Abstract
Sulfated glycoproteins extracted for the first time from the Moroccan green seaweed Codium decorticatum were investigated for their ability to induce a natural defense metabolism in the roots and the upper leaves of tomato seedlings. The crude (AGB) and the purified fractions (AGP) were characterized chemically (Colorimetric assays) and structurally (SEC-MALS, GC-EI/MS, ATR-FTIR). The elicitor aqueous solutions (1 g/L) were applied by foliar spray and syringe infiltration into the internodal middle of 45-day-old tomato seedlings. Phenylalanine ammonia-lyase (PAL) activity, polyphenols, and lignin contents were measured in the roots and the leaves after 0 h, 12, 24, 48, and 72 h of treatment. The AGB and AGP extracts contained 37.67% and 48.38% of the total carbohydrates, respectively, and were mainly composed of galactose, glucose, arabinose, and a minor amount of xylose and rhamnose. They were characterized by an important molecular weight (Mw) > of 2000 × 103 g·mol−1 and a high degree of sulfation and protein (12–23% (w/w)), indicating that the extracted polysaccharides could be an arabinogalactan-rich protein present in the cell wall of the green seaweed C. decorticatum. Both crude and purified fractions exhibited an elicitor effect by inducing the PAL activity, the accumulation of phenolic compounds and lignin contents in the roots and the leaves of tomato seedlings. These responses were systemic in both the methods used (injection and foliar spray) and were mobilized throughout tissues that are not directly treated (roots and/or leaves). Regarding the elicitor activities, AGB and AGP presented globally similar patterns, which revealed the importance of crude extracts in the stimulation of plant immunity. These results suggest the new application of sulfated glycoprotein isolated from green seaweed in agriculture as inducers of natural defenses of plants.
1. Introduction
Macroalgae constitute an interesting marine biomass rich in diverse bioactive compounds, such as proteins (1–50% dry weight), lipids (0.3–5% dry weight), and carbohydrates (15–76% dry weight) [1]. More recently, marine polysaccharides present in the cell wall and the extracellular matrix of seaweed (alginate, agar, carrageenans, fucoidan, and laminaran) are the most extensively exploited components in cosmetics, nutraceutical, pharmacology, chemistry, and sustainable agriculture [2]. Polysaccharides derived from macroalgae are largely reported as bio-elicitors capable of inducing the natural defenses of plants to provide protection against a wide range of phytopathogens [3].
Sulfated carbohydrates (SPs) are most commonly used for diverse biological activities; for example, it has anticoagulant, antioxidant, antilipidemic, antithrombotic, antiviral, antihyperlipidemic, and immuno-inflammatory effects, as well as elicitor effects [4,5]. They are found predominantly as fucoidans, carrageenans, and ulvans in red, brown, and green algae [6]. The sulfated fucans, ulvans, and galactans are widely studied in crop protection [3,5,7,8]. The green algae contain a range of sulfated homo and heteropolysaccharides that are still rarely explored for their elicitor activities in agriculture. The ulvans (8 to 29% of the algal dry weight) are recognized by their high degree of sulfation [9]. These SPs are usually the most phycocolloid studied in various fields [9,10]. Recently, the SPs from the genus Codium were found to be an interesting bioactive compound, particularly used for medical purposes [11]. Currently, the Codium genus (125 species) is classified as the second group of algae rich in SPs, after the Ulva genus. Codium fragile ssp. fragile (formerly Codium fragile ssp. Tomentosoides) is the most invasive green algae in the world [12,13]. The cell walls of the Codium species are mainly composed of sulfated mannans, arabinans, galactans, and arabinogalactan-rich proteins (AGPs) [14,15,16].
AGPs, also known as arabinogalactan type II (AG type II), are cell wall glycoproteins belonging to the hydroxyproline-rich glycoproteins (HRGPs) family. They are found principally in plants, some microorganisms, and have recently been detected in the seagrass species Zostera marina L. [17,18,19]. Arabinogalactans from the green seaweed Codium seem to be almost identical to terrestrial plants [16]. They are structurally characterized by the presence of a β-(1,3) and/or β-(1,6)- linked d-galactopyranose chain attached to hydroxyproline (Hyp) residues [17,18]. The non-reducing ends often consist of a main chain of l-arabinofuranose, l-arabinopyranose, d-mannose, d-xylose, d-glucose, l-rhamnose, l-fucose, d-glucosamine, and d-glucuronic acid [17,19,20]. Concerning the Codium genus, the AGPs have been detected for the first time in C. fragile, which were composed mainly of linear (1→4)-β- d-mannans, pyruvylated arabinogalactan sulfates (pAGS), and low amounts of HRGP [16]. A similar composition has been reported in the cell wall glycoproteins of C. vermilara [21]. Several studies have focused on the pharmaceutical applications of AGPs, but no previous research has investigated the use of these compounds in sustainable agriculture, especially as inducers of the natural defenses of plants. This approach is one of the new promising alternatives to chemical pesticides that focus on the use of natural products initiating the defense mechanisms leading to the stimulation of plant resistance against a wide range of phytopathogens.
Codium decorticatum (formerly Codium elongatum) is a dark green alga belonging to the family of Codiales, which grows on hard substrate in the lower mediolittoral zone. On the Moroccan coast, this species exhibits massive growth and large distribution. Despite their richness in bioactive compounds, they are not valorized locally [22]. As far as we know, the AGPs have never been isolated from C. decorticatum or used as a bio-elicitor of the natural defenses of plants. Therefore, the main aim of this work is to determine the structure of the cell wall glycoprotein of the Moroccan green seaweed C. decorticatum and to investigate, for the first time, their ability to induce natural defense mechanisms, through the measurement of the phenylalanine ammonia-lyase (PAL) activity, the accumulation of phenolic compounds, and the lignin contents in the roots and the leaves of tomato seedlings.
2. Materials and Methods
2.1. Collection of C. decorticatum Samples
The seaweed samples were collected by hand, during July 2019, at low tide, from the intertidal zone of the Moroccan Atlantic coast in the south of El Jadida city (Sidi Bouzid station: Latitude 32° 150′ to 33°150′; longitude 7°550′ to 9°150′) and placed into an adequate container before being transported to the laboratory. After being washed thoroughly with distilled water, the samples were oven-dried at 50 °C and then milled in fine powder.
2.2. Extraction and Purification of Sulfated Polysaccharides from C. decorticatum
One hundred of the algal powder was initially treated with 96° ethanol at room temperature for 24 h under constant stirring to remove impurities (pigments, lipids, and terpenoids). The obtained residue was dried in the oven at 50 °C and soaked in 20 volumes (w/v) of distilled water for 48 h under stirring at room temperature. After centrifugation (7000 g, 20 min), the supernatant was concentrated and precipitated with 3 volumes of 96° ethanol and left overnight at −20 °C. The lyophilized fraction constitutes the crude polysaccharides (AGB). On the other side, a purified fraction (AGP) was obtained by ultrafiltration using Vivaflow 50R-200 with a membrane of 100 kDa MWCO. This system involves the desalting and removal of components with a molecular size of less than 100 kDa. The final solution was then freeze-dried for 72 h (Figure 1).
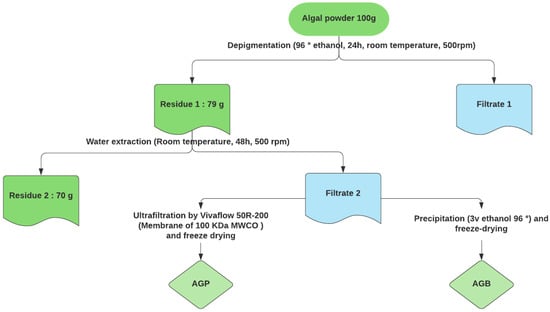
Figure 1.
Process for extraction and purification of sulfated glycoproteins from C. decorticatum.
2.3. Chemical Composition
Polysaccharide fractions were analyzed for their carbohydrates, proteins, sulfates, and polyphenol contents.
Total sugars were estimated by the Dubois assay using d-glucose as standard [23]. Neutral sugars were determined by the resorcinol sulfuric acid method and quantified according to the corrective formula described by Monsigny et al. [24]. The uronic acids were measured with m-hydroxydiphenyl (m-HDP) assay [25]. The d-galactose and d-glucuronic acid were used as standards for neutral and acid carbohydrates, respectively.
