Abstract
Several metal oxide compounds, especially those containing metals possessing several valence states, are able to absorb or release oxygen under suitable thermodynamic conditions. Such behavior is found often in systems containing oxides of transition metals. It is important to note that the equilibrium oxidation level of those metal oxides can depend on the aggregation state, which may significantly impede crystal growth processes from the melt. If during the melt growth of such oxide crystals, the average valence state of the oxides is different in the molten and solid state, then crystallization is connected with the absorption of free oxygen from the ambient gas, or with the release of free oxygen into it. This phenomenon can be detected by simultaneous DTA/TG measurements and can deteriorate the stability of crystal growth. This holds especially if the average valence in the solid is smaller than in the melt, because oxygen release can lead to bubble formation at the crystallization front.
1. Introduction
1.1. Oxide Bulk Crystal Growth
The first oxide material from which crystals were grown on an industrial scale is -quartz (SiO). Quartz crystals are still of high technical relevance; their growth technique, as described in 1953 by Walker [1], relies on hydrothermal solutions. However, the equilibria between solvents and solutes will not be in the focus of this article. Rather, equilibria between oxides in the solid (crystalline) and molten state will be described, which are important for the growth of crystals from the melt, e.g., using the Czochralski, Bridgman, or float-zone methods [2,3]. Here, the Czochralski method is demonstrated first for CaWO, which is now often used for different materials such as LiNbO, YAG (YAlO) and other garnets, and partially for -AlO (usually named sapphire, but “corundum” would be more accurate) [4]. These materials can be grown in different atmospheres, ranging from air to “pure” argon or nitrogen with ca. 99.9…99.999% purity. Independently of these different conditions, the constituent metal cations prevail almost completely in one oxidation state. Here, these are Ca, W, Li, Nb, Y, or Al, respectively.
Some other substances, especially many transparent conducting oxides (TCO’s), behave differently: crystals of -GaO (melting point °C, [5]) and ZnO ( °C, [6]) can be grown only if a sufficiently high oxygen partial pressure prevails in the atmosphere. With current FactSage data [7] one calculates that at their melting points, GaO (sol/liq) is stable for bar, and ZnO (sol/liq) for bar. If falls below these limits, Ga or Zn is reduced to the corresponding metal, which occurs here in a gaseous state. On the other side, sufficiently high will always stabilize Ga or Zn and, hence, GaO or ZnO because no higher oxidation state exists. The opposite holds for the melt growth of Ti doped AlO crystals (“Ti:sapphire”) because Ti is the lowest possible valence state of titanium under all conditions, where AlO can be grown from the melt. Hence, should be as low as possible under realistic experimental conditions, e.g., by introducing graphite parts in the growth setup [8,9].
The situation is more complicated if an intermediate oxidation state is desired. This is the case, for example, for wüstite FeO and olivine (Fe, Mg)SiO[10]—both containing Fe. These phases are stable only in a corridor , and the lower and upper limits rise with temperature T. It was demonstrated, however, that also under such conditions, “dynamic atmospheres” containing mixtures of CO and CO often can deliver some , which stabilizes the desired oxidation state over a wide T range [11,12]. Thermogravimetric measurements with aegirine (NaFeSiO) nanocatalysts showed mass losses up to 25% during heating to 700 °C in air. The mass loss decreased significantly if the material was doped with Ni, which substitutes for Fe as well as for Na. Even if such high mass loss results mainly from the desorption of volatiles from the surface, contributions of changing Fe valence are possible [13].
Several oxide materials with an intermediate oxidation state received attention as prospective ionic electrical conductors. In a series of publications, Han et al. demonstrated this for some mixed ionic–electronic conducting (MIEC) oxides. These oxides are typically based on the perovskite structure, or are derived from it by additional Ruddlesden–Popper-like layers in the crystal structure. Perovskites of the type LaSrCoFeO, as hollow fibers, can separate oxygen from gas mixtures [14,15,16]. Ruddlesden–Popper-like phases such as Mo-doped PrNiO and LaNiMoO showed partially enhanced stability and efficiency in gas mixtures containing carbon dioxide [17,18].
This paper intends to show that, unfortunately, the stabilization of the desired oxidation state in the solid phase is sometimes insufficient for obtaining a stable growth process for bulk crystals. Problems can arise if significantly different oxidation states of metal ions prevail in the melt, and in the crystal which is grown from that melt. Calcium ferrite CaFeO is introduced in the following subsection as a paragon in detail, and some more examples are presented later in brief.
