Abstract
To detect residual pre-proinsulin (PPI) in recombinant human insulin production, an analytical method based on double-antibody sandwich ELISA was developed in this study. The BALB/c mice were immunized with PPI, and the hybridomas secreting anti-PPI monoclonal antibodies were obtained using the conventional cell fusion technique and ELISA screening. We purified the antibody using a Protein G gel column and identified its purity by SDS-PAGE. The sandwich ELISA was used to explore the pairing effect, and the specificity of the paired antibody was determined. We selected a paired antibody with relatively good specificity to establish sandwich ELISA, constructed a quantitative curve, and evaluated the accuracy and sensitivity of the method. Six anti-PPI monoclonal antibodies were obtained, named P1, P2, P3, P4, P5 and P6, of which P5 had the highest titer value. The sandwich ELISA method was established with P5 for plating and P2 as detection antibodies. The linear range of the quantitative curve of PPI by sandwich ELISA was 0.645 to 82.5 pg/mL, the recovery was 95% and the detection limit was 3.06 pg/mL. In this study, we prepared six anti-PPI monoclonal antibodies and established the sandwich ELISA method to detect PPI in process control and product release control for recombinant human insulin production.
1. Introduction
Diabetes mellitus, commonly known as diabetes, is a metabolic disease characterized by high blood glucose levels due to defective insulin secretion or impaired insulin action []. Diabetes includes mainly Type I and Type II diabetes, and is one of the major diseases that threaten human health and life. Type I diabetes is characterized by a loss of the ability of pancreatic beta cells to secrete insulin, and patients rely on insulin therapy to maintain their lives. Type II diabetes, which accounts for approximately 90% of adult-onset diabetes, is caused by a combination of insulin secretion, insulin resistance, hepatic glucose output and lipid disorders []. Insulin can regulate glucose metabolism and control blood glucose balance and is the main drug for treating diabetes []. In addition, insulin therapy has shown significant advantages over anti-diabetic drugs in both type I and type II diabetes []. Insulin, originally produced as preproinsulin, is transformed into a proinsulin by proteolytic action and finally into the active polypeptide hormone, insulin []. Recently, developments in recombinant DNA technology and protein engineering have led to the increasing availability of human insulins and modified insulin analogues with improved pharmacokinetic properties []. Most of the insulins currently used in clinical trials are formed by recombinant DNA expression in E. coli or yeast to form inactive pre-proinsulin (PPI), subsequently enzymatically cleaved and purified to obtain high purity insulin. The efficient and stable expression of PPI is a prerequisite for obtaining high-yield insulin [,]. PPI, although low in molecular weight (only 15 kD), remains immunogenic and its prolonged presence can produce antibodies and thereby reduce the pharmacological effects of insulin []. However, undigested and unremoved PPI seriously affects the quality and safety of insulin [,]. Therefore, the accurate detection of PPI expression and residues in insulin is very critical and urgent in the quality control of insulin biosynthesis.
According to the European Pharmacopeia, PPI in insulin must be detected and controlled; however, no specific detection method has been proposed. Currently, the commonly used methods include radioimmunoassay and high-performance liquid chromatography (HPLC) [,,]. Radioimmunoassay has been gradually eliminated due to inadequate prices, pollution to the environment and harm to human health []. High-performance liquid chromatography is often used to detect the PPI content in the purification process; however, its poor sensitivity leads to its inability to meet the requirements for detecting PPI residues in the final product [].
Enzyme-linked immunosorbent assays have high sensitivity and can be used to detect trace amounts of PPI. However, due to the large differences in the contents of PPI produced by different processes, using a universal ELISA kit for determination is currently impossible. In this study, a PPI molecule consists of the A-chain of insulin, B-chain of insulin, a guide peptide (SOD) and a -Lys-Arg- linker. After trypsin digestion, SOD and single-chain Lys-Arg-insulin (sIKR) were produced. As the enzyme digestion proceeded, the junction of the A-chain and arginine was cleaved, but only the remaining B-chain was linked to Arg-Lys-, and the free A-chain was correctly paired with the B-chain to form Arg-Lys-insulin (IKR). The IKR was turned into lys-insulin (IK) after the arginine residue was removed by carboxypeptidase B, and the lysine residue in IK was further removed to form insulin with the correct spatial conformation. To explore a highly targeted and sensitive method, we prepared six anti-PII monoclonal antibodies using the hybridoma technique and preliminarily established double-antibody sandwich ELISA to determine the PPI residue in recombinant human insulin.
