Abstract
When present in high concentrations in red wine, 4-ethylphenol (4-EP) and 4-ethylguaiacol (4-EG) are responsible for the introduction of unpleasant aromas, which causes wine depreciation. The work presented concerns the performance of textural and chemical-modified activated carbons (ACs), produced from coconuts shells, in the treatment of spoiled wines. ACs were submitted to basic and acid treatment, by impregnation into solutions containing NaOH and HNO3, respectively. Modified ACs showed only a small, but noticeable, increase in apparent surface area and micropore volume when compared to the original AC. However, the surface chemistry underwent significant changes. The ability of modified ACs to remove 4-EP and 4-EG, which cause the off-flavor known as “Brett character”, from wine-like solutions has been successfully achieved. On the systems studied, 4-EG was retained in greater extension, but 4-EP was retained more strongly on the surface of the ACs. Ethanol was found to compete with 4-EP and 4-EG for the adsorptive centres. However, when 4-EP and 4-EG were present in the same solution, the addition of ethanol promoted a cooperative effect and favoured the adsorption of both compounds. It should be noted that the modified ACs were able to eliminate 4-EP and 4-EG to levels below their sensory perception thresholds referred for red wine.
1. Introduction
Accidental or unexpected chemical impurities can spoil wine during grape ripening, fermentation and bottling, and at the end of the process during storage and transportation. Grapes used for winemaking possess different yeast species, such as Saccharomyces cerevisiae, Dekkera, and Brettanomyces [1,2]. When present, Brettanomyces and Dekkera yeasts produce a variety of volatile phenolic compounds that are critical for the sensory properties of wine. Brettanomyces bruxellensis has been identified as one of the main yeasts responsible for oenological spoilage due to its ability to produce 4-EP and 4-EG during the winemaking process [1].
Phenolic compounds affect wine colour, the taste of bitterness, and astringency [3,4] and are also critical in long ageing, especially in red wine [5]. When present in high concentrations, 4-EP and 4-EG are responsible for the introduction of unpleasant aromas [6], which causes wine depreciation [6,7,8,9,10,11,12,13,14]. When present at concentrations below the perception thresholds, they add complexity to the wine, but the sensorial perception is not affected [15]. The perception thresholds for 4-EP and 4-EG in wine vary between 230 and 650 μg L−1 and 33 to 135 μg L−1, respectively [8,15].
Various treatments have been investigated for their capacity to inhibit Brettanomyces growth, passing from the addition of sulphite, commercial β-glucanase, dimethyl dicarbonate, and chitosan, or a combination of processes that end with the physical removals of Brettanomyces cells [10].
However, in the presence of contaminated wines, it is necessary to apply measures to restore the wine’s health and quality. It is necessary to implement removal methods able to eliminate the phenolic compounds that are responsible for wine deterioration.
Among the different treatments tested to treat damaged wines, the mixing of spoiled with clean wine is highlighted. Molecularly imprinted polymers and esterified cellulose polymers were reported to remove between 20 and 30% of 4-EP and 4-EG from aqueous solutions [16]. Polyaniline-based compounds were identified to reduce almost 68% of 4-EP, 50% of 4-EG and over 40% of all phenolic compounds, from wine-like model solutions [17]. Reverse osmosis [18] and adsorption on different adsorbents have also been successively tested for the removal of 4-EP and 4-EG [14,18,19,20].
Among the additives used to treat wine, the addition of chitosan was described as effective in the reduction in B. Bruxellenis viable cells under winemaking conditions [21]. Filipe-Ribeiro, Cosme, and Nunes (2018) concluded that fungal chitosan with a high deacetylation degree could remove negative volatile phenols and simultaneously increase the fruity and floral notes [9]. However, Elmacı et al. (2015) reported that chitosan had a retarding effect on alcoholic fermentation. The authors state that the use of 0.6 g L−1 of chitosan affects the chemical composition of wine through the increase in the volatile acidity, such as acetic acid, which results in a vinegar taste [22]. Colangelo et al. (2018) reported that the addition of chitosan does not have a real impact on the removal of polyphenols [23]. However, chitosan was accepted to treat wine, in 2009, by the Organisation Internationale de la Vigne et du Vin and in 2010 by the European Union [23,24].
To identify new additives to improve the quality of wine, Milheiro et al. (2017) reported the use of eight adsorbents in the removal of 4-EP and 4-EG from red wines. AC was considered the most efficient adsorbent, with a reduction of 57% of 4-EP and 4-EG at the levels used [25]. Another work reported the use of seven ACs, with different characteristics, in the removal of 4-EP and 4-EG from red wines. All ACs significantly reduced levels of 4-EP and 4-EG to a maximum of 73%. It should be noted that some wineries recommend the use of doses between 0.015 and 0.24 g L−1 of charcoal to treat wine with low concentrations of phenolic compounds, and between 0.12 and 0.96 g L−1 for wine with high levels of phenolic compounds [17]. However, some authors have reported a negative impact associated with the removal of some aromatic compounds [1]. Among the methods mentioned, none presents a performance of 100%, and the search for new additives or adsorbent materials is on the rise.
