Abstract
The identification of concerning high levels of pyrrolizidine alkaloids (PAs) in a wide variety of food products has raised the occurrence of these natural toxins as one of the main current issues of the food safety field. Consequently, a regulation with maximum concentration levels of these alkaloids has recently been published to monitor their occurrence in several foodstuffs. According to legislation, the analytical methodologies developed for their determination must include multiresidue extractions with high selectivity and sensitivity, as a set of 21 + 14 PAs should be simultaneously monitored. However, the multiresidue extraction of these alkaloids is a difficult task due to the high complexity of food and feed samples. Accordingly, although solid-phase extraction is still the technique most widely used for sample preparation, the QuEChERS method can be a suitable alternative for the simultaneous determination of multiple analytes, providing green extraction and clean-up of samples in a quick and cost-effective way. Hence, this review proposes an overview about the QuEChERS concept and its evolution through different modifications that have broadened its applicability over time, focusing mainly on its application regarding the determination of PAs in food and feed, including the revision of published works within the last 11 years.
1. Introduction
In recent years, the awareness about natural toxins of plant origin in food and feed, such as pyrrolizidine alkaloids (PAs), has risen as one of the main issues of food safety. PAs are probably the most widely extended natural plant toxins, as more than 600 different chemical structures of these alkaloids have been identified from over more than 6000 plant species, being the main PA-producing plants belonging to the families Asteraceae, Fabaceae, Boraginaceae, Orchidaceae and Apocynaceae [1]. In this sense, these alkaloids can be introduced into the food chain from different vegetables and botanical sources. In some cases, these PA-producing plants are directly consumed by animals (through forage) and humans (e.g., borage, salads, food supplements, teas and herbal infusions made of PAs producing plants, such as chamomile or rooibos, etc.). However, currently, the major sources of PA consumption in animal and humans seem to be feed and plant-derived products contaminated with PA-producing plants, as many of these plants grow in fields as weeds, leading to the contamination of food crops [2,3]. Accordingly, several different contamination paths have been reported, such as cross-contamination during harvesting processes, natural horizontal transfer through soil, as well as food fraud and adulteration [2,3,4]. As a consequence, many food alerts have notified in the last years high levels of these alkaloids in a wide variety of food products: spices and aromatic herbs (57% of the food alerts notified), teas and herbal teas (15% of the food alerts notified), food supplements (13% of the food alerts notified), herbs (8% of the food alerts notified), pollen (7% of the food alerts notified) and honey (1% of the food alerts notified) [2,5]. Nonetheless, these alkaloids have also been detected in other plant-derived products, such as cereals, flours and salads, as well as in some animal-derived products such as milk and dairy products, eggs, meat and meat products [2,6,7,8,9]. However, contamination of PAs in products of animal origin is less frequent, and the concentration levels found of these alkaloids are often low in this type of foodstuff. The intake of PAs represents a potential health risk, as they are known to produce both acute and chronic effects. In this sense, the ingestion of these alkaloids is mainly associated with liver damage (hepatic veno-occlusive disease (HVOD), liver cirrhosis and liver failure), but they can also produce genotoxic and carcinogenic effects at long-term exposure. Some of them (monocrotaline, riddelliine, and lasiocarpine) have been classified as potential carcinogens to humans (Group 2B) by the International Agency for Research on Cancer (IARC) [10,11,12,13]. Therefore, due to the wide spread of these alkaloids in a large variety of food products and the potential health risk that their frequent intake may entail for consumers, the analytical control of these alkaloids in food and feed is of utmost importance and constitutes a matter of interest. For this reason, in December 2020, the European Commission published a regulation amending Regulation (EC) No. 1881/2006 to monitor the occurrence of PAs in some food products [14]. This regulation sets maximum total concentration levels of PAs (ranging from 1.0 to 1000 μg/kg) in different foodstuffs, including: tea and herbal infusions, herbal food supplements, pollen-based food supplements, pollen, pollen products, dried herbs and cumin seeds [14]. Moreover, according to this legislation, every analytical methodology used to monitor these contaminants in food or feed must include the analysis of 21 PAs (including their N-oxide forms, PANOs). At the same time, 14 additional PAs can be considered if the chromatographic method employed enables the individual separation and identification of them without coelution problems, as they are isomers of one or more of the previous mentioned 21 PAs that are known to co-elute with some of them [14]. The coelution of these isomers is one of the main issues in the analysis of PAs. Consequently, powerful and efficient methods are required to perform the determination of PAs, which must include multiresidue extraction with high selectivity and sensitivity, as well as being quick and environmentally friendly procedures. However, the multiresidue extraction of these natural toxins is a difficult task, as they can be subjected to multiple matrix interferences that hamper their extraction and identification due to the high complexity of food and feed samples. In this sense, the QuEChERS (acronym of quick, easy, cheap, effective, rugged and safe) strategy can be a suitable approach for the determination of multiple analytes at the same time, as it enables the simultaneous green extraction and clean-up of samples before their instrumental analysis by gas chromatography (GC) or liquid chromatography (LC) [15,16].
The QuEChERS method was first proposed by Anastassiades et al. (2003) [17] and afterward validated by Lehotay et al. (2005) [18] for the simultaneous multiresidue extraction of a wide variety of pesticides (covering a broad range from non-polar to polar compounds) from fruit and vegetable samples. Since then, this method has gained great popularity, expanding its application range to other matrices and analytes due to its inherent advantages (quick, cheap, simple and user-friendly) and high throughput extraction efficiency [15,16]. However, despite its multiple advantages and compliance with the green analytical chemistry (GAC) principles, this method has scarcely been applied in the determination of PAs in contrast with other extraction and purification conventional methods, such as solid-phase extraction (SPE) [2,13]. In this sense, despite that conventional SPE requires more time and reagents to be performed, 58% of the published works that carry out the determination of PAs in food and feed samples used this technique, followed by 29% of works that apply the QuEChERS strategy, whereas only 13% of the articles only perform solid–liquid or liquid–liquid extraction (depending on the sample) without clean-up or purification steps (Figure 1a). Nevertheless, a slight increase in the number of works published using QuEChERS for the determination of PAs has been observed in recent years (Figure 1b). Although the general trend shows great fluctuations over the last 11 years in its application (Figure 1b), it is expected that its increase will continue in the coming years.

Figure 1.
(a) Overview of the main extraction methods used for pyrrolizidine alkaloids from food and feed, and (b) evolution of the number of published articles using QuEChERS for the determination of pyrrolizidine alkaloids in food and feed samples over the last 11 years (2011–2022). Data obtained from Scopus, Web of Science and Google search engines up to March 2022. LLE: liquid–liquid extraction; SLE: solid–liquid extraction; SPE: solid–phase extraction.
Accordingly, this review aims to give an overview about the QuEChERS concept and its evolution through different modifications that have improved and extended its applicability over time, mainly focusing on its application in the determination of PAs in food and feed samples. For this purpose, the analytical procedures published that have employed this strategy for the determination of these alkaloids in food and feed over the last 11 years (from 2011 to 2022) are reviewed. Likewise, expected future outlooks within the next years are also included.
2. Basis of the QuEChERS Method
The QueChERS was designed as a multiresidue approach for the determination of multiple analytes (more than 200 pesticides) at the same time, involving simultaneous extraction and clean-up of samples (particularly, fruit and vegetables) [17]. This method is based on the dispersion of salts (salting-out effect) to extract and isolate a wide variety of analytes from complex matrices in addition to the subsequent clean-up of the sample extract obtained. In this sense, there are two clear steps in this approach:
- (i)
- Extraction step based on partitioning via salting-out extraction, achieving an equilibrium between an aqueous and an organic phase.
- (ii)
- Clean-up step carried out by dispersive solid-phase extraction (dSPE) using different sorbent materials and salts to remove matrix interferences.