2. Materials and Methods
2.1. Materials
Experimental animals were 6- to 8-week-old BALB/c mice. Penicillin, HAT (50×), HT, PEG1450, incomplete Freund’s adjuvant and streptomycin were purchased from Sigma-Aldrich (Burlington, MA, USA). DMEM medium and Fetal bovine serum was purchased from Gibco (Thermo Fisher, Waltham, MA, USA). Horseradish peroxidase-labeled sheep anti-mouse IgG (HRP-sheep anti-mouse IgG) was purchased from Frdbio (Wuhan, China). Cell dissociation buffer (0.25% pancreatin and 0.02% EDTA) was prepared for use. The reagents for purifying antibodies, including antibody elution buffer, neutralization buffer, preservation buffer, PBS buffer (pH 7.4) and sodium azide were self-made.
The reagents for detecting ELISA in the establishment of the double-antibody sandwich method are as follows: ELISA coating solution, washing solution (PBST), 3,3′,5′-tetramethylbenzidine (hereinafter referred to as TMB) substrate reaction solution, sealing solution, universal diluent and termination solution. They were all self-made.
The PPI of animal immune antigens, PPI of antigens coated indirectly by ELISA method, PPI standard in double-antibody sandwich method, SOD, sIKR, IKR and IK were collected from the PPI in the production process of the recombinant human insulin and its digested intermediate product. After purification, their purity was over 95%. The insulin and PPI are manufactured at Hefei Tianmai Biotechnology Development Company.
2.2. Preparation, Purification and Marking of Monoclonal Antibodies
The 6- to 8-week-old BALB/c mice were immunized with PPI as the immunogen, and blood was taken as a negative serum control before immunization. The initial immunization dose was 100 μg/mouse. The PPI was emulsified with the same volume of ferrin complete adjuvant and then immunized the mice and then immunized mice at an interval of 2 weeks. The serum antibody titer of mice was detected 14 days after the third immunization. The mice with relatively high titer were immunized by intraperitoneal injection of immunogen 100 μg/mouse three days before cell fusion. Then, the blood was sacrificed, and the immune spleen cells were isolated. The immune spleen cells and the prepared mouse myeloma cells SP2/0 were fused. Positive monoclonal hybridoma cells were screened by ELISA. After three subclones and a completely positive ELISA test, expand the culture and construct the cell line. The screened hybridoma cells stably secreting target antibodies were inoculated in BALB/c mice aged 6 to 8 weeks at 1 to 5 × 106/mouse with incomplete first adjuvant. When the abdomens of the mice were significantly enlarged, and the skin being touched by hands was tense, the ascites were collected with a 10-mL needle. The ascites was centrifuged, the supernatant was collected, and the titer was determined. The antibody was purified with a Protein G gel column, and the purified monoclonal antibody(mAb) was identified by SDS-PAGE gel electrophoresis. The obtained monoclonal antibody was diluted to 1 mg/mL with 0.1 M PBS buffer (pH 7.4), and the interfering substance was removed by ultrafiltration. Water-soluble biotin was added, and the reaction was performed at room temperature for 1 h. The free biotin was removed by ultrafiltration.
2.3. Double Antibody Sandwich ELISA Screening for Paired Antibodies
The purified monoclonal antibody was diluted to 1 μg/mL with PBS buffer (pH 7.4) and were separately encapsulated in the enzyme-labeled plates at 100 μL/well. Then, block with 2% bovine serum albumin solution at 37 °C. Wash three times with tbst after 60 min. After that, PPI with a final concentration of 1 μg/mL was added to each well and incubated at 37 °C for 60 min. After three times of TBST washing, use the microplate reader to detect. In addition, 1% BSA was used as the blank control. According to the checkerboard method, each monoclonal antibody labeled with biotin was added at 100 μL/pore, placed at 37 °C for 60 min and washed with TBST three times. Streptavidin HRP was added at 100 μL/pore, placed at 37 °C for 60 min and washed with TBST three times. Then, the TMB color rendering solution was added to avoid light for 5 min. Finally, the terminal solution was added, and the absorbance value at 450 nm wavelength was read. The sample hole with an absorbance value of more than 2.1 times the absorbance value of the blank control hole was considered a positive detection hole.
