High-Pressure Technologies for the Recovery of Bioactive Molecules from Agro-Industrial Waste
Abstract
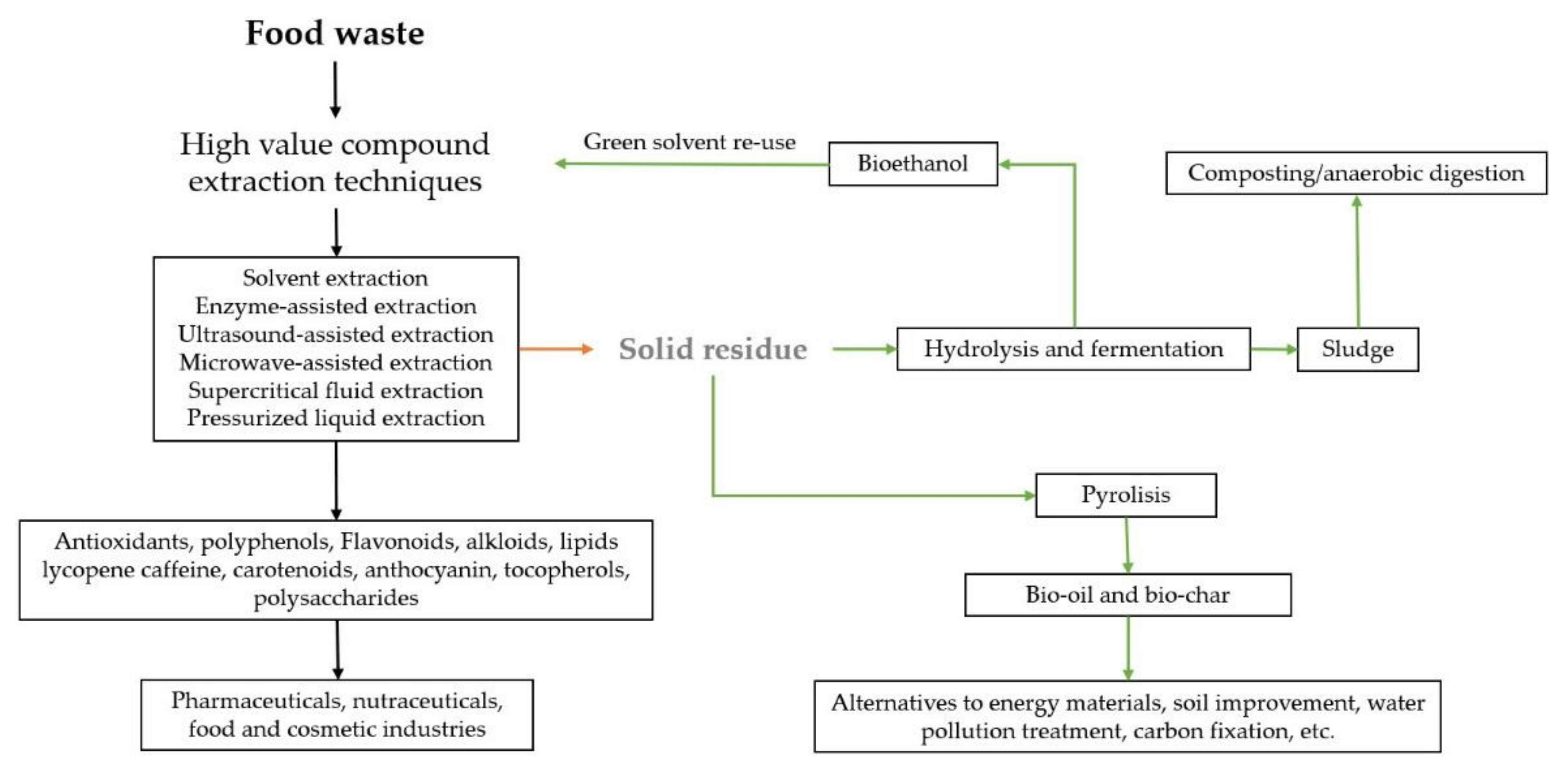
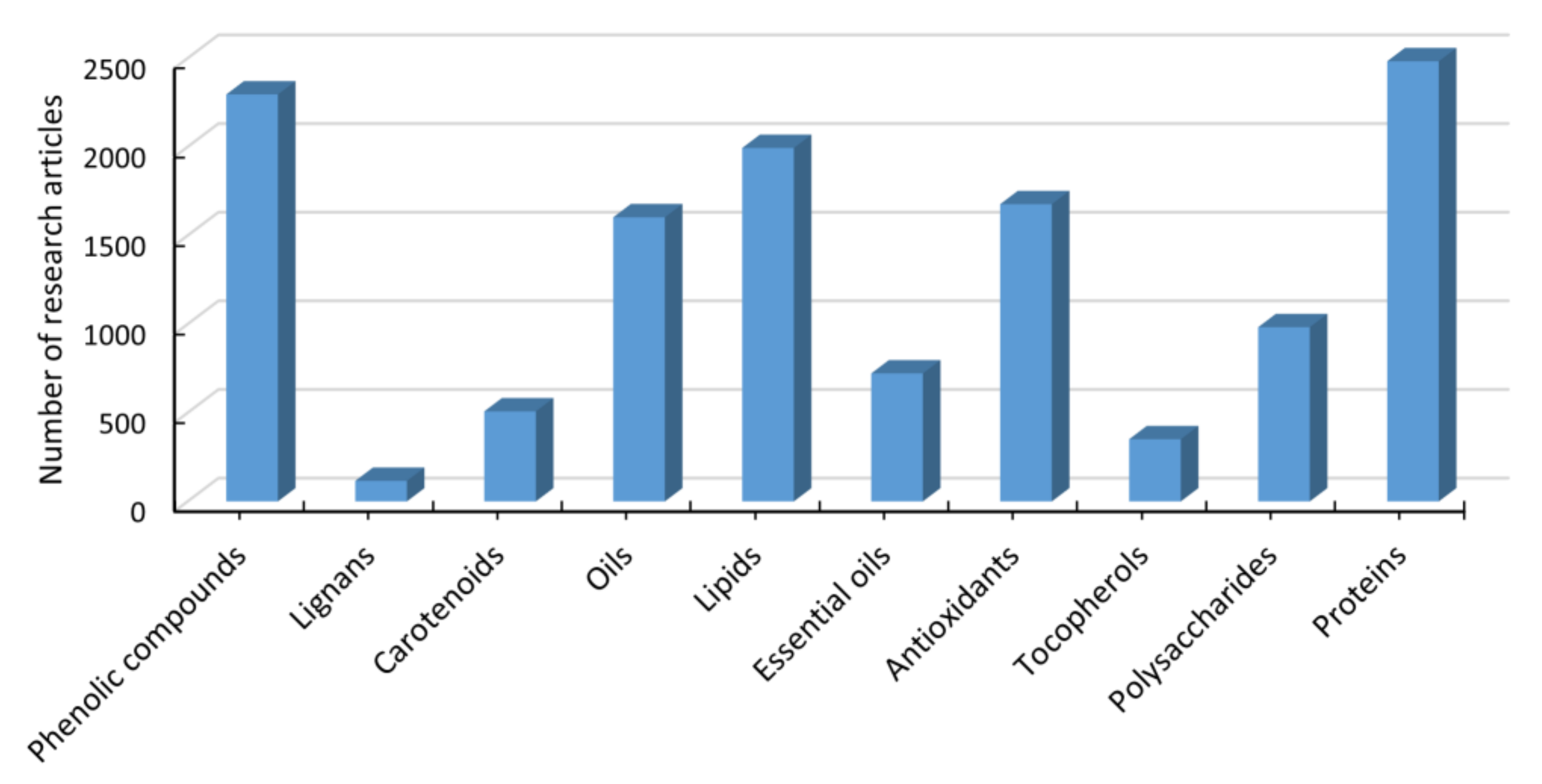
:1. Introduction
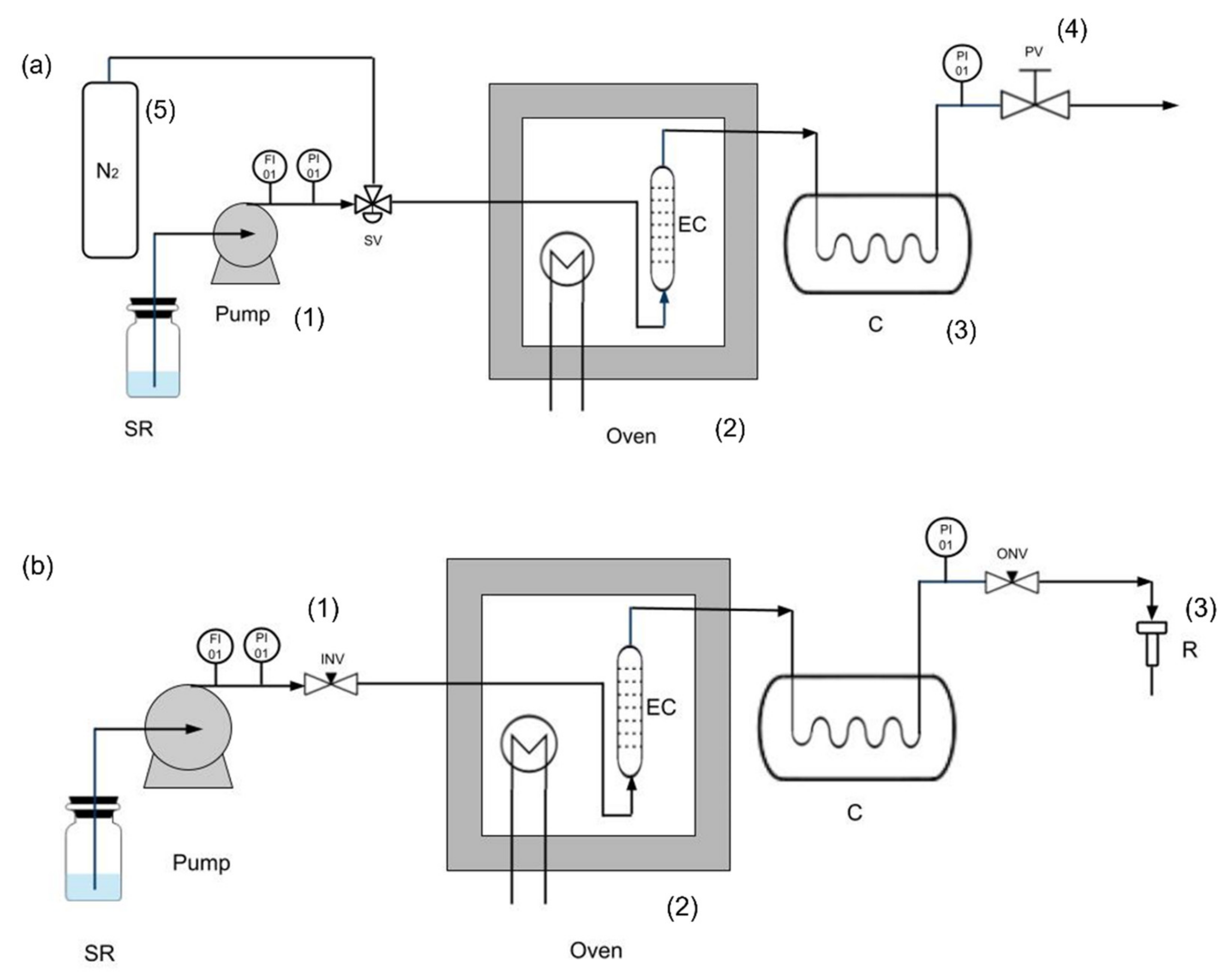
2. Pressurized Liquid Extraction Processes
2.1. Effect of Operative Parameters
2.2. PLE of Bioactive Compounds from Waste
2.2.1. Proteins
2.2.2. Polysaccharides
2.2.3. Lipids
2.2.4. Polyphenolic Compounds
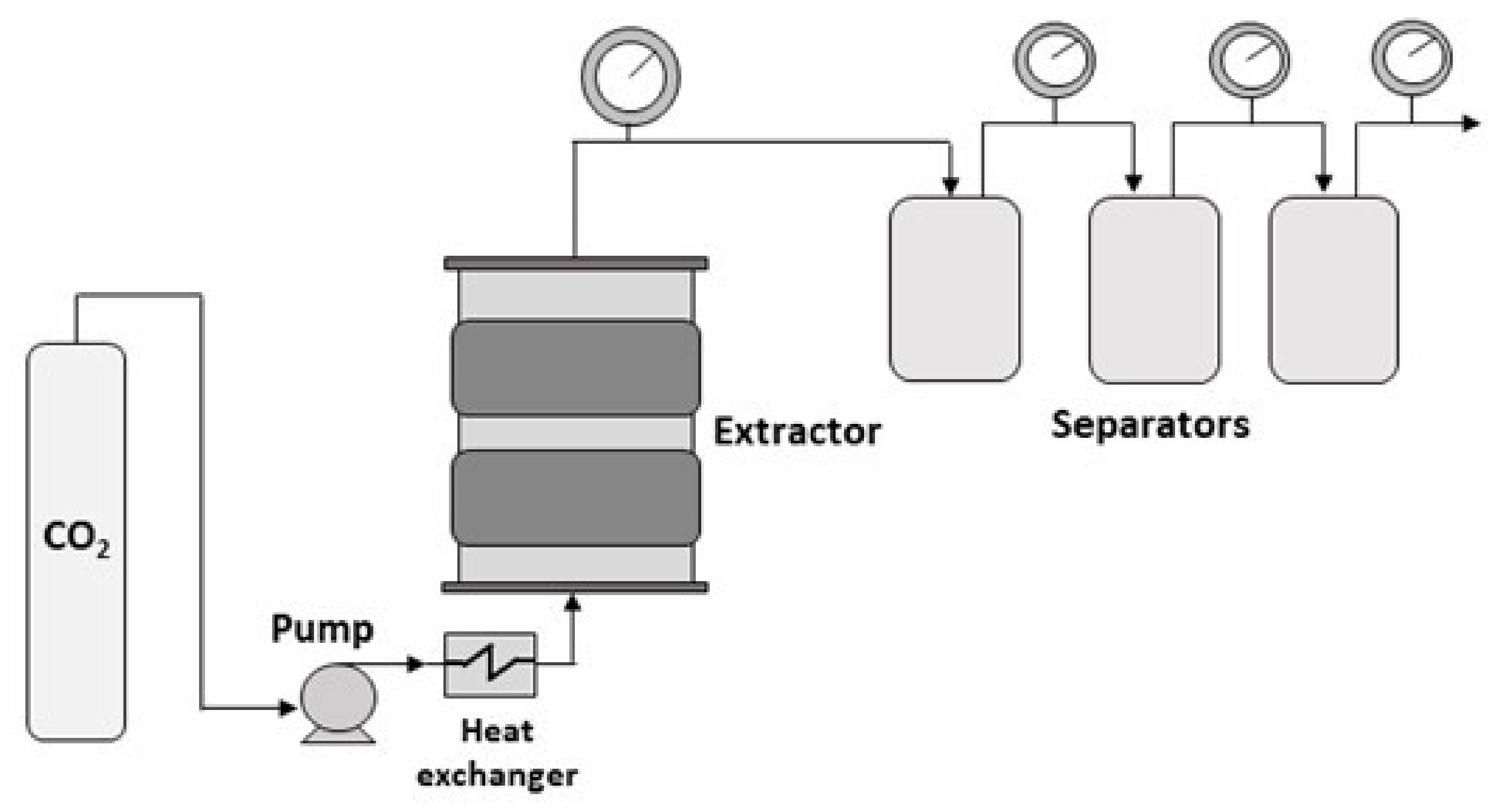
3. Supercritical Fluid Extraction
3.1. Effect of the Operating Parameters
3.2. SFE of Bioactive Compounds from Waste
3.2.1. Lipids
3.2.2. Carotenoids
4. Combined Extractions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beltrán-Ramírez, F.; Orona-Tamayo, D.; Cornejo-Corona, I.; González-Cervantes, J.L.N.; de Jesús Esparza-Claudio, J.; Quintana-Rodríguez, E. Agro-Industrial Waste Revalorization: The Growing Biorefinery. In Biomass Bioenergy-Recent Trends Future Challenges; Abomohra, A., Ed.; IntechOpen: London, UK, 2019; pp. 83–103. [Google Scholar] [CrossRef] [Green Version]
- Fierascu, R.C.; Fierascu, I.; Avramescu, S.M.; Sieniawska, E. Recovery of Natural Antioxidants from Agro-Industrial Side Streams through Advanced Extraction Techniques. Molecules 2019, 24, 4212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, D.T.V.; Zabot, G.L.; Reyes, F.G.R.; Iglesias, A.H.; Martínez, J. Integration of pressurized liquids and ultrasound in the extraction of bioactive compounds from passion fruit rinds: Impact on phenolic yield, extraction kinetics and technical-economic evaluation. Innov. Food Sci. Emerg. Technol. 2021, 67, 102549. [Google Scholar] [CrossRef]
- Morini, S. Global Food Losses and Food Waste—Extent, Causes and Prevention; Food and Agriculture Organization of the United Nations: Roma, Italy, 2011; p. 37. [Google Scholar]
- Pettinato, M. Spent Coffee Grounds Valorization by Green and Innovative Extraction Technologies: Process Optimization and Product Stabilization for Industrial Purposes; University of Genoa: Genova, Italy, 2019. [Google Scholar]
- Belwal, T.; Chemat, F.; Venskutonis, P.R.; Cravotto, G.; Jaiswal, D.K.; Bhatt, I.D.; Devkota, H.P.; Luo, Z. Recent advances in scaling-up of non-conventional extraction techniques: Learning from successes and failures. TrAC Trends Anal. Chem. 2020, 127, 115895. [Google Scholar] [CrossRef]
- Khaw, K.Y.; Parat, M.O.; Shaw, P.N.; Falconer, J.R. Solvent Supercritical Fluid Technologies to Extract Bioactive Compounds from Natural Sources: A Review. Molecules 2017, 22, 1186. [Google Scholar] [CrossRef]
- Andreu, V.; Picó, Y. Pressurized liquid extraction of organic contaminants in environmental and food samples. TrAC Trends Anal. Chem. 2019, 118, 709–721. [Google Scholar] [CrossRef]
- Carabias-Martinez, R.; Rodriguez-Gonzalo, E.; Revilla-Ruiz, P.; Hernandez-Mendez, J. Pressurized liquid extraction in the analysis of food and biological samples. J. Chromatogr. A 2005, 1089, 1–17. [Google Scholar] [CrossRef]
- Dobrincic, A.; Balbino, S.; Zoric, Z.; Pedisic, S.; Bursac Kovacevic, D.; Elez Garofulic, I.; Dragovic-Uzelac, V. Advanced Technologies for the Extraction of Marine Brown Algal Polysaccharides. Mar. Drugs 2020, 18, 168. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef]
- Richter, B.E.; Jones, B.A.; Ezzell, J.L.; Porter, N.L. Accelerated Solvent Extraction: A Technique for Sample Preparation. Angew. Chem. 1996, 68, 1033–1039. [Google Scholar] [CrossRef]
- Hawthorne, S.B.; Grabanski, C.B.; Martin, E.; Miller, D.J. Comparisons of Soxhlet extraction, pressurized liquid extraction, supercritical fluid extraction and subcritical water extraction for environmental solids: Recovery, selectivity and effects on sample matrix. J. Chromatogr. A 2000, 892, 421–433. [Google Scholar] [CrossRef]
- Schantz, M.M. Pressurized liquid extraction in environmental analysis. Anal. Bioanal. Chem. 2006, 386, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, L.; Williams, E.S.; Brooks, B.W.; Usenko, S. Development and application of a novel method for high-throughput determination of PCDD/Fs and PCBs in sediments. Environ. Toxicol. Chem. 2014, 33, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.H.; Kammers, J.C.; Silva, A.P.; Oliveira, J.V.; Hense, H. Antioxidant and antibacterial compounds from feijoa leaf extracts obtained by pressurized liquid extraction and supercritical fluid extraction. Food Chem. 2021, 344, 128620. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; del Pilar Sánchez-Camargo, A.; Cifuentes, A.; Ibáñez, E. Plants, seaweeds, microalgae and food by-products as natural sources of functional ingredients obtained using pressurized liquid extraction and supercritical fluid extraction. TrAC Trends Anal. Chem. 2015, 71, 26–38. [Google Scholar] [CrossRef]
- Ruiz-Domínguez, M.C.; Fuentes, J.L.; Mendiola, J.A.; Cerezal-Mezquita, P.; Morales, J.; Vílchez, C.; Ibáñez, E. Bioprospecting of cyanobacterium in Chilean coastal desert, Geitlerinema sp. molecular identification and pressurized liquid extraction of bioactive compounds. Food Bioprod. Process. 2021, 128, 227–239. [Google Scholar] [CrossRef]
- Gfrerer, M.; Chen, S.; Lankmayr, E.P.; Quan, X.; Yang, F. Comparison of different extraction techniques for the determination of chlorinated pesticides in animal feed. Anal. Bioanal. Chem. 2004, 378, 1861–1867. [Google Scholar] [CrossRef]
- Celeiro, M.; Llompart, M.; Lamas, J.P.; Lores, M.; Garcia-Jares, C.; Dagnac, T. Determination of fungicides in white grape bagasse by pressurized liquid extraction and gas chromatography tandem mass spectrometry. J. Chromatogr. A 2014, 1343, 18–25. [Google Scholar] [CrossRef]
- Sumere, B.R.; de Souza, M.C.; Dos Santos, M.P.; Bezerra, R.M.N.; da Cunha, D.T.; Martinez, J.; Rostagno, M.A. Combining pressurized liquids with ultrasound to improve the extraction of phenolic compounds from pomegranate peel (Punica granatum L.). Ultrason. Sonochem. 2018, 48, 151–162. [Google Scholar] [CrossRef]
- Santos, M.P.; Souza, M.C.; Sumere, B.R.; da Silva, L.C.; Cunha, D.T.; Bezerra, R.M.N.; Rostagno, M.A. Extraction of bioactive compounds from pomegranate peel (Punica granatum L.) with pressurized liquids assisted by ultrasound combined with an expansion gas. Ultrason. Sonochem. 2019, 54, 11–17. [Google Scholar] [CrossRef]
