Picrasma quassioides (D.DON) Benn. Ethanolic Extract Improves Atopic Dermatitis and Hyperactivity Disorder in DNCB-Treated BALB/c Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Herbal Ethanolic Extracts of Picrasma quassioides (D.Don) Benn. Bark
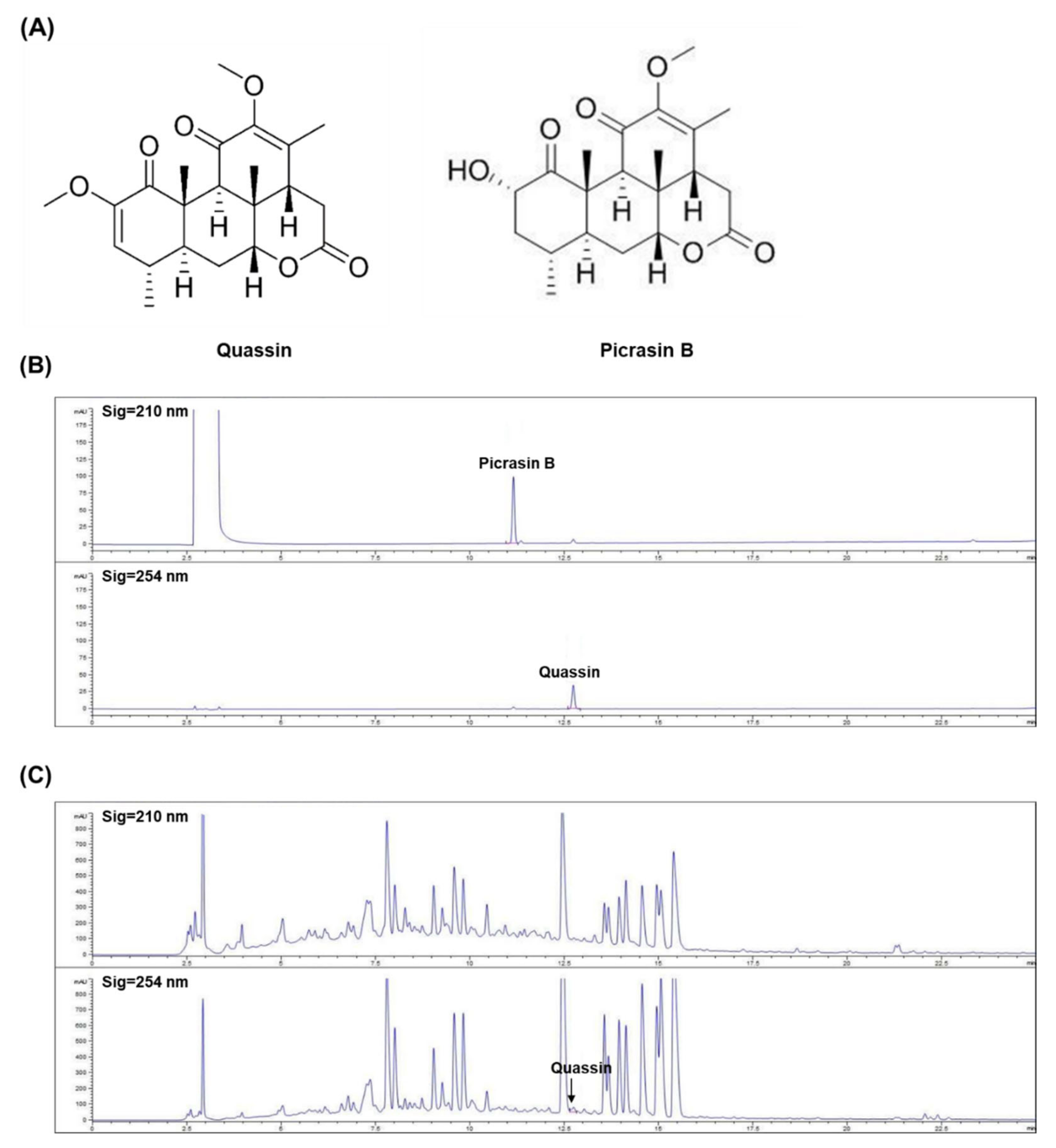
2.3. High-Performance Liquid Chromatography (HPLC) Analysis
2.4. Animal Experiments
2.5. Open Field Test (OFT)
2.6. Elevated Plus Maze (EPM)
2.7. Histological Analysis
2.8. Cell Culture and Treatments
2.9. Cell Viability Assay
2.10. Enzyme-Linked Immunosorbent Assay (ELISA)
2.11. Statistical Analysis
3. Results
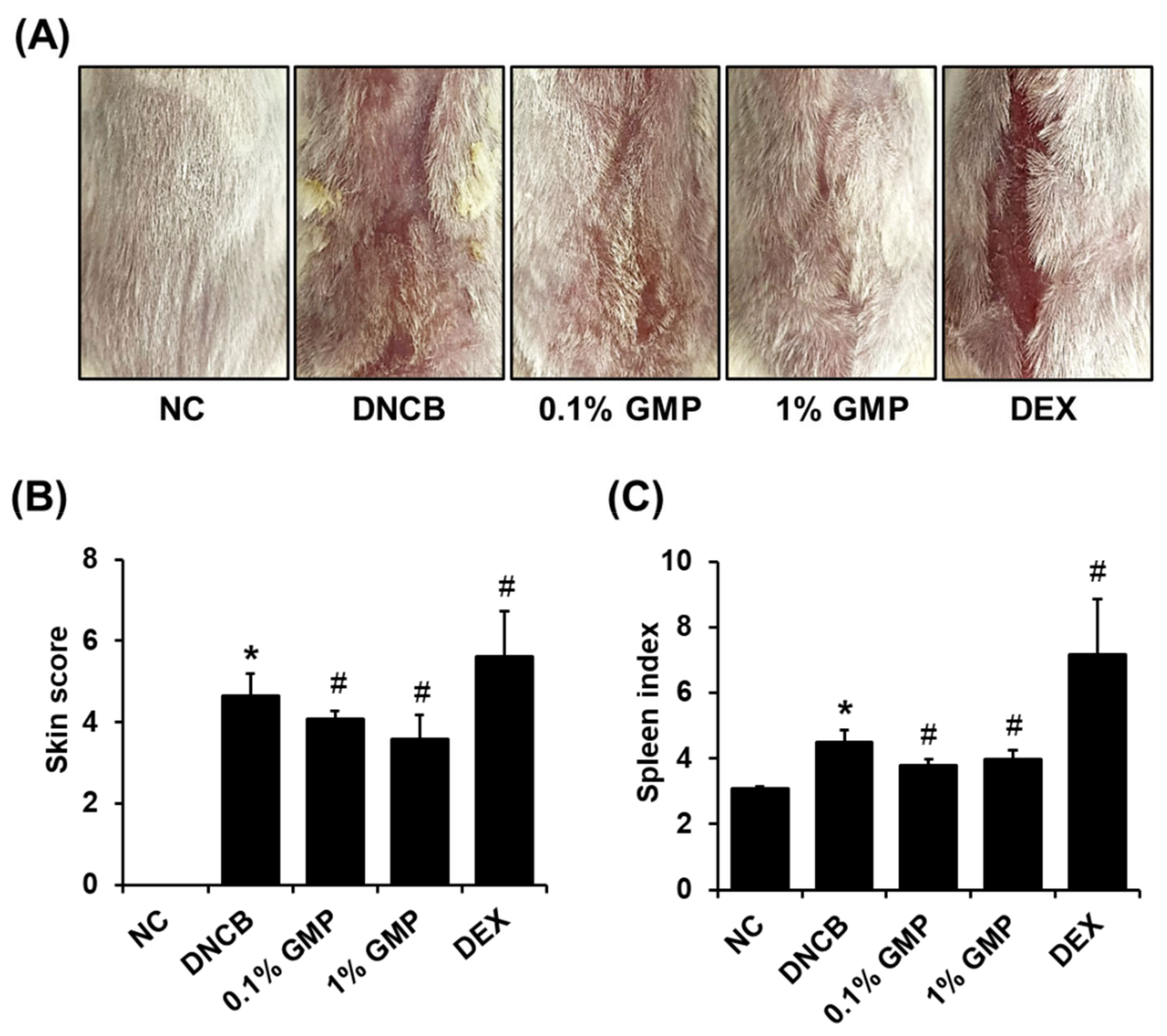
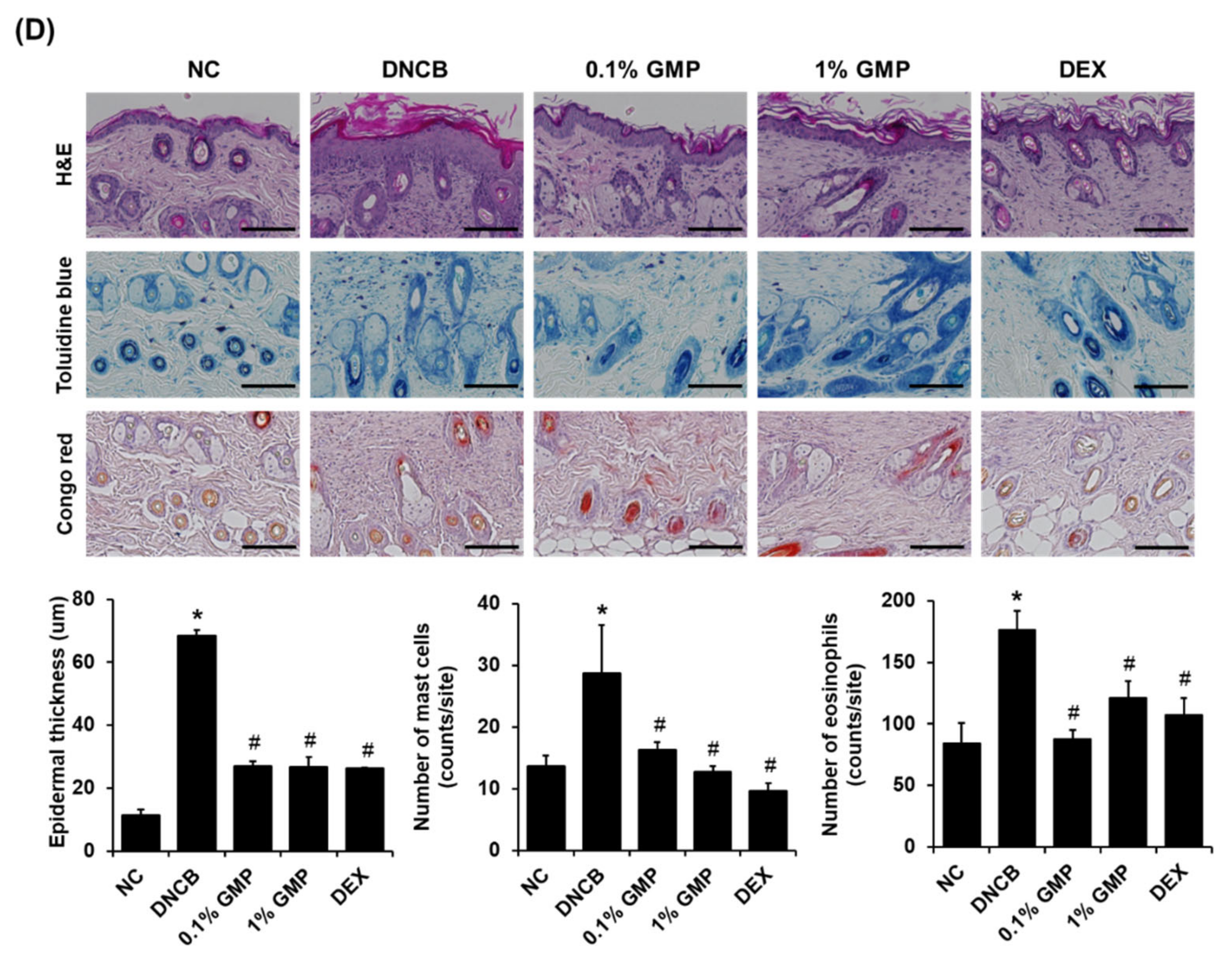
3.1. Effect of GMP on Skin Lesions in DNCB-Treated BALB/c Mice
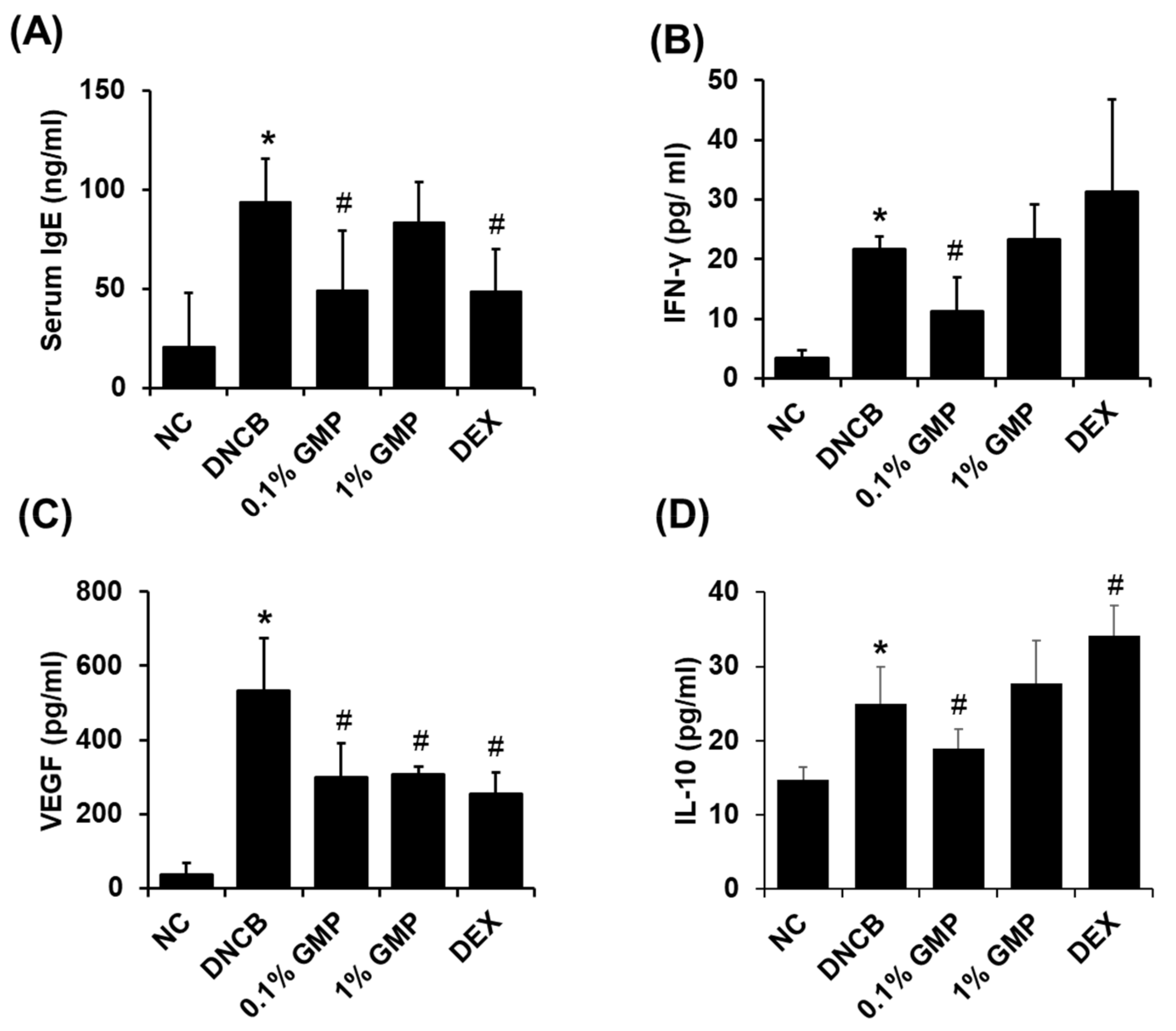
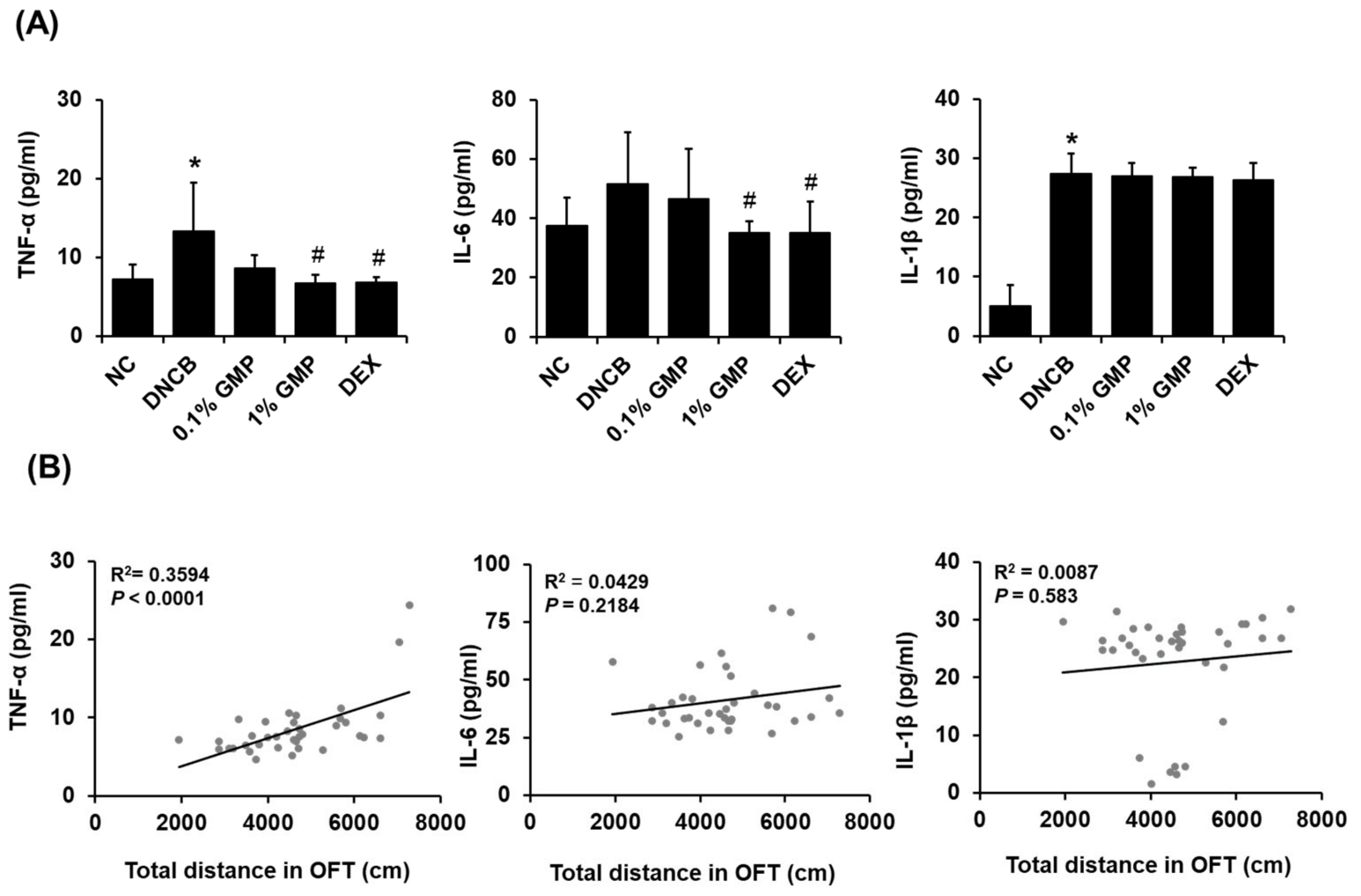
3.2. Effect of GMP on Serum IgE Levels and Inflammatory Cytokine Levels in Skin Lesions in DNCB-Treated BALB/c Mice
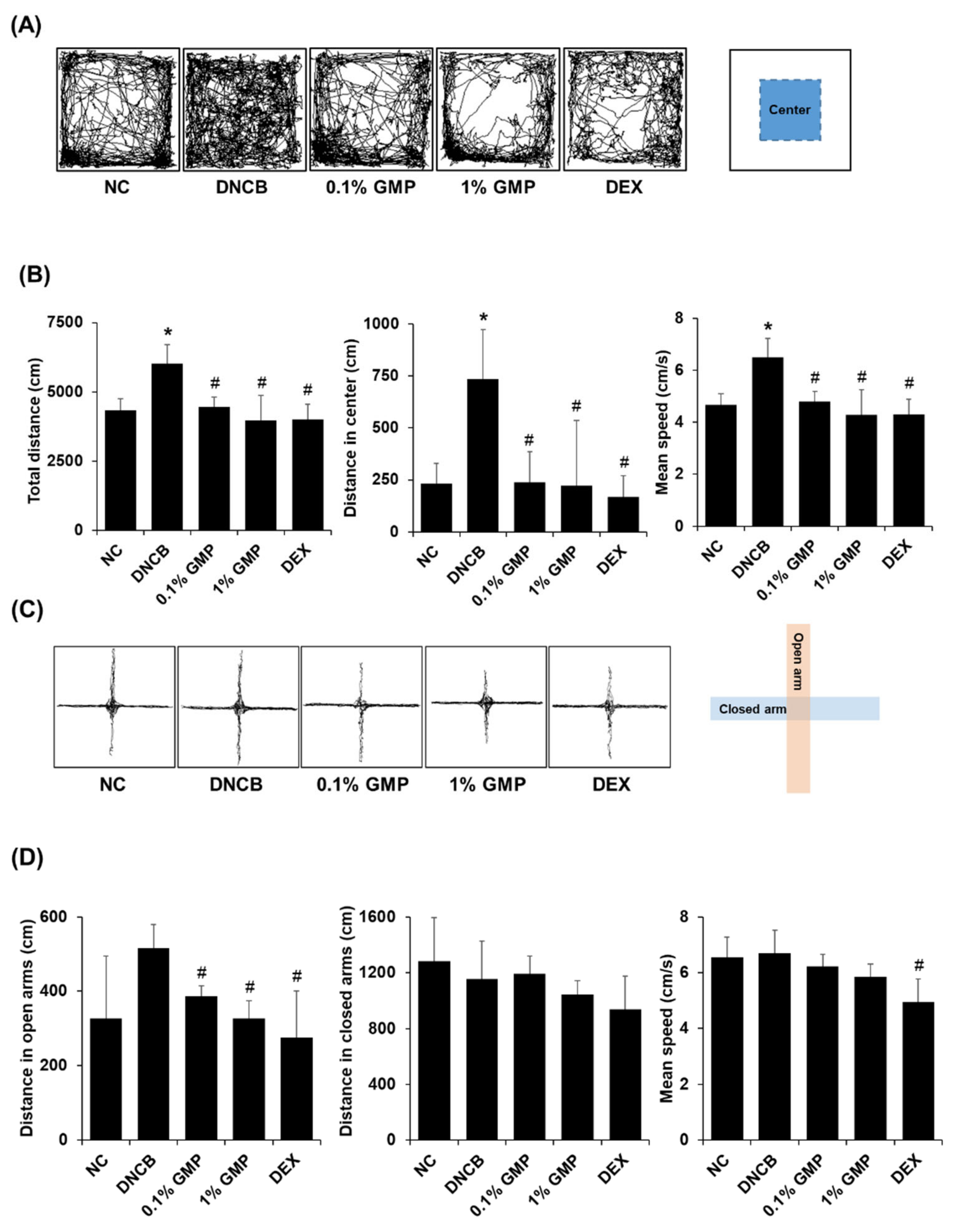
3.3. Effect of GMP on the Behavioral Changes in DNCB-Treated BALB/c Mice
3.4. Effect of GMP on Neuroinflammation in DNCB-Treated BALB/c Mice
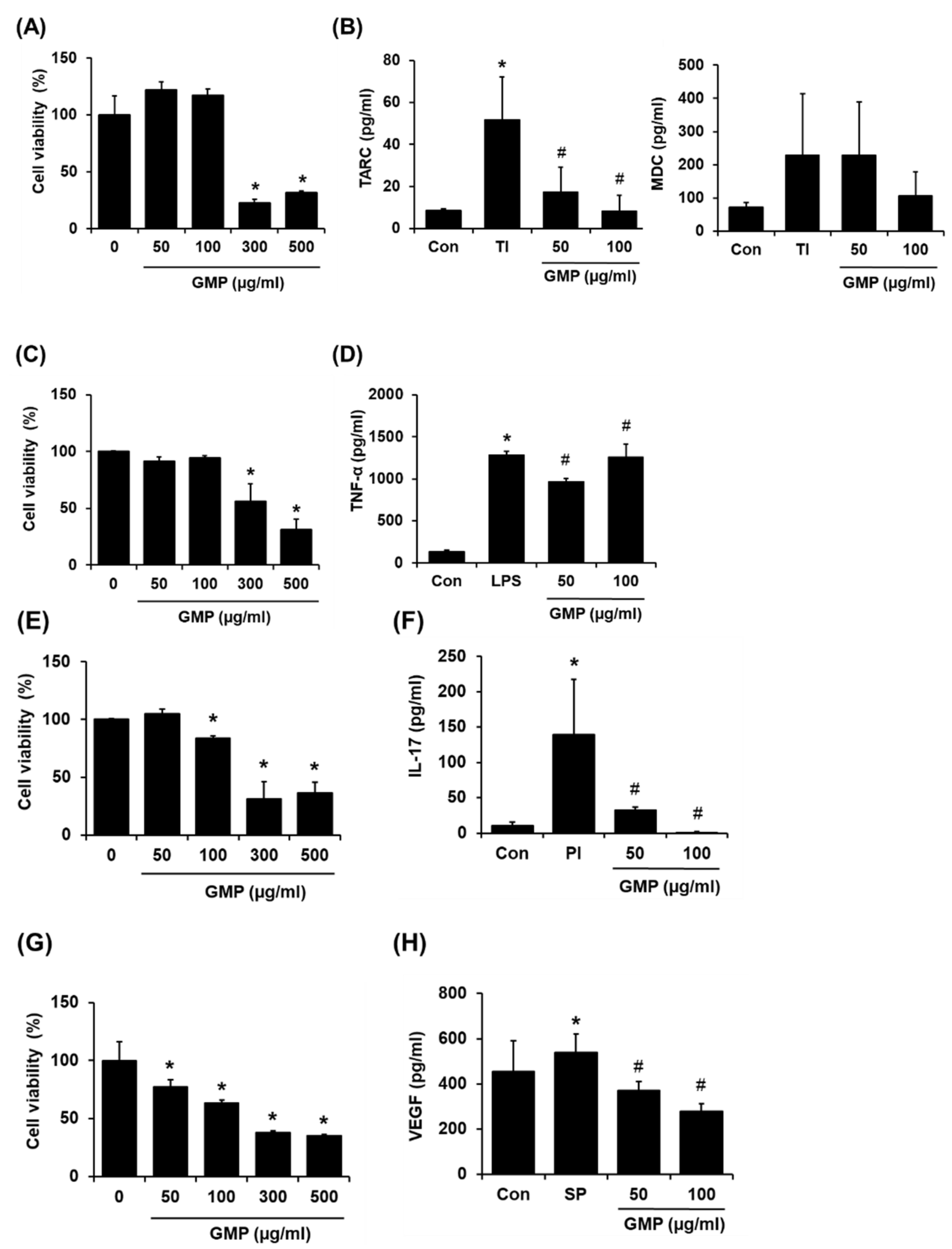
3.5. Effect of GMP on Inflammatory Responses In Vitro
3.6. Quantification of Chemical Constituents in GMP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shin, J.E.; Jeon, Y.H.; Yang, H.J.; Pyun, B.Y. Comparison of Clinical Severity and Laboratory Results between Atopic and Non-atopic Eczema in Children. Pediatr. Allergy Respir. Dis. 2008, 18, 219–227. [Google Scholar]
- Kang, W.S.; Jeoung, S.M.; Xu, X.; Lee, J.E.; Ryu, S.H.; Ahn, D.H. Anti-Atopic Effect of DNCB-Induced Mouse in Chondrus canaliculatus Ethanol Extracts. J. Korean Soc. Food Sci. Nutr. 2019, 49, 482–483. [Google Scholar]