Commercial ACs are primarily produced from coal, coconut shell and wood, but with the increase in the AC market and the production of a diversity of waste from agricultural activities, new precursors have been tested. The efficiency of ACs in removing compounds from the liquid phase is due to their high, apparent surface area, porous volume and pore size distribution, conjugated with surface chemistry and fast adsorption kinetics [7,25,26,27,28,29,30,31]. The main objective of this work is to explore the effect of chemical and textural modifications, induced in commercial ACs produced from coconut shells, on their performance, as adsorbents, in the simultaneous removal of 4-ethylphenol and 4-ethylguaiacol from wine-like solutions.
2. Materials and Methods
2.1. Materials
Four commercial ACs, 3 produced from coconut shells and 1 from bituminous coal, were tested, as received and after being submitted to textural and chemical modifications, on the 4-EP and 4-EG removal, from wine-like solutions. The assays were made from solutions with one or both compounds in the mixture and the presence or absence of 13% v/v of ethanol. The adsorbents were four commercial ACs, one from Panreac, one from Merck, and two from Norit (Norit 1240W and Norit 1240X). The AC from Merck was submitted to textural and chemical modification processes to improve its adsorption capacity concerning both phenolic compounds.
The 4-EP and 4-EG were both from Aldrich, with purity greater than 99%. The HPLC-grade acetonitrile was supplied by Merck, the phosphoric acid was from Sigma-Aldrich and the purified water was obtained from a Milli-Q water purification system from Interface. All solutions and solvents used in the high-performance liquid chromatography (HPLC) were previously filtered through 0.45-µm membranes from Millipore.
2.2. Methods
2.2.1. Adsorbents’ Textural and Chemical Characterisation
All ACs were texturally and chemically characterised by nitrogen adsorption, at 77 K, and elemental analysis by the Servicio de Apoyo à l’Investigación, at the Servicio de Análisis Elemental y Molecular the l’Universidad d’ Extremadura.
The pH at the point of zero charge (pH pzc) was determined by mass titration using 7 w/v% suspensions of ACs in a 0.1 mol L−1 solution of sodium nitrate (NaNO3, > 99.5%, Riedel), as proposed by Noh and Schwarz in 1990, whose adaptation was previously described [29]. The surface functional groups were identified by Fourier Transform Infrared (FTIR) using a Perkin Elmer Spectrum, Two FT-IR Spectrophotometer. Solid discs were prepared using potassium bromide (KBr) as a diluent, in which the ACs were dispersed in a ratio of 1:750 (w/w, AC:KBr). The spectra were obtained using a resolution of 4 cm−1 and 20 scans, between 4000 and 450 cm−1.
2.2.2. Textural and Chemical Modification
The AC, from Merck, which was produced from biomass, such as coconut shells, presented a well-developed porous structure and a neutral character identified by a point of zero charge near 7. The AC prepared from coconuts shells was selected to be submitted to acid and basic treatments to evaluate the influence of these modifications on the textural and chemical properties and the performance of 4-EP and 4-EG removals from wine-like solutions. The modifications were conducted based on a procedure previously used on other ACs [29].
2.2.3. AC Modification with Nitric Acid
For this purpose, 3 g of AC from Merck were placed on 150 mL of HNO3 solution, 1.5 mol L−1 (10% (m/v) and 3 mol L−1 (20% (m/v) which was heated to 363 K, for 3 h, under agitation. The AC was then washed with distilled water, until the supernatant had a pH near to the distilled water. This was filtered and dried at 383 K, for two days. The modified samples were named Merck-HNO3-10 and Merck-HNO3-20.
2.2.4. AC Modification with Sodium Hydroxide
The basic treatment of ACs produces a positive surface charge which is useful for adsorbing negatively charged species from liquid solutions. In this sense, the Merck AC was modified through the impregnation in sodium hydroxide solutions, the concentrations of which were 0.5, 2.5 and 5.0 mol L−1. For this purpose, 3 g of Merck AC were placed on 150 mL of NaOH solution, under constant agitation, for 3 h, at room temperature. The AC was washed with distilled water and dried at 383 K, for two days. The samples obtained were named Merck NaOH-0.5, 2.5 and 5 (mol L−1).
After basic and acid treatments, the ACs were textural and chemically analyzed based on the nitrogen adsorption, conducted at 77 K, elemental analysis, FITR and determination of the pHpzc, as described previously.
2.2.5. Kinetic and Adsorption Studies of 4-Ethylphenol and 4-Ethylguaiacol
In an Erlenmeyer, 10 mg of AC were weighed, to which 25 mL of an aqueous solution containing 4-EP or 4-EG was added with concentrations varying between 0.05 and 1.5 mmol L−1. The range in concentrations of 4-EP and 4-EG used allowed the remaining amount in the solution (after removal by ACs) to fall below the limits of the perception thresholds in wine. The Erlenmeyer flasks, with a stopper, were placed under continuous agitation (150 revolutions per minute) in a thermostated shaker bath, at room temperature, for different times, until a maximum of 48 h. The quantity of phenolic compounds adsorbed on the ACs was obtained from the difference between the initial and measured concentration, after an equilibrium time.