- Rostagno, M.A.; Palma, M.; Barroso, C.G. Pressurized liquid extraction of isoflavones from soybeans. Anal. Chim. Acta 2004, 522, 169–177. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, P.; Li, S.P.; Wang, Y.T.; Tu, P.F. Optimization of pressurized liquid extraction of five major flavanoids from Lysimachia clethroide. J. Pharm. Biomed. Anal. 2007, 43, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Barriada-Pereira, M.; Gonzalez-Castro, M.J.; Muniategui-Lorenzo, S.; Lopez-Mahia, P.; Prada-Rodriguez, D.; Fernandez-Fernandez, E. Comparison of pressurized liquid extraction and microwave assisted extraction for the determination of organochlorine pesticides in vegetables. Talanta 2007, 71, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Kawasaki, T.; Takemura, A.; Fukutsu, N.; Kishi, N.; Kusu, F. Identification of degradation products in loxoprofen sodium adhesive tapes by liquid chromatography-mass spectrometry and dynamic pressurized liquid extraction-solid-phase extraction coupled to liquid chromatography-nuclear magnetic resonance spectroscopy. J. Chromatogr. A 2008, 1208, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Luque-García, J.L.; Luque de Castro, M.D. Comparison of the static, dynamic and static-dynamic pressurised liquid extraction modes for the removal of nitrated polycyclic aromatic hydrocarbons from soil with on-line filtration-preconcentration. J. Chromatogr. A 2003, 1010, 129–140. [Google Scholar] [CrossRef]
- Pitipanapong, J.; Chitprasert, S.; Goto, M.; Jiratchariyakul, W.; Sasaki, M.; Shotipruk, A. New approach for extraction of charantin from Momordica charantia with pressurized liquid extraction. Sep. Purif. Technol. 2007, 52, 416–422. [Google Scholar] [CrossRef]
- Wicker, A.P.; Carlton, D.D., Jr.; Tanaka, K.; Nishimura, M.; Chen, V.; Ogura, T.; Hedgepeth, W.; Schug, K.A. On-line supercritical fluid extraction-supercritical fluid chromatography-mass spectrometry of polycyclic aromatic hydrocarbons in soil. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1086, 82–88. [Google Scholar] [CrossRef]
- Naviglio, D. Naviglio’s Principle and Presentation of an Innovative Solid–Liquid Extraction Technology: Extractor Naviglio®. Anal. Lett. 2003, 36, 1647–1659. [Google Scholar] [CrossRef]
- Naviglio, D.; Pizzolongo, F.; Ferrara, L.; Aragòn, A.; Santini, A. Extraction of pure lycopene from industrial tomato by-products in water using a new high-pressure process. J. Sci. Food Agric. 2008, 88, 2414–2420. [Google Scholar] [CrossRef]
- Aliakbarian, B.; Casazza, A.A.; Perego, P. Valorization of olive oil solid waste using high pressure–high temperature reactor. Food Chem. 2011, 128, 704–710. [Google Scholar] [CrossRef]
- Pettinato, M.; Trucillo, P.; Campardelli, R.; Perego, P.; Reverchon, E. Bioactives extraction from spent coffee grounds and liposome encapsulation by a combination of green technologies. Chem. Eng. Process.-Process Intensif. 2020, 151, 107911. [Google Scholar] [CrossRef]
- Hu, W.; Chen, S.; Wu, D.; Zhu, K.; Ye, X. Manosonication assisted extraction and characterization of pectin from different citrus peel wastes. Food Hydrocoll. 2021, 121, 106952. [Google Scholar] [CrossRef]
- Chienthavorn, O.; Insuan, W. Superheated Water Extraction of Lime Peel: A Comparison with Conventional Methods. Anal. Lett. 2004, 37, 2393–2409. [Google Scholar] [CrossRef]
- Ding, W.-H.; Fann, J.C.H. Determination of linear alkylbenzenesulfonates in sediments using pressurized liquid extraction and ion-pair derivatization gas chromatography-mass spectrometry. Anal. Chim. Acta 2000, 408, 291–297. [Google Scholar] [CrossRef]
- Herrera, M.C.; Prados-Rosales, R.C.; Luque-García, J.L.; Luque de Castro, M.D. Static–dynamic pressurized hot water extraction coupled to on-line filtration–solid-phase extraction–high-performance liquid chromatography–post-column derivatization–fluorescence detection for the analysis of N-methylcarbamates in foods. Anal. Chim. Acta 2002, 463, 189–197. [Google Scholar] [CrossRef]
- Dobrincic, A.; Pedisic, S.; Zoric, Z.; Jurin, M.; Roje, M.; Coz-Rakovac, R.; Dragovic-Uzelac, V. Microwave Assisted Extraction and Pressurized Liquid Extraction of Sulfated Polysaccharides from Fucus virsoides and Cystoseira barbata. Foods 2021, 10, 1481. [Google Scholar] [CrossRef]
- Vazquez-Roig, P.; Picó, Y. Pressurized liquid extraction of organic contaminants in environmental and food samples. TrAC Trends Anal. Chem. 2015, 71, 55–64. [Google Scholar] [CrossRef]
- Herbst, G.; Hamerski, F.; Errico, M.; Corazza, M.L. Pressurized liquid extraction of brewer’s spent grain: Kinetics and crude extracts characterization. J. Ind. Eng. Chem. 2021, 102, 370–383. [Google Scholar] [CrossRef]
- Pronyk, C.; Mazza, G. Design and scale-up of pressurized fluid extractors for food and bioproducts. J. Food Eng. 2009, 95, 215–226. [Google Scholar] [CrossRef]
- Osorio-Tobón, J.F.; Carvalho, P.I.N.; Rostagno, M.A.; Petenate, A.J.; Meireles, M.A.A. Extraction of curcuminoids from deflavored turmeric (Curcuma longa L.) using pressurized liquids: Process integration and economic evaluation. J. Supercrit. Fluids 2014, 95, 167–174. [Google Scholar] [CrossRef]
- Nathia-Neves, G.; Tarone, A.G.; Tosi, M.M.; Marostica Junior, M.R.; Meireles, M.A.A. Extraction of bioactive compounds from genipap (Genipa americana L.) by pressurized ethanol: Iridoids, phenolic content and antioxidant activity. Food Res. Int. 2017, 102, 595–604. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, S.; Wang, Y.; Li, L. Screening solvents to extract phenol from aqueous solutions by the COSMO-SAC model and extraction process simulation. Fluid Phase Equilibria 2017, 451, 12–24. [Google Scholar] [CrossRef]
- Aliakbarian, B.; Fathi, A.; Perego, P.; Dehghani, F. Extraction of antioxidants from winery wastes using subcritical water. J. Supercrit. Fluids 2012, 65, 18–24. [Google Scholar] [CrossRef]
- Nastić, N.; Švarc-Gajić, J.; Delerue-Matos, C.; Morais, S.; Barroso, M.F.; Moreira, M.M. Subcritical water extraction of antioxidants from mountain germander (Teucrium montanum L.). J. Supercrit. Fluids 2018, 138, 200–206. [Google Scholar] [CrossRef] [Green Version]
- da Silva, M.F.; Casazza, A.A.; Ferrari, P.F.; Aliakbarian, B.; Converti, A.; Bezerra, R.P.; Porto, A.L.F.; Perego, P. Recovery of phenolic compounds of food concern from Arthrospira platensis by green extraction techniques. Algal Res. 2017, 25, 391–401. [Google Scholar] [CrossRef]
- Pettinato, M.; Casazza, A.A.; Ferrari, P.F.; Palombo, D.; Perego, P. Eco-sustainable recovery of antioxidants from spent coffee grounds by microwave-assisted extraction: Process optimization, kinetic modeling and biological validation. Food Bioprod. Process. 2019, 114, 31–42. [Google Scholar] [CrossRef]
- Li, J.; Pettinato, M.; Casazza, A.A.; Perego, P. A Comprehensive Optimization of Ultrasound-Assisted Extraction for Lycopene Recovery from Tomato Waste and Encapsulation by Spray Drying. Processes 2022, 10, 308. [Google Scholar] [CrossRef]
- Nunes, A.N.; Roda, A.; Gouveia, L.F.; Fernández, N.; Bronze, M.R.; Matias, A.A. Astaxanthin Extraction from Marine Crustacean Waste Streams: An Integrate Approach between Microwaves and Supercritical Fluids. ACS Sustain. Chem. Eng. 2021, 9, 3050–3059. [Google Scholar] [CrossRef]
- Roy, V.C.; Getachew, A.T.; Cho, Y.-J.; Park, J.-S.; Chun, B.-S. Recovery and bio-potentialities of astaxanthin-rich oil from shrimp (Penaeus monodon) waste and mackerel (Scomberomous niphonius) skin using concurrent supercritical CO2 extraction. J. Supercrit. Fluids 2020, 159, 104773. [Google Scholar] [CrossRef]