- Ahn, J.-Y.; Im, L.-R.; Kim, J.-H.; Park, J.-H.; Kim, D.-K.; Lee, Y.-M. Effects of Rumecis Radix Water Extract on Development of Atopic Dermatitis in BALB/c Mice. Korean J. Pharmacogn. 2009, 40, 218–223. [Google Scholar]
- Chen, Y.; Xian, Y.F.; Loo, S.; Lai, Z.; Chan, W.Y.; Liu, L.; Lin, Z.X. Huang-Lian-Jie-Du extract ameliorates atopic dermatitis-like skin lesions induced by 2,4-dinitrobenzene in mice via suppression of MAPKs and NF-κB pathways. J. Ethnopharmacol. 2020, 249, 112367. [Google Scholar] [CrossRef] [PubMed]
- Walsh, L.J.; Trinchieri, G.; Waldorf, H.A.; Whitaker, D.; Murphy, G.F. Human dermal mast cells contain and release tumor necrosis factor alpha, which induces endothelial leukocyte adhesion molecule 1. Proc. Natl. Acad. Sci. USA 1991, 88, 4220–4224. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K. Role of Mast Cells in Allergic Inflammation and Innate Immunity. Korean J. Pediatr. 2004, 47, 1137–1141. [Google Scholar]
- Chieosilapatham, P.; Kiatsurayanon, C.; Umehara, Y.; Trujillo-Paez, J.V.; Peng, G.; Yue, H.; Nguyen, L.T.H.; Niyonsaba, F. Keratinocytes: Innate immune cells in atopic dermatitis. Clin. Exp. Immunol. 2021, 204, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Hänel, K.H.; Cornelissen, C.; Lüscher, B.; Baron, J.M. Cytokines and the skin barrier. Int J. Mol. Sci 2013, 14, 6720–6745. [Google Scholar] [CrossRef]
- Yaghmaie, P.; Koudelka, C.W.; Simpson, E.L. Mental health comorbidity in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2013, 131, 428–433. [Google Scholar] [CrossRef]
- Strom, M.A.; Fishbein, A.B.; Paller, A.S.; Silverberg, J.I. Association between atopic dermatitis and attention deficit hyperactivity disorder in U.S. children and adults. Br. J. Dermatol. 2016, 175, 920–929. [Google Scholar] [CrossRef]