The sulfate content of the polysaccharides was determined by the BaCl2/gelatin turbidimetric method after hydrolysis with HCl (2 N) at 100 °C for 2 h. The potassium sulfate was used as the standard [26].
Proteins were estimated according to the Lowry method using bovine serum albumin (BSA) as the standard [27]. The evaluation of polyphenol contents was carried out by the Folin-Ciocalteu assay, and the results were expressed as gallic acid equivalents [28].
2.4. HPSEC-MALLS Analysis
Samples were prepared (1 g/L) in 0.1 M LiNO3 and then incubated at room temperature for 24 h under stirring. Solutions were filtrated through a 0.45 µm regenerated cellulose membrane filter and then analyzed for 120 min on a SEC-MALS-dRI-UV-280 nm system using an 804–806 column at 0.5 mL/min with an injection volume of 100 µL. The Intrinsic viscosity, the number average molecular weight (Mn), the weight average molecular weight (Mw), and the polydispersity index (Đ) were estimated using dn/dc = 0.150 mL/g.
2.5. Monosaccharide Compositions Analysis by GC-EI/MS
Dry fractions AGP and AGB (10 g/L) were treated with 2 M trifluoroacetic acid (TFA) and heated at 120 °C for 90 min under agitation in a dry bath. The TFA was discarded under a nitrogen blowdown dry evaporator (60 °C), and then the hydrolysates were washed twice with methanol to remove excess acid. Dry samples were dissolved in a mixture of pyridine and N,O-Bis (trimethylsilyl) trifluoroacetamide (BSTFA) (v/v), mixed with a vortex, then incubated for 2 h under stirring at room temperature. After evaporation, the trimethylsilyl-O-glycosides were resuspended into dichloromethane to be injected into the GC-MS system. The standard monosaccharides were treated with the same conditions as before. Analysis was carried out on a GCMS-GP2020 NX instrument from Shimadzu, using an OPTIMA-1MS (30 m, 0.25 mm, 0.25 μm) column with a constant flow rate of 1.75 mL/min. The helium pressure was set at 7.8 psi, the injection volume at 1 µL, and the split ratio at 50: 1. The oven program was started at 100 °C for 3 min; afterward, the temperature was increased at 8 °C/min to 200 °C for 1 min and finally at 5 °C/min to 215 °C. The mass spectra were obtained by the electronic impact of 70 eV applying the following parameters: injector temperature 250 °C, ion source temperature 150 °C, and the target ion range m/z 40–800 [29]. The data analysis was performed using MestReNova 7.1. software (Mestrelab Research, Escondido, CA, USA), and the results were expressed in a molar ratio (%).
2.6. ATR-FTIR Spectroscopy
Fourier Transform Infrared (FTIR) spectroscopies of the crude extract (AGB) and the purified fraction (AGP) of C. decorticatum, were analyzed with a VERTEX 70 FTIR instrument using an ATR A225 diamante. The IR spectra of dried polysaccharides were recorded after 50 scans at room temperature and referenced against the air in the wavenumber range of 500–4000 cm−1. The data analysis was performed using OPUS 7.2 software (Bruker, Billerica, MA, USA).
2.7. Elicitor Applications
The sterile seeds of tomato (Var. Campbell 33) were germinated in individual pots containing peat substrate for three weeks in a growth room at 30 °C, with a photoperiod of 16/8 h (day/night). The young seedlings were then transplanted into pots containing a mixture of soil and peat (v/v) and grown under greenhouse conditions. The polysaccharide aqueous solutions (1 g/L, pH 6) were applied to 45-day-old tomato seedlings by foliar spray until run-off (20 mL) and syringe infiltration (10 µL) into the internodal middle of plants. The control plants were treated with distilled water. The roots and two apical leaves (n and n − 1) of four tomato seedlings were collected after 0, 12, 24, 48, and 72 h for each treatment group.
2.8. Phenylalanine Ammonia Lyase (PAL) Activity and Protein Assays
The determination of PAL activity was carried out according to the Liu et al. method with slight modifications [30]. Briefly, the root and the leaf tissues of tomato seedlings previously ground were treated by a buffer solution containing 100 mM boric acid, pH 6, 1 mM of ethylenediaminetetraacetic acid (EDTA), and 1% of insoluble Polyvinylpolypyrrolidone (PVPP). After centrifugation (10,000 g, 30 min, 4 °C), 200 µL of enzyme extracts were added to 1 mL of boric acid (100 mM) and 200 µL of l-phenylalanine (20 mM). The solutions were incubated for 60 min at 30 °C and the reaction was stopped by adding HCl (6 N). The absorbance was read at 280 nm and the results were expressed in µmol of trans-cinnamic acid/mg proteins/h/mg of dry weight (DW) using an extinction coefficient of 9116 L·mol−1 cm−1 [31]. The protein assays were performed according to the Bradford method, using bovine serum albumin (BSA) as the standard [32].
2.9. Extraction and Purification of Total Phenolic Compounds
The polyphenols were extracted according to the method described by El Modafar et al. [33]. Briefly, samples were ground in liquid nitrogen in the presence of acetone 80°, the homogenates were then incubated in an ultrasonic bath for 30 min at 4 °C and centrifuged at 10,000 g for 15 min at 4 °C. The recovered supernatants were evaporated, then depigmented and delipidated by adding 1 mL of distilled water and ½ v of petroleum ether. The obtained aqueous phases were treated with 2% of metaphosphoric acid and ethyl acetate (v/v), and then the phenolic extracts were evaporated and dissolved in acetone 80°.
The estimation of total phenolic compounds was performed according to the Budini et al. method [34] with slight modifications. One hundred microliters of purified polyphenols were added to Folin Ciocalteu reagent (1/3) and sodium carbonate (20%). Afterward, the solutions were incubated at 40 °C for 30 min. The absorbances were read at 760 nm, and the results were expressed in µg of gallic acid/g of dry weight (DW).
2.10. Extraction and Quantification of Lignin Content
Root and leaf tissues were macerated in the presence of acetone 80°, then the homogenates were centrifuged at 7000 g for 20 min. The recovered pellets were dried at room temperature and treated with HCl (2 M) and thioglycolic acid (1 M). The mixtures were heated at 100 °C for 8 h and centrifuged at 7000 g for 20 min at 4 °C. The supernatants were discarded and the pellets were washed with distilled water to remove residual acids and then resuspended in NaOH (1 M). After incubation (20 °C, 18 h) and centrifugation (7000 g, 20 min, 4 °C), the supernatants were recuperated and treated with HCl (12 N). Afterward, the samples were left overnight at 4 °C in order to precipitate the thioglycolic acid-lignin complex and then dissolved in NaOH (1 M). The absorbances were measured at 280 nm, and the lignin contents were expressed in A280/g of dry weight (DW) [35].
2.11. Statistical Analysis
The data analysis was performed using the ANOVA test in SPSS version 25.0 software, and the results were presented as mean value ± Standard error (SE). Statistical differences were considered significant at p < 0.05.
3. Results
3.1. Extraction Yield and Chemical Composition of AGB and AGP Fractions
The AGB and AGP polysaccharides were extracted from the green seaweed C. decorticatum from the Moroccan coast of El Jadida city. The extraction was carried out using water and then precipitated by ethanol to obtain the crude polysaccharides (AGB). For the purified fractions (AGP), ultrafiltration was performed using a cut-off membrane of 100 kDa in order to discard all particles with molecular weight < 100 kDa. As illustrated in Table 1, the extraction yield of AGB (11%) was higher than that obtained for AGP fractions (5%).

Table 1.
Chemical analysis and extraction yields of AGB and AGP fractions from green seaweed C. decorticatum.
The chemical compositions of crude and purified polysaccharides are summarized in Table 1. AGB and AGP fractions contained 37.67% and 48.38% of total carbohydrates, respectively—mainly neutral sugars—37.56% for AGB, and 45.68% for AGP, with a minor amount of uronic acids (<5%). The presence of a high degree of sulfation and proteins, in the range of 12–23%, indicated that the extracted polysaccharides belonged to sulfated glycoproteins in the cell wall of C. decorticatum.
3.2. Molecular Weight Analysis of AGB and AGP Polysaccharides by SEC-MALLS
The molecular mass distribution of extracted polysaccharides was determined by High-Performance Size-Exclusion Chromatography coupled with on-line Multi-angle Laser Light Scattering (HPSEC-MALLS). AGB and AGP were characterized by Mn of 711 × 103 g·mol−1 and 1295 × 103 g·mol−1, respectively, and Mw of 2173 × 103 g·mol−1 for the crude fraction and 2042 × 103 g·mol−1 for the purified polysaccharides (Table 2).