- Ferrari, P.F.; Pettinato, M.; Casazza, A.A.; De Negri Atanasio, G.; Palombo, D.; Perego, P. Polyphenols from Nerone Gold 26/6, a new pigmented rice, via non-conventional extractions: Antioxidant properties and biological validation. J. Chem. Technol. Biotechnol. 2021, 96, 1691–1699. [Google Scholar] [CrossRef]
- Spennati, E.; Mirizadeh, S.; Casazza, A.A.; Solisio, C.; Converti, A. Chlorella vulgaris and Arthrospira platensis growth in a continuous membrane photobioreactor using industrial winery wastewater. Algal Res. 2021, 60, 102519. [Google Scholar] [CrossRef]
- Aliakbarian, B.; Casazza, A.A.; Montoya, E.J.O.; Convert, A. Valorisation of Olive Oil Solid Wastes: Valuable Compounds Recovery Using High Pressure- High Temperature. J. Biotechnol. 2010, 150, 332. [Google Scholar] [CrossRef]
- Casazza, A.A.; Pettinato, M.; Perego, P. Polyphenols from apple skins: A study on microwave-assisted extraction optimization and exhausted solid characterization. Sep. Purif. Technol. 2020, 240, 116640. [Google Scholar] [CrossRef]
- Šalplachta, J.; Hohnová, B. Pressurized hot water extraction of proteins from Sambucus nigra L. branches. Ind. Crops Prod. 2017, 108, 312–315. [Google Scholar] [CrossRef]
- Celiktas, M.S.; Kirsch, C.; Smirnova, I. Cascade processing of wheat bran through a biorefinery approach. Energy Convers. Manag. 2014, 84, 633–639. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, M.; Carrillo, C.; Zhu, Z.; Brncic, M.; Berrada, H.; Barba, F.J. Impact of Pressurized Liquid Extraction and pH on Protein Yield, Changes in Molecular Size Distribution and Antioxidant Compounds Recovery from Spirulina. Foods 2021, 10, 2153. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, B.; Pallares, N.; Berrada, H.; Barba, F.J. Development of Antioxidant Protein Extracts from Gilthead Sea Bream (Sparus aurata) Side Streams Assisted by Pressurized Liquid Extraction (PLE). Mar. Drugs 2021, 19, 199. [Google Scholar] [CrossRef]
- de la Fuente, B.; Pallares, N.; Barba, F.J.; Berrada, H. An Integrated Approach for the Valorization of Sea Bass (Dicentrarchus labrax) Side Streams: Evaluation of Contaminants and Development of Antioxidant Protein Extracts by Pressurized Liquid Extraction. Foods 2021, 10, 546. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, B.; Pallarés, N.; Berrada, H.; Barba, F.J. Salmon (Salmo salar) Side Streams as a Bioresource to Obtain Potential Antioxidant Peptides after Applying Pressurized Liquid Extraction (PLE). Mar. Drugs 2021, 19, 323. [Google Scholar] [CrossRef]
- Hernández-Corroto, E.; Plaza, M.; Marina, M.L.; García, M.C. Sustainable extraction of proteins and bioactive substances from pomegranate peel (Punica granatum L.) using pressurized liquids and deep eutectic solvents. Innov. Food Sci. Emerg. Technol. 2020, 60, 102314. [Google Scholar] [CrossRef]
- Saravana, P.S.; Cho, Y.J.; Park, Y.B.; Woo, H.C.; Chun, B.S. Structural, antioxidant, and emulsifying activities of fucoidan from Saccharina japonica using pressurized liquid extraction. Carbohydr. Polym. 2016, 153, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Wang, S.F.; Zhao, J.; Li, S.P. Effects of extraction methods on immunology activity and chemical profiles of Lycium barbarum polysaccharides. J. Pharm. Biomed. Anal. 2020, 185, 113219. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Guo, B.L.; Li, S.P.; Zhang, Q.W.; Tu, P.F.; Wang, Y.T. Simultaneous determination of 15 flavonoids in Epimedium using pressurized liquid extraction and high-performance liquid chromatography. J. Chromatogr. A 2007, 1163, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Saravana, P.S.; Tilahun, A.; Gerenew, C.; Tri, V.D.; Kim, N.H.; Kim, G.-D.; Woo, H.-C.; Chun, B.-S. Subcritical water extraction of fucoidan from Saccharina japonica: Optimization, characterization and biological studies. J. Appl. Phycol. 2017, 30, 579–590. [Google Scholar] [CrossRef]
- Tejedor-Calvo, E.; Morales, D.; Marco, P.; Sanchez, S.; Garcia-Barreda, S.; Smiderle, F.R.; Iacomini, M.; Villalva, M.; Santoyo, S.; Soler-Rivas, C. Screening of bioactive compounds in truffles and evaluation of pressurized liquid extractions (PLE) to obtain fractions with biological activities. Food Res. Int. 2020, 132, 109054. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wen, C.; Chen, M.; Gu, J.; Zhou, J.; Duan, Y.; Zhang, H.; Ma, H. Antioxidant activities of Sagittaria sagittifolia L. polysaccharides with subcritical water extraction. Int. J. Biol. Macromol. 2019, 134, 172–179. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Gu, J.; Ji, C.; Duan, Y.; Zhang, H. Effects of subcritical water extraction microenvironment on the structure and biological activities of polysaccharides from Lentinus edodes. Int. J. Biol. Macromol. 2019, 123, 1002–1011. [Google Scholar] [CrossRef]
- Mirpoor, S.F.; Varriale, S.; Porta, R.; Naviglio, D.; Spennato, M.; Gardossi, L.; Giosafatto, C.V.L.; Pezzella, C. A biorefinery approach for the conversion of Cynara cardunculus biomass to active films. Food Hydrocoll. 2021, 122, 107099. [Google Scholar] [CrossRef]
- Avanza, M.V.; Álvarez-Rivera, G.; Cifuentes, A.; Mendiola, J.A.; Ibáñez, E. Phytochemical and Functional Characterization of Phenolic Compounds from Cowpea (Vigna unguiculata (L.) Walp.) Obtained by Green Extraction Technologies. Agronomy 2021, 11, 162. [Google Scholar] [CrossRef]
- Sumampouw, G.A.; Jacobsen, C.; Getachew, A.T. Optimization of phenolic antioxidants extraction from Fucus vesiculosus by pressurized liquid extraction. J. Appl. Phycol. 2021, 33, 1195–1207. [Google Scholar] [CrossRef]
- Paini, M.; Casazza, A.A.; Aliakbarian, B.; Perego, P.; Binello, A.; Cravotto, G. Influence of ethanol/water ratio in ultrasound and high-pressure/high-temperature phenolic compound extraction from agri-food waste. Int. J. Food Sci. Technol. 2016, 51, 349–358. [Google Scholar] [CrossRef]
- Xu, H.; Wang, W.; Liu, X.; Yuan, F.; Gao, Y. Antioxidative phenolics obtained from spent coffee grounds (Coffea arabica L.) by subcritical water extraction. Ind. Crops Prod. 2015, 76, 946–954. [Google Scholar] [CrossRef]
- Casazza, A.A.; Aliakbarian, B.; Sannita, E.; Perego, P. High-pressure high-temperature extraction of phenolic compounds from grape skins. Int. J. Food Sci. Technol. 2012, 47, 399–405. [Google Scholar] [CrossRef]
- de Aguiar, A.C.; Osorio-Tobón, J.F.; Viganó, J.; Martínez, J. Economic evaluation of supercritical fluid and pressurized liquid extraction to obtain phytonutrients from biquinho pepper: Analysis of single and sequential-stage processes. J. Supercrit. Fluids 2020, 165, 104935. [Google Scholar] [CrossRef]
- Croxatto Vega, G.; Sohn, J.; Voogt, J.; Birkved, M.; Olsen, S.I.; Nilsson, A.E. Insights from combining techno-economic and life cycle assessment—A case study of polyphenol extraction from red wine pomace. Resour. Conserv. Recycl. 2021, 167, 105318. [Google Scholar] [CrossRef]