- Zhou, R.Y.; Wang, J.J.; Sun, J.C.; You, Y.; Ying, J.N.; Han, X.M. Attention deficit hyperactivity disorder may be a highly inflammation and immune-associated disease (Review). Mol. Med. Rep. 2017, 16, 5071–5077. [Google Scholar] [CrossRef] [PubMed]
- Leffa, D.T.; Torres, I.L.S.; Rohde, L.A. A Review on the Role of Inflammation in Attention-Deficit/Hyperactivity Disorder. Neuroimmunomodulation 2018, 25, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Costa-Pinto, F.A.; Basso, A.S.; Britto, L.R.; Malucelli, B.E.; Russo, M. Avoidance behavior and neural correlates of allergen exposure in a murine model of asthma. Brain Behav. Immun. 2005, 19, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Lerch, J.; Greenstein, D.; Sharp, W.; Clasen, L.; Evans, A.; Giedd, J.; Castellanos, F.X.; Rapoport, J. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry 2006, 63, 540–549. [Google Scholar] [CrossRef]
- Buske-Kirschbaum, A.; Trikojat, K.; Tesch, F.; Schmitt, J.; Roessner, V.; Luksch, H.; Rösen-Wolff, A.; Plessow, F. Altered hypothalamus-pituitary-adrenal axis function: A relevant factor in the comorbidity of atopic eczema and attention deficit/hyperactivity disorder? Psychoneuroendocrinology 2019, 105, 178–186. [Google Scholar] [CrossRef]
- Jung, Y.S.; Eun, C.S.; Jung, Y.T.; Kim, H.J.; Yu, M.H. Anti-Inflammatory Effects of Picrasma Quassioides (D.DON) BENN Leaves Extracts. Korean Soc. Life Sci. 2013, 23, 629–636. [Google Scholar]
- Jin, Y.-S.; Yin, Y.; Shin, T.-H.; Sa, J.-H.; Wang, M.-H. Studies for Component Analysis and Biological Evaluation in Picrasma quassioides (D.Don) Benn. Extract. Korean J. Pharmacogn. 2006, 37, 37–41. [Google Scholar]