Table 2.
Determination of molecular weight of sulfated glycoprotein extracted from C. decorticatum.
The dispersion characterization of molar masses in polysaccharide samples exhibited a polydispersity index of 3.1 for AGB and 1.6 for AGP. This indicated that the purified fractions are monodisperse polymers, meaning that the samples are composed of a single macromolecular species with slight degradation during the extraction and purification processes. However, the crude extracts seemed to be a polydisperse polymer with Đ > 2, indicating the presence of macromolecular species of different molar masses. The measurement of the intrinsic viscosity revealed that AGB represented a [η] value of 266.7 mL·g−1 and 560.6 mL·g−1 for AGP (Table 2).
3.3. Monosaccharide Compositions of AGB and AGP Fractions
The monosaccharide compositions were performed by GC-EI/MS analysis. Based on the chromatograms (Figure 2), the molar ratio (%) was calculated, and the results were summarized in Table 3. The sulfated glycoproteins were essentially composed of 46.07% and 51.55% of galactose, 38.78% and 24.56% of glucose, and 12.08% and 17.32% of arabinose, for AGB and AGP, respectively, with minor amounts of xylose and rhamnose.
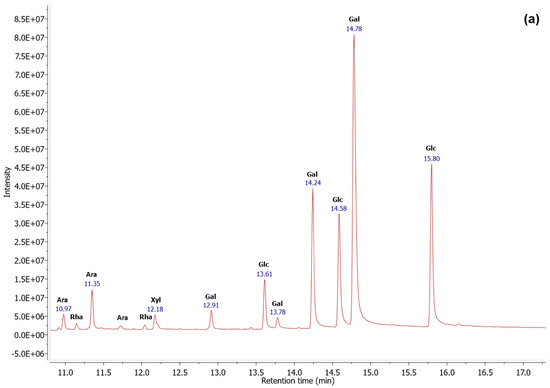
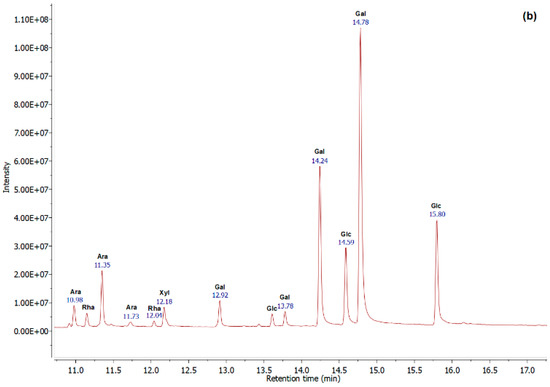
Figure 2.
GC-EI/MS profiles of the sulfated glycoproteins AGB (a) and AGP (b), obtained using OPTIMA-1MS (30 m, 0.25 mm, 0.25 μm) column at a flow rate of 1.75 mL/min.

Table 3.
Monosaccharide compositions determined by GC-EI/MS of sulfated glycoproteins AGB and AGP extracted from the green seaweed C. decorticatum.
The chemical analysis and the monosaccharide compositions of the sulfated glycoproteins extracted from C. decorticatum indicate that the studied polysaccharides seem to be a sulfated arabinogalactan type II (AGPs).
3.4. Fourier Transform Infrared Spectroscopy
The FTIR spectroscopy was performed to identify the functional groups of polysaccharide samples (Figure 3). The IR spectrum of isolated crude and purified fractions showed bands at ≈3270–3300 cm−1 and 2920 cm−1, indicating the presence of -OH and -CH stretching, respectively [36]. The intense band in the range of 1620–1631 cm−1 might correspond to amide I, which is mostly a carbonyl (C-O) stretching. The peak at 1537 and 1541 cm−1 could be attributed to the amide II (N-H) band. The signals extending from 1449 to 1377 cm−1 might contribute to the amide III bands [37,38], and absorption in the range of 1141–1216 cm−1 was attributed to the sulfate ester functional groups. The strong signal at ≈1019–1022 cm−1 indicated the stretching vibrations of sulphoxides (S=O). The wavenumber at 930–934 cm−1 could correspond to the absorbance of galactopyranose [39]. Around 840 cm−1 to 850 cm−1, the signals indicate the sulfate location bonded in the axial or equatorial positions [39]. The intense band in the range of 570–594 cm−1 is characteristic of the bending vibration of C-O-SO3. Based on the peak intensities, the AGB and AGP fractions contained a significant content of sulfate groups, indicating that the extracts were confirmed as sulfated polysaccharides.
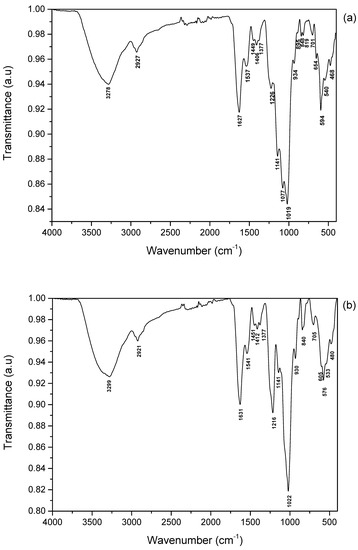
Figure 3.
ATR-FTIR spectra of sulfated glycoproteins from C. decorticatum, AGB (a), and AGP (b).
3.5. Effect of AGB and AGP Fractions on the Induction of PAL Activity in the Roots and the Leaves of Tomato Seedlings
AGB and AGP extracts were applied using two modes of treatment, foliar spray, and syringe infiltration of 1 g/L of elicitors. Control plants were treated with water. The PAL activity was measured in the roots and two apical leaves (n and n − 1) of 45-day-old tomato seedlings. The sulfated glycoproteins significantly induced the PAL activity in the roots and the leaves of treated plants, depending on the incubation period (p < 0.05). After the injection of AGB, the PAL activity showed an upward trend in the leaves from 24 h, achieving a peak value 1.6-fold higher after 72 h. In response to AGP, the activity in the leaf tissue was induced after 24 h and 72 h (Figure 4a). In the roots, levels rose slowly after 48 h and 72 h after the treatment by both fractions (Figure 4b). Based on this, both fractions exhibited considerable induction of the PAL activity. The crude fractions (AGB) showed an elicitor capacity more effective in the leaf tissue; however, the purified polysaccharides (AGP) exhibited an efficient activity in the roots of tomato seedlings (Figure 4).
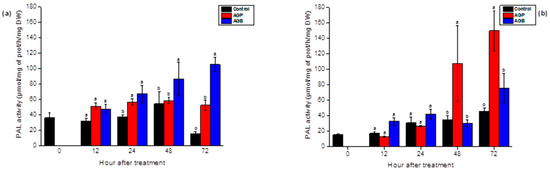
Figure 4.
Stimulation of PAL activity in the leaves (a) and the roots (b) of tomato seedlings after injection (10 µL) of AGB and AGP fractions (1 g/L). Each value is the mean of four repetitions ± SE. The letters (a, b, o) indicate significant differences between treatments (Control, AGB, and AGP) in each point time using Tukey-HSD test, at (p < 0.05). Treatments presented with the same letter are not significantly different.
The foliar spray of the AGB and AGP extracts revealed globally similar responses to PAL activity. As illustrated in Figure 5, a significant increase was observed after 72 h of treatment by both sulfated polysaccharides in the leaves and the roots. After 48 h, the elicitor activity was more important in the roots in responses to AGP treatment. In the leaves, the stimulation of the PAL was precocious after the injection of elicitors (24 h) compared to foliar spray. The sulfated glycoproteins showed fluctuations in the elicitor effect depending on the elicitation method (injection and foliar spray) (Figure 4 and Figure 5).
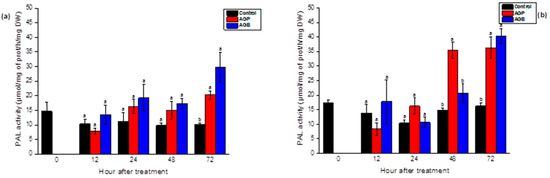
Figure 5.
Stimulation of PAL activity in the leaves (a) and the roots (b) of tomato seedlings after foliar spray (20 mL) of AGB and AGP fractions (1 g/L). Each value is the mean of four repetitions ± SE. The letters (a, b) indicate significant differences between treatments (Control, AGB, and AGP) in each point time using a Tukey-HSD test at (p < 0.05). Treatments presented with the same letter are not significantly different.