The original QuEChERS procedure involves using 10 g of sample, 10 mL of organic solvent (acetonitrile, ACN), 5 g of partitioning salts (4 g MgSO4 and 1 g NaCl) and 175 mg of clean-up sorbents (25 mg primary secondary amine (PSA) and 150 mg MgSO4) [17]. Accordingly, the sample is first subjected to a solid–liquid extraction (SLE) with ACN carried out by manual shaking and followed by the salt partitioning step with MgSO4 and NaCl to promote water partition from the organic layer and its dehydration. The addition of these salts enables a decrease in the solubility of polar compounds in the aqueous phase and removal of water from the organic phase. To achieve efficient and homogeneous interaction among the salts, the organic solvent and the sample, a stirring process followed by centrifugation is carried out, which allows for the separation of both phases. Afterward, an aliquot of the supernatant corresponding to the organic phase is recovered for the subsequent dSPE clean-up step. PSA is a weak anion exchange sorbent; thus, it can interact strongly with the acid matrix interferents that may have been co-extracted during the process, such as sugars, fatty acids and organic acids, promoting their elimination from the ACN phase. Conversely, the addition of MgSO4 in the clean-up step removes the residual water content in the extract and improves the interaction of the above-mentioned matrix interferences on the PSA sorbent, leading to a final extract with less polarity due to the precipitation of the polar interferents which improves their retention in the PSA sorbent. After a brief shaking and centrifugation, the supernatant is recovered and can be directly analyzed by GC or LC [15,16,17,18]. Figure 2 shows the schematic layout of the original QuEChERS procedure.
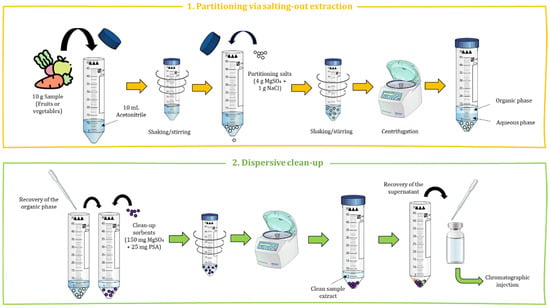
Figure 2.
Schematic layout of the original QuEChERS procedure with its two differentiated steps.
Nevertheless, since its origin, despite its high efficiency, the QuEChERS concept has evolved to be adapted to other analytes and matrices. In this sense, due to its flexibility, several modifications have been successfully introduced in its key parameters in order to improve its extraction efficiency, to spread its potential application to a wide range of matrices and to achieve simultaneous extraction of multiple compounds belonging to different chemical families [15,16]. Some of the most notable modifications in the QuEChERS procedure are depicted in Figure 3.
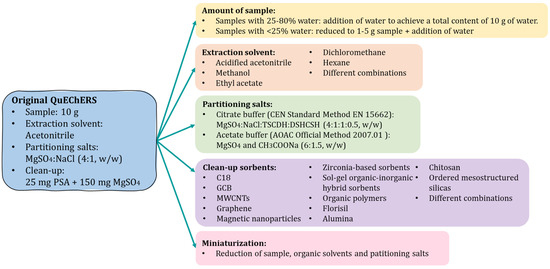
Figure 3.
Most notable modifications of the original QuEChERS procedure. PSA: primary secondary amine; TSCDH: trisodium citrate dihydrate; DSHCSH: disodium hydrogen citrate sesquihydrate; C18: octadecylsilane, GCB: graphitized carbon black; MWCNTs: multiwalled carbon nanotubes.
3. Evolution of the Original QuEChERS Method and Its Application to the Determination of Pyrrolizidine Alkaloids in Food and Feed Samples
Table 1 summarizes the different QuEChERS strategies carried out within the last 11 years for the extraction and analysis of PAs and PANOs in different food and feed products. As it can be observed, this strategy has been mainly applied to the determination of PAs in honey samples, followed by the analysis of herb samples (highlighting oregano) and (herbal) teas (including both the dry product and the beverage) (Figure 4 and Table 1), likewise, its application to cereal samples, such as wheat, sorghum and quinoa. In contrast, to a lesser extent, it has been applied to the analysis of legumes (pea and soy) and vegetables (leek) (Table 1 and Figure 4). It has also been used in products employed as food supplements, such as pollen, and in feed and forage samples (Table 1 and Figure 4).

Table 1.
Application of modified QuEChERS to the determination of pyrrolizidine alkaloids in food and feed samples.
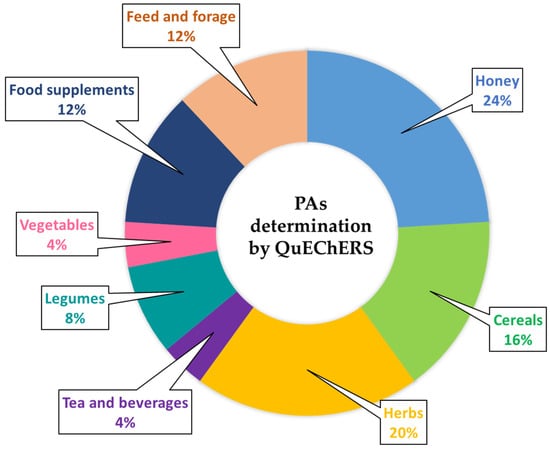
Figure 4.
Overview of the different food and feed matrices used as samples for the determination of pyrrolizidine alkaloids by modified QuEChERS method over the last 11 years (2011–2022). Data obtained from Scopus, Web of Science and Google search engines up to March 2022.
Due to the multiple advantages of the QuEChERS method (simplicity, cost-effectiveness and ability to perform multiple extraction of different analytes), many of the works reviewed carried out the simultaneous co-analysis of PAs with other compounds, such as other plant toxins (mainly tropane alkaloids, among others such as glycoalkaloids, isoquinoline alkaloids, ergot alkaloids, opium alkaloids, etc.), pesticides, mycotoxins, drugs, phytoestrogens, etc. [19,23,29,30,31,32,33,34,35]. However, despite its advantageous properties, the QuEChERS procedure has been scarcely employed for the analysis of PAs compared to SPE, as previously mentioned (Figure 1). Nonetheless, its application to the analysis of PAs is expected to increase significantly in the coming years, as it is quicker and more environmentally friendly than conventional SPE. In this context, Martinello et al. (2017) compared both types of extraction techniques for the analysis of nine PAs in honey samples, concluding that SPE, besides being more tedious and time-consuming than QuEChERS, also provided worse recovery values for the analytes [19].