- Lee, J.; Gong, Y.-X.; Jeong, H.; Seo, H.; Xie, D.-P.; Sun, H.-N.; Kwon, T. Pharmacological effects of Picrasma quassioides (D. Don) Benn for inflammation, cancer and neuroprotection (Review). Exp. Ther. Med. 2021, 22, 1357. [Google Scholar] [CrossRef]
- Shin, N.R.; Shin, I.S.; Jeon, C.M.; Hong, J.M.; Oh, S.R.; Hahn, K.W.; Ahn, K.S. Inhibitory effects of Picrasma quassioides (D.Don) Benn. on airway inflammation in a murine model of allergic asthma. Mol. Med. Rep. 2014, 10, 1495–1500. [Google Scholar] [CrossRef]
- Ryu, I.-H.; Cho, H.-B.; Kim, S.-B.; Seo, Y.-J.; Choi, C.-M. The inhibitory effect of Picrasmae Lignum on inflammatory responses. Korean J. Obstet. Gynecol. 2011, 24, 1–14. [Google Scholar]
- Kanda, N.; Hoashi, T.; Saeki, H. The Roles of Sex Hormones in the Course of Atopic Dermatitis. Int. J. Mol. Sci. 2019, 20, 4660. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, J.I.; Barbarot, S.; Gadkari, A.; Simpson, E.L.; Weidinger, S.; Mina-Osorio, P.; Rossi, A.B.; Brignoli, L.; Saba, G.; Guillemin, I. Atopic dermatitis in the pediatric population: A cross-sectional, international epidemiologic study. Ann. Allergy Asthma Immunol. 2021, 126, 417–428.e412. [Google Scholar] [CrossRef] [PubMed]
- Barbarot, S.; Auziere, S.; Gadkari, A.; Girolomoni, G.; Puig, L.; Simpson, E.; Margolis, D.; de Bruin-Weller, M.; Eckert, L. Epidemiology of atopic dermatitis in adults: Results from an international survey. Allergy 2018, 73, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Paller, A.; Jaworski, J.C.; Simpson, E.L.; Boguniewicz, M.; Russell, J.J.; Block, J.K.; Tofte, S.; Dunn, J.D.; Feldman, S.R.; Clark, A.R.; et al. Major Comorbidities of Atopic Dermatitis: Beyond Allergic Disorders. Am. J. Clin. Dermatol. 2018, 19, 821–838. [Google Scholar] [CrossRef]