3.6. Effect of AGB and AGP Fractions on the Accumulation of Phenolic Compounds in the Roots and the Leave Tissues of Tomato Seedlings
The accumulation of polyphenols in the leaves and the roots of tomato plants was significantly (p < 0.05) triggered after treatment by AGB and AGP compared to the control. These responses depended on the incubation period. Compared to the control, the injection of AGB strongly increased the levels of polyphenols, reaching peak values 5 and 2 times higher in the leaves and the roots, respectively, after 48 h (Figure 6). In response to AGP infiltration, the total phenolic compounds rose sharply after 12 h and 48 h in the leaves, and after 12 and 24 h of treatment in the roots (Figure 6).
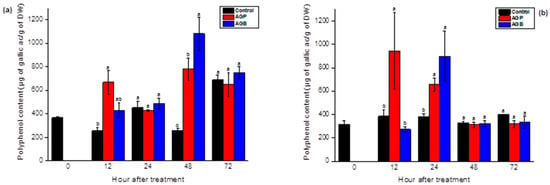
Figure 6.
Accumulation of phenolic compounds in the leaves (a) and the roots (b) of tomato seedlings after injection (10 µL) of AGB and AGP fractions (1 g/L). Each value is the mean of four repetitions ± SE. The letters (a, b, o) indicate significant differences between treatments (Control, AGB, and AGP) in each point time using the Tukey-HSD test, at (p < 0.05). Treatments presented with the same letter are not significantly different.
After foliar treatments, both sulfated polysaccharides showed a substantial accumulation of total polyphenols in the leaves after 12 h of incubation, to achieve a significant drop in the following days. For plants treated by AGB fractions, levels hit the lowest point after 24 h and 48 h of treatment compared to the control (Figure 7a). Nevertheless, the synthesis of these metabolites was mobilized to the root tissue, reaching higher values after 24 h and 48 h in response to AGP application and after 48 h in the plants treated by AGB extracts (Figure 7b). Compared to the AGB, the elicitor capacity of AGP was more efficient in the roots (Figure 7b). Globally, the AGB fractions showed a considerable increase of polyphenols in the leaves, whereas the AGP showed an important accumulation of these metabolites in the roots (Figure 7 and Figure 8).
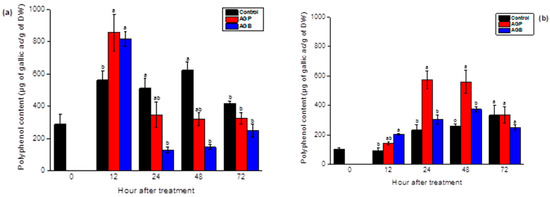
Figure 7.
Accumulation of phenolic compounds in the leaves (a) and the roots (b) of tomato seedlings after foliar spray (20 mL) of AGB and AGP fractions (1 g/L). Each value is the mean of four repetitions ± SE. The letters (a, b, o) indicate significant differences between treatments (Control, AGB, and AGP) in each point time using the Tukey-HSD test, at (p < 0.05). Treatments presented with the same letter are not significantly different.
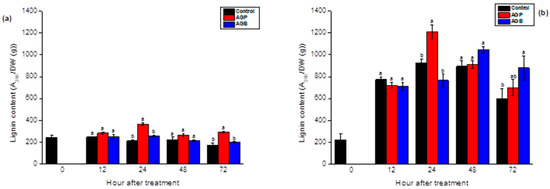
Figure 8.
Lignin content in the leaves (a) and the roots (b) of tomato seedlings after injection (10 µL) of AGB and AGP fractions (1 g/L). Each value is the mean of four repetitions ± SE. The letters (a, b) indicate significant differences between treatments (Control, AGB, and AGP) in each point time using the Tukey-HSD test, at (p < 0.05). Treatments presented with the same letter are not significantly different.
3.7. Effect of AGB and AGP Fractions on the Lignin Content in the Root and the Leaf Tissues of Tomato Seedlings
The AGB and AGP fractions showed significant elicitor activity by stimulating the lignin synthesis in the leaves and the roots of the 45-day-old tomato seedlings. The responses were related to the incubation period (p < 0.05). The lignin contents were initially increased in the leaves at 24 h and 72 h after the injection of purified polysaccharides (AGP) (Figure 8a). These metabolites were promoted in the roots after 24 h and 72 h in response to AGP and AGB, respectively (Figure 8b). The AGB did not show a significant response in the leaves. The treatment by AGP fractions exhibited an elicitor activity more effective in the leaves and the roots compared to crude extracts (AGB).
The foliar spray of purified extracts (AGP) exhibited an accumulation of lignin polymers after 48 h and 72 h of incubation. Levels were enhanced slowly (72 h) after the elicitation by AGB, which were slightly lower compared to AGP (Figure 9a). In the roots, the responses were more promoted for both sulfated glycoprotein fractions. The values initially rose from 12 h and remained higher throughout the following days, to reach the control levels after 72 h of treatment (Figure 9b). The lignin contents were more induced in the roots compared to the leaves (Figure 8 and Figure 9).
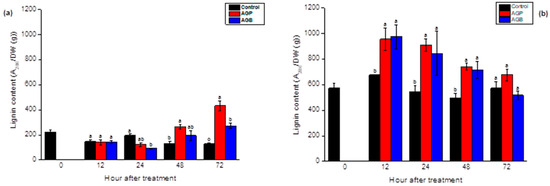
Figure 9.
Lignin content in the leaves (a) and the roots (b) of tomato seedlings after foliar spray (20 mL) of AGB and AGP fractions (1 g/L). Each value is the mean of four repetitions ± SE. The letters (a, b, o) indicate significant differences between treatments (Control, AGB, and AGP) in each point time, using the Tukey-HSD test, at (p < 0.05). Treatments presented with the same letter are not significantly different.
4. Discussion
The induction of the natural defenses of plants in response to polysaccharides from marine seaweed is one of the new promising alternative strategies in crop protection. Fucans, fucoidans [7], alginates [40], and laminarins [41] from the Phaeophyceae, Ulvans, and glucuronan [5] from the Chlorophyceae, and carrageenans [42] from the Rhodophyceae are the principal carbohydrates studied for their elicitor activities to control plant disease. A recent review has discussed the application of polysaccharides derived from macroalgae in crop protection [43]. The present work shows for the first time the ability of sulfated glycoproteins extracted from the Moroccan green seaweed C. decorticatum to enhance the natural defense mechanisms of tomato seedlings.
The crude and purified polysaccharides AGB and AGP were extracted from C. decorticatum with deionized water. The purification was carried out by ultrafiltration using 100 kDa molecular weight cut-off membranes. The yield of AGB and AGP fractions was about 11% and 5% (w/w). The chemical and structural analysis revealed the presence of a significant rate of proteins and sulfates (Table 1) and the presence of galactose, arabinose, and glucose as the principal constitutive monosaccharides with a minor amount of xylose and rhamnose (Table 3). The sulfated glycoproteins extracts were characterized by a high molecular weight (Mw) (>of 2000 kDa) (Table 2). Additionally, the FTIR spectroscopy has confirmed the presence of sulfates and proteins in both extracts by a strong signal extending from 1000 to 1600 cm−1. Basically, the proteins are known by the presence of bands between 1720 and 1600 cm−1 (intense peaks at 1627 cm−1 for AGB and 1631 cm−1 for AGP) corresponding to the amide I, amide II band, ranging from 1600 and 1500 cm−1 (peaks at 1537 and 1541 cm−1 for AGB and AGP, respectively), and the amide III band between 1450 and 1200 cm−1 (peaks extending from 1449 cm−1 to 1377 cm−1 for both AGB and AGP) [37,38]. This result ties approximately with previous work reported by Zhou et al., wherein the quality of AGPs extracted from green tea were studied [38]. Based on these, the AGB and AGP fractions seem to be a sulfated arabinogalactan-rich protein in the cell wall of the green seaweed C. decorticatum.