Conversely, some authors carried out a first extraction with H2SO4 followed by the addition of zinc dust prior to the QuEChERS method. This first step is performed with the aim of reducing the PANOs to their corresponding PAs form (which is their tertiary base form) [19,20,34]. For this reason, in these works, only PAs are determined in the honey and pollen samples, while PANOs are not determined nor quantified. This reduction procedure is more indicated for the analysis of PAs by GC, as PANOs cannot be analyzed with this technique because they are unstable at the temperatures needed for volatilization [2,37,38]. Therefore, as PAs and PANOs are both toxicologically important and both forms need to be included in the analytical determination of these alkaloids [1,14], this reduction procedure can be carried out to ensure the extraction and determination of both types. However, in all the works reviewed for the determination of PAs by QuEChERS extraction, none of them used GC for the instrumental analysis of the sample extracts obtained after the QuEChERS procedure (Table 1). In contrast, all of them used LC coupled to tandem mass spectrometry (LC-MS/MS), which it is generally preferred over GC, as no derivatization of PAs is required; thus, sample preparation is easier and quicker [2]. Moreover, LC-MS/MS achieves the individual detection and quantification of both PAs and PANOs. Therefore, one of the reasons to use the zinc reduction strategy with LC analysis may be the acquisition of less commercial standards to perform the total quantification of PAs, as it avoids acquiring commercial standards for PANOs [2]. However, one drawback of this reduction procedure is that it does not perform individual analysis of PAs, and consequently, it is not possible to determine the contamination profile of PAs and PANOs in the samples or the origin of the contamination source. Moreover, the reduction step increases the time of the analytical method, as it requires 1 h and 30 min [20], which makes the extraction process more tedious and time-consuming. Regarding the LC-MS/MS instrumental analysis of the works reviewed, it is observed that all of them used electrospray ionization (ESI) in positive ion mode as ionization source (Table 1). Nonetheless, some works also indicated the simultaneous determination in both positive and negative ion mode [30,31,32]. However, the reason is that in these works, in addition to PAs, other compounds are also included in the chromatographic analysis, as previously indicated (e.g., plant toxins, mycotoxins, pesticides, phytoestrogens, etc.). Thus, the ionization in negative ion mode is employed for the identification of these other analytes. Additionally, most of the works reviewed used multiple reaction monitoring (MRM) as detection mode (Table 1), as it is common for all types of analytes when MS/MS is performed. In a lesser extent, other authors have used high resolution mass spectrometry (HRMS) as a detection mode (Table 1), since they used a Q-Orbitrap as mass spectrometry analyzer, which is suitable for this type of detection. Moreover, in general, other compounds different from PAs are also simultaneously co-analyzed in the chromatographic method of these works. Therefore, the HRMS mode is suitable for this purpose, as it detects a high number of compounds with tentative analysis. Conversely, only one work carried out the analysis with selected ion monitoring (SIM) as detection mode, since a single quadrupole (Q) was used as a mass spectrometry analyzer for the detection [20].
Regarding the QuEChERS method, it can be observed that none of the works published in the literature for the determination of PAs in food and feed have carried out the original QuEChERS procedure, since they all present one or more modifications (Table 1). Nonetheless, despite the modifications made, good extraction efficiency has been achieved for the extraction of PAs, as in general satisfactory recovery values have been achieved with adequate sensitivity (Table 1). Some of the most notable modifications in the QuEChERS procedure and their application to the determination of PAs are described below.
3.1. Modifications in the Amount of Sample
As it has been indicated, the original QuEChERS method requires large amount of sample (10 g) [17]. However, in order to spread its applicability to different matrices other than fruits and vegetables, some modifications have been suggested depending on the nature of the samples. In this sense, samples with a water content between 25–80%, such as bananas, require the addition of water before the initial extraction step to achieve a total content of 10 g of water (if 10 g of sample are employed). Conversely, in samples with a water content lower than 25%, such as cereals, dried fruits, honey, spices, aromatic herbs, flours, etc., the initial amount of sample has to be reduced to 1–5 g depending on the load of matrix interferences co-extracted and expected in the final extracts, and they require the addition of water before their extraction [39]. Table 2 shows the proposal recommended for the amount of sample and the addition of water depending on the sample nature in some food products.

Table 2.
Proposal of the amount of sample and water addition to perform QuEChERS depending on the type of sample (data obtained and adapted from reference [39]).
Reviewing the works published in the literature that performed QuEChERS for the multiresidue analysis of PAs in food and feed (Table 1), in general, lower amounts of samples are used than the ones proposed in Table 2 for products with contents of water below 80% and 25%. Nonetheless, the addition of water before the extraction with the organic solvent is carried out and consider by the authors (Table 1). For instance, Guo et al. (2022) only used 1 g of honey and evaluated the addition of different amounts of water (0.5, 1, 2 and 3 mL) to the samples before the QuEChERS extraction [23]. It was revealed that 0.5 mL were not enough, as it seemed that honey was not completely dispersed. Conversely, the response of some PAs, such as seneciphylline, decreased when the volume of water added was higher than 1 mL, due to the water solubility of these compounds. [23] Therefore, despite the recommendation to add water to honey samples in a 2:1 ratio, respectively (Table 2), in this work the best results were achieved with a 1:1 ratio [23].
3.2. Modifications in the Extraction Solvent
In the original QuEChERS method, the extraction solvent proposed is ACN, as it extracts a broad range of organic compounds without leading to co-extraction of lipophilic materials [17]. Moreover, this organic solvent is less polluting than others, such as methanol, and it promotes protein precipitation, which can be considered a first clean-up step in complex matrices, such as food and feed. Nevertheless, over time, many solvent modifications have been proposed in this procedure to improve the extraction efficiency of different analytes from diverse matrices. The most common is to use acidified ACN with some type of acid, such as formic acid (the most extended), acetic acid or citric acid. Another common practice is to use mixtures of ACN with other solvents, such as water, methanol, ethyl acetate, dichloromethane or hexane. In other cases, ACN can be substitute by other organic solvents, such as methanol, or even used combinations of different solvents without ACN [15,16].
Accordingly, in the case of PAs, several authors have chosen to perform the first extraction step of the QuEChERS procedure with acidified ACN, mainly using different percentages of formic acid ranging from 0.1% to 2% (Table 1). For instance, Kaczynski et al. (2020) compared the extraction of PAs with non-acidified ACN and with ACN acidified with 1% formic acid [26]. The results revealed that the recoveries of the targeted PAs evaluated improved 6-18% when ACN was acidified. Likewise, Mol et al. (2011) also used acidified ACN to extract 14 PAs and PANOs from different products (food supplements, honey and feed), but using in this case 1% acetic acid [30]. Conversely, some authors have also used mixtures of ACN with other solvents for the extraction of PAs in the QuEChERS procedure (Table 1). In this sense, León et al. (2022) combined ACN with an acidified aqueous solution with 0.5% formic acid (75:25, v/v) for the extraction of 28 PAs and PANOs from dried teas and herbs [25]. One of the main reasons why authors selected acidified polar solvents or acidified aqueous solutions is due to the great polarity of PANOs, as is essential to simultaneously extract both PAs and PANOs from the samples [40]. Vaclavik et al. (2014) observed that the pH of the matrices influenced the extraction efficiency of basic analytes such as PAs and PANOs [29]. In this sense, matrices with pH values >5 did not provide acceptable recovery values, whereas in food matrices with pH values <4.5, good recoveries were achieved. For this reason, they decided to add 2% formic acid to the extraction solvent, observing an improvement in the recovery of those problematic analytes in the less acidic food matrices [29]. As the authors stated, the extraction at lower pH probably prevented or at least minimized the interaction between the analytes and the matrix through their charged functional groups. However, in contrast, Qie et al. (2021) achieved the best extraction of PAs from forage grass under basic conditions, by using a mixture of ACN with an aqueous solution of ammonium carbonate (84:16, v/v) at pH 8.5 [35]. Nonetheless, in this case, only five PAs were used as target analytes (retrorsine, senkirkine, seneciphylline, monocrotaline and senecionine), and no PANOs were included in the determination. In this work, three solvent mixtures with different pH values were tested, including ACN:aqueous solution of ammonium carbonate (200 mg/L) (84:16, v/v) with pH 8.5, methanol:phosphoric acid (0.25%) (40:60, v/v) with pH 2.2, and ACN:ammonium acetate (10 mM) (10:20, v/v) with pH 6.5. The worst results were achieved using the mixture of methanol and phosphoric acid at pH 2.2, as recovery values achieved were lower than 60% in all the targeted analytes [35]. The recoveries improved with the mixture of ACN:ammonium acetate at pH 6.5, but the best results were provided with the mixture ACN:ammonium carbonate at pH 8.5, achieving recovery values in the range of 72–96% for all the analytes [35]. Regarding the substitution of ACN as an extraction solvent, Dzuman et al. (2020) performed the QuEChERS extraction of 33 PAs and PANOs from sorghum, oregano, and mixed herbal tea samples using a combination of methanol:water:formic acid (60:39.6:0.4, v/v), achieving good analytical performance for all the compounds analyzed [33].