1.2. Calcium Ferrite as Model Substance
The orthorhombic “brownmillerite-like” crystal structure of calcium ferrite CaFeO was described first by Bertaut [19] in the space group with lattice parameters Å, Å, and Å. The structure refinement by Berggren [20] used another setting of the same space group #62 with lattice parameters Å, Å, Å. In the structure, the Fe ions are coordinated by O, in alternately distorted tetrahedral and distorted octahedral environments, and form chains along the b axis. Figure 1 shows one unit cell, drawn with data from Redhammer et al. [21], where the chemical composition was found to be CaFeO. The structure is occasionally described as an ordered oxygen-deficient perovskite. One can take as the lattice parameters for this slightly distorted ordered perovskite, and this structure was reported to be stable up to at least 1100 °C [22]. Recently, it was shown, however, that the structure is incommensurately modulated already at 827 °C [23]. It should be noted that the compound CaFeO appears also as the mineral Srebrodolskite [24]. In the last years, CaFeO received rising interest as a potential component of anodes in Li-ion batteries, as well as a catalyst for the steam-iron process to produce hydrogen [25,26].
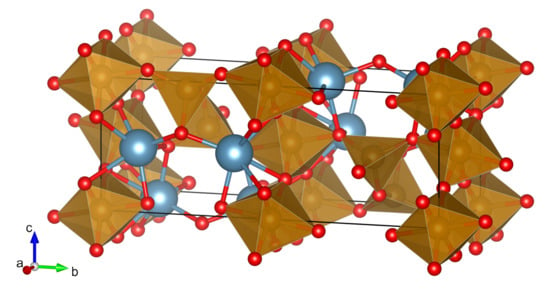
Figure 1.
In the unit cell of CaFeO, Fe ions in octahedral and tetrahedral coordination are alternating along the b axis. (Ca—blue, O—red) [21,27].
The formation of CaFeO from powders of CaCO and different iron oxides (FeO, FeO, FeO) was investigated by Jeon et al. [28]. Ceretti et al. [29] succeeded to grow CaFeO single crystals of good quality from the melt by the optical float zone (OFZ) technique, which is a strong indication for congruent melting. For this growth method, a polycrystalline stoichiometric feed rod is moved through a hot zone, which is heated by the focused radiation of lamps; Cerreti et al. used two 500 W halogen lamps for heating. (More details for the OFZ method can be found elsewhere [3]). Increased oxygen mobility in the crystals above ca. 450 °C was observed by thermogravimetric (TG) measurements of O enriched material in O atmosphere. The observed mass loss was finished at ca. 600 °C and was attributed to the O/O exchange. Congruent melting of CaFeO in air or pure oxygen at 1449 °C was also observed by Phillips and Muan [30]; but in Figure 1 of their article, these authors presented also an alternative phase diagram CaO–FeO, where this phase melts peritectically already at ca. 1435 °C under the release of solid CaO and an iron oxide rich melt.
In the following, thermoanalytic measurements and thermodynamic calculations for a x CaO + FeO mixture with fixed composition are presented. This is slightly more rich in CaO, compared to the CaFeO phase (), and near the CaO/CaFeO eutectic, which was reported by Phillips and Muan [30] at and °C. Irrespective of whether CaFeO shows congruent or incongruent melting, it is the major phase in solids with .
2. Experiments and Results
Simultaneous differential thermal analysis (DTA) and thermogravimetry (TG) was performed in a NETZSCH STA 449 F3 “Jupiter” with “type S” thermocouples and platinum crucibles using heating/cooling rates of K/min. The samples were prepared by mixing appropriate quantities of CaCO and FeO powders of ≥99.99% purity in a mortar. To avoid charging errors, the metal oxide assay of both chemicals was preliminarily controlled as separate DTA/TG measurements [31].
The thermal analyzer establishes, via mass flow controllers, three independent gas flows that are unified in the sample chamber. The first “protective gas” flow was held constant at 20 mL/min argon, and to this were added either 50 mL/min Ar or 50 mL/min O, which sets an overall gas flow rate of 70 mL/min for all measurements. From this, one obtains for the latter measurements bar. For the measurements in (20 + 50) mL/min Ar, the rest of the impurities of the nominally 99.999% pure Ar sets a lower limit, and can be estimated on a level of bar in agreement with earlier investigations of the Fe–O system [10]. Equilibrium phase formation was performed in a first DTA/TG cycle, and the results of the second heating runs are shown in Figure 2.
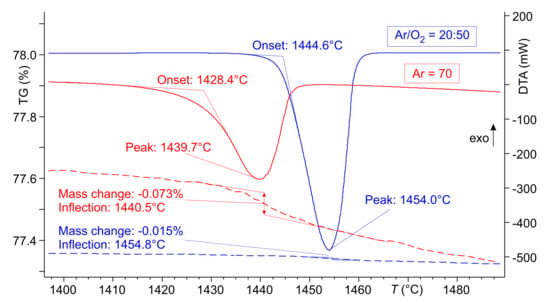
Figure 2.