One of the features that distinguishes seaweed from other resources is its large content of sulfated polysaccharides (SPs). In contrast to brown and red seaweed, green algae are still insufficiently explored as a source of SPs. The Ulvans derived from Ulva sp. are the most studied SPs because of their widespread application in agriculture, human health, and biomaterials [10]. Recently, attention has been devoted to the investigation of the SPs derived from the Codium genus of the order Bryopsidales to hunt for different SPs present in green seaweed. The chemical and structural analysis of the cell wall polysaccharides from Codium sp. has shown the presence of three different types of SPs, sulfated galactans, sulfated arabinans, and sulfated mannans, as well as hydroxyproline-rich glycoproteins (HRGPs), also known as AGPs [11]. Love and Percival (1964) have explored for the first time the cell wall polysaccharides of C. fragile, and they have found that the cold and hot water extracts contained galactose and arabinose as major sugars with minor amounts of glucose, mannose, xylose, and rhamnose [44]. The yield of different fractions was in the range of 0.6–5%. In addition, the extract, which was considered sulfated arabinogalactans, contained 22% of sulfates and 25% of proteins [44]. These chemical and structural features are approximately similar to those provided in the present study. It was reported in another work that the polysaccharides extracted with water at room temperature from C. fragile (Argentina) were composed mostly of galactose and arabinose with minor quantities of xylose, mannose, and glucose, and with a high content of sulfates (20.6%) and proteins (11.3%). This indicates the occurrence of low amounts HRGP epitopes as well as pyruvate arabinogalactan sulfates (pAGS) [16]. Similar cell wall structures have been found in the green seaweed C. vermilara, which was composed of small amounts of hydroxyproline-rich glycoprotein-like (HRGP-L) [21]. The yield, structure, chemical composition, and content of polysaccharides in seaweed varied among each species, depending on the age, season, geographic location, and climatic conditions, as well as the extraction processes [45]. Conventional extraction of the cell wall SPs from Codium sp. is typically performed using water as a solvent under specific temperature conditions for several hours. As discussed earlier, when the aqueous extraction was performed at room temperature, sulfate AGPs were the main obtained polysaccharides from C. fragile; however, when hot water was used (i.e., 90 °C for 3 h), the major components were sulfated mannans [16]. The extraction of sulfated mannans with hot water was also confirmed in previous works using C. vermilara [14] and C. fragile [46]. The difference in the monosaccharide composition of Codium sp. extracts seems to be related to the extraction procedure; thus, the sulfated arabinogalactans and arabinans seem to have a high solubility in water at room temperature and the sulfated mannans in hot aqueous temperature [11,46]. It was reported that the Mw of water-soluble polysaccharides isolated from C. fragile was in the range of 148–4879 kDa, including a subfraction with an Mw of 2017 kDa [46]. These are in accordance with the Mw of the AGB and AGP fractions from C. decorticatum obtained in this work. However, several studies revealed that the SPs extracted from C. vermilara, C. fragile, and C. isthmocladum were characterized by a low Mw, ranging from 10 to 140 kDa [6,11,14,47,48]. The green seaweed Ulva sp. is commonly known as a source of ulvans; nevertheless, Přerovská et al. reported for the first time the presence of AGP-like glycoproteins in Ulva lactuca with an Mw ranging from 100 to 250 kDa [49]. The structural features of cell wall polysaccharides from C. decorticatum collected along the Patagonic coast (Argentina) revealed the presence of 6.9% of soluble and 32.9% of fibrillar carbohydrates [50]. Four water soluble polysaccharides were detected, as well as pyruvylated and sulfated galactans, sulfated arabinans, and mannans with a low degree of sulfation. Besides this, HRGPs were co-localized with the fibrillar components [50]. In line with the present work, a glycoprotein (GLP) has been isolated from C. decorticatum (India) containing 36.24% of carbohydrates [51]. It was characterized by a molecular mass of 48 kDa and the presence of rhamnose, galactose, glucose, and mannose as the main monosaccharides [51]. Compared to the terrestrial plants, the Mw of the type II arabinogalactans varied from 10.4 to 3780 kDa [19]. The glycoproteins purified from C. decorticatum showed some similarity in terms of chemical and structural compositions with the AG-type II found in land plants [19]. Therefore, the green alga C. decorticatum could be exploited as a source of sulfated arabinogalactans-rich protein and might be an alternative to terrestrial plants, due to its abundance, the important yield of cell wall AGPs, and the facility of the extraction procedure, which uses only water as an extractant.
In order to investigate the elicitor capacities of the sulfated glycoproteins isolated from C. decorticatum, the crude and purified extracts (AGB and AGP) were foliar sprayed and injected in 45-day-old tomato seedlings. The results showed that both extracts induced the natural defense mechanisms by enhancing the PAL activity, the phenolic compounds, and the lignin contents in the upper leaves (n and n − 1) and the roots depending on the incubation period. PAL is the key enzyme associated with the natural defenses of plants; it is one of the most studied enzymes in plant secondary metabolisms, and it catalyzes the first reaction of the phenylpropanoid pathway through the deamination of phenylalanine to trans-cinnamic acid, which is an essential precursor to the biosynthesis of the majority of polyphenols and lignin [52]. Lignin is a complex polymer derived from the polymerization of monolignols in the presence of peroxidases and laccases. Lignin is involved in plant resistance to pathogens by providing rigidity to the cell walls to avoid permeability to phytopathogens [53]. To the best of our knowledge, this is the first report indicating the ability of sulfated arabinogalactans-rich protein isolated from the green seaweed C. decorticatum to act as a potent elicitor of the natural defenses of plants. It has been previously reported that aqueous (hot/cold) extracts from C. decorticatum, harvested from the Karachi coast, were found to be active elicitors capable of inducing browning and secondary metabolites in chickpea tissues [54,55]. The extracted polysaccharides were characterized by the presence of glucose, rhamnose, arabinose, xylose, fucose, and glucuronic and galacturonic acids as the main monosaccharides, with a low degree of sulfates (<4 to 8%) and a negligible amount of proteins (0.5%) [55]. These features are completely different from those found in this work using the same species, which illustrates the influence of the geographic location and the extraction processes, as discussed above.
At present, alginates, fucoidans, laminarins, carrageenans, glucuronans, and ulvans are the main polysaccharides, isolated from marine macroalgae, that have been reported as bio-elicitors of defense mechanisms in diverse plants [3,5,7,8,40,41,56]. Currently, the only polysaccharide from the macroalgae released in the market as an elicitor is laminarin from the brown algae Laminaria digitata, known as Iodus 40 [57]. This product is solely used to control the powdery mildew on wheat in the field [58]. As has been previously reported in the literature, glycoproteins (GP) isolated from several microorganisms are known as elicitors and are capable of stimulating plant defense responses. A peptide-galactoglucomannan isolated from the cell wall of the fungal plant pathogen Cladosporium fulvum has enhanced the accumulation of phytoalexins in treated tomato leaves and fruits. The monosaccharide composition was mannose, galactose, and traces of glucose, and the protein moiety was rich in alanine, asparagine/aspartic acid, glutamine/glutamic acid, proline, serine, and threonine [59]. GPs extracted from other fungi, namely Verticillium dahliae, Phytophthora palmivora, Phytophthora megasperma, Botrytis cinerea, and Alternaria tenuissima, have been found to induce phytoalexin formation in cotton cell suspension cultures [60], expression of defense genes, and accumulations of H2O2, SA, scopoletin (Scp), and abscisic acid (ABA) in rubber, tobacco, and tomato leaves [61,62,63,64]. On the other hand, GPs derived from bacteria have also exhibited plant immunity-inducing effects [65].
In this study, the tomato seedlings were treated by two different modes of application: the injection in the midstem, in which the molecules are penetrated directly into the plant system, and the foliar spray, in which elicitors are uptaken by both the stomata and cuticle of the leaves. Whatever the elicitor application, the responses followed globally similar patterns and were mobilized throughout the plant. The PAL activity, the phenolic compounds, and the lignin contents were promoted in roots after foliar application and in both roots and leaves after infiltration of elicitors in tomato plants, which were not directly treated. In this case, the responses were systemic and seemed to be related to the salicylic acid (SA). This form of defense reaction, known also as systemic acquired resistance (SAR), is a type of induced resistance wherein the SA signaling pathway is involved [66]. A previous study has shown systemic effects after syringe infiltration of polysaccharides and oligosaccharides from the green seaweed Ulva lactuca in tomato plants. These responses were accompanied by the accumulation of salicylic acid in the leaves located above and below the elicitation site [5]. The application of these elicitors to plant foliage is a practical method in the field and could be used to protect against foliar, stem, and vascular diseases, since the responses are expressed in the shoot and root systems of the plant. Furthermore, the foliar application is more prompt and target-oriented than other modes of application such as soil application because the molecules are directly in contact with the plant without dilution or degradation that can happen in soil.