3.3. Modifications in the Partitioning Salts
In the original QuEChERS method, the first partitioning step via salting-out extraction was performed under unbuffered conditions, only using MgSO4 and NaCl as partitioning salts in a 4:1 ratio, respectively [17]. Over time, it has been observed that under these conditions, the determination of some analytes may be affected, as they can be sensitive to degradation at high or low pH values [15,16]. Consequently, this has led to the introduction of buffers in the QuEChERS procedure, as they can be useful to enhance the extraction efficiency of wider groups of analytes and matrices. In this sense, two main buffered procedures have been officially proposed, based on citrate or acetate buffers. Both buffered options have been extensively used and evaluated in the scientific community; thus, in consequence, they have given rise to two official methods: the CEN Standard Method EN 15,662 (the citrate buffer) [41] and the AOAC Official Method 2007.01 (the acetate buffer) [42]. The citrate buffer (relatively low buffering capacity), apart from the MgSO4 and NaCl, also includes the addition of trisodium citrate dihydrate and disodium hydrogen citrate sesquihydrate. Accordingly, the four salts have to be added in a 4:1:1:0.5 ratio (w/w) [41]. In contrast, the acetate buffer (strong buffering capacity) involves the use of MgSO4 and sodium acetate in a 6:1.5 ratio (w/w) [42]. Both buffered options keep the pH constant around 5–5.5 during the extraction procedure, which is a pH value that in general provides satisfactory recovery values (usually higher than 70%) for acid-sensitive compounds without degradation of base-sensitive compounds [15]. Other buffered systems, such as the phosphate buffer, have been proposed for more alkaline extractions [15]. However, they are not contemplated as official methods, and they have not been reported for the analysis of PAs by QuEChERS (Table 1). In the QuEChERS extraction of PAs, the most extended practice is the use of citrate buffer in the salting-out extraction step (50% of the works reviewed) (Table 1). Moreover, some authors have reduced the amount of the salts used in this step but maintaining the 4:1:1:0.5 proportion (w/w), achieving satisfactory recovery values [21,24,27,28]. In contrast, only two works (11% of the works reviewed) carried out the QuEChERS procedure for the extraction of PAs using the acetate buffer (Table 1). Leon et al. (2022) evaluated the two official buffer methods for the extraction of 28 PAs/PANOs from dried teas and herbs, and better results were achieved with the acetate buffer [25]. Likewise, Mol et al. (2011) also used the acetate buffer strategy for the extraction of 11 PAs and PANOs from food supplements, feed and honey, but reducing the amount of salts employed (4 g of MgSO4 and 1 g of sodium acetate), keeping the 6:1.5 ratio [30]. Conversely, 22% of the works reviewed performed the salting-out extraction step of PAs as in the original QuEChERS procedure, using 4 g of MgSO4 and 1 g of NaCl, also achieving good results (Table 1). Some authors have also evaluated the amount of NaCl added [22,23]. MgSO4 can promote the distribution of analytes in the organic phase mainly by absorbing water, thus retaining some undesirable polar compounds from the sample matrix, such as sugars, improving the salting-out effect of the organic extraction solvent. However, it can also decrease the response for some water-soluble compounds, such as in the case of some PAs and mainly PANOs. Conversely, NaCl controls the polarity of the extraction solvents, increasing the selectivity of the process. Accordingly, saturated aqueous solutions of NaCl would increase the solubility of the analytes in the organic phase. With this premise, Sixto et al. (2019) evaluated increasing the amount of NaCl from 1 to 2 g to promote the salting out effect with the aim of improving the recovery values of the PANOs [22]. However, the recovery values were not affected by this modification. Something similar was observed by Guo et al. (2022) [23]. In this work, the amount of NaCl added was evaluated in the range of 0.5–3 g. The response of the analytes improved when NaCl was added from 0.5 to 1 g, but it decreased when 3 g was used. Therefore, 1 g was chosen for the extraction. Moreover, the addition of MgSO4 was omitted in order to avoid reducing the response of the most water-soluble PAs and PANOs [23]. In contrast, other authors have directly decided to omit the salting-out procedure in the QuEChERS extraction of PAs to avoid these drawbacks (Table 1).
3.4. Modifications in the Clean-Up Sorbents
Concerning the second dispersive clean-up step in the QuEChERS procedure, the original procedure proposes using 25 mg of PSA and 150 mg of MgSO4 [17]. PSA removes the acid matrix interferents co-extracted during the first step of the process (such as organic polar acids, polar pigments, sugar, fatty acids) due to its weak anion exchange properties, while MgSO4 eliminates the water of the sample extract and reduces its polarity, thus leading to precipitation of polar interferents and improving their retention in the PSA sorbent [15,16,17,18]. However, PSA is sometimes not able to remove excessive interferences in complex matrices [43]. For this reason, over the years, the QuEChERS procedure has been modified by the introduction of many different types of clean-up sorbent materials to improve the extraction efficiency [15,16]. The main ones have been silica-bonded octadecylsilane (C18) and graphitized carbon black (GCB), which have been used in combination with PSA or separately [15,16]. C18 is suitable for the effective removal of non-polar interfering substances such as lipids, and its combination with PSA is indicative of a more efficient clean-up procedure of fatty and complex matrices. Conversely, GCB is particularly effective in removing pigments, namely carotenoids and chlorophyll; thus, it is suitable for vegetables (carrots, red sweet pepper, spinach, rucola, lettuce) and plant-based products (e.g., teas and aromatic herbs) [15,16,39]. However, one of the drawbacks of GCB is that it may have affinity for compounds with planar structures; thus, there is risk to loose analytes with this type of structure when this sorbent is used in the dSPE clean-up step [15,16,39]. Nonetheless, alternative sorbents are continuously being proposed to improve and broad the range of application of the QuEChERS approach. In this context, many researchers have searched for and evaluated other novel clean-up sorbents for QuEChERS, such as: multiwalled carbon nanotubes (MWCNTs), magnetic nanoparticles, zirconia-based sorbents, sol-gel organic-inorganic hybrid sorbents, organic polymers, florisil, alumina, chitosan, molecularly imprinted polymers, ordered mesostructured silicas, graphene, etc. [15,28,43,44,45,46,47,48,49,50]. Likewise, over time, the amount used of these clean-up sorbents has also been modified from the initial amounts proposed.