DTA (full lines) and TG (dashed lines) of CaO–FeO mixtures with 70% CaO. Sample masses were 65–70 mg, atmosphere was Ar (99.999% purity) or an Ar/O mixture (flow rates given in mL/min.)
Both measurements show one endothermal DTA melting peak which is broader, and is shifted to lower T in the “pure” Ar atmosphere. In both cases, the melting process is accompanied by a mass loss which is marginal (0.015%) in the oxygen-rich atmosphere and almost five times larger in Ar. The inflection points in both TG curves are in fairly good agreement with the endothermal minimum positions of the DTA curves. This means that the melting process is obviously directly related to the mass loss.
The experimental results are confirmed by the FactSage [7] calculations that are shown in Figure 3. These curves were obtained by calculating the equilibria between the starting mixture of CaO and FeO (1 mole in total) in an excess of the corresponding atmosphere (7 mole gas in total) in 1 K steps. In both cases, CaFeO is the dominating solid phase at 1380 °C, accompanied by a smaller amount of Fe-saturated CaO, which is designated in the following as CaO(ss). CaFeO is known as a stoichiometric line compound without significant variation of the oxygen stoichiometry [29]. Hence, the small iron content (a few percent, [32]) in the minority phase CaO(ss) is expected to be the only carrier of Fe in the solids.
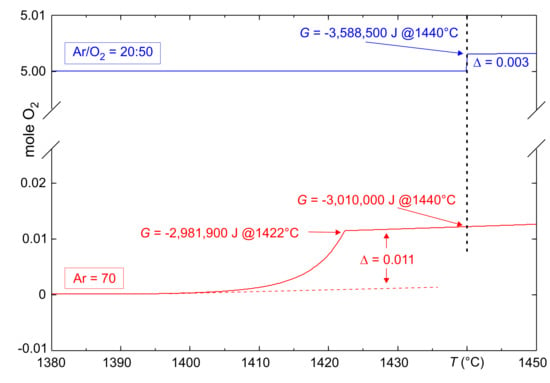
Figure 3.
Calculated equilibrium of 0.7 mol CaO + 0.3 mol FeO in 5 mol O + 2 mol Ar, or in 7 mol Ar, respectively. The curves show the total amount of O in the atmosphere. Total Gibbs energy G of the system given at selected points.
The situation is different for the liquid phase, where under accessible conditions, always an equilibrium between Fe and Fe prevails. From the Ellingham diagrams of the Fe–O system (cf. [10,33]) it is obvious that the liquid FeO “slag” phase always contains both valence states of iron, and their ratio depends on T and . Gurry and Darken [34] measured in a CaO/FeO melt with 36.6 mol-% CaO that was held for 1 h at 1574 °C a Fe/Fe ratio of ≈19. Recently, Bykova et al. [35] showed that even under very high temperatures, K and pressures GPa FeO does not melt stoichiometrically; rather, mixed valence oxides of a series nFeOFeO were found.
Solid FeO will convert under ambient pressure to a molten phase between ≈1500…1600 °C, under the release of oxygen. This process can be described as a chemical reaction
and it is obvious that the amount of Fe which is formed during this melting process depends on in the surrounding atmosphere. This is confirmed by the lower experimental mass loss for the blue TG curve in Figure 2, and is in agreement with the calculated smaller oxygen release for the blue curve in Figure 3.
The vertical dashed line in Figure 3 marks °C, where in both cases, the whole system is molten. At the melting points (1422 °C for the red curve, 1440 °C for the blue curve), G for the solid and liquid phases is identical. At 1440 °C, G is more negative for the blue curve, because there, iron remains also in the melt, preferably as Fe. For the red curve, partial reduction according to Equation (1) takes place, which is endothermal. At this temperature, the reduction of 1 mole pure FeO requires 346,840 J. This major contribution is supplemented other effects (e.g., different specific heat capacity), which are not treated here in detail.
3. Discussion
3.1. Calcium Ferrite
The DTA curves in Figure 2 show that the sample melts in Ar at lower T than in the Ar/O mixture. The melting peak is broader in the oxygen deficient gas flow. Fortunately, reliable thermodynamic data on the Ca–Fe–O ternary system are available, e.g., from the assessment by Hillert et al. [32]. Under the given experimental conditions, only calcium iron(III) ferrites are stable because calcium iron(II,III) ferrites, such as CaFeO (“CWF”), are formed only for lower , e.g., in atmospheres containing hydrogen [36]. The samples with 70% CaO that were used in Figure 2 contain CaO(ss) as the second solid phase, as indicated above.