To simulate real wine, acidic solutions (pH = 3) containing 4-EG, 4-EP, and 13% v/v of ethanol were prepared. The adsorption capacity of the ACs for 4-EP and 4-EG, in the presence of 13% v/v of ethanol, was evaluated following the procedure described above. The first quantification tests were conducted by UV/VIS spectrophotometry on a UV-Visible Nicolet Evolution 300 apparatus, from Thermo Electron Corporation. Furthermore, the 4-EP was quantified at 221 and 271 nm and the 4-EG at 234 and 294 nm. Spectrophotometric analysis is commonly used because it is environmentally friendly, simple, rapid, and low-cost. However, for more accurate results, and when both compounds were present in the mixture, their quantification was achieved using high-performance liquid chromatography (HPLC) equipment. This was an Agilent 1100 system, from Agilent Technologies, Germany, equipped with a UV detector and a manual injector Rheodyne 7725i, controlled by HP Chemstation software. The experimental conditions used on the HPLC were optimised. Three flow rates were tested (0.8, 1.2 and 1.5 mL min−1) of acetonitrile and acidified water, varying between 25 and 75%. The 4-EP and 4-EG quantification was performed using an external pattern, presenting an excellent correlation coefficient (R > 0.999), for concentrations ranging between 0 and 1.5 mmol L−1. The best results were obtained at 278 nm, with an isocratic elution, with a mobile phase comprised of acetonitrile/ultrapure water/phosphoric acid (750/240/1, v/v). The assays were made in triplicate and under these conditions, the retention times were 7.8 and 8.5 min, for 4-EG and 4-EP, respectively. The method used in HPLC was validated for its selectivity, linearity range, detection and quantification limits, reproducibility, precision, and accuracy.
3. Results and Discussion
3.1. Textural and Chemical Characterisation of Activated Carbons
The four original ACs, after being submitted to acid and basic treatments, were texturally characterised by N2 adsorption, at 77 K. Representative isotherms are shown in Figure 1. The isotherms obtained on the modified ACs are presented in Figures S1 and S2 (Supplementary Information). The textural characteristics, obtained by the application of the αs, DR and BET methods, of the N2 adsorption isotherms are presented in Table 1.
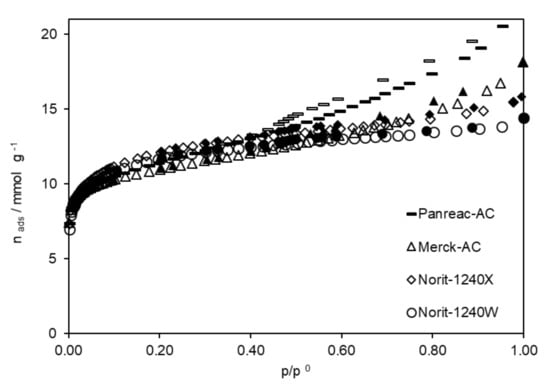
Figure 1.
Nitrogen adsorption isotherms obtained, at 77 K, on the original ACs. (Adsorption branch—open symbols and desorption branch—close symbols).

Table 1.
Chemical and textural characteristics, obtained by application of the αs, DR and BET methods, to N2 adsorption isotherms, obtained on different ACs as received and after being submitted to acid or basic treatments.
The four ACs presented close textural properties, with apparent superficial areas varying between 927 and 975 m2 g−1, micropores volumes varying from 0. 22 and 0.23 cm3 g−1 and mean pore size varying from 0.97 and 1.11 nm. Textural properties do not seem to be very determining in the performance of ACs in the removal of phenolic compounds. However, the physical shape of the ACs influences their performance in future applications. It should be noted that the Norit ACs were in granular form and the Merck and Panreac ACs were powdered.
The four ACs originally presented a basic character, with a pHpzc varying between 7.27 and 10.49. Merck’s AC presented a pHpzc closest to seven, and for this reason, it was chosen to undergo acid and basic treatments. The Merck AC treated with NaOH presented a high basic character (pHpzc from 7.76 to 8.67), which is helpful for the removal of phenolic compounds from the liquid phase [32,33].
The elemental analysis did not reveal any significant change in the ACs modified with NaOH. However, the ACs treated with HNO3 presented a noteworthy reduction in the C content, varying from 85.2%, in the original AC, to 70.5% and 71.8%, in the modified ACs. An increase in the oxygen percentage, which was reflected in an increase in the acid functional groups, on the ACs surface, offset this reduction.
FTIR analysis also confirmed the presence of acidic groups, on the surface of the ACs modified with nitric acid (Figures S3 and S4, in Supplementary Information). All of the spectra are almost similar in the absorption region, between 3200–3600 cm−1. The principal bands and peaks are related to alkyl groups, namely CH3, CH2 and CH, more specifically with anti-symmetric and symmetric stretching, in the range 2800–3000 cm−1. The band found in the original ACs in the region of 3400 cm−1 was attributed to an O-H stretching mode of the hydroxyl group in alcohols, phenols or absorbed water. This peak was slightly broader in the modified ACs, in which the band shifted slightly, to 3450–3500 cm−1. The peak near 1700 cm−1, present on the ACs modified with HNO3, indicated C=O groups, in mode stretch, or the carboxyl group, responsible for their pronounced acid character. Simultaneously, the intensity of the band at 1410 cm−1 increased due to the presence of a lactone structure. To summarise, as a result of the nitric acid treatment, the quinone functional groups were reduced and carboxylic acid groups were introduced.