In the case of PAs, many different types of clean-up sorbents, as well as combinations of them, have been used in the QuEChERS procedure (Table 1). For instance, three works performed the clean-up step with the original combination of PSA and MgSO4, but modifying in some cases the original amounts (Table 1). However, no other sorbents or combinations of them were tested in these works. Conversely, other authors used the combination C18 and MgSO4 for the clean-up extraction of PAs from herbal dietary supplements, leek, wheat and tea (Table 1) [29,31]. In both works, it was observed that the C18 sorbent has great potential for the removal of non-polar co-extractants, slightly reducing matrix effects without affecting the recovery of the analytes [29,31]. These sorbents have also been used individually and combined in the QuEChERS clean-up step for the determination of PAs. In this sense, Dzuman et al. (2020) evaluated the individual use of PSA, C18 and Z-Sep as clean-up sorbents for sorghum, oregano and mixed herbal tea matrices [33]. It was concluded that 100 mg of any of these sorbents is feasible to achieve good recovery of the targeted analytes, as no recovery losses were detected [33]. Likewise, PSA has been individually used for the clean-up of honey, herbal beverages and forage grass with satisfactory results (Table 1). In these works, the authors evaluated and compared the PSA with different types of sorbents. For instance, Guo et al. (2022), compared PSA with C18, EMR (enhanced matrix removal lipid, is a sorbent that combines hydrophobic interactions and size exclusion between the long aliphatic chain of lipids and the sorbent, increasing the efficiency of lipid removal from the matrix) and two zirconia-based sorbents (Z-Sep and Z-Sep+) for the clean-up of honey and herbal beverage samples [23]. The results revealed that PSA provided better results than the other sorbents tested, although similar values were obtained with Z-Sep+ and EMR sorbents [23]. Moreover, the amount of PSA used was evaluated (25, 50, 100 mg) and compared with 50 mg PSA + 150 MgSO4. Worse results were obtained with the addition of MgSO4, while 50 mg provided the best results, although no big differences were observed among the other amounts of PSA tested [23]. In contrast, Kempf et al. (2011) only employed MgSO4 in the clean-up step for honey samples, achieving satisfactory recovery values [21]. Conversely, Qie et al. (2021) evaluated the use of 50 mg of PSA individually, and its combination with C18 (25 mg PSA + 25 mg of C18) and GCB (25 mg PSA + 25 mg GCB) for the purification of forage grass samples [35]. The best recovery values (>80%) of PAs were achieved when PSA was used individually, whereas they were lower than 81% when PSA was combined with the other two clean-up sorbents. The worst results were achieved with the combination PSA + GCB, for which recoveries were lower than 70%. This fact was attributed to the great affinity of GCB for planar structures, which some alkaloids may present [35]. Conversely, the combination of PSA, C18 and MgSO4 has been proven for clean-up of bottle tea, tea leaves and honey (Table 1). Martinello et al. (2014) evaluated three different combinations of sorbents: PSA + MgSO4, PSA + MgSO4 + C18 and PSA + MgSO4 + Chlorofiltr® (a resin sorbent used as an alternative to GCB to remove chlorophyll without loss of compounds with planar structures) [20]. The best results were achieved with the combination PSA + MgSO4 + C18 for the clean-up of honey samples. For their part, León et al. (2022) recently tried different types of clean-up sorbents (PSA, MgSO4, C18 and GBC) in varying amounts for the QuEChERS extraction of dried teas and herb samples, achieving the best results with high amounts of the four previous sorbents (400, 1200, 400 and 400 mg, respectively) [25].
Some authors have tried other less frequent clean-up sorbents for the QuEChERS determination of PAs. For instance, Kaczynski et al. (2020) first tried the combination of PSA + MgSO4 + C18 + GCB for the clean-up procedure of herb samples, achieving acceptable matrix effect values for 77% of the targeted PAs evaluated [26]. However, strong signal suppression was observed for some compounds (europine N-oxide, intermedine, jacobine N-oxide, senecivernine and senecivernine N-oxide), and recovery values were below 70% for almost the half of the compounds analyzed, mainly the PANOs. In contrast, when individually using graphene as a clean-up sorbent, the recoveries significantly improved [26], although they remained lower than 70% for some analytes (Table 1). More recently, Izcara et al. (2022) proposed for the first time the use of ordered mesostructured silicas as clean-up sorbents for the QuEChERS method in aromatic herb samples [28]. Ordered mesostructured silicas are a type of sol-gel material with advanced textural properties, such as: high surface area, controllable particle size, large pore volume, well-defined pore distribution, excellent chemical, thermal and mechanical stability, among others [51]. However, their most advantageous property is that their surface can be easily modified with many different types of ligands that can tailor their physical and chemical properties to specific applications [51]. In this sense, they can be specifically designed to display different chemical properties in adsorption processes, such as the clean-up step in the QuEChERS procedure. In this work, two ordered mesostructured silicas were prepared, one of them without surface modification (LP-MS) and other with its surface modified with amino groups (LP-MS-NH2+) [28]. Both materials were evaluated as clean-up sorbents and compared with PSA under same conditions. The results revealed that in general matrix effects were stronger with PSA than with the ordered mesostructured silicas, which suggested that the clean-up efficiency of these materials was higher than the one provided by PSA. Among the two ordered mesostructured silicas, LP-MS-NH2+ seemed to be the most effective clean-up sorbent, as fewer analytes presented matrix interferences when using this material [28].
Conversely, several authors decided to omit the clean-up step of the QuEChERs procedure to reduce the operating time or to avoid losses of analytes as observed in previous findings with SPE cartridges (Table 1). However, this may not be suitable, as this step is important to reduce matrix interferences and enhance the sensitivity and detection of the analytes. Moreover, in the QuEChERS procedure, a salting-out extraction is carried out with different salts, which are convenient to remove before chromatographic analysis and mass spectrometry detection. In this sense, when mass spectrometry is used for detection, it is not convenient to inject sample extracts without a previous clean-up or purification procedure, especially if there are matrix interferences or salts, as they can foul the ionization source and decrease the sensitivity of the equipment, leading to more frequent and thorough expensive maintenance of the detector. Likewise, the presence of salts can produce precipitation phenomena in the chromatographic column, reducing its lifetime. Moreover, the recovery values achieved in these works were in general lower than the ones achieved in the works that included the clean-up step (Table 1).
3.5. Miniaturization of the QuEChERS Procedure
Currently, one important trend in the analytical chemistry field is the miniaturization of conventional extraction methods to develop environmentally friendly methodologies, which mainly involve minimum consumption of solvents and samples in order to comply with the GAC principles [52,53,54,55,56]. Based on this, miniaturization is one of the modifications that the QuEChERS procedure has experienced in the last years [15]. For instance, several authors have proposed its miniaturization for the determination of a wide variety of analytes in a broad range of matrices, such as for the simultaneous analysis of perchlorate and bromate in fruits and vegetables [57], the determination of antibiotics in human urine and serum [58], the determination of zearalenone in cereals [59], the multicomponent extraction of phenolic compounds in baby foods [60], the analysis of psychotropic drugs in blood and serum [61], the simultaneous determination of pesticides in human serum [62] and in odonata nymphs [63], and the extraction of bisphenol A from human urine samples [64]. Regarding PAs, two works have successfully proposed the miniaturization of the QuEChERS strategy in different aromatic herb samples, including oregano, thyme, rosemary, basil and herbs de Provence [27,28]. In both works, satisfactory recovery results were achieved, and the methods were properly validated fulfilling the criteria set in the validation guidelines. Miniaturization was achieved by reducing, by ten times, the amount of sample, the volume of solvents and the amount of partitioning salts employed according to the original QuEChERS procedure (Table 1). Accordingly, for the determination of 21 PAs and PANOs in oregano samples, the miniaturized QuEChERS procedure proposed using 0.2 g of dry oregano, 1 mL of ACN as extraction solvent, 0.65 g of partitioning salts (keeping the proportion 4:1:1:0.5 of MgSO4:NaCl: trisodium citrate dihydrate:disodium hydrogen citrate sesquihydrate) and 175 mg of clean-up sorbents [27]. For thyme, basil, rosemary and herbs de Provence samples, the miniaturized QuEChERS procedure was reduced in the same way; however, a second extraction cycle with 0.5 mL of ACN before the clean-up step and an elution step with 250 µL of ACN after the clean-up step were required to improve the extraction efficiency of some analytes, leading to 0.2 g of dry herb sample, 1.75 mL of ACN as extraction solvent, 0.65 g of partitioning salts (keeping the proportion 4:1:1:0.5 of MgSO4:NaCl: trisodium citrate dihydrate:disodium hydrogen citrate sesquihydrate) and 175 mg of clean-up sorbents [28]. In comparison to the original QuEChERS method, these miniaturized procedures produce minimal waste amounts and involve considerably less sample, partitioning salts and solvent amounts. Moreover, the good analytical performance of the two procedures highlights the possibility to successfully miniaturize the QuEChERS strategy, leading to improved cost-effective and environmentally friendly microextraction methods, which meet the GAC principles.