2.4. Specific Evaluation of Paired Antibody
The coating antibody corresponding to the positive detection hole and the biotin-labeled antibody were used as the solid-phase antibody and the detection antibody, respectively. Then, 1 μg/mL of PPI, SOD, sIKR, IKR and IK were added to the detection hole, and 1% BSA was used as the blank control to test the specificity of each paired antibody according to the method described in method 2.2.
2.5. Construction of Quantitative Curve
The determined optimum solid-phase antibodies were coated in the enzyme-labeled plate. Then, 100 μL PPI with the concentrations of 82.5 pg/mL, 41.25 pg/mL, 20.65 pg/mL, 10.31 pg/mL, 5.16 pg/mL, 2.58 pg/mL, 1.29 pg/mL, 0.645 pg/mL and 0 pg/mL were added, respectively. They were placed at 37 °C for 60 min and washed with TBST three times. Next, 1 μg/mL of optimum detection antibody was added at 100 μL/pore, and the double-antibody sandwich ELISA was performed according to the method described in 1.2.2 with three multiple holes per PPI concentration. With the average OD450 value of each concentration as the ordinate and the PPI concentration as the abscissa, the standard curve was fitted with Skanlt RE (Thermo, Varioskan LUX, Waltham, MA, USA) to determine the linear range and quantitative equation of the detection.
2.6. Accuracy and Sensitivity Evaluation
In order to evaluate the accuracy of our established double antibody sandwich ELISA method, we further determined the recovery rate. First, recombinant human insulin was accurately weighed and dilutions at a concentration of 2 μg/mL were prepared. Next, PPI standards were prepared at concentrations of 82.5 pg/mL, 20.58 pg/mL and 5.16 pg/mL. Afterwards, 2 μg/mL of recombinant human insulin solution was mixed with equal volumes of different concentrations of PPI standards to prepare QC samples with final concentrations of 41.25 pg/mL, 10.31 pg/mL and 2.58 pg/mL. The samples to be tested were diluted from 2 μg/mL of recombinant human insulin solution to 1 μg/mL of solution. Finally, the standard, the sample to be tested and the QC sample were added to the coated ELISA plate, and 3 replicate wells of each sample were used as blank control with the universal dilution solution, and the assay was performed according to the ELISA method established above. The accuracy was expressed as the recovery rate. The mean value of the measured concentration of the control sample was subtracted from the mean value of the measured concentration of the sample to be tested, and the ratio of the difference to the theoretical added concentration was the recovery rate.
The absorbance values of 12 blank samples, i. e., those of the general dilutions at 450 nm wavelength, were determined by the double-antibody sandwich ELISA method. The average absorbance value of the measured samples plus the absorbance value obtained by three times standard deviation was substituted into the quantitative equation.
2.7. Statistical Analysis
Data were presented as mean ± standard deviation (SD). Statistical significance was assessed by one-way or two-way ANOVA via GraphPad Prism software (Prism version 9, San Diego, CA, USA). p-value < 0.05 was considered as statistically significant.
3. Results
3.1. Preparation and Purification of Monoclonal Antibody
Hybridoma cells were prepared using PPI-immunized mice. After multiple rounds of screening and three subclones, six cell lines capable of stably secreting anti-PPI monoclonal antibodies were obtained, namely P1, P2, P3, P4, P5 and P6. In subsequent studies, each monoclonal antibody was named after its source cell line. The selected cell lines were injected into the peritoneal cavities of the mice at 1 to 5 × 106/only to prepare ascites, and the collected ascites supernatant was measured with titer (Figure 1). The results showed that P5 had the highest titer, and the titer values of P1 and P3 were the lowest. However, the titer value of every single group was above 1:7.29 × 105, which can be further studied.