- Xu, Y.-C.; Wang, J.-P.; Zhu, W.-J.; Li, P. Childhood atopic dermatitis as a precursor for developing attention deficit/hyperactivity disorder. Int. J. Immunopathol. Pharmacol. 2020, 34, 2058738420962902. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Sanders, S.; Doust, J.; Beller, E.; Glasziou, P. Prevalence of attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. Pediatrics 2015, 135, e994–e1001. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J.; Shin, S.; Cho, A.; Heo, Y. Molecular Mechanism of Atopic Dermatitis Induction Following Sensitization and Challenge with 2,4-Dinitrochlorobenzene in Mouse Skin Tissue. Toxicol. Res. 2018, 34, 7–12. [Google Scholar] [CrossRef]
- Nomura, T.; Honda, T.; Kabashima, K. Multipolarity of cytokine axes in the pathogenesis of atopic dermatitis in terms of age, race, species, disease stage and biomarkers. Int. Immunol. 2018, 30, 419–428. [Google Scholar] [CrossRef]
- Kawakami, T.; Ando, T.; Kimura, M.; Wilson, B.S.; Kawakami, Y. Mast cells in atopic dermatitis. Curr. Opin. Immunol. 2009, 21, 666–678. [Google Scholar] [CrossRef]
- Amin, K. The role of mast cells in allergic inflammation. Respir. Med. 2012, 106, 9–14. [Google Scholar] [CrossRef]
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast Cell: A Multi-Functional Master Cell. Front. Immunol. 2016, 6, 620. [Google Scholar] [CrossRef] [PubMed]
- Kasraie, S.; Werfel, T. Role of Macrophages in the Pathogenesis of Atopic Dermatitis. Mediators Inflamm. 2013, 2013, 942375. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, T.; Skabytska, Y.; Kaesler, S.; Volz, T. Regulation of T Cell Immunity in Atopic Dermatitis by Microbes: The Yin and Yang of Cutaneous Inflammation. Front. Immunol. 2015, 6, 353. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.M.; Bieber, T. Atopic dermatitis. Lancet 2003, 361, 151–160. [Google Scholar] [CrossRef]