4. Conclusions and Future Outlooks
Sample preparation is a crucial step in any analytical method involving the determination of contaminants in complex samples, such as food and feed. In this sense, although SPE is still the sample preparation technique of choice for the extraction and purification of PAs, the QuEChERS method can be a suitable alternative, as it simultaneously performs multiresidue extraction and clean-up procedures. This strategy, besides allowing for the determination of multiple analytes at the same time, has proven to be faster, simpler, cheaper and more environmentally friendly than conventional SPE, providing the same or even better extraction efficiency than this sample preparation technique. However, in the last years, the QuEChERS method has scarcely been applied to the determination of PAs. Nonetheless, due to its inherent multiple advantages and its high throughput efficiency, its application in the determination of PAs is expected to increase significantly in the forthcoming years. Moreover, the requirement established in the recent PAs legislation to carry out multiresidue analytical methods will probably contribute to this increase.
Conversely, due to the great flexibility of the QuEChERS method, it has been possible to improve and adapt its applicability to different analytes and matrices other than those initially designed in its origin, such as its application to the determination of PAs in a wide variety of food and feed samples. As it has been described, it is possible to introduce a large number of modifications in its key parameters to improve the extraction efficiency of the method depending on the sample and the analytes. In this sense, there is a wide variety of extraction solvents and clean-up sorbents that can be used individually or in combination, leading to endless options to optimize the method and achieve satisfactory results. Moreover, it has been observed that many of the works published that performed the QuEChERS extraction for the determination of PAs in food and feed samples also carried out at the same time the co-analysis of other contaminants, mainly plant toxins, pesticides, mycotoxins, drugs and phytoestrogens, with high extraction efficiency. This is interesting, as this strategy provides enough potential to monitor simultaneously in a single extraction the occurrence of different types of organic contaminants belonging to different chemical families, which involves a great advance and improvement in the food safety field.
Finally, it has been proven that it is possible to carry out the miniaturization of the QuEChERS procedure, achieving satisfactory results in the determination of PAs. Several works have demonstrated that it is possible to reduce the amounts of sample, organic solvents and partitioning salts employed without losing extraction efficiency. These features enable great reduction in time, costs and wastes, leading to the development of improved cost-effective and environmentally friendly analytical methodologies that comply with the current GAC principles. Therefore, this opens a huge research window in the coming years to focus on the development of miniaturized strategies that increase the number of analytes to be extracted and extend their applicability to other food matrices with the aim of contributing to ensure the food safety of consumers.
Author Contributions
Conceptualization, I.S. and S.M.-Z.; methodology, N.C.; software, N.C.; formal analysis, N.C.; investigation, N.C.; resources, I.S.; data curation, N.C.; writing—original draft preparation, N.C.; writing—review and editing, N.C., S.M.-Z. and I.S.; visualization, I.S. and S.M.-Z.; supervision, I.S. and S.M.-Z.; project administration, I.S.; funding acquisition, I.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by MCIU/AEI/FEDER, UE, project number RTI2018-094558-B-I00.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- European Food Safety Authority. Scientific opinion on pyrrolizidine alkaloids in food and feed. EFSA J. 2011, 9, 1–134. [Google Scholar]
- Casado, N.; Morante-Zarcero, S.; Sierra, I. The concerning food safety issue of pyrrolizidine alkaloids: An overview. Trends Food. Sci. Technol. 2022, 120, 123–139. [Google Scholar] [CrossRef]
- Schrenk, D.; Gao, L.; Lin, G.; Mahony, C.; Mulder, P.P.; Peijnenburg, A.; Pfuhler, S.; Ivonne, M.C.M.; Rietjens, I.M.C.M.; Rutz, L.; et al. Pyrrolizidine alkaloids in food and phytomedicine: Occurrence, exposure, toxicity, mechanisms, and risk assessment-A review. Food Chem. Toxicol. 2020, 136, 111107. [Google Scholar] [CrossRef] [PubMed]
- Selmar, D.; Wittke, C.; Beck-von Wolffersdorff, I.; Klier, B.; Lewerenz, L.; Kleinwächter, M.; Nowak, M. Transfer of pyrrolizidine alkaloids between living plants: A disregarded source of contaminations. Environ. Pollut. 2019, 248, 456–461. [Google Scholar] [CrossRef]
- RASFF Portal. Food and Feed Safety Alerts. Available online: https://ec.europa.eu/food/safety/rasff-food-and-feed-safety-alerts/rasff-portal_es (accessed on 11 March 2022).
- Mulder, P.P.; de Witte, S.L.; Stoopen, G.M.; van der Meulen, J.; van Wikselaar, P.G.; Gruys, E.; Groot, M.J.; Hoogenboom, R.L.A.P. Transfer of pyrrolizidine alkaloids from various herbs to eggs and meat in laying hens. Food Addit. Contam. Part A 2016, 33, 1826–1839. [Google Scholar] [CrossRef]
- Mulder, P.P.; Sánchez, P.L.; These, A.; Preiss-Weigert, A.; Castellari, M. Occurrence of pyrrolizidine alkaloids in food. EFSA Support. Publ. 2015, 12, 859E. [Google Scholar] [CrossRef]
- Mulder, P.P.; López, P.; Castelari, M.; Bodi, D.; Ronczka, S.; Preiss-Weigert, A.; These, A. Occurrence of pyrrolizidine alkaloids in animal-and plant-derived food: Results of a survey across Europe. Food Addit. Contam. Part A 2018, 35, 118–133. [Google Scholar] [CrossRef] [Green Version]
- Picron, J.F. Pyrrolizidine Alkaloids in Food…the Good, the Bad and the Ugly! Labinfo n° 18, RTF Étiqueté EndNote XML RIS. 2019. Available online: https://www.sciensano.be/fr/biblio/pyrrolizidine-alkaloids-food-good-bad-and-ugly (accessed on 4 April 2022).
- Dusemund, B.; Nowak, N.; Sommerfeld, C.; Lindtner, O.; Schäfer, B.; Lampen, A. Risk assessment of pyrrolizidine alkaloids in food of plant and animal origin. Food Chem. Toxicol. 2018, 115, 63–72. [Google Scholar] [CrossRef]
- Xu, J.; Wang, W.; Yang, X.; Xiong, A.; Yang, L.; Wang, Z. Pyrrolizidine alkaloids: An update on their metabolism and hepatotoxicity mechanism. Liver Res. 2019, 3, 176–184. [Google Scholar] [CrossRef]
- Letsyo, E.; Jerz, G.; Winterhalter, P.; Beuerle, T. Toxic pyrrolizidine alkaloids in herbal medicines commonly used in Ghana. J. Ethnopharmacol. 2017, 202, 154–161. [Google Scholar] [CrossRef]
- Rivera-Pérez, A.; Romero-González, R.; Garrido-Frenich, A. Determination and occurrence of alkenylbenzenes, pyrrolizidine and tropane alkaloids in spices, herbs, teas, and other plant-derived food products using chromatographic methods: Review from 2010–2020. Food Rev. Int. 2021, in press. [Google Scholar] [CrossRef]
- COMMISSION REGULATION (EU) 2020/2040 of 11 December 2020 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Pyrrolizidine Alkaloids in Certain Foodstuffs. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020R2040&from=ES (accessed on 11 March 2022).