Comparing the elicitor activities of the crude (AGB) and purified (AGP) extracts from C. decorticatum, the results have not shown a considerable difference in responses. Both fractions presented the same monosaccharide composition (Gal, Glc, Ara, Xyl, and Rha) and the same Mw (>to 2000 kDa), as well as an important amount of proteins and sulfates in both fractions AGB (>to 20% (w/w)) and AGP (< to 14% (w/w)). The carbohydrates, the molecular weight, and the presence of proteins and sulfates groups in this polymer seemed to be the main factors influencing the eliciting responses. To further understand this, additional studies are required. Several studies have demonstrated that the sulfate groups of some polysaccharides and oligosaccharides have the main role in various biochemical and biological activities. In tobacco and Arabidopsis thaliana, the treatment with sulfated laminarin induced an SA signaling pathway, leading to resistance against Tobacco mosaic virus infection compared to the native unsulfated laminarin [67]. In tomato seedlings, the desulfation of ulvans extracted from Ulva lactuca reduced the PAL activity in the leaves of treated plants. Compared to glucuronan, an unsulfated polysaccharide, the sulfated ulvans exhibited a more efficient elicitor effect, this difference in responses was related to the sequences of aldobiuronic acids and particularly the sulfate substituent [5]. In contrast, Caillot et al. have investigated the effects of sulfation degree variation of oligoglucuronans on H2O2 production and defense-related genes induction in grapevine cells; they found that the sulfated oligoglucuronans did not possess any significant induction of the oxidative burst whatever their degrees of polymerization [68]. On the other side, Schaffrath et al. have demonstrated the importance of carbohydrate moiety in the elicitor capacity. After the digestion of the glycoprotein from Pyricularia oryzae with pronase, the activity was not affected; however, when the molecule was altered with sodium periodate, the elicitor capacity was totally suppressed, indicating that the responses were associated with the saccharide part of the polymer [69]. As well as this, the elicitor responses of a high molecular weight glycoprotein isolated from Colletotrichum falcatum, were found to reside in the carbohydrate moiety [70]. Otherwise, others have shown that in some glycoprotein elicitors, only the protein moiety is responsible for the induction of the natural defenses of plants [60,71]. This difference could be attributed to many factors, such as plant species, monosaccharides, amino acid compositions, or the molecular weight of the molecules.
As discussed above, elicitation by the crude and the purified extract of C. decorticatum manifested globally similar responses in tomato seedlings. These results showed the importance of crude extracts in the stimulation of plant immunity. In this case, the purification steps could be skipped to avoid the yield and the algal raw material losses, as well as to minimize the process costing. The high purity of the molecule is essential for structural determination in order to investigate its biological properties and bring to light new elicitors with particular features. Using the aqueous extraction of polysaccharides is a simple and green optimization method. Additionally, it can decrease the yield loss of polysaccharides and ensure that there are no changes to its physical and chemical characteristics. For industry, this extraction process seemed to be adequate, since it fulfills all the criteria needed, such as being time-saving and cost-effective. Several studies have demonstrated the elicitor potential of aqueous extract from marine seaweed. A crude extract prepared from Ulva armoricana, essentially enriched in ulvans, has enhanced resistance against three powdery mildew pathogens on the common bean, grapevine, and cucumber [72]. Extracts of the brown alga Ascophyllum nodosum are the most commonly explored in agricultural commercial production, and are used to improve plant tolerances to abiotic and biotic stresses, to promote plant growth, and to ameliorate the root and microorganism interactions [73].
5. Conclusions
In this work, sulfated glycoproteins, identified as sulfated arabinogalactan-rich protein, was extracted for the first time from the Moroccan green seaweed C. decorticatum. This molecule was characterized by high molecular weight and a significant degree of sulfates and proteins. The application of the crude (AGB) and the purified extracts (AGP) revealed their capacity to trigger the natural defense mechanisms by inducing PAL activity, the accumulation of polyphenols, and the lignin content in the roots and the upper leaves of tomato seedlings. These responses were systemic and were mobilized beyond the plant tissues after foliar spray and infiltration of elicitors. These results highlighted the novel elicitor potential of the sulfated glycoproteins isolated from seaweed, as well as their new application in agriculture. The hunt for new bioactive compounds from marine algae becomes very noticeable and challenging, which can lead to the discovery of new drugs with various applications.
Author Contributions
Conceptualization, M.A., Z.E.A.-T., C.D. and C.E.M.; methodology, M.A., Z.E.A.-T., C.D., D.L.C., C.R. and C.E.M.; software, M.A.; validation, M.A., Z.E.A.-T., C.D. and C.E.M.; formal analysis, M.A., Z.E.A.-T., C.D. and C.E.M.; investigation, M.A., Z.E.A.-T., C.D. and C.E.M.; resources, M.A., Z.B., M.D.O.E.-H. and H.R.; data curation, M.A.; writing—original draft preparation, M.A.; writing—review and editing, M.A., Z.E.A.-T., C.D., C.E.M., G.P., P.D. and P.M.; visualization, M.A., Z.E.A.-T., C.D., I.F., S.A. and C.E.M.; supervision, Z.E.A.-T., C.D. and C.E.M.; project administration, G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the French Ministry of Europe and Foreign Affairs and the Moroccan, Algerian and Tunisian Ministries of Higher Education and Scientific Research, within the framework of the Hubert Curien program (PHC Maghreb), grant number 19MAG36, and by the National Centre for Scientific and Technical Research (CNRST Morocco), grant number 4UCA2018.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bedoux, G.; Hardouin, K.; Burlot, A.S.; Bourgougnon, N. Bioactive Components from Seaweeds. In Sea Plants; Academic Press: Cambridge, MA, USA, 2014; pp. 345–378. [Google Scholar]
- Rioux, L.-E.; Turgeon, S.L. Seaweed carbohydrates. In Seaweed Sustainability; Academic Press: Cambridge, MA, USA, 2015; pp. 141–192. [Google Scholar]
- Vera, J.; Castro, J.; Gonzalez, A.; Moenne, A. Seaweed polysaccharides and derived oligosaccharides stimulate defense responses and protection against pathogens in plants. Mar. Drugs 2011, 9, 2514–2525. [Google Scholar] [CrossRef] [PubMed]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Modafar, C.; Elgadda, M.; El Boutachfaiti, R.; Abouraicha, E.; Zehhar, N.; Petit, E.; El Alaoui-Talibi, Z.; Courtois, B.; Courtois, J. Induction of natural defence accompanied by salicylic acid-dependant systemic acquired resistance in tomato seedlings in response to bioelicitors isolated from green algae. Sci. Hortic. 2012, 138, 55–63. [Google Scholar] [CrossRef]
- Farias, E.H.; Pomin, V.H.; Valente, A.P.; Nader, H.B.; Rocha, H.A.; Mourao, P.A. A preponderantly 4-sulfated, 3-linked galactan from the green alga Codium isthmocladum. Glycobiology 2008, 18, 250–259. [Google Scholar] [CrossRef] [Green Version]
- Bouissil, S.; Alaoui-Talibi, Z.E.; Pierre, G.; Rchid, H.; Michaud, P.; Delattre, C.; El Modafar, C. Fucoidans of Moroccan Brown Seaweed as Elicitors of Natural Defenses in Date Palm Roots. Mar. Drugs 2020, 18, 596. [Google Scholar] [CrossRef] [PubMed]