- van den Bogaard, E.H.; Tjabringa, G.S.; Joosten, I.; Vonk-Bergers, M.; van Rijssen, E.; Tijssen, H.J.; Erkens, M.; Schalkwijk, J.; Koenen, H. Crosstalk between keratinocytes and T cells in a 3D microenvironment: A model to study inflammatory skin diseases. J. Investig. Dermatol. 2014, 134, 719–727. [Google Scholar] [CrossRef]
- Nakazato, J.; Kishida, M.; Kuroiwa, R.; Fujiwara, J.; Shimoda, M.; Shinomiya, N. Serum levels of Th2 chemokines, CCL17, CCL22, and CCL27, were the important markers of severity in infantile atopic dermatitis. Pediatr. Allergy Immunol. 2008, 19, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.X.; Xu Landén, N. New insights into T cells and their signature cytokines in atopic dermatitis. IUBMB Life 2015, 67, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Orciani, M.; Campanati, A.; Caffarini, M.; Ganzetti, G.; Consales, V.; Lucarini, G.; Offidani, A.; Di Primio, R. T helper (Th)1, Th17 and Th2 imbalance in mesenchymal stem cells of adult patients with atopic dermatitis: At the origin of the problem. Br. J. Dermatol. 2017, 176, 1569–1576. [Google Scholar] [CrossRef]
- Nakajima, S.; Kitoh, A.; Egawa, G.; Natsuaki, Y.; Nakamizo, S.; Moniaga, C.S.; Otsuka, A.; Honda, T.; Hanakawa, S.; Amano, W.; et al. IL-17A as an inducer for Th2 immune responses in murine atopic dermatitis models. J. Investig. Dermatol. 2014, 134, 2122–2130. [Google Scholar] [CrossRef]
- Milovanovic, M.; Drozdenko, G.; Weise, C.; Babina, M.; Worm, M. Interleukin-17A promotes IgE production in human B cells. J. Investig. Dermatol. 2010, 130, 2621–2628. [Google Scholar] [CrossRef]