The melting point of CaO (≈2900 °C) is far above the melting point of CaFeO, and correspondingly, the eutectic point between both phases is very close to the latter compound. This leads to interesting results if one calculates the phase equilibria between the end members FeO and CaO under different .
Both graphs in Figure 4 should not be labeled “phase diagrams” because the end members are not able to form all intermediate phases—in this case, FeO. The upper panel (a) was calculated for a constant bar, which corresponds to the flow of 20 mL/min Ar + 50 mL/min O. Here, three intermediate calcium iron(III) ferrites exist, and CaFeO melts congruently at 1445 °C. The extrapolated onset of the blue melting peak of the 70% mixture in Figure 2 is very close to this melting point, and to the eutectic temperature at 1440 °C. (The small difference cannot be resolved in the DTA curve.) It is interesting to note that the “FeO+slag” phase field on the left side extends only to ca. 1440 °C because at higher T, the iron(II,III) oxide FeO is formed.
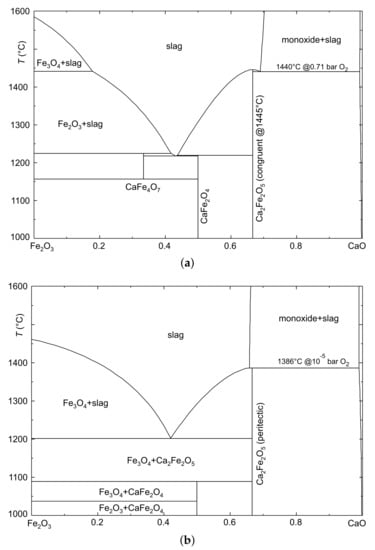
Figure 4.
Isopleth sections CaO–FeO of the system FeO–FeO–CaO, calculated for different atmospheres with FactSage [7]. (a) Calculation for bar: CaFeO melts congruently, and the eutectic toward CaO is at °C. (b) Calculation for bar: CaFeO melts incongruently under the release of CaO(ss) at °C.
It is not possible to give exact data for the oxygen partial pressure that prevails during the measurements in “pure” argon. The value bar that was used for the calculation of Figure 4b should be considered a lower limit, which is delivered by the contamination of the Ar gas of nominally 99.999% purity with air. However, additional oxygen could be added by minor leaks of the DTA apparatus, or by gases emanating from the sample itself, such as CO from the calcination of CaCO.
The stability range of the CaFeO phase, which contains a high amount of Fe, is very limited already for 0.71 bar oxygen pressure (Figure 4a, [32]). Under the significantly lower in Figure 4b, this phase is not stable at all, and also the highest temperature, where both calcium iron(III) ferrites are stable, is decreased. For CaFeO, this results in incongruent melting under the release of CaO(ss), and the melting process can be described by a reaction
which relates the mass loss O to the amount of x FeO in the melt. For the red TG curve in Figure 2, showing a mass loss of 0.073% upon melting, one calculates with Equation (2) . This means that ≈1.2% of the iron ions are reduced to Fe. This is significantly less than that measured by Gurry and Darken (≈5%, [34]). However, they performed the following:
- Their CaO/FeO melt contained only 36.6% CaO, but the CaO concentration of the samples in this study was on the same CaO/FeO scale at 53.8%. The higher CaO content is known to stabilize Fe [30].
3.2. Other Examples
Reversible mass changes during the melting and crystallization of oxide compounds were reported occasionally in the literature:
- The rare-earth calcium oxyborates YCaO(BO) and GdCaO(BO) gain 0.009% or 0.014% mass, respectively, upon melting in air [37].
- If mixtures of MoO and VO are molten together, a phase 6 MoO 0.5 VO 4 VO = VMoO is formed, which contains V and V. If the mixtures are molten in air, the mass rises due to oxidation of V to V. The reverse effect occurs during crystallization. If molten or cooled in Ar, the mass remains constant [38,39]. It should be noted that also in some ABO-type materials, molybdenum plays a role in oxygen storage and transport [17,18].
- The color of SrPrGaO (SPG) crystals depends sensitively on the growth atmosphere. Only if the atmosphere is virtually free of O, or if grown crystals are annealed under reducing conditions (e.g., in forming gas), the crystals are green from Pr. Already, several ppm O result in dark red/brownish coloring. It seems that the darker color is related to partial oxidation to Pr; however, this explanation is disputed. Nevertheless, incorporation of interstitial oxygen is proven. With simultaneous DTA/TG measurements, it was found that melting is accompanied by a positive TG step, and the magnitude of this step (≲0.2%) is almost proportional to the oxygen concentration in the atmosphere during the measurement [40,41].