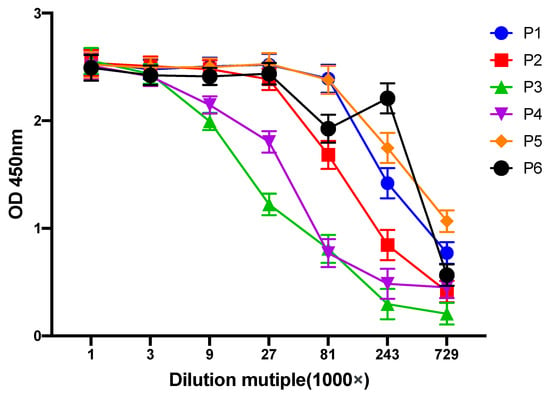
Figure 1.
Titers of monoclonal antibodies against PPI.
Protein G column was used for affinity chromatography of monoclonal antibody P1, P2, P3, P4, P5 and P6, and purified monoclonal antibody was detected by reducing SDS-PAGE (Figure 2). The results showed that each monoclonal antibody had high purity and no miscellaneous protein except immunoglobulin light chain and heavy chain.
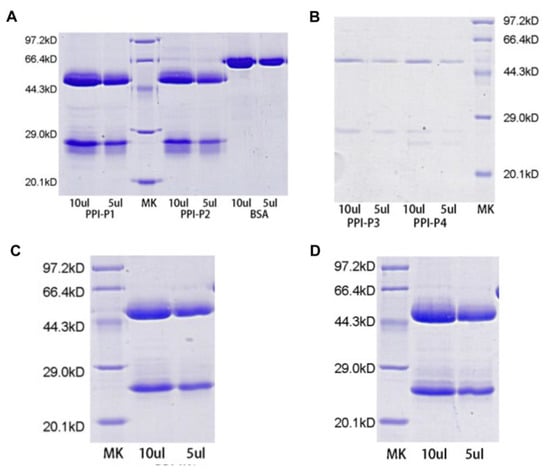
Figure 2.
Purification of monoclonal antibodies against PPI. (A) Reduced SDS-PAGE showing purification of monoclonal antibodies P1 and P2. 1: 10 μL of P1; 2: 5 μL of P1; 3: 10 μL of P2; 4: 5 μL of P2; (B) P3 and P4. 1: 10 μL of P3; 2: 5 μL of P3; 3: 10 μL of P4; 4: 5 μL of P4; (C) P5. 1: 10 μL of P5; 2: 5 μL of P5; (D) P6. 1: 10 μL of P6; 2: 5 μL of P6; M: molecular weight mass standards (kDa).
3.2. Double Antibody Sandwich ELISA Screening for Paired Antibodies
P1, P2, P3, P4, P5 and P6 were successively used as solid-phase antibody packages in the enzyme-labeled plate, PPI was added as an antigen, and 1% BSA as blank control. A biotin-labeled monoclonal antibody was added for double-antibody sandwich ELISA detection. The antibody in the detection hole with an absorbance value 2.1 times greater than that of the blank hole was used as the positive paired antibody (Table 1). The results showed that when P5 was used as the solid-phase antibody, biotin-labeled P1, P2, P3, P4 and P6 could be used as detection antibodies. P2 was not successfully paired as a solid-phase antibody. P1, P3, P4 and P6 could only be detected with biotin-labeled P5 as solid-phase antibodies.

Table 1.
Screening of paired antibodies.
3.3. Specific Evaluation of Paired Antibody
Using P1, P3, P4 and P6 as solid-phase antibodies and the intermediates SOD, sIKR, IKR and IK digested by PPI and P5 as antigens, we determined the specificity of each paired antibody (Figure 3A). The results showed that when P3 was used as the solid-phase antibody, the absorbance value of the blank control was the highest, and the paired antibody did not react with each antigen; when P4 was used as the solid-phase antibody, the paired antibody could react with PPI, sIKR and IKR, and the specificity was poor; when P1 was used as the solid-phase antibody, the paired antibody only reacted with PPI, but the absorptivity value was only 0.293; when P6 was used as the solid-phase antibody, the paired antibody produced immune responses to both PPI and sIKR, and the absorbance value of sIKR was much higher than that of PPI (0.58).