- Perestrelo, R.; Silva, P.; Porto-Figueira, P.; Pereira, J.A.; Silva, C.; Medina, S.; Câmara, J.S. QuEChERS-Fundamentals, relevant improvements, applications and future trends. Anal. Chim. Acta 2019, 1070, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Bruzzoniti, M.C.; Checchini, L.; De Carlo, R.M.; Orlandini, S.; Rivoira, L.; Del Bubba, M. QuEChERS sample preparation for the determination of pesticides and other organic residues in environmental matrices: A critical review. Anal. Bioanal. Chem. 2014, 406, 4089–4116. [Google Scholar] [CrossRef] [PubMed]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [Green Version]
- Lehotay, S.J.; Kok, A.D.; Hiemstra, M.; Bodegraven, P.V. Validation of a fast and easy method for the determination of residues from 229 pesticides in fruits and vegetables using gas and liquid chromatography and mass spectrometric detection. J. AOAC Int. 2005, 88, 595–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinello, M.; Borin, A.; Stella, R.; Bovo, D.; Biancotto, G.; Gallina, A.; Mutinelli, F. Development and validation of a QuEChERS method coupled to liquid chromatography and high resolution mass spectrometry to determine pyrrolizidine and tropane alkaloids in honey. Food Chem. 2017, 234, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Martinello, M.; Cristofoli, C.; Gallina, A.; Mutinelli, F. Easy and rapid method for the quantitative determination of pyrrolizidine alkaloids in honey by ultra performance liquid chromatography-mass spectrometry: An evaluation in commercial honey. Food Control 2014, 37, 146–152. [Google Scholar] [CrossRef]
- Kempf, M.; Wittig, M.; Reinhard, A.; Von der Ohe, K.; Blacquière, T.; Raezke, K.P.; Michel, R.; Schreier, P.; Beuerle, T. Pyrrolizidine alkaloids in honey: Comparison of analytical methods. Food Addit. Contam. Part A 2011, 28, 332–347. [Google Scholar] [CrossRef] [Green Version]
- Sixto, A.; Niell, S.; Heinzen, H. Straightforward determination of pyrrolizidine alkaloids in honey through simplified methanol extraction (QuPPE) and LC-MS/MS modes. ACS Omega 2019, 4, 22632–22637. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.; Yang, Y.; Li, J.; Shao, B.; Zhang, J. Screening for plant toxins in honey and herbal beverage by ultrahigh-performance liquid chromatography-ion mobility-quadrupole time of flight mass spectrometry. Am. J. Anal. Chem. 2022, 13, 108–134. [Google Scholar] [CrossRef]
- Takatsuji, Y.; Kakitani, A.; Nagatomi, Y.; Harayama, K.; Suzuki, K. A novel method for the detection of pyrrolizidine alkaloids in bottled tea and tea leaves by LC-MS/MS. Jpn. J. Food Chem. Saf. 2018, 25, 97–104. [Google Scholar]
- León, N.; Miralles, P.; Yusà, V.; Coscollà, C. A green analytical method for the simultaneous determination of 30 tropane and pyrrolizidine alkaloids and their N-oxides in teas and herbs for infusions by LC-Q-Orbitrap HRMS. J. Chromatogr. A 2022, 1666, 462835. [Google Scholar] [CrossRef] [PubMed]
- Kaczyński, P.; Łozowicka, B. A novel approach for fast and simple determination pyrrolizidine alkaloids in herbs by ultrasound-assisted dispersive solid phase extraction method coupled to liquid chromatography–tandem mass spectrometry. J. Pharm. Biomed. Anal. 2020, 187, 113351. [Google Scholar] [CrossRef] [PubMed]
- Izcara, S.; Casado, N.; Morante-Zarcero, S.; Sierra, I. A miniaturized QuEChERS method combined with ultrahigh liquid chromatography coupled to tandem mass spectrometry for the analysis of pyrrolizidine alkaloids in oregano samples. Foods 2020, 9, 1319. [Google Scholar] [CrossRef]
- Izcara, S.; Casado, N.; Morante-Zarcero, S.; Pérez-Quintanilla, D.; Sierra, I. Miniaturized and modified QuEChERS method with mesostructured silica as clean-up sorbent for pyrrolizidine alkaloids determination in aromatic herbs. Food Chem. 2022, 380, 132189. [Google Scholar] [CrossRef]
- Vaclavik, L.; Krynitsky, A.J.; Rader, J.I. Targeted analysis of multiple pharmaceuticals, plant toxins and other secondary metabolites in herbal dietary supplements by ultra-high performance liquid chromatography–quadrupole-orbital ion trap mass spectrometry. Anal. Chim. Acta 2014, 810, 45–60. [Google Scholar] [CrossRef]
- Mol, H.G.J.; Van Dam, R.C.J.; Zomer, P.; Mulder, P.P. Screening of plant toxins in food, feed and botanicals using full-scan high-resolution (Orbitrap) mass spectrometry. Food Addit. Contam. Part A 2011, 28, 1405–1423. [Google Scholar] [CrossRef]
- Dzuman, Z.; Zachariasova, M.; Veprikova, Z.; Godula, M.; Hajslova, J. Multianalyte high performance liquid chromatography coupled to high resolution tandem mass spectrometry method for control of pesticide residues, mycotoxins, and pyrrolizidine alkaloids. Anal. Chim. Acta 2015, 863, 29–40. [Google Scholar] [CrossRef]
- Bessaire, T.; Ernest, M.; Christinat, N.; Carrères, B.; Panchaud, A.; Badoud, F. High resolution mass spectrometry workflow for the analysis of food contaminants: Application to plant toxins, mycotoxins and phytoestrogens in plant-based ingredients. Food Addit. Contam. Part A 2021, 38, 978–996. [Google Scholar] [CrossRef]
- Dzuman, Z.; Jonatova, P.; Stranska-Zachariasova, M.; Prusova, N.; Brabenec, O.; Novakova, A.; Fenclova, M.; Hajslova, J. Development of a new LC-MS method for accurate and sensitive determination of 33 pyrrolizidine and 21 tropane alkaloids in plant-based food matrices. Anal. Bioanal. Chem. 2020, 412, 7155–7167. [Google Scholar] [CrossRef]
- Martinello, M.; Manzinello, C.; Gallina, A.; Mutinelli, F. In-house validation and application of UHPLC-MS/MS method for the quantification of pyrrolizidine and tropane alkaloids in commercial honey bee-collected pollen, teas and herbal infusions purchased on Italian market in 2019–2020 referring to recent European Union regulations. Int. J. Food Sci. Technol. 2022, in press. [Google Scholar]
- Qie, M.; Li, S.; Guo, C.; Yang, S.; Zhao, Y. Study of the occurrence of toxic alkaloids in forage grass by liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2021, 1654, 462463. [Google Scholar] [CrossRef]
- Bolechová, M.; Čáslavský, J.; Pospíchalová, M.; Kosubová, P. UPLC–MS/MS method for determination of selected pyrrolizidine alkaloids in feed. Food Chem. 2015, 170, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Mandić, B.M.; Vlajić, M.D.; Trifunović, S.S.; Simić, M.R.; Vujisić, L.V.; VuČković, I.M.; Novaković, M.M.; Nikolić-Mandić, S.D.; Tešević, V.V.; Vajs, V.V.; et al. Optimisation of isolation procedure for pyrrolizidine alkaloids from Rindera umbellata Bunge. Nat. Prod. Res. 2015, 29, 887–890. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Liu, Y.; Zhu, L.; Ji, H.; Song, X.; Guo, H.; Yi, T. Determination and regulation of hepatotoxic pyrrolizidine alkaloids in food: A critical review of recent research. Food Chem. Toxicol. 2018, 119, 50–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- QuEChERS CVUA Stuttgart. A Mini-Multiresidue Method for the Analysis of Pesticide Residues in Low-Fat Products. Available online: https://www.quechers.com/pdf/reality.pdf (accessed on 4 April 2022).
- Crews, C.; Berthiller, F.; Krska, R. Update on analytical methods for toxic pyrrolizidine alkaloids. Anal. Bioanal. Chem. 2010, 396, 327–338. [Google Scholar] [CrossRef]
- UNE-EN 15662:2009. Foods of Plant Origin—Determination of Pesticide Residues Using GC-MS and/or LC-MS/MS Following Acetonitrile Extraction/Partitioning and Clean-Up by Dispersive SPE—QuEChERS-Method. 2009. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0043515 (accessed on 4 April 2022).