- Cardoso, L.C.; Serrano, C.M.; Quintero, E.T.; Lopez, C.P.; Antezana, R.M.; Martinez de la Ossa, E.J. High pressure extraction of antioxidants from Solanum stenotomun peel. Molecules 2013, 18, 3137–3151. [Google Scholar] [CrossRef] [Green Version]
- Kuzmanovića, M.; Tišmaa, M.; Bucić-Kojića, A.; Casazzab, A.A.; Marco Painib, B.A.; Perego, P. High–Pressure and Temperature Extraction of Phenolic Compounds from Corn Silage. Chem. Eng. Traansactions 2015, 43, 133–138. [Google Scholar] [CrossRef]
- Silva, M.F.d.; Pettinato, M.; Casazza, A.A.; Maciel, M.I.S.; Perego, P. Design and evaluation of non-conventional extraction for bioactive compounds recovery from spent coffee (Coffea arabica L.) grounds. Chem. Eng. Res. Des. 2022, 177, 418–430. [Google Scholar] [CrossRef]
- Yan, Z.; Zhang, H.; Dzah, C.S.; Zhang, J.; Diao, C.; Ma, H.; Duan, Y. Subcritical water extraction, identification, antioxidant and antiproliferative activity of polyphenols from lotus seedpod. Sep. Purif. Technol. 2020, 236, 116217. [Google Scholar] [CrossRef]
- Ben Hamissa, A.M.; Seffen, M.; Aliakbarian, B.; Casazza, A.A.; Perego, P.; Converti, A. Phenolics extraction from Agave americana (L.) leaves using high-temperature, high-pressure reactor. Food Bioprod. Process. 2012, 90, 17–21. [Google Scholar] [CrossRef]
- Duba, K.S.; Casazza, A.A.; Mohamed, H.B.; Perego, P.; Fiori, L. Extraction of polyphenols from grape skins and defatted grape seeds using subcritical water: Experiments and modeling. Food Bioprod. Process. 2015, 94, 29–38. [Google Scholar] [CrossRef]
- De Marco, A.; Luongo, G.; Di Marino, C.; De Tommaso, G.; Di Fabio, G.; Zarrelli, A. Silymarin from Silybum marianum by Naviglio’s extractor: A new and very efficient approach. Nat. Prod. Res. 2021, 35, 2621–2627. [Google Scholar] [CrossRef] [PubMed]
- Çam, M.; Hışıl, Y. Pressurised water extraction of polyphenols from pomegranate peels. Food Chem. 2010, 123, 878–885. [Google Scholar] [CrossRef]
- Pojić, M.; Mišan, A.; Tiwari, B. Eco-innovative technologies for extraction of proteins for human consumption from renewable protein sources of plant origin. Trends Food Sci. Technol. 2018, 75, 93–104. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Potkule, J.; Verma, R.; Punia, S.; Mahapatra, A.; Belwal, T.; Dahuja, A.; Joshi, S.; Berwal, M.K.; et al. Advances in the plant protein extraction: Mechanism and recommendations. Food Hydrocoll. 2021, 115, 106595. [Google Scholar] [CrossRef]
- Contreras, M.D.M.; Lama-Munoz, A.; Manuel Gutierrez-Perez, J.; Espinola, F.; Moya, M.; Castro, E. Protein extraction from agri-food residues for integration in biorefinery: Potential techniques and current status. Bioresour. Technol. 2019, 280, 459–477. [Google Scholar] [CrossRef] [PubMed]
- Shi, L. Bioactivities, isolation and purification methods of polysaccharides from natural products: A review. Int. J. Biol. Macromol. 2016, 92, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Feng, J.; Sun, P.; Xiang, N.; Lu, B.; Qiu, D. Recent advances in improving stability of food emulsion by plant polysaccharides. Food Res. Int. 2020, 137, 109376. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Huang, G. Preparation and antioxidant activities of important traditional plant polysaccharides. Int. J. Biol. Macromol. 2018, 111, 780–786. [Google Scholar] [CrossRef]
- Ying, M.; Yu, Q.; Zheng, B.; Wang, H.; Wang, J.; Chen, S.; Nie, S.; Xie, M. Cultured Cordyceps sinensis polysaccharides modulate intestinal mucosal immunity and gut microbiota in cyclophosphamide-treated mice. Carbohydr. Polym. 2020, 235, 115957. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, B.; Tang, C.; Gou, Y.; Chen, H.; Wang, Y.; Jin, C.; Liu, J.; Niu, F.; Kan, J.; et al. Characterization, antioxidant activity and hepatoprotective effect of purple sweetpotato polysaccharides. Int. J. Biol. Macromol. 2018, 115, 69–76. [Google Scholar] [CrossRef]
- Yan, T.; Nian, T.; Liao, Z.; Xiao, F.; Wu, B.; Bi, K.; He, B.; Jia, Y. Antidepressant effects of a polysaccharide from okra (Abelmoschus esculentus (L) Moench) by anti-inflammation and rebalancing the gut microbiota. Int. J. Biol. Macromol. 2020, 144, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.S.A.; Naveed, M.; Jost, N. Polysaccharides; Classification, Chemical Properties, and Future Perspective Applications in Fields of Pharmacology and Biological Medicine (A Review of Current Applications and Upcoming Potentialities). J. Polym. Environ. 2021, 29, 2359–2371. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Huang, G. Extraction, separation, modification, structural characterization, and antioxidant activity of plant polysaccharides. Chem. Biol. Drug Des. 2020, 96, 1209–1222. [Google Scholar] [CrossRef]
- Leong, Y.K.; Yang, F.C.; Chang, J.S. Extraction of polysaccharides from edible mushrooms: Emerging technologies and recent advances. Carbohydr. Polym. 2021, 251, 117006. [Google Scholar] [CrossRef]
- Yang, B.; Wu, Q.; Luo, Y.; Yang, Q.; Wei, X.; Kan, J. High-pressure ultrasonic-assisted extraction of polysaccharides from Hovenia dulcis: Extraction, structure, antioxidant activity and hypoglycemic. Int. J. Biol. Macromol. 2019, 137, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Luthria, D.; Biswas, R.; Natarajan, S. Comparison of extraction solvents and techniques used for the assay of isoflavones from soybean. Food Chem. 2007, 105, 325–333. [Google Scholar] [CrossRef]
- Xie, J.J.; Lu, J.; Qian, Z.M.; Yu, Y.; Duan, J.A.; Li, S.P. Optimization and comparison of five methods for extraction of coniferyl ferulate from Angelica sinensis. Molecules 2009, 14, 555–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, G.L.; Maciel, L.G.; Mazzutti, S.; Barbi, R.C.T.; Ribani, R.H.; Ferreira, S.R.S.; Block, J.M. Sequential green extractions based on supercritical carbon dioxide and pressurized ethanol for the recovery of lipids and phenolics from Pachira aquatica seeds. J. Clean. Prod. 2021, 306, 127223. [Google Scholar] [CrossRef]
- Berrada, H.; Borrull, F.; Font, G.; Marce, R.M. Determination of macrolide antibiotics in meat and fish using pressurized liquid extraction and liquid chromatography-mass spectrometry. J. Chromatogr. A 2008, 1208, 83–89. [Google Scholar] [CrossRef]
- Khan, M.R.; Busquets, R.; Santos, F.J.; Puignou, L. New method for the analysis of heterocyclic amines in meat extracts using pressurised liquid extraction and liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2008, 1194, 155–160. [Google Scholar] [CrossRef]
- Bosellia, E.; Velazcoa, V.; Cabonib, M.F.; Lerckera, G. Pressurized liquid extraction of lipids for the determination of oxysterols in egg-containing food. J. Chromatogr. A 2001, 917, 239–244. [Google Scholar] [CrossRef]