- Recently Wolff et al. reported crystal growth experiments from the melt with delafossite-type crystals CuMeO (Me = Al, Fe) [42,43]. It turned out that only small millimeter-sized crystallites of CuAlO could be obtained. The experiments with CuFeO, in contrast, were more successful, and single crystalline OFZ bars with diameters up to 10 mm and several centimeters in length were obtained. It is shown in the next section that this very different behavior of isotype crystals with similar melting temperatures around 1200 °C results from the different oxidation levels of the ions in the liquid and solid phases: the melts contain in both cases Cu and Cu, and both solids only Cu. This requires the reduction in Cu ions during crystallization. The valence state of Al does not change during the crystallization of CuAlO. For CuFeO, in contrast, the melt contains besides the incorporated Fe also significant amounts of Fe which must be oxidized.
- More recently, it was shown that also during the crystallization of BiCuO and BiPdO, the mass rises, due to oxygen incorporation and partial oxidation of Cu or Pd to the two-valent state. BiCuO single crystals up to 6 mm in diameter and 7 cm in length were obtained by OFZ [44].
4. Conclusions
Many transition metals can occur in a variety of oxidation states, depending on the oxygen concentration in the atmosphere and on temperature. Molten oxides usually contain more than one of these oxidation states because the simultaneous occurrence of several valences favors the mixture entropically. In contrast, solids contain often (but not always) only one valence for each element.
If during oxide crystal growth from the melt, the average valence states in the melt and in the crystal are different, free oxygen molecules must be either absorbed from the ambient gas, or must be released. Taking this into account, the terms “congruent melting” and “peritectic melting” become disputable: “congruent melting” means in the closer sense that the solid and the melt in equilibrium possess an identical chemical composition; and “peritectic melting” means that a solid converts, upon heating, to a melt with a different composition under the release of another solid. In both cases, the gas phase should not, per definition, be involved. However, changing the valence of ions requires involvement of the gas phase. In particular, the exchange of O with the gas phase can lead to bubbles, which destabilize or inhibit crystal growth [45,46,47,48,49].
Figure 5a demonstrates this for the case where the average oxidation level of the crystal is higher, compared to the melt. Continuously, the melt must absorb O(gas) from the atmosphere to facilitate the Me/Me equilibrium in the melt, because the latter ion is permanently incorporated into the crystal. Possibly, this limits the maximum growth rate, but nevertheless, the growth of crystals with a high oxidation state, such as the initial example CaFeO[29], or BiCuO [44], is possible without severe problems.
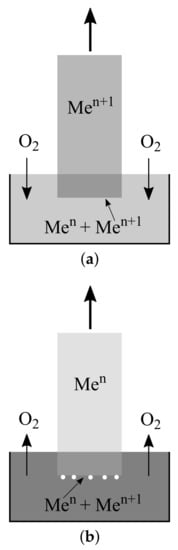
Figure 5.
Growth of a metal oxide crystal with integer valence of the metal Me from a melt with different valences, Me and Me. A darker color means a higher oxidation level. (a) The crystal contains the higher valence and only Me is incorporated from the melt. The melt depletion by this ion must be compensated by the oxidation of Me ions. (b) The crystal contains the lower valence and only Me is incorporated from the melt. The melt depletion by this ion must be compensated by a reduction in Me ions, under the production of free oxygen. This will occur preferably at the crystallization front, and can lead there to bubbles (white circles).
Figure 5b shows the opposite case where the average oxidation level of the crystal is lower than that of the melt. During growth, Me is incorporated into the crystal, and readjustment of the Me/Me equilibrium in the melt requires permanent reduction in the latter. Melt depletion by Me will occur at the crystallization front, and consequently, the formation of O gas bubbles is expected there. The volume of these bubbles can be estimated from Equation (1): the reduction in 1 mole Me to Me produces moles O. Assuming an ideal gas, this corresponds to, for example, 1700 K to as much as 35 litres. In the case of iron (atomic mass 55.8 g/mol), every gram of reduced iron delivers ≈ 630 mL O at this temperature. Wolff et al. described recently “almost peritectic” melting processes, where a solid phase decomposes to a melt and another solid phase under the compulsive exchange with the gas phase, as “exaperitectic”, derived from the Greek (to exhaust).
As a conclusion, it is recommended to adjust (if possible) the atmosphere for crystal growth experiments with critical valence changes during crystallization in such a way that these changes between the solid and liquid phases remain small, e.g., by reactive components, such as CO [11]. The measurement of mass changes during melting/crystallization in a simultaneous DTA/TG measurement is a useful tool to detect such critical valence changes.