3.2. Adsorption of 4-Ethylphenol and 4-Ethylguaiacol
Four commercial ACs, produced from coconut shell, were first tested, as received, in the removal of 4-EP and 4-EG from the liquid phase. In the first tests, the amount of 4-EP and 4-EG removed by the ACs was evaluated by spectrophotometric UV/VIS analysis. The 4-EP was analyzed at 221 and 271 nm and the 4-EG at 234 and 294 nm. To obtain more accuracy and reproducibility, in the following tests, the 4-EP and 4-EG quantification was conducted by HPLC, using the conditions stated in the experimental section.
The influence of the pH solution, the concentration of each compound, equilibrium time, textural characteristics, and physical form of the ACs (power and granulated), on the phenolic compound’s removals was evaluated. The increase in the solution acidity promotes the adsorption of 4-EP and 4-EG, primarily when the ACs presented a basic surface character; this is in agreement with previous work, which included phenol and para-nitrophenol adsorption on ACs [32]. The decision to perform the adsorption of 4-EP and 4-EG in an acid solution was also supported by the fact that wines are acidic, presenting a pH closer to 3.
3.2.1. Kinetic Studies of 4-Ethylphenol and 4-Ethylguaiacol
The kinetic curves exhibited an initial stage in which the adsorption of 4-EP and 4-EG was fast, followed by a second step, in which the uptake steadily increases up to equilibrium concentrations. The single-component kinetic provides information regarding the mechanism involved and the rate-controlling step on the 4-EP and 4-EG adsorption [2].
The removal of 4-EP and 4-EG reached 75 ± 2% and 72 ± 2% of the maximum amount removed within 3 h. The relatively slow adsorption of 4-EG might be a result of its higher solubility in water as well as its possessing the largest molecular size, compared to the 4-EP. As reported in a previous paper, an attractive adsorbent for organic compound removals must exhibit a large volume of micropores with widths of approximately 1.5 times the kinetic diameter of the target adsorbate [26]. The four commercial ACs present a mean pore varying between 0.97 and 1.11 nm, and 4-EG and 4-EP present an area of 29.5 and 20.2 Å2, respectively. The 4-EG could find resistance, to some extent, to enter the narrow micropores.
With the Merck AC, only 7 h were needed for both phenolic compounds to achieve their thermodynamic equilibrium. In order to facilitate and quicken laboratory work, and as in any industry, “time is money”, an equilibrium time of seven hours was chosen to carry out the remaining tests. However, results not shown here revealed that an increase in the equilibrium time, up to 48 h, allowed an increase in the 4-EP and 4-EG maximum adsorption quantities, mostly on granular ACs, such as those from Norit.
3.2.2. Kinetic Studies of 4-Ethylphenol and 4-Ethylguaiacol on Granular and Powder ACs
The influence of the ACs physical forms on the 4-EP and 4-EG removal from the aqueous phase was evaluated. For this purpose, AC Norit 1240W was used in a granulated and powdered form. When it was placed in solutions with high concentrations of both phenolic compounds, both ACs forms (granular and powder) exhibited almost the same maximum adsorption capacity.
In the diluted solutions of 4-EP and 4-EG, the Norit AC in powder form presented a maximum adsorption capacity of more than double that displayed by the granular form (0.33 and 0.15 mmol g−1, respectively). These results highlight the external transport contribution, as a limiting step, to the phenomenon of adsorption on dilute solutions. When using granular AC, the adsorptive molecules (4-EP and 4-EG) were too far from direct contact with the inner surface, and adsorption has no place, which does not happen when it was sprayed. Nevertheless, when AC powder is used in diluted solutions containing a deficient mixture, diffusion has been referred to as the limiting step in the adsorption process [26].
3.2.3. 4-Ethylphenol and 4-Ethylguaiacol Removals from Like-Wine Solutions
The adsorption isotherms of 4-EP and 4-EG were obtained on four untreated commercial ACs. In the dilute solutions, the ACs from Panreac and Merck adsorbed comparable quantities of 4-EP and 4-EG, as presented in Figure S2 (Supplementary Information). However, these amounts are quite superior to those achieved by the Norit granular ACs. From the concentrated solutions, (>0.2 mmol L−1) the maximum adsorption capacity of all of the ACs concerning 4-EG was always superior to 4-EP, as shown in Table 2. At high concentrations, 4-EG was adsorbed in greater extension, but the respective isotherm showed a more open knee. This allows us to state that 4-EP was adsorbed with greater affinity than 4-EG.

Table 2.
Adsorption capacity of the original ACs, concerning 4-EP and 4-EG, in the absence and presence of 13% (v/v) of ethanol, at pH = 3.
The maximum adsorption capacity of the four ACs was also evaluated, vis-à-vis the two phenolic compounds, in the presence of 13% v/v of ethanol. From the data presented in Table 2, the presence of direct competition between ethanol and both phenolic compounds by the active centres is evident. These results are in line with the published results [19].