- Lund, M.; Duedahl-Olesen, L.; Christensen, J.H. Extraction of polycyclic aromatic hydrocarbons from smoked fish using pressurized liquid extraction with integrated fat removal. Talanta 2009, 79, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Catchpole, O.; Moreno, T.; Montañes, F.; Tallon, S. Perspectives on processing of high value lipids using supercritical fluids. J. Supercrit. Fluids 2018, 134, 260–268. [Google Scholar] [CrossRef]
- Villagrasa, M.; Guillamon, M.; Navarro, A.; Eljarrat, E.; Barcelo, D. Development of a pressurized liquid extraction-solid-phase extraction followed by liquid chromatography-electrospray ionization tandem mass spectrometry method for the quantitative determination of benzoxazolinones and their degradation products in agricultural soil. J. Chromatogr. A 2008, 1179, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Onofrejova, L.; Vasickova, J.; Klejdus, B.; Stratil, P.; Misurcova, L.; Kracmar, S.; Kopecky, J.; Vacek, J. Bioactive phenols in algae: The application of pressurized-liquid and solid-phase extraction techniques. J. Pharm. Biomed. Anal. 2010, 51, 464–470. [Google Scholar] [CrossRef]
- Andujar-Ortiz, I.; Pozo-Bayon, M.A.; Garcia-Ruiz, A.; Moreno-Arribas, M.V. Role of specific components from commercial inactive dry yeast winemaking preparations on the growth of wine lactic acid bacteria. J. Agric. Food Chem. 2010, 58, 8392–8399. [Google Scholar] [CrossRef]
- Gómez-Ariza, J.L.; Caro de la Torre, M.A.; Giráldez, I.; Morales, E. Speciation analysis of selenium compounds in yeasts using pressurised liquid extraction and liquid chromatography–microwave-assisted digestion–hydride generation–atomic fluorescence spectrometry. Anal. Chim. Acta 2004, 524, 305–314. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- He, Y.; Huang, Z.; Zhong, C.; Guo, Z.; Chen, B. Pressurized liquid extraction with ethanol as a green and efficient technology to lipid extraction of Isochrysis biomass. Bioresour. Technol. 2019, 293, 122049. [Google Scholar] [CrossRef]
- Cescut, J.; Severac, E.; Molina-Jouve, C.; Uribelarrea, J.L. Optimizing pressurized liquid extraction of microbial lipids using the response surface method. J. Chromatogr. A 2011, 1218, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Bermejo, D.; Calvo, M.V.; Castro-Gomez, P.; Fornari, T.; Fontecha, J. Production of omega 3-rich oils from underutilized chia seeds. Comparison between supercritical fluid and pressurized liquid extraction methods. Food Res. Int. 2019, 115, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green extraction of natural products. Origins, current status, and future challenges. TrAC Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Mazzutti, S.; Pedrosa, R.C.; Salvador Ferreira, S.R. Green processes in Foodomics. Supercritical Fluid Extraction of Bioactives. In Comprehensive Foodomics; Elsevier: Sergipe, Brazil, 2021; pp. 725–743. [Google Scholar]
- Quintin, D.; Garcia-Gomez, P.; Ayuso, M.; Sanmartin, A.M. Active biocompounds to improve food nutritional value. Trends Food Sci. Technol. 2019, 84, 19–21. [Google Scholar] [CrossRef]
- Osorio-Tobon, J.F. Recent advances and comparisons of conventional and alternative extraction techniques of phenolic compounds. J. Food Sci. Technol. 2020, 57, 4299–4315. [Google Scholar] [CrossRef] [PubMed]
- Temelli, F. Perspectives on supercritical fluid processing of fats and oils. J. Supercrit. Fluids 2009, 47, 583–590. [Google Scholar] [CrossRef]
- Wianowska, D.; Gil, M. Critical approach to PLE technique application in the analysis of secondary metabolites in plants. TrAC Trends Anal. Chem. 2019, 114, 314–325. [Google Scholar] [CrossRef]
- Hogervorst Cvejić, J.; Atanacković Krstonošić, M.; Bursać, M.; Miljić, U. Polyphenols. In Nutraceutical and Functional Food Components; Academic Press: Novi Sad, Serbia, 2017; pp. 203–258. [Google Scholar]
- Socrier, L.; Quero, A.; Verdu, M.; Song, Y.; Molinie, R.; Mathiron, D.; Pilard, S.; Mesnard, F.; Morandat, S. Flax phenolic compounds as inhibitors of lipid oxidation: Elucidation of their mechanisms of action. Food Chem. 2019, 274, 651–658. [Google Scholar] [CrossRef]
- Yousefian, M.; Shakour, N.; Hosseinzadeh, H.; Hayes, A.W.; Hadizadeh, F.; Karimi, G. The natural phenolic compounds as modulators of NADPH oxidases in hypertension. Phytomedicine 2019, 55, 200–213. [Google Scholar] [CrossRef]
- Luna-Guevara, M.L.; Luna-Guevara, J.J.; Hernández-Carranza, P.; Ruíz-Espinosa, H.; Ochoa-Velasco, C.E. Phenolic Compounds: A Good Choice Against Chronic Degenerative Diseases. Stud. Nat. Prod. Chem. 2018, 59, 79–108. [Google Scholar]
- Ullah, M.; Uddin, Z.; Song, Y.H.; Li, Z.P.; Kim, J.Y.; Ban, Y.J.; Park, K.H. Bacterial neuraminidase inhibition by phenolic compounds from Usnea longissima. S. Afr. J. Bot. 2019, 120, 326–330. [Google Scholar] [CrossRef]
- Chen, L.; Gnanaraj, C.; Arulselvan, P.; El-Seedi, H.; Teng, H. A review on advanced microencapsulation technology to enhance bioavailability of phenolic compounds: Based on its activity in the treatment of Type 2 Diabetes. Trends Food Sci. Technol. 2019, 85, 149–162. [Google Scholar] [CrossRef]
- Martini, S.; Conte, A.; Tagliazucchi, D. Bioaccessibility, bioactivity and cell metabolism of dark chocolate phenolic compounds after in vitro gastro-intestinal digestion. J. Funct. Foods 2018, 49, 424–436. [Google Scholar] [CrossRef] [Green Version]
- Galanakis, C.M.; Tsatalas, P.; Charalambous, Z.; Galanakis, I.M. Control of microbial growth in bakery products fortified with polyphenols recovered from olive mill wastewater. Environ. Technol. Innov. 2018, 10, 1–15. [Google Scholar] [CrossRef]
- Basanta, M.F.; Rojas, A.M.; Martinefski, M.R.; Tripodi, V.P.; De’Nobili, M.D.; Fissore, E.N. Cherry (Prunus avium) phenolic compounds for antioxidant preservation at food interfaces. J. Food Eng. 2018, 239, 15–25. [Google Scholar] [CrossRef]
- Wong-Paz, J.E.; Muñiz-Márquez, D.B.; Aguilar-Zárate, P.; Ascacio-Valdés, J.A.; Cruz, K.; Reyes-Luna, C.; Rodríguez, R.; Aguilar, C.N. Extraction of Bioactive Phenolic Compounds by Alternative Technologies. In Ingredients Extraction by Physicochemical Methods in Food; Academic Press: Cambridge, MA, USA, 2017; pp. 229–252. [Google Scholar]
- Caldas, T.W.; Mazza, K.E.L.; Teles, A.S.C.; Mattos, G.N.; Brígida, A.I.S.; Conte-Junior, C.A.; Borguini, R.G.; Godoy, R.L.O.; Cabral, L.M.C.; Tonon, R.V. Phenolic compounds recovery from grape skin using conventional and non-conventional extraction methods. Ind. Crops Prod. 2018, 111, 86–91. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H. Microwave-assisted extraction of phenolics from Hibiscus sabdariffa calyces: Kinetic modelling and process intensification. Ind. Crops Prod. 2019, 137, 528–535. [Google Scholar] [CrossRef]
- Battistella Lasta, H.F.; Lentz, L.; Gonçalves Rodrigues, L.G.; Mezzomo, N.; Vitali, L.; Salvador Ferreira, S.R. Pressurized liquid extraction applied for the recovery of phenolic compounds from beetroot waste. Biocatal. Agric. Biotechnol. 2019, 21, 101353. [Google Scholar] [CrossRef]