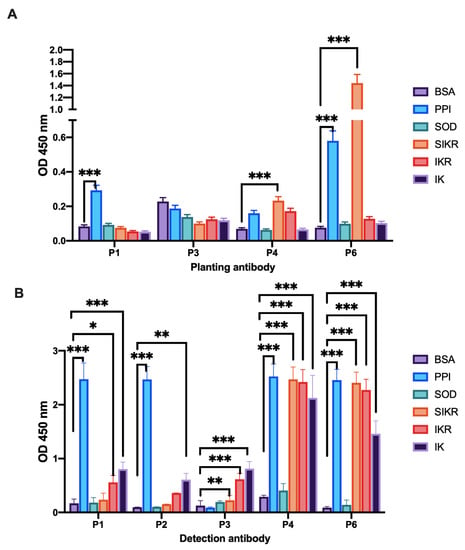
Figure 3.
Specificity evaluation of paired antibody. (A) Plating antibodies are listed below the X-axis, detection antibodies is P5. (B) Plating antibodies is P5, detection antibodies are listed below the X-axis. * p < 0.05; ** p < 0.01; *** p < 0.001.
Then, P5 was used as the solid-phase antibody, and PPI and its digested intermediates SOD, IK, IKR and sIKR were used as antigens. P1, P2, P3, P4 and P6 were used as detection antibodies to determine the specificity of each paired antibody (Figure 3B). The results showed that the paired antibodies were less specific when P4 and P6 were used as detection antibodies, and that they were immunoreactive with PPI, sIKR, IKR and IK and had similar absorbance values. When P3 is used as the detection antibody, IK has the highest absorbance value and does not immunoreact with PPI. However, when P1 and P2 were used as detection antibodies, they were positive for the paired antibodies IKR and IK, with absorbance values of 0.56 and 0.81 measured for P1 and P2 against IKR, respectively. These results show that the absorbance values of P1 and P2 are much lower than the PPI absorbance values for the IKR and IK reactions, and therefore do not interfere with the PPI reactions. Therefore, we used P5 as the solid phase antibody and P2 as the detection antibody in our subsequent experiments.
3.4. Construction of Quantitative Curves
With P5 as the solid-phase antibody, PPI standard solutions with a series of concentrations were added, and biotin-labeled P2 was used as the detection antibody to determine PPI by double-antibody sandwich ELISA. Using OD 450 nm and PPI concentration as vertical and horizontal coordinates, respectively, four-parameter fitting was carried out, and the standard curve was drawn (Figure 4). We obtained the fitting curve equation as follows: y = 7.92579 + [(0.130957 − 7.92579)/(1 + (x/96.6272) ^1.51852)] (R2 = 1). The detection method had a linear range of 0.645–82.5 pg/mL.
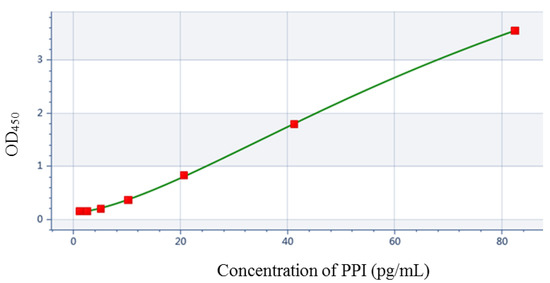
Figure 4.
Quantitative curve of PPI.
3.5. Accuracy and Sensitivity Evaluation of PPI Method for Double Antibody Sandwich ELISA
In order to test the recovery of the quality control samples, three concentrations of high (41.25 pg/mL), medium (10.31 pg/mL) and low (2.58 pg/mL) samples were tested and the results are shown in Table 2. The results showed that the PPI concentration measured in 1 μg/mL of recombinant human insulin solution was 0.638 pg/mL and the recoveries obtained for the high, medium and low-quality control samples were 91.4%, 88.62% and 95.41%, respectively, which indicated that the established method was accurate.

Table 2.
Recoveries of PPI from the samples fortified with different amounts of PPI.