- Abouraïcha, E.; El Alaoui-Talibi, Z.; El Boutachfaiti, R.; Petit, E.; Courtois, B.; Courtois, J.; El Modafar, C. Induction of natural defense and protection against Penicillium expansum and Botrytis cinerea in apple fruit in response to bioelicitors isolated from green algae. Sci. Hortic. 2015, 181, 121–128. [Google Scholar] [CrossRef]
- Lahaye, M.; Robic, A.J.B. Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Kidgell, J.T.; Magnusson, M.; de Nys, R.; Glasson, C.R.K. Ulvan: A systematic review of extraction, composition and function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Fernández, P.V.; Arata, P.X.; Ciancia, M. Polysaccharides from Codium Species. In Sea Plants; Academic Press: Cambridge, MA, USA, 2014; pp. 253–278. [Google Scholar]
- Provan, J.; Murphy, S.; Maggs, C.A. Tracking the invasive history of the green alga Codium fragile ssp. tomentosoides. Mol. Ecol. 2005, 14, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, X.; Wu, H.; Liu, R. Overview on biological activities and molecular characteristics of sulfated polysaccharides from marine green algae in recent years. Mar. Drugs 2014, 12, 4984–5020. [Google Scholar] [CrossRef] [PubMed]
- Fernández, P.V.; Estevez, J.M.; Cerezo, A.S.; Ciancia, M. Sulfated β-d-mannan from green seaweed Codium vermilara. Carbohydr. Polym. 2012, 87, 916–919. [Google Scholar] [CrossRef]
- Fernandez, P.V.; Quintana, I.; Cerezo, A.S.; Caramelo, J.J.; Pol-Fachin, L.; Verli, H.; Estevez, J.M.; Ciancia, M. Anticoagulant activity of a unique sulfated pyranosic (1->3)-beta-l-arabinan through direct interaction with thrombin. J. Biol. Chem. 2013, 288, 223–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estevez, J.M.; Fernandez, P.V.; Kasulin, L.; Dupree, P.; Ciancia, M. Chemical and in situ characterization of macromolecular components of the cell walls from the green seaweed Codium fragile. Glycobiology 2009, 19, 212–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villa-Rivera, M.G.; Cano-Camacho, H.; Lopez-Romero, E.; Zavala-Paramo, M.G. The Role of Arabinogalactan Type II Degradation in Plant-Microbe Interactions. Front. Microbiol. 2021, 12, 730543. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, L.; Shafee, T.; Johnson, K.L.; Bacic, A.; Classen, B. Arabinogalactan-proteins of Zostera marina L. contain unique glycan structures and provide insight into adaption processes to saline environments. Sci. Rep. 2020, 10, 8232. [Google Scholar] [CrossRef]
- Saeidy, S.; Petera, B.; Pierre, G.; Fenoradosoa, T.A.; Djomdi, D.; Michaud, P.; Delattre, C. Plants arabinogalactans: From structures to physico-chemical and biological properties. Biotechnol. Adv. 2021, 53, 107771. [Google Scholar] [CrossRef]
- Nagel, A.; Conrad, J.; Leitenberger, M.; Carle, R.; Neidhart, S. Structural studies of the arabinogalactans in Mangifera indica L. fruit exudate. Food Hydrocoll. 2016, 61, 555–566. [Google Scholar] [CrossRef]
- Fernández, P.V.; Ciancia, M.; Miravalles, A.B.; Estevez, J.M. Cell-Wall Polymer Mapping in the Coenocytic Macroalga Codium Vermilara (Bryopsidales, Chlorophyta)1. J. Phycol. 2010, 46, 456–465. [Google Scholar] [CrossRef]
- Abou Oualid, H.; Abdellaoui, Y.; Laabd, M.; El Ouardi, M.; Brahmi, Y.; Iazza, M.; Abou Oualid, J. Eco-Efficient Green Seaweed Codium decorticatum Biosorbent for Textile Dyes: Characterization, Mechanism, Recyclability, and RSM Optimization. ACS Omega 2020, 5, 22192–22207. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.; Hamilton, J.; Rebers, P.; Smith, F.J.N. A colorimetric method for the determination of sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef]
- Monsigny, M.; Petit, C.; Roche, A.-C. Colorimetric determination of neutral sugars by a resorcinol sulfuric acid micromethod. Anal. Biochem. 1988, 175, 525–530. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G.J.A.b. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Dodgson, K.; Price, R.J.B.J. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef] [Green Version]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.C.; De Mello, J.C.P. Application and analysis of the folin ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef] [Green Version]
- Pierre, G.; Graber, M.; Rafiliposon, B.A.; Dupuy, C.; Orvain, F.; De Crignis, M.; Maugard, T. Biochemical composition and changes of extracellular polysaccharides (ECPS) produced during microphytobenthic biofilm development (Marennes-Oléron, France). Microb. Ecol. 2012, 63, 157–169. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Jiang, W.; Bi, Y.; Luo, Y. Postharvest BTH treatment induces resistance of peach (Prunus persica L. cv. Jiubao) fruit to infection by Penicillium expansum and enhances activity of fruit defense mechanisms. Postharvest Biol. Technol. 2005, 35, 263–269. [Google Scholar] [CrossRef]
- Hendrikse, N.M.; Holmberg Larsson, A.; Svensson Gelius, S.; Kuprin, S.; Nordling, E.; Syren, P.O. Exploring the therapeutic potential of modern and ancestral phenylalanine/tyrosine ammonia-lyases as supplementary treatment of hereditary tyrosinemia. Sci. Rep. 2020, 10, 1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- El Modafar, C.; Tantaoui, A.; El Boustani, E. Changes in cell wall-bound phenolic compounds and lignin in roots of date palm cultivars differing in susceptibility to Fusarium oxysporum f. sp albedinis. J. Phytopathol. 2000, 148, 405–411. [Google Scholar] [CrossRef]
- Budini, R.; Tonelli, D.; Girotti, S.J.J.o.A.; Chemistry, F. Analysis of total phenols using the Prussian blue method. J. Agric. Food Chem. 1980, 28, 1236–1238. [Google Scholar] [CrossRef]
- El Modafar, C.; El Boustani, E. Cell wall-bound phenolic acid and lignin contents in date palm as related to its resistance to Fusarium oxysporum. Biol. Plant 2001, 44, 125–130. [Google Scholar] [CrossRef]
- Petera, B.; Delattre, C.; Pierre, G.; Wadouachi, A.; Elboutachfaiti, R.; Engel, E.; Poughon, L.; Michaud, P.; Fenoradosoa, T.A. Characterization of arabinogalactan-rich mucilage from Cereus triangularis cladodes. Carbohydr. Polym. 2015, 127, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Mallamace, F.; Corsaro, C.; Mallamace, D.; Vasi, S.; Vasi, C.; Dugo, G. The role of water in protein’s behavior: The two dynamical crossovers studied by NMR and FTIR techniques. Comput. Struct. Biotechnol. J. 2015, 13, 33–37. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.L.; Sun, P.N.; Bucheli, P.; Huang, T.H.; Wang, D. FT-IR methodology for quality control of arabinogalactan protein (AGP) extracted from green tea (Camellia sinensis). J. Agric. Food Chem. 2009, 57, 5121–5128. [Google Scholar] [CrossRef]
- Li, N.; Mao, W.; Yan, M.; Liu, X.; Xia, Z.; Wang, S.; Xiao, B.; Chen, C.; Zhang, L.; Cao, S. Structural characterization and anticoagulant activity of a sulfated polysaccharide from the green alga Codium divaricatum. Carbohydr. Polym. 2015, 121, 175–182. [Google Scholar] [CrossRef]
- Aitouguinane, M.; Bouissil, S.; Mouhoub, A.; Rchid, H.; Fendri, I.; Abdelkafi, S.; Ould El-Hadj, M.D.; Boual, Z.; Dubessay, P.; Gardarin, C.; et al. Induction of Natural Defenses in Tomato Seedlings by Using Alginate and Oligoalginates Derivatives Extracted from Moroccan Brown Algae. Mar. Drugs 2020, 18, 521. [Google Scholar] [CrossRef]
- Xin, Z.; Cai, X.; Chen, S.; Luo, Z.; Bian, L.; Li, Z.; Ge, L.; Chen, Z. A Disease Resistance Elicitor Laminarin Enhances Tea Defense against a Piercing Herbivore Empoasca (Matsumurasca) onukii Matsuda. Sci. Rep. 2019, 9, 814. [Google Scholar] [CrossRef]
- Sangha, J.S.; Khan, W.; Ji, X.; Zhang, J.; Mills, A.A.; Critchley, A.T.; Prithiviraj, B. Carrageenans, sulphated polysaccharides of red seaweeds, differentially affect Arabidopsis thaliana resistance to Trichoplusia ni (cabbage looper). PLoS ONE 2011, 6, e26834. [Google Scholar] [CrossRef]