- Romanos, M.; Gerlach, M.; Warnke, A.; Schmitt, J. Association of attention-deficit/hyperactivity disorder and atopic eczema modified by sleep disturbance in a large population-based sample. J. Epidemiol. Community Health 2010, 64, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Buske-Kirschbaum, A.; Schmitt, J.; Plessow, F.; Romanos, M.; Weidinger, S.; Roessner, V. Psychoendocrine and psychoneuroimmunological mechanisms in the comorbidity of atopic eczema and attention deficit/hyperactivity disorder. Psychoneuroendocrinology 2013, 38, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.A.; Nigg, J.T.; Sullivan, E.L. Neuroinflammation as a risk factor for attention deficit hyperactivity disorder. Pharmacol. Biochem. Behav. 2019, 182, 22–34. [Google Scholar] [CrossRef]
- Song, Y.; Lu, M.; Yuan, H.; Chen, T.; Han, X. Mast cell-mediated neuroinflammation may have a role in attention deficit hyperactivity disorder (Review). Exp. Ther. Med. 2020, 20, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Corona, J.C. Role of Oxidative Stress and Neuroinflammation in Attention-Deficit/Hyperactivity Disorder. Antioxidants 2020, 9, 1039. [Google Scholar] [CrossRef]
- Hong, J.E.; Park, M.-c.; Kang, S.-J.; Yang, G.-J.; Jo, E.H. New Interpretation on MyeonGu of Baekho-Tang Text of Shanghanlun through Case Reports; The Physiological Society of Korean Medicine and the Society of Pathology in Korean Medicine: Seoul, Korea, 2020. [Google Scholar]
- Lim, K.-M.; Song, J.-H.; Choi, J.-H.; Kim, J.-H.; Jung, M.-Y.; Park, S.-Y. Effects of Three Types of GagamBangpungtongseong-san(Except Talcum) on the Atopic Dermatitis in Mice. J. Korean Med. Ophthalmol. Otolaryngol. Dermatol. 2020, 33, 1–26. [Google Scholar]