According to the method of sensitivity determination, the OD450 values of 12 blank samples, i.e., sample dilutions, were determined. The average value obtained by adding three times standard deviation was 0.172, and the minimum detection limit was calculated to be 3.06 pg/mL, meeting the requirement of PPI detection sensitivity in recombinant human insulin.
4. Discussion
Diabetes is a chronic and metabolic disease that occurs when the pancreas does not produce enough insulin or when the body is unable to use the insulin produced effectively []. When diabetes is left uncontrolled or poorly controlled over time, it can lead to increased atherosclerosis throughout the body, resulting in atherosclerotic disease. At present, there is no eradication of diabetes, but there are various treatments that can be used to help control it. Medication is divided into two categories: the first is oral medication, including sulphonylureas, biguanides, alpha glucosidase inhibitors, insulin sensitizers and glargine insulin promoters; the second is insulin therapy. Insulin preparations include animal insulin, human insulin and insulin analogues []. The disadvantages of drug treatment, however, are the development of resistance and weight gain. In addition to the use of medication, exercise therapy is more recommended and can increase physical activity to improve the body’s sensitivity to insulin. In addition, diet is the basis of treatment for all types of diabetes, and some patients can control mild diabetes with diet alone []. When patients with uropathy are unable to control their blood sugar through diet, exercise and other medications, or when medications cannot be used, then insulin therapy is necessary. Insulin precursors are produced in the synthesis of recombinant insulin and the precursor proteins inevitably trigger immunogenic interference with the effectiveness of insulin therapy. Therefore, the detection and quality control of insulin precursors is necessary and important. To address this issue, we have developed an ELISA method for the rapid and highly sensitive detection of insulin precursors.
In all of European pharmacopeia, US pharmacopeia and Chinese pharmacopeia, to control the production process of insulin precursors was proposed, indicating that detecting insulin precursors has become an indispensable link in process control and final product release. The process control is mostly performed by a proper HPLC method []. However, because of the low content of precursor material in the final product, and the high sensitivity of the detection method, detecting insulin precursors in the product release control is difficult for each enterprise. Both insulin and insulin analogues are recombinantly expressed as insulin precursors and are obtained by enzymatic purification. For the determination of insulin precursors, the US Pharmacopoeia sets a limit of detection (10 ng/mg) but does not give a specific method, whereas European Pharmacopoeia recommends the use of radioimmunoassay (10 ppm), but this method requires high instrumentation and radioactive contamination. Sulena et al. used the RP-HPlC method for the determination of insulin precursors, but the low sensitivity clearly does not meet the requirements of the relevant [].
Enzyme-linked immunosorbent assays have high sensitivity, but they are mostly used to detect natural pro-insulin in serum [,]. There are many kinds of intermediate products and similar molecular structures when the precursor substances in insulin are detected, easily leading to cross reactions; in addition, the high concentration of insulin in the detection samples tends to interfere with the detection results. In this study, the purified insulin precursor was collected in the production process of recombinant human insulin in a Hefei Tianmai biology company as an antigen to prepare monoclonal antibodies.
In order to establish a double antibody sandwich ELISA for the detection of insulin precursors, the monoclonal antibodies obtained after purification were screened against each other to obtain the best antibody pairing, and subsequently, the specificity of the paired antibodies was assayed to screen out the antibody combinations with good specificity. In addition, the accuracy and sensitivity of the method were verified. Compared with the sensitivity of the method established by Leng et al., our method was significantly improved; the insulin interference was excluded in the accuracy verification [], indicating that we initially established a promising detection method for insulin precursors.
The insulin precursors used in this study were expressed in the form of fusion proteins. The antibodies obtained from their screening as antigens and the established double antibody sandwich ELISA assay cannot be applied to the insulin precursors produced by different production processes, but this can be used as a reference for further research and the establishment of ELISA assays for insulin precursors of different production processes.
5. Conclusions
In summary, to address a series of problems such as difficulties in the detection of insulin precursors, unstable methods and poor sensitivity in the current production of bio-recombinant human insulin, we developed a double antibody sandwich ELISA method for the efficient detection of human insulin precursors by preparing high quality and high purity antibodies to insulin precursor proteins. Our results show that the method can not only improve the sensitivity of the assay, but also precisely exclude the interference of insulin on the accuracy of the assay, which can be used as a more ideal assay in the quality control of human recombinant insulin manufacturing, providing assurance for the efficacy and safety of human recombinant insulin products.