In the presence of 13% v/v of ethanol, the amount of 4-EP adsorbed was 63%, 67%, 70% and 84% of the amount adsorbed in the absence of ethanol, in the AC from Panreac, Merck, Norit 1240X and 1240W, respectively. In the AC from Panreac and Merck, the amount of 4-EG adsorbed was only 48% and 63%, respectively. However, with both granular ACs from Norit, the amount of 4-EG adsorbed was less than 20% of the amount of 4-EG adsorbed in the absence of ethanol. The difference between the maximum amount of each phenolic compound adsorbed highlights the greater affinity between the ACs and 4-EP.
To increase the adsorptive capacities of ACs regarding 4-EG and 4-EP, Merck AC was submitted to different acid/basic treatments. The modified ACs were tested on the removal of 4-EP and 4-EG from an aqueous solution or a wine-like solution (containing 13% v/v of ethanol). The respective isotherms are presented in Figure 2 and Figure 3.
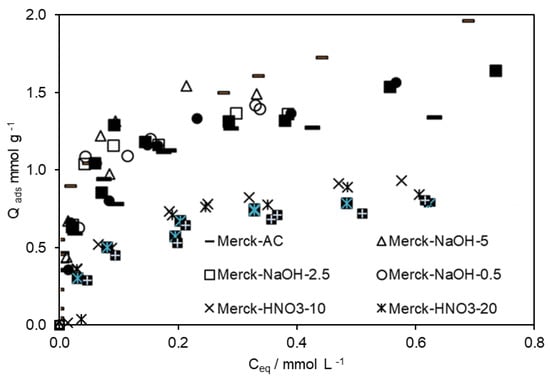
Figure 2.
Adsorption isotherms of 4-EG (open symbols), and 4-EP (closed symbols), obtained on modified ACs, in the absence of ethanol.
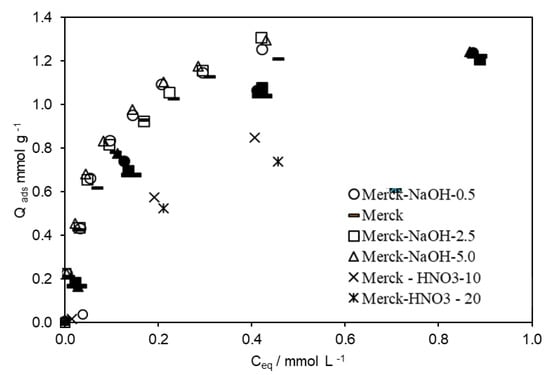
Figure 3.
Adsorption isotherms of 4-EG (open symbols) and 4-EP (closed symbols) obtained on modified ACs, in the presence of 13% (v/v) ethanol.
In the initial stage of the adsorption processes, there were a lot of active sites on the ACs surface and the strongly and weakly adsorbed components took the active sites easily, in agreement with published data [33]. However, for the same equilibrium concentration, the amount of 4-EG adsorbed by each AC was always higher than the 4-EP. However, the maximum amounts of 4-EP and 4-EG adsorbed were higher in the absence of ethanol, highlighting the competitiveness of ethanol by adsorption sites.
The data presented in Table 3 allow us to conclude that the basic groups on the ACs surface favour the adsorption of 4-EP, but do not significantly impact the adsorption of 4-EG. Here, again, ethanol competes with 4-EP and 4-EG for the active centers.

Table 3.
Adsorption capacity of the modified ACs concerning 4-EP ad 4-EG, obtained in the absence or presence of 13% v/v of ethanol, at an equilibrium concentration of 0.6 mmol L−1.
The results obtained from the ACs submitted to acidic treatments were not promising; however, this agrees with the results found in the literature [34]. Thus, it is possible to infer that ACs’ surface chemistry is the foremost control factor in the 4-EG and 4-EP adsorption process, to the detriment of the textural properties.
3.2.4. Joint Removal of 4-Ethylphenol and 4-Ethylguaiacol from Like-Wine Solutions
When 4-EP and 4-EG were present in the same solution, significant differences were observed, as shown in Figure 2 and Figure 4. The sequence of both phenolic compounds’ absorption was similar to that obtained with the individual compound, even though the preferential adsorption of 4-EG, compared to 4-EP, was now more obvious. The sum of the maximum adsorption capacity of both compounds was similar to that obtained for 4-EG when it was alone in the solution.
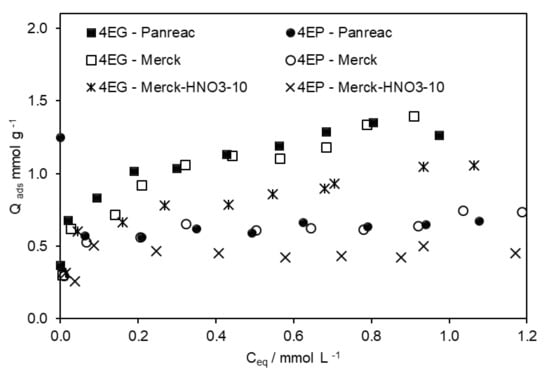
Figure 4.
Adsorption isotherms of 4-EG and 4-EP obtained on different ACs, from solutions where both compounds were present, without ethanol.
When the adsorption of both compounds was performed in a wine-like solution, with 13% v/v of ethanol, their respective maximum adsorption capacity was inferior to that found when they were alone in the solution (Figure 3 and Figure 5). The sum of 4-EP and 4-EG was higher than the amount of 4-EG adsorbed on the respective AC when it was alone in the solution. Ethanol seems to promote a cooperative effect that favours the adsorption of both compounds when they are mixed. These outcomes are very promising, as it is expected that with real wine, the results obtained will be similar or even better. The comparison between the 4-EP and 4-EG isotherms obtained in different media is presented in Figure S5 (Supplementary Information).