- Okiyama, D.C.G.; Soares, I.D.; Cuevas, M.S.; Crevelin, E.J.; Moraes, L.A.B.; Melo, M.P.; Oliveira, A.L.; Rodrigues, C.E.C. Pressurized liquid extraction of flavanols and alkaloids from cocoa bean shell using ethanol as solvent. Food Res. Int. 2018, 114, 20–29. [Google Scholar] [CrossRef]
- Shang, Y.F.; Xu, J.L.; Lee, W.J.; Um, B.H. Antioxidative polyphenolics obtained from spent coffee grounds by pressurized liquid extraction. S. Afr. J. Bot. 2017, 109, 75–80. [Google Scholar] [CrossRef]
- Posadino, A.M.; Biosa, G.; Zayed, H.; Abou-Saleh, H.; Cossu, A.; Nasrallah, G.K.; Giordo, R.; Pagnozzi, D.; Porcu, M.C.; Pretti, L.; et al. Protective Effect of Cyclically Pressurized Solid(-)Liquid Extraction Polyphenols from Cagnulari Grape Pomace on Oxidative Endothelial Cell Death. Molecules 2018, 23, 2105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casazza, A.A.; Aliakbarian, B.; Mantegna, S.; Cravotto, G.; Perego, P. Extraction of phenolics from Vitis vinifera wastes using non-conventional techniques. J. Food Eng. 2010, 100, 50–55. [Google Scholar] [CrossRef]
- Hubert, P.; Vitzthum, O.G. Fluid Extraction of Hops, Spices, and Tobacco with Supercritical Gases. Angew. Chem. 1978, 17, 710–715. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I. Supercritical fluid extraction and fractionation of natural matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- Herrero, M.; Cifuentes, A.; Ibanez, E. Sub- and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae: A review. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef] [Green Version]
- Reverchon, E. Supercritical fluid extraction and fractionation of essential oils and related products. J. Supercrit. Fluids 1997, 10, 1–37. [Google Scholar] [CrossRef]
- Lang, Q.; Wai, C.M. Supercritical fluid extraction in herbal and natural product studies—A practical review. Talanta 2001, 53, 771–782. [Google Scholar] [CrossRef]
- Pourmortazavi, S.M.; Hajimirsadeghi, S.S. Supercritical fluid extraction in plant essential and volatile oil analysis. J. Chromatogr. A 2007, 1163, 2–24. [Google Scholar] [CrossRef] [PubMed]
- Romano, R.; Aiello, A.; Pizzolongo, F.; Rispoli, A.; De Luca, L.; Masi, P. Characterisation of oleoresins extracted from tomato waste by liquid and supercritical carbon dioxide. Int. J. Food Sci. Technol. 2020, 55, 3334–3342. [Google Scholar] [CrossRef]
- Kuvendziev, S.; Lisichkov, K.; Zeković, Z.; Marinkovski, M. Artificial neural network modelling of supercritical fluid CO2 extraction of polyunsaturated fatty acids from common carp (Cyprinus carpio L.) viscera. J. Supercrit. Fluids 2014, 92, 242–248. [Google Scholar] [CrossRef]
- Obregón Tinoco, H.; Huayta Socantaype, F.; Cardenas Toro, F. Optimización del Proceso de Extracción por Fluidos Supercríticos en la Obtención de Aceite de Semillas de Uva con el Empleo de la Metodología Taguchi y Superficie de Respuesta. In Proceedings of the 16th LACCEI International Multi-Conference for Engineering, Education, and Technology: “Innovation in Education and Inclusion, Lima, Peru, 18–20 July 2018; p. 268. [Google Scholar]
- Galarce-Bustos, O.; Fernandez-Ponce, M.T.; Montes, A.; Pereyra, C.; Casas, L.; Mantell, C.; Aranda, M. Usage of supercritical fluid techniques to obtain bioactive alkaloid-rich extracts from cherimoya peel and leaves: Extract profiles and their correlation with antioxidant properties and acetylcholinesterase and alpha-glucosidase inhibitory activities. Food Funct. 2020, 11, 4224–4235. [Google Scholar] [CrossRef] [PubMed]
- Santana, Á.L.; Albarelli, J.Q.; Santos, D.T.; Souza, R.; Machado, N.T.; Araújo, M.E.; Meireles, M.A.A. Kinetic behavior, mathematical modeling, and economic evaluation of extracts obtained by supercritical fluid extraction from defatted assaí waste. Food Bioprod. Process. 2018, 107, 25–35. [Google Scholar] [CrossRef]
- Vági, E.; Simándi, B.; Vásárhelyiné, K.P.; Daood, H.; Kéry, Á.; Doleschall, F.; Nagy, B. Supercritical carbon dioxide extraction of carotenoids, tocopherols and sitosterols from industrial tomato by-products. J. Supercrit. Fluids 2007, 40, 218–226. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Attard, T.M.; Chen, S.S.; Tsang, D.C.W.; Hunt, A.J.; Jérôme, F.; Ok, Y.S.; Poon, C.S. Supercritical Carbon Dioxide Extraction of Value-Added Products and Thermochemical Synthesis of Platform Chemicals from Food Waste. ACS Sustain. Chem. Eng. 2018, 7, 2821–2829. [Google Scholar] [CrossRef]
- Campalani, C.; Amadio, E.; Zanini, S.; Dall’Acqua, S.; Panozzo, M.; Ferrari, S.; De Nadai, G.; Francescato, S.; Selva, M.; Perosa, A. Supercritical CO2 as a green solvent for the circular economy: Extraction of fatty acids from fruit pomace. J. CO2 Util. 2020, 41, 101259. [Google Scholar] [CrossRef]
- Rosa, A.; Era, B.; Masala, C.; Nieddu, M.; Scano, P.; Fais, A.; Porcedda, S.; Piras, A. Supercritical CO2 Extraction of Waste Citrus Seeds: Chemical Composition, Nutritional and Biological Properties of Edible Fixed Oils. Eur. J. Lipid Sci. Technol. 2019, 121, 1800502. [Google Scholar] [CrossRef]
- Castillo-Herrera, G.A.; Farías-Álvarez, L.J.; García-Fajardo, J.A.; Delgado-Saucedo, J.I.; Puebla-Pérez, A.M.; Lugo-Cervantes, E. Bioactive extracts of Citrus aurantifolia swingle seeds obtained by supercritical CO2 and organic solvents comparing its cytotoxic activity against L5178Y leukemia lymphoblasts. J. Supercrit. Fluids 2015, 101, 81–86. [Google Scholar] [CrossRef]
- Moulehi, I.; Bourgou, S.; Ourghemmi, I.; Tounsi, M.S. Variety and ripening impact on phenolic composition and antioxidant activity of mandarin (Citrus reticulate Blanco) and bitter orange (Citrus aurantium L.) seeds extracts. Ind. Crops Prod. 2012, 39, 74–80. [Google Scholar] [CrossRef]
- Dorado Achicanoy, D.; Hurtado Benavides, A.; Martinez-Correa, H.A. Study of supercritical CO2 extraction of tamarillo (Cyphomandra Betacea) seed oil containing high added value compounds. Electrophoresis 2018, 39, 1917–1925. [Google Scholar] [CrossRef]
- Salgın, U.; Salgın, S.; Ekici, D.D.; UludaĿ, G. Oil recovery in rosehip seeds from food plant waste products using supercritical CO2 extraction. J. Supercrit. Fluids 2016, 118, 194–202. [Google Scholar] [CrossRef]
- Larrauri, J.A.; Rupérez, P.; Borroto, B.; Saura-Calixto, F. Mango Peels as a New Tropical Fibre: Preparation and Characterization. Chem. Lwt-Food Sci. Technol. 1996, 29, 729–733. [Google Scholar] [CrossRef]
- Louaer, M.; Zermane, A.; Larkeche, O.; Meniai, A.H. Experimental study and optimization of the extraction of Algerian date stones oil (Phoenix dactylifera L.) using supercritical carbon dioxide. J. Food Process Eng. 2019, 42, 13049. [Google Scholar] [CrossRef]
- Kayathi, A.; Chakrabarti, P.P.; Bonfim-Rocha, L.; Cardozo-Filho, L.; Jegatheesan, V. Selective extraction of polar lipids of mango kernel using Supercritical Carbon dioxide (SC-CO2) extraction: Process optimization of extract yield/phosphorous content and economic evaluation. Chemosphere 2020, 260, 127639. [Google Scholar] [CrossRef] [PubMed]