Author Contributions
P.W. and Z.Z. (Zhiming Zheng) conceived the idea and supervised the research. Z.Z. (Zhu Zhu) and H.W. designed and performed the experiments, analyzed and interpreted data, performed statistical analysis and wrote the manuscript. L.W. and Z.W. provided technical assistance in the experiments. All authors have read and agreed to the published version of the manuscript.
Funding
This work was sponsored by financial support from the Major Projects of Science and Technology of Anhui Province (202103a06020003), China National Key Research and Development Program (2019YFA0904300 and 2019YFA0904304).
Institutional Review Board Statement
Animal experiments were approved by the Animal Care and Use Committee, Hefei Institutes of Physical Science, Chinese Academy of Sciences (Number: DW-2020-36).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to a partner wanting to continue the research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mathieu, C.; Martens, P.-J.; Vangoitsenhoven, R. One Hundred Years of Insulin Therapy. Nat. Rev. Endocrinol. 2021, 17, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Wondmkun, Y.T. Obesity, Insulin Resistance, and Type 2 Diabetes: Associations and Therapeutic Implications. Diabetes Metab. Syndr. Obes. Target. 2020, 13, 3611–3616. [Google Scholar] [CrossRef] [PubMed]
- Ruttenberg, M.A. Human Insulin: Facile Synthesis by Modification of Porcine Insulin. Science 1972, 177, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, A.; Duvoor, C.; Dendi, V.S.R.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical Review of Antidiabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front. Endocrinol. 2017, 8, 6. [Google Scholar] [CrossRef]
- Patil, N.H.; Devarajan, P.V. Colloid and Interface Science in Pharmaceutical Research and Development; Elsevier: Amsterdam, The Netherlands, 2014; pp. 411–442. [Google Scholar] [CrossRef]
- Landgraf, W.; Germany, M.A.D.D.; Frankfurt, S.A.; Sandow, J.; Germany, P. Centre of Pharmacology, Johann-Wolfgang-Goethe University, Frankfurt/Main, Recombinant Human Insulins–Clinical Efficacy and Safety in Diabetes Therapy. Eur. Endocrinol. 2016, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Govender, K.; Naicker, T.; Lin, J.; Baijnath, S.; Chuturgoon, A.A.; Abdul, N.S.; Docrat, T.; Kruger, H.G.; Govender, T. A Novel and More Efficient Biosynthesis Approach for Human Insulin Production in Escherichia coli (E. coli). Amb. Express. 2020, 10, 43. [Google Scholar] [CrossRef]
- Bhoria, S.; Yadav, J.; Yadav, H.; Chaudhary, D.; Jaiwal, R.; Jaiwal, P.K. Current Advances and Future Prospects in Production of Recombinant Insulin and Other Proteins to Treat Diabetes Mellitus. Biotechnol. Lett. 2022, 44, 643–669. [Google Scholar] [CrossRef]
- Luo, Z.; Fu, J.P.; Li, N.; Liu, Z.X.; Qin, T.; Zhang, X.L.; Nie, P. Immunogenic Proteins and Their Vaccine Development Potential Evaluation in Outer Membrane Proteins (OMPs) of Flavobacterium Columnare. Aquac. Fish. 2016, 1, 1–8. [Google Scholar] [CrossRef]
- Riggs, A.D. Making, Cloning and Expression of Human Insulin Genes in Bacteria: The Path to Humulin. Endocr. Rev. 2020, 42, bnaa029. [Google Scholar] [CrossRef]
- Zieliński, M.; Romanik-Chruścielewska, A.; Mikiewicz, D.; Łukasiewicz, N.; Sokołowska, I.; Antosik, J.; Sobolewska-Ruta, A.; Bierczyńska-Krzysik, A.; Zaleski, P.; Płucienniczak, A. Expression and Purification of Recombinant Human Insulin from E. coli 20 Strain. Protein. Expres. Purif. 2019, 157, 63–69. [Google Scholar] [CrossRef]