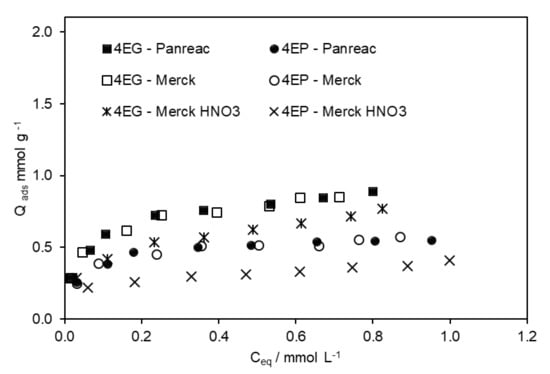
Figure 5.
Adsorption isotherms of 4-EG and 4-EP were obtained on different ACs, from solutions where both compounds were present, in the presence of 13% v/v of ethanol.
The 4-EP and 4-EG isotherms were adjusted with Langmuir and Freundlich equations. These equations allow us to obtain some of the most common parameters used to evaluate the performance of ACs against adsorptives [27,35]. Table 4 shows all of the parameters obtained for 4-EP and 4-EG when they were alone or mixed in solution, in the absence or presence of 13% v/v of ethanol, applying the Langmuir and Freundlich models.

Table 4.
Adsorption isotherm parameters of 4-EP and 4-EG onto different ACs under different experimental conditions. nmax—maximum adsorption capacity, taken directly from the isotherm (mmol g−1), nmL—monolayer capacity (mmol g−1), KL—Langmuir constant (mmol g−1) KF (mmol g−1 (dm3 mmol−1)1/nF) and nF—are constant and exponent of Freundlich.
The values of the monolayer capacity (nmL) were very similar to the experimental maximum adsorption capacities for both compounds. However, the adjustment to the Langmuir equation was less consistent in the region of high concentrations. From the data presented in Table 4, it is possible to conclude that the affinity between both phenolic compounds and the ACs surface has changed. The presence of 13% v/v of ethanol promotes a decrease in the affinity between each compound and the ACs. When 4-EP and 4-EG were present in the same solution, the interaction between 4-EP and the ACs surface seemed to strengthen compared with its affinity when it was alone in the solution. Surprisingly, when 4-EP and 4-EG were present in the same solution, with 13% v/v of ethanol, an increase in the surface affinity was noticed with both compounds. The data included in Table 4 attest to a significant increase in the KL values, which agrees with the increase in the interactions between adsorbent and adsorbate.
Good adjustments were obtained using the Freundlich equation. However, the correlations were lower than those obtained through the Langmuir equation. The constants of Freundlich (KF) were very similar to the experimental maximum adsorption capacities. On the other hand, the Freundlich exponent (nF) took values between 2.15 and 9.86, which indicates the presence of favorable adsorption. On Merck-HNO3-10, the nF values were slightly lower than those determined on other ACs, indicating that the adsorption was less favourable. The nF values were higher when both phenolic compounds were present in the same solution, and even higher in the presence of ethanol, reinforcing the cooperative interactions.
4. Conclusions
Four commercial ACs were successfully tested for the removal of 4-EP and 4-EG from the aqueous phase and ethanol-containing wine-like solutions. To improve the AC capacity for the removal of 4-EP and 4-EG, AC from Merck has been chemically modified through its impregnation into NaOH and HNO3 solutions. The textural properties of the modified ACs presented only minor changes, however, the chemical surface changed significantly, as illustrated in the FTIR spectrum and through the change of pHpzc.
In the systems studied, when only one or both compounds were present in the same solution, 4-EG was adsorbed to a greater extent, but 4-EP was retained to a greater extent. Ethanol had a competing effect on the adsorption of 4-EG and 4-EP when only one compound was present in the solution. Thus, when both compounds were present in the same solution, the presence of ethanol appeared to promote a cooperative effect and favour the adsorption of both compounds. These results are very promising as the use of modified ACs with a basic character show a better performance in the removal of 4-EG and 4-EP in the presence of ethanol. ACs with similar properties may be a solution for the removal of these unpleasant compounds from red wine, up to a level below the perception threshold. However, it is necessary to deepen the knowledge of the removal efficiency of 4-EP and 4-EG and their effects on red wine quality.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app122211754/s1, Figure S1: Nitrogen adsorption isotherms obtained, at 77 K, on the commercial AC, from Merck, submitted to basic treatments with NaOH; Figure S2: Nitrogen adsorption obtained, at 77 K, on the commercial AC, from Merck, submitted to treatments with HNO3; Figure S3: FTIR spectra of AC from Merck, as received and after submitted to acid treatments with HNO3; Figure S4: FTIR spectra of AC from Merck, as received and after submitted to basic treatments with NaOH; Figure S5: Adsorption isotherms of 4-EP and 4-EG obtained from solutions where the phenolic compounds were isolated or present in a mixture, (a) Panreac; (b) Merck; (c) Merck-HNO3-10.