- Bhattacharjee, S.; Gupta, G.; Bhattacharya, P.; Mukherjee, A.; Mujumdar, S.B.; Pal, A.; Majumdar, S. Quassin alters the immunological patterns of murine macrophages through generation of nitric oxide to exert antileishmanial activity. J. Antimicrob. Chemother. 2009, 63, 317–324. [Google Scholar] [CrossRef][Green Version]
- He, C.; Wang, Y.; Yang, T.; Wang, H.; Liao, H.; Liang, D. Quassinoids with Insecticidal Activity against Diaphorina citri Kuwayama and Neuroprotective Activities from Picrasma quassioides. J. Agric. Food Chem. 2020, 68, 117–127. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, M.-J.; Nguyen, L.T.H.; Shin, H.-M.; Yang, I.-J. Picrasma quassioides (D.DON) Benn. Ethanolic Extract Improves Atopic Dermatitis and Hyperactivity Disorder in DNCB-Treated BALB/c Mice. Appl. Sci. 2022, 12, 2032. https://doi.org/10.3390/app12042032
Choi M-J, Nguyen LTH, Shin H-M, Yang I-J. Picrasma quassioides (D.DON) Benn. Ethanolic Extract Improves Atopic Dermatitis and Hyperactivity Disorder in DNCB-Treated BALB/c Mice. Applied Sciences. 2022; 12(4):2032. https://doi.org/10.3390/app12042032
Chicago/Turabian StyleChoi, Min-Jin, Ly Thi Huong Nguyen, Heung-Mook Shin, and In-Jun Yang. 2022. "Picrasma quassioides (D.DON) Benn. Ethanolic Extract Improves Atopic Dermatitis and Hyperactivity Disorder in DNCB-Treated BALB/c Mice" Applied Sciences 12, no. 4: 2032. https://doi.org/10.3390/app12042032
APA StyleChoi, M.-J., Nguyen, L. T. H., Shin, H.-M., & Yang, I.-J. (2022). Picrasma quassioides (D.DON) Benn. Ethanolic Extract Improves Atopic Dermatitis and Hyperactivity Disorder in DNCB-Treated BALB/c Mice. Applied Sciences, 12(4), 2032. https://doi.org/10.3390/app12042032