- Asai, S.; Žáková, L.; Selicharová, I.; Marek, A.; Jiráček, J. A Radioligand Receptor Binding Assay for Measuring of Insulin Secreted by MIN6 Cells after Stimulation with Glucose, Arginine, Ornithine, Dopamine, and Serotonin. Anal. Bioanal. Chem. 2021, 413, 4531–4543. [Google Scholar] [CrossRef] [PubMed]
- Hazra, P.; Sreenivas, S.; Venkatesan, K.; Patale, M.B.; Chatterjee, A.; Ramprabu, N.; Shaikh, A.M.; Kusumanchi, M. A Novel Peptide Design Aids in the Expression and Its Simplified Process of Manufacturing of Insulin Glargine in Pichia Pastoris. Appl. Microbiol. Biot. 2021, 105, 3061–3074. [Google Scholar] [CrossRef] [PubMed]
- Siew, Y.Y.; Rai, A.; Pek, H.B.; Ow, D.S.-W.; Zhang, W. New and Efficient Purification Process for Recombinant Human Insulin Produced in Escherichia Coli. Appl. Microbiol. Biot. 2021, 105, 9137–9151. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Prinyawiwatkul, W.; Xu, Z. Insulin: A Review of Analytical Methods. Analyst 2019, 144, 4139–4148. [Google Scholar] [CrossRef]
- Kaki, S.B.; Prasad, A.N.; Chintagunta, A.D.; Dirisala, V.R.; Kumar, N.S.S.; Naidu, S.J.K.; Ramesh, B. Industrial Scale Production of Recombinant Human Insulin Using Escherichia Coli BL-21. Iran. J. Sci. Technol. Trans. Sci. 2022, 46, 373–383. [Google Scholar] [CrossRef]
- Association, A.D. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2006, 29, s43–s48. [Google Scholar] [CrossRef]
- Harrigan, R.A.; Nathan, M.S.; Beattie, P. Oral Agents for the Treatment of Type 2 Diabetes Mellitus: Pharmacology, Toxicity, and Treatment. Ann. Emerg. Med. 2001, 38, 68–78. [Google Scholar] [CrossRef]
- Klein, S.; Burke, L.E.; Bray, G.A.; Blair, S.; Allison, D.B.; Pi-Sunyer, X.; Hong, Y.; Eckel, R.H.; Metabolism, A.H.A.C. on N., Physical Activity, and Clinical Implications of Obesity with Specific Focus on Cardiovascular Disease. Circulation 2004, 110, 2952–2967. [Google Scholar] [CrossRef]
- Siew, Y.Y.; Zhang, W. Downstream Processing of Recombinant Human Insulin and Its Analogues Production from E. Coli Inclusion Bodies. Bioresour. Bioprocess. 2021, 8, 65. [Google Scholar] [CrossRef]
- Polez, S.; Origi, D.; Zahariev, S.; Guarnaccia, C.; Tisminetzky, S.G.; Skoko, N.; Baralle, M. A Simplified and Efficient Process for Insulin Production in Pichia Pastoris. PLoS ONE 2016, 11, e0167207. [Google Scholar] [CrossRef]
- Ramaswamy, S.G.; Nayak, V.G.; Jha, S.K.; Hegde, V.; Waichale, V.S.; Melarkode, R.; Chirmule, N.; Rao, A.U.; Sengupta, N. Development and Validation of an Electrochemiluminescent ELISA for Quantitation of Oral Insulin Tregopil in Diabetes Mellitus Serum. Bioanalysis 2017, 9, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.; Lee, E.-J.; Ahn, S.M.; Kim, G.; Lee, S.H.; Ji, D.-Y.; Kang, J.-T. Production of Genetically Modified Pigs Expressing Human Insulin and C-Peptide as a Source of Islets for Xenotransplantation. Transg. Res. 2019, 28, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Leng, C.; Li, Q.; Wu, F.; Chen, L.; Su, P. Detection of the Single-Chain Precursor in the Production and Purification Process of Recombinant Human Insulin. Monoclon. Antibod. Immunodiagn. Immunother. 2013, 32, 255–261. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).