- Agarwal, P.K.; Dangariya, M.; Agarwal, P. Seaweed extracts: Potential biodegradable, environmentally friendly resources for regulating plant defence. Algal Res. 2021, 58, 102363. [Google Scholar] [CrossRef]
- Love, J.; Percival, E. 632. The polysaccharides of the green seaweed Codium fragile. Part II. The water-soluble sulphated polysaccharides. J. Chem. Soc. 1964, 3338–3345. [Google Scholar] [CrossRef]
- Dobrincic, A.; Balbino, S.; Zoric, Z.; Pedisic, S.; Bursac Kovacevic, D.; Elez Garofulic, I.; Dragovic-Uzelac, V. Advanced Technologies for the Extraction of Marine Brown Algal Polysaccharides. Mar. Drugs 2020, 18, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabarsa, M.; Karnjanapratum, S.; Cho, M.; Kim, J.K.; You, S. Molecular characteristics and biological activities of anionic macromolecules from Codium fragile. Int. J. Biol. Macromol. 2013, 59, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ohta, Y.; Lee, J.-B.; Hayashi, K.; Hayashi, T.J.B.; Bulletin, P. Isolation of sulfated galactan from Codium fragile and its antiviral effect. Pharm. Bull. 2009, 32, 892–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabry, D.A.; Cordeiro, S.L.; Ferreira Silva, C.H.; Cunha Farias, E.H.; Sassaki, G.L.; Nader, H.B.; Oliveira Rocha, H.A. Pharmacological prospection and structural characterization of two purified sulfated and pyruvylated homogalactans from green algae Codium isthmocladum. Carbohydr. Polym. 2019, 222, 115010. [Google Scholar] [CrossRef]
- Přerovská, T.; Henke, S.; Bleha, R.; Spiwok, V.; Gillarová, S.; Yvin, J.C.; Ferrières, V.; Nguema-Ona, E.; Lipovová, P. Arabinogalactan-like Glycoproteins from Ulva lactuca (Chlorophyta) Show Unique Features Compared to Land Plants AGPs. J. Phycol. 2021, 57, 619–635. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, P.V.; Raffo, M.P.; Alberghina, J.; Ciancia, M. Polysaccharides from the green seaweed Codium decorticatum. Structure and cell wall distribution. Carbohydr. Polym. 2015, 117, 836–844. [Google Scholar] [CrossRef]
- Senthilkumar, D.; Jayanthi, S. Partial characterization and anticancer activities of purified glycoprotein extracted from green seaweed Codium decorticatum. J. Funct. Foods 2016, 25, 323–332. [Google Scholar] [CrossRef]
- Walters, D. Plant Defense: Warding Off Attack by Pathogens, Pests and Vertebrate Herbivores; Wiley-Blackwell: Hoboken, NJ, USA, 2011. [Google Scholar]
- Pilate, G.; Dejardin, A.; Leple, J.-C. Field Trials with Lignin–Modified Transgenic Trees. In Lignins—Biosynthesis, Biodegradation and Bioengineering; Academic Press: Cambridge, MA, USA, 2012; pp. 1–36. [Google Scholar]
- Bi, F.; Iqbal, S.E. Dose dependent and time course elicitor activity of Codium elongatum and Ulva lactulus (green algae) of Karachi coast. Pak. J. Bot. 2003, 35, 511–518. [Google Scholar]
- Bi, F.; Iqbal, S. Studies on aqueous extracts of three green algae. J. Pak. J. Bot. 1999, 31, 193–198. [Google Scholar]
- Abouraïcha, E.F.; El Alaoui-Talibi, Z.; Tadlaoui-Ouafi, A.; El Boutachfaiti, R.; Petit, E.; Douira, A.; Courtois, B.; Courtois, J.; El Modafar, C. Glucuronan and oligoglucuronans isolated from green algae activate natural defense responses in apple fruit and reduce postharvest blue and gray mold decay. J. Appl. Phycol. 2016, 29, 471–480. [Google Scholar] [CrossRef]
- Renard-Merlier, D.; Randoux, B.; Nowak, E.; Farcy, F.; Durand, R.; Reignault, P. Iodus 40, salicylic acid, heptanoyl salicylic acid and trehalose exhibit different efficacies and defence targets during a wheat/powdery mildew interaction. Phytochemistry 2007, 68, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Baez, R.V. Lipid Metabolism; BoD-Books on Demand, InTech: Rijeka, Croatia, 2013. [Google Scholar]
- De Wit, P.; Kodde, E. Further characterization and cultivar-specificity of glycoprotein elicitors from culture filtrates and cell walls of Cladosporium fulvum (syn. Fulvia fulva). Physiol. Mol. 1981, 18, 297–314. [Google Scholar] [CrossRef]
- Davis, D.; Low, P.; Heinstein, P.J.P.; Pathology, M.P. Purification of a glycoprotein elicitor of phytoalexin formation fromVerticillium dahliae. Physiol. Mol. 1998, 52, 259–273. [Google Scholar] [CrossRef]
- Pettongkhao, S.; Churngchow, N. Novel Cell Death-Inducing Elicitors from Phytophthora palmivora Promote Infection on Hevea brasiliensis. Phytopathology 2019, 109, 1769–1778. [Google Scholar] [CrossRef] [PubMed]
- Baillieul, F.; Genetet, I.; Kopp, M.; Saindrenan, P.; Fritig, B.; Kauffmann, S. A new elicitor of the hypersensitive response in tobacco: A fungal glycoprotein elicits cell death, expression of defence genes, production of salicylic acid, and induction of systemic acquired resistance. Plant J. 1995, 8, 551–560. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Qiu, D.; Zeng, H.; Guo, L.; Yang, X. BcGs1, a glycoprotein from Botrytis cinerea, elicits defence response and improves disease resistance in host plants. Biochem. Biophys. Res. Commun. 2015, 457, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Qiu, D.; Zeng, H.; Yuan, J.; Mao, J. Purification and characterization of a glycoprotein elicitor from Alternaria tenuissima. World J. Microbiol. Biotechnol. 2009, 25, 2035–2042. [Google Scholar] [CrossRef]
- Han, L.; Sun, Y.; Zhou, X.; Hao, X.; Wu, M.; Zhang, X.; Feng, J. A novel glycoprotein from Streptomyces sp. triggers early responses of plant defense. Pestic. Biochem. Physiol. 2021, 171, 104719. [Google Scholar] [CrossRef]
- Choudhary, D.K.; Prakash, A.; Johri, B.N. Induced systemic resistance (ISR) in plants: Mechanism of action. Indian J. Microbiol. 2007, 47, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Menard, R.; Alban, S.; de Ruffray, P.; Jamois, F.; Franz, G.; Fritig, B.; Yvin, J.C.; Kauffmann, S. Beta-1,3 glucan sulfate, but not beta-1,3 glucan, induces the salicylic acid signaling pathway in tobacco and Arabidopsis. Plant Cell 2004, 16, 3020–3032. [Google Scholar] [CrossRef] [Green Version]
- Caillot, S.; Rat, S.; Tavernier, M.-L.; Michaud, P.; Kovensky, J.; Wadouachi, A.; Clément, C.; Baillieul, F.; Petit, E. Native and sulfated oligoglucuronans as elicitors of defence-related responses inducing protection against Botrytis cinerea of Vitis vinifera. Carbohydr. Polym. 2012, 87, 1728–1736. [Google Scholar] [CrossRef]
- Schaffrath, U.; Scheinpflug, H.; Reisener, H.J.P.; Pathology, M.P. An elicitor from Pyricularia oryzae induces resistance responses in rice: Isolation, characterization and physiological properties. Physiol. Mol. 1995, 46, 293–307. [Google Scholar] [CrossRef]
- Sundar, A.R.; Velazhahan, R.; Vidhyasekaran, P. A glycoprotein elicitor isolated from Colletotrichum falcatum induces defense mechanisms in sugarcane leaves and suspension-cultured cells/Ein Glycoprotein aus Colletotrichum falcatum induziert als Elicitor Abwehrmechanismen in Zuckerrohrblättern und Zellkulturen. Plant Dis. Prot. 2002, 109, 601–611. [Google Scholar]
- Parker, J.E.; Schulte, W.; Hahlbrock, K.; Scheel, D. An extracellular glycoprotein from Phytophthora megasperma f. sp. Glycinea elicits phytoalexin synthesis in cultured parsley cells and protoplasts. Mol. Plant Microbe Interact 1991, 4, 19–27. [Google Scholar] [CrossRef]
- Jaulneau, V.; Lafitte, C.; Corio-Costet, M.-F.; Stadnik, M.J.; Salamagne, S.; Briand, X.; Esquerré-Tugayé, M.-T.; Dumas, B. An Ulva armoricana extract protects plants against three powdery mildew pathogens. Eur. J. Plant Pathol. 2011, 131, 393–401. [Google Scholar] [CrossRef]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-Based Biostimulants: Sustainable Applications in Agriculture for the Stimulation of Plant Growth, Stress Tolerance, and Disease Management. Front. Plant Sci. 2019, 10, 655. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).