- Murthy, P.S.; Madhava Naidu, M. Sustainable management of coffee industry by-products and value addition—A review. Resour. Conserv. Recycl. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- Ahangari, B.; Sargolzaei, J. Extraction of lipids from spent coffee grounds using organic solvents and supercritical carbon dioxide. J. Food Process. Preserv. 2013, 37, 1014–1021. [Google Scholar] [CrossRef]
- Nasti, R.; Galeazzi, A.; Marzorati, S.; Zaccheria, F.; Ravasio, N.; Bozzano, G.L.; Manenti, F.; Verotta, L. Valorisation of Coffee Roasting By-Products: Recovery of Silverskin Fat By Supercritical CO2 Extraction. Waste Biomass Valorization 2021, 12, 6021–6033. [Google Scholar] [CrossRef]
- Ferdosh, S.; Sarker, M.Z.I.; Abd Rahman, N.N.N.; Selamat, J.; Karim, M.R.; Razak, T.A.; Abd Kadir, M.O. Fish Oil Recovery from Viscera of Indian Mackerel (Rastrelliger kanagurta) by Supercritical Fluid: An Optimization Approach. J. Chin. Chem. Soc. 2012, 59, 1421–1429. [Google Scholar] [CrossRef]
- Franklin, E.C.; Haq, M.; Roy, V.C.; Park, J.S.; Chun, B.S. Supercritical CO2 extraction and quality comparison of lipids from Yellowtail fish (Seriola quinqueradiata) waste in different conditions. J. Food Process. Preserv. 2020, 44, e14892. [Google Scholar] [CrossRef]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- de Andrade Lima, M.; Kestekoglou, I.; Charalampopoulos, D.; Chatzifragkou, A. Supercritical Fluid Extraction of Carotenoids from Vegetable Waste Matrices. Molecules 2019, 24, 466. [Google Scholar] [CrossRef] [Green Version]
- Baysal, T.; Ersus, S.; Starmans, D.A.J. Supercritical CO2 Extraction of â-Carotene and Lycopene from Tomato Paste Waste. J. Agric. Food Chem. 2000, 48, 5507–5511. [Google Scholar] [CrossRef] [PubMed]
- Sabio, E.; Lozano, M.; de Espinosa, V.M.; Mendes, R.L.; Pereira, A.P.; Palavra, A.F.; Coelho, J.A. Lycopene and â-Carotene Extraction from Tomato Processing Waste Using Supercritical CO2. Ind. Eng. Chem. Res. 2003, 42, 6641–6646. [Google Scholar] [CrossRef]
- Longo, C.; Leo, L.; Leone, A. Carotenoids, fatty acid composition and heat stability of supercritical carbon dioxide-extracted-oleoresins. Int. J. Mol. Sci. 2012, 13, 4233–4254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derrien, M.; Aghabararnejad, M.; Gosselin, A.; Desjardins, Y.; Angers, P.; Boumghar, Y. Optimization of supercritical carbon dioxide extraction of lutein and chlorophyll from spinach by-products using response surface methodology. Lwt 2018, 93, 79–87. [Google Scholar] [CrossRef]
- Quitain, A.T.; Kai, T.; Sasaki, M.; Goto, M. Supercritical carbon dioxide extraction of fucoxanthin from Undaria pinnatifida. J. Agric. Food Chem. 2013, 61, 5792–5797. [Google Scholar] [CrossRef]
- Bendif, H.; Adouni, K.; Miara, M.D.; Baranauskiene, R.; Kraujalis, P.; Venskutonis, P.R.; Nabavi, S.M.; Maggi, F. Essential oils (EOs), pressurized liquid extracts (PLE) and carbon dioxide supercritical fluid extracts (SFE-CO2) from Algerian Thymus munbyanus as valuable sources of antioxidants to be used on an industrial level. Food Chem. 2018, 260, 289–298. [Google Scholar] [CrossRef]
- Gil-Ramírez, A.; Aldars-García, L.; Palanisamy, M.; Jiverdeanu, R.M.; Ruiz-Rodríguez, A.; Marín, F.R.; Reglero, G.; Soler-Rivas, C. Sterol enriched fractions obtained from Agaricus bisporus fruiting bodies and by-products by compressed fluid technologies (PLE and SFE). Innov. Food Sci. Emerg. Technol. 2013, 18, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Quispe-Fuentes, I.; Uribe, E.; López, J.; Contreras, D.; Poblete, J. A study of dried mandarin (Clementina orogrande) peel applying supercritical carbon dioxide using co-solvent: Influence on oil extraction, phenolic compounds, and antioxidant activity. J. Food Process. Preserv. 2021, 46, e16116. [Google Scholar] [CrossRef]
- Castro-Vargas, H.I.; Baumann, W.; Ferreira, S.R.S.; Parada-Alfonso, F. Valorization of papaya (Carica papaya L.) agroindustrial waste through the recovery of phenolic antioxidants by supercritical fluid extraction. J. Food Sci. Technol. 2019, 56, 3055–3066. [Google Scholar] [CrossRef]
- Romano, R.; Aiello, A.; Meca, G.; De Luca, L.; Pizzolongo, F.; Masi, P. Recovery of bioactive compounds from walnut (Juglans regia L.) green husk by supercritical carbon dioxide extraction. Int. J. Food Sci. Technol. 2021, 56, 4658–4668. [Google Scholar] [CrossRef]
- Caballero, A.S.; Romero-García, J.M.; Castro, E.; Cardona, C.A. Supercritical fluid extraction for enhancing polyphenolic compounds production from olive waste extracts. J. Chem. Technol. Biotechnol. 2019, 95, 356–362. [Google Scholar] [CrossRef]
- Jafarian Asl, P.; Niazmand, R.; Yahyavi, F. Extraction of phytosterols and tocopherols from rapeseed oil waste by supercritical CO2 plus co-solvent: A comparison with conventional solvent extraction. Heliyon 2020, 6, e03592. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.M.; Guedes, J.A.C.; de Brito, E.S.; Ferreira, S.R.S. Valorization of tamarind seeds using high-pressure extraction methods to obtain rich fractions in fatty acid and phenolic compounds. J. Supercrit. Fluids 2022, 183, 105556. [Google Scholar] [CrossRef]
- Viganó, J.; Zabot, G.L.; Martínez, J. Supercritical fluid and pressurized liquid extractions of phytonutrients from passion fruit by-products: Economic evaluation of sequential multi-stage and single-stage processes. J. Supercrit. Fluids 2017, 122, 88–98. [Google Scholar] [CrossRef]
- Tamkutė, L.; Liepuoniūtė, R.; Pukalskienė, M.; Venskutonis, P.R. Recovery of valuable lipophilic and polyphenolic fractions from cranberry pomace by consecutive supercritical CO2 and pressurized liquid extraction. J. Supercrit. Fluids 2020, 159, 104755. [Google Scholar] [CrossRef]
- Mazzutti, S.; Rodrigues, L.G.G.; Mezzomo, N.; Venturi, V.; Ferreira, S.R.S. Integrated green-based processes using supercritical CO2 and pressurized ethanol applied to recover antioxidant compouds from cocoa (Theobroma cacao) bean hulls. J. Supercrit. Fluids 2018, 135, 52–59. [Google Scholar] [CrossRef]
- Kraujalienė, V.; Pukalskas, A.; Venskutonis, P.R. Biorefining of goldenrod (Solidago virgaurea L.) leaves by supercritical fluid and pressurized liquid extraction and evaluation of antioxidant properties and main phytochemicals in the fractions and plant material. J. Funct. Foods 2017, 37, 200–208. [Google Scholar] [CrossRef]
- Villanueva Bermejo, D.; Ibáñez, E.; Reglero, G.; Turner, C.; Fornari, T.; Rodriguez-Meizoso, I. High catechins/low caffeine powder from green tea leaves by pressurized liquid extraction and supercritical antisolvent precipitation. Sep. Purif. Technol. 2015, 148, 49–56. [Google Scholar] [CrossRef]
- Ferreira, W.S.; Viganó, J.; Veggi, P.C. Economic assessment of emerging extraction techniques: Hybridization of high-pressure extraction and low-frequency ultrasound to produce piceatannol-rich extract from passion fruit bagasse. Chem. Eng. Process.-Process Intensif. 2022, 174, 108850. [Google Scholar] [CrossRef]