Author Contributions
Conceptualization, D.K.; investigation, N.W. and D.K.; writing, D.K. and N.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This article has passed the institutional review board procedure of Leibniz-Institut für Kristallzüchtung (IKZ) Berlin, Germany.
Acknowledgments
The authors are indebted to Zbigniew Galazka for reading the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Walker, A.C. Hydrothermal synthesis of quartz crystals. J. Am. Ceram. Soc. 1953, 36, 250–256. [Google Scholar] [CrossRef]
- Sarukura, N.; Nawata, T.; Ishibashi, H.; Ishii, M.; Fukuda, T. Czochralski growth of oxides and fluorides. In Handbook of Crystal Growth, 2nd ed.; Rudolph, P., Ed.; Elsevier: Boston, MA, USA, 2015; pp. 131–168. [Google Scholar] [CrossRef]
- Dabkowska, H.A.; Da̧bkowski, A.B.; Hermann, R.; Priede, J.; Gerbeth, G. Floating zone growth of oxides and metallic alloys. In Handbook of Crystal Growth (Second Edition); Rudolph, P., Ed.; Elsevier: Boston, MA, USA, 2015; pp. 281–329. [Google Scholar] [CrossRef]
- Brandle, C.D. Czochralski growth of oxides. J. Cryst. Growth 2004, 264, 593–604. [Google Scholar] [CrossRef]
- Tomm, Y.; Reiche, P.; Klimm, D.; Fukuda, T. Czochralski grown Ga2O3 crystals. J. Cryst. Growth 2000, 220, 510–514. [Google Scholar] [CrossRef]
- Klimm, D.; Ganschow, S.; Schulz, D.; Fornari, R. The growth of ZnO crystals from the melt. J. Cryst. Growth 2008, 310, 3009–3013. [Google Scholar] [CrossRef]
- GTT Technologies, Kaiserstr. 100, 52134 Herzogenrath, Germany. FactSage 8.1. 2021. Available online: http://www.factsage.com/ (accessed on 1 October 2021).
- Uecker, R.; Klimm, D.; Ganschow, S.; Reiche, P.; Bertram, R.; Roßberg, M.; Fornari, R. Czochralski growth of Ti:sapphire laser crystals. Optically Based Materials and Optically Based Biological and Chemical Sensing for Defence II; International Society for Optics and Photonics (SPIE): Bellingham, WA, USA, 2005; Volume 5990, pp. 53–61. [Google Scholar] [CrossRef]
- Xuan, L. Thermodynamic and Kinetic Model of Point Defect Distributions during Ti:sapphire Growth. Ph.D. Thesis, SIMaP-INP-UGA, Grenoble, France, 2021. [Google Scholar]
- Klimm, D.; Ganschow, S. The control of iron oxidation state during FeO and olivine crystal growth. J. Cryst. Growth 2005, 275, e849–e854. [Google Scholar] [CrossRef][Green Version]
- Klimm, D.; Ganschow, S.; Schulz, D.; Bertram, R.; Uecker, R.; Reiche, P.; Fornari, R. Growth of oxide compounds under dynamic atmosphere composition. J. Cryst. Growth 2009, 311, 534–536. [Google Scholar] [CrossRef]
- Ganschow, S.; Schulz, D.; Klimm, D.; Bertram, R.; Uecker, R. Application of predominance diagrams in melt growth of oxides. Cryst. Res. Technol. 2010, 45, 1219–1224. [Google Scholar] [CrossRef]
- Sebakhy, K.O.; Vitale, G.; Pereira-Almao, P. Dispersed Ni-doped aegirine nanocatalysts for the selective hydrogenation of olefinic molecules. ACS Appl. Nano Mater. 2018, 1, 6269–6280. [Google Scholar] [CrossRef]
- Han, N.; Zhang, C.; Tan, X.; Wang, Z.; Kawi, S.; Liu, S. Re-evaluation ofLa0.6Sr0.4Co0.2Fe0.8O3−δ hollow fiber membranes for oxygen separation after long-term storage of five and ten years. J. Membr. Sci. 2019, 587, 117180. [Google Scholar] [CrossRef]
- Han, N.; Zhang, W.; Guo, W.; Xie, S.; Zhang, C.; Zhang, X.; Fransaer, J.; Liu, S. Novel oxygen permeable hollow fiber perovskite membrane with surface wrinkles. Sep. Purif. Technol. 2021, 261, 118295. [Google Scholar] [CrossRef]