Author Contributions
Conceptualization, I.P.d.P.C. and P.A.M.M.; methodology, I.P.d.P.C. and P.A.M.M.; software, P.A.M.M.; validation, I.P.d.P.C. and P.A.M.M.; formal analysis, I.P.d.P.C. and P.A.M.M.; investigation, I.D.M., V.P. and J.J.; resources, P.A.M.M.; data curation, I.D.M., V.P. and J.J.; writing—original draft preparation, I.D.M., V.P. and J.J.; writing—review and editing, I.P.d.P.C. and P.A.M.M.; visualization, I.P.d.P.C.; supervision, I.P.d.P.C. and P.A.M.M.; project administration, I.P.d.P.C.; funding acquisition, P.A.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by FCT (Project UID/QUI/0619/2016) with National (OE) funds.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Milheiro, J.; Filipe-Ribeiro, L.; Vilela, A.; Cosme, F.; Nunes, F.M. 4-Ethylphenol, 4-ethylguaiacol and 4-ethylcatechol in red wines: Microbial formation, prevention, remediation and overview of analytical approaches. Crit. Rev. Food Sci. Nutr. 2019, 59, 1367–1391. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Gong, Q.; Chen, Z.; Wang, W.D.; Huang, Q.; Song, S.; Wang, X. Adsorption and competition investigation of phenolic compounds on the solid-liquid interface of three-dimensional foam-like graphene oxide. Chem. Eng. J. 2019, 378, 122085. [Google Scholar] [CrossRef]
- Ferrer-Gallego, R.; Hernández-Hierro, J.M.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Sensory evaluation of bitterness and astringency sub-qualities of wine phenolic compounds: Synergistic effect and modulation by aromas. Food Res. Int. 2022, 62, 1100–1107. [Google Scholar] [CrossRef]
- Paissoni, M.A.; Bitelli, G.; Vilanova, M.; Montanini, C.; Segade, S.R.; Rolle, L.; Giacosa, S. Relative impact of oenological tannins in model solutions and red wine according to phenolic, antioxidant, and sensory traits. Food Res. Int. 2022, 157, 111203. [Google Scholar] [CrossRef]
- Malfeito-Ferreira, M. Two Decades of “Horse Sweat” Taint and Brettanomyces Yeasts in Wine: Where do We Stand Now? Beverages 2018, 4, 32. [Google Scholar] [CrossRef]
- Schumaker, M.R.; Chandra, M.; Malfeito-Ferreira, M.; Ross, C.F. Influence of Brettanomyces ethylphenols on red wine aroma evaluated by consumers in the United States and Portugal. Food Res. Int. 2017, 100, 161–167. [Google Scholar] [CrossRef][Green Version]
- Aliakbarian, B.; Casazza, A.A.; Perego, P. Kinetic and isotherm modelling of the adsorption of phenolic compounds from olive mill water onto activated carbon. Food Technol. Biotechnol. 2015, 53, 207–214. [Google Scholar] [CrossRef]
- Caboni, P.; Sarais, G.; Cabras, M.; Angioni, A. Determination of 4-ethylphenol and 4-ethylguaiacol in wines by LC-MS-MS and HPLC-DAD-fluorescence. J. Agric. Food Chem. 2007, 55, 7288–7293. [Google Scholar] [CrossRef]
- Filipe-Ribeiro, L.; Cosme, F.; Nunes, F.M. Reducing the negative sensory impact of volatile phenols in red wine with different chitosans: Effect of structure on efficiency. Food Chem. 2017, 242, 591–600. [Google Scholar] [CrossRef]
- Schumaker, M.R.; Diako, C.; Castura, J.C.; Edwards, C.G.; Ross, C.F. Influence of wine composition on consumer perception and acceptance of Brettanomyces metabolites using temporal check-all-that-apply methodology. Food Res. Int. 2019, 116, 963–972. [Google Scholar] [CrossRef]
- Šućur, S.; Čadež, N.; Košmerl, T. Volatile phenols in wine: Control measures of Brettanomyces/Dekkera yeasts. Acta Agric. Slov. 2016, 107, 453–472. [Google Scholar] [CrossRef]
- Oelofse, A.; Malherbe, S.; Pretorius, I.S.; Du Toit, M. Preliminary evaluation of infrared spectroscopy for the differentiation of Brettanomyces bruxellensis strains isolated from red wines. Int. J. Food Microbiol. 2010, 143, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Steensels, J.; Daenen, L.; Malcorps, P.; Derdelinckx, G.; Verachtert, H.; Verstrepen, K.J. Brettanomyces yeasts—From spoilage organisms to valuable contributors to industrial fermentations. Int. J. Food Microbiol. 2015, 206, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Filipe-Ribeiro, L.; Milheiro, J.; Matos, C.C.; Cosme, F.; Nunes, F.M. Reduction of 4-ethylphenol and 4-ethylguaiacol in red wine by activated carbons with different physicochemical characteristics: Impact on wine quality. Food Chem. 2017, 229, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Malfeito-Ferreira, M. Yeasts and wine off-flavours: A technological perspective. Ann. Microbiol. 2011, 61, 95–102. [Google Scholar] [CrossRef]