- Trucillo, P.; Campardelli, R.; De Marco, I. Environmental and Sustainability Analysis of a Supercritical Carbon Dioxide-Assisted Process for Pharmaceutical Applications. Processes 2021, 9, 1788. [Google Scholar] [CrossRef]
| Compounds | Source | Extraction Solvent | Operating Conditions | Sample Weight Treated | Extraction Method | Ref |
|---|---|---|---|---|---|---|
| Proteins | Sambucus nigra L. branches | water | 50 °C and 15 MPa for 5 min | 4 g | PHWE | [56] |
| Proteins | Wheat bran | water | 80 °C, pH 9.3, and 30 bar for 30 min | 15 g | SWE | [57] |
| Proteins | Spirulina | water | 40 C, pH 4, and 103.4 bar for 10 min | 500 mg | PLE | [58] |
| Proteins | Sea Bream (Sparus aurata, Salmo salar and Dicentrarchus labrax) Side Streams | water | 1500 psi; 20 °C, pH 7, 5 min for muscle; 60 °C, pH 4, 15 min for heads; 50 °C, pH 7, 15 min for viscera; 55 °C, pH 7, 5 min for skin; 60 °C, pH 7, 15 min for tailfins | 6 g | PLE | [59,60,61] |
| Proteins | Pomegranate peel (Punica granatum L.) | 70% (v/v) EtOH | 120 °C, 1500 psi static extraction time, 3 min; extraction time, 12 min | 10 g | PLE | [62] |
| Proteins | Saccharina japonica | water (1.13–2.40%) | 140 °C and 50 bar for 5 min | 10 g | PLE | [63] |
| Fucoidan | Saccharina japonica | NaOH (4.15%) | 140 °C and 50 bar for 5 min | 2 g | PLE | [63] |
| L. barbarum polysaccharides | Fruit of Lycium barbarum | water | 100 °C and 1500 psi for 20 min | 250 mg | PLE | [64,65] |
| Fucoidan | Saccharina japonica | water | 127.01 °C, 80 bar, S/L ratio of 0.04 g mL−1, AS of 300 rpm, for 11.98 min | <20 g | SWE | [66] |
| β-glucan | Tuber melanosporum | water and 100% EtOH | 180 °C and 16.7 MPa for 30 min | 500 mg | PLE | [67] |
| Polysaccharides | Fucus virsoides and Cystoseira barbata | water and 0.1 M H2SO4 | 140 °C and 1500 psi for 15 min | 1 g | PLE | [38] |
| Polysaccharides | Sagittaria sagittifolia L. | water | 170 °C, L/S of 7 mL/g for 16 min | 1 g | SWE | [68] |
| Polysaccharides | Lentinus edodes | water | 150 °C for 15 min | 1 g | SWE | [69] |
| Gallic acid | Cardoon leaf | 100% EtOH | room temperature, 9 bar, static phase 2 min; dynamic phase 2 min | 40 g | Naviglio® method | [70] |
| Gallic acid | Cowpea | 50% (v/v) EtOH | 170 °C, 10.34 MPa for 10 min | 5 g | PLE | [71] |
| Gallic acid | Fucus vesiculosus | 58.65% (v/v) EtOH | 137.18 °C for 4.68 min | 1 g | PLE | [72] |
| Gallic acid | Grape marc of Croatina cultivar and olive pomace | 75% (v/v) EtOH | 180 °C for 90 min | 5 g | HPTE | [73] |
| Gallic acid | Spent coffee (Coffea arabica L.) | water | 110–190 °C; time: 15–75 min; solid-to-liquid ratio: 1:10–1:70, w/v | 4 g | SWE | [74] |
| Gallic acid | Grape skins | methanol | 150 °C for 270 min | <10 g | HPTE | [75] |
| Gallic acid | Biquinho pepper | 75% wt ethanol | inlet and outlet temperatures of 190 °C and 88 °C, respectively, 10 MPa | <5 kg | PLE | [76] |
| Gallic acid | Red wine pomace | 37.5%: 37.5%: 25% water: EtOH: CO2 | 80 °C, 25 solvent to DW ratio (kg/kg DW), 100 bar for 30 min | <5 kg | PLE | [77] |
| Polyphenolic compounds | Solanum stenotomun Peel | 80% (v/v) of ethanol in water acidified to pH 2.6 with acetic acid | 65 °C, 100 bar | 10 g/min | PLE | [78] |
| Polyphenolic compounds | Saccharina japonica | NaOH (1.54–3.64%) | 140 °C and 50 bar for 5 min | 10 g | PLE | [63] |
| Polyphenolic compounds | Corn Silage | 10% (v/v) EtOH | 180 °C, L/S ratio 20 for 120 min | <10 g | HPTE | [79] |
| Caffeic acid | Spent coffee (Coffea arabica L.) | 50% (v/v) EtOH | 150 °C | 4 g | HPTE | [80] |
| Caffeic acid | Spent coffee (Coffea canephora) | 54% (v/v) EtOH | L/S ratio of 10 mL/g, 150 °C for 60 min | <10 g | HPTE | [48] |
| Polyphenolic compounds | Lotus seedpod | water | 140 °C, L/S ratio of 70 mL/g for 20 min | <10 g | SWE | [81] |
| Polyphenolic compounds | Agave americana leaves | water | 150 °C and 10 bar for 240 min | <10 g | HPTE | [82] |
| Polyphenolic compounds | Grape skins and defatted grape seeds | water | 80–120 °C,10 MPa, 2–5 mL/min, for 2 h | 65 g | SWE | [83] |
| Polyphenolic compounds | Silybum marianum | EtOH | over half an hour | <10 g | Navi-glio® method | [84] |
| Polyphenolic compounds | Pomegranate peels | water | 40 °C and 102.1 atm for 5 min | 10 g | PWE | [85] |
| Carotenoid | Source | Sample Weight Treated | EtOH % | P Bar | T °C | t min | Recovery % | Ref |
|---|---|---|---|---|---|---|---|---|
| α-carotene | Apricot peels | 5 g | 15.5 | 350 | 59 | 30 | 97.9 | [167] |
| β-carotene | Tomato paste waste | 60 g | 5 | 300 | 65 | 120 | 50 | [168] |
| β-carotene | Tomato processing waste | 40–50 g | / | 300 | 80 | / | 88 | [169] |
| β-carotene | Sweet potato peels | 5 g | 15.5 | 350 | 59 | 30 | 99.8 | [167] |
| β-carotene | Tomato peels | 5 g | 15.5 | 350 | 59 | 30 | 96.9 | [167] |
| β-carotene | Apricot peels | 5 g | 15.5 | 350 | 59 | 30 | 99 | [167] |
| β-carotene | Pumpkin peels | 5 g | 15.5 | 350 | 59 | 30 | 88.2 | [167] |
| β-carotene | Peach peels | 5 g | 15.5 | 350 | 59 | 30 | 99.2 | [167] |
| lycopene | Tomato paste waste | 60 g | 5 | 300 | 55 | 120 | 54 | [168] |
| lycopene | Tomato processing waste | 40–50 g | / | 300 | 80 | / | 80 | [169] |
| lycopene | Tomato processing waste | 2 kg | / | 450 | 70 | 420 | 75 | [170] |
| lycopene | Tomato peels | 5 g | 15.5 | 350 | 59 | 30 | 92.5 | [167] |
| lycopene | Apricot peels | 5 g | 15.5 | 350 | 59 | 30 | 83.2 | [167] |
| lutein | Spinach by-products | 25 g | 10 | 390 | 56 | 215 | 72 | [171] |
| lutein | Sweet potato peels | 5 g | 15.5 | 350 | 59 | 30 | 99.8 | [167] |
| lutein | Peach peels | 5 g | 15.5 | 350 | 59 | 30 | 75.3 | [167] |
| chlorophyll | Spinach by-products | 25 g | 10 | 390 | 56 | 215 | 50 | [171] |
| astaxanthin | Crustacean waste | 25 g | 13 | 200 | 40 | 180 | 62 | [50] |
| astaxanthin | Shrimp waste | 60 g | / | 250 | 45 | 120 | 47 | [51] |
| fucoxanthin | Brown seaweed waste | 56 g | / | 400 | 40 | 180 | 80 | [172] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Pettinato, M.; Campardelli, R.; De Marco, I.; Perego, P. High-Pressure Technologies for the Recovery of Bioactive Molecules from Agro-Industrial Waste. Appl. Sci. 2022, 12, 3642. https://doi.org/10.3390/app12073642
Li J, Pettinato M, Campardelli R, De Marco I, Perego P. High-Pressure Technologies for the Recovery of Bioactive Molecules from Agro-Industrial Waste. Applied Sciences. 2022; 12(7):3642. https://doi.org/10.3390/app12073642
Chicago/Turabian StyleLi, Junyang, Margherita Pettinato, Roberta Campardelli, Iolanda De Marco, and Patrizia Perego. 2022. "High-Pressure Technologies for the Recovery of Bioactive Molecules from Agro-Industrial Waste" Applied Sciences 12, no. 7: 3642. https://doi.org/10.3390/app12073642
APA StyleLi, J., Pettinato, M., Campardelli, R., De Marco, I., & Perego, P. (2022). High-Pressure Technologies for the Recovery of Bioactive Molecules from Agro-Industrial Waste. Applied Sciences, 12(7), 3642. https://doi.org/10.3390/app12073642