- Han, N.; Shen, Z.; Zhao, X.; Chen, R.; Thakur, V.K. Perovskite oxides for oxygen transport: Chemistry and material horizons. Sci. Total Environ. 2022, 806, 151213. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Wei, Q.; Zhang, S.; Yang, N.; Liu, S. Rational design via tailoring Mo content in La2Ni1−xMoxO4+δ to improve oxygen permeation properties in CO2 atmosphere. J. Alloys Compds. 2019, 806, 153–162. [Google Scholar] [CrossRef]
- Han, N.; Guo, X.; Cheng, J.; Liu, P.; Zhang, S.; Huang, S.; Rowles, M.R.; Fransaer, J.; Liu, S. Inhibiting in situ phase transition in Ruddlesden-Popper perovskite via tailoring bond hybridization and its application in oxygen permeation. Matter 2021, 4, 1720–1734. [Google Scholar] [CrossRef]
- Bertaut, E.F.; Blum, P.; Sagnières, A. Structure du ferrite bicalcique et de la brownmillerite. Acta Cryst. 1959, 12, 149–159. [Google Scholar] [CrossRef]
- Berggren, J. Refinement of the crystal structure of dicalcium ferrite, Ca2Fe2O5. Acta Chem. Scand. 1971, 25, 3616–3624. [Google Scholar] [CrossRef]
- Redhammer, G.J.; Tippelt, G.; Roth, G.; Amthauer, G. Structural variations in the brownmillerite series Ca2(Fe2−xAlx)O5: Single-crystal X-ray diffraction at 25 °C and high-temperature X-ray powder diffraction (25 °C≤ T ≤ 1000 °C). Am. Mineral. 2004, 89, 405–420. [Google Scholar] [CrossRef]
- Shin, S.; Yonemura, M.; Ikawa, H. Crystallographic properties of Ca2Fe2O5. Difference in crystallographic properties of brownmillerite-like compounds, Ca2Fe2O5 and Sr2Fe2O5, at elevated temperatures. Bull. Chem. Soc. Jpn. 1979, 52, 947–948. [Google Scholar] [CrossRef]
- Krüger, H.; Kahlenberg, V. Incommensurately modulated ordering of tetrahedral chains in Ca2Fe2O5 at elevated temperatures. Acta Cryst. B: Struct. Sci. 2005, 61, 656–662. [Google Scholar] [CrossRef]
- Chesnokov, B.V.; Bazhenova, L.F. Srebrodolskite Ca2Fe2O5—A new mineral. Zap. VseSoyuznogo Mineral. Obshchestva 1985, 114, 195–199. Available online: https://rruff.info/uploads/ZVMO114N2_195.pdf (accessed on 1 October 2021). (In Russian).
- Sharma, N.; Shaju, K.M.; Subba Rao, G.V.; Chowdari, B.V.R. Mixed oxides Ca2Fe2O5 and Ca2Co2O5 as anode materials for Li-ion batteries. Electrochim. Acta 2004, 49, 1035–1043. [Google Scholar] [CrossRef]
- Chan, M.S.C.; Liu, W.; Ismail, M.; Yang, Y.; Scott, S.A.; Dennis, J.S. Improving hydrogen yields, and hydrogen:steam ratio in the chemical looping production of hydrogen using Ca2Fe2O5. Chem. Eng. J. 2016, 296, 406–411. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Cryst. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Jeon, J.; Jung, S.M.; Sasaki, Y. Formation of calcium ferrites under controlled oxygen potentials at 1273 K. ISIJ Int. 2010, 50, 1064–1070. [Google Scholar] [CrossRef]
- Ceretti, M.; Corallini, S.; Paulus, W. Influence of phase transformations on crystal growth of stoichiometric brownmillerite oxides: Sr2ScGaO5 and Ca2Fe2O5. Crystals 2016, 6, 146. [Google Scholar] [CrossRef]
- Phillips, B.; Muan, A. Phase equilibria in the system CaO–iron oxide in air and at 1 atm. O2 Pressure. J. Amer. Ceram. Soc. 1958, 41, 445–454. [Google Scholar] [CrossRef]
- Bertram, R.; Klimm, D. Assay measurements of oxide materials by thermogravimetry and ICP-OES. Thermochim. Acta 2004, 419, 189–193. [Google Scholar] [CrossRef]
- Hillert, M.; Selleby, M.; Sundman, B. An assessment of the Ca–Fe–O system. Met. Trans. A 1990, 21, 2759–2776. [Google Scholar] [CrossRef]