- Larcher, R.; Puecher, C.; Rohregger, S.; Malacarne, M.; Nicolini, G. 4-Ethylphenol and 4-ethylguaiacol depletion in wine using esterified cellulose. Food Chem. 2012, 132, 2126–2130. [Google Scholar] [CrossRef]
- Carrasco-Sánchez, V.; John, A.; Marican, A.; Santos, L.S.; Laurie, V.F. Removal of 4-ethylphenol and 4-ethylguaiacol with polyaniline-based compounds in wine-like model solutions and red Wine. Molecules 2015, 20, 14312–14325. [Google Scholar] [CrossRef]
- Suárez, R.; Suárez-Lepe, J.A.; Morata, A.; Calderón, F. The production of ethylphenols in wine by yeasts of the genera Brettanomyces and Dekkera: A review. Food Chem. 2007, 102, 10–21. [Google Scholar] [CrossRef]
- Madureira, J.; Melo, R.; Verde, S.C.; Matos, I.; Bernardo, M.; Noronha, J.P.; Fonseca, I.M. Recovery of phenolic compounds from multi-component solution by a synthesized activated carbon using resorcinol and formaldehyde. Water Sci. Technol. 2018, 77, 456–466. [Google Scholar] [CrossRef]
- Da̧browski, A.; Podkościelny, P.; Hubicki, Z.; Barczak, M. Adsorption of phenolic compounds by activated carbon—A critical review. Chemosphere 2005, 58, 1049–1070. [Google Scholar] [CrossRef]
- Taillandier, P.; Joannis-Cassan, C.; Jentzer, J.B.; Gautier, S.; Sieczkowski, N.; Granes, D.; Brandam, C. Effect of a fungal chitosan preparation on Brettanomyces bruxellensis, a wine contaminant. J. Appl. Microbiol. 2015, 118, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Elmacı, S.B.; Gulgor, G.K.; Tokatlı, M.; Erten, H.; Isci, A.; Ozcelik, F. Effectiveness of chitosan against wine-related microorganisms. Antonie Van Leeuwenhoek 2015, 107, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, D.; Torchio, F.; De Faveri, D.M.; Lambri, M. The use of chitosan as alternative to bentonite for wine fining: Effects on heat-stability, proteins, organic acids, colour, and volatile compounds in an aromatic white wine. Food Chem. 2018, 264, 301–309. [Google Scholar] [CrossRef] [PubMed]
- European Union (EU). Commission regulation (EU) 53/2011 of 21 January 2011. Off. J. Eur. Union. 2011, 22, L19/1–L19/6. [Google Scholar]
- Milheiro, J.; Filipe-Ribeiro, L.; Cosme, F.; Nunes, F.M. A simple, cheap and reliable method for control of 4-ethylphenol and 4-ethylguaiacol in red wines. Screening of fining agents for reducing volatile phenols levels in red wines. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2017, 1041–1042, 183–190. [Google Scholar] [CrossRef]
- Belo, C.R.; Cansado, I.; Mourão, P. Synthetic polymers blend used in the production of high activated carbon for pesticides removals from liquid phase. Environ. Technol. 2017, 38, 285–296. [Google Scholar] [CrossRef]
- Mourão, P.A.M.; Cansado, I.P.P.; Carrott, P.J.M.; Ribeiro Carrott, M.M.L. Designing activated carbons from natural and synthetic raw materials for pollutants adsorption. Mater. Sci. Forum 2010, 636–637, 1404–1409. [Google Scholar] [CrossRef]
- Cansado, I.P.P.; Belo, C.R.; Mourão, P.A.M. Valorisation of Tectona Grandis tree sawdust through the production of high activated carbon for environment applications. Bioresour. Technol. 2018, 249, 328–333. [Google Scholar] [CrossRef]
- Cansado, I.P.P.; Galacho, C.; Nunes, A.S.; Carrott, M.L.R.; Carrott, P.J.M. Adsorption properties of activated carbons prepared from recycled pet in the removal of organic pollutants from aqueous solutions. Adsorpt. Sci. Technol. 2010, 28, 807–821. [Google Scholar] [CrossRef]
- Gokce, Y.; Aktas, Z. Nitric acid modification of activated carbon produced from waste tea and adsorption of methylene blue and phenol. Appl. Surf. Sci. 2014, 313, 352–359. [Google Scholar] [CrossRef]
- Lorenc-Grabowska, E. Effect of micropore size distribution on phenol adsorption on steam activated carbons. Adsorption 2016, 22, 599–607. [Google Scholar] [CrossRef]
- Cansado, I.P.P.; Mourão, P.A.M.; Falcão, A.I.; Ribeiro Carrott, M.L.; Carrott, P.J.M. The influence of the activated carbon post-treatment on the phenolic compounds removal. Fuel Process. Technol. 2012, 103, 64–70. [Google Scholar] [CrossRef]
- Sulaymon, A.H.; Ahmed, K.W. Competitive adsorption of furfural and phenolic compounds onto activated carbon in fixed bed column. Environ. Sci. Technol. 2008, 42, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Soudani, N.; Souissi-Najar, S.; Ouederni, A. Influence of nitric acid concentration on characteristics of olive stone based activated carbon. Chin. J. Chem. Eng. 2013, 21, 1425–1430. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).