Anemia in Preschool Children with Severe Early Childhood Caries: A Case–Control Study. Paediatr. Child Health 2018, 23, e40–e45. [Google Scholar] [CrossRef] [PubMed]
- Olczak-Kowalczyk, D.; Kaczmarek, U.; Gozdowski, D.; Turska-Szybka, A. Association of Parental-Reported Vitamin D Supplementation with Dental Caries of 3-Year-Old Children in Poland: A Cross-Sectional Study. Clin. Oral Investig. 2021, 25, 6147–6158. [Google Scholar] [CrossRef]
- Schroth, R.J.; Rabbani, R.; Loewen, G.; Moffatt, M.E. Vitamin D and Dental Caries in Children. J. Dent. Res. 2016, 95, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Navarro, C.L.A.; Grgic, O.; Trajanoska, K.; van der Tas, J.T.; Rivadeneira, F.; Wolvius, E.B.; Voortman, T.; Kragt, L. Associations Between Prenatal, Perinatal, and Early Childhood Vitamin D Status and Risk of Dental Caries at 6 Years. J. Nutr. 2021, 151, 1993–2000. [Google Scholar] [CrossRef]
- Gyll, J.; Ridell, K.; Öhlund, I.; Karlsland Åkeson, P.; Johansson, I.; Lif Holgerson, P. Vitamin D Status and Dental Caries in Healthy Swedish Children. Nutr. J. 2018, 17, 11. [Google Scholar] [CrossRef]
- Kühnisch, J.; Thiering, E.; Heinrich-Weltzien, R.; Hellwig, E.; Hickel, R.; Heinrich, J. Fluoride/Vitamin D Tablet Supplementation in Infants—Effects on Dental Health after 10 Years. Clin. Oral Investig. 2017, 21, 2283–2290. [Google Scholar] [CrossRef]
- Carvalho Silva, C.; Gavinha, S.; Manso, M.C.; Rodrigues, R.; Martins, S.; Guimarães, J.T.; Santos, A.C.; Melo, P. Serum Levels of Vitamin D and Dental Caries in 7-Year-Old Children in Porto Metropolitan Area. Nutrients 2021, 13, 166. [Google Scholar] [CrossRef]
- Wójcik, D.; Szalewski, L.; Pietryka-Michałowska, E.; Borowicz, J.; Pels, E.; Beń-Skowronek, I. Vitamin D 3 and Dental Caries in Children with Growth Hormone Deficiency. Int. J. Endocrinol. 2019, 2019, 2172137. [Google Scholar] [CrossRef] [PubMed]
- Akinkugbe, A.A.; Moreno, O.; Brickhouse, T.H. Serum Cotinine, Vitamin D Exposure Levels and Dental Caries Experience in U.S. Adolescents. Community Dent. Oral Epidemiol. 2018, 47, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-J.; Lee, H.-S.; Ju, H.-J.; Na, J.-Y.; Oh, H.-W. A Cross-Sectional Study on the Association between Vitamin D Levels and Caries in the Permanent Dentition of Korean Children. BMC Oral Health 2018, 18, 43. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Zhou, Y.; Shi, J. The Association between Serum 25-Hydroxyvitamin D Levels and Dental Caries in US Adults. Oral Dis. 2020, 26, 1537–1547. [Google Scholar] [CrossRef]
- Hujoel, P.P. Vitamin D and Dental Caries in Controlled Clinical Trials: Systematic Review and Meta-Analysis. Nutr. Rev. 2013, 71, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhou, F.; Xu, H. Circulating Vitamin C and D Concentrations and Risk of Dental Caries and Periodontitis: A Mendelian Randomization Study. J. Clin. Periodontol. 2022, 49, 335–344. [Google Scholar] [CrossRef]
- Dodhia, S.A.; West, N.X.; Thomas, S.J.; Timpson , N.J.; Johansson, I.; Lif Holgerson, P.; Dudding, T.; Haworth, S. Examining the causal association between 25-hydroxyvitamin D and caries in children and adults: A two-sample Mendelian randomization approach. Wellcome Open Res. 2021, 5, 281. [Google Scholar] [CrossRef]
- Karnaki, P.; Katsas, K.; Diamantis, D.V.; Riza, E.; Rosen, M.S.; Antoniadou, M.; Gil-Salmerón, A.; Grabovac, I.; Linou, A. Dental Health, Caries Perception and Sense of Discrimination among Migrants and Refugees in Europe: Results from the Mig-HealthCare Project. Appl. Sci. 2022, 12, 9294. [Google Scholar] [CrossRef]
- Bernabé, E.; Sheiham, A. Age, period and cohort trends in caries of permanent teeth in four developed countries. Am. J. Public Health 2014, 104, e115–e121. [Google Scholar] [CrossRef]
- Schwendicke, F.; Dörfer, C.E.; Schlattmann, P.; Foster Page, L.; Thomson, W.M.; Paris, S. Socioeconomic inequality and caries: A systematic review and meta-analysis. J. Dent. Res. 2015, 94, 10–18. [Google Scholar] [CrossRef]
- Stennett, M.; Tsakos, G. The impact of the COVID-19 pandemic on oral health inequalities and access to oral healthcare in England. Br. Dent. J. 2022, 232, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Soares, G.H.; Pereira, N.F.; Biazevic, M.G.; Braga, M.M.; Michel-Crosato, E. Dental caries in South American Indigenous peoples: A systematic review. Community Dent. Oral Epidemiol. 2019, 47, 142–152. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Global Oral Health Status Report. WHO Highlights Oral Health Neglect Affecting nearly Half of the World’s Population. Available online: https://www.who.int/news/item/18-11-2022-who-highlights-oral-health-neglect-affecting-nearly-half-of-the-world-s-population (accessed on 25 March 2023).
- Di Spigna, G.; Del Puente, A.; Covelli, B.; Abete, E.; Varriale, E.; Salzano, S.; Postiglione, L. Vitamin D receptor polymorphisms as tool for early screening of severe bone loss in women patients with rheumatoid arthritis. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4664–4669. [Google Scholar] [PubMed]
- Di Francia, R.; Amitrano, F.; De Lucia, D. Evaluation of genotyping methods and costs for VDR polymorphisms. Letter to the editor. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1–3. Available online: https://www.europeanreview.org/wp/wp-content/uploads/1-3-Evaluation-of-genotyping-methods-and-costs-for-VDR-polymorphisms.pdf (accessed on 25 March 2023).
- Zampetti, P.; Scribante, A. Historical and bibliometric notes on the use of fluoride in caries prevention. Eur. J. Pediatr. Dent. 2020, 21, 148–152. [Google Scholar]
- Khanduri, N.; Kurup, D.; Mitra, M. Quantitative evaluation of remineralizing potential of three agents on artificially demineralized human enamel using scanning electron microscopy imaging and energy-dispersive analytical X-ray element analysis: An in vitro study. Dent. Res. J. 2020, 17, 366–372. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).