- Klimm, D. Thermal Analysis and Thermodynamics in Materials Science; De Gruyter STEM: Berlin, Germany, 2022. [Google Scholar]
- Gurry, R.W.; Darken, L.S. The composition of CaO–FeO–Fe2O3 and MnO–FeO–Fe2O3 melts at several oxygen pressures in the vicinity of 1600°. J. Amer. Chem. Soc. 1950, 72, 3906–3910. [Google Scholar] [CrossRef]
- Bykova, E.; Dubrovinsky, L.; Dubrovinskaia, N.; Bykov, M.; McCammon, C.; Ovsyannikov, S.V.; Liermann, H.P.; Kupenko, I.; Chumakov, A.I.; Rüffer, R.; et al. Structural complexity of simple Fe2O3 at high pressures and temperatures. Nat. Commun. 2016, 7, 10661. [Google Scholar] [CrossRef]
- Berger, C.M.; Mahmoud, A.; Hermann, R.P.; Braun, W.; Yazhenskikh, E.; Sohn, Y.J.; Menzler, N.H.; Guillon, O.; Bram, M. Calcium-iron oxide as energy storage medium in rechargeable oxide batteries. J. Am. Ceram. Soc. 2016, 99, 4083–4092. [Google Scholar] [CrossRef]
- Klimm, D.; Bertram, R.; Ganschow, S.; Doerschel, J. Phase separation in oxide-borate mixed systems. J. Chem. Eng. Data 2003, 48, 120–123. [Google Scholar] [CrossRef]
- Slade, R.C.T.; Ramanan, A.; West, B.C.; Prince, E. The structure of V9Mo6O40 determined by powder neutron diffraction. J. Solid State Chem. 1989, 82, 65–69. [Google Scholar] [CrossRef]
- Klimm, D.; Orlinski, K.; Pawlak, D.A. Self-organized V–Mo oxide fibers by the micro-pulling down method. Mater. Res. Soc. Symp. Proc. 2010, 1223, 1209–1223. [Google Scholar] [CrossRef]
- Uecker, R.; Reiche, P.; Ganschow, S.; Uecker, D.C.; Schultze, D. Investigation of crystal growth of SrPrGaO4 and SrLaGaO4. Acta Phys. Pol. A 1997, 92, 23–34. [Google Scholar] [CrossRef]
- Pajaczkowska, A.; Novoselov, A.; Klimm, D.; Talik, E.; Uecker, R. Does valency of Pr ions influence color of SrPrGaO4 single crystals? Cryst. Growth Des. 2004, 4, 497–501. [Google Scholar] [CrossRef]
- Wolff, N.; Klimm, D.; Siche, D. Thermodynamic investigations on the growth of CuAlO2 delafossite crystals. J. Solid State Chem. 2018, 258, 495–500. [Google Scholar] [CrossRef]
- Wolff, N.; Schwaigert, T.; Siche, D.; Schlom, D.G.; Klimm, D. Growth of CuFeO2 single crystals by the optical floating-zone technique. J. Cryst. Growth 2020, 532, 125426. [Google Scholar] [CrossRef]
- Wolff, N.; Klimm, D.; Habicht, K.; Fritsch, K. Crystal growth and thermodynamic investigation of Bi2M2+O4 (M = Pd, Cu). CrystEngComm 2021, 23, 3230–3238. [Google Scholar] [CrossRef]
- Nielsen, J.W.; Blank, S.L. Crystal growth and phase equilibrium studies in the system (R.E.)2O3–Fe2O3. J. Cryst. Growth 1972, 13–14, 702–705. [Google Scholar] [CrossRef]
- Shimura, F.; Fujino, Y. Crystal growth and fundamental properties of LiNb1−yTayO3. J. Cryst. Growth 1977, 38, 293–302. [Google Scholar] [CrossRef]
- Behr, G.; Löser, W.; Apostu, M.O.; Gruner, W.; Hücker, M.; Schramm, L.; Souptel, D.; Teresiak, A.; Werner, J. Floating zone growth of CuO under elevated oxygen pressure and its relevance for the crystal growth of cuprates. Cryst. Res. Technol. 2005, 40, 21–25. [Google Scholar] [CrossRef]
- Li, Q.J.; Xu, L.M.; Fan, C.; Zhang, F.B.; Lv, Y.Y.; Ni, B.; Zhao, Z.Y.; Sun, X.F. Single crystal growth of the pyrochlores R2Ti2O7 (R = rare earth) by the optical floating-zone method. J. Cryst. Growth 2013, 377, 96–100. [Google Scholar] [CrossRef]
- Wizent, N.; Leps, N.; Behr, G.; Klingeler, R.; Büchner, B.; Löser, W. The effect of process parameters on floating zone crystal growth of selected cuprates. J. Cryst. Growth 2014, 401, 596–600. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).