- AOAC Official Method 2007.01 Pesticide Residues in Foods by Acetonitrile Extraction and Partitioning with Magnesium Sulfate. 2007. Available online: https://nucleus.iaea.org/sites/fcris/Shared%20Documents/SOP/AOAC_2007_01.pdf (accessed on 4 April 2022).
- Oellig, C.; Schmid, S. Polyethyleneimine as weak anionic exchanger adsorbent for clean-up in pesticide residue analysis of fruits and vegetables. J. Chromatogr. A 2019, 1597, 9–17. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, L.; Zhou, L.; Zhang, F.; Kang, S.; Pan, C. Multi-walled carbon nanotubes as alternative reversed-dispersive solid phase extraction materials in pesticide multi-residue analysis with QuEChERS method. J. Chromatogr. A 2012, 1225, 17–25. [Google Scholar] [CrossRef]
- Han, Y.; Zou, N.; Song, L.; Li, Y.; Qin, Y.; Liu, S.; Li, X.; Pan, C. Simultaneous determination of 70 pesticide residues in leek, leaf lettuce and garland chrysanthemum using modified QuEChERS method with multi-walled carbon nanotubes as reversed-dispersive solid-phase extraction materials. J. Chromatogr. B 2015, 1005, 56–64. [Google Scholar] [CrossRef]
- Uclés, A.; López, S.H.; Hernando, M.D.; Rosal, R.; Ferrer, C.; Fernández-Alba, A.R. Application of zirconium dioxide nanoparticle sorbent for the clean-up step in post-harvest pesticide residue analysis. Talanta 2015, 144, 51–61. [Google Scholar] [CrossRef]
- Li, Y.F.; Qiao, L.Q.; Li, F.W.; Ding, Y.; Yang, Z.J.; Wang, M.L. Determination of multiple pesticides in fruits and vegetables using a modified quick, easy, cheap, effective, rugged and safe method with magnetic nanoparticles and gas chromatography tandem mass spectrometry. J. Chromatogr. A 2014, 1361, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.B.; Ding, J.; Zheng, S.J.; Yu, Q.W.; Yuan, B.F.; Feng, Y.Q. Magnetic “one-step” quick, easy, cheap, effective, rugged and safe method for the fast determination of pesticide residues in freshly squeezed juice. J. Chromatogr. A 2015, 1398, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.; Lesueur, C. Comparing d-SPE sorbents of the QuEChERS extraction method and EMR-lipid for the determination of polycyclic aromatic hydrocarbons (PAH4) in food of animal and plant origin. Food Anal. Methods 2017, 10, 2111–2124. [Google Scholar] [CrossRef]
- Omar, M.M.A.; Ibrahim, W.A.W.; Elbashir, A.A. Sol–gel hybrid methyltrimethoxysilane–tetraethoxysilane as a new dispersive solid-phase extraction material for acrylamide determination in food with direct gas chromatography–mass spectrometry analysis. Food Chem. 2014, 158, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Casado, N.; Pérez-Quintanilla, D.; Morante-Zarcero, S.; Sierra, I. Current development and applications of ordered mesoporous silicas and other sol gel silica-based materials in food sample preparation for xenobiotics analysis. Trends Anal. Chem. 2017, 88, 167–184. [Google Scholar] [CrossRef]
- Casado, N.; Morante-Zarcero, S.; Pérez-Quintanilla, D.; Câmara, J.S.; Sierra, I. Two novel strategies in food sample preparation for the analysis of dietary polyphenols: Micro-extraction techniques and new silica-based sorbent materials. Trends Food Sci. Technol. 2020, 98, 167–180. [Google Scholar] [CrossRef]
- Casado, N.; Gañán, J.; Morante-Zarcero, S.; Sierra, I. New advanced materials and sorbent-based microextraction techniques as strategies in sample preparation to improve the determination of natural toxins in food samples. Molecules 2020, 25, 702. [Google Scholar] [CrossRef] [Green Version]
- Pereira, J.A.M.; Casado, N.; Porto-Figueira, P.; Câmara, J.S. The Potential of microextraction techniques for the analysis of bioactive compounds. Food Front. Nutr. 2022, 9, 825519. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Szczepańska, N.; Owczarek, K.; Namieśnik, J. Miniaturized solid phase extraction. In Green Extraction Techniques: Principles, Advances and Applications; Ibañez, E., Cifuentes, A., Eds.; Comprehensive Analytical Chemistry Elsevier: Amsterdam, The Netherlands, 2017; Volume 76, pp. 279–318. [Google Scholar]
- Filippou, O.; Bitas, D.; Samanidou, V. Green approaches in sample preparation of bioanalytical samples prior to chromatographic analysis. J. Chromatogr. B 2017, 1043, 44–62. [Google Scholar] [CrossRef]
- Dong, H.; Xiao, K.; Xian, Y.; Wu, Y.; Zhu, L. A novel approach for simultaneous analysis of perchlorate (ClO4−) and bromate (BrO3−) in fruits and vegetables using modified QuEChERS combined with ultrahigh performance liquid chromatography-tandem mass spectrometry. Food Chem. 2019, 270, 196–203. [Google Scholar] [CrossRef]
- Muhammad, N.; Rahman, A.; Younis, M.A.; Subhani, Q.; Shehzad, K.; Cui, H.; Zhu, Y. Porous SnO2 nanoparticles based ion chromatographic determination of non-fluorescent antibiotic (chloramphenicol) in complex samples. Sci. Rep. 2018, 8, 12327. [Google Scholar] [CrossRef] [PubMed]
- Porto-Figueira, P.; Camacho, I.; Câmara, J.S. Exploring the potentialities of an improved ultrasound-assisted quick, easy, cheap, effective, rugged, and safe-based extraction technique combined with ultrahigh pressure liquid chromatography fluorescence detection for determination of Zearalenone in cereals. J. Chromatogr. A 2015, 1408, 187–196. [Google Scholar] [PubMed]
- Casado, N.; Perestrelo, R.; Silva, C.L.; Sierra, I.; Câmara, J.S. An improved and miniaturized analytical strategy based on μ-QuEChERS for isolation of polyphenols. A powerful approach for quality control of baby foods. Microchem. J. 2018, 139, 110–118. [Google Scholar] [CrossRef]
- Pouliopoulos, A.; Tsakelidou, E.; Krokos, A.; Gika, H.G.; Theodoridis, G.; Raikos, N. Quantification of 15 psychotropic drugs in serum and postmortem blood samples after a modified mini-QuEChERS by UHPLC–MS-MS. J. Anal. Toxicol. 2018, 42, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Lee, J.; Lee, J.; Lee, J.; Kim, E.; Liu, K.H.; Lee, H.S.; Kim, J.H. Validation of a multiresidue analysis method for 379 pesticides in human serum using liquid chromatography–tandem mass spectrometry. J. Agric. Food Chem. 2018, 66, 3550–3560. [Google Scholar] [CrossRef]
- Jesús, F.; Hladki, R.; Gérez, N.; Besil, N.; Niell, S.; Fernández, G.; Heinzen, H.; Cesio, M.V. Miniaturized QuEChERS based methodology for multiresidue determination of pesticides in odonate nymphs as ecosystem biomonitors. Talanta 2018, 178, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Correia-Sá, L.; Norberto, S.; Delerue-Matos, C.; Calhau, C.; Domingues, V.F. Micro-QuEChERS extraction coupled to GC–MS for a fast determination of Bisphenol A in human urine. J. Chromatogr. B 2018, 1072, 9–